Transforming Growth Factor-β Induced Warburg-Like Metabolic Reprogramming May Underpin the Development of Peritoneal Endometriosis (original) (raw)
Abstract
Context:
TGF-β is believed to play a major role in the etiology of peritoneal endometriosis. In tumors, TGF-β induces the metabolic conversion of glucose to lactate via glycolysis, a process referred to as the “Warburg effect.” Lactate increases cell invasion, angiogenesis, and immune suppression, all crucial steps in the development of endometriosis.
Objective:
The aim of this study was to determine whether TGF-β induces a “Warburg-like” effect in peritoneal endometriosis.
Design:
The study was informed by human tissue analysis and cel culture.
Setting:
The study was conducted at the university research institute.
Patients or Other Participants:
We studied women undergoing surgical investigation for endometriosis.
Interventions:
Concentrations of lactate and TGF-β1 in peritoneal fluid (n = 16) were measured by commercial assay. Expression of genes implicated in glycolysis was measured in endometrial and peritoneal biopsies (n = 31) by quantitative RT-PCR and immunohistochemistry. The effect of TGF-β1 on primary human peritoneal mesothelial cells (n = 6) and immortalized mesothelial (MeT-5A) cells (n = 3) was assessed by quantitative RT-PCR, Western blot, and commercial assays.
Main Outcome Measures:
Lactate, TGF-β1, and markers of glycolysis were measured.
Results:
Concentrations of lactate in peritoneal fluid paralleled those of TGF-β1, being significantly higher in women with endometriosis compared to women without (P < .05). Endometriosis lesions expressed higher levels of glycolysis-associated genes HIF1A, PDK1, and LDHA than eutopic endometrium, and adjacent peritoneum had higher levels of HIF1A and SLC2A1 than peritoneum from women without disease (P < .05 to P < .001). Exposure of mesothelial cells to TGF-β1 increased production of lactate (P < .05), increased HIF1A mRNA (P < .05), and protein, and increased concentrations of mRNAs encoded by glycolysis-associated genes (LDHA, _PDK_1, SLC2A1; P < .05).
Conclusions:
A change in the metabolic phenotype of endometriosis lesions and peritoneal mesothelium in women with endometriosis may favor development of endometriosis.
Endometriosis is a nonmalignant disorder defined as the presence of endometrial tissue outside the uterus, most commonly on the pelvic peritoneum, in close association with the peritoneal mesothelial cells (PMCs) (1). It is thought to affect 6–10% of women of reproductive age and is associated with chronic pelvic pain, dysmenorrhea, and infertility (2). Endometriosis is currently managed surgically or medically, but symptoms can recur in up to 75% of surgical cases, and available medical treatments have undesirable side effects (3).
The major obstacle in the development of new agents for the treatment of endometriosis is our limited understanding of its etiology. A widely accepted hypothesis is that retrograde menstruation through the fallopian tube is a means for the transfer of endometrial tissue into the peritoneal cavity, where it implants on the mesothelium of the peritoneum (4). However, the reason this occurs in some women and not in others is not known. To date, most research into the etiology of endometriosis has focused on changes within eutopic and ectopic endometrium, but there are limited data on the role of the peritoneum (5).
The TGF-β superfamily is thought to play a major role in the development of endometriosis (6). In studies using a mouse model of endometriosis, TGF-β-null females develop fewer and smaller peritoneal endometriosis lesions than their wild-type counterparts (7). In women, expression of TGF-β superfamily members and their signaling components appear to be dysregulated in the eutopic endometrium in women with endometriosis (8), and concentrations of TGF-β in peritoneal fluid are increased in women with endometriosis compared to women without disease (9–11). However, the underlying mechanism(s) influenced by the actions of TGF-β remains poorly understood.
It is recognized that endometriosis and tumorigenesis share clear parallels (12–14). During tumorigenesis, TGF-β can induce a shift in cell metabolism from mitochondrial oxidative phosphorylation to aerobic glycolysis, known as the “Warburg effect” (15–17). Neighboring cells of tumors are also programmed to use aerobic glycolysis by TGF-β1, and this process generates lactate, which feeds adjacent tumor cells, establishing an integrated metabolic tumor microenvironment (16, 18). Overproduction of lactate increases cell invasion, angiogenesis, and immune suppression, all crucial steps in the development of tumors and known regulators of endometriosis (19).
One key factor in this pathway is hypoxia inducible factor-1 (HIF-1), a heterodimer composed of HIF-1α (HIF1A) and HIF-1β subunits (20). HIF-1β is constitutively expressed in cells, whereas HIF-1α is hydroxylated at two proline residues before binding the von Hippel-Lindau protein for ubiquitination and proteasomal degradation in the presence of oxygen. Because oxygen is needed for the hydroxylation, hypoxic conditions lead to HIF-1α stabilization, and thus activity (20). HIF-1α protein has previously been detected in normoxic conditions. The mechanism for this normoxic stabilization remains unclear but may occur through various signaling pathways that block HIF-1α ubiquitination (21) or by increased HIF1A gene expression or translation enabling protein production at a greater rate than proteasomal degradation (22–25). HIF-1α is a key mediator of glycolysis and induces the expression of several key genes in the glycolysis pathway. These include lactate dehydrogenase A (LDHA), which converts pyruvate to lactate, and pyruvate dehydrogenase kinase 1 (PDK1), which suppresses mitochondrial oxidative metabolism by inhibiting pyruvate dehydrogenase and the conversion of pyruvate to acetyl coenzyme A needed for the tricarboxylic acid cycle as well as glucose transporter 1 (SLC2A1), which is responsible for increased glucose uptake by the cell (26, 27).
Studies have shown HIF-1α to be expressed in endometriosis lesions, and this expression is significantly increased compared to matched eutopic endometrium and healthy control endometrium (28, 29). Although these studies refer to the transient hypoxia experienced by shed ectopic endometrial cells during lesion development (28, 30, 31), to date no link has been reported between increased concentrations of TGF-β in the peritoneal fluid and HIF1A expression.
The aim of our study was to determine whether there are changes in the metabolic phenotype and production of lactate in the peritoneum of women with endometriosis, similar to the Warburg effect reported in cancers, which would provide an environment favorable for the maintenance and growth of lesions. First, we correlated levels of TGF-β and lactate in the peritoneal fluid of women with and without endometriosis. We then examined expression of glycolysis genes (including HIF1A, LDHA, PDK1, and SLC2A1) in endometrial, peritoneal, and endometriosis lesion biopsies from women with and without endometriosis. Finally, we examined the effect of TGF-β1 on lactate production and expression of glycolysis markers in primary human peritoneal and immortalized mesothelial (MeT-5A) cells.
Subjects and Methods
Subjects
Ethical approval for this study was obtained from the Lothian Research Ethics Committee (LREC 11/AL/0376), with informed written consent obtained from all patients. All of the women included in this study underwent surgery for investigation of chronic pelvic pain to identify underlying endometriosis. The “control” group of women had no evidence of endometriosis at laparoscopy, nor was there evidence of any other underlying pelvic pathology to explain their painful symptoms (eg, adhesions). None of the women were taking hormonal contraceptives, and they all had regular 21- to 35-day menstrual cycles. Women were operated on once during this study. Peritoneal fluid, primary PMCs, peritoneal biopsies, endometrial biopsies, and endometriosis lesion biopsies were obtained at the beginning of surgery.
In women with endometriosis (n = 15), we recovered endometriotic lesions (for study and histological confirmation of disease) and peritoneum adjacent and distal to lesions (for study and histological conformation of the absence of disease) and endometrial biopsies. In women without endometriosis (n = 16), we biopsied the peritoneum at locations within the abdomen at similar locations to those obtained from patients (for study and histological confirmation of the absence of disease). The tissues were collected according to the Endometriosis Phenome and Biobanking Harmonisation Project (EPHect) guidelines (http://endometriosisfoundation.org/ephect/).
Peritoneal biopsies and endometriosis lesion biopsies were divided into two portions; one was immersed in RNAlater at 4°C for 24 hours and frozen at −80°C for RNA extraction, and the other portion was fixed in 4% neutral-buffered formalin for 24 hours at 4°C, followed by storage in 70% ethanol, and subsequent embedding in paraffin wax for later immunohistochemistry. Peritoneal fluid was collected from women with endometriosis (n = 8) and women without endometriosis (n = 8) at the beginning of surgery. Primary PMCs were collected from women with and without endometriosis (n = 6) as previously described by gentle scraping of the pelvic mesothelium with a Tao brush, followed by vigorously agitating in 15 mL of serum-containing culture media to dislodge cells (QC Sciences) (32). We found no difference in response of primary cells to TGF-β when comparing the 2 groups.
Cycle staging
Cycle phase was determined by examination of endometrial biopsies (stained with hematoxylin and eosin) collected at the same time as the peritoneal fluid samples. An expert histopathologist who used Noyes' criteria to determine cycle phase examined the samples. Cycle phase was also confirmed by the measurement of serum estradiol and progesterone levels together with day of the cycle.
Establishment of cell culture
Cells were cultured as previously described (33) in HOSE I media, 40% Media 199, 40% MCDB 105, and supplemented with 15% fetal bovine serum, 0.5% penicillin/streptomycin, and 1% L-glutamine (Life Technologies Inc and Sigma Chemical Co).
The MeT-5A cell line (CRL-9444; ATCC) was originally established by transfecting normal human mesothelial cells from the plural cavity with a plasmid containing Simian virus (SV40) early region DNA, and it expresses SV40 large T antigen (ECACC) (10, 11). MeT-5A cells were grown in Iscove's Modified Dulbecco's Media supplemented with 10% fetal bovine serum and 1% L-glutamine (Life Technologies Inc).
Experimental treatments of primary PMCs and MeT-5A cells
Cells were plated at either 1.5 × 105 in a 12-well plate for primary PMCs or 2 × 105 in a 12-well plate for MeT-5A cells and left to seed for 12 hours before serum starving for 24 hours.
Cells were exposed to physiological levels (34) of recombinant human TGF-β1 (2 ng/mL) as previously described (10, 11) for between 3 and 48 hours (PeproTech EC Ltd and R&D Systems). Cells were harvested in RLT buffer containing 0.01% β-mercaptoethanol and stored at −80°C until RNA extraction (QIAGEN). For protein analysis, cells were harvested in NuPage LDS sample buffer diluted 1:4 with added dithiothreitol and Halt protease-phosphatase inhibitor cocktail (Life Technologies Inc and Thermo Fisher Scientific). Lysates were prepared by removal of DNA contamination in a QIAshredder spin column (QIAGEN). Media was removed and stored at −80°C for analysis.
Lactate assay
Lactate concentration was determined using a commercial kit (Randox Laboratories Ltd) adapted for use on a Cobas Fara centrifugal analyzer (Roche Diagnostics Ltd). The minimal detectable concentration with an acceptable level of precision was determined as 0.15 mmol/L, and the test was linear up to an L-lactate concentration of 19 mmol/L. The within-batch coefficient of variation (CV) was 0.9%, and the between-batch CV was 2.7% at a value of 1.26 mmol/L.
TGF-β1 immunoassay
A TGF-β1 ELISA was performed using a Human TGF-β1 Quantikine Kit (DB100B; R&D Systems) according to the manufacturer's instructions. Samples were diluted 1:2, and latent TGF-β1 was activated to the immunoreactive form using 1 m HCl and neutralized with 1.2 m NaOH/0.5 m HEPES buffer before assaying. Samples were assayed in duplicate, and after development assays were measured on a Lab Systems Multiscan EX Microplate reader at 450 nm with wavelength correction at 540 nm. Values were determined by standard curve analysis with a detection limit of 16 pg/mL and linear range from 16 to 1000 pg/mL. Intra-assay CV is 2.5%, and the between-batch CV is 8.3% for cell culture supernatants.
Cell proliferation assay
Cell proliferation was measured using CyQuant cell proliferation assay kit (Invitrogen) according to the manufacturer's instructions. Cells were pretreated with Ribonuclease A (Sigma Chemical Co) to remove any contaminating RNA before assay. Plates were measured on FluroStar Optima at 480 nm. Cell number was calculated by a standard curve with an assay linear detection range of between 50 and 50 000 cells.
Immunostaining
Paraffin-embedded peritoneal biopsies and endometriosis lesion biopsies were cut into 5-μm sections, mounted onto electrostatically charged microscope slides, dewaxed, and rehydrated (VWR International Ltd). Antigen retrieval was preformed by pressure cooking slides for 5 minutes in 10 mm Tris 1 mm EDTA (pH 9). Slides were blocked for 30 minutes in normal horse serum diluted 1:6 in Tris-buffered saline with 0.05% Tween 20 before incubation with primary antibody (Biosera). Primary antibodies included: HIF-1α, 1:100 (10790; Santa Cruz Biotechnology); glucose transporter-1 (GLUT-1), 1:1000 (ab652; Abcam Inc); PDK-1, 1:2000 (NBP1-96065; Novus Biologicals); LDHA, 1:1000 (ab52488; Abcam Inc); or rabbit IgG (X0903; Dako). Sections were washed in TBS-T20 and incubated for 30 minutes with species-specific impress kit (Vector Laboratories). Sections were then washed, incubated for 5 minutes with 3,3′-diaminobenzidine (Vector Laboratories), counterstained with hematoxylin, dehydrated, mounted, and visualized by light microscopy. Images were captured using an Olympus Provis microscope equipped with a Kodak DCS330 camera (Olympus Optical Co and Kodak Ltd). A total of 12 peritoneal biopsies, four endometriosis lesions, and four endometrial biopsies were examined using immunohistochemistry.
For cell culture immunofluorescence, cells were fixed in ice-cold methanol for 15 minutes before being washed in TBS-T20, then blocked in normal horse serum as above. Cells were incubated with mouse anti-HIF-1α (2.5 μg/mL, 610958; BD Biosciences) or equimolar concentrations of mouse IgG (2.5 μg/mL, M7894; Sigma Chemical Co) at 4°C overnight. Cells were washed in TBS-T20 and incubated for 30 minutes with species-specific impress kit (Vector Laboratories). After washes in TBS, sections were incubated with Tyramide and counterstained with DAPI, before visualizing with wide field microscopy (PerkinElmer). Images were captured using an Axiovert 200M equipped with an Axiocam HRc camera (Carl Zeiss). A total of three MeT-5A cultures were examined using immunofluorescence.
RNA preparation and cDNA synthesis
RNA was extracted using the RNeasy Mini kit with on-column DNaseI digestion (QIAGEN) according to the manufacturer's instructions. First-strand cDNA synthesis was performed using Superscript VILO Master Mix (Life Technologies Inc) according to the manufacturer's instructions.
Quantitative RT-PCR analysis
mRNA transcripts were quantified relative to the appropriate housekeeping gene, GAPDH, as determined by geNorm assay (Primerdesign Ltd). Reactions were performed using an ABI Prism 7900 Fast system under standard running conditions with brilliant III ultrafast SYBR green QPCR master mix (Applied Biosystems and Agilent). Melt curves were analyzed to confirm specific products with prevalidated primers and quantification using the 2−ΔCt or the 2−ΔΔCt method after normalization against controls (Primerdesign Ltd).
Immunoblotting
Cell lysates (at a concentration of 200000 cells/200 μL, with 10 μL loaded onto each lane) were resolved on NuPage Novex 4–12% Bis-Tris polyacrylamide gels (Life Technologies Inc) under reducing conditions with NuPage MOPS SDS running buffer, according to the manufacturer's instructions. Proteins were transferred to a polyvinylidene difluoride membrane using a semidry blotter and blocked with 5% milk powder in Tris-buffered saline with 0.1% Tween 20 (TBS-T20). The membrane was incubated with mouse anti-GAPDH (0.5 μg/mL, G9545; Sigma Chemical Co) in TBS-T20 and 5% milk for 2 hours at room temperature, before incubating with anti-HIF-1α (1 μg/mL, 610958; BD Biosciences) at 4°C overnight in TBS-T20 and 5% milk overnight at 4°C. After this, membranes were washed and incubated with species-specific impress kit 1:10 000 (Vector Laboratories Ltd) in TBS-T20 and 5% milk for 1 hour at room temperature. The membrane was washed and incubated with Tyramide for 30 minutes and imaged with Fujifilm FLA-5100 Fluorescent Image Analyzer (PerkinElmer and Fugifilm Ltd).
Statistical analysis
All results are expressed as mean ± SEM of a minimum of three independent experiments. ELISA and lactate assay data were analyzed using unpaired Student's t test and were log-transformed before Pearson correlation testing. Quantitative RT-PCR, cell proliferation, and cell cytotoxicity were analyzed using paired and unpaired Student's t tests, as appropriate. All statistical results were generated using GraphPad PRISM version 5 statistical software (GraphPad Software, Inc), and P < .05 was considered significant.
Results
Lactate concentrations are increased in the peritoneal fluid of women with endometriosis, and these correlate positively with peritoneal fluid TGF-β1 levels
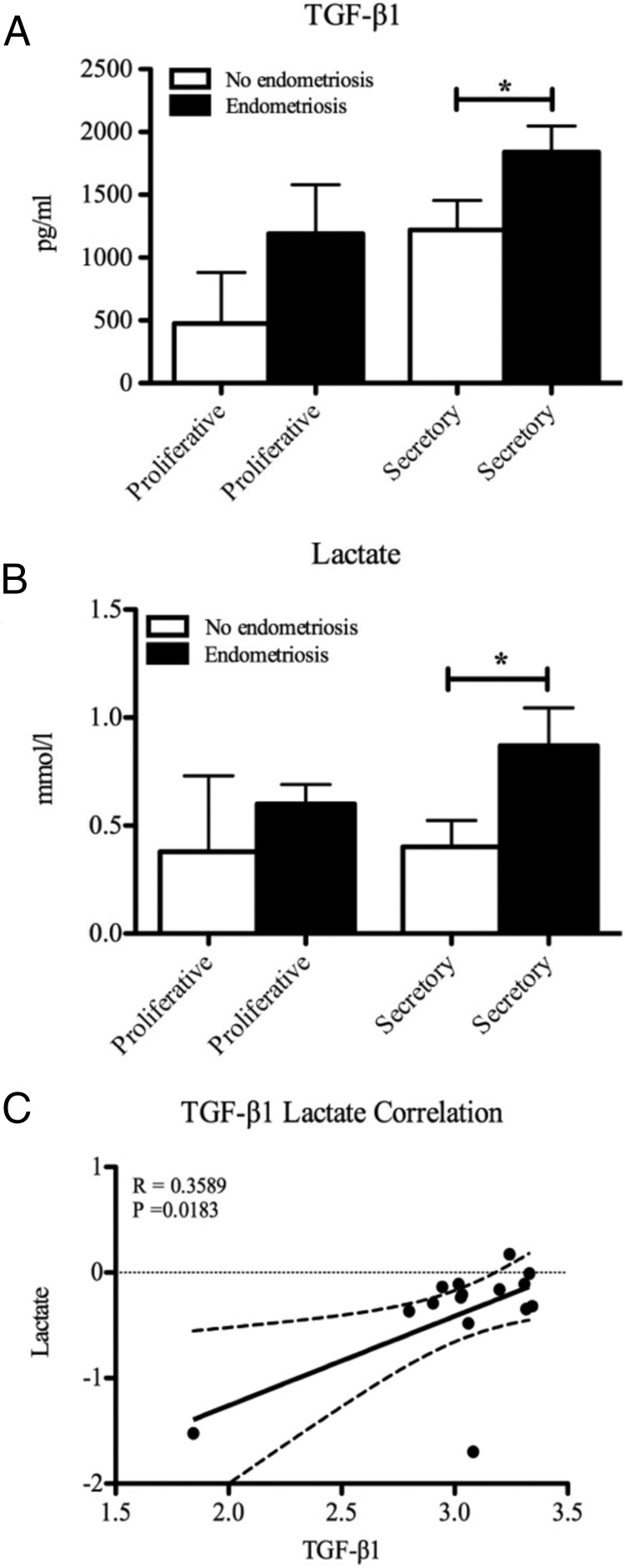
Because TGF-β is known to be increased in the peritoneal fluid of women with endometriosis (16) and TGF-β1 is known to increase lactate levels (16), we investigated whether TGF-β1 concentrations correlated with those of lactate in the peritoneal fluid of women with and without endometriosis. TGF-β1 concentrations (Figure 1A) and lactate levels (Figure 1B) were significantly increased in the peritoneal fluid of women with endometriosis compared to women without endometriosis in the secretory phase of the cycle. The same sample set was used for both assays, and we found a significant positive correlation between concentrations of TGF-β1 and lactate in the peritoneal fluid of women with and without endometriosis (Figure 1C).
Figure 1.
A and B, TGF-β1 (A) and lactate (B) levels were increased in peritoneal fluid from women with endometriosis compared to women without endometriosis during the secretory phase of the menstrual cycle. C, There was a significant positive correlation across the menstrual cycle between levels of TGF-β1 and lactate in women with and without endometriosis. *, P < .05; R2 = 0.3589 (n = 2 each proliferative group, n = 6 each secretory group).
Aerobic glycolysis marker expression is increased in endometriosis lesions compared to eutopic endometrium
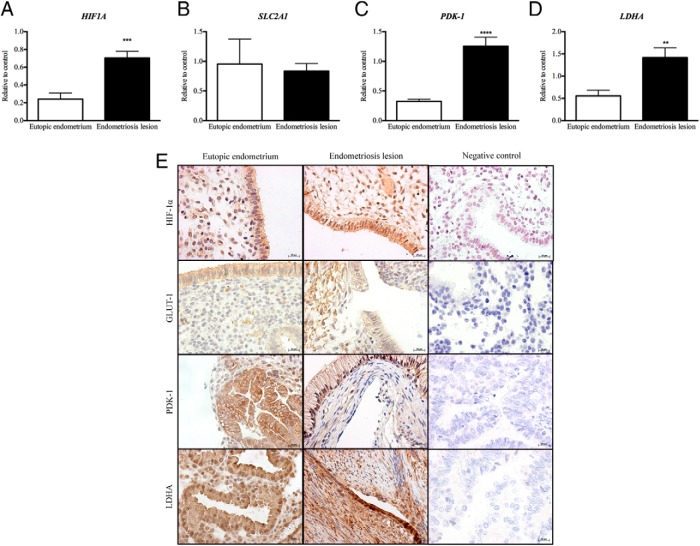
To investigate whether endometriosis lesions use aerobic glycolysis as a means of metabolism, we measured expression of glycolysis markers in endometriosis lesions compared to eutopic endometrium from women with endometriosis. Concentrations of mRNAs encoded by HIF1A, PDK1, and LDHA were significantly increased in endometriosis lesions when compared to eutopic endometrium from women with endometriosis (Figure 2, A, C, and D). However, no change was found in the expression of SLC2A1 (Figure 2B). Endometriosis lesions stained positive for HIF1A, PDK-1, LDHA, and GLUT-1 in the glandular epithelium and surrounding stromal cells (Figure 2E). No staining was observed in the negative control sections (Figure 2E).
Figure 2.
HIF1A (A), PDK1 (C), and LDHA (D) mRNA concentrations were significantly increased in endometriosis lesions when compared to endometrium from women with endometriosis. B, There was no significant change in SLC2A1 mRNA expression between endometriosis lesions and endometrium from women with disease. E, HIF-1α, GLUT-1, PDK-1, and LDHA were expressed in glandular epithelial cells and surrounding stromal cells of endometriosis lesions and endometrium. There was no staining in sections probed with control antibodies. **, P < .01; ***, P < .001; ****, P < .0001 (n = 7 each group mRNA analysis, n = 2 each group immunohistochemical analysis). Original magnification, ×400.
Aerobic glycolysis marker expression is increased in the peritoneum of women with endometriosis compared to women without endometriosis
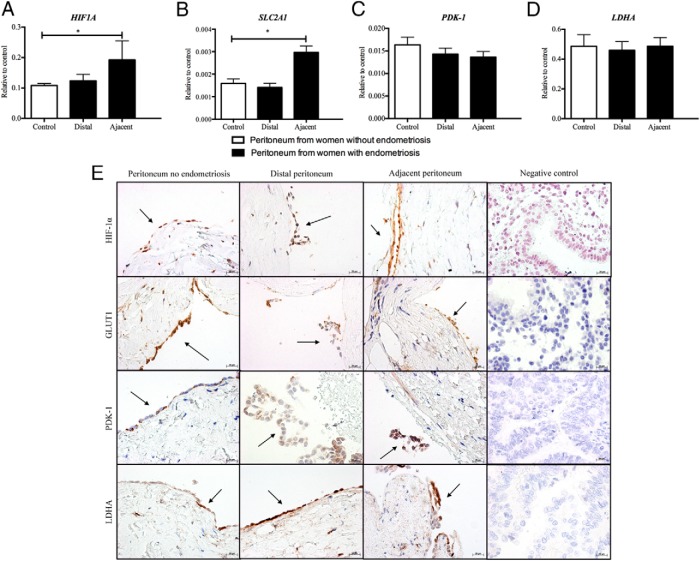
To investigate whether the peritoneum is a source of lactate, expression of genes associated with the regulation of aerobic glycolysis was quantified in peritoneal biopsies from women with and without endometriosis. Concentrations of mRNAs encoded by HIF1A and SLC2A1 were significantly increased in peritoneal biopsies adjacent to endometriosis lesions when compared to peritoneum from women without endometriosis (Figure 3, A and B). There was no difference in either HIF1A or SLC2A1 mRNA concentrations between peritoneal biopsies distal to endometriosis lesions and peritoneum from women without endometriosis. mRNAs encoded by LDHA and PDK1 were detected in all samples, but expression did not change between women with and without endometriosis, nor did it change between sites (Figure 3, C and D). HIF1A, PDK-1, LDHA, and GLUT-1 protein could be localized to mesothelial cells and some stromal cells in peritoneal biopsies in the presence or absence of endometriosis (Figure 3E). No staining was observed in the negative control sections, and no endometriosis was detected in peritoneum adjacent to lesions (Figure 3E).
Figure 3.
A and B, HIF1A (A) and SLC2A1 (B) mRNA concentrations were significantly increased in peritoneum adjacent to endometriosis lesions when compared to peritoneum of women without disease. There was no significant change in HIF1A and SLC2A1 expression from sites of peritoneum distal to endometriosis lesions and peritoneum from women without endometriosis. C and D, PDK1 (C) and LDHA (D) were expressed at all sites of peritoneum biopsied; however, there was no change in expression between women with and without endometriosis. E, HIF-1α, GLUT-1, PDK-1, and LDHA were expressed in PMCs of peritoneum from women with and without endometriosis. There was no staining in sections probed with control antibodies. *, P < .05 (n = 13 peritoneal control, n = 11 peritoneum distal, and n = 4 peritoneal adjacent, for mRNA analysis; n = 2 in each group for immunohistochemical analysis). Original magnification, ×400.
TGF-β1 increases lactate production in primary human PMCs and immortalized mesothelial cells (MeT-5A)
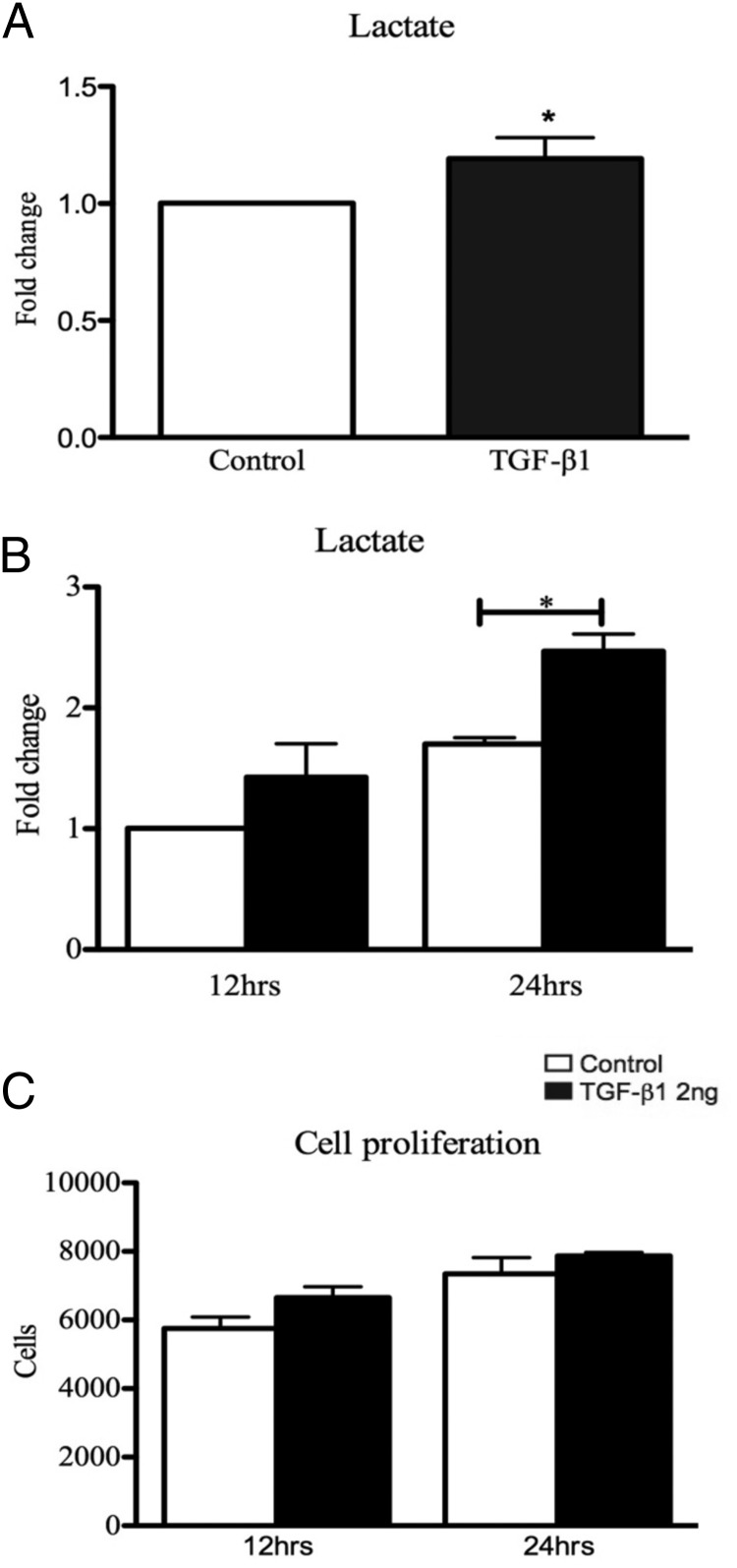
To determine whether TGF-β1 increases the production of lactate in mesothelial cells, primary human PMCs and an immortalized mesothelial cell line (MeT-5a) were treated with a physiological concentration of TGF-β1 (10, 11). Cell culture supernatants from PMCs treated with 2 ng/mL TGF-β1 for 12 hours produced significantly higher levels of lactate than control untreated PMCs (Figure 4A). MeT-5A mesothelial cells also showed significantly increased expression of lactate when treated with 2 ng/mL TGF-β1 at 24 hours (Figure 4B). Because increasing amounts of lactate can be associated with increasing cell turnover, we tested the cell proliferation of MeT-5A cells in culture when treated with and without TGF-β1. We saw no changes in cell number between TGF-β1-treated cells and control cells at 12 and 24 hours (Figure 4C).
Figure 4.
PMCs and MeT-5A cells were exposed to 2 ng/mL TGF-β1 for 12 hours or for 12 and 24 hours, respectively. A and B, TGF-β1 treatment resulted in a significant increase in lactate expression at 12 hours in PMCs (A) and 24 hours in MeT-5A cells (B). Lactate levels significantly increased over time in both the TGF-β1 treatment and the control group. C, Exposure of MeT-5A cells to TGF-β1 (2 ng/mL) showed no change in cell number compared with control. *, P < .05 (n = 5 PMCs, n = 3 MeT-5a).
TGF-β1 increases HIF-1α mRNA concentration and increases HIF-1α protein levels in PMCs
To address the question of how TGF-β1 increases lactate levels in the peritoneal fluid of women with endometriosis, we assessed the effects of TGF-β1 on HIF1A expression and HIF1A stabilization in vitro. TGF-β1 significantly increased HIF1A mRNA concentrations in primary PMCs (Figure 5A) and in MeT-5A cells after 12 hours of treatment (Figure 5B). Immunofluorescent staining of MeT-5A cells showed HIF1A protein to be increased on treatment with TGF-β1 after 24 hours with HIF1A localized to the nucleus in these cells (Figure 5C). MeT-5A cells treated with TGF-β1 for 24 hours showed increased concentrations on Western blots, consistent with protein stabilization (Figure 5D). Taken together, these data suggest that TGF-β1 up-regulates transcription of HIF1A and increases HIF-1α protein in PMCs.
Figure 5.
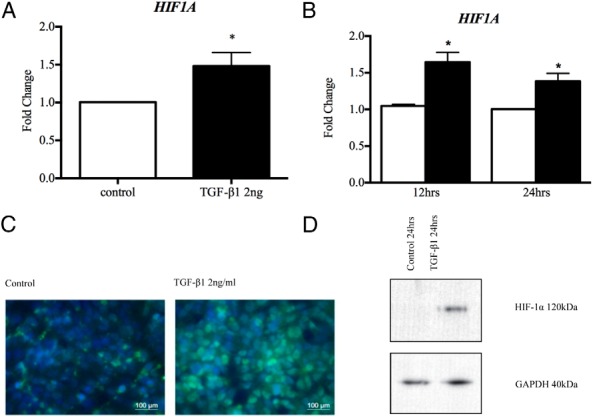
A and B, TGF-β1 treatment (2 ng/mL) significantly up-regulated HIF-1α gene expression in PMCs (A) and MeT-5A cells (B) after 12 hours. C, MeT-5A cells treated with TGF-β1 for 24 hours showed increased HIF-1α levels and localization within the nucleus by green immunofluorescence staining. D, MeT-5A cells treated with TGF-β1 in normoxic conditions for 24 and 48 hours showed stable expression of HIF-1a protein compared to controls. *, P < .05 (n = 6 PMCs, n = 3 MeT-5A).
TGF-β1 increases concentrations of mRNAs encoded by HIF-1α target genes involved in glycolysis in primary human PMCs and MeT-5A cells
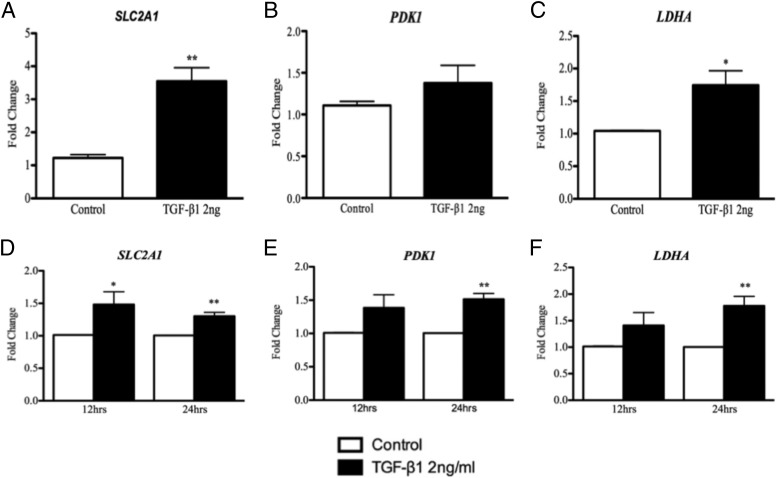
To investigate the role of HIF-1α in PMC metabolism, we analyzed expression of several HIF-1α target genes involved in glycolysis: LDHA, PDK-1, and SLC2A1. As shown in Figure 6, A–C, concentrations of mRNAs encoded by LDHA and SLC2A1 but not PDK1 are significantly increased in primary human PMCs by TGF-β1 at 12 hours. As shown in Figure 6, D and E, expression of LDHA, PDK1, and SLC2A1 was increased in MeT-5A cells by TGF-β1.
Figure 6.
TGF-β1 treatment (2 ng/mL) up-regulated LDHA and SLC2A1 mRNA expression at 12 hours in PMCs (A and C) and at 24 hours in MeT-5A cells (D and F). PDK-1 was up-regulated by TGF-β1 in PMCs at 12 hours (B), but this was not significant; however, PDK-1 was significantly up-regulated by TGF-β1 in MeT-5A cells at 24 hours (E). *, P < .05; **, P < .001 (n = 6 PMCs, n = 3 MeT-5A cells).
Discussion
We present novel data demonstrating that there are metabolic changes in endometriosis lesions and the surrounding peritoneum, consistent with the induction of a Warburg-like effect reminiscent of the one that occurs during tumorigenesis. We have shown that lactate, a by-product of glycolysis, is increased in the peritoneal fluid of women with endometriosis and that lactate concentrations positively correlate with increasing TGF-β1 levels in the peritoneal fluid. In addition, we have shown that metabolic drivers HIF1A, PDK1, and LDHA are increased in endometriosis lesions, and HIF1A and SLC2A1 are increased in peritoneum adjacent to endometriosis lesions, suggesting a metabolic change similar to that of the Warburg effect. We have also shown that TGF-β1 increases expression of key enzymes in the glycolysis pathway and lactate production in PMCs that may provide a high-energy metabolite to feed-forward to the endometriotic cells in a paracrine fashion to promote endometriosis lesion development.
TGF-β1 levels were significantly increased within the peritoneal fluid of women with endometriosis when compared to women without during the secretory phase. These results correspond to several studies within the literature demonstrating TGF-β and TGF-β1 levels to be increased in women with endometriosis and that TGF-β levels are cyclically regulated within the peritoneal fluid (9–11). We have shown that lactate concentrations positively correlated with those of TGF-β within the peritoneal fluid across the menstrual cycle. Lactate is a metabolic by-product of glycolysis, and increasing lactate concentrations can increase cell migration and invasion, angiogenesis, and immune escape during tumorigenesis, all of which are also implicated in the development of endometriosis (19). Increased lactate may therefore help facilitate ectopic endometrial cell survival and lesion establishment in a similar fashion to that of cancer cell metastasis. Furthermore, increasing lactate concentrations can lower pH and can activate latent TGF-β1, and this may initiate a positive feedback loop for the increasing TGF-β1 and lactate levels. Acid activation of the TGF-β ligand by increasing amounts of lactate has been shown to induce myofibroblast differentiation, which may lead to epithelial to mesenchymal transition within the adjacent peritoneum or lesions, again facilitating ectopic endometrial cell invasion (36, 37). Importantly, because levels of lactate have been shown to be positively correlated with the incidence of metastases (19), in vivo assessment of lactate may have utility as a biomarker for endometriosis and its response to treatment.
Endometriosis lesions expressed significantly higher levels of HIF1, PDK1, and LDHA mRNA when compared to eutopic endometrium from women with endometriosis. This observation suggests that endometriosis lesions undergo a change in metabolism, which is consistent with aerobic glycolysis and the Warburg effect seen in tumorigenesis, despite being established lesions with their own vascular and presumed normoxia (38). We have also shown that the peritoneum adjacent, but not distal, to endometriosis lesions had significantly increased expression of HIF1A and SLC2A1 compared to peritoneum from women without endometriosis; however, we saw no significant changes in gene expression of PDK1 or LDHA between the groups. This lack of difference is likely due to the fact that the peritoneal biopsies and endometriosis lesions contain additional cell types other than mesothelial cells (eg, endometrial epithelium and stroma, adipocytes) that likely express these genes in different amounts. Further analysis of peritoneal biopsies demonstrated HIF-1α, GLUT-1, PDK-1, and LDHA to be localized to the peritoneal mesothelium, and in vitro analysis of primary PMCs displayed significant increased expression of all markers. We propose that the peritoneum adjacent to lesions may have been reprogrammed to use glycolysis as a means of energy production and that lactate produced by peritoneal cells could be taken up by adjacent endometriotic cells in a feed-forward mechanism to facilitate the establishment of an integrated microenvironment at sites of peritoneal endometriosis that promotes its establishment and progression. In support of this, electron microscopy studies have also described unusually small mitochondria within lesions, consistent with glycolysis (39). Similarly, increased glycolysis is a common feature of all cancers, even in normoxia, and this is thought to be an important early event during tumorigenesis (40). Recent studies have described cells adjacent to tumors to produce increasing lactate concentrations, which are thought to drive tumor development, known as “the reverse Warburg effect” (18). Given the parallels already known between tumorigenesis and endometriosis (12–14), these observations provide additional data to support the hypothesis that these pathologies share similar underlying mechanisms.
In addition, we have shown that TGF-β1 is able to increase HIF1A mRNA expression and HIF-1α protein in both primary human PMCs and MeT-5A cells. TGF-β1 is known to induce stable HIF-1α expression in normoxic conditions by overcoming the degradation pathway (35); however, there are reports of increased rates of transcription or translation overcoming the protein degradation in normoxic conditions (22–25). We have not investigated the mechanism of HIF-1α protein presence because this is outside the scope of the paper. The increase we see may be due to increased transcription, translation, or stabilization.
HIF-1α is a transcription factor that has already been shown to directly regulate key genes involved in the glycolysis pathway (19). These genes include glucose transporters such as SLC2A1, and enzymes involved conversion of pyruvate to lactate (LDHA) and inhibition of mitochondrial oxidative metabolism through inhibition of pyruvate dehydrogenase by PDK1 (36). Our cell culture analysis showed increased concentrations of mRNAs encoded by LDHA, PDK1, and SLC2A1 in primary human PMCs and in MeT-5A cells after 24 hours of TGF-β1 treatment, suggesting that altered expression of these genes may follow HIF-1α stabilization. This suggestion is also supported by the observation that there was no detectable HIF-1α protein in MeT-5A cells at 12 hours by Western blot (data not shown). These results demonstrate that TGF-β1 is regulating genes that are involved in the glycolysis pathway and are HIF-1α regulated in the PMCs.
Our data show for the first time that lactate is significantly increased in the peritoneal fluid of women with endometriosis and that this correlates to increasing levels of TGF-β1. Taken together with our observation that both the endometriosis lesions and peritoneum adjacent to endometriosis lesions express significantly higher levels of glycolysis markers, these results suggest a possible change in the metabolic phenotype, similar to that of the Warburg effect seen in tumorigenesis. Increasing lactate production by peritoneal tissue and within the peritoneal fluid may “feed” ectopic endometrial cells, allowing them to survive, implant, and invade into the peritoneum, resulting in endometriosis lesions. These results may inform new biomarker strategies, such as in vivo lactate assessment (18), and treatment targets for endometriosis, such as HIF-1 inhibitors (20).
Acknowledgments
We are grateful to Mrs Helen Dewart and Mrs Ann Doust for patient recruitment and sample collection; Dr Forbes Howie for assay development; Prof Steve Hillier for use of the MeT-5A cell line; Mr Bob Morris, Mrs Frances Collins, Ms Arantza Esnal-Zufiurre, and Mrs Jean Wade for technical support and advice; Mrs Sheila Milne for secretarial support; and Mr Ronnie Grant and Mr Jeremy Tavener for graphics support.
This work was funded by a Wellbeing of Women research Grant R42533 awarded to A.W.H., J.K.B., P.T.K.S., and W.C.D.V.J.Y. receives grant support from the Federation of Women Graduates (134225) and a PhD studentship from the College of Medicine and Veterinary Medicine at the University of Edinburgh.
Disclosure Summary: The authors declare they have no conflicts of interest.
Footnotes
Abbreviations:
CV
coefficient of variation
GLUT
glucose transporter
HIF-1
hypoxia inducible factor-1
LDHA
lactate dehydrogenase A
PDK1
pyruvate dehydrogenase kinase 1
PMC
peritoneal mesothelial cell.
References
- 1.Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27:1292–1299. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson TZ, Duffy JM, Barlow D, Koninckx PR, Garry R. Laparoscopic surgery for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2009;4:CD001300. [DOI] [PubMed] [Google Scholar]
- 4.Sampson J. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–469. [Google Scholar]
- 5.Young VJ, Brown JK, Saunders PT, Horne AW. The role of the peritoneum in the pathogenesis of endometriosis. Hum Reprod Update. 2013;19:558–569. [DOI] [PubMed] [Google Scholar]
- 6.Omwandho CO, Konrad L, Halis G, Oehmke F, Tinneberg HR. Role of TGF-βs in normal human endometrium and endometriosis. Hum Reprod. 2010;25:101–109. [DOI] [PubMed] [Google Scholar]
- 7.Hull ML, Johan MZ, Hodge WL, Robertson SA, Ingman WV. Host-derived TGFB1 deficiency suppresses lesion development in a mouse model of endometriosis. Am J Pathol. 2012;180:880–887. [DOI] [PubMed] [Google Scholar]
- 8.Johnson MC, Torres M, Alves A, et al. Augmented cell survival in eutopic endometrium from women with endometriosis: expression of c-myc, TGF-β1 and bax genes. Reprod Biol Endocrinol. 2005;3:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pizzo A, Salmeri FM, Ardita FV, Sofo V, Tripepi M, Marsico S. Behaviour of cytokine levels in serum and peritoneal fluid of women with endometriosis. Gynecol Obstet Invest. 2002;54(2):82–87. [DOI] [PubMed] [Google Scholar]
- 10.Oosterlynck DJ, Meuleman C, Waer M, Koninckx PR. Transforming growth factor-β activity is increased in peritoneal fluid from women with endometriosis. Obstet Gynecol. 1994;83:287–292. [PubMed] [Google Scholar]
- 11.Küpker W, Schultze-Mosgau A, Diedrich K. Paracrine changes in the peritoneal environment of women with endometriosis. Hum Reprod Update. 1998;4:719–723. [DOI] [PubMed] [Google Scholar]
- 12.Li MQ, Hou XF, Lv SJ, et al. CD82 gene suppression in endometrial stromal cells leads to increase of the cell invasiveness in the endometriotic milieu. J Mol Endocrinol. 2011;47:195–208. [DOI] [PubMed] [Google Scholar]
- 13.Shaco-Levy R, Sharabi S, Benharroch D, Piura B, Sion-Vardy N. Matrix metalloproteinases 2 and 9, E-cadherin, and β-catenin expression in endometriosis, low-grade endometrial carcinoma and non-neoplastic eutopic endometrium. Eur J Obstet Gynecol Reprod Biol. 2008;139:226–232. [DOI] [PubMed] [Google Scholar]
- 14.Flores I, Rivera E, Ruiz LA, Santiago OI, Vernon MW, Appleyard CB. Molecular profiling of experimental endometriosis identified gene expression patterns in common with human disease. Fertil Steril. 2007;87:1180–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fosslien E. Cancer morphogenesis: role of mitochondrial failure. Ann Clin Lab Sci. 2008;38(4):307–329. [PubMed] [Google Scholar]
- 16.Guido C, Whitaker-Menezes D, Capparelli C, et al. Metabolic reprogramming of cancer-associated fibroblasts by TGF-β drives tumor growth: connecting TGF-β signaling with “Warburg-like” cancer metabolism and L-lactate production. Cell Cycle. 2012;11:3019–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fosslien E. Cancer morphogenesis: role of mitochondrial failure. Ann Clin Lab Sci. 2008;38:307–329. [PubMed] [Google Scholar]
- 18.Pavlides S, Whitaker-Menezes D, Castello-Cros R, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. [DOI] [PubMed] [Google Scholar]
- 19.Hirschhaeuser F, Sattler UG, Mueller-Klieser W. Lactate: a metabolic key player in cancer. Cancer Res. 2011;71:6921–6925. [DOI] [PubMed] [Google Scholar]
- 20.Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basu RK, Hubchak S, Hayashida T, Runyan CE, Schumacker PT, Schnaper HW. Interdependence of HIF-1α and TGF-β/Smad3 signaling in /Loxic and hypoxic renal epithelial cell collagen expression. Am J Physiol Renal Physiol. 2011;300:F898–F905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haddad JJ, Land SC. A non-hypoxic, ROS-sensitive pathway mediates TNF-α-dependent regulation of HIF-1α. FEBS Lett. 2001;505:269–274. [DOI] [PubMed] [Google Scholar]
- 23.Blouin CC, Pagé EL, Soucy GM, Richard DE. Hypoxic gene activation by lipopolysaccharide in macrophages: implication of hypoxia-inducible factor 1α. Blood. 2004;103:1124–1130. [DOI] [PubMed] [Google Scholar]
- 24.Fukuda R, Kelly B, Semenza GL. Vascular endothelial growth factor gene expression in colon cancer cells exposed to prostaglandin E2 is mediated by hypoxia-inducible factor 1. Cancer Res. 2003;63:2330–2334. [PubMed] [Google Scholar]
- 25.Frede S, Freitag P, Otto T, Heilmaier C, Fandrey J. The proinflammatory cytokine interleukin 1β and hypoxia cooperatively induce the expression of adrenomedullin in ovarian carcinoma cells through hypoxia inducible factor 1 activation. Cancer Res. 2005;65:4690–4697. [DOI] [PubMed] [Google Scholar]
- 26.Tsai YP, Wu KJ. Hypoxia-regulated target genes implicated in tumor metastasis. J Biomed Sci. 2012;19:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wigfield SM, Winter SC, Giatromanolaki A, Taylor J, Koukourakis ML, Harris AL. PDK-1 regulates lactate production in hypoxia and is associated with poor prognosis in head and neck squamous cancer. Br J Cancer. 2008;98:1975–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren X, He YL, Pan SL, Peng DX. Expression of hypoxia-inducible factor-1α in endometriosis [in Chinese]. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27:538–540. [PubMed] [Google Scholar]
- 29.Wu MH, Chen KF, Lin SC, Lgu CW, Tsai SJ. Aberrant expression of leptin in human endometriotic stromal cells is induced by elevated levels of hypoxia inducible factor-1α. Am J Pathol. 2007;170:590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becker CM, Rohwer N, Funakoshi T, et al. 2-Methoxyestradiol inhibits hypoxia-inducible factor-1α and suppresses growth of lesions in a mouse model of endometriosis. Am J Pathol. 2008;172:534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imesch P, Samartzis EP, Schneider M, Fink D, Fedier A. Inhibition of transcription, expression, and secretion of the vascular epithelial growth factor in human epithelial endometriotic cells by romidepsin. Fertil Steril. 2011;95:1579–1583. [DOI] [PubMed] [Google Scholar]
- 32.Fegan KS, Rae MT, Critchley HO, Hillier SG. Anti-inflammatory steroid signalling in the human peritoneum. J Endocrinol. 2008;196:369–376. [DOI] [PubMed] [Google Scholar]
- 33.Ke Y, Reddel RR, Gerwin BI, et al. Establishment of a human in vitro mesothelial cell model system for investigating mechanisms of asbestos-induced mesothelioma. Am J Pathol. 1989;134:979–991. [PMC free article] [PubMed] [Google Scholar]
- 34.Knight PA, Brown JK, Wright SH, Thornton EM, Pate JA, Miller HR. Aberrant mucosal mast cell protease expression in the enteric epithelium of nematode-infected mice lacking the integrin αvβ6, a transforming growth factor-β1 activator. Am J Pathol. 2007;171:1237–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao Y, Liu X, Zhang W, et al. TGF-β repression of Id2 induces apoptosis in gut epithelial cells. Oncogene. 2009;28:1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kottmann RM, Kulkarni AA, Smolnycki KA, et al. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-β. Am J Respir Crit Care Med. 2012;186:740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuzaki S, Darcha C. Epithelial to mesenchymal transition-like and mesenchymal to epithelial transition-like processes might be involved in the pathogenesis of pelvic endometriosis. Hum Reprod. 2012;27:712–721. [DOI] [PubMed] [Google Scholar]
- 38.McLaren J. Vascular endothelial growth factor and endometriotic angiogenesis. Hum Reprod Update. 2000;6:45–55. [DOI] [PubMed] [Google Scholar]
- 39.Jones CJ, Nardo LG, Litta P, Fazleabas AT. Ultrastructure of ectopic peritoneal lesions from women with endometriosis, including observations on the contribution of coelomic mesothelium. Reprod Sci. 2009;16:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeBerardinis RJ. Is cancer a disease of abnormal cellular metabolism? New angles on an old idea. Genet Med. 2008;10:767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]