Dynein-mediated Vesicle Transport Controls Intracellular Salmonella Replication (original) (raw)
Abstract
Salmonella typhimurium survives and replicates intracellular in a membrane-bound compartment, the _Salmonella_-containing vacuole (SCV). In HeLa cells, the SCV matures through interactions with the endocytic pathway, but Salmonella avoids fusion with mature lysosomes. The exact mechanism of the inhibition of phagolysosomal fusion is not understood. Rab GTPases control several proteins involved in membrane fusion and vesicular transport. The small GTPase Rab7 regulates the transport of and fusion between late endosomes and lysosomes and associates with the SCV. We show that the Rab7 GTPase cycle is not affected on the SCV. We then manipulated a pathway downstream of the small GTPase Rab7 in HeLa cells infected with Salmonella. Expression of the Rab7 effector RILP induces recruitment of the dynein/dynactin motor complex to the SCV. Subsequently, SCV fuse with lysosomes. As a result, the intracellular replication of Salmonella is inhibited. Activation of dynein-mediated vesicle transport can thus control intracellular survival of Salmonella.
INTRODUCTION
Salmonella typhimurium species are gram-negative facultative intracellular pathogens. Salmonella causes diseases ranging from gastroenteritis to typhoid fever, depending on the serotype and the host. In epithelial cells, Salmonella survives and replicates in a membrane-bound compartment, the _Salmonella_-containing vacuole (SCV) (Meresse et al., 1999b). Intracellular replication starts after a lag phase of ∼4 h (Garcia-del Portillo et al., 1993). The SCV acquires the vacuolar ATPase pump and lysosomal glycoproteins, such as LAMP-1 (Meresse et al., 1999a; Steele-Mortimer et al., 1999). In contrast to phagosomes containing latex beads (Desjardins et al., 1994; Garcia-del Portillo and Finlay, 1995), the SCV segregates from the normal endocytic pathway and does not acquire the mannose-6-phosphate receptor and lysosomal hydrolases such as cathepsin D and L (Garcia-del Portillo and Finlay, 1995; Meresse et al., 1999a). By avoiding fusion with mature cathepsin D- and L-containing lysosomes, Salmonella is probably protected from degradation (Meresse et al., 1999b). Prevention of fusion with mature lysosomes depends on the ability of the pathogen to modulate the interaction of the phagosome with elements of the host's endocytic pathway (Holden, 2002).
Members of the Rab family of small GTPases are regulators of the host endocytic pathway. Numerous Rab proteins have been described, and each Rab member localizes to a specific compartment (Stenmark and Olkkonen, 2001). By interacting with one or more effector proteins, Rab proteins regulate specific downstream functions such as membrane transport and fusion (Novick and Zerial, 1997; Somsel Rodman and Wandinger-Ness, 2000). For example, Rab5 regulates fusion between early endosomes and motility of these compartments along microtubules (Gorvel et al., 1991; Barbieri et al., 1994). Rab7 acts more downstream in the endocytic pathway, regulating transport to late endosomes (Feng et al., 1995; Press et al., 1998) and regulating transport of and fusion between late endosomes and lysosomes (Meresse et al., 1995; Vitelli et al., 1997; Bucci et al., 2000). In HeLa cells, Rab7 interacts with the SCV and is involved in its maturation (Meresse et al., 1999a).
We show that the Rab7 GTPase cycle is identical on late endocytic structures and the SCV by using fluorescent recovery after photobleaching (FRAP) experiments. Apparently, the mere presence of Rab7 is not sufficient to regulate fusion between mature lysosomes and the SCV in HeLa cells. We have shown previously that the Rab7 effector RILP (Cantalupo et al., 2001; Jordens et al., 2001) recruits the minus-end–directed motor complex dynein/dynactin to late endosomal/lysosomal compartments, thereby facilitating their transport toward the microtubule organizing center (Jordens et al., 2001). RILP and the dynein motor also have been implicated in the regulation of fusion between latex-beads containing phagosomes and lysosomes (Harrison et al., 2003). Moreover, the dynein motor transports Chlamydiae inclusions, although it is unclear how this is regulated (Grieshaber et al., 2003).
Here, we studied whether manipulation of motor-mediated transport of the SCV to lysosomes during the early phase of infection can modulate intracellular replication. Therefore, the Rab7 effector RILP was expressed during the lag phase of Salmonella infection. Expression of RILP induced the recruitment of the minus-end motor dynein to the SCV. Subsequently, SCV were rapidly transported toward the minus-end of microtubules where they fused with lysosomes. As a result, the intracellular replication of Salmonella in HeLa cells was impaired. For the first time, a motor protein (dynein) is implemented in the control of intracellular growth of a pathogen, Salmonella.
EXPERIMENTAL PROCEDURES
DNA Constructs
Wild-type and mutant Rab7 cDNA, a kind gift from P. Chavier (Meresse et al., 1995; Meresse et al., 1999a), were subcloned into pcDNA3 (Invitrogen, Carlsbad, CA) with an N-terminal myc-tag for immunodetection. To generate the green fluorescent protein (GFP)-tagged constructs, the ATG start codon of RILP, RILP-ΔN, and Rab7 were eliminated by polymerase chain reaction, and the fragments were cloned into pEGFP-C1 (BD Biosciences Clontech, Palo Alto, CA). cDNA encoding p50dynamitin (Echeverri et al., 1996) and RILP-ΔN were subcloned in the eukaryotic expression vector pCMVβ with an N-terminal VSV-tag as described previously (Wubbolts et al., 1999). cDNA encoding GFP-p50dynamitin was a kind gift from C. Hoogenraad (Hoogenraad et al., 2001; Matanis et al., 2002). RILP was subcloned into the eukaryotic expression vector pcDNA3 (Invitrogen) with a C-terminal myc-tag. All constructs were sequence verified.
Cell Culture
HeLa cells were maintained in Iscoves medium (Invitrogen) supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were transiently transfected with GFP-Rab7 by using Genejammer transfection reagent according to the manufacturer's protocol (Stratagene, La Jolla, CA). Briefly, 4 μg of DNA and 24 μl of Genejammer reagent were used to transfect 3 × 106 cells. Fresh medium was added after 24 h and the cells were further incubated for at least 24 h before infection.
Bacterial Strains, Growth Conditions, and Infections
The mouse virulent strains S. typhimurium SL1344, GFP-S. typhimurium SL1344, and GFP-S. typhimurium 12023 (a kind gift from S. Meresse; Meresse et al., 1999a) were used. Bacteria were grown in Luria-Bertani (LB) media at 37°C in 5% CO2. HeLa cells were seeded on glass coverslips at a density of 6 × 106 cells/10-cm dish or 3 × 106 cells/6-cm dish. Cells were cultured at 37°C in 5% CO2 for 48 h in Iscoves media without penicillin/streptomycin. Bacteria were grown overnight at 37°C while shaking, subcultured at a dilution of 1:33 in fresh LB media, and incubated at 37°C while shaking for 3.5 h. Cells were infected with 100 bacteria/cell (unless otherwise indicated) in Iscoves media without antibiotics for 30 min at 37°C in 5% CO2. Infected cells were washed four to six times and incubated for 1 h in media containing 50 μg/ml gentamicin (Invitrogen). Infected cells were subsequently incubated for the indicated time points in media containing 10 μg/ml gentamicin.
Microinjection
Infected cells seeded on glass coverslips at a confluence of 30–60% were used. Cells were injected on a heated xy stage of an Olympus XL70 microscope equipped with an Eppendorf manipulator 5171/transjector 5246 system. Approximately 100–200 cells were injected with cDNA at a final concentration of 100–200 ng/μl (unless indicated otherwise) in microinjection buffer containing 120 mM K-glutamate, 40 mM KCl, 1 mM MgCl2, 1 mM EGTA, 200 μM CaCl2, 10 mM HEPES, 40 mM mannitol, pH 7.2. Fluorescein isothiocyanate (FITC)-Dextran 3000 mW 1 mg/ml (Molecular Probes, Eugene, OR) was used as a microinjection marker for live imaging. When indicated, H2B-GFP cDNA (100 ng/μl) was used as an injection marker. Cells were subsequently cultured at 37°C in 5% CO2 and analyzed at the indicated time points. Fixation was either in 3.7% formaldehyde for 15 min followed by permeabilization with 0.1% Triton X-100 or in methanol (-20°C) for 2 min. Fixed cells were immunostained with the indicated antibodies in phosphate-buffered saline + 0.5% bovine serum albumin (BSA) and analyzed by confocal laser scanning microscopy (CLSM).
Confocal Laser Scanning Microscopy
Confocal analysis was performed using a Leica TCS SP confocal laser scanning microscope equipped with an Argon/Krypton laser (Leica Microsystems, Heidelberg, Germany). Green fluorescence was detected at λ > 515 nm after excitation at λ = 488 nm. For dual analyses, green fluorescence was detected at 520–560 nm. Red fluorochromes were excited at λ = 568 nm and detected at λ > 585 nm. Triple analysis was performed using Texas-Red excited at λ = 568 nm and detected at 600–620 nm, and Cy5 was excited at λ = 633 and detected at λ > 660 nm. All experiments presented were repeated several times on different days, and results were consistent and reproducible. The results from different experiments were calculated and presented as mean percentage ± SE.
SCV Fusion with SR101 or DQ Red BSA-labeled Lysosomes
HeLa cells seeded on glass coverslips were labeled with sulforhodamine 101 (SR101: 50 μg/ml) (Molecular Probes) by a 30-min pulse at 37°C in 5% CO2. The labeling media were removed, and the cells were washed extensively and incubated for at least 2 h before infections were started, according to the protocol described using GFP-S. typhimurium SL1344. After incubation with 50 μg/ml gentamicin for 1 h, cells were cultured in media containing 10 μg/ml gentamicin. Infected cells were then microinjected with cDNA coding for RILP, and FITC-dextran was used as an injection marker. One hour after injection, fusion between SCV- and SR101-loaded lysosomes was studied using living cell analysis with time-lapse confocal microscopy. Fusion between SCV- and SR101-loaded lysosomes was followed in cells injected with RILP cDNA and control cells for ∼4 h. The protocol described above was also followed to study the interaction of the SCV with DQ Red BSA-positive vesicles. Except that DQ Red BSA was added to the cells before the imaging, ∼1 h after microinjection. DQ Red BSA is dequenched after proteolysis in lysosomes (DQ Red BSA 10 μg/ml) (Molecular Probes).
FRAP
FRAP experiments were performed as described previously (Reits et al., 2000; Jordens et al., 2001). Briefly, HeLa cells transiently expressing GFP-Rab7 were seeded on coverslips and infected. Approximately 24 h after infection, SCV containing elongated bacteria were bleached for 1 s by a high-intensity laser beam. Subsequently, the recovery of fluorescence in the bleached spot was quantified. As a control Rab7-positive late endosomes and lysosomes were simultaneously bleached in the same cell or in noninfected control cells. SCV and control vesicles were visualized by LysoTracker Red (Molecular Probes). SCV and control late endosomes and lysosomes with little lateral movement were selected. Because they were not completely stationary their position was tracked and corrected by a program written in Matlab (Mathworks, Natick, MA) and the half-time of fluorescence recovery (_t_1/2) was determined. The experiments were repeated several times on different days.
Isolation of the SCV
HeLa cells (2–3 × 107) cells infected with GFP-S. typhimurium SL1344 for 3 h or noninfected HeLa cells were washed four times with phosphate-buffered saline. Cells were harvested by scraping and diluted in 1 ml of homogenization buffer HB (20 mM 250 mM sucrose, Tris, pH 7.5, and a cocktail of protease inhibitors (Complete; Roche Diagnostics, Indianapolis, IN) and homogenized with a ball-bearing homogenizer (ball size 8.008). Nuclei and debris were removed by low-speed centrifugation (100 × g, 3 min). The resulting post-nuclear supernatant was layered on top of a stepwise sucrose gradient containing layers of 0.4/0.6/1.0/1.2/1.6/2.0 M sucrose prepared in HB. The samples were centrifuged in a Beckman SW 40 Ti for 2 h at 28,000 rpm at 4°C. Fractions of 0.5 ml were taken from the top, and proteins were recovered by trichloroacetic acid precipitation before analysis by 12% SDS-PAGE and Western blotting. The sucrose concentration of the fractions was measured after the run with a refractomer (PmT Tamson) and is indicated in the figure. Lysosomal fractions were determined by assaying for β-hexosaminidase activity in all sucrose gradient fractions. The position of LAMP-1, cathepsin D, RILP, and Rab7 were detected using specific antibodies. The position of the SCV was determined using anti-GFP antibodies.
Antibodies and Reagents
The following antibodies were used: rabbit polyclonal anti-CD63 (Vennegoor et al., 1985), mouse monoclonal anti-p150glued (BD Transduction Laboratories, Lexington, KY), mouse monoclonal p50dynamitin (BD Transduction Laboratories), rabbit polyclonal anti-Rab7 (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal anti-kinesin heavy chain (kind gift from R. Vallee, Columbia University, New York, NY), rabbit polyclonal anti-RILP (Jordens et al., 2001), rabbit polyclonal anti-GFP (van Ham et al., 1997). Mouse anti-LAMP-1 (BD Transduction Laboratories), goat polyclonal anti-cathepsin D (Santa Cruz Biotechnology), mouse anti-EEA1 (BD Transduction Laboratories), and mouse monoclonal anti-myc (9E10). Rabbit polyclonal anti-S. typhimurium lipopolysaccharide (LPS) (Difco, Detroit, MI) and mouse monoclonal 1E6 anti-S. typhimurium LPS (Biodesign International, Kennebunk, ME). FITC- and Texas Red (Molecular Probes)-conjugated mouse and rabbit secondary antibodies were used.
RESULTS
The SCV Recruits Functional Rab7 in HeLa Cells
S. typhimurium strains 12023 and SL1344 were used to study the intracellular fate of Salmonella. The S. typhimurium SL1344 strain forms elongated bacteria when replicating, due to a lack of septation in some cell types (Martinez-Lorenzo et al., 2001). These elongated bacteria reside in a membrane-bound vacuole, called the SCV, with the same characteristics as for other Salmonella strains. Although they acquire late-endosomal/lysosomal markers such as LAMP-1 and CD63, they exclude hydrolases that reside in mature lysosomes, protecting them from degradation (Martinez-Lorenzo et al., 2001). The intracellular formation of elongated bacteria was used to visualize intracellular bacterial replication.
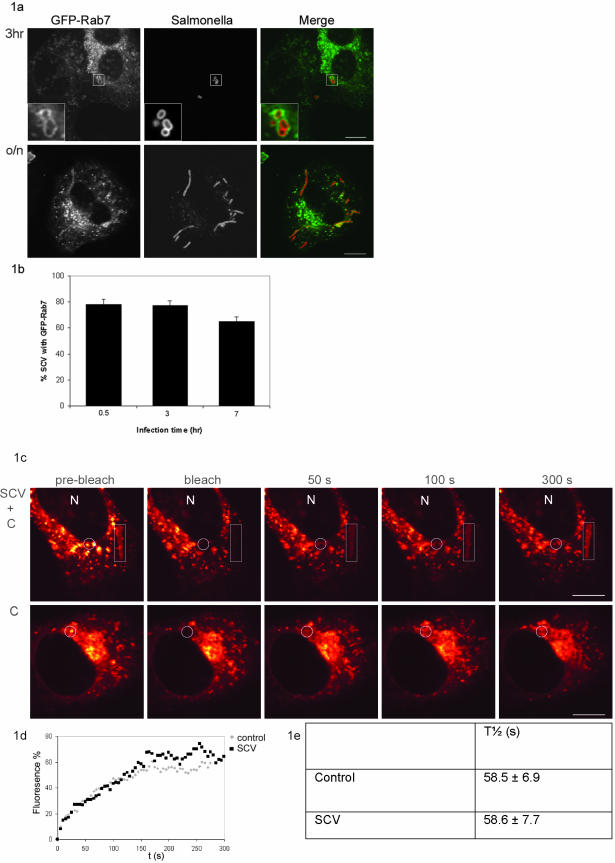
To study the interaction of Rab7 with the SCV, HeLa cells were transiently transfected with GFP-Rab7. At various time points after infection, the interaction between Rab7 and the SCV was visualized by CLSM and subsequently quantified. Colocalization of GFP-Rab7 with the SCV was already observed 30 min after infection. Figure 1a shows a representative image 3 h after infection. Approximately 80% of the SCV recruited detectable amounts of GFP-Rab7 at all indicated time points (Figure 1b) with ∼70% at time point 7 h. The elongated bacteria were detected after overnight culture and accumulated GFP-Rab7 in 70–90% of SCV (Figure 1a; our unpublished data). This indicates that Rab7 is present at the membrane of SCV. This was further confirmed using the active GTPase-deficient mutant of Rab7, Rab7Q67L. Rab7Q67L was recruited to the membrane of the SCV containing elongated bacteria. Expression of this mutant did not interfere with intracellular replication of the bacteria because elongated bacteria were still formed after o/n culture (our unpublished data).
Figure 1.
SCV recruits functional Rab7. (A) Confocal fluorescence analysis of HeLa cells transiently transfected with GFP-Rab7 and infected with S. typhimurium SL1344. The SCV recruited GFP-Rab7 after 30 min of infection. Representative confocal images at 3 h and after o/n culture are shown. The insets in the top panels show a coat of GFP-Rab7 on the SCV that is representative for the accumulation of Rab7 observed at all time points. The majority of the SCV contained elongated bacteria after o/n culture, still colocalizing with GFP-Rab7 (bottom). Left, GFP-Rab7; middle, anti-Salmonella LPS; and right, merge. Bar, 10 μm. (B) Quantification of three experiments for colocalization of the SCV with GFP-Rab7. Values are given as mean percentage of colocalization ± SE. t = 0.5 h is the time point immediately after the 30 min infection (for each time point 100 cells were counted). (C) To determine whether the Rab7 recruited to the SCV is functional, the GTPase cycle of Rab7 was studied using FRAP. Representative examples of GFP-Rab7 cells are shown in a glow-over/under representation. Images are shown before bleach (prebleach), immediately after bleach and at three time points after bleach. The bleached area and the nucleus (N) are indicated. Top, SCV indicated with a rectangle and control late endocytic structures in the circle were bleached. Bottom, noninfected control cell was bleached in the circle. Bar, 10 μm. (D) Representative curves of fluorescent recovery in the bleached spot/area. The fluorescence (percentage) was related to the initial fluorescence set at 100%. t = 0 s is the first image after the bleach. (E) Quantification of the recovery time (_t_1/2) deduced from the curves including the SE. There is no difference between the _t_1/2 of SCV and late endocytic structures (control) even within the same cell. (F) Sucrose gradient fractionation profile of vesicles from cells infected with GFP-S. typhimurium SL1344. Lysosomes were detected by β-hexosaminidase activity and mature cathepsin D staining. The density (molar concentration sucrose) of the corresponding fractions measured with a refractomer is indicated (top). The localization of the SCV was determined with anti-GFP antibodies. LAMP-1, Rab7, and RILP localization were analyzed with specific antibodies. Bottom, corresponding Western blots for LAMP-1, RILP, Rab7, and GFP. The localization of the soluble fraction, lysosomes, and the SCV are indicated.
Small GTPases such as Rab7 cycle between an active membrane-bound and an inactive mainly cytosolic state. We previously used FRAP to visualize the Rab7 cycle in GFP-Rab7–expressing cells. After bleaching the fluorescence of a small portion of vesicles, the recovery of fluorescence in this bleached spot was followed by time-lapse CLSM. Recovery of fluorescence can only occur when membrane-bound bleached GFP-Rab7 dissociates from the membrane and is replaced by fluorescent GFP-Rab7 from the cytosol. Therefore, the fluorescence recovery in the bleached spot represents the Rab7 activation cycle (Jordens et al., 2001).
FRAP was used to study the Rab7 cycle on the SCV and to determine whether Salmonella alters the Rab7 activation cycle on the SCV. HeLa cells were transiently transfected with GFP-Rab7, and ∼24 h after infection the SCV containing elongated bacteria were bleached. Representative images before and after the bleach are shown in Figure 1c. Recovery of fluorescence was plotted in a recovery curve (Figure 1d). From the recovery curve, the recovery time (_t_1/2, the time in which 50% of the fluorescence was recovered in the bleached spot) of the SCV was determined. Vesicles and elongated structures were always completely bleached to prevent recovery of fluorescence by lateral diffusion of GFP-Rab7 on the membrane of the same vesicle or elongated structures. Because lateral diffusion is considerably faster than the normal Rab7 cycle, partial bleaching could be easily distinguished from complete bleaching of the structures (our unpublished data).
No difference was observed in the cycle time between late endocytic structures in infected cells and in noninfected cells (Figure 1e, control). The cycle time of GFP-Rab7 on late endocytic structures was 58.5 ± 6.9 s; the SCV showed a recovery time of 58.6 ± 7.7 s (Figure 1e). These data indicate that the Rab7 cycle is comparable between SCV and late endocytic compartments, even within the same cell. Thus, Salmonella does not alter the Rab7 GTPase cycle on the SCV.
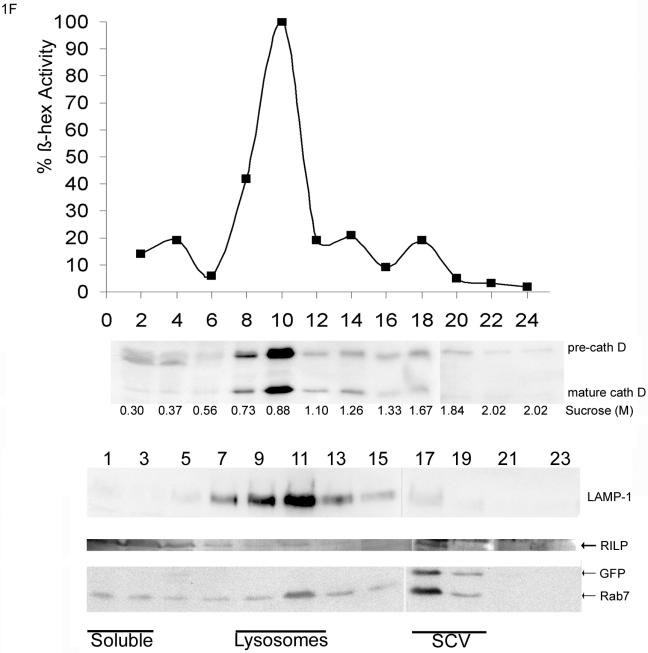
To test whether the Rab7 effector RILP could be recruited to SCV in HeLa cells, subcellular fractions were analyzed (Figure 1f). HeLa cells infected with GFP-S. typhimurium SL1344 or noninfected were homogenized, and the post-nuclear supernatant was separated by sucrose density centrifugation. Fractions were subsequently taken from top and analyzed by SDS-PAGE and Western blotting. The position of the early endosomes was determined by immunostaining for EEA1. EEA1 migrated in fraction 1–5 (our unpublished data). The position of the lysosomes was determined by β-hexosaminidase activity. Lysosomes were detected around fraction 8–11 in both control (our unpublished data) and infected cells (Figure 1f); mature cathepsin D also comigrated in these fractions. The position of the SCV was determined by immunostaining with anti-GFP. SCV run at a higher density than late endocytic structures around fraction 17–19. Mostly immature cathepsin D (precath D) comigrated with the SCV. Rab7 comigrated with cathepsin D and LAMP-1 in the late endocytic fraction (fraction 11), but also comigrated at a higher density with the SCV. A major portion of RILP was present in the soluble fraction (top fractions 1–5) and a small portion comigrated with Rab7 in the late endocytic fractions and with Rab7 in the SCV-containing GFP fractions. These data suggest that the SCV, like late endocytic structures, sequester both Rab7 and RILP but mainly contain the immature form of cathepsin D.
The Rab7 Effector RILP Blocks Replication of Salmonella in HeLa Cells
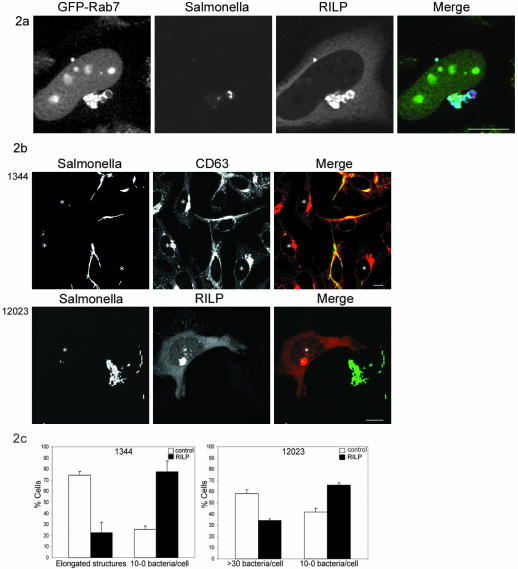
Although Rab7 and RILP are both present on late endocytic structures, overexpression of RILP is required for efficient dynein motor recruitment to these compartments (Jordens et al., 2001). To test whether this is also occurring for the SCV and whether this may change the fate of intracellular Salmonella, we overexpressed RILP at several time points after infection by microinjection of RILP cDNA. Intracellular Salmonella replication was followed by the formation of elongated structures for the SL1344 strain. Ectopically expressed RILP was sequestered to the SCV where it colocalized with Rab7 (Figure 2a). When RILP was expressed 2–4 h after Salmonella uptake, before bacterial replication had started, a marked reduction in Salmonella replication was observed after o/n culture. RILP expression resulted in less elongated bacteria and more single bacteria per cell (Figure 2b).
Figure 2.
Expression of RILP results in a replication block of Salmonella. (A) Confocal analysis of infected cells expressing GFP-Rab7 microinjected with RILP and H2B-GFP. From left to right, GFP-Rab7 and H2B-GFP, Salmonella SL1344 labeled with anti-LPS-Texas Red, RILP, and the merge. Bar, 10 μm. (B) Confocal analysis of cells infected with either SL1344 (top) or the 12023 Salmonella strain (bottom) and microinjected with RILP. Cells microinjected with RILP cDNA (and H2B-GFP cDNA as injection marker) show clustering of late endosomes and lysosomes. In these cells, the elongated bacteria are not formed after o/n incubation (top). Note that elongated bacteria occurred in control cells. Microinjected cells are indicated with an asterisk. Left, H2B-GFP and Salmonella (stained with anti-LPS) are shown. Middle, CD63 staining. Right, merge. Bar, 10 μm. Bottom, cells infected with the Salmonella 12023 strain. Microinjected cells are indicated with an asterisk. Left, Salmonella 12023 strain. Middle, RILP. Right, merge. Bar, 10 μm. (C) Quantification of five individual experiments (n = 30–40 microinjected cells and n = 100 control cells). Left, mean percentage of SCV-containing elongated bacteria or 10–0 single bacteria are indicated ± SE for both RILP and control cells infected with the Salmonella SL1344 strain. Right, SCV containing >30 bacteria/cell or 10–0 single bacteria/cell are indicated ± SE for both RILP and control cells infected with the Salmonella 12023 strain.
Quantification of the confocal images of five individual experiments showed that 75% of the SCV contained elongated bacteria, which was reduced to 23% when RILP was expressed (Figure 2c). Seventy-seven percent of the RILP-expressing cells contained a small number of single bacteria per cell (10–0 bacteria/cell), compared with 25% in control cells not ectopically expressing RILP (Figure 2c). Timing of RILP expression was critical because expression of RILP 6–8 h or longer after infection resulted in SCV containing elongated bacteria, as observed for control cells (our unpublished data). This suggests that RILP should be expressed before Salmonella starts replicating and segregates from the endocytic pathway. As a control, microinjection of H2B-GFP cDNA did not effect the intracellular replication (our unpublished data).
A significant reduction also could be observed when the S. typhimurium 12023 strain was used. This strain can replicate normally inside HeLa cells, although the high load of intracellular bacteria can lead to apoptosis in a subset of the cells. Reducing the mode of infection to <50 bacteria per cell results in a heterogenic population after overnight incubation, with the majority of the cells containing replicating bacteria (Figure 2c). When RILP was expressed the number of cells containing >30 bacteria per cell decreased by one-half. The number of cells containing <10 bugs per cell increased as a result of RILP expression, indicating that bacterial replication was also reduced in cells infected with the S. typhimurium 12023 strain and expressing RILP (Figure 2, b and c). It should be noted that our experimental protocol does not allow quantification of the RILP effect by plating assays, because RILP had to be expressed in a rather narrow window of time (2–4 h after infection), which could only be achieved by microinjection of cDNA.
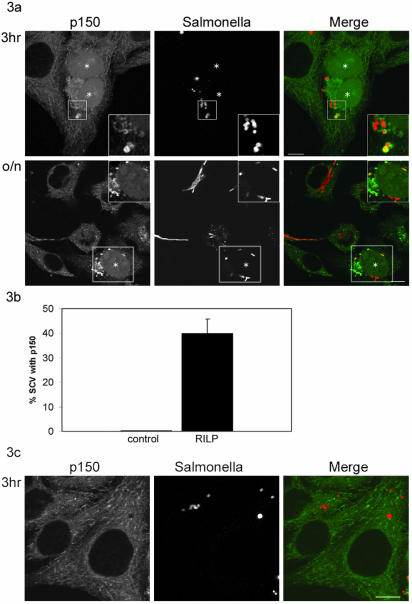
SCV Recruits the Minus-End–directed Dynein Motor after RILP Expression
To examine whether the dynein motor also could be recruited to the SCV following RILP expression, the interaction between the endogenous minus-end–directed motor dynein and the SCV was analyzed. Dynein recruitment was visualized by immunolabeling cells with antibodies recognizing p150glued, a subunit of the dynein/dynactin complex. Figure 3a shows that p150glued was recruited to the SCV upon RILP expression. Note that in some cases the SCV was more intensively labeled with p150glued than late endocytic structures in the same cell. No significant difference in motor recruitment was observed between cells expressing RILP for 1, 3, and 7 h or o/n. In all cases, the p150glued motor subunit could be detected on ∼40% of the SCV (Figure 3b). Importantly, p150glued was never observed on the SCV in control cells (Figure 3c; representative image 3 h after infection).
Figure 3.
The SCV recruits the minus end motor dynein. (A) Confocal analysis of cells immunolabeled for the dynein/dynactin subunit p150glued. The images presented are representative for the colocalization of p150glued and the SCV at time points 1, 3, and 7 h and o/n after infection. At all time points a significant colocalization was observed in the cells microinjected with cDNA encoding RILP (and H2B-GFP cDNA as injection marker), whereas no colocalization was observed in control cells. Insets (top) show the area of the microinjected cell depicted in the box. After o/n incubation after infection, the control cells showed formation of the elongated bacteria. RILP-expressing cells contained a small number of single bacteria, which colocalized with p150glued (bottom). Insets of the microinjected cell are shown in the bottom panels. Injected cells are indicated with an asterisk. Left, p150glued and H2B-GFP. Middle, Salmonella. Right, merge. Bar, 10 μm. (B) Quantification of colocalization between the SCV and p150glued of six individual experiments 3 h after infection. These data represent 50 microinjected cells and 100 control cells. Mean ± SEs are indicated. (C) Confocal analysis of control cells immunolabeled with p150glued 3 h after infection. Left, p150glued. Middle, Salmonella. Right, merge. Bar, 10 μm.
Similar results were obtained with p50dynamitin, another subunit of the dynein/dynactin complex and with the Salmonella 12023 strain (our unpublished data). Recruitment of the opposite, plus-end motor kinesin could not be observed at any time point (our unpublished data). These data show that RILP specifically recruits the dynein/dynactin motor toward Rab7-containing compartments, including the SCV.
Dynein/Dynactin Motor Activity Inhibits Salmonella Replication
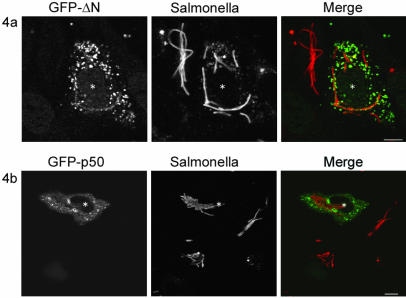
The C-terminal one-half of RILP (RILP-ΔN) binds to active GTP-bound Rab7 but cannot recruit the minus-end motor dynein (Jordens et al., 2001). Consequently, late endosomal/lysosomal compartments move toward the cell periphery. Thus, in contrast to RILP-expressing cells, in cells expressing GFP-RILP-ΔN the late endosomes and lysosomes are dispersed instead of clustered (Figure 4a). Importantly, when GFP-RILP-ΔN was expressed 2–4 h after infection, no reduction of elongated bacteria was observed after o/n incubation (Figure 4a). Instead the GFP-RILP-ΔN–expressing cells showed formation of elongated bacteria similar to control cells. These data indicate that the RILP-induced dynein motor recruitment inhibits Salmonella replication.
Figure 4.
Recruitment of functional dynein motor is necessary for the replication block. (A) Microinjection of GFP-RILP-ΔN cDNA results in dispersion of late endosomes/lysosomes and formation of elongated bacteria. Microinjected cells are indicated by an asterisk. Left, GFP-RILP-ΔN. Middle, Salmonella SL1344 stained with anti-LPS antibodies. Right, merge. Bar, 10 μm. (B) Disruption of the minus-end dynein motor can overcome the RILP-induced replication block. A 10-fold molar excess GFP-p50dynamitin cDNA was microinjected together with RILP cDNA, to uncouple RILP action from dynein motor recruitment. Confocal images show GFP-p50dynamitin–containing vesicles that relocated to the cell periphery. These cells contained elongated bacteria. Left, GFP-p50dynamitin. Middle, Salmonella SL1344. Right, merge. Bar, 10 μm.
We further investigated whether the recruited dynein/dynactin motor complexes were functionally involved in the inhibition of bacterial replication. Therefore, RILP expression was combined with inactivation of the dynein motor by overexpressing GFP-p50dynamitin by simultaneous microinjection of cDNA for both proteins. Overexpression of p50dynamitin dissociates the dynactin complex, thereby uncoupling the dynein motor from its cargo (Burkhardt et al., 1997). In cells coexpressing p50dynamitin and RILP, the p50dynamitin subunit of the dynein/dynactin motor complex is still recruited to late endosomes/lysosomes by RILP (Jordens et al., 2001) (Figure 4b).
Coexpression of RILP with excess GFP-p50dynamitin reversed the late endosomal/lysosomal clustering normally induced by RILP (Figure 4b). More importantly, the inhibitory effect of RILP on intracellular growth of Salmonella is reversed as well, and elongated structures are formed similar to control cells. GFP-p50dynamitin localized to the dispersed late endosomes and lysosomes, indicating that RILP was expressed as well. This was confirmed in a reciprocal experiment using GFP-RILP with VSV-tagged p50dynamitin (our unpublished data). These data imply that both the recruitment and the activity of the minus-end dynein motor are critical for the inhibition of bacterial replication.
Expression of RILP Induces SCV Transport to and Subsequent Fusion with Lysosomes
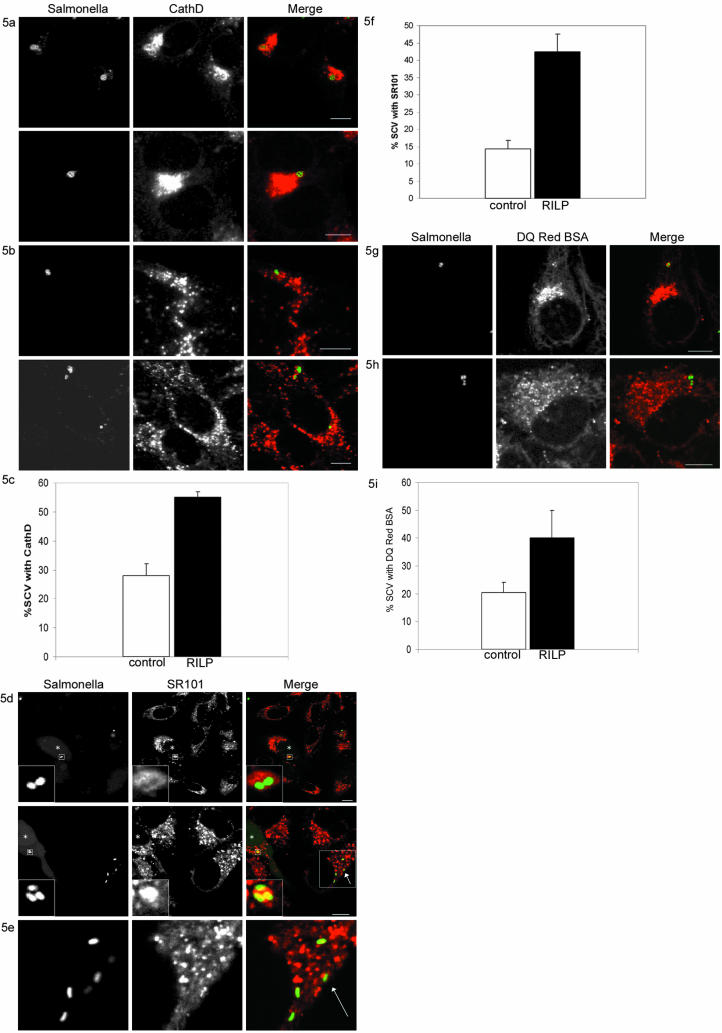
Besides regulating vesicle transport (Jordens et al., 2001), Rab7 is a key regulator of fusion between late endosomes and lysosomes (Meresse et al., 1995; Bucci et al., 2000). The RILP-induced motor recruitment might target the bacteria for degradation by increasing transport to the microtubule minus-end, where the lysosomes reside, and induce fusion with lysosomes at this site. The interaction between lysosomes and the SCV was visualized with an antibody recognizing the aspartic proteinase cathepsin D. Cathepsin D is involved in degradation of proteins in the lysosomal compartment. In control cells, some of the SCV recruited cathepsin D (Figure 5, b and c). This increased significantly when RILP was expressed for 3–4 h (Figure 5, a and c), indicating that RILP expression enhanced fusion with lysosomes.
Figure 5.
RILP-induced motor recruitment increases fusion with lysosomes. (A) Infected cells microinjected with RILP cDNA showing cathepsin D accumulation on the SCV. Left, Salmonella SL1344. Middle, cathepsin D. Right, merge. Bar, 10 μm. (B) Control cells immunostained for cathepsin D. Left, Salmonella SL1344. Middle, cathepsin D. Right, merge. Bar 10 μm. (C) Quantification of cathepsin D accumulation on the SCV of three individual experiments 3–4 h after infection. These data represent 40 microinjected cells and 80 control cells. Mean ± SEs are indicated. (D) Confocal analysis of living HeLa cells labeled with SR101, infected with GFP-SL1344 Salmonella and microinjected with RILP cDNA. Cells were followed for ∼4 h at 37°C. Microinjected cells were visualized using FITC-dextran as an injection marker. Microinjected cells accumulated SR101 in the SCV. Insets are shown of these areas. Left, GFP-SL1344 Salmonella and injected cells with FITC-dextran. Middle, SR101 labeling of lysosomes. Right, merge. Bar, 10 μm. Injected cells are indicated by an asterisk. (E) Zoom in of the area indicated with an arrow in Figure 5d, bottom right (merge). In this area, the SCV in the nonmicroinjected cell showed no SR101 accumulation. Left, GFP-SL1344 Salmonella. Middle, SR101 labeling of lysosomes. Right, merge. (F) Quantification of seven independent experiments. The mean percentage of colocalization between SCV and SR101 is presented ± SE in control (n = 90) and RILP-expressing cells (n = 80). (G) Confocal analysis of living cells microinjected with RILP cDNA and labeled with DQ Red BSA which is a fluid phase marker that is dequenched upon proteolytic activity. Cells were followed for ∼4 h at 37°C. Microinjected cells were visualized using FITC-dextran as an injection marker. Left, GFP-SL1344 Salmonella and injected cells with FITC-dextran. Middle, DQ Red BSA labeling. Right, merge. Bar, 10 μm. (H) Confocal analysis of control cells labeled with DQ Red BSA. Left, GFP-SL1344 Salmonella. Middle, DQ Red BSA labeling. Right, merge. Bar, 10 μm. (I) Quantification of colocalization between the SCV and DQ Red BSA of two individual experiments. The data represent 50 microinjected cells and 100 control cells. Mean ± SEs are indicated.
To further test this RILP-induced fusion with lysosomes, cells were preloaded with the fluid phase marker SR101 for 30 min followed by a 2-h incubation to allow the fluorophore to accumulate in lysosomes. Cells were then infected with Salmonella SL1344 and RILP was expressed 2–4 h after infection. One hour after microinjection of RILP, living cells expressing RILP and control cells were followed using time-lapse confocal microscopy. Cells microinjected with RILP showed a rapid accumulation of SR101 in 42.5 ± 5.0% of the SCV (Figure 5, d and f). In control cells, only 14.0 ± 2.4% of the SCV accumulated SR101 (Figure 5, e and f). Similar results were obtained for the 12023 strain (our unpublished data). As a control, RILP-ΔN was expressed and then the SCV accumulated SR101 similar to control cells (our unpublished data). Moreover, similar results were obtained with DQ Red BSA, a strong self-quenched fluid phase marker that is dequenched upon proteolytic digestion. In control cells, only few of the SCV accumulated dequenched DQ Red BSA (Figure 5, h and i), which was significantly increased (2-fold) upon RILP expression (Figure 5, g and i).
Together, these results indicate that the minus-end motor dynein directs transport of the SCV to sites where lysosomes reside. Here, fusion between the two compartments can occur. Due to this fusion, Salmonella is rapidly targeted to lysosomes where it may be degraded.
DISCUSSION
S. typhimurium resides in a specialized vacuole inside host cells that does not fuse with mature lysosomes (Garcia-del Portillo and Finlay, 1995; Meresse et al., 1999a). Although various Salmonella proteins have been identified that could interfere with lysosomal fusion, it is unclear which host cell systems are targeted. Furthermore, it remains unclear how host cells can clear Salmonella infections. Our study shows that manipulation of a pathway downstream of Rab7, through its effector RILP, induces the recruitment of the dynein motor to the SCV. This results in transport of the SCV to lysosomes, where Salmonella growth is inhibited.
The small GTPases Rab5 and Rab7 are regulators of different transport and fusion steps in the endocytic pathway (Novick and Zerial, 1997; Somsel Rodman and Wandinger-Ness, 2000). Phagosomes containing bacteria or inert particles acquire Rab5 and Rab7 sequentially when they mature along the endocytic pathway (Desjardins et al., 1994). At the final stage of the endocytic pathway, bacteria are degraded in mature lysosomes that contain hydrolases. Therefore, several bacteria interfere with the host's endosomal pathway to avoid degradation in lysosomes (Knodler et al., 2001). For example, retention of Rab5 on the phagosome and failure to acquire Rab7 protects M. tuberculosis from degradation (Via et al., 1997; Kelley and Schorey, 2003). In murine macrophages, fusion with early endosomes and retention of Rab5 on the SCV is important in preventing transport to lysosomes as well (Hashim et al., 2000; Mukherjee et al., 2001). However, studies in other cells, including HeLa, showed that the SCV does acquire Rab7. In HeLa cells, expression of a dominant-negative form of Rab7 delayed the maturation of the SCV, suggesting that Rab7 is involved in the maturation of the SCV (Meresse et al., 1999a).
Although Rab7 interacts with the SCV in HeLa cells (Martinez-Lorenzo et al., 2001; Meresse et al., 1999a), there is no fusion with mature lysosomes. For other intracellular bacteria, it has also been shown that targeting of Rab7 to phagosomes is not sufficient for phagolysosomal fusion. For instance, targeting of Rab7 to phagosomes containing Mycobacterium tuberculosis or Legionella pneumophila could not induce fusion with lysosomes (Clemens et al., 2000; Kelley and Schorey, 2003). Moreover, using phosphatidylinositol 3-kinase inhibitors, Vieira et al. (2003) could induce a maturation block due to prolonged Rab5 activity on phagosomes containing latex beads. In these cells, recruitment to and activation of Rab7 on the phagosomes was not sufficient to overcome the maturation block (Vieira et al., 2003).
We confirmed that Rab7 is targeted to the SCV in HeLa cells. Using transient expression of GFP-Rab7, we could show that Rab7 is present on the SCV during all phases of infection, and not only during the early phases of infection (Meresse et al., 1999a). By using FRAP, the Rab7 activation cycle on the SCV was compared with the cycle on late endocytic structures, even within the same cell. This is considerably more complicated, if not impossible, when compartments have to be isolated for biochemical analysis. Our experiments showed that the Rab7 activation cycle on the SCV was identical to that on late endocytic structures, even within the same cell. Thus, Salmonella does not affect the Rab7 activation cycle on the SCV or late endocytic structures in our experimental system.
The role of Rab7 in regulation of late endosomal/lysosomal transport and fusion has been widely studied. However, some controversy remains about the exact localization of Rab7. Rab7 was reported exclusively located on late endosomes (Feng et al., 1995; Meresse et al., 1995), whereas others placed Rab7 on late endosomes and lysosomes (Bucci et al., 2000). Considering markers that define late endosomes and lysosomes, Rab7 is located on mannose-6-phosphate receptor-containing structures, a marker for late endosomes (Feng et al., 1995; Meresse et al., 1995), as well as on cathepsin D-positive structures, a marker for lysosomes (Bucci et al., 2000). However, thus far most reports used ectopic expression of Rab7 constructs, which might result in a broader distribution. We show that expression of the Rab7 effector RILP results in clustering of late endosomes as well as lysosomes, because the clustered compartments are cathepsin D, SR101, and LysoTracker positive (Figure 5, a–f) (Cantalupo et al., 2001; Jordens et al., 2001). Because RILP binds to, and regulates transport of, compartments containing (endogenous) Rab7, this implies that endogenous Rab7 should be located on both compartments (see also Figure 5, a–f).
Because Rab7 is crucial in regulating late endosomal/lysosomal fusion, modulating a pathway downstream of Rab7 could affect the intracellular fate of Salmonella. We therefore tested whether the Rab7 effector RILP could be recruited to the SCV. Because endogenous RILP cannot be detected using immunofluorescence, SCV separated by density gradient centrifugation were analyzed. RILP comigrated with Rab7 on the SCV and with late endocytic fractions. RILP is a limiting factor in controlling dynein motor recruitment to late endosomes and lysosomes, because ectopic expression of RILP enhances this motor recruitment and activates minus-end transport of late endosomes and lysosomes (Jordens et al., 2001). To test whether RILP expression causes similar effects on the SCV or affects intracellular survival of Salmonella, cells infected with Salmonella were microinjected with RILP. Ectopic expression of RILP inhibited intracellular replication of two Salmonella strains. Under normal conditions endogenous RILP is recruited to the SCV (Figure 1f). However this level of expression may be insufficient to affect Salmonella infections. Because ectopic expression of RILP can affect intracellular Salmonella replication, it might be that RILP or RILP activity is a direct target of Salmonella, but this is unclear.
How does RILP affect intracellular replication of Salmonella? It has been shown that phagosomes move along microtubules in a bidirectional manner by using the microtubule motors dynein and kinesin. This microtubule-based transport is required for phagosomal maturation because it facilitates fusion between phagosomes and endocytic compartments (Blocker et al., 1996). Previously, we showed that RILP recruits the dynein motor to Rab7-containing late endosomes and lysosomes, thereby regulating minus-end transport of these compartments (Jordens et al., 2001). Here, we show that expression of RILP induces recruitment of the minus-end motor dynein to the SCV and blocks intracellular Salmonella replication. This is the result of dynein motor activity because concomitant inactivation of the dynein/dynactin motor could overcome the RILP-mediated block in replication of Salmonella. Increased movement of the SCV in RILP-expressing cells could not be imaged due to phototoxicity. RILP expression only inhibited intracellular Salmonella growth during the early intracellular phase (2–4 h after infection). Expression of RILP 8 h or later after infection does not inhibit intracellular growth of Salmonella. Later, during the infection, the SCV is segregated from the endocytic pathway (Garvis et al., 2001), rendering it not susceptible to RILP-induced dynein-mediated transport to lysosomes.
Our data suggest that transport of the SCV to lysosomes is one of the limiting factors in host cells for intracellular survival of Salmonella. Because lysosomes are usually located in the perinuclear area, close to the microtubule minus-end, transport of the SCV to this site has to precede fusion with lysosomes. Indeed, we show that dynein recruitment resulted in an increased fusion of the SCV with lysosomes and inhibition of Salmonella growth. RILP induced dynein-mediated transport of the SCV, thus controls intracellular Salmonella replication during the early phase of infection. Motor proteins are novel host components involved in controlling intracellular survival of Salmonella and possibly other intracellular pathogens.
Acknowledgments
We thank Stéphane Méresse for the Salmonella strains, and Lauran Oomen and Lenny Brocks for excellent assistance with confocal microscopy. We thank Tom Ottenhoff for support. This study was supported by grants from NWO MW 901-06-096 and Zon MW PGS 912-03-026.
References
- Barbieri, M.A., Li, G., Colombo, M.I., and Stahl, P.D. (1994). Rab5, an early acting endosomal GTPase, supports in vitro endosome fusion without GTP hydrolysis. J. Biol. Chem. 269, 18720-18722. [PubMed] [Google Scholar]
- Blocker, A., Severin, F.F., Habermann, A., Hyman, A.A., Griffiths, G., and Burkhardt, J.K. (1996). Microtubule-associated protein-dependent binding of phagosomes to microtubules. J. Biol. Chem., 271, 3803-3811. [DOI] [PubMed] [Google Scholar]
- Bucci, C., Thomsen, P., Nicoziani, P., McCarthy, J., and van Deurs, B. (2000). Rab 7, a key to lysosome biogenesis. Mol. Biol. Cell 11, 467-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt, J.K., Echeverri, C.J., Nilsson, T., and Vallee, R.B. (1997). Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 139, 469-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalupo, G., Alifano, P., Roberti, V., Bruni, C.B., and Bucci, C. (2001). Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. EMBO J. 20, 683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens, D.L., Lee, B.Y., and Horwitz, M.A. (2000). Mycobacterium tuberculosis and Legionella pneumophila phagosomes exhibit arrested maturation despite acquisition of Rab7. Infect. Immun. 68, 5154-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins, M., Celis, J.E., van Meer, G., Dieplinger, H., Jahraus, A., Griffiths, G., and Huber, L.A. (1994). Molecular characterization of phagosomes. J. Biol. Chem. 269, 32194-32200. [PubMed] [Google Scholar]
- Echeverri, C.J., Paschal, B.M., Vaughan, K.T., and Vallee, R.B. (1996). Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 132, 617-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Y., Press, B., and Wandinger-Ness, A. (1995). Rab 7, an important regulator of late endocytic membrane traffic. J. Cell Biol. 131, 1435-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-del Portillo, F., and Finlay, B.B. (1995). Targeting of Salmonella typhimurium to vesicles containing lysosomal membrane glycoproteins bypasses compartments with mannose 6-phosphate receptors. J. Cell Biol. 129, 81-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-del Portillo, F., Zwick, M.B., Leung, K.Y., and Finlay, B.B. (1993). Intracellular replication of Salmonella within epithelial cells is associated with filamentous structures containing lysosomal membrane glycoproteins. Infect. Agents Dis. 2, 227-231. [PubMed] [Google Scholar]
- Garvis, S.G., Beuzon, C.R., and Holden, D.W. (2001). A role for the PhoP/Q regulon in inhibition of fusion between lysosomes and Salmonella-containing vacuoles in macrophages. Cell Microbiol. 3, 731-744. [DOI] [PubMed] [Google Scholar]
- Gorvel, J.P., Chavrier, P., Zerial, M., and Gruenberg, J. (1991). rab5 controls early endosome fusion in vitro. Cell 64, 915-925. [DOI] [PubMed] [Google Scholar]
- Grieshaber, S.S., Grieshaber, N.A., and Hackstadt, T. (2003). Chlamydia trachomatis uses host cell dynein to traffic to the microtubule-organizing center in a p50 dynamitin-independent process. J. Cell Sci. 116, 3793-3802. [DOI] [PubMed] [Google Scholar]
- Harrison, R.E., Bucci, C., Vieira, O.V., Schroer, T.A., and Grinstein, S. (2003). Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: role of Rab7 and RILP. Mol. Cell. Biol. 23, 6494-6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashim, S., Mukherjee, K., Raje, M., Basu, S.K., and Mukhopadhyay, A. (2000). Live Salmonella modulate expression of Rab proteins to persist in a specialized compartment and escape transport to lysosomes. J. Biol. Chem. 275, 16281-16288. [DOI] [PubMed] [Google Scholar]
- Holden, D.W. (2002). Trafficking of the Salmonella vacuole in macrophages. Traffic 3, 161-169. [DOI] [PubMed] [Google Scholar]
- Hoogenraad, C.C., Akhmanova, A., Howell, S.A., Dortland, B.R., De Zeeuw, C.I., Willemsen, R., Visser, P., Grosveld, F., and Galjart, N. (2001). Mammalian Golgi-associated Bicaudal-D2 functions in the dynein-dynactin pathway by interacting with these complexes. EMBO J. 20, 4041-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordens, I., Fernandez-Borja, M., Marsman, M., Dusseljee, S., Janssen, L., Calafat, J., Janssen, H., Wubbolts, R., and Neefjes, J. (2001). The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr. Biol. 11, 1680-1685. [DOI] [PubMed] [Google Scholar]
- Kelley, V.A., and Schorey, J.S. (2003). Mycobacterium's arrest of phagosome maturation in macrophages requires Rab5 activity and accessibility to iron. Mol. Biol. Cell, 14, 3366-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodler, L.A., Celli, J., and Finlay, B.B. (2001). Pathogenic trickery: deception of host cell processes. Nat. Rev. Mol. Cell. Biol. 2, 578-588. [DOI] [PubMed] [Google Scholar]
- Martinez-Lorenzo, M.J., Meresse, S., de Chastellier, C., and Gorvel, J.P. (2001). Unusual intracellular trafficking of Salmonella typhimurium in human melanoma cells. Cell Microbiol. 3, 407-416. [DOI] [PubMed] [Google Scholar]
- Matanis, T., et al. (2002). Bicaudal-D regulates COPI-independent Golgi-ER transport by recruiting the dynein-dynactin motor complex. Nat. Cell Biol. 4, 986-992. [DOI] [PubMed] [Google Scholar]
- Meresse, S., Gorvel, J.P., and Chavrier, P. (1995). The rab7 GTPase resides on a vesicular compartment connected to lysosomes. J. Cell Sci. 108, 3349-3358. [DOI] [PubMed] [Google Scholar]
- Meresse, S., Steele-Mortimer, O., Finlay, B.B., and Gorvel, J.P. (1999a). The rab7 GTPase controls the maturation of _Salmonella typhimurium_-containing vacuoles in HeLa cells. EMBO J. 18, 4394-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meresse, S., Steele-Mortimer, O., Moreno, E., Desjardins, M., Finlay, B., and Gorvel, J.P. (1999b). Controlling the maturation of pathogen-containing vacuoles: a matter of life and death. Nat. Cell Biol. 1, E183-E188. [DOI] [PubMed] [Google Scholar]
- Mukherjee, K., Parashuraman, S., Raje, M., and Mukhopadhyay, A. (2001). SopE acts as an Rab5-specific nucleotide exchange factor and recruits non-prenylated Rab5 on Salmonella-containing phagosomes to promote fusion with early endosomes. J. Biol. Chem. 276, 23607-23615. [DOI] [PubMed] [Google Scholar]
- Novick, P., and Zerial, M. (1997). The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol. 9, 496-504. [DOI] [PubMed] [Google Scholar]
- Press, B., Feng, Y., Hoflack, B., and Wandinger-Ness, A. (1998). Mutant Rab7 causes the accumulation of cathepsin D and cation-independent mannose 6-phosphate receptor in an early endocytic compartment. J. Cell Biol. 140, 1075-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reits, E.A., Vos, J.C., Gromme, M., and Neefjes, J. (2000). The major substrates for TAP in vivo are derived from newly synthesized proteins. Nature 404, 774-778. [DOI] [PubMed] [Google Scholar]
- Somsel Rodman, J., and Wandinger-Ness, A. (2000). Rab GTPases coordinate endocytosis. J. Cell Sci. 113, 183-192. [DOI] [PubMed] [Google Scholar]
- Steele-Mortimer, O., Meresse, S., Gorvel, J.P., Toh, B.H., and Finlay, B.B. (1999). Biogenesis of Salmonella typhimurium-containing vacuoles in epithelial cells involves interactions with the early endocytic pathway. Cell Microbiol. 1, 33-49. [DOI] [PubMed] [Google Scholar]
- Stenmark, H. and Olkkonen, V.M. (2001) The Rab GTPase family. Genome Biol. 2, REVIEWS3007. [DOI] [PMC free article] [PubMed]
- van Ham, S.M., et al. (1997). HLA-DO is a negative modulator of HLA-DM-mediated MHC class II peptide loading. Curr. Biol., 7, 950-957. [DOI] [PubMed] [Google Scholar]
- Vennegoor, C., Calafat, J., Hageman, P., van Buitenen, F., Janssen, H., Kolk, A., and Rumke, P. (1985). Biochemical characterization and cellular localization of a formalin-resistant melanoma-associated antigen reacting with monoclonal antibody NKI/C-3. Int. J. Cancer 35, 287-295. [DOI] [PubMed] [Google Scholar]
- Via, L.E., Deretic, D., Ulmer, R.J., Hibler, N.S., Huber, L.A., and Deretic, V. (1997). Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J. Biol. Chem. 272, 13326-13331. [DOI] [PubMed] [Google Scholar]
- Vieira, O.V., Bucci, C., Harrison, R.E., Trimble, W.S., Lanzetti, L., Gruenberg, J., Schreiber, A.D., Stahl, P.D., and Grinstein, S. (2003). Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase. Mol. Cell. Biol. 23, 2501-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitelli, R., Santillo, M., Lattero, D., Chiariello, M., Bifulco, M., Bruni, C.B., and Bucci, C. (1997). Role of the small GTPase Rab7 in the late endocytic pathway. J. Biol. Chem. 272, 4391-4397. [DOI] [PubMed] [Google Scholar]
- Wubbolts, R., Fernandez-Borja, M., Jordens, I., Reits, E., Dusseljee, S., Echeverri, C., Vallee, R.B., and Neefjes, J. (1999) Opposing motor activities of dynein and kinesin determine retention and transport of M.H.C. class II-containing compartments. J. Cell Sci. 112, 785-795. [DOI] [PubMed] [Google Scholar]