Underwater Photosynthesis in Flooded Terrestrial Plants: A Matter of Leaf Plasticity (original) (raw)
Abstract
• Background Flooding causes substantial stress for terrestrial plants, particularly if the floodwater completely submerges the shoot. The main problems during submergence are shortage of oxygen due to the slow diffusion rates of gases in water, and depletion of carbohydrates, which is the substrate for respiration. These two factors together lead to loss of biomass and eventually death of the submerged plants. Although conditions under water are unfavourable with respect to light and carbon dioxide supply, photosynthesis may provide both oxygen and carbohydrates, resulting in continuation of aerobic respiration.
• Scope This review focuses on evidence in the literature that photosynthesis contributes to survival of terrestrial plants during complete submergence. Furthermore, we discuss relevant morphological and physiological responses of the shoot of terrestrial plant species that enable the positive effects of light on underwater plant performance.
• Conclusions Light increases the survival of terrestrial plants under water, indicating that photosynthesis commonly occurs under these submerged conditions. Such underwater photosynthesis increases both internal oxygen concentrations and carbohydrate contents, compared with plants submerged in the dark, and thereby alleviates the adverse effects of flooding. Additionally, several terrestrial species show high plasticity with respect to their leaf development. In a number of species, leaf morphology changes in response to submergence, probably to facilitate underwater gas exchange. Such increased gas exchange may result in higher assimilation rates, and lower carbon dioxide compensation points under water, which is particularly important at the low carbon dioxide concentrations observed in the field. As a result of higher internal carbon dioxide concentrations in submergence-acclimated plants, underwater photorespiration rates are expected to be lower than in non-acclimated plants. Furthermore, the regulatory mechanisms that induce the switch from terrestrial to submergence-acclimated leaves may be controlled by the same pathways as described for heterophyllous aquatic plants.
Keywords: Flooding, gas exchange, heterophylly, hormonal regulation, leaf morphology, phenotypic plasticity, photorespiration, photosynthesis, Rumex, submergence, survival, wetlands
INTRODUCTION
Complete submergence imposes considerable stress on plant functioning, predominantly by way of oxygen deprivation, and rapidly results in loss of biomass and ultimately in death of many plant species. However, not all species are equally vulnerable to submergence, and flooding therefore results in distinct distribution limits of plant species along the vertical elevation gradient of river floodplains (Sýkora et al., 1988; Lenssen et al., 1999; Silvertown et al., 1999; Bockelmann et al., 2002). As expected, flooding-sensitive species are generally restricted to high elevated sites in these floodplains where floods are rare, whereas most species growing at low elevated and more frequently flooded habitats are tolerant to flooding. Although this relationship may seem trivial, experimental data were not available until recently, when van Eck et al. (2004) found a strong relationhip between the LT50 of species, i.e. the time at which 50% of the plants had perished upon experimental submergence, and their vertical distribution in a floodplain. In particular, the depth and the duration of the floods appear strongly to determine the chances of plant survival (Klimešová, 1994; Blom and Voesenek, 1996; Toner and Keddy, 1997; van de Steeg and Blom, 1998; Casanova and Brock, 2000), even in flood-tolerant species.
Plants have evolved a number of mechanisms that are considered to reduce the negative effects of submergence, and which include both metabolical and morphological plasticity (Armstrong et al., 1994_a_; Vartapetian and Jackson, 1997). Many of the traits of flood-tolerant plants are directed to amelioration of oxygen availability. A well-described example is elongation of the shoot (reviewed in Voesenek et al., 2004), either by increased growth of petioles and lamina (e.g. in Rumex palustris; Voesenek et al., 2003) or by stem elongation (e.g. in rice; Oryza sativa; Kende et al., 1998), which can ultimately restore the contact of the plant with the atmosphere. Once oxygen enters the shoot, within-plant diffusion is enhanced by longitudinal air channels (aerenchyma) in shoot and roots (Visser et al., 1996; Jackson and Armstrong, 1999; Colmer, 2003) and by development of a gas-tight barrier in the roots to prevent oxygen from diffusing into the anaerobic soil (Armstrong 1979; Colmer et al., 1998; Visser et al., 2000). Voesenek et al. (2004) showed, however, that only a subset of flooding-tolerant plant species was capable of significant shoot elongation. These species generally inhabit poorly drained habitats, where floodwater may remain stagnant for a substantial period of the growing season, and shoot elongation is at these sites an efficient solution to avoid oxygen deficiency. Many species, on the other hand, experience submerged conditions that are too deep for the shoot to reach the surface. A straightforward way to reduce shortage of both oxygen and carbohydrates under such conditions would be the continuation of photosynthesis under water. As photosynthesis produces both oxygen and carbohydrates, it might alleviate stress considerably in completely submerged plants.
Our aim is to provide an overview of current knowledge on the importance of underwater photosynthesis for the survival of submerged terrestrial plants. The main factors that change in the underwater environment will be briefly discussed, after which we will summarize the effects of photosynthesis on internal oxygen concentrations.
Furthermore, we will focus on the morphological acclimation of the shoot that is often found in submerged plants, which is important for gas exchange between the leaves and the floodwater, and thus crucial for underwater photosynthesis. The relevance of these changes in leaf phenotype for plant performance will be shown by comparing them with the leaves of heterophyllous aquatic and amphibious species. The latter groups also show high plasticity in leaf morphology and have leaves specialized for photosynthesis in either air or water. We will conclude the review with some perspectives of what we believe are important questions that remain to be solved to increase our understanding of the importance and function of underwater photosynthesis further.
SUBMERGED CONDITIONS LIMIT THE AVAILABILITY OF OXYGEN, LIGHT AND CARBON
Submergence severely inhibits gas exchange between the plant and the environment due to a 104 times lower diffusion rate of gases in water than in air (Jackson, 1985). As a result of this hampered gas exchange, oxygen concentrations within the submerged plant may fall in darkness (Stünzi and Kende, 1989; Rijnders et al., 2000). Such low oxygen levels will then limit aerobic respiration and other essential oxygen-dependent processes (Armstrong and Gaynard, 1976; Laan et al., 1990). Anaerobic metabolic pathways, such as fermentation, may partly compensate the low ATP yield from impaired aerobic metabolism (Perata and Alpi, 1993; Gibbs and Greenway, 2003), but these pathways are far less efficient than aerobic respiration and thus reduce the pool of carbohydrate reserves rapidly (Laan and Blom, 1990; Guglielminetti et al., 1997). Submergence-induced oxygen deficiency in terrestrial plants is, therefore, inevitably accompanied by energy and carbohydrate deficits.
Light quantity and quality are also different under water, compared with the conditions above water. Aquatic environments are generally considered to be shaded environments, since light is attenuated by surface reflection, back-scattering, and absorption by water and suspended particles (Holmes and Klein, 1987; Sand-Jensen, 1989). This particularly applies to river water, in which the load of suspended sediment is often very high, and thus light transmission is poor. For example, median transmission in the river Rhine is <1% in a flood of 1 m depth, even at the lowest levels of suspended loads observed during flooding (Vervuren et al., 2003). Similarly, seasonally flooded rice fields may also suffer from turbid conditions, with light penetrating <0·4 m deep into the floodwater (Ram et al., 2002). Such low light conditions result in a particularly unfavourable environment for underwater photosynthesis.
Additionally, photosynthesis will not only be limited by light during flooding, but also the availability of carbon dioxide is severely limited. Although this gas is approx. 28 times more soluble in water than oxygen, the slow diffusion rate in water will greatly hamper uptake rates by the leaves compared with those in air. Boundary layers around the leaves are likely to be several orders greater in water than in air, particularly in stagnant or slow flowing water (Smith and Walker, 1980). Additionally, carbon dioxide levels fluctuate strongly in time, in both diurnal and yearly cycles, with higher concentrations typically present at night and in the colder season, because of temperature effects on the solubility of gasses in water (Maberly, 1985). Floodwater may contain higher carbon dioxide concentrations if the pH is sufficiently low, but both rice fields (Setter et al., 1987) and river forelands (van den Brink et al., 1993) are commonly submerged with water containing little carbon dioxide, e.g. in the range of 3–100 µm.
LIGHT IMPROVES SURVIVAL DURING SUBMERGENCE
Because of the unfavourable light conditions and carbon dioxide concentrations described above, potentials for underwater photosynthesis would be expected to be very low in terrestrial plants. However, an increasing body of evidence points to a beneficial effect of light on plant survival. Vervuren et al. (2003) tested the survival of various floodplain species during prolonged submergence in different light environments. In all species tested, higher light conditions resulted in improved survival, independent from their flooding tolerance. Remarkable was the response of Rumex crispus, a perennial grassland species, whose survival increased from 4 months in nearly dark conditions (0·4 µmol PAR m−2 s−1; day/night length 16 h/8 h) to >2 years in rather low light conditions (17 µmol PAR m−2 s−1, day/night length 16 h/8 h). Clearly, not much light is needed to maintain surviving plant tissues, although total biomass usually decreases considerably under such conditions. Similar but less extreme responses were found for three other Rumex species (Nabben et al., 1999), some terrestrial Ranunculus species (He et al., 1999), a set of other river-accompanying species (Blom et al., 1994) and rice (Adkins et al., 1990; Ito et al., 1999; Ram et al., 2002). Even tall helophyte (Clevering et al., 1995; Armstrong et al., 1999) and tree species (Siebel et al., 1998) may profit from underwater photosynthesis, when they are submerged during their establishment phase.
Improved survival of submerged plants in the presence of light was found to correlate with the carbohydrate status of the plants. Internal concentrations of soluble carbohydrates and starch in the shoots of submerged rice decreased in both light and shaded (i.e. 75 % less light) conditions, but submerged plants in full light always contained more carbohydrates (30–160 %, depending on the cultivar) than those submerged in shade (Ram et al., 2002). Rumex crispus showed a similar response, although in this case the total starch content, rather than the concentration, was considerably higher (approx. 70 %) in light-grown submerged plants (Laan and Blom, 1990). Such differences in carbohydrate levels are likely to result from carbon fixation and subsequent sucrose production, which in turn fuels respiration and thereby decreases the demand on stored carbon (i.e. starch). On the other hand, oxygen produced by photosynthesis may improve the aeration status of submerged plant organs (see the following section), and thus enable continued aerobic respiration, which is far more efficient in terms of carbohydrate use per unit ATP produced than anaerobic metabolism (Gibbs and Greenway, 2003). In this way, the depletion of the carbohydrate storage will also be slowed down considerably.
LIGHT INCREASES THE INTERNAL OXYGEN CONCENTRATIONS IN SUBMERGED PLANTS
As shown above, light, and therefore presumably underwater photosynthesis, may determine the survival of terrestrial plant species when flooded. Oxygen deficiency is commonly considered to be the main stress factor under these conditions, and one would therefore expect that one important result from photosynthesis is an improved oxygen status in the submerged leaves. Then, if the porosity of the tissues is sufficiently high, oxygen may be able to diffuse throughout the plant, including the root system. Such a mechanism has been shown for submerged Eriophorum angustifolium plants (Gaynard and Armstrong, 1987), where photosynthetically produced oxygen contributed to radial oxygen loss (ROL) from the roots to the sediment. Similarly, rice (Waters et al., 1989), sea grasses (Pedersen et al., 1998, 2004) and amphibious isoetid species (Pedersen et al., 1995; Sorrell, 2004) showed substantially higher oxygen concentrations in the rhizosphere during the light period. Also, hydroponically grown Rumex maritimus plants that were completely submerged in the light showed lower oxygen uptake rates from the medium surrounding the roots compared with dark-submerged plants, due to the additional supply of photosynthetically derived oxygen (Laan and Blom, 1990). These indirect methods have in common that they all clearly point to increased oxygen concentrations in submerged plants when these were photosynthesizing, although they do not predict the actual internal oxygen concentration.
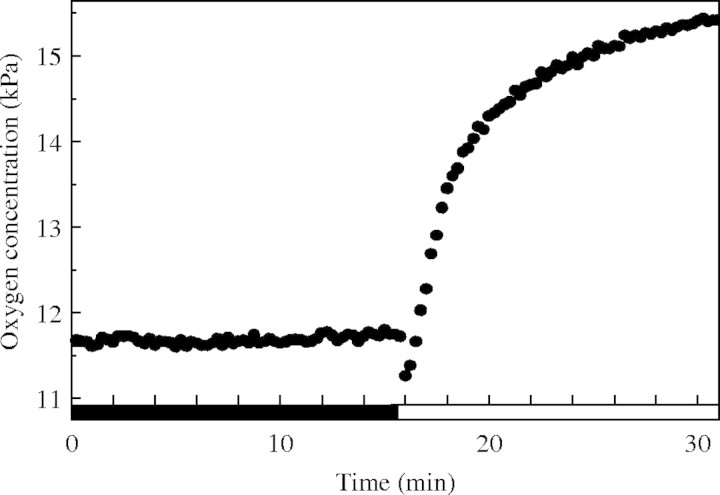
A more direct approach was used by Rijnders et al. (2000) and Mommer et al. (2004), who measured the internal oxygen concentration in the petioles of submerged R. palustris plants and found increased concentrations in the light (Fig. 1), even in the presence of very limited amounts of dissolved carbon dioxide in the floodwater (Mommer et al., 2004). The internal oxygen concentrations observed in the study of Mommer et al. (2004) were more than sufficient to maintain aerobic respiration in the shoot in light, but also in darkness, indicating that hypoxic conditions are not always as prevalent as reported in the past. Putatively, such relatively high oxygen concentrations in the shoot provide a source to maintain at least part of the root system well aerated (Armstrong et al., 1994_b_; Sand-Jensen et al., 2005).
Fig. 1.
Internal oxygen concentrations in the petiole of a submerged Rumex palustris plant, in the dark (closed bar) and in the light (open bar). Oxygen concentrations were measured with a microelectrode inserted in the petiole in close proximity to the leaf lamina (cf. Mommer et al., 2004). Light conditions were saturating (450 µmol PAR m−2 s−1 at the leaf level), and external dissolved carbon dioxide concentration was low (8 µm), at a temperature of 20 °C.
CARBON DIOXIDE IS AN IMPORTANT LIMITING FACTOR FOR UNDERWATER PHOTOSYNTHESIS
As stated in a previous section, underwater photosynthesis will not only be limited by light, but also by a severely reduced inorganic carbon supply compared with photosynthesis above water, due to slower carbon dioxide diffusion rates (Bowes, 1987; Madsen and Sand-Jensen, 1994). Furthermore, the development of larger stagnant boundary layers around the leaves (Smith and Walker, 1980; Jones et al., 2000) reduces carbon dioxide availability for photosynthesis under water even further. In terrestrial plants, which are not specialized for an aquatic life, underwater photosynthesis is, therefore, characterized by relatively low photosynthesis rates, high carbon dioxide compensation points and low uptake efficiency of carbon dioxide due to high diffusion resistance (Maberly and Madsen, 1998; Sand-Jensen and Frost-Christensen, 1999).
True aquatic plant species often have thin, highly dissected leaves, a morphology which is believed to be directed to the optimization of gas exchange underwater (Sculthorpe, 1967; Rascio et al., 1999). These ‘aquatic’ leaves, such as in aquatic Ranunculus species (Bruni et al., 1996) and Elodea nuttallii (Jones et al., 2000), do not possess stomata. Instead, cuticles of these leaves are minimized or even lacking and, therefore, underwater gas exchange most probably occurs via the epidermal cells and cuticle layer.
Many aquatic plants not only rely on their highly specialized growth forms, but have also developed additional carbon dioxide-concentrating mechanisms, which enhance carbon gain under water (Bowes and Salvucci, 1989; Keeley and Santamaria, 1992; Maberly and Madsen, 2002). The most widespread mechanism to increase carbon dioxide availability is the ability to use 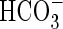 in photosynthesis (Allen and Spence, 1981; Prins and Elzenga, 1989; Madsen, 1993). This may be achieved by proton extrusion at one side of the leaf, thereby lowering the pH and thus shifting the inorganic carbon equilibrium in favour of carbon dioxide over
in photosynthesis (Allen and Spence, 1981; Prins and Elzenga, 1989; Madsen, 1993). This may be achieved by proton extrusion at one side of the leaf, thereby lowering the pH and thus shifting the inorganic carbon equilibrium in favour of carbon dioxide over 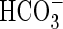 (Prins et al., 1982; Lara et al., 2002). Alternatively,
(Prins et al., 1982; Lara et al., 2002). Alternatively, 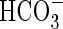 itself may also be actively taken up (Elzenga and Prins, 1989; Lara et al., 2002). The use of
itself may also be actively taken up (Elzenga and Prins, 1989; Lara et al., 2002). The use of 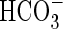 is a carbon-concentrating mechanism, and often coupled to a C4 metabolism, as has been reported for Hydrilla verticillata (Holaday and Bowes, 1980; Spencer et al., 1996; Magnin et al., 1997; Reiskind et al., 1997), Elodea canadensis (Elzenga and Prins, 1989) and Egeria densa (Browse et al., 1979; Casati et al., 2000). This type of metabolism generally relies on a spatial separation between the C3 and C4 carboxylating enzymes, but the characteristic Kranz or bundle sheath anatomy observed in terrestrial plants (Lambers et al., 1998) is most often lacking in aquatic species (Magnin et al., 1997; Reiskind et al., 1997). Separation between the C3 and C4 carboxylating enzymes in aquatic species appears to occur at the cellular level at the chloroplasts (Reiskind et al., 1997; Casati et al., 2000; Rao et al., 2002).
is a carbon-concentrating mechanism, and often coupled to a C4 metabolism, as has been reported for Hydrilla verticillata (Holaday and Bowes, 1980; Spencer et al., 1996; Magnin et al., 1997; Reiskind et al., 1997), Elodea canadensis (Elzenga and Prins, 1989) and Egeria densa (Browse et al., 1979; Casati et al., 2000). This type of metabolism generally relies on a spatial separation between the C3 and C4 carboxylating enzymes, but the characteristic Kranz or bundle sheath anatomy observed in terrestrial plants (Lambers et al., 1998) is most often lacking in aquatic species (Magnin et al., 1997; Reiskind et al., 1997). Separation between the C3 and C4 carboxylating enzymes in aquatic species appears to occur at the cellular level at the chloroplasts (Reiskind et al., 1997; Casati et al., 2000; Rao et al., 2002).
Another alternative carboxylation pathway is crassulacean acid metabolism (CAM), which is also observed in drought-adapted plant species. This alternative carboxylation pathway has a separation of the two carboxylation steps in time. CAM has only been observed in isoetids, such as Lobelia dortmanna and Littorella uniflora (Robe and Griffiths, 1990; Madsen et al., 2002). These species can use carbon dioxide from the sediment, which contains high carbon dioxide concentrations as a result of microbial respiration (Wium-Andersen, 1971; Roelofs et al., 1984; Pedersen et al., 1995). This carbon dioxide diffuses from the soil into the roots and then further follows the concentration gradient into the shoot via aerenchymatous tissue. Future research will need to prove if these mechanisms can be induced in terrestrial wetland plants.
HETEROPHYLLY AS A STRATEGY TO INCREASE CARBON DIOXIDE UPTAKE IN CONTRASTING ENVIRONMENTS
Several aquatic and amphibious species growing in the transition from water to land, e.g. various Potamogeton species (Frost-Christensen and Sand-Jensen, 1995), L. uniflora (Hostrup and Wiegleb, 1991; Robe and Griffiths, 1998) and Ranunculus species (Bruni et al., 1996; Garbey et al., 2004), show remarkable plasticity in leaf form, specialized for photosynthesis either in air or under water (Maberly and Spence, 1989; Sand-Jensen and Frost-Christensen, 1999). In order to acclimate successfully to flooding and improve underwater gas exchange, submerged terrestrial plants probably need to develop leaves with a fundamentally different morphology and anatomy. It is an intriguing question whether terrestrial plants can employ heterophyllous strategies similar to aquatic and amphibious species.
As mentioned above, typical aquatic-like leaves have a specialized leaf form with filamentous, dissected leaves with few or no stomata, which is entirely different from the terrestrial form (Sculthorpe, 1967). Most aquatic leaves of amphibious plants, however, are simply more elongated and thinner and have a higher specific leaf area (SLA) than terrestrial leaves (Nielsen, 1993; Frost-Christensen and Sand-Jensen, 1995). Measurements on the terrestrial plant R. palustris also showed elongated leaves (Fig. 2) and an increased SLA (Mommer et al., 2005), indicating decreased thickness and a relatively increased gas exchange area (Mommer et al., 2004).
Fig. 2.

Morphology of a control (left) and submerged (right) Rumex palustris plant. Submergence took place during 14 d in 0·7 m deep containers with circulating clear tap water, with light intensities of 100 µmol PAR m−2 s−1 at leaf level and a 16 h/8 h day/night cycle; temperature 20 °C. Plants were 52 d old. Scale bar = 50 mm.
Leaf plasticity does not only occur at the level of species, but the ability to express differential leaf anatomy under different environmental conditions may also vary among populations of a single species (Lynn and Waldren, 2001; Lenssen et al., 2004). Lynn and Waldren (2002) showed that populations of Ranunculus repens from frequently flooded habitats have a highly dissected leaf type, which was more favourable for underwater photosynthesis. In the case of another terrestrial Ranunculus species, R. flammula, the one population that had little leaf plasticity performed extremely poorly in a survival experiment under submerged conditions (10 % survival) compared with populations that could change their leaf morphology in response to submergence (75–100 % survival) (Cook and Johnson, 1967).
Aquatic leaf type formation in heterophyllous amphibious plants has been claimed to be regulated by the plant hormones ethylene and abscisic acid (ABA) (Kuwabara et al., 2001; Minorsky, 2003). Leaves were narrow and contained fewer stomata when Ludwigia arcuata was submerged or when treated with ethylene (Kuwabara et al., 2003). However, the ethylene concentrations needed to mimic the submergence response fully were exceptionally high (>50 µL L−1) and may not be reached under submerged conditions [given the data provided in the same paper, and the concentrations of 4–5 µL L−1 ethylene in submerged _Rumex_ plants found by Voesenek et al. (1993_a_)]. Exogenous supply of ABA was able to counteract the submergence response and switched on terrestrial leaf formation in Marsilea quadrifolia (Lin and Yang, 1999) and L. arcuata (Kuwabara et al., 2003). Hsu et al. (2001) confirmed these results and, moreover, showed that the ABA response was correlated with a differential expression pattern of ABA-induced ABRH (ABA-responsive heterophylly) genes in Marsilea.
Contrasting evidence for ABA action originates from work on Egeria densa, where application of exogenous ABA induced expression of C4-like biochemical traits (Casati et al., 2000). Thus, although ABA induces the terrestrial leaf morphology in Marsilea and Ludwigia, it induces the ‘aquatic’ photosynthesis type in Egeria. To increase complexity even further, Eleocharis vivipara also showed induction of C4 photosynthesis by ABA (Ueno, 1998), but in this species the C4 traits were expressed in air instead of under water, where it showed C3 characteristics (Ueno, 2001). We conclude that ABA is likely to play an important role in inducing a heterophyllous switch (with many details still needing to be elucidated), but it remains difficult to extrapolate its role in leaf anatomy to its role in photosynthetic metabolism. Interestingly, ABA, in an interplay with ethylene, is also a key player in submergence-induced elongation growth responses in the terrestrial species R. palustris and rice (Kende et al., 1998; Voesenek et al., 2003), but this work focused on petioles and stems rather than on the leaf lamina. It therefore remains to be clarified whether these plant hormones are also responsible for changes in leaf morphology of submerged terrestrial plants.
LEAF ACCLIMATION ENHANCES GAS EXCHANGE FROM THE WATER COLUMN INTO THE LEAVES
Attempts to relate underwater photosynthesis to flooding tolerance have failed when non-acclimated plants were investigated at high carbon dioxide concentrations (Voesenek et al., 1993_b_; He et al., 1999; Vervuren et al., 1999; Mommer et al., 2005). We suggest, therefore, that it is not the photosynthesis capacity under water per se that determines survival under water, but photosynthetic performance under more natural conditions, where carbon dioxide availability is limited and thus low gas diffusion resistance becomes more important.
The degree to which plants are able to conduct underwater photosynthesis largely depends on the gas exchange capacity of their leaves under water. The development of new, acclimated leaves may therefore be crucial for survival under water. We observed that flooding-tolerant species generally continued to develop new leaves during complete submergence, whereas flooding-intolerant species, such as Daucus carota, were hardly able to develop new leaves under water (Fig. 3A). This inability of flooding-intolerant species to produce new leaves is probably related to shortage of energy, as illustrated by van Eck et al. (2005), who showed that intolerant species such as D. carota were unable to access stored carbohydrates in the taproot. Furthermore, internal aeration in these species may be poor and thus limits underwater plant performance. Flooding-tolerant species had different patterns of leaf formation under water. Rumex palustris showed a continuous turnover of leaves, compensating the loss of older leaves by formation of new acclimated leaves (Fig. 3B), whereas other species, such as Mentha aquatica, had much lower turnover rates, but also continued leaf development (Fig. 3C). Another flood-tolerant species, Oenanthe aquatica, even developed highly dissected leaves under water (L. Mommer unpubl. res.), strongly resembling the submerged leaves of some aquatic heterophyllous Ranunculus species (Bruni et al., 1996; Rascio et al., 1999; Germ and Gaberscik, 2003; Garbey et al., 2004).
Fig. 3.
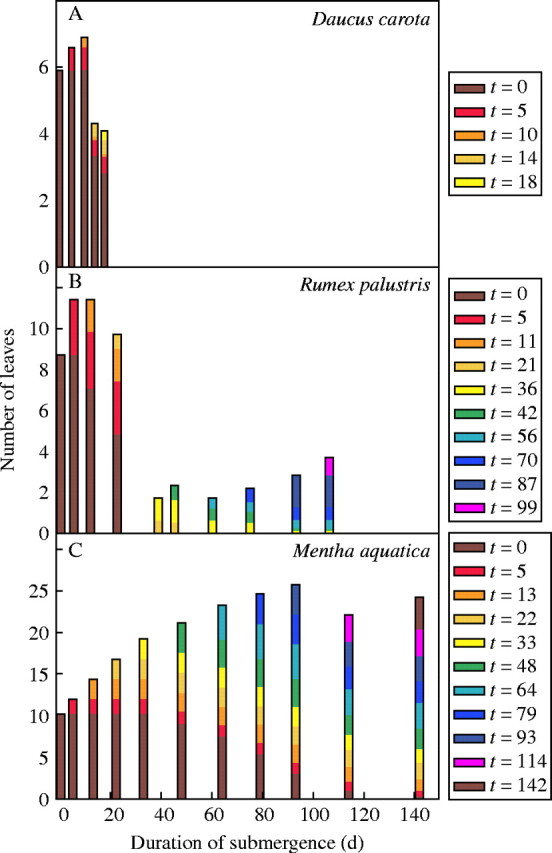
Leaf longevity of submerged plants of three plant species contrasting in flooding tolerance. Species presented are (A) _Daucus carota_—flooding-intolerant; (B) _Rumex palustris_—flooding-tolerant; (C) _Mentha aquatica_—flooding-tolerant. New leaves present at the subsequent censuses (t = x days) are represented by different colours. Leaves present at the onset of submergence are indicated as t = 0. Conditions of submergence were similar to those given at Fig. 2, but with lower light conditions (30 µmol PAR m−2 s−1). Measurements were performed on ten plants per species. Standard errors were typically 5 % of the mean.
The amount of data on the effect of leaf acclimation on the internal gas concentration is very limited. Experiments with microelectrodes measuring internal oxygen concentrations within the petioles of submerged R. palustris plants showed that, even in the dark when the only source of oxygen is uptake from the floodwater, the internal oxygen concentrations were considerably higher in submergence-acclimated plants than in non-acclimated plants (Mommer et al., 2004). This passive diffusion of oxygen from the water column into the plant has been observed previously for aquatic macrophytes such as L. uniflora and L. dortmanna (Sand-Jensen et al., 1982) and seagrasses (Pedersen et al., 1998; Greve et al., 2003; Pedersen et al., 2004). It was remarkable that the internal oxygen concentrations of petioles of submergence-acclimated Rumex plants were almost similar to the oxygen concentrations of the water column (Mommer et al., 2004). This shows clearly that shoot acclimation to submergence is particularly functional with respect to gas exchange capacity between the water column and the plant.
The data of Mommer et al. (2004) contrast to some extent with experiments of Rijnders et al. (2000) and Stünzi and Kende (1989), where internal oxygen concentrations decreased rapidly upon submergence. This contrast shows the importance of boundary layers under water. Oxygen concentrations remained stable and relatively high when plants were submerged in a stirred solution (Mommer et al., 2004), whereas, without stirring, oxygen concentrations fell rapidly (Stünzi and Kende, 1989; Rijnders et al., 2000).
The data described above suggest that shoot acclimation to submergence involves a reduction of the diffusion resistance to gases, which increases not only diffusion of oxygen into the plant, but also the influx of carbon dioxide, which enhances underwater photosynthesis. Such reduced gas diffusion resistance resulted in aquatic leaves of amphibious plant species exhibiting increased underwater photosynthesis rates and a higher carbon dioxide affinity compared with their terrestrial counterparts (Frost-Christensen and Sand-Jensen, 1992, 1995; Nielsen, 1993). The major factor determining carbon dioxide uptake efficiency under water is considered to be cuticle resistance (Frost-Christensen et al., 2003). Frost-Christensen et al. (2003) showed that aquatic leaves of five amphibious species had a reduced cuticle thickness compared with terrestrial leaves, and an accompanying reduced diffusional resistance for gases such as oxygen. Hoffmann-Benning and Kende (1992) did not find differences in cuticle and epidermal cell wall thickness of submerged, elongated deepwater rice stem segments compared with non-flooded internodes, indicating that cuticle and cell wall synthesis can keep pace with the fast elongation growth (up to 5 mm h−1) that takes place in this and other terrestrial wetland species. However, 14C-labelled palmitic acid fed to fast elongating stem segments was incorporated into cutin polymers with different composition, as shown after reductive hydrolysis of the cuticle and subsequent fractioning on thin-layer chromatography (TLC) (Hoffmann-Benning and Kende, 1992). This analysis indicated that, tentatively, increased stem elongation was accompanied by a promotion of cutin monomer hydroxylation. Possibly, such changes in cuticle composition also take place in submerged leaves, thereby adding to a decrease in gas diffusion resistance.
Next to differences in diffusion resistance, differences at the biochemical level of photosynthesis have also been observed between the leaf types of amphibious plants, and therefore may also occur in submerged terrestrial plants. Chlorophyll contents (Frost-Christensen and Sand-Jensen, 1992; Nielsen, 1993), as well as concentrations of the carboxylation enzymes Rubisco and phosphoenolpyruvate carboxylase (Farmer et al., 1986; Beer et al., 1991), are lower in aquatic compared with terrestrial leaves of amphibious plants.
SUBMERGENCE LEADS POTENTIALLY TO HIGH PHOTORESPIRATION RATES
Underwater photosynthesis in terrestrial plants may be characterized by high photorespiration rates, as reduced gas diffusion rates under water will lead to relatively low internal carbon dioxide concentrations compared with the internal oxygen concentrations in the presence of light (Maberly and Spence, 1989; Jahnke et al., 1991). High photorespiration rates may cause loss of assimilated carbon, which would add to the scarcity of carbon in submerged conditions.
Many aquatic macrophytes, however, such as E. nuttallii and E. densa do not suffer from high photorespiration rates, since they have low carbon dioxide compensation points or can even alter these under unfavourable (i.e. very low carbon dioxide) conditions as a result of their carbon-concentrating mechanisms (Van et al., 1976; Salvucci and Bowes, 1981; Bowes et al., 2002). This has been shown with enzyme assays and labelling studies of the photorespiratory cycle and the coupled C4 metabolism (Hough, 1974; Salvucci and Bowes, 1983). Direct measurements of underwater photorespiration rates are lacking up to now. To our knowledge, the only data available on amphibious or terrestrial leaves are those of Lloyd et al. (1977), who showed that if the oxygen concentration doubles in water-saturated air, photosynthesis decreases by 50 % and thus photorespiration has increased. We suggest that submergence-acclimated leaves of terrestrial plant species will have decreased underwater photorespiration rates, because of the much higher CO2 diffusion into these leaves relative to non-acclimated leaves.
PERSPECTIVES
As this review has shown, underwater photosynthesis is vital for survival of terrestrial plants during conditions of deep floods. Even rather low light conditions already result in increased survival. Changes in leaf morphology upon submergence, which at least partly compensate the unfavourable gas exchange conditions under water by reducing the gas diffusion resistance, increase underwater photosynthesis rates, and also decrease photorespiration rates.
It would be particularly interesting to elucidate through which mechanisms the morphological changes of the leaves decrease the resistance to gas exchange. It could simply be the larger leaf surface area to volume ratio that increases the relative flux of carbon dioxide and oxygen from the water column into the plant. However, if the development of submergence-acclimated leaves is similar to heterophylly in aquatic and amphibious plants, terrestrial plants may be able to decrease cell wall and cuticle thickness in response to submergence, and even change the composition of the cuticle in order to decrease gas diffusion resistance (cf. Frost-Christensen et al., 2003). Furthermore, acclimation to submergence may involve not only diffusion resistance, but also biochemical processes in the photosynthetic apparatus.
It is not yet known how the formation of submerged leaf types is induced in terrestrial plants. Data from aquatic and amphibious plants suggest the hormone ABA, and possibly ethylene, to be key players in morphological, anatomical and photosynthetic (biochemical) changes upon submergence (e.g. Kuwabara et al., 2001, 2003; Minorsky, 2003). Interestingly, these hormones are also essential components of signalling cascades leading to enhanced shoot elongation during submergence in some terrestrial species (Kende et al., 1998; Voesenek et al., 2003). Incorporating plant hormones into underwater photosynthesis research in terrestrial plants will be a promising avenue of research to explore how changes in underwater gas exchange capacity of terrestrial plants are regulated.
Acknowledgments
We thank Ronald Pierik and Hans de Kroon for suggestions on drafts of the manuscript. Dick van Aalst produced the photograph of Figure 2.
LITERATURE CITED
- Adkins SW, Shiraishi T, McComb JA.1990. Submergence tolerance of rice—a new glasshouse method for the experimental submergence of plants. Physiologia Plantarum 80: 642–646. [Google Scholar]
- Allen ED, Spence DHN.1981. The differential ability of aquatic plants to utilize the inorganic carbon supply in the fresh waters. New Phytologist 87: 269–283. [Google Scholar]
- Armstrong W.1979. Aeration in higher plants. In: Woolhouse HW, ed. Advances in botanical research. London: Academic Press, 226–328. [Google Scholar]
- Armstrong W, Gaynard TJ.1976. The critical oxygen pressures for respiration in higher plants. Physiologia Plantarum 37: 200–206. [DOI] [PubMed] [Google Scholar]
- Armstrong W, Brändle R, Jackson MB.1994a . Mechanisms of flood tolerance in plants. Acta Botanica Neerlandica 43: 307–358. [Google Scholar]
- Armstrong W, Strange ME, Cringle S, Beckett PM.1994b . Microelectrode and modelling study of oxygen distribution in roots. Annals of Botany 74: 287–299. [Google Scholar]
- Armstrong J, Afreen-Zobayed F, Blyth S, Armstrong W.1999.Phragmites australis: effects of shoot submergence on seedling growth and survival and radial oxygen loss from roots. Aquatic Botany 64: 275–289. [Google Scholar]
- Beer S, Sand-Jensen K, Madsen TV, Nielsen SL.1991. The carboxylase activity of Rubisco and the photosynthetic performance in aquatic plants. Oecologia 87: 429–434. [DOI] [PubMed] [Google Scholar]
- Blom CWPM, Voesenek LACJ.1996. Flooding: the survival strategies of plants. Tree 11: 290–295. [DOI] [PubMed] [Google Scholar]
- Blom CWPM, Voesenek LACJ, Banga M, Engelaar WMHG, Rijnders JGHM, Van de Steeg HM, et al .1994. Physiological ecology of riverside species: adaptive responses of plants to submergence. Annals of Botany 74: 253–263. [Google Scholar]
- Bockelmann AC, Bakker JP, Neuhaus R, Lage J.2002. The relation between vegetation zonation, elevation and inundation frequency in a Wadden Sea salt marsh. Aquatic Botany 73: 211–221. [Google Scholar]
- Bowes G.1987. Aquatic plant photosynthesis: strategies that enhance carbon gain. In: Crawford RMM, ed. Plant life in aquatic and amphibious habitats. Oxford: Blackwell Scientific Publishers, 79–98. [Google Scholar]
- Bowes G, Salvucci E.1989. Plasticity in the photosynthetic carbon metabolism of submerged aquatic macrophytes. Aquatic Botany 34: 233–266. [Google Scholar]
- Bowes G, Rao SK, Estavillo GM, Reiskind JB.2002. C-4 mechanisms in aquatic angiosperms: comparisons with terrestrial C-4 systems. Functional Plant Biology 29: 379–392. [DOI] [PubMed] [Google Scholar]
- van den Brink FWB, de Leeuw JPM, van der Velde G, Verheggen GM.1993. Impact of hydrology on the chemistry and phytoplankton development in floodplain lakes along the lower Rhine and Meuse. Biogeochemistry 19: 103–128. [Google Scholar]
- Browse JA, Dromgoole FI, Brown JMA.1979. Photosynthesis in the aquatic macrophyte Egeria densa 3. Gas exchange studies. Australian Jounal of Plant Physiology 6: 499–512. [Google Scholar]
- Bruni NC, Young JP, Dengler NC.1996. Leaf developmental plasticity of Ranunculus flabellaris in response to terrestrial and submerged environments. Canadian Journal of Botany 74: 823–837. [Google Scholar]
- Casanova MT, Brock MA.2000. How do depth, duration and frequency of flooding influence the establishment of wetland communities? Plant Ecology 147: 237–250. [Google Scholar]
- Casati P, Lara MV, Andreo CS.2000. Induction of a C-4-like mechanism of CO2 fixation in Egeria densa, a submersed aquatic species. Plant Physiology 123: 1611–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevering OA, van Vierssen W, Blom CWPM.1995. Growth, photosynthesis and carbohydrate utilization in submerged Scirpus maritimus L. during spring growth. New Phytologist 130: 105–116. [Google Scholar]
- Colmer TD, Gibberd MR, Wiengweera A, Tinh TK.1998. The barrier to radial oxygen loss from roots of rice (Oryza sativa L.) is induced by growth in stagnant solution. Journal of Experimental Botany 49: 1431–1436. [Google Scholar]
- Colmer TD.2003. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell and Environment 26: 17–36. [Google Scholar]
- Cook SA, Johnson MP.1967. Adaptation to heterogeneous environments. 1. Variation in heterophylly in Ranunculus flammula L. Evolution 22: 496–516. [DOI] [PubMed] [Google Scholar]
- van Eck WHJM, Van de Steeg HM, Blom CWPM, de Kroon H.2004. Is tolerance to summer flooding correlated with distribution patterns in river floodplains? A comparative study of 20 terrestrial grassland species. Oikos 107: 393–405. [Google Scholar]
- van Eck WHJM, Lenssen JPM, Rengelink RHJ, Blom CWPM, de Kroon H.2005. An experimental assessment of the effects of plant seasonal status, water temperature and oxygen concentration on plant response to flooding. Aquatic Botany 81: 253–264. [Google Scholar]
- Elzenga JTM, Prins HBA.1989. Light-induced polar pH changes in leaves of Elodea canadensis 1. Effects of carbon concentration and light intensity. Plant Physiology 91: 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer AM, Maberly SC, Bowes G.1986. Activities of carboxylation enzymes in freshwater macrophytes. Journal of Experimental Botany 37: 1568–1573. [Google Scholar]
- Frost-Christensen H, Sand-Jensen K.1992. The quantum efficiency of photosynthesis in macroalgae and submerged angiosperms. Oecologia 91: 377–384. [DOI] [PubMed] [Google Scholar]
- Frost-Christensen H, Sand-Jensen K.1995. Comparative kinetics of photosynthesis in floating and submerged Potamogeton leaves. Aquatic Botany 51: 121–134. [Google Scholar]
- Frost-Christensen H, Bolt Jørgensen L, Floto F.2003. Species specificity of resistance to oxygen diffusion in thin cuticular membranes from amphibious plants. Plant Cell and Environment 26: 561–569. [DOI] [PubMed] [Google Scholar]
- Garbey C, Thiebaut G, Muller S.2004. Morphological plasticity of a spreading aquatic macrophyte, Ranunculus peltatus, in response to environmental variables. Plant Ecology 173: 125–137. [Google Scholar]
- Gaynard TJ, Armstrong W.1987. Some aspects of internal plant aeration in amphibious habitats. In: Crawford RMM, ed. Plant life in aquatic and amphibious habitats. Oxford: Blackwell Scientific Publishers, 303–320. [Google Scholar]
- Germ M, Gaberscik A.2003. Comparison of aerial and submerged leaves in two amphibious species, Myosotis scorpioides and _Ranunculus trichophyllus_Photosynthetica 41: 91–96. [Google Scholar]
- Gibbs J, Greenway H.2003. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Functional Plant Biology 30: 1–47. [DOI] [PubMed] [Google Scholar]
- Greve TM, Borum J, Pedersen O.2003. Meristematic oxygen variability in eelgrass (Zostera marina). Limnology and Oceanography 48: 210–216. [Google Scholar]
- Guglielminetti L, Wu Y, Boschi E, Yamaguchi J, Favati A, Vergara M, et al .1997. Effects of anoxia on sucrose degrading enzymes in cereal seeds. Journal of Plant Physiology 150: 251–258. [Google Scholar]
- He JB, Bögemann GM, Van de Steeg HM, Rijnders JHGM, Voesenek LACJ, Blom CWPM.1999. Survival tactics of Ranunculus species in river floodplains. Oecologia 118: 1–8. [DOI] [PubMed] [Google Scholar]
- Hoffmann-Benning S, Kende H.1992. On the role of abscisic acid and gibberellin in the regulation of growth in rice. Plant Physiology 99: 1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaday AS, Bowes G.1980. C4 acid metabolism and dark CO2 fixation in a submersed aquatic macrophyte (Hydrilla verticillata). Plant Physiology 65: 331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MG, Klein WH.1987. The light and temperature environments. In: Crawford RMM, ed. Plant life in aquatic and amphibious habitats. Oxford: Blackwell Scientific Publishers, 3–22. [Google Scholar]
- Hostrup O, Wiegleb G.1991. Anatomy of leaves of submerged and emergent forms of Littorella uniflora (L.) Ascherson. Aquatic Botany 39: 195–209. [Google Scholar]
- Hough RA.1974. Photorespiration and productivity in submersed aquatic vascular plants. Limnology and Oceanography 19: 912–927. [Google Scholar]
- Hsu TC, Liu HC, Wang JS, Chen RW, Wang YC, Lin BL.2001. Early genes responsive to abscisic acid during heterophyllous induction in _Marsilea quadrifolia_Plant Molecular Biology 47: 703–715. [DOI] [PubMed] [Google Scholar]
- Ito O, Ella E, Kawano N.1999. Physiological basis of submergence tolerance in rainfed lowland rice ecosystem. Field Crops Research 64: 75–90. [Google Scholar]
- Jackson MB.1985. Ethylene and responses of plants to soil waterlogging and submergence. Annual Review of Plant Physiology 36: 145–174. [Google Scholar]
- Jackson MB, Armstrong W.1999. Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biology 1: 274–287. [Google Scholar]
- Jahnke LS, Eighmy TT, Fagerberg WR.1991. Studies of Elodea nuttallii grown under photorespiratory conditions. 1. Photosynthetic characteristics. Plant Cell and Environment 14: 147–156. [Google Scholar]
- Jones JI, Eaton JW, Hardwick K.2000. The effect of changing environmental variables in the surrounding water on the physiology of _Elodea nuttalli_Aquatic Botany 66: 115–129. [Google Scholar]
- Keeley JE, Santamaría L.1992. Carbon: freshwater plants. Plant Cell and Environment 15: 1021–1035. [Google Scholar]
- Kende H, Van der Knaap E, Cho H-T.1998. Deepwater rice: a model plant to study stem elongation. Plant Physiology 118: 1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimešová J.1994. The effects of timing and duration of floods on growth of young plants of Phalaris arundinacea L. and Urtica dioica L.:an experimental study. Aquatic Botany 48: 21–29. [Google Scholar]
- Kuwabara A, Tsukaya H, Nagata T.2001. Identification of factors that cause heterophylly in Ludwigia arcuata Walt. (Onagraceae). Plant Biology 3: 670. [Google Scholar]
- Kuwabara A, Ikegami K, Koshiba T, Nagata T.2003. Effects of ethylene and abscisic acid upon heterophylly in Ludwigia arcuata (Onagraceae). Planta 217: 880–887. [DOI] [PubMed] [Google Scholar]
- Laan P, Blom CWPM.1990. Growth and survival responses of Rumex species to flooded and submerged conditions: the importance of shoot elongation, underwater photosynthesis and reserve carbohydrates. Journal of Experimental Botany 41: 775–783. [Google Scholar]
- Laan P, Tosserams M, Blom CWPM, Veen BW.1990. Internal oxygen transport in Rumex species and its significance for respiration under hypoxic conditions. Plant and Soil 122: 39–46. [Google Scholar]
- Lambers H, Chapin FS, Pons TL.1998.Plant physiological ecology. New York: Springer. [Google Scholar]
- Lara MV, Casati P, Andreo CS.2002. CO2-concentrating mechanisms in Egeria densa, a submersed aquatic plant. Physiologia Plantarum 115: 487–495. [DOI] [PubMed] [Google Scholar]
- Lenssen JPM, Menting FBJ, Van der Putten WH, Blom CWPM.1999. Control of plant species richness and zonation of functional groups along a freshwater flooding gradient. Oikos 86: 523–534. [Google Scholar]
- Lenssen JPM, van Kleunen M, Fischer M, de Kroon H.2004. Local adaptation of the clonal plant Ranunculus reptans to flooding along a small-scale gradient. Journal of Ecology 92: 696–706. [Google Scholar]
- Lin BL, Yang WJ.1999. Blue light and abscisic acid independently induce heterophyllous switch in _Marsilea quadrifolia_Plant Physiology 119: 429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd NDH, Canvin DT, Bristow JM.1977. Photosynthesis and photorespiration in submerged aquatic vascular plants. Canadian Journal of Botany 55: 3001–3005. [Google Scholar]
- Lynn DE, Waldren S.2001. Morphological variation in populations of Ranunculus repens from the temporary limestone lakes (Turloughs) in the West of Ireland. Annals of Botany 87: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn DE, Waldren S.2002. Physiological variation in populations of Ranunculus repens L. (creeping buttercup) from the temporary limestone lakes (turloughs) in the west of Ireland. Annals of Botany 89: 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maberly SC.1985. Photosynthesis by Fontinalis antipyretica 2. Assessment of environmental factors limiting photosynthsis and production. New Phytologist 100: 141–155. [Google Scholar]
- Maberly SC, Madsen TV.1998. Affinity for CO2 in relation to the ability of freshwater macrophytes to use /article/back/ref-list/ref/citation/inline-formula
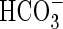 Functional Ecology 12: 99–106. [Google Scholar]
Functional Ecology 12: 99–106. [Google Scholar] - Maberly SC, Madsen TV.2002. Freshwater angiosperm carbon concentrating mechanisms: processes and patterns. Functional Plant Biology 29: 393–405. [DOI] [PubMed] [Google Scholar]
- Maberly SC, Spence DHN.1989. Photosynthesis and photorespiration in freshwater organisms: amphibious plants. Aquatic Botany 34: 267–286. [Google Scholar]
- Madsen TV.1993. Growth and photosynthetic acclimation by Ranunculus aquatilis L. in response to inorganic carbon availability. New Phytologist 707–715. [DOI] [PubMed] [Google Scholar]
- Madsen TV, Sand-Jensen K.1994. The interactive effects of light and inorganic carbon on aquatic plant growth. Plant Cell and Environment 17: 955–962. [Google Scholar]
- Madsen TV, Olesen B, Bagger J.2002. Carbon acquisition and carbon dynamics by aquatic isoetids. Aquatic Botany 73: 351–371. [Google Scholar]
- Magnin NC, Cooley BA, Reiskind JB, Bowes G.1997. Regulation and localization of key enzymes during the induction of Kranz-less, C-4-type photosynthesis in _Hydrilla verticillata_Plant Physiology 115: 1681–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minorsky PV.2003. The hot and the classic. Plant Physiology 133: 1671–1672. [Google Scholar]
- Mommer L, Pedersen O, Visser EJW.2004. Acclimation of a terrestrial plant to submergence facilitates gas exchange under water. Plant Cell and Environment 27: 1281–1287. [Google Scholar]
- Mommer L, de Kroon H, Pierik R, Bögemann GM, Visser EJW.2005. A functional comparison of acclimation to shade and submergence in two terrestrial plant species. _New Phytologist_167: 197–206. [DOI] [PubMed] [Google Scholar]
- Nabben RHM, Blom CWPM, Voesenek LACJ.1999. Resistance to complete submergence in Rumex species with different life histories: the influence of plant size and light. New Phytologist 144: 313–321. [Google Scholar]
- Nielsen SL.1993. A comparison of aerial and submerged photosynthesis in some Danish amphibious plants. Aquatic Botany 27–40. [Google Scholar]
- Pedersen O, Sand-Jensen K, Revsbech NP.1995. Diel pulses of O2 and CO2 in in sandy lake sediments inhabited by _Lobelia dortmanna_Ecology 76: 1536–1545. [Google Scholar]
- Pedersen O, Borum J, Duarte CM, Fortes MD.1998. Oxygen dynamics in the rhizosphere of _Cymodocea rotundata_Marine Ecology Progress Series 169: 283–288. [Google Scholar]
- Pedersen O, Binzer T, Borum J.2004. Sulfide intrusion in eelgrass (Zostera marina L.). Plant Cell and Environment 27: 595–602. [Google Scholar]
- Perata P, Alpi A.1993. Plant responses to anaerobiosis. Plant Science 93: 1–17. [Google Scholar]
- Prins HBA, Snel JFH, Zanstra PE, Helder RJ.1982. The mechanism of bicarbonate assimililation by the polar leaves of Potamogeton and Elodea: CO2 concentrations at the leaf surface. Plant Cell and Environment 5: 207–214. [Google Scholar]
- Prins HBA, Elzenga JTM.1989. Bicarbonate utilization: function and mechanism. Aquatic Botany 34: 59–83. [Google Scholar]
- Ram PC, Singh BB, Singh AK, Ram P, Singh PN, Singh HP, et al .2002. Submergence tolerance in rainfed lowland rice: physiological basis and prospects for cultivar improvement through marker-aided breeding. Field Crops Research 76: 131–152. [Google Scholar]
- Rao SK, Magnin NC, Reiskind JB, Bowes G.2002. Photosynthetic and other phosphoenolpyruvate carboxylase isoforms in the single-cell, facultative C-4 system of _Hydrilla verticillata_Plant Physiology 130: 876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascio N, Cuccato F, Dalla Vecchia F, La Rocca N, Larcher W.1999. Structural and functional features of the leaves of Ranunculus trichophyllus Chaix., a freshwater submerged macrophyte. Plant Cell and Environment 22: 205–212. [Google Scholar]
- Reiskind JB, Madsen TV, VanGinkel LC, Bowes G.1997. Evidence that inducible C-4-type photosynthesis is a chloroplastic CO2-concentrating mechanism in Hydrilla, a submersed monocot. Plant Cell and Environment 20: 211–220. [Google Scholar]
- Rijnders JGHM, Armstrong W, Darwent MJ, Blom CWPM, Voesenek LACJ.2000. The role of oxygen in submergence-induced petiole elongation in Rumex palustris: in situ measurements of oxygen in petioles of intact plants using micro-electrodes. New Phytologist 147: 479–504. [DOI] [PubMed] [Google Scholar]
- Robe WE, Griffiths H.1990. Photosynthesis of Littorella uniflora grown under two PAR regimes: C3 and CAM gas exchange and the regulation of internal CO2 and O2 concentrations. Oecologia 85: 128–136. [DOI] [PubMed] [Google Scholar]
- Robe WE, Griffiths H.1998. Adaptations for an amphibious life: changes in leaf morphology, growth rate, carbon and nitrogen investment, and reproduction during adjustment to emersion by the freshwater macrophyte _Littorella uniflora_New Phytologist 140: 9–23. [Google Scholar]
- Roelofs JGM, Schuurkes JAAR, Smits AJM.1984. Impact of acidification and eutrophication on macrophyte communities in soft waters: 2. Experimental studies. Aquatic Botany 18: 398–411. [Google Scholar]
- Salvucci E, Bowes G.1981. Induction of reduced photorespiratory activity in submersed and amphibious aquatic macrophytes. Plant Physiology 67: 335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci ME, Bowes G.1983. Two photosynthetic mechanisms mediating the low photorespiratory state in submersed aquatic angiosperms. Plant Physiology 73: 488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand-Jensen K.1989. Environmental variables and their effect on photosynthesis of aquatic plant communities. General features of aquatic photosynthesis. Aquatic Botany 34: 5–25. [Google Scholar]
- Sand-Jensen K, Frost-Christensen H.1999. Plant growth and photosynthesis in the transition zone between land and stream. Aquatic Botany 63: 23–35. [Google Scholar]
- Sand-Jensen K, Prahl C, Stokholm H.1982. Oxygen release from roots of submerged aquatic macrophytes. Oikos 38: 349–354. [Google Scholar]
- Sand-Jensen K, Pedersen O, Binzer T, Borum J.2005. Contrasting oxygen dynamics in the freshwater isoetid Lobelia dortmanna and the marine seagrass _Zostera marina_Annals of Botany 96: 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculthorpe CD.1967.The biology of aquatic vascular plants. London: Edward Arnold. [Google Scholar]
- Setter TL, Kupkanchankul T, Kupkanchankul K, Bhekasut P, Wiengweera A, Greenway H.1987. Concentrations of CO2 and O2 in floodwater and in internodal lacunae of floating rice growing at 1–2 metre water depths. Plant Cell and Environment 10: 767–776. [Google Scholar]
- Siebel HN, van Wijk M, Blom CWPM.1998. Can tree seedlings survive increased flood levels of rivers? Acta Botanica Neerlandica 47: 219–230. [Google Scholar]
- Silvertown J, Dodd ME, Gowing DJG, Mountford JO.1999. Hydrologically defined niches reveal a basis for species richness in plant communities. Nature 400: 61–63. [Google Scholar]
- Smith FA, Walker NA.1980. Photosynthesis by aquatic plants: effects of unstirred layers in relation to assimilation of CO2 and /article/back/ref-list/ref/citation/inline-formula
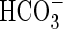 and to carbon isotopic discrimination. New Phytologist 86: 245–259. [Google Scholar]
and to carbon isotopic discrimination. New Phytologist 86: 245–259. [Google Scholar] - Sorrell BK.2004. Regulation of root anaerobiosis and carbon translocation by light and root aeration in _Isoetes alpinus_Plant Cell and Environment 27: 1102–1111. [Google Scholar]
- Spencer WE, Wetzel RG, Teeri J.1996. Photosynthetic phenotype plasticity and the role of phosphoenolpyruvate carboxylase in _Hydrilla verticillata_Plant Science 118: 1–9. [Google Scholar]
- van de Steeg HM, Blom CWPM.1998. Impact of hydrology on floodplain vegetation in the lower Rhine system: implications for nature conservation and nature development. In: Nienhuis PH, Leuven RSEW, Ragas AMJ, eds. New concepts for sustainable management of river basins. Leiden, The Netherlands: Backhuys Publishers, 131–144. [Google Scholar]
- Stünzi JT, Kende H.1989. Gas composition in the internal air spaces of deepwater rice in relation to growth induced by submergence. Plant Cell Physiology 30: 49–56. [Google Scholar]
- Sýkora KV, Scheper E, van der Zee F.1988. Inundation and the distribution of plant communities on Dutch river dikes. Acta Botanica Neerlandica 37: 279–290. [Google Scholar]
- Toner M, Keddy P.1997. River hydrology and riparian wetlands: a predictive model for ecological assembly. Ecological Applications 7: 236–246. [Google Scholar]
- Ueno O.1998. Induction of Kranz anatomy and C-4-like biochemical characteristics in a submerged amphibious plant by abscisic acid. Plant Cell 10: 571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno O.2001. Environmental regulation of C3 and C4 differentiation in the amphibious sedge _Eleocharis vivipara_Plant Physiology 127: 1524–1532. [PMC free article] [PubMed] [Google Scholar]
- Van TK, Haller WT, Bowes G.1976. Comparison of the photosynthetic characteristics of three submersed aquatic plants. Plant Physiology 58: 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartapetian BB, Jackson MB.1997. Plant adaptations to anaerobic stress. Annals of Botany 79: 3–20. [Google Scholar]
- Vervuren PJA, Beurskens SMJH, Blom CWPM.1999. Light acclimation, CO2 response and long-term capacity of underwater photosynthesis in three terrestrial plant species. Plant Cell and Environment 22: 959–968. [Google Scholar]
- Vervuren PJA, Blom CWPM, De Kroon H.2003. Extreme flooding events on the Rhine and the survival and distribution of riparian plant species. Journal of Ecology 91: 135–146. [Google Scholar]
- Visser EJW, Blom CWPM, Voesenek LACJ.1996. Flooding-induced adventitious rooting in Rumex: morphology and development in an ecological perspective. Acta Botanica Neerlandica 45: 17–28. [Google Scholar]
- Visser EJW, Colmer TD, Blom CWPM, Voesenek LACJ.2000. Changes in growth, porosity, and radial oxygen loss from adventitious roots of selected mono- and dicotyledonous wetland species with contrasting types of aerenchyma. Plant Cell and Environment 23: 1237–1245. [Google Scholar]
- Voesenek LACJ, Banga M, Thier R, Mudde CM, Harren FJM, Barendse GWM, et al .1993. Submergence-induced ethylene synthesis, entrapment, and growth in two plant species with contrasting flooding resistances. Plant Physiology 103: 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LACJ, Van Oorschot FJMM, Smits AJM, Blom CWPM.1993. The role of flooding resistance in the establishment of Rumex seedlings in river flood plains. Functional Ecology 7: 105–114. [Google Scholar]
- Voesenek LACJ, Benschop JJ, Bou J, Cox MCH, Groeneveld HW, Millenaar FF, et al .2003. Interactions between plant hormones regulate submergence-induced shoot elongation in the flooding tolerant dicot _Rumex palustris_Annals of Botany 91: 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LACJ, Rijnders JHGM, Peeters AJM, Van de Steeg HM, de Kroon H.2004. Plant hormones regulate fast shoot elongation under water: from genes to communities. Ecology 85: 16–27. [Google Scholar]
- Waters I, Armstrong W, Thompson CJ, Setter TL, Adkins S, Gibbs J, et al .1989. Diurnal changes in radial oxygen loss and ethanol metabolism in roots of submerged and non-submerged rice seedlings. New Phytologist 113: 439–451. [Google Scholar]
- Wium-Andersen S.1971. Photosynthetic uptake of free CO2 by the roots of _Lobelia dortmanna_Physiologia Plantarum 25: 245–248. [Google Scholar]