Stable expression of HIF-1α in tubular epithelial cells promotes interstitial fibrosis (original) (raw)
Abstract
Chronic hypoxia accelerates renal fibrosis. The chief mediator of the hypoxic response is hypoxia-inducible factor 1 (HIF-1) and its oxygen-sensitive component HIF-1α. HIF-1 regulates a wide variety of genes, some of which are closely associated with tissue fibrosis. To determine the specific role of HIF-1 in renal fibrosis, we generated a knockout mouse in which tubular epithelial expression of von Hippel-Lindau tumor suppressor (VHL), which acts as a ubiquitin ligase to promote proteolysis of HIF-1α, was targeted. We investigated the effect of VHL deletion (i.e., stable expression of HIF-1α) histologically and used the anti-HIF-1α agent [3-(5′-hydroxymethyl-2′-furyl)-1-benzyl indazole] (YC-1) to test whether inhibition of HIF-1α could represent a novel approach to treating renal fibrosis. The area of renal fibrosis was significantly increased in a 5/6 renal ablation model of VHL−/− mice and in all VHL−/− mice at least 60 wk of age. Injection of YC-1 inhibited the progression of renal fibrosis in unilateral ureteral obstruction model mice. In conclusion, HIF-1α appears to be a critical contributor to the progression of renal fibrosis and could be a useful target for its treatment.
Keywords: hypoxia, HIF-1, VHL, fibrosis, fibroblast-specific protein 1
peritubular capillary loss due to glomerular injury reduces the oxygen supply to the interstitium, leading to chronic interstitial and tubular cell hypoxia (5, 41). Such hypoxia is known to precede the development of interstitial fibrosis (24, 27, 29), but the mechanism underlying that response remains unclear. Recent advances in molecular biology have enabled identification of a family of hypoxia-inducible transcription factors (HIFs), which are central regulators of hypoxic responses (34). HIF-1, for example, is a heterodimer composed of a constitutively expressed HIF-1β subunit and an O2-regulated HIF-1α subunit. HIF-2α, another HIF subunit, appears to have overlapping but distinct specificities with respect to physiological inducers and to target gene activation in the kidney: while HIF-2α is expressed mainly in glomerular cells, peritubular fibroblasts, and endothelial cells, HIF-1α is expressed throughout the kidney and plays a central role in the hypoxic responses of tubular epithelial cells (7, 33, 37). Indeed, hypoxia-inducible gene expression in primary renal proximal tubular epithelial cells is almost completely blocked by inactivation of HIF-1α, suggesting that their response to hypoxia is largely dependent on HIF-1α (9).
HIF-1 stimulates the expression of vasculogenic genes such as erythropoietin (EPO) and vascular endothelial growth factor (VEGF) to maintain oxygen delivery and to protect cells from ischemia. In this respect, HIF-1 would appear to exert a beneficial effect on renal tissues (1, 3). On the other hand, HIF-1 also induces expression of profibrogenic genes like tissue inhibitor of metalloproteinase 1 (TIMP1), connective tissue growth factor (CTGF), and plasminogen activator inhibitor 1 (PAI-1) (9, 16, 27). It is thus likely that by upregulating these profibrogenic factors, HIF-1 accelerates tissue fibrosis. Recently, we found that HIF-1 also enhances epithelial-to-mesenchymal transition (EMT) in vitro and that genetic ablation of HIF-1α inhibited the development of tubulointerstitial fibrosis in vivo (10). To further explore the in vivo function of HIFs in a mouse model of renal fibrosis, we have now generated a knockout mouse in which tubular epithelial expression of von Hippel-Lindau tumor suppressor (VHL) was targeted. Under normoxic conditions, VHL acts as a ubiquitin ligase to promote degradation of HIF-1α (34). As HIF-1α was stable in the tubular epithelial cells of our VHL−/− mice, even under normoxic conditions, we were able to estimate its direct effect on the progression of renal injury. We also examined the potential of HIF-1α to serve as a target for antifibrosis therapy by assessing the extent to which the anti-HIF-1α agent [3-(5′-hydroxymethyl-2′-furyl)-1-benzyl indazole] (2, 39) (YC-1) could inhibit the progression of renal fibrosis.
MATERIALS AND METHODS
Transfection of mutant HIF-1α into murine proximal tubular epithelial cells.
A line of murine proximal tubular epithelial cells (MCT) were maintained in DMEM (Nacalai Tesque, Kyoto, Japan) supplemented with 10% fetal calf serum, 10 U/ml penicillin, and 10 μg/ml streptomycin at 37°C under a humidified 5% CO2 atmosphere. To construct eukaryotic expression vectors encoding a stable mouse HIF-1α mutant (mtHIF-1α; P402A/P577A/N813A), site-directed mutagenesis was carried out to induce mutation of the codons for residues Pro402, Pro577, and Asn813. After gene transfer to MCT by electroporation using Nucleofector (Amaxa, Cologne, Germany), the levels of expression of HIF-1α protein were determined by Western blotting. Whereas endogenous HIF-1α was degraded completely after 30 min in culture under normoxic conditions (20% O2), levels of mtHIF-1α protein remained stable (data not shown). After culturing the transfectants for 24 h under normoxic conditions (20% O2), real-time PCR was carried out to assess mRNA expression (see below).
RNA isolation and real-time PCR analysis.
Total RNA was prepared using RNA-Bee-RNA isolation reagent (Tel-Test) according to the manufacturer's protocol. Two micrograms of RNA were reverse transcribed in a 20-μl reaction volume, after which a 1-μl sample of the resultant cDNA was used for PCR. Amplification was carried out with an ABI Prism 7700 Sequence Detection System (Applied Biosystems, Tokyo, Japan) using either SYBR Green Mastermix (Qiagen, Tokyo, Japan) or Taqman PCR Master Mix (Applied Biosystems). The following SYBR Green primers were used to amplify specific target genes: for fibroblast-specific protein 1 (FSP1), 5′-GAGGAGGCCCTGGATGTAAT-3′ (forward) and 5′-CTTCATTGTCCCTGTTGCTG-3′ (reverse); for vascular endothelial growth factor (VEGF), 5′-CCACGTCAGAGAGCAACATCA-3′ (forward) and 5′-TCATCTCTCCTATGTGCTGGCTTT-3′ (reverse); for Bnip3 5′-CATGTCGCAGAGCGGGGA-3′ (forward) and 5′-GTCACAGTGAGAACTCTTG-3′ (reverse); for phosphoglycerate kinase 1 (PGK), 5′-GCCTTGATCCTTTGGTTGTTTG-3′ (forward) and 5′-GGAAGCGGGTCGTGATGA-3′ (reverse); for type 1 collagen, 5′-CCCGCCGATGTCGCTAT-3′ (forward) and 5′-GCTACGCTGTTCTTGCAGTGAT-3′ (reverse); for lysyl oxidase (LOX), 5′-CCACAGCATGGACGAATTCA-3′ (forward) and 5′-AGCTTGCTTTGTGGCCTTCA-3′ (reverse); for fibronectin, 5′-GATGCACCGATTGTCAACAG-3′ (forward) and 5′-ACTCTGATCAGCATGGACCA-3′ (reverse); and for plasminogen activator inhibitor 1 (PAI-1), 5′-GGCTTCATGCCCCACTTCTTC-3′ (forward) and 5′-ATTCACCAGCACCAGGCGTGT-3′ (reverse). Taqman probe sets for 18S, connective tissue growth factor (CTGF), and transforming growth factor β1 (TGF-β1) were purchased from Applied Biosystems. 18S was used to normalize the mRNA levels for all genes.
Generation of mouse strains and experimental animal protocols.
The generation, characterization and genotyping of the mouse strains used in this study have all been described previously (8, 13, 31). All procedures involving mice were carried out in accordance with the National Institutes of Health guidelines for the care use of live animals and were approved by the Nara Medical University Animal Care Committees.
Eight-week-old mice (VHL−/− mice: n = 8, 4 males and 4 females; VHL+/+ mice: n = 8, 4 males and 4 females) underwent subtotal nephrectomy (Nx). After induction of anesthesia, the left flank was opened through a small incision, and two-thirds of the left kidney was cut off. Two weeks later, the right kidney was removed after ligation of the renal artery and ureter with 2-0 suture. Remnant kidneys were harvested for immunohistochemical analysis 20 wk after the second operation.
Unilateral ureteral obstruction (UUO) was performed in 8- to 10-wk-old C57BL/6 mice as described previously (11). UUO mice received a daily intraperitoneal injection of either vehicle (DMSO; n = 9, 4 males and 5 females) or YC-1 (30 μg/g; Wako Pure Chemical, Osaka, Japan; n = 8, 3 males and 5 females). Administration was started the day after the operation and continued until the day before death. On day 10, both the obstructed and unobstructed kidneys were harvested for immunohistochemical analysis.
Renal function.
Twenty-four-hour urine samples were collected 4, 8, and 20 wk post-Nx using metabolic cages. Blood samples were taken before death after week 20. Urinary albumin was determined by nephelometry utilizing rabbit anti-mouse albumin. Urinary and serum creatinine were measured enzymatically. Blood urea nitrogen (BUN) was determined by the urease/GLDH method.
Histology.
Two-micrometer-thick paraffin sections were used for Masson's trichrome staining and for most immunohistochemical analyses; analysis of type 1 collagen was carried out using 4-μm-thick frozen sections. The following primary antibodies were employed: mouse monoclonal anti- HIF-1α (α67, 1:50 dilution, Novus Biologicals, Littleton, CO), rabbit polyclonal anti-FSP1 (1:5,000 dilution) (13), and rabbit polyclonal anti-type 1 collagen (1:100 dilution, Chemicon, Temecula, CA). For HIF-1α, target antigen retrieval entailed pressure cooking for 15 min in antigen retrieval solution (pH 9.0; Nichirei Bioscience, Tokyo, Japan); for FSP1, it entailed incubation in 400 μg/ml proteinase K (DakoCytomation, CA) for 5 min at room temperature. After endogenous peroxidase activity was blocked with hydrogen peroxide, the sections were incubated with anti-HIF-1α antibody for 2 h at 37°C or with anti-FSP1 or anti-type 1 collagen antibody for 1 h at room temperature. HRP-conjugated secondary antibody was used to detect the primary antibodies. The staining was then developed with diaminobenzidine tetrahydrochloride. Hematoxylin was used as a counterstain.
Under a light microscope at ×100 magnification, 8–10 nonoverlapping fields/kidney section were captured and transferred to the screen of a computer. The number of FSP1+ fibroblasts was counted in each field and then averaged. The area positively stained for type 1 collagen was calculated using AnalySIS image analysis software (Soft Imaging System, Munster, Germany).
Statistics.
All data are expressed as means ± SE. Wilcoxon tests were used to evaluate differences between groups. Values of P < 0.05 were considered significant.
RESULTS
Stable transfection of a nondegradable HIF-1α mutant induces upregulation of target genes in mouse tubular epithelial cells.
HIFs are stable within cells under hypoxic conditions, enabling them to be translocated to the nucleus, where they activate transcription of target genes. As HIF-1 is the dominant HIF in tubular epithelial cells (9), our first aim was to determine whether stabilization of HIF-1α is, by itself, sufficient to upregulate gene expression in those cells. For this purpose, we generated an expression vector encoding a nondegradable mtHIF-1α in which the key proline and asparagine residues responsible for HIF-1α degradation were replaced with alanines. We transfected the mtHIF-1α construct into MCT cells, a tubular epithelial cell line, and examined target gene expression under normoxic conditions. We found that mtHIF-1α transfection induced significant expression of Bnip3, a representative HIF-1 target gene in tubular epithelial cells, as well as several fibrogenic factors, including FSP1, a key marker of EMT (12, 13, 28, 36); CTGF, the main growth factor involved in the synthesis of ECM; LOX, a critical mediator involved in cell motility (4, 20); and fibronectin, the dominant ECM component in tubulointerstitial fibrosis (Fig. 1). Expression of type 1 collagen also tended to be elevated, but the effect was not statistically significant.
Fig. 1.

Effects of hypoxia-inducible factor-1α (HIF-1α) on tubular epithelial cells in vitro. Stable transfection of a nondegradable HIF-1α mutant induces upregulation of target genes. Transfection of mtHIF-1α into murine proximal tubular epithelial cells (MCT) upregulates expression of Bnip3 and representative fibrogenic genes. Values are means ± SE of 3 experiments. *P < 0.05 vs. empty vector.
Stable expression of HIF-1α in kidneys of VHL−/− mice.
To confirm the deletion of VHL and subsequent stabilization of HIF-1α in the proximal tubule of VHL knockout (VHL−/−) mice, we immunostained the kidneys of eight 8-wk-old VHL−/− mice (4 males and 4 females) and five of their VHL+/+ littermates (2 males and 3 females) for HIF-1α. Pronounced HIF-1α expression was detected in cortical tubular epithelial cells of VHL−/− mice, but little or no HIF-1α was detected in the renal cortex or medulla in VHL+/+ mice (Fig. 2_A_). Moreover, to confirm that the stabilized HIF-1α acts as a functional transcriptional factor and induces target gene expression, we extracted total RNA from the renal cortex, and transcription of Bnip3, VEGF, and PGK, three classic HIF-1α transcriptional targets, was analyzed by real-time PCR. As shown in Fig. 2_B_, expression of all three target genes was significantly enhanced in VHL−/− mice.
Fig. 2.

Stable expression of HIF-1α in kidneys of von Hippel-Lindau tumor suppressor knockout (VHL−/−) mice. A: immunostaining shows a clear nuclear staining pattern for HIF-1α in a VHL−/− kidney, whereas no staining was observed in a VHL+/+ kidney. Original magnification, ×100. B: real-time PCR analysis shows significant elevation of HIF-1 target genes in the kidneys of VHL−/− mice. Values are means ± SE (VHL+/+, n = 5; VHL−/−, n = 8). *P < 0.05, **P < 0.01 vs. VHL+/+ mice.
VHL deletion in tubular epithelial cells promotes development of interstitial fibrosis in the 5/6 renal ablation model.
To assess the role of HIF-1α in the pathogenesis and progression of renal fibrosis, we generated the 5/6 renal ablation model (Nx) in VHL−/− and VHL+/+ mice. As reported previously, Nx alone rarely led to the development of interstitial fibrosis in mice (18, 21). In preliminary studies, the histological features of the remnant kidney were evaluated 10 and 16 wk post-Nx, and there were no obvious pathological findings after 10 wk in either the VHL+/+ or VHL−/− kidneys. Mild fibrosis that was limited to the peritubular area of the proximal tubule was detected in VHL−/− kidneys 16 wk post-Nx, although no fibrosis was observed in VHL+/+ kidneys (data not shown). By 20 wk post-Nx; however, fibrosis was widespread in the interstitium of VHL−/− kidneys, with significant accumulation of type 1 collagen. By contrast, little or no fibrosis was detected in VHL+/+ kidneys (Fig. 3_A_), so that the percentage of fractional area of type 1 collagen was increased 1.7-fold in VHL−/− kidneys, compared with VHL+/+ kidneys (Fig. 3_B_). Moreover, a large number of FSP1+ cells, which are the most reliable marker for the progression of interstitial fibrosis (26), had accumulated in the interstitium of the VHL−/− kidneys but not the VHL+/+ kidneys (Fig. 3, A and B). The numbers of FSP1-positive proximal tubular epithelial cells was also increased in VHL−/− kidneys compared with VHL+/+ kidneys (Fig. 3_B_). We also analyzed the renal histology in male and female mice separately and obtained similar results from both (Supplementary Fig. 1; all supplementary material is available in the online version of this article on the journal Web site).
Fig. 3.

Development of interstitial fibrosis in the remnant kidneys of VHL−/− mice. A: Masson's trichrome staining (top) and immunostaining of type 1 collagen (middle) and fibroblast-specific protein 1 (FSP1; bottom) reveal the presence of interstitial fibrosis in VHL−/− kidneys 20 wk post-5/6 renal ablation (Nx). Original magnification, ×100. B: area of type 1 collagen staining, the number of FSP1+ cells, and the number of FSP1+ proximal tubular epithelial cells (TECs) were significantly increased in VHL−/− kidneys 20 wk post-Nx. C: levels of urinary albumin/creatinine ratio (Ualb/Ucr ratio) were significantly elevated in VHL−/− mice. D: real-time PCR analysis of fibrosis-related gene expression showed significant upregulation of FSP1, type 1 collagen (Coll I), transforming growth factor (TGF)-β1, and plasminogen activator inhibitor 1 (PAI-1). Values are means ± SE of 8 mice/group. *P < 0.05, **P < 0.01 vs. VHL+/+ mice.
Renal function was assessed by measuring the levels of albumin in the mice's urine 4, 8, and 20 wk post-Nx and serum creatinine and BUN 20 wk post-Nx. After normalization of urinary albumin to urinary creatinine, the resultant albumin/creatinine ratio was significantly higher in VHL−/− than VHL+/+ mice, which may reflect reabsorption injury caused by the interstitial fibrosis and tubular damage (Fig. 3_C_). On the other hand, serum creatinine and BUN were not elevated significantly in VHL−/− mice (serum creatinine, 0.29 ± 0.03 mg/dl in VHL+/+ vs. 0.34 ± 0.04 mg/dl in VHL−/−; BUN, 60.8 ± 7.1 mg/dl in VHL+/+ vs. 70.5 ± 5.8 mg/dl in VHL−/−).
We next analyzed the renal transcription of key genes associated with fibrosis. The levels of mRNA encoding type 1 collagen and FSP1 were significantly higher in VHL−/− kidneys than VHL+/+ kidneys, which was consistent with our histological findings. Similarly, levels of PAI-1 mRNA were significantly higher in VHL−/− kidneys than VHL+/+ kidneys, and although the difference is not significant, levels of CTGF and LOX mRNA also tended to be higher in VHL−/− kidneys. In addition, expression of the cytokine TGF-β1, a key mediator of EMT and fibrosis, also was enhanced in VHL−/− kidneys (Fig. 3_D_). By contrast, body weights (30.6 ± 3.2 g in VHL+/+ vs. 29.9 ± 1.4 g in VHL−/−) and systolic blood pressures (113.6 ± 11.7 mmHg in VHL+/+ vs. 117.4 ± 5.2 mmHg in VHL−/−) did not differ between VHL−/− and VHL+/+ mice.
VHL deletion in tubular epithelial cells promotes spontaneous development of interstitial fibrosis in aged VHL−/− mice.
To assess the long-term effects of HIF-1α stabilization in vivo, we examined the histology of kidneys from aged VHL−/− mice. Although no significant interstitial fibrosis was observed in any VHL−/− mice at 48 wk of age (8 males and 12 females), marked interstitial fibrosis had developed in all VHL−/− mice by 60 wk (eight 60-wk-old and four 96-wk-old females, five 60-wk-old and two 96-wk-old males), whereas no or a small interstitial fibrosis was observed in age matched VHL+/+ mice (four 60-wk-old and two 96-wk-old females, five 60-wk-old and two 96-wk-old males) (Fig. 4).
Fig. 4.
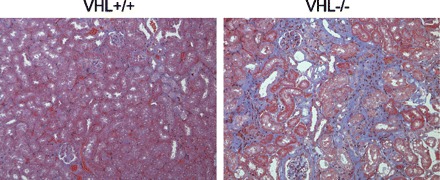
Development of interstitial fibrosis in aged VHL−/− mice. Masson's trichrome staining reveals the presence of interstitial fibrosis in aged VHL−/− mice. Original magnification, ×100.
Blocking HIF-1α ameliorates interstitial fibrosis in UUO kidneys.
We investigated the extent to which blocking HIF-1α would affect the development of interstitial fibrosis using UUO model mice treated with either vehicle (DMSO) or YC-1 (30 μg/g). We found that YC-1-treated mice showed significantly less collagen accumulation than vehicle-treated controls (from 37.2 ± 1.4 to 28.0 ± 1.6%) (Fig. 5, A and B). The total numbers of FSP1+ cells and numbers of FSP1+ tubular epithelial cells were also diminished in the YC-1-treated group (total FSP1+ cells, 124.4 ± 6.4 cells/field in vehicle-treated group and 84.2 ± 5.1 cells/field in YC-1-treated group; FSP1+ tubular epithelial cells, 6.5 ± 0.50 cells/field in vehicle-treated group and 4.3 ± 0.37 cells/field in YC-1-treated group) (Fig. 5_B_). Similar results were obtained when male and female mice were analyzed separately (Supplementary Fig. 2). This suggests that HIF-1α inhibition suppresses the progression of renal fibrosis and that HIF-1α may be a useful therapeutic target for the treatment of renal fibrosis.
Fig. 5.

Amelioration of interstitial fibrosis in YC-1-treated unilateral ureteral obstruction (UUO) kidneys. A: Masson's trichrome staining (top) and immunostaining for type 1 collagen (middle) and FSP1 (bottom) show that interstitial fibrosis can be diminished by treating mice with an anti-HIF-1α agent (YC-1). Original magnification, ×100. B: area of type 1 collagen staining, the number of FSP1+ cells, and the number of FSP1+ proximal TECs were significantly diminished in YC-1-treated mice. Values are means ± SE (vehicle, n = 9; YC-1, n = 8). **P < 0.01 vs. vehicle-treated mice.
DISCUSSION
Several studies have shown that hypoxia may promote fibrosis via EMT when cells are cultured under hypoxic conditions. However, hypoxia upregulates numerous genes, making it difficult to selectively investigate the role played by HIF-1α. We therefore generated stable HIF-1α mutants and examined the changes in the expression of representative fibrogenic genes caused by HIF-1α stabilization. Similar mutations have already been shown to block degradation of HIF-1α in vivo and in vitro and to activate downstream gene expression (15, 22, 30). We also confirmed that the HIF-1α mutant is stable even in normoxic condition, and our in vitro experiments indicate that stabilization of HIF-1α facilitates fibrosis through upregulation of fibrogenic gene expression, even under normoxic conditions.
We also examined the effect of HIF-1α on renal fibrosis in vivo using a knockout mouse in which tubular epithelial expression of VHL was deleted. VHL is a component of the E3 ubiquitin ligase, which targets proteins for degradation in the proteosome. In the presence of oxygen, prolyl hydroxylase domain enzymes hydroxylate HIFs, enabling them to interact with VHL, which results in their ubiquitination and subsequent proteosomal degradation. Bearing in mind that loss of VHL function leads to stabilization of HIFs, we used Cre-loxP-mediated gene targeting to selectively delete the VHL gene from the proximal tubule. Mice carrying the Cre recombinase transgene driven by the γ-glutamyl transpeptidase (γ-GT) promoter were crossed with mice carrying VHL 2 lox alleles. The γ-GT promoter is strongly activated in proximal tubular epithelial cells (13), and the expressed Cre recombinase splices out VHL, eliminating its expression. Nuclear localization of HIF-1α and upregulation of target genes in VHL−/− mice indicated the successful deletion of VHL in our system. To confirm the in vivo effect of HIF-1α stabilization, we utilized the 5/6 renal ablation (Nx) model in which interstitial fibrosis is rarely observed in wild-type kidneys. We found that HIF-1α was undetectable in tubular epithelial cells from wild-type kidneys 4, 8, and 20 wk post-Nx, indicating that the post-Nx kidney was not sufficiently hypoxic to block prolyl hydroxylase domain enzyme activity in wild-type mice. However, VHL deletion led to the development of interstitial fibrosis with upregulation of fibrogenic factors in the post-Nx kidney, suggesting that HIF-1α stabilization is the critical factor in the development of renal fibrosis and subsequent renal failure.
Significant spontaneous progression of fibrosis was not observed in VHL−/− mice until they were more than 48 wk of age, suggesting that, as in the Nx model, additional factors (e.g., renin-angiotensin-aldosterone system activation) are required to initiate early development of fibrosis. Nonetheless, the fact that the area of interstitial fibrosis was significantly increased in aged mice (at least 60 wk) indicates that more prolonged stimulation with HIF-1α alone may be sufficient to elicit tubulointerstitial fibrosis. Thus long-term stabilization of HIF-1α induced by chronic hypoxia may be associated with the progression of human nephritis. Consistent with that idea, interstitial fibrosis often progresses very slowly in human IgA nephropathy, sometimes developing over a period of more than 20 years.
Although degradation of HIF-1α is the best documented function of VHL products (pVHL) (14), several HIF-1-independent functions of pVHL were recently reported (19, 38, 40). The present study does not rule out such HIF-1-independent effects of pVHL on renal fibrosis; however, our recent report showing that HIF-1 knockout ameliorates the progression of interstitial fibrosis supports the idea that HIF-1α stabilization is the most critical factor for the progression of chronic interstitial fibrosis (10). A variety of pathological conditions can generate a state of hypoxia in the kidney, including loss of glomeruli, overproduction of angiotensin II and aldosterone, inhibition of nitric oxide synthesis, and renal anemia. HIF-1α stabilization could be the common phenomenon linking these pathological conditions to development of interstitial fibrosis. Interstitial fibrosis itself disrupts peritubular capillary formation, worsening hypoxia, and inhibiting the degradation of HIF-1α. There is thus a vicious cycle that promotes interstitial fibrosis under hypoxic conditions.
Rankin et al. (32) previously showed that ∼20% of mice with conditional VHL inactivation develop renal cysts, a pathological finding associated with renal cell carcinoma. In the present study, however, no cyst formation was seen in any of the knockout mice, even at a very advanced age (older than 96 wk). This discrepancy may reflect a difference in the promoter used to control the Cre-recombinase expression. Whereas in earlier studies the phosphoenolpyruvate carboxykinase (PEPCK) promoter was used, we used the γ-GT promoter in the present study. In 100% of VHL mutant mice driven by the PEPCK promoter, development of polycythemia with increased EPO expression in the liver was observed. Thus cyst formation may be associated with increased EPO levels and polycythemia. Although it was commonly thought that preneoplastic renal cysts originate from the proximal tubule (6, 25), more recent studies of Tamm-Horsfall protein expression suggest that early lesions are more frequently derived from the distal tubule (23). Therefore, the cellular origin of renal cysts remains controversial. The absence of cyst formation in our knockout mice may be consistent with the idea that preneoplastic cysts originate in the distal tubule.
Although our findings clearly indicate that HIF-1α promotes interstitial fibrosis both in vitro and in vivo, it remains unclear whether inhibition of HIF-1α could serve as a novel therapeutic strategy to prevent or treat tubulointerstitial fibrosis. Because HIFs exert protective effects against ischemic renal injury by ameliorating oxidative stress (17), it is possible that their inhibition would promote hypoxia and oxidative stress in the renal interstitium. We therefore examined whether blocking HIF-1α would ameliorate the progression of interstitial fibrosis using a UUO model in which hypoxia was detected in tubular epithelial cells as early as 24 h after ligation. The inhibitory effect of an anti-HIF-1 agent on the progression of renal fibrosis is consistent with our hypothesis that HIF-1 is a harmful factor that promotes the development and progression of interstitial fibrosis.
In conclusion, the results of the present study show that hypoxia and the resultant stabilization of HIF-1α play pivotal roles in the development of tubulointerstitial fibrosis. They also suggest that HIF-1α could be a useful therapeutic target for the treatment of renal fibrosis. In fact, a number of HIF-1 inhibitors have already been developed for the treatment of metastatic cancer (35). Perhaps these agents could also be utilized for the treatment of tubulointerstitial fibrosis.
GRANTS
This work was supported in part by research Grants 17590839 (M. Iwano) and 19590960 (M. Iwano) from the Ministry of Education and Science of Japan and by Grants-in-Aid for the Research Group on Progressive Renal Diseases from the Ministry of Health, Labor, and Welfare of Japan.
Acknowledgments
We thank Hiromi Ohura, Fumika Kunda, and Miyako Sakaida of the Nara Medical University for excellent technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “_advertisement_” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bernhardt WM**, Campean V, Kany S, Jurgensen JS, Weidemann A, Warnecke C, Arend M, Klaus S, Gunzler V, Amann K, Willam C, Wiesener MS, Eckardt KU.** Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J Am Soc Nephrol 17: 1970–1978, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Chun YS**, Yeo EJ, Choi E, Teng CM, Bae JM, Kim MS, Park JW.** Inhibitory effect of YC-1 on the hypoxic induction of erythropoietin and vascular endothelial growth factor in Hep3B cells. Biochem Pharmacol 61: 947–954, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Eckardt KU**, Bernhardt WM, Weidemann A, Warnecke C, Rosenberger C, Wiesener MS, Willam C.** Role of hypoxia in the pathogenesis of renal disease. Kidney Int Suppl S46–S51, 2005. [DOI] [PubMed]
- 4.Erler JT**, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ.** Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440: 1222–1226, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Fine LG**, Bandyopadhay D, Norman JT, Suranyi A, Streitman K, Pal A, Nyari T, Retz C, Foidart JM, Schaaps JP, Kovacs L.** Is there a common mechanism for the progression of different types of renal diseases other than proteinuria? Towards the unifying theme of chronic hypoxia fetal renal artery flow and renal echogenicity in the chronically hypoxic state. Kidney Int Suppl 75: S22–S26, 2000. [PubMed] [Google Scholar]
- 6.Grone HJ**, Weber K, Helmchen U, Osborn M.** Villin—a marker of brush border differentiation and cellular origin in human renal cell carcinoma. Am J Pathol 124: 294–302, 1986. [PMC free article] [PubMed] [Google Scholar]
- 7.Haase VH**.** Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol 291: F271–F281, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haase VH**, Glickman JN, Socolovsky M, Jaenisch R.** Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc Natl Acad Sci USA 98: 1583–1588, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins DF**, Biju MP, Akai Y, Wutz A, Johnson RS, Haase VH.** Hypoxic induction of Ctgf is directly mediated by Hif-1. Am J Physiol Renal Physiol 287: F1223–F1232, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Higgins DF**, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH.** Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest 117: 3810–3820, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwano M**, Fischer A, Okada H, Plieth D, Xue C, Danoff TM, Neilson EG.** Conditional abatement of tissue fibrosis using nucleoside analogs to selectively corrupt DNA replication in transgenic fibroblasts. Mol Ther 3: 149–159, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Iwano M**, Neilson EG.** Mechanisms of tubulointerstitial fibrosis. Curr Opin Nephrol Hypertens 13: 279–284, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Iwano M**, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG.** Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapitsinou PP**, Haase VH.** The VHL tumor suppressor and HIF: insights from genetic studies in mice. Cell Death Differ 15: 650–659, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly BD**, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL.** Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res 93: 1074–1081, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Kietzmann T**, Roth U, Jungermann K.** Induction of the plasminogen activator inhibitor-1 gene expression by mild hypoxia via a hypoxia response element binding the hypoxia-inducible factor-1 in rat hepatocytes. Blood 94: 4177–4185, 1999. [PubMed] [Google Scholar]
- 17.Kojima I**, Tanaka T, Inagi R, Kato H, Yamashita T, Sakiyama A, Ohneda O, Takeda N, Sata M, Miyata T, Fujita T, Nangaku M.** Protective role of hypoxia-inducible factor-2alpha against ischemic damage and oxidative stress in the kidney. J Am Soc Nephrol 18: 1218–1226, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Kren S**, Hostetter TH.** The course of the remnant kidney model in mice. Kidney Int 56: 333–337, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Kurban G**, Hudon V, Duplan E, Ohh M, Pause A.** Characterization of a von Hippel-Lindau pathway involved in extracellular matrix remodeling, cell invasion, and angiogenesis. Cancer Res 66: 1313–1319, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Lucero HA**, Kagan HM.** Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci 63: 2304–2316, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma LJ**, Fogo AB.** Model of robust induction of glomerulosclerosis in mice: importance of genetic background. Kidney Int 64: 350–355, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Manalo DJ**, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL.** Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105: 659–669, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Mandriota SJ**, Turner KJ, Davies DR, Murray PG, Morgan NV, Sowter HM, Wykoff CC, Maher ER, Harris AL, Ratcliffe PJ, Maxwell PH.** HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell 1: 459–468, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Manotham K**, Tanaka T, Matsumoto M, Ohse T, Miyata T, Inagi R, Kurokawa K, Fujita T, Nangaku M.** Evidence of tubular hypoxia in the early phase in the remnant kidney model. J Am Soc Nephrol 15: 1277–1288, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Motzer RJ**, Bander NH, Nanus DM.** Renal-cell carcinoma. N Engl J Med 335: 865–875, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Nishitani Y**, Iwano M, Yamaguchi Y, Harada K, Nakatani K, Akai Y, Nishino T, Shiiki H, Kanauchi M, Saito Y, Neilson EG.** Fibroblast-specific protein 1 is a specific prognostic marker for renal survival in patients with IgAN. Kidney Int 68: 1078–1085, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Norman JT**, Clark IM, Garcia PL.** Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int 58: 2351–2366, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Okada H**, Danoff TM, Kalluri R, Neilson EG.** Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol Renal Physiol 273: F563–F574, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Orphanides C**, Fine LG, Norman JT.** Hypoxia stimulates proximal tubular cell matrix production via a TGF-beta1-independent mechanism. Kidney Int 52: 637–647, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Patel TH**, Kimura H, Weiss CR, Semenza GL, Hofmann LV.** Constitutively active HIF-1alpha improves perfusion and arterial remodeling in an endovascular model of limb ischemia. Cardiovasc Res 68: 144–154, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Rankin EB**, Higgins DF, Walisser JA, Johnson RS, Bradfield CA, Haase VH.** Inactivation of the arylhydrocarbon receptor nuclear translocator (Arnt) suppresses von Hippel-Lindau disease-associated vascular tumors in mice. Mol Cell Biol 25: 3163–3172, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rankin EB**, Tomaszewski JE, Haase VH.** Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res 66: 2576–2583, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberger C**, Mandriota S, Jurgensen JS, Wiesener MS, Horstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, Eckardt KU.** Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol 13: 1721–1732, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Schofield CJ**, Ratcliffe PJ.** Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol 5: 343–354, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Semenza GL**.** Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3: 721–732, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Strutz F**, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG.** Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 130: 393–405, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiesener MS**, Jurgensen JS, Rosenberger C, Scholze CK, Horstrup JH, Warnecke C, Mandriota S, Bechmann I, Frei UA, Pugh CW, Ratcliffe PJ, Bachmann S, Maxwell PH, Eckardt KU.** Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J 17: 271–273, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Yang H**, Minamishima YA, Yan Q, Schlisio S, Ebert BL, Zhang X, Zhang L, Kim WY, Olumi AF, Kaelin WG Jr.** pVHL acts as an adaptor to promote the inhibitory phosphorylation of the NF-kappaB agonist Card9 by CK2. Mol Cell 28: 15–27, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeo EJ**, Chun YS, Cho YS, Kim J, Lee JC, Kim MS, Park JW.** YC-1: a potential anticancer drug targeting hypoxia-inducible factor 1. J Natl Cancer Inst 95: 516–525, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Young AP**, Schlisio S, Minamishima YA, Zhang Q, Li L, Grisanzio C, Signoretti S, Kaelin WG Jr.** VHL loss actuates a HIF-independent senescence programme mediated by Rb and p400. Nat Cell Biol 10: 361–369, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Zhang B**, Liang X, Shi W, Ye Z, He C, Hu X, Liu S.** Role of impaired peritubular capillary and hypoxia in progressive interstitial fibrosis after 56 subtotal nephrectomy of rats. Nephrology (Carlton) 10: 351–357, 2005. [DOI] [PubMed] [Google Scholar]