Proliferation and migration of hepatoblastoma cells are mediated by IRS-4 via PI3K/Akt pathways (original) (raw)
. 2014 Oct 15;7(10):3763–3769.
Abstract
Levels of IRS-4 (insulin receptor substrate-4) in hepatoblastoma cells from the patients, HepG2 and HuH-6 cell lines were measured by Western Blot analysis, and they were all found significantly higher than those of normal hepatic cells. When the HepG2 and HuH-6 cells cultivated in vitro were treated with IRS-4 and infected with recombinant adenovirus containing IRS-4 gene, the proliferation and migration of the cells were significantly improved. Meanwhile, the expressions of PI3K (phosphatidylinositol 3’-kinase), pS473 Akt (Protein Kinase B) and pThr308 Akt were positively regulated by IRS-4 overexpression. These results indicated that IRS-4 was very likely to be involved in PI3K/Akt path way, which was further confirmed by the fact that the PI3K/Akt blocker LY294002 attenuated the proliferation and migration of the hepatoblastoma cells. In conclusion, IRS-4 enhanced the proliferation and migration of the hepatoblastoma cells via PI3K/Akt pathways.
Keywords: Hepatoblastoma, insulin receptor substrate-4, PI3K/Akt pathway, proliferation, migration
Introduction
Hepatoblastoma (HBL), the most common primary pediatric liver tumor, is usually diagnosed during the first three years of life [1]. It originates in liver primordium embryonic cells, and it’s a type of malignant tumor of epithelial origin. HBL was firstly reported by Walter in 1896 [2], which also named as liver embryonic carcinoma or pediatric hepatocellular carcinoma [3]. It is usually solitary and varied in size. The disease has rapid development, high grade malignancy and multiple differentiation paths [4,5]. What’s worse, after its widespread metastasis through lymph and blood circulation, many metastatic sites will be suffering, such as lymph gland, lung, enterocoelia and brain. At present, the main therapy for HBL is exairesis of diseased tissue along with chemotherapy [6]. Recent pediatric cancer epidemiological studies report that the average annual percent have been increasing in incidence of HB during the last 30 years [1].
Insulin receptor substrates (IRSs), which refer to those substrates that react to insulin receptor tyrosine kinase, have a dozen of phosphorylatable tyrosine sites. The phosphorylated IRSs are able to bond to and activate their down-stream effectors. Presently, IRSs family is found to have 4 members, IRS-1, -2, -3 and -4 [7], but their functions in tissue distribution, subcellular localization, integration with insulin and interaction with proteins containing SH2 domain vary from one another. They are the key intermediary molecules in insulin signaling transduction system, participating in a variety of hormones- or cytokines-mediated signal transductions as well as their core effects on maintenance of the basic function of cells like growth, survive and substance metabolism [8,9].
In hepatoblastoma cell lines, the expression level of IRS-4 was significantly increased, showing intimate relationship with tumor growth [10]. It has been well known that PI3K (phosphatidylinositol 3’-kinase)/Akt (Protein Kinase B) pathway plays a very important role in the proliferation of lots of the tumor cells. As for hepatoblastoma cells, this role is not an exception [11]. However, the relation between IRS-4 and cell proliferation and migration in hepatoblastoma cells is still not clear. So, we proposed and authenticated the following assumptions that IRS-4 improves the proliferation and migration of hepatoblastoma cells by targeting PI3K/Akt signaling.
Materials and methods
Tissue and cell samples
Fresh hepatoblastoma and corresponding normal liver tissues that 5 cm away from the edge of the hepatoblastoma tissue were taken from the patients with hepatoblastoma who had not received any chemotherapy or radiotherapy prior to surgery, and then snap-frozen in liquid nitrogen immediately after resection and kept at -80°C. Hepatoblastoma cell lines HepG2 and HuH-6, purchased from Tongpai Biological Technology Co., Ltd. (Shanghai, China), were respectively cultured in DMEM and RPMI-1640 media with 10% FBS (fetal bovine serum), 100 IU/ml penicillin and 100 μg/ml streptomycin at 37°C in a 5% CO2 humidified incubator. The cells were subcultured every 2 or 3 days.
Western blot
Tissue and cell were lysed in accordance with the method described by Cuevas et al. [12]. Referring to the report by Tsuruzoe et al. [13], proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. The blots were blocked with 3% BSA (Bull Serum Albumin) in TBS (triethanolamine-buffered saline) buffer, incubated with primary antibodies (anti-β-actin, anti-IRS-4, anti-Akt, anti-pS473 Akt and anti-pThr308 Akt antibodies) in TBS with 2% BSA in it, and then incubated with secondary antibodies conjugated with horseradish peroxidase. The immunoreactive bands were visualized by ECL (enhanced chemiluminescence) detection kit (Pierce Chemical Co., Rockford, USA) and captured on X-ray film.
Cell proliferation assay
The proliferation of HepG2 and HuH-6 cells was assessed using a cell proliferation ELISA, BrdUrd colorimetric assay kit (Roche Applied Science, USA) according to the manufacturer’s protocol. The cells at a concentration of 1.5 × 105/ml were initially inoculated in a 35 mm culture dish. After the cells were incubated 37°C for 40 min, they were counted under a hemocytometer (Shanghai Medical Optical Instrument Plant, Shanghai, China) under an optical microscope (Shanghai Optical Instruments Factory, China).
Cell migration assay
Migration assay described in the report by Wu et al. [14] was adopted. Twenty four-well transwell chambers with 8.0-μm-pore polycarbonate filter inserts (Nalge Nunc International, Rochester, NY) were used. HepG2 and HuH-6 cells were seeded at a concentration of 1 × 105 individuals per well in complete medium. After the incubation for 48 h, cells migrated to the other side of the membrane were stained with 0.3% crystal violet. The cells in the upper side of the insert membrane were rubbed with a cotton swab. Cell migration was determined by counting whole cell numbers at single filter with optical microscopy at × 100 magnification.
Infection of hepatoblastoma cells by recombinant adenovirus encoding IRS-4
HumanIRS4 adenovirus (Vector Biolabs, Philadelphia, USA) were maintained in packaging cell line 293. According to the literature [15], the recombinant adenovirus was amplified to produce high titer virus stocks of about 0.5 to 1 × 109 pfu/ml. Amplified viruses titration were determined by the limit dilution assay and then used for infection at a MOI (multiplicity of infection) of 50 pfu/cell.
Statistical analysis
Data were expressed as mean ± SE (standard error). Comparisons between two groups were analyzed by Student’s paired t-test. P < 0.05 was considered statistically significant.
Results
Expression of IRS-4 in hepatoblastoma cells was upregulated
Compared with control (normal hepatocytes), IRS-4 in hepatoblastoma cells were all significantly at promoted levels from extremely low ones, not only in tumor tissue of patients with hepatoblastoma, but also in the cell lines HepG2 and HuH-6 (Figure 1).
Figure 1.
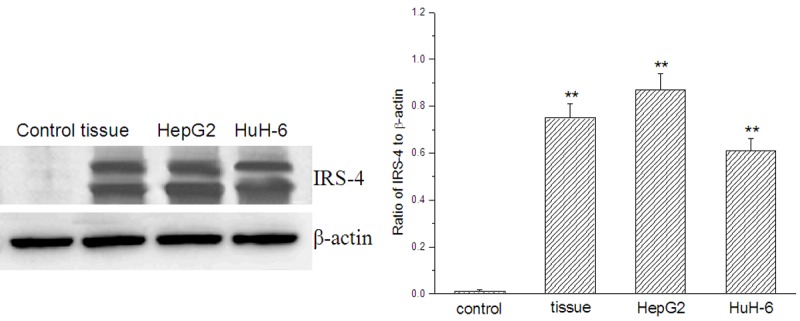
Expression of IRS-4 in tumor tissue and hepatoblastoma cell lines HepG2 and HuH-6 analyzed by Western Blot; control: normal hepatocytes; tissue: tumor tissue of patients with hepatoblastoma; compared with the control, **p < 0.01.
The proliferation and migration of HepG2 and HuH-6 cells were improved by IRS-4.
Compared with the cells treated with PBS, the proliferation of HepG2 and HuH-6 cells were both significantly improved to different extents after the cells were treated with IRS-4 (Figure 2A). Their migration behaved in the similar way as well (Figure 2B).
Figure 2.
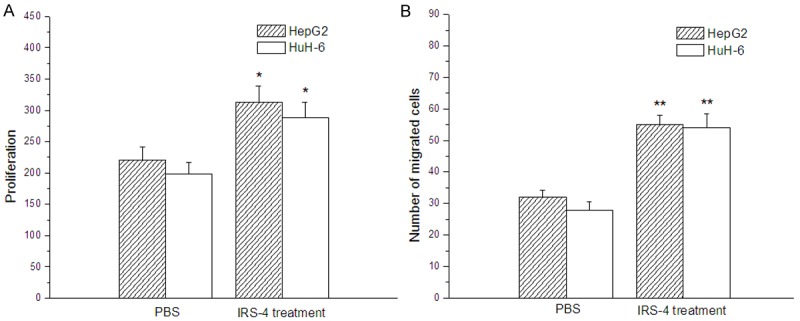
Effects of IRS-4 on the proliferation and migration of hepatoblastoma cells HepG2 and HuH-6 evaluated by cell proliferation and cell migration assays; compared with the hepatoblastoma cells treated with PBS, *p < 0.05, **p < 0.01.
The expressions of PI3K and Akt were upregulated by overexpression of IRS-4
Compared with the non-infected hepatoblastoma cells, expressions of PI3K, pThr 308 Akt and pS473 Akt were all very evidently upregulated both in HepG2 and HuH-6 cells infected with recombinant adenovirus containing IRS-4 gene (Figure 3). The results indicated the expressions of PI3K and Akt were all upregulated by overexpression of IRS-4, which meant that PI3K/Akt signaling was enhanced by overexpressed IRS-4.
Figure 3.
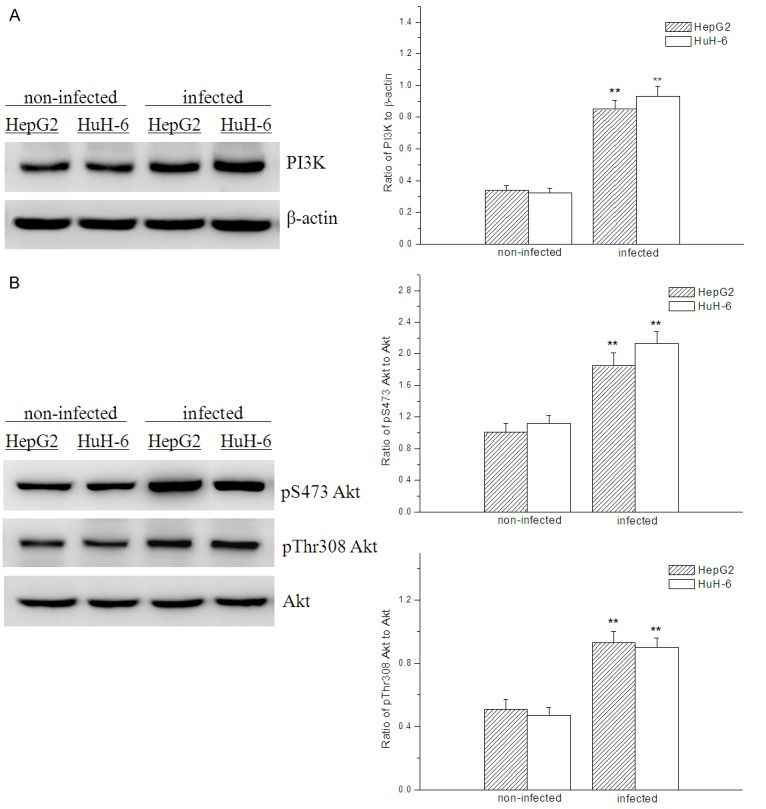
Effect of IRS-4 overexpression on expressions of PI3K, pS473 Akt and pThr 308 Akt evaluated by Western Blot analysis; compared with the non-infected hepatoblastoma cells, **p < 0.01.
PI3K/Akt blocker LY294002 attenuated the proliferation and migration of HepG2 and HuH-6 cells
When HepG2 and HuH-6 cells were treated with LY294002 (10 μM), PI3K/Akt pathway in them was very markedly blocked (Figure 4A), their proliferations in vitro were therefore significantly interfered and dropped down (Figure 4B), and the numbers of migrated cells in HepG2 and HuH-6 cells were significantly reduced (Figure 4C). However, the proliferation and migration of the tumor cells was not totally stopped but still performed.
Figure 4.
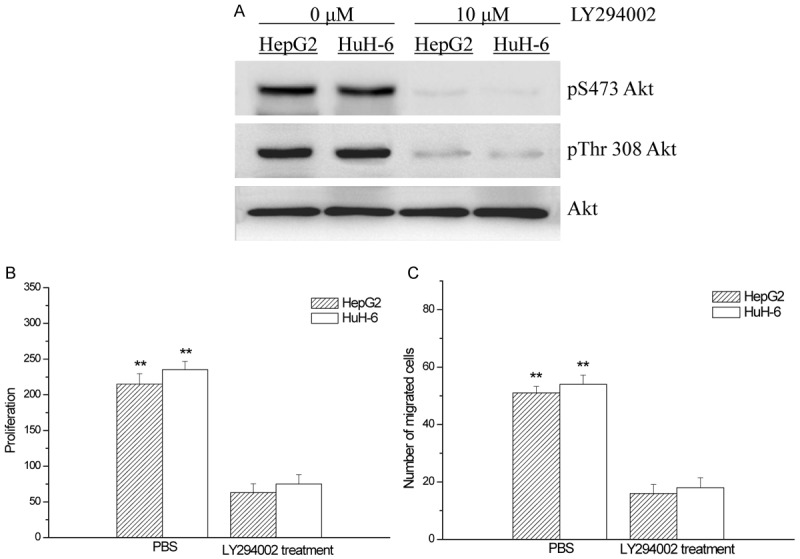
Effects of PI3K/Akt blocker LY294002 on PI3K/Akt pathways (A), the proliferation (B) and migration (C) of HepG2 and HuH-6 cells; compared with the hepatoblastoma cells treated with PBS, **p < 0.01.
Discussion
IRS-4 is a member in the family of cytoplasmic adaptors, which has been reported to couple activation of the insulin receptor to downstream PI3K/Akt and Ras signaling pathways [16,17]. Although IRS-4 is found at a relatively low level in normal human liver tissue, it is highly expressed in the tumors of patients who are suffering from hepatocellular carcinoma [18], including hepatoblastoma (Figure 1). It has been reported that highly expressed IRS-4 had proliferative effects on human tumor cells, such as hepatoblastoma cells HepG2 [19,20]. The similar effect was also observed in HepG2 and HuH-6 cells by our investigation (Figure 2).
It is well known that PI3K/Akt signaling pathway plays a critical role in the proliferation and migration of hepatocellular carcinoma cells [21], and its activation is essential in hepatoblastoma survival [11], which may potentiate tumors’ life process [22]. Interacting with SH2-containing proteins such as PI3K [23], IRS-4 has been proved to be involved in PI3K/Akt pathway, and knockdown of IRS-4 gene will inhibit cell proliferation [19,24]. In our present study, we found PI3K and phosphorylated Akt (pS473 Akt and pThr308 Akt) were upregulated in hepatoblastoma cell lines that were infected with recombinant adenovirus encoding IRS-4 (Figure 3), suggesting that IRS-4 might somehow trigger a further activation of PI3K/Akt pathway [24]. So we supposed the overexpression of IRS-4 triggered and resulted in the enhancement of PI3K/Akt signal transduction, thus led to the improvement of proliferation and migration of hepatoblastoma cells. If the above viewpoint was valid, cell proliferation and migration would be inhibited in hepatoblastoma cells overexpressing IRS-4, when PI3K/Akt was blocked by the blocker LY294002. As a result, the proliferation and migration of hepatoblastoma cells in vitro were attenuated even if in the presence of overexpressed IRS-4 (Figure 4).
Based on the above, it could be concluded that PI3K/Akt signaling was very impotant in IRS-4 mediated cell proliferation and migration, which was consistent with the results reported by Hoxhaj et al. [24]. Taking more into account, it probably was not the unique pathway participated in the proliferation and migration. Some other signal transductions, which could provide alternative options for the proliferation and migration of the tumor cells when PI3K/Akt signaling was blocked, were also activated or enhanced by IRS-4 [12]. So, this may account for why proliferation and migration still went on when PI3K/Akt pathway was blocked.
As for the exact mechanism about PI3K/Akt signaling in IRS-4 mediated cell proliferation and migration, although still unclear, we pointed out two directions, which will be confirmed in our future study. Primarily, it was inferred that IGF-1 was probably taking part in the beginning of the whole transduction system as well as some crucial down-stream signaling molecules, such as ERK and p70S6K [12,23,25]. Secondarily, complicated correlations in tumor cells between IRS-4 and other members of IRS family are controversially reported [13,25,26]. But whatever the correlations really will be, the related signaling pathways mutually interact each other, and these interactions may show us what the exact mechanism is.
To sum up, IRS-4 could mediate PI3K/Akt signaling transduction in hepatoblastoma cells, therefore it enhanced the cell proliferation and migration.
References
- 1.Czauderna P, Lopez-Terrada D, Hiyama E, Häberle B, Malogolowkin MH, Meyers RL. Hepatoblastoma state of the art: pathology, genetics, risk stratification, and chemotherapy. Curr Opini Pediatr. 2014;26:19–28. doi: 10.1097/MOP.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 2.Heywood G, Burgart LJ, Nagorney DM. Ossifying malignant mixed epithelial and stromal tumor of the liver. Cancer. 2002;94:1018–1022. [PubMed] [Google Scholar]
- 3.Broughan TA, Esquivel CO, Vogt DP, Griffin GC, Norris DG. Pretransplant chemotherapy in pediatric hepatocellular carcinoma. J Pediatr Surg. 1994;29:1319–1322. doi: 10.1016/0022-3468(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 4.Herzog CE, Andrassy RJ, Eftekhari F. Childhood cancers: hepatoblastoma. Oncologist. 2000;5:445–453. doi: 10.1634/theoncologist.5-6-445. [DOI] [PubMed] [Google Scholar]
- 5.Zsíros J, Maibach R, Shafford E, Brugieres L, Brock P, Czauderna P, Roebuck D, Childs M, Zimmermann A, Laithier V. Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: final results of the SIOPEL-3HR study. J. Clin. Oncol. 2010;28:2584–2590. doi: 10.1200/JCO.2009.22.4857. [DOI] [PubMed] [Google Scholar]
- 6.Perilongo G, Malogolowkin M, Feusner J. Hepatoblastoma clinical research: Lessons learned and future challenges. Pediatr Blood Cancer. 2012;59:818–821. doi: 10.1002/pbc.24217. [DOI] [PubMed] [Google Scholar]
- 7.Ozoe A, Sone M, Fukushima T, Kataoka N, Chida K, Asano T, Hakuno F, Takahashi S. Insulin receptor substrate-1 associates with small nucleolar RNA which contributes to ribosome biogenesis. Front Endocrinol. 2014;5:24. doi: 10.3389/fendo.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo S. Decoding insulin resistance and metabolic syndrome for promising therapeutic intervention. J Endocrinol. 2014;220:E1–E3. doi: 10.1530/JOE-13-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz M, Velasco E, Kumate J. Intracellular signals intervening in glucose control. Gac Med Mex. 2001;137:135–146. [PubMed] [Google Scholar]
- 10.Cuevas EP, Escribano O, Monserrat J, Martínez-Botas J, Sánchez MG, Chiloeches A, Hernández-Breijo B, Sánchez-Alonso V, Román ID, Fernández-Moreno MD. RNAi-mediated silencing of insulin receptor substrate-4 enhances actinomycin D-and tumor necrosis factor-α-induced cell death in hepatocarcinoma cancer cell lines. J Cell Biochem. 2009;108:1292–1301. doi: 10.1002/jcb.22359. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann W, Küchler J, Koch A, Friedrichs N, Waha A, Endl E, Czerwitzki J, Metzger D, Steiner S, Wurst P. Activation of phosphatidylinositol-3’-kinase/AKT signaling is essential in hepatoblastoma survival. Clin Cancer Res. 2009;15:4538–4545. doi: 10.1158/1078-0432.CCR-08-2878. [DOI] [PubMed] [Google Scholar]
- 12.Cuevas EP, Escribano O, Chiloeches A, Ramirez Rubio S, Román ID, Fernández-Moreno MD, Guijarro LG. Role of insulin receptor substrate-4 in IGF-I-stimulated HEPG2 proliferation. J Hepatol. 2007;46:1089–1098. doi: 10.1016/j.jhep.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 13.Tsuruzoe K, Emkey R, Kriauciunas KM, Ueki K, Kahn CR. Insulin receptor substrate 3 (IRS-3) and IRS-4 impair IRS-1-and IRS-2-mediated signaling. Mol Cell Biol. 2001;21:26–38. doi: 10.1128/MCB.21.1.26-38.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu WS, Tsai RK, Chang CH, Wang S, Wu JR, Chang YX. Reactive oxygen species mediated sustained activation of protein kinase C α and extracellular signal-regulated kinase for migration of human hepatoma cell Hepg2. Mol Cancer Res. 2006;4:747–758. doi: 10.1158/1541-7786.MCR-06-0096. [DOI] [PubMed] [Google Scholar]
- 15.Dubourdeau M, Miyamura T, Matsuura Y, Alric L, Pipy B, Rousseau D. Infection of HepG2 cells with recombinant adenovirus encoding the HCV core protein induces p21WAF1 down-regulation-effect of transforming growth factor β. J Hepatol. 2002;37:486–492. doi: 10.1016/s0168-8278(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 16.Wauman J, De Smet AS, Catteeuw D, Belsham D, Tavernier J. Insulin receptor substrate 4 couples the leptin receptor to multiple signaling pathways. Mol Endocrinol. 2008;22:965–977. doi: 10.1210/me.2007-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo Y, Yang C, Jin C, Xie R, Wang F, McKeehan WL. Novel phosphotyrosine targets of FGFR2IIIb signaling. Cell Signal. 2009;21:1370–1378. doi: 10.1016/j.cellsig.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cantarini MC, de la Monte SM, Pang M, Tong M, D’Errico A, Trevisani F, Wands JR. Aspartyl-asparagyl β hydroxylase over-expression in human hepatoma is linked to activation of insulin-like growth factor and notch signaling mechanisms. Hepatology. 2006;44:446–457. doi: 10.1002/hep.21272. [DOI] [PubMed] [Google Scholar]
- 19.Fantin VR, Keller SR, Lienhard GE, Wang LM. Insulin receptor substrate 4 supports insulin-and interleukin 4-stimulated proliferation of hematopoietic cells. Biochem Biophy Res Commun. 1999;260:718–723. doi: 10.1006/bbrc.1999.0967. [DOI] [PubMed] [Google Scholar]
- 20.Villarreal RS, Forneris ML, Uranga RM, Salvador GA, Ciuffo GM. Role of IRS-4 in PI3-K activation by insulin in HepG2 cells, modulation by angiotensin II. Regul Pept. 2010;161:67–72. doi: 10.1016/j.regpep.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, Anania FA. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67:2497–2507. doi: 10.1158/0008-5472.CAN-06-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annual review of pathology. 2009;4:127. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qu BH, Karas M, Koval A, LeRoith D. Insulin receptor substrate-4 enhances insulin-like growth factor-I-induced cell proliferation. J Biol Chem. 1999;274:31179–31184. doi: 10.1074/jbc.274.44.31179. [DOI] [PubMed] [Google Scholar]
- 24.Hoxhaj G, Dissanayake K, MacKintosh C. Effect of IRS4 Levels on PI 3-Kinase Signalling. PloS One. 2013;8:e73327. doi: 10.1371/journal.pone.0073327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boissan M, Beurel E, Wendum D, Rey C, Lécluse Y, Housset C, Lacombe ML, Desbois-Mouthon C. Overexpression of insulin receptor substrate-2 in human and murine hepatocellular carcinoma. Am J Pathol. 2005;167:869–877. doi: 10.1016/S0002-9440(10)62058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka S, Wands JR. Insulin receptor substrate 1 overexpression in human hepatocellular carcinoma cells prevents transforming growth factor β1-induced apoptosis. Cancer Res. 1996;56:3391–3394. [PubMed] [Google Scholar]