Thermogenic Capacity Is Antagonistically Regulated in Classical Brown and White Subcutaneous Fat Depots by High Fat Diet and Endurance Training in Rats: IMPACT ON WHOLE-BODY ENERGY EXPENDITURE (original) (raw)
Background: Brown adipose tissue (BAT) is important for cold- and diet-induced thermogenesis.
Results: Obesity and chronic exercise antagonistically regulate thermogenic capacity of BAT and subcutaneous white fat (SC WAT).
Conclusion: Endurance exercise reduces thermogenic capacity in classical BAT while increasing it in the SC WAT.
Significance: Browning of the SC WAT may be potentially used to treat obesity.
Keywords: Adipose Tissue Metabolism; AMP-activated Kinase (AMPK); Exercise; Obesity; Peroxisome Proliferator-activated Receptor γ Coactivator 1-α (PGC-1a) (PPARGC1A); UCP-1, FNDC5, Irisin, Brown Adipocytes, ATGL
Abstract
This study investigated the regulation of thermogenic capacity in classical brown adipose tissue (BAT) and subcutaneous inguinal (SC Ing) white adipose tissue (WAT) and how it affects whole-body energy expenditure in sedentary and endurance-trained rats fed ad libitum either low fat or high fat (HF) diets. Analysis of tissue mass, PGC-1α and UCP-1 content, the presence of multilocular adipocytes, and palmitate oxidation revealed that a HF diet increased the thermogenic capacity of the interscapular and aortic brown adipose tissues, whereas exercise markedly suppressed it. Conversely, exercise induced browning of the SC Ing WAT. This effect was attenuated by a HF diet. Endurance training neither affected skeletal muscle FNDC5 content nor circulating irisin, but it increased FNDC5 content in SC Ing WAT. This suggests that locally produced FNDC5 rather than circulating irisin mediated the exercise-induced browning effect on this fat tissue. Importantly, despite reducing the thermogenic capacity of classical BAT, exercise increased whole-body energy expenditure during the dark cycle. Therefore, browning of subcutaneous WAT likely exerted a compensatory effect and raised whole-body energy expenditure in endurance-trained rats. Based on these novel findings, we propose that exercise-induced browning of the subcutaneous WAT provides an alternative mechanism that reduces thermogenic capacity in core areas and increases it in peripheral body regions. This could allow the organism to adjust its metabolic rate to accommodate diet-induced thermogenesis while simultaneously coping with the stress of chronically increased heat production through exercise.
Introduction
Adipose tissue can be broadly classified into white and brown (1). Although WAT3 is specialized to store energy, BAT has a great capacity to dissipate energy in the form of heat (2). In its activated state, BAT utilizes glucose and fatty acids for heat production via the mitochondrial uncoupling protein-1 (UCP-1), thereby reducing the availability of substrate for storage in WAT. It is now recognized that adult humans have significant amounts of inducible BAT (3–6). In fact, two populations of these inducible brown fat cells have been identified as follows: classical brown and “beige“ adipocytes (7, 8). The former displays all the features of the typical brown fat cell found in rodents and is activated upon cold exposure. The latter does not seem to have the same developmental origin as classical BAT, but it can be induced to acquire a “brown-like” thermogenic phenotype and potentially increase whole-body energy expenditure (8).
It has been suggested that humans have a population of quiescent beige adipocytes dispersed within the WAT (particularly in the SC WAT) that can be recruited to promote energy dissipation (8) and therefore can be targeted for the treatment of major metabolic disorders such as obesity and type 2 diabetes. In this context, it has been reported that chronic endurance exercise (9, 10) has the ability to promote the expression of thermogenic genes in white adipocytes (“browning” of the WAT) and increase whole-body energy expenditure. Such effects have been proposed to be induced by a myokine released during exercise (irisin) derived from the cleavage of fibronectin domain-containing protein 5 (FNDC5) that promotes browning of the SC WAT (9). These findings established a direct novel relationship between endurance exercise and browning of WAT with important potential implications for the regulation of whole-body energy homeostasis. However, the contribution of exercise-induced WAT browning to whole-body energy expenditure has been questioned. Particularly, because exercise is thermogenic in itself, it seems counterintuitive that it would augment heat production by conferring brown-like features to WAT. Also, it has been reported that oxidation of pyruvate, α-ketoglutarate, palmitoylcarnitine, and succinate were reduced by at least 50% in mitochondria isolated from interscapular BAT (iBAT) of rats subjected to treadmill running for 5–6 weeks when compared with sedentary counterparts (11, 12).
These studies provide evidence that chronic endurance exercise actually reduces thermogenic activity in classical BAT. Based on these findings, it seems that chronic endurance exercise exerts antagonistic effects on thermogenesis in classical BAT versus SC WAT. Currently, limited information is available with regard to the thermogenic capacity of classical BAT in comparison with SC WAT under conditions of chronic endurance exercise. Additionally, even though the extent and mechanisms by which exercise-induced browning of WAT affects whole-body energy expenditure are of great interest, they remain largely undetermined. Therefore, the aim of this study was to determine the effects of chronic endurance exercise on thermogenic capacity in classical BAT and SC WAT and the impact on whole-body energy expenditure. To accomplish this, we assessed alterations in major molecular determinants of thermogenesis in classical brown and SC Ing WAT, as well as circulating irisin and FNDC5 content in skeletal muscle and SC WAT. Because classical BAT plays an important role in diet-induced thermogenesis (DIT) and regulation of whole-body energy homeostasis (13–15), we also assessed molecular markers of thermogenesis (PGC-1α and UCP-1 content) and palmitate oxidation in iBAT and aortic BAT (aBAT), as well as in SC WAT from sedentary and chronically endurance-trained rats fed either LF or HF diets. Here, we provide a detailed analysis of the physiological and molecular mechanisms by which thermogenic capacity is regulated in classical BAT and SC WAT and at the whole-body level under diet-induced obesity and chronic endurance exercise conditions.
EXPERIMENTAL PROCEDURES
Reagents
Fatty acid-free bovine serum albumin (BSA), l-carnitine, CoA, and palmitic acid were obtained from Sigma. DTT, ATP, ADP, and nicotinamide adenine dinucleotide phosphate (NADP) were obtained from BioShop Canada Inc. (Burlington, Ontario, Canada). [1-14C]Palmitic acid was from GE Healthcare. The irisin kit (catalog no. 067-29) was from Phoenix Pharmaceuticals, Inc. (Burlingame, CA). Protease (cOmplete ULTRA Tablets) and phosphatase (PhosStop) inhibitors were from Roche Diagnostics. Specific antibodies against ATGL, AMPK, P-AMPKα (Thr-172), and β-actin were purchased from Cell Signaling Technology Inc. (Beverly, MA). The PGC-1α antibody was from Millipore (Temecula, CA), and the antibodies against UCP-1, FNDC5, and GAPDH were purchased from Abcam (Cambridge, MA).
Animals, Selection Protocol, and Diet
Male albino rats from the Wistar strain (Charles River Laboratories, Montreal, Quebec, Canada) weighing ∼200 g (initial weight) were maintained at a constant temperature (23 °C), with a fixed 12-h light/12-h dark cycle. The protocol containing all animal procedures described in this study was specifically approved by the Committee on the Ethics of Animal Experiments of York University (York University Animal Care Committee, permit number 2011-14) and performed strictly in accordance with the York University Animal Care Committee guidelines. All surgery was performed under ketamine/xylazine anesthesia, and all efforts were made to minimize suffering. Prior to assigning animals to each experimental group, each rat was subjected to a screening exercise protocol to determine the ones unwilling to exercise. The screening protocol consisted of three separate treadmill exercise sessions, each starting with a 5-min warm-up period with constant inclination and speed set at 5% and 10 m/min, respectively. Subsequently, the inclination was increased to 10% and maintained constant, although the speed was increased by 2 m/min every 2 min up to 30 m/min. Rats that did not run beyond the speed of 20 m/min for at least 20 min all 3 days of the selection period were excluded from the study. Only 10% of the animals did not meet the inclusion criteria. The selected animals were then randomly divided into four groups as follows: 1) sedentary fed a LF diet (Sed LF); 2) endurance-trained fed a LF diet (Ex LF); 3) sedentary fed a HF diet (Sed HF); and 4) endurance-trained fed a HF diet (Ex HF). The animals were fed ad libitum purified ingredient diets from Research Diets Inc. Rats on the LF diet groups were provided with food containing 10, 70, and 20% of the total calories from fat, carbohydrate (sucrose levels matching the HF diet), and protein, respectively (catalog no. D12450J). The HF diet groups were provided with food containing 59.9, 20.1, and 20% of the total calories from fat, carbohydrate, and protein, respectively (catalog no. D12492).
Determination of Organ Mass and Lean Body Mass (LBM)
Body composition was assessed as described previously (16). Briefly, at the end of the study, Sed and Ex rats were weighed, anesthetized (ketamine/xylazine 0.4 and 8 mg per 100 g of body weight), decapitated, and exsanguinated. A longitudinal anterior skin incision from neck to tail was then made. A scalpel was used to detach the entire skin consisting of fur and SC WAT from the carcass of each animal. At this point, the SC Ing fat depot was carefully excised and weighed. A similar procedure was carried out to remove the skin from the head. The head and body skins were weighed separately. The iBAT was removed, thoroughly trimmed of any visual white adipose tissue present, and weighed. The abdominal and thoracic cavities were then incised longitudinally, and the internal organs were exposed. The aBAT was excised along with the liver, kidneys, and heart and individually weighed. The remaining abdominal organs as well as the lungs were all accounted for as viscera. Next, the epididymal and retroperitoneal fat depots were removed and weighed. The mass of the remaining carcass consisting of skeletal muscle and bones combined with the skinned head, liver, heart, and kidneys was used as LBM (16).
Determination of Irisin in the Serum
Irisin was determined by using ELISA kits from Phoenix Pharmaceuticals, Inc. Blood was extracted under resting conditions at week 8 of the study and also immediately after exercise at week 6 of the study. For the determination of resting irisin, blood was collected from the saphenous vein 24 h after the last bout of exercise in the fed state. For the determination of irisin immediately after exercise, blood was collected at week 6 with all animals (including the sedentary groups) immediately after running for 1 h at 70–85% of peak VO2.
Peak Oxygen Consumption (Peak VO2) and Training Protocol
To set the initial training intensity and to adjust it as the animals improved their running ability, peak VO2 tests were conducted at weeks 0, 2, 4, and 6 of the study. Specially designed treadmills connected to the comprehensive laboratory animal monitoring system (CLAMS) from Columbus Instruments (Columbus, OH) were used to apply an exercise protocol of incremental workloads to determine peak VO2 in rats. To accomplish that, all rats were placed on the treadmill, and VO2 was continuously monitored under resting (after 15–20 min of being in the treadmill chamber) and exercising conditions. After recording resting VO2 values, the rats were exposed to a 5-min warm-up period (10 m/min, 0% inclination). Subsequently, treadmill speed was progressively increased (2 m/min every 2 min) until exhaustion was reached (characterized by the rats remaining on the shocking grid for 5 consecutive seconds) or at the point at which increments in speed were not accompanied by increases in VO2, and respiratory exchange ratio approached the value of 1. Treadmill inclination was increased to 5% in stage 1 and to 10% in stage 2 and then maintained constant until the end of the test. Rats in the endurance training groups were exposed to treadmill running at 75–85% of peak VO2, 1 h/day, 5 days/week for 8 weeks. To ensure equal conditions between the sedentary and endurance-trained groups, all rats were placed on the treadmill simultaneously. The treadmill speed for sedentary animals was kept at 1–2 m/min during the entire duration (1 h) of the training session. Treadmill speed was adjusted every 2 weeks to maintain the exercise intensity between 75 and 85% of peak VO2 throughout the study. For weeks 1 and 2, the training sessions started with a warm-up period (3 min at 12 m/min, 0% inclination, followed by 2 min at 14 m/min, 5% inclination). Treadmill inclination was then increased to 10% and maintained constant, and the speed was progressively increased in a manner that by the 20th min all animals had reached the 75–85% peak VO2 training range and were then exercised at that intensity for the remaining 40 min. The animals quickly adapted to the training protocol. Thus, for weeks 3–8 the warm-up lasted only 2 min and started at 24 m/min and 10% inclination with the speed being progressively increased every 2 min. This was done in a manner that the training range was reached within 10 min of the start and then maintained for the remaining 50 min of each training session. The average treadmill speed required to maintain the training intensity also increased from 24 m/min (week 1) to 38 m/min (week 4) and then to 42 m/min at week 8. With this training regimen, the total weekly mileage increased from 6.57 km at week 1 to 11.09 km at week 4, reaching 11.98 km at week 8. The intensity, frequency, and volume of exercise chosen here have been previously demonstrated to significantly increase peak VO2 in rats (17). This training protocol is also compatible with exercise prescriptions used in humans to improve cardiovascular fitness (18).
Adipose Tissue Morphology
Morphological analysis was performed using light microscopy as described previously (19) with a few modifications. Briefly, upon extraction of the fat pads, small samples (∼50–100 mg) of iBAT and aBAT, as well as of SC Ing and epididymal (Epid) white adipose tissues, were removed. For the SC Ing fat depot, two samples were collected as follows: one with a visible brownish appearance localized within the central portion of the tissue, and another clearly white in appearance localized to the proximal (upper) and distal (lower) extremities of the SC Ing fat depot. All fat samples were fixed in 4% paraformaldehyde, 0.1 m phosphate buffer solution, pH 7.4, for 24 h at room temperature. After fixation, tissue samples were washed (three times) and stored in 70% ethanol. Samples were subsequently sent to the Toronto Centre for Phenogenomics (Toronto, Ontario, Canada) where they were embedded in paraffin blocks, sectioned, and processed for hematoxylin and eosin staining. Stained samples were viewed using a Nikon Eclipse TiE inverted microscope (Nikon Canada, Mississauga, Ontario, Canada) under ×10 and ×20 magnification. Average adipocyte area was determined by two independent investigators who measured the area of 150 cells in three randomly selected fields of view for each animal. This was done to prevent the biased selection of cells for measurement. Area was determined by NIS-elements basic research imaging software (Nikon Canada, Mississauga, Ontario, Canada), and images were captured with a digital Nikon DS-QI1Mc camera (Nikon Canada, Mississauga, Ontario, Canada).
Western Blot Determination of UCP-1, PGC-1α, AMPK, Phospho-AMPK, ATGL, FNDC5, GAPDH, and β-Actin
Immediately after extraction, tissues were snap-frozen in liquid nitrogen and homogenized in buffer containing 25 mm Tris-HCl, 25 mm NaCl, 1 mm MgCl2, 2.7 mm KCl, 1% Nonidet P-40, and protease (cOmplete ULTRA Tablets) and phosphatase (PhosStop) inhibitors, pH 7.4. Sample homogenates were then transferred to microtubes and centrifuged (16,000 × g for 10 min at 4 °C), and the infranatant was collected. An aliquot of the tissue lysates was used to determine the concentration of protein in each sample by the Bradford method. Before loading onto SDS-polyacrylamide gels, the samples were diluted 1:1 (v/v) with Laemmli sample buffer (62.5 mm Tris-HCl, pH 6.8, 2% (w/v) SDS, 50 mm DTT, 0.01% (w/v) bromphenol blue). To determine the total and phosphorylated (Thr-172) forms of AMPK (62 kDa), aliquots containing 80 μg of protein were subjected to SDS-PAGE and then transferred to polyvinyldifluoride (PVDF) membranes (Bio-Rad). To determine ATGL (54 kDa) and UCP-1 (33 kDa) contents in SC Ing fat, aliquots containing 50 μg of protein were loaded onto the gels. Aliquots containing 25 μg of protein were used to probe for UCP-1 content in lysates from iBAT and aBAT. All primary antibodies were at 1:1000 dilution. The blots were scanned, and the density of each band of interest was determined using the ImageJ program. Western blot data for ATGL, FNDC5 (22 kDa), PGC-1α (∼113 kDa), and UCP-1 were expressed as arbitrary units. The values were obtained by dividing the density of the band of interest by that of either β-actin (45 kDa) or GAPDH (36 kDa) (as indicated in the figure legends) from the same blot. Similarly, P-AMPK data were normalized by total AMPK.
Palmitate Oxidation
Samples of soleus (Sol) and extensor digitorum longus (EDL) muscles (100 mg), iBAT and aBAT (100 mg), and SC Ing fat (300 mg) were extracted and thoroughly minced in 200 μl of ice-cold SETH buffer (300 mm sucrose, 2 mm EDTA, and 10 mm Tris-HCl, pH 7.4). Additional SETH buffer was added to yield a 20-fold (w/v) diluted minced tissue sample. The solution was then homogenized in an ice-cold Potter-Elvehjem glass homogenizer (10–12 passes across ∼30 s). Subsequently, 400 μl of tissue homogenates were transferred to plastic scintillation vials containing 1.6 ml of the reaction mixture (150 mm sucrose, 5 mm MgCl2, 30 mm KCl, 30 mm potassium phosphate buffer, 2 mm EDTA, 2 mm ADP, 15 mm Tris-HCl, 1% BSA, 0.75 mm palmitate, 1 mm carnitine, 0.025 mm CoA, pH 7.4) containing 0.2 μCi/ml [1-14C]palmitic acid. Cold and labeled palmitate were complexed with fatty acid-free BSA prior to adding to the reaction mixture. The rates of palmitate oxidation by Sol and EDL muscles, iBAT, aBAT, and SC Ing fat homogenates were measured by the production of 14CO2 from [1-14C]palmitic acid. The flasks where tissue homogenates were incubated had a centered isolated well containing a loosely folded piece of filter paper moistened with 0.2 ml of 2-phenylethylamine/methanol (1:1, v/v). After the 1-h incubation period, the media were acidified with 0.2 ml of H2SO4 (5 n), and the flasks were maintained sealed at 37 °C for an additional 1 h for collection of the released 14CO2. Subsequently, the filter papers were carefully removed and transferred to scintillation vials for radioactivity counting (20).
Determination of in Vivo Metabolic Parameters
The CLAMS was used to perform all automated in vivo determinations as described previously (16). Briefly, the CLAMS measures oxygen consumption (VO2), carbon dioxide production (VCO2), and respiratory exchange ratio. Each cage is also equipped with a system of infrared beams that detects animal movement in the x and z axes, which was used to determine spontaneous ambulatory activity. Energy expenditure (heat) was calculated by multiplying the calorific value (CV = 3.815 + 1.232 × respiratory exchange ratio) by VO2. Measurements using the CLAMS were performed after 8 weeks of diet and exercise interventions. The animals were placed in the CLAMS at 11:00 a.m. and 24 h after the last exercise training session. The 1st h of data collected in the CLAMS was discarded, because it is the time required for the rats to acclimatize to the cage environment (16). The rats were monitored for a 24-h period encompassing the light (07:00–19:00 h) and dark (19:00–07:00 h) cycles.
Statistical Analyses
The significance of differences between two groups was determined by two-tailed Student's unpaired or paired t tests and for multiple comparisons by either one-way or two-way analysis of variance (ANOVA) as indicated. The Bonferroni post hoc multiple comparison test was used when differences were identified. The Graph Pad Prism 5 software was used for all statistical analyses.
RESULTS
Body Mass, LBM, and Adiposity
Body mass of the Ex LF and Ex HF rats was ∼12% lower than the Sed LF and Sed HF controls (Table 1). Body mass of Sed HF rats was 8% higher than that of Sed LF rats, although this did not reach statistical significance (Table 1). LBM of Ex LF and Ex HF rats was also reduced by ∼7% when compared with Sed LF and Sed HF controls (Table 1). In Ex LF and Ex HF animals, the masses of the Epid, SC Ing, and Retro fat pads were significantly reduced by 33 and 30%, 40 and 26%, and by 42 and 37%, respectively, when compared with Sed LF and Sed HF controls (Table 1). As expected, adiposity was significantly increased by HF feeding, which was demonstrated by 1.73-, 1.3-, and 1.86-fold increases in the masses of the Epid, Sc Ing, and Retro fat pads of Sed HF when compared with Sed LF rats (Table 1). Conversely, endurance training prevented the increase in adiposity induced by HF feeding. In fact, the masses of the Epid, Sc Ing, and Retro fat pads of Ex HF rats were similar to those of Sed LF rats (Table 1).
TABLE 1.
Effects of chronic endurance training on body mass, adiposity, and LBM
Total body mass, Epid, SC Ing, and Retro fat masses and LBM were measured at the end of the study.
| Sed LF | Ex LF | Sed HF | Ex HF | |
|---|---|---|---|---|
| Body mass (g) | 481.50 ± 8.54 | 428.43 ± 10.29a | 524.20 ± 8.77 | 464.89 ± 9.73a |
| Fat pad mass (g) | ||||
| Epid | 8.16 ± 0.55 | 5.49 ± 0.36b | 14.09 ± 0.77c | 9.83 ± 0.93d |
| SC Ing | 11.41 ± 0.67 | 6.79 ± 0.32b | 14.89 ± 0.87c | 11.00 ± 0.85d |
| Retro | 6.78 ± 0.78 | 3.94 ± 0.23b | 12.61 ± 0.85c | 7.95 ± 0.78d |
| LBM (g) | 294.30 ± 6.14 | 271.60 ± 5.22a | 295.20 ± 4.2 | 273.80 ± 5.6a |
iBAT Mass and Assessment of Unilocular Droplet Area
iBAT mass significantly increased (1.84-fold) in Sed HF when compared with Sed LF rats (Fig. 1, A and C). Chronic endurance exercise significantly reduced iBAT mass by 39% when comparing Sed LF and Ex LF (558.26 ± 47.34 versus 338.23 ± 27.12 mg) and by 26% when comparing Sed HF and Ex HF (1028.39 ± 58.55 versus 756.66 ± 39.84 mg) rats (Fig. 1, A and C). Microscopic analysis revealed that iBAT of Sed LF and Sed HF rats contained essentially multilocular brown adipocytes (Fig. 1B), although the area of unilocular adipocytes was ∼47% higher in the latter than the former (Fig. 1D). Interestingly, the iBAT of Ex LF and Ex HF rats was occupied by much larger unilocular lipid droplets resembling white adipocytes (Fig. 1B). In fact, it was found that the area of unilocular adipocytes present in iBAT was 3-fold higher in the Ex LF than Sed LF rats (768.6 ± 15.08 versus 256.1 ± 10.69 μm2) and 1.84-fold higher in Ex HF than Sed HF (689.8 ± 27.04 versus 375.5 ± 8.99 μm2) rats (Fig. 1D).
FIGURE 1.
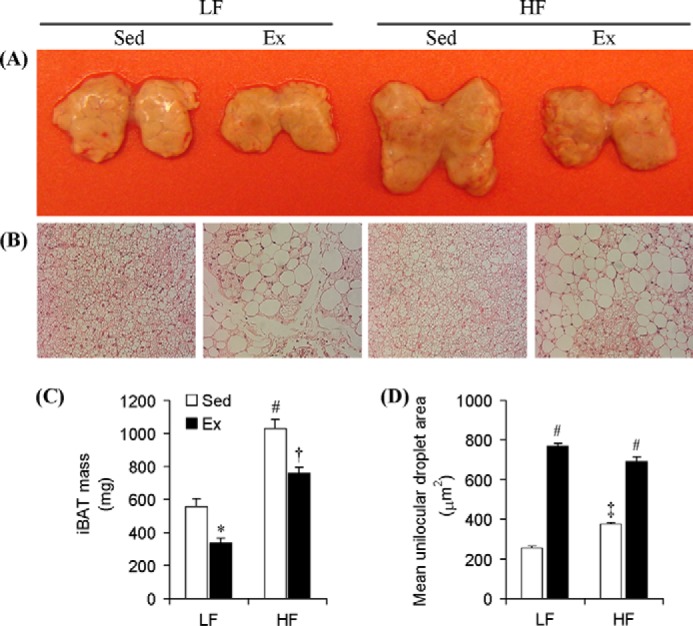
Chronic endurance training and HF diet exert antagonistic effects on mass and unilocular lipid content in the iBAT of rats. A, picture of iBATs dissected from Sed and Ex rats fed either LF or HF diets at week 8. B, respective microscopic images (×20 magnification) of H&E staining of iBAT samples from all groups of animals. Average iBAT mass (C) and unilocular adipocyte area (D) are present within the iBAT of all four groups of animals. *, p < 0.05 versus Sed LF; #, p < 0.05 versus Sed LF, Ex LF, and Ex HF; †, p < 0.05 versus Ex LF and Sed HF; ‡, p < 0.05 versus all other conditions. Two-way ANOVA (n = 8).
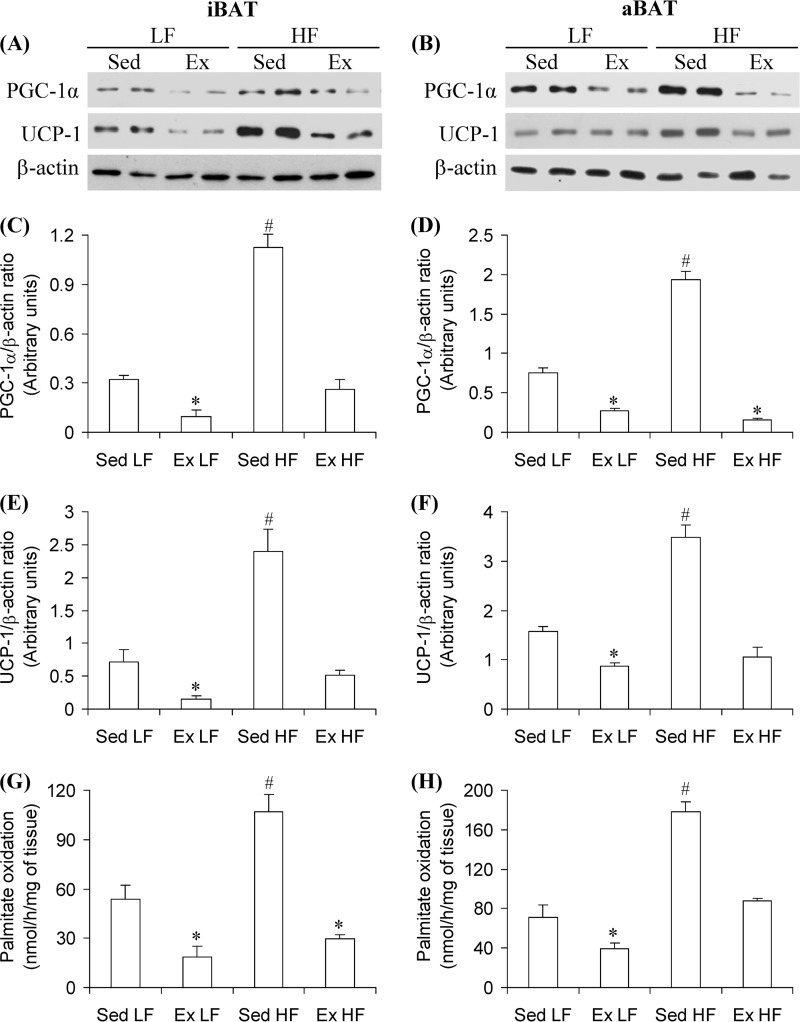
PGC-1α and UCP-1 Content and Palmitate Oxidation in iBAT and aBAT
Western blotting analysis revealed that in iBAT and aBAT of Sed HF rats, the contents of PGC-1α and UCP-1 were significantly increased by 3.5- and 2.55-fold and by 3.38- and 2.21-fold when compared with Sed LF rats, respectively (Fig. 2, A–F). Conversely, PGC-1α content was reduced by 69 and 63% and UCP-1 by 79 and 45% in the iBAT and aBAT of Ex LF rats, respectively, when compared with Sed LF controls. Furthermore, chronic endurance exercise completely prevented the HF diet-induced increase in PGC-1α and UCP-1 in iBAT and aBAT (Fig. 2, A–F). These effects were accompanied by increased palmitate oxidation in iBAT (2-fold) and aBAT (2.5-fold) of Sed HF rats when compared with Sed LF controls, whereas marked reductions were found in palmitate oxidation in iBAT (65 and 72%) and aBAT (45 and 51%) of Ex LF and Ex HF when compared with Sed LF and Sed HF, respectively (Fig. 2, G and H). These findings indicate that HF feeding increases and chronic endurance exercise reduces thermogenic capacity in iBAT and aBAT.
FIGURE 2.
Chronic endurance training reduces and HF diet increases PGC-1α and UCP-1 content, as well as palmitate oxidation in the iBAT and aBAT, respectively, brown adipose tissues. iBAT and aBAT were extracted from Sed or 8-week Ex rats fed either a LF or a HF diet. Representative blots (A and B) and densitometric analysis of PGC-1α (C and D) and UCP-1 (E and F) contents and the assessment of palmitate oxidation (G and H) in iBAT and aBAT, respectively, are shown. β-Actin was used as loading control. *, p < 0.05 versus Sed LF; #, p < 0.05 versus all other conditions. One-way ANOVA (n = 8).
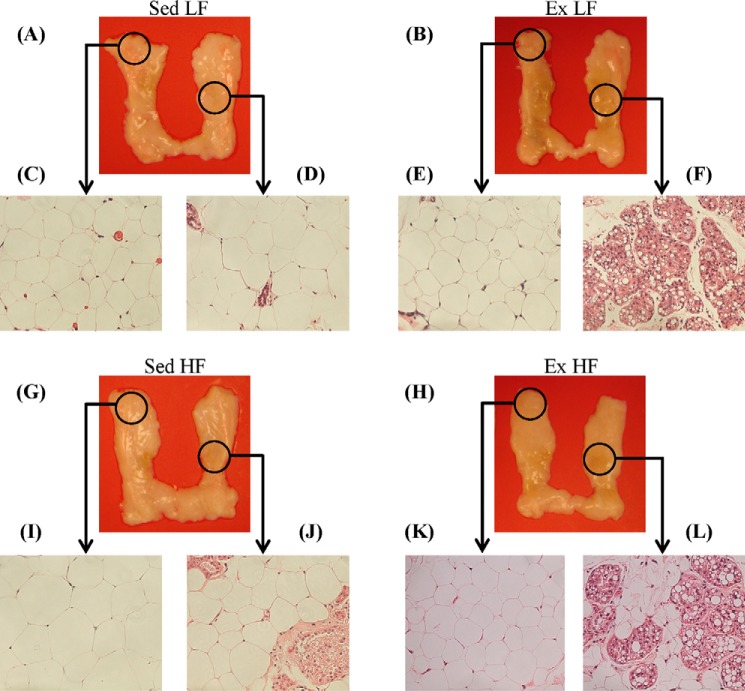
Morphological Analysis and Mean Adipocyte Area of the SC Ing Fat Depot
The initial visual impression of the SC Ing fat depot indicated that the middle region of the tissue was browner in animals exposed to chronic endurance training than in sedentary controls, although the upper and lower extremities conserved their white appearance (Fig. 3, A and B and G and H). To determine whether this was because the SC Ing fat depot was acquiring a brown-like phenotype, samples of the upper extremities and the middle regions of the tissue were used for H&E staining (Fig. 3, A and B and G and H). It was found that the middle and the upper extremity regions of the SC Ing fat from Sed LF (Fig. 3, C and D) and Sed HF (Fig. 3, I and J) rats contained essentially unilocular adipocytes typical of WAT. Interestingly, the middle region of the SC Ing fat depot from Ex LF (Fig. 3, E and F) and Ex HF (Fig. 3, K and L) rats were occupied by a large number of multilocular adipocytes typical of BAT, although the upper extremities of this tissue in Ex LF and Ex HF rats were occupied by unilocular adipocytes. Furthermore, when comparing the middle regions of the SC Ing fat depot between Ex LF and Ex HF, it was found that the former contained a much larger area occupied by multilocular brown-like adipocytes than the latter (Fig. 3, F and L). These findings suggested that although chronic endurance training induced browning of the SC Ing fat, HF feeding attenuated this effect. It was also found that the mean adipocyte area of the upper extremity of the SC Ing fat pad was significantly increased (1.66-fold) in Sed HF compared with Sed LF controls. Also, this variable was significantly reduced by 35% in Ex LF rats and by 65% in Ex HF when compared with Sed LF and Sed HF, respectively (Fig. 4A). In the middle region of the SC Ing fat depot, the mean adipocyte area was also reduced by 30% in Ex LF rats and by 36% in Ex HF when compared with Sed LF and Sed HF, respectively (Fig. 4B). However, this variable did not differ between Sed HF and Sed LF rats (Fig. 4B). These findings indicated that adipocytes located in the middle region of the SC Ing fat pad were resistant to HF-induced hypertrophy, which is compatible with the increased presence of multilocular brown-like adipocytes in this region.
FIGURE 3.
Chronic endurance training induces browning and increases the number of multilocular adipocytes in the SC Ing fat depot of LF- and HF-fed rats. Pictures of left and right SC Ing fat depots from Sed and Ex rats fed either a LF (A and B) or a HF (G and H) diet for 8 weeks. Samples of the upper extremities and middle regions of the SC Ing fat depots, as indicated by the black circles, were used for H&E staining and microscopy analyses. Representative images (×20 magnification) of adipocytes from Sed (C and D) and Ex (E and F) rats fed a LF diet or from Sed (I and J) and Ex (K and L) rats fed a HF diet are shown.
FIGURE 4.
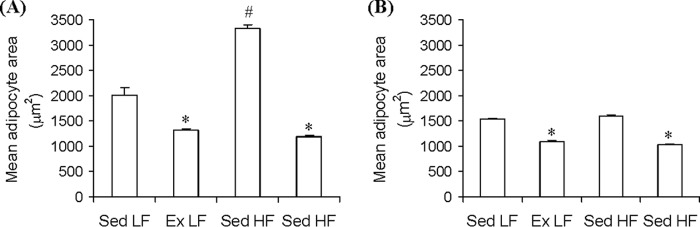
Chronic endurance training reduces unilocular adipocyte area in the upper (A) and middle (B) regions of the SC Ing fat depots, whereas a HF diet increases unilocular adipocyte area only in the upper region. Samples of the upper and middle regions of the SC Ing fat depot (as indicated in Fig. 3) from Sed and Ex rats fed either a LF or a HF diet for 8 weeks were extracted for microscopic analysis and determination of mean unilocular adipocyte area. *, p < 0.05 versus Sed LF; #, p < 0.05 versus all other conditions, One-way ANOVA (n = 8).
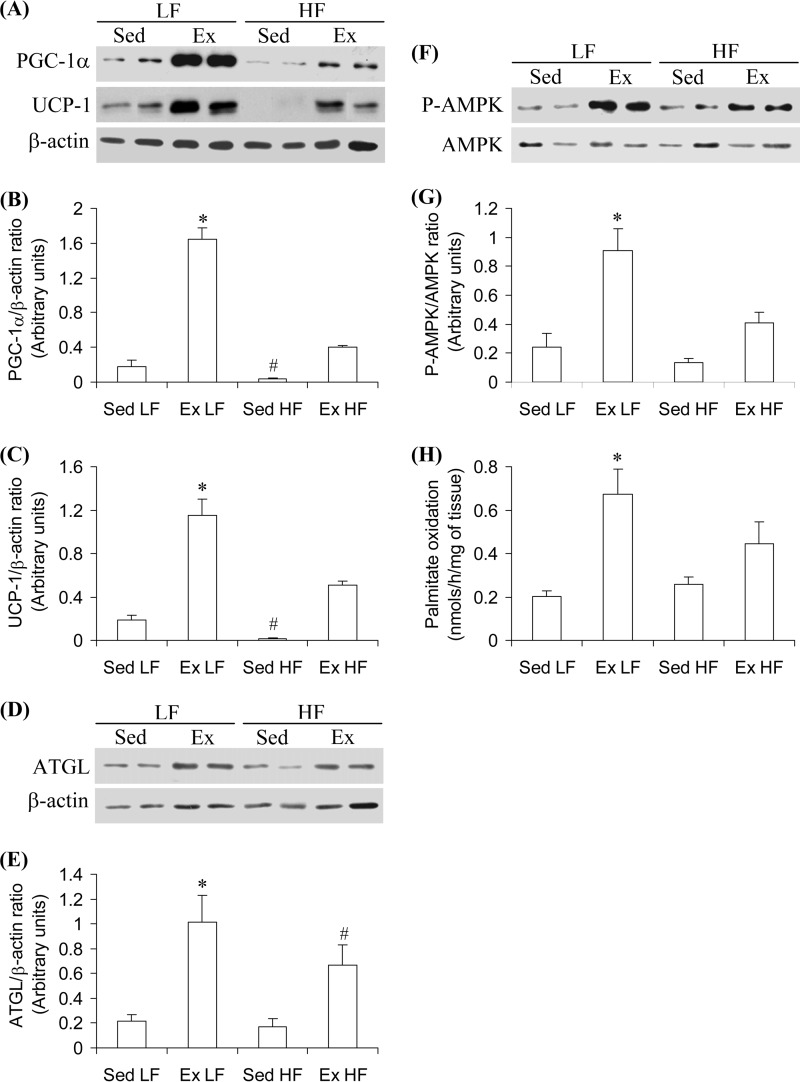
PGC-1α, UCP-1, and ATGL Content, AMPK Phosphorylation, and Palmitate Oxidation in the SC Ing Fat Depot
To test whether or not the middle region of the SC Ing fat pad actually contained functional features of thermogenic brown adipocytes, we measured the content of proteins involved in thermogenesis and the oxidative capacity of the tissue. It was found that PGC-1α, UCP-1, and ATGL content, AMPK phosphorylation, and palmitate oxidation were increased by 9.1-fold (Fig. 5, A and B), 6.13-fold (Fig. 5, A and C), 4.84-fold (Fig. 5, D and E), 3.8-fold (Fig. 5, F and G), and 3.35-fold, respectively, in the middle region of the SC Ing fat pad of Ex LF when compared with Sed LF controls. In Sed HF rats, the content of PGC-1α was reduced by 78%, and UCP-1 was almost undetectable. No alterations were found for ATGL content, AMPK phosphorylation, and palmitate oxidation in the middle region of the SC Ing fat pad of Sed HF rats when compared with Sed LF controls. In Ex HF rats, PGC-1α, UCP-1, and ATGL content, AMPK phosphorylation, and palmitate oxidation also increased but to a much lower extent (2.28-, 2.9-, 3.18-, 1.71-, and 2.25-fold, respectively) than in Ex LF rats when compared with Sed LF controls (Fig. 5, A–H). These findings indicate that the exercise-induced appearance of multilocular brown-like adipocytes within the middle region of the SC Ing fat pad was also accompanied by functional thermogenic adaptive responses and that HF feeding attenuated these effects.
FIGURE 5.
Chronic endurance training increases and HF diet reduces PGC-1α (A and B), UCP-1 (A and C), and ATGL (D and E) contents, as well as AMPK phosphorylation (F and G) and palmitate oxidation (H) in the SC Ing fat depot. The middle region of the SC Ing fat was extracted from Sed or 8-week Ex rats fed either an LF or a HF diet. β-Actin was used as loading control. *, p < 0.05 versus Sed LF; #, p < 0.05 versus all other conditions. One-way ANOVA (n = 8).
UCP-1 Content in Visceral Fat
To test whether exercise-induced browning effects were specific to the SC Ing fat depot or also took place in other white fat depots, we measured UCP-1 content in the Epid and Retro (Fig. 6) fat pads. UCP-1 was not detected in these fat pads extracted from Sed LF, Ex LF, Sed HF, and Ex HF rats (Fig. 6). These findings indicated that the SC Ing fat was the only WAT depot that underwent browning under chronic exercise conditions.
FIGURE 6.
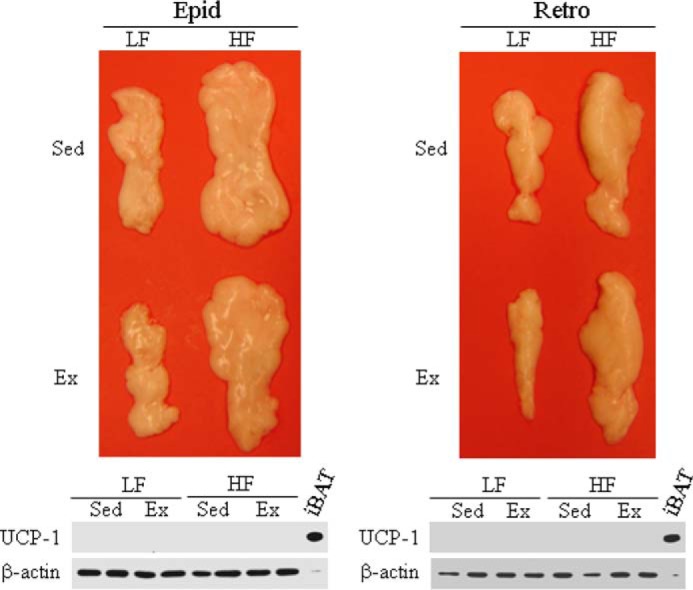
Neither Ex nor HF diet affect UCP1 content in the Epid and Retro fat pads. The Epid and Retro fat pads were excised from Sed and 8-week endurance-trained rats fed either a HF or a LF diet. The whole tissues were used for the determination by Western blot of UCP-1 content. β-Actin was used as loading control. A sample containing 10 μg of protein extracted from the iBAT was used as a positive control for UCP-1. All samples from Epid and Retro fat pads contained 50 μg of protein.
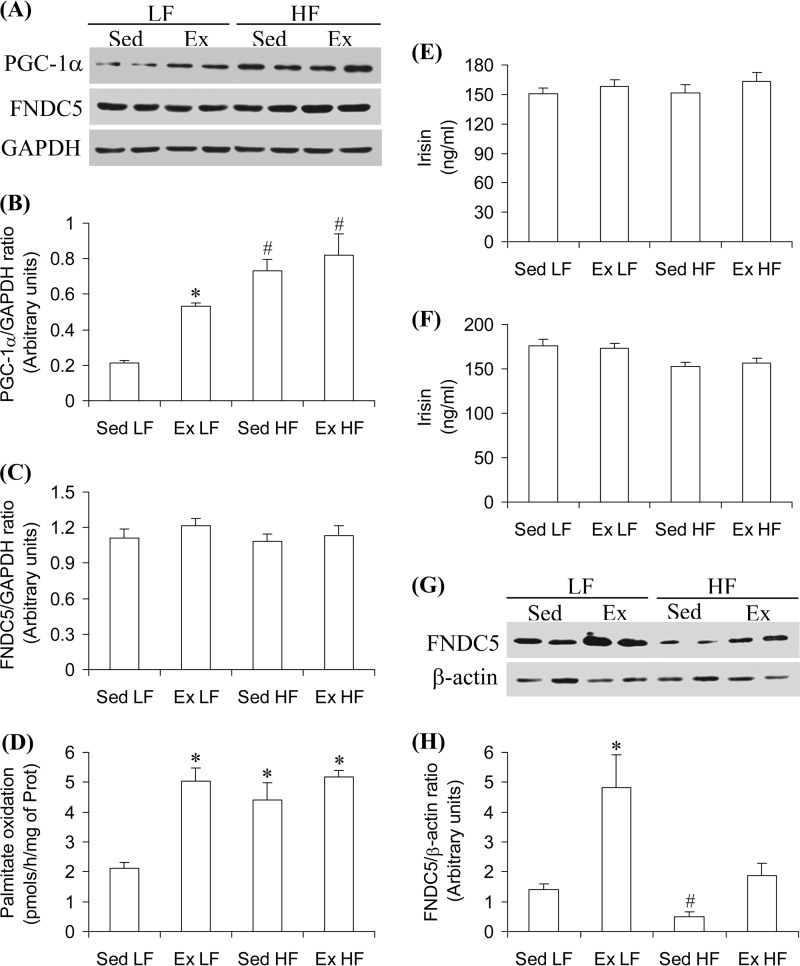
PGC-1α and FNDC5 Content and Palmitate Oxidation in Soleus Muscles
PGC-1α content in soleus muscles from Ex LF, Sed HF, and Ex HF rats increased by 2.5-, 3.45-, and 3.85-fold, respectively, when compared with Sed LF rats (Fig. 7, A and B). No alterations in FNDC5 content were observed in Sol muscles from any of the HF and/or Ex conditions (Fig. 7, A and B). When compared with Sed LF, palmitate oxidation in Sol muscles increased by 2.4-fold in Ex LF, 2.1-fold in Sed HF, and 2.45-fold in Ex HF rats (Fig. 7D). Similar findings for PGC-1α, FNDC5 content, and for palmitate oxidation were also obtained with EDL muscles (data not shown). These findings indicate that chronic endurance exercise caused a very robust training effect in skeletal muscles; however, it did not alter FNDC5 content in this tissue.
FIGURE 7.
Chronic endurance training increases PGC-1α and FNDC5 content in SC Ing fat, but neither affects soleus muscle FNDC5 content nor circulating irisin levels. Soleus muscles were extracted from Sed and 8-week Ex rats fed either a LF or a HF diet and assayed for PGC-1α (A and B) and FNDC5 content (A and C), as well as palmitate oxidation (D). Irisin was measured in the plasma under resting conditions 24 h after the last bout of exercise (E) and immediately after exercise at week 6 (F). The middle region of the SC Ing fat depot was used for the determination of FNDC5 content (G and H). β-Actin and GAPDH were used as loading control. *, p < 0.05 versus Sed LF; #, p < 0.05 versus all other conditions. One-way ANOVA (n = 8).
Circulating Irisin and FNDC5 Content in Sc Ing Fat
After 8 weeks of the diet and exercise interventions, circulating levels of irisin under resting conditions were similar among all groups (Fig. 7E). When measured immediately after exercise at week 6, circulating irisin did not differ among the four groups of animals either (Fig. 7F). Irisin levels in the serum of HF-fed animals immediately after exercise were slightly lower than those rats fed a LF diet; however, this was not statistically significant. FNDC5 content was significantly increased (3.4-fold) in the SC Ing fat depot of Ex LF rats, and the content in Sed HF was markedly reduced (65%) (Fig. 7, G and H). Exercise attenuated the effect of HF diet and raised FNDC5 content in the SC Ing fat of Ex HF rats to values similar to those of Sed LF controls (Fig. 7, G and H).
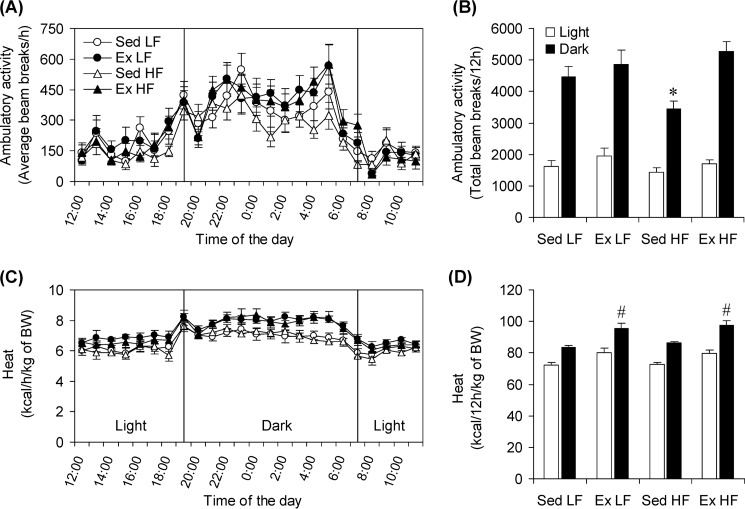
Ambulatory Activity and Energy Expenditure
Ambulatory activity during the light cycle did not differ significantly among the groups; however, during the dark cycle this variable was 23, 29, and 35% lower in Sed HF rats than Sed LF, Ex LF, and Ex HF rats, respectively (Fig. 8, A and B). Analysis of energy expenditure expressed in kilocalories/kg of body weight revealed that this variable did not differ among the four groups during the light cycle, but it was significantly increased by 14.2% in Ex LF rats and by 16.8% during the dark cycle in Ex HF rats when compared with Sed LF controls (Fig. 8, C and D). Also, during the dark cycle, energy expenditure was 13.3% higher in Ex HF than Sed HF rats (Fig. 8, C and D).
FIGURE 8.
Effects of chronic Ex and HF diet on spontaneous physical activity and whole-body energy expenditure. Ambulatory activity (A and B) and energy expenditure (C and D) were measured after the last bout of exercise at the end of the 8-week endurance training period. The rats were allowed to rest for 24 h prior to being placed in the CLAMS. *, p < 0.05 versus Sed LF; #, p < 0.05 versus all other conditions. Two-way ANOVA (n = 18).
DISCUSSION
Here, we provide novel evidence that thermogenesis is antagonistically regulated under conditions of chronic endurance exercise and energy surplus (HF diet) in classical brown fat depots and SC Ing WAT. This is supported by our observations that in Sed HF rats iBAT and aBAT tissue mass, PGC-1α and UCP-1 contents, and palmitate oxidation significantly increased, whereas in Ex LF rats these variables were markedly reduced. Conversely, in the SC Ing fat depot of Ex LF rats PGC-1α, UCP-1, and ATGL contents, AMPK phosphorylation as well as palmitate oxidation were robustly increased, whereas HF feeding attenuated these effects. These antagonistic effects of exercise and HF diet on thermogenesis in brown and WAT depots were also compatible with our findings that a large number of unilocular adipocytes typically found in WAT accumulated in iBAT and aBAT from Ex LF and Ex HF rats, although the SC Ing fat depot of Ex LF and Ex HF rats was enriched with brown-like UCP-1-positive multilocular adipocytes. Furthermore, specific areas within the SC Ing fat depot were more responsive to the browning effect of chronic endurance training than others. In fact, the exercise-induced increases in the number of multilocular adipocytes, UCP-1 content, and rate of palmitate oxidation were found in the middle region of the SC Ing fat but not at the proximal and distal extremities of this fat depot. Additionally, in Sed HF rats, the mean adipocyte area in the extremities of the SC Ing fat depot increased, whereas the middle region of this tissue remained unchanged. This indicates that the middle region of the SC Ing fat depot contains adipocytes that are resistant to hypertrophy under conditions of energy surplus, which is compatible with the site-specific differences in thermogenic capacity that we have found within the SC Ing fat depot in rats. Studies in mice have indeed reported major depot- and strain-specific differences in UCP-1 expression, indicating that the SC WAT is more prone to acquiring a brown-like phenotype through transdifferentiation than other visceral fat depots upon cold exposure (21). Here, we show that the exercise-induced browning of WAT is also depot-specific in rats, because increased UCP-1 content and the presence of multilocular adipocytes were found in the SC Ing fat pad but not in the Epid and Retro fat depots of these animals.
It has been reported that moderate to high intensity endurance training increases sympathetic nervous system activity and circulating catecholamines when compared before and after training under conditions of same relative intensity (22, 23). Importantly, catecholamines are well known for inducing BAT thermogenesis (24, 25). Throughout our study, training intensity was kept constant (70–85% VO2 max), so circulating catecholamines are expected to have been consistently increased during exercise bouts in our endurance-trained rats. Despite that, thermogenic capacities in classical BAT and SC Ing fat were regulated in an opposite fashion. It appears that chronic exposure to increased heat production through exercise overrides catecholamine-induced nonshivering thermogenesis in core regions (iBAT and aBAT) but not in specific fat depots located peripherally. The mechanism underlying these effects is unknown. However, this could be conferred by adaptive responses that affect local catecholamine release and distribution of α- and β-adrenergic receptors, as well as by central nervous system-mediated regulatory responses that drive sympathetic activity in a tissue-specific manner. Also, it has been recently demonstrated that brown fat activation can occur via a nonadrenergic activation mechanism (26). Therefore, in our study, other exercise-induced factors could have promoted browning of the SC Ing fat independently of catecholamines.
In this context, previous studies have suggested that exercise-induced browning of the SC Ing fat depot is mediated by the release of a PGC-1α-dependent myokine named irisin, which is proposed to be derived from the cleavage of the ectodomain of the FNDC5 receptor under exercising conditions (9). Therefore, we tested whether this could also be the case in this study. We found that the exercise training protocol and HF diet both caused robust increases in PGC-1α content and palmitate oxidation in Sol and EDL muscles. However, we did not detect any significant alterations in FNDC5 content in these muscles. Furthermore, circulating levels of irisin under resting conditions were not affected by either chronic endurance training or HF feeding. It could be that irisin increased transiently after exercise, and values obtained under resting conditions 24 h after the last bout of exercise could have missed such an effect. Therefore, we also measured circulating irisin immediately after exercise at week 6 of the study, but again, no significant differences were found among the four groups of animals. Our findings are consistent with other studies reporting a lack of muscle FNDC5 and irisin regulation under exercising conditions in humans (27–29). The discrepancy of our findings with those that do report an increase in circulating irisin with exercise in either humans or rodents (9, 30–34) could derive from methodological differences (rodent species, mode, intensity, and duration of exercise, as well as timing of blood sampling) that exist among the studies.
Because FNDC5 and irisin have been reported to be also expressed and released by adipocytes (35, 36), we measured FNDC5 content in the SC Ing fat depot. Similarly to what was found for PGC-1α and UCP-1, chronic endurance exercise caused a significant increase in FNDC5 content, whereas HF diet markedly reduced it in the SC Ing fat depot. These findings suggest that locally produced FNDC5 rather than circulating irisin could have mediated the browning effect of chronic endurance exercise on SC Ing fat. Recent crystal structure analysis and biochemical characterization studies have predicted that a tight dimerization of the FNDC5 ectodomain may form intra- and/or intercellular dimers at the cell surface, which could lead to autocrine or paracrine signaling independently of cleavage and release of irisin in the circulation (37). In this context, the increase and reduction in FNDC5 content found in the SC Ing fat depot of Ex LF and Sed HF rats, respectively, could provide a mechanism by which exercise induced a marked browning effect in LF-fed rats, whereas HF feeding attenuated this effect.
Exercise is thermogenic in itself, and a large amount of heat is actually produced as a consequence of muscle contractions. Thus, a reduction in BAT activity is expected to occur under conditions of repeated chronic endurance exercise. In fact, previous studies have reported that the tissue mass and Ucp-1 mRNA levels of iBAT were markedly reduced in rodents exposed to chronic endurance training (38). Other studies have also demonstrated that chronic endurance exercise reduces the thermogenic activity of iBAT (11, 12, 39, 40). Besides cold-induced thermogenesis, previous studies in rats have consistently demonstrated that structural and functional alterations in iBAT are also directly related to DIT and the regulation of energy balance (13–15, 31, 41–44). In mice, UCP-1 ablation abolished DIT, and the animals developed obesity when living at thermoneutrality (44). In humans, it has also been reported that glucose uptake in BAT increases after a meal, which indicates a role for BAT in reducing metabolic efficiency (45). In this context, our findings that chronic endurance exercise reduces tissue mass and thermogenic capacity in iBAT and aBAT are compatible with a compensatory adaptive response that down-regulates thermogenesis in an animal that is regularly exposed to exercise-induced increased heat production. Also, our findings of increased iBAT and aBAT mass and thermogenic capacity in Sed HF rats are consistent with increased DIT in a sedentary animal that is chronically exposed to energy surplus and increased adiposity.
The question that arises is why does chronic endurance exercise induce browning of the SC Ing fat depot if heat production is already increased by muscle contractions? It has been proposed that exercise-induced browning of the SC WAT could have evolved from shivering-related muscle contractions, serving to augment brown fat thermogenesis under conditions of cold exposure (33). The rats in our study were not exposed to cold stress, and the typically thermogenic iBAT and aBAT had their masses, UCP-1 content, and rate of fatty acid oxidation markedly reduced in animals exposed to chronic endurance training. Therefore, it seems unlikely that exercise-induced browning of the SC Ing fat depot took place to enhance BAT thermogenesis in these animals. Instead, it appears to be more compatible with an adaptive response to chronic endurance exercise that provides an alternative mechanism for the regulation of whole-body energy balance when DIT and cold-induced thermogenesis through classical BAT is impaired. This is consistent with our in vivo findings that whole-body dark-cycle energy expenditure was increased by 14.2% in Ex LF rats and by 16.8% in Ex HF rats when compared with Sed LF rats. This occurred despite the reductions in tissue mass, UCP-1 content, and palmitate oxidation in iBAT and aBAT in Ex LF and Ex HF rats. We cannot attribute these effects solely to browning of the SC Ing fat pad in these animals; however, it seems to have at least partially contributed to increase whole-body energy expenditure in chronically endurance-trained rats. This is supported by the fact that thermogenic capacity in iBAT and aBAT was markedly attenuated in Ex LF and Ex HF rats, yet whole-body energy expenditure was increased in these animals when compared with Sed LF rats. This suggests that an alternative mechanism, likely browning of SC WAT, exerted a compensatory effect and raised whole-body energy expenditure in these animals. Importantly, dark cycle spontaneous physical activity was 23–35% lower in Sed HF rats than in the other groups of animals, and endurance training completely reversed this effect. In fact, spontaneous physical activity of Ex HF rats in the dark cycle was the highest among the groups, although not statistically significant from those of Sed LF and Ex LF rats. Because energy expenditure did not differ between Sed LF and Sed HF rats despite lower ambulatory activity in the latter than the former, it appears that alterations in dark cycle spontaneous physical activity was not the major force driving the increase in energy expenditure found in chronically endurance-trained rats. This again provides support to a compensatory increase in thermogenesis through browning of SC Ing WAT.
In conclusion, this study provides novel evidence that thermogenic capacity is antagonistically regulated in classical brown and white adipose tissues by chronic endurance training and HF diet. These adaptive responses indicate that DIT, which normally depends on increased classical BAT activity in core areas, is shifted by chronic endurance exercise toward more superficial regions. This is characterized by a reduction in thermogenic capacity of classical BAT accompanied by browning of SC WAT. This may serve to allow the organism to make adjustments in whole-body energy expenditure by activating thermogenesis in peripheral tissues while simultaneously coping with the stress of exercise-induced heat production in core regions of the body. Our in vivo findings also provide evidence that the induction of browning in SC WAT may have an important impact on whole-body energy expenditure and be potentially used as a therapeutic approach to treat obesity and its related metabolic disorders.
Acknowledgment
We thank Diane Sepa-Kishi for helping with the organization of references.
*
This work was supported by Natural Sciences and Engineering Research Council of Canada Grant 311818-2011 (to R. B. C.).
3
The abbreviations used are:
WAT
white adipose tissue
AMPK
AMP-activated protein kinase
ATGL
adipose triglyceride lipase
CLAMS
comprehensive laboratory animal monitoring system
DIT
diet-induced thermogenesis
BAT
brown adipose tissue
aBAT
aortic BAT
SC WAT
subcutaneous WAT
Sed
sedentary
Ex
endurance-trained
HF
high fat
LF
low fat
SC Ing
subcutaneous inguinal
Epid
epididymal
Retro
retroperitoneal
ANOVA
analysis of variance
iBAT
interscapular BAT
LBM
lean body mass
Sol
soleus
EDL
extensor digitorum longus.
REFERENCES
- 1.Cinti S. (2009) Transdifferentiation properties of adipocytes in the adipose organ. Am. J. Physiol. Endocrinol. Metab. 297, E977–E986 [DOI] [PubMed] [Google Scholar]
- 2.Cannon B., Nedergaard J. (2004) Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 [DOI] [PubMed] [Google Scholar]
- 3.van Marken Lichtenbelt W. D., Vanhommerig J. W., Smulders N. M., Drossaerts J. M., Kemerink G. J., Bouvy N. D., Schrauwen P., Teule G. J. (2009) Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508 [DOI] [PubMed] [Google Scholar]
- 4.Virtanen K. A., Lidell M. E., Orava J., Heglind M., Westergren R., Niemi T., Taittonen M., Laine J., Savisto N.-J., Enerbäck S., Nuutila P. (2009) Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360, 1518–1525 [DOI] [PubMed] [Google Scholar]
- 5.Ouellet V., Labbé S. M., Blondin D. P., Phoenix S., Guérin B., Haman F., Turcotte E. E., Richard D., Carpentier A. C. (2012) Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J. Clin. Invest. 122, 545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cypess A. M., White A. P., Vernochet C., Schulz T. J., Xue R., Sass C. A., Huang T. L., Roberts-Toler C., Weiner L. S., Sze C., Chacko A. T., Deschamps L. N., Herder L. M., Truchan N., Glasgow A. L., Holman A. R., Gavrila A., Hasselgren P.-O., Mori M. A., Molla M., Tseng Y.-H. (2013) Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat. Med. 19, 635–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lidell M. E., Betz M. J., Dahlqvist Leinhard O., Heglind M., Elander L., Slawik M., Mussack T., Nilsson D., Romu T., Nuutila P., Virtanen K. A., Beuschlein F., Persson A., Borga M., Enerbäck S. (2013) Evidence for two types of brown adipose tissue in humans. Nat. Med. 19, 631–634 [DOI] [PubMed] [Google Scholar]
- 8.Wu J., Cohen P., Spiegelman B. M. (2013) Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 27, 234–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boström P., Wu J., Jedrychowski M. P., Korde A., Ye L., Lo J. C., Rasbach K. A., Boström E. A., Choi J. H., Long J. Z., Kajimura S., Zingaretti M. C., Vind B. F., Tu H., Cinti S., Højlund K., Gygi S. P., Spiegelman B. M. (2012) A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Matteis R., Lucertini F., Guescini M., Polidori E., Zeppa S., Stocchi V., Cinti S., Cuppini R. (2013) Exercise as a new physiological stimulus for brown adipose tissue activity. Nutr. Metab. Cardiovasc. Dis. 23, 582–590 [DOI] [PubMed] [Google Scholar]
- 11.Gohil K., Henderson S., Terblanche S. E., Brooks G. A., Packer L. (1984) Effects of training and exhaustive exercise on the mitochondrial oxidative capacity of brown adipose tissue. Biosci. Rep. 4, 987–993 [DOI] [PubMed] [Google Scholar]
- 12.Terblanche S. E., Gohil K., Packer L., Henderson S., Brooks G. A. (2001) The effects of endurance training and exhaustive exercise on mitochondrial enzymes in tissues of the rat (Rattus norvegicus). Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 128, 889–896 [DOI] [PubMed] [Google Scholar]
- 13.Rothwell N. J., Stock M. J. (1979) A role for brown adipose tissue in diet-induced thermogenesis. Nature 281, 31–35 [DOI] [PubMed] [Google Scholar]
- 14.Young J. B., Saville E., Rothwell N. J., Stock M. J., Landsberg L. (1982) Effect of diet and cold exposure on norepinephrine turnover in brown adipose tissue of the rat. J. Clin. Invest. 69, 1061–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz J. H., Young J. B., Landsberg L. (1983) Effect of dietary fat on sympathetic nervous system activity in the rat. J. Clin. Invest. 72, 361–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araujo R. L., Andrade B. M., Padrón A. S., Gaidhu M. P., Perry R. L., Carvalho D. P., Ceddia R. B. (2010) High-fat diet increases thyrotropin and oxygen consumption without altering circulating 3,5,3′-triiodothyronine (T3) and thyroxine in rats: the role of iodothyronine deiodinases, reverse T3 production, and whole-body fat oxidation. Endocrinology 151, 3460–3469 [DOI] [PubMed] [Google Scholar]
- 17.Wisløff U., Helgerud J., Kemi O. J., Ellingsen O. (2001) Intensity-controlled treadmill running in rats: VO(2 max) and cardiac hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 280, H1301–H1310 [DOI] [PubMed] [Google Scholar]
- 18.Pescatello L., Arena R., Riebe D., Thompson P. D. (eds) (2014) American College of Sports Medicine's Guideline for Exercise Testing and Prescription, 9th Ed., pp. 162–193, Wolters Kluwer Health; Lippincott WIlliams & Wilkins [Google Scholar]
- 19.Gaidhu M. P., Frontini A., Hung S., Pistor K., Cinti S., Ceddia R. B. (2011) Chronic AMP-kinase activation with AICAR reduces adiposity by remodeling adipocyte metabolism and increasing leptin sensitivity. J. Lipid Res. 52, 1702–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vitzel K. F., Bikopoulos G., Hung S., Pistor K. E., Patterson J. D., Curi R., Ceddia R. B. (2013) Chronic treatment with the AMP-kinase activator AICAR increases glycogen storage and fatty acid oxidation in skeletal muscles but does not reduce hyperglucagonemia and hyperglycemia in insulin deficient rats. PLoS One 8, e62190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbatelli G., Murano I., Madsen L., Hao Q., Jimenez M., Kristiansen K., Giacobino J. P., De Matteis R., Cinti S. (2010) The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 298, E1244–E1253 [DOI] [PubMed] [Google Scholar]
- 22.Winder W. W., Hagberg J. M., Hickson R. C., Ehsani A. A., McLane J. A. (1978) Time course of sympathoadrenal adaptation to endurance exercise training in man. J. Appl. Physiol. 45, 370–374 [DOI] [PubMed] [Google Scholar]
- 23.Péronnet F., Cléroux J., Perrault H., Cousineau D., de Champlain J., Nadeau R. (1981) Plasma norepinephrine response to exercise before and after training in humans. J. Appl. Physiol. 51, 812–815 [DOI] [PubMed] [Google Scholar]
- 24.Bachman E. S., Dhillon H., Zhang C.-Y., Cinti S., Bianco A. C., Kobilka B. K., Lowell B. B. (2002) βAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297, 843–845 [DOI] [PubMed] [Google Scholar]
- 25.Lowell B. B., Bachman E. S. (2003) β-Adrenergic receptors, diet-induced thermogenesis, and obesity. J. Biol. Chem. 278, 29385–29388 [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y., Guo H., Deis J. A., Mashek M. G., Zhao M., Ariyakumar D., Armien A. G., Bernlohr D. A., Mashek D. G., Chen X. (2014) Lipocalin 2 regulates brown fat activation via a nonadrenergic activation mechanism. J. Biol. Chem. 289, 22063–22077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pekkala S., Wiklund P. K., Hulmi J. J., Ahtiainen J. P., Horttanainen M., Pöllänen E., Mäkelä K. A., Kainulainen H., Häkkinen K., Nyman K., Alén M., Herzig K.-H., Cheng S. (2013) Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J. Physiol. 591, 5393–5400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raschke S., Elsen M., Gassenhuber H., Sommerfeld M., Schwahn U., Brockmann B., Jung R., Wisløff U., Tjønna A. E., Raastad T., Hallén J., Norheim F., Drevon C. A., Romacho T., Eckardt K., Eckel J. (2013) Evidence against a beneficial effect of irisin in humans. PLoS One 8, e73680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norheim F., Langleite T. M., Hjorth M., Holen T., Kielland A., Stadheim H. K., Gulseth H. L., Birkeland K. I., Jensen J., Drevon C. A. (2014) The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 281, 739–749 [DOI] [PubMed] [Google Scholar]
- 30.Huh J. Y., Panagiotou G., Mougios V., Brinkoetter M., Vamvini M. T., Schneider B. E., Mantzoros C. S. (2012) FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 61, 1725–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aydin S., Kuloglu T., Aydin S., Eren M. N., Celik A., Yilmaz M., Kalayci M., Sahin İ., Gungor O., Gurel A., Ogeturk M., Dabak O. (2014) Cardiac, skeletal muscle and serum irisin responses to with or without water exercise in young and old male rats: cardiac muscle produces more irisin than skeletal muscle. Peptides 52, 68–73 [DOI] [PubMed] [Google Scholar]
- 32.Kraemer R. R., Shockett P., Webb N. D., Shah U., Castracane V. D. (2014) A transient elevated irisin blood concentration in response to prolonged, moderate aerobic exercise in young men and women. Horm. Metab. Res. 46, 150–154 [DOI] [PubMed] [Google Scholar]
- 33.Lee P., Linderman J. D., Smith S., Brychta R. J., Wang J., Idelson C., Perron R. M., Werner C. D., Phan G. Q., Kammula U. S., Kebebew E., Pacak K., Chen K. Y., Celi F. S. (2014) Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 19, 302–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brenmoehl J., Albrecht E., Komolka K., Schering L., Langhammer M., Hoeflich A., Maak S. (2014) Irisin is elevated in skeletal muscle and serum of mice immediately after acute exercise. Int. J. Biol. Sci. 10, 338–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roca-Rivada A., Castelao C., Senin L. L., Landrove M. O., Baltar J., Belén Crujeiras A., Seoane L. M., Casanueva F. F., Pardo M. (2013) FNDC5/irisin is not only a myokine but also an adipokine. PLoS One 8, e60563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreno-Navarrete J. M., Ortega F., Serrano M., Guerra E., Pardo G., Tinahones F., Ricart W., Fernández-Real J. M. (2013) Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 98, E769–E778 [DOI] [PubMed] [Google Scholar]
- 37.Schumacher M. A., Chinnam N., Ohashi T., Shah R. S., Erickson H. P. (2013) The structure of irisin reveals a novel intersubunit β-sheet fibronectin type III (FNIII) dimer: implications for receptor activation. J. Biol. Chem. 288, 33738–33744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamashita H., Yamamoto M., Sato Y., Izawa T., Komabayashi T., Saito D., Ohno H. (1993) Effect of running training on uncoupling protein mRNA expression in rat brown adipose tissue. Int. J. Biometeorol. 37, 61–64 [DOI] [PubMed] [Google Scholar]
- 39.Larue-Achagiotis C., Rieth N., Goubern M., Laury M. C., Louis-Sylvestre J. (1995) Exercise-training reduces BAT thermogenesis in rats. Physiol. Behav. 57, 1013–1017 [DOI] [PubMed] [Google Scholar]
- 40.Arnold J., Richard D. (1987) Exercise during intermittent cold exposure prevents acclimation to cold rats. J. Physiol. 390, 45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young J. B., Landsberg L. (1983) Diminished sympathetic nervous system activity in genetically obese (ob/ob) mouse. Am. J. Physiol. 245, E148–E154 [DOI] [PubMed] [Google Scholar]
- 42.Landsberg L., Young J. B. (1981) Diet-induced changes in sympathoadrenal activity: implications for thermogenesis. Life Sci. 28, 1801–1819 [DOI] [PubMed] [Google Scholar]
- 43.Rappaport E. B., Young J. B., Landsberg L. (1982) Initiation, duration and dissipation of diet-induced changes in sympathetic nervous system activity in the rat. Metabolism 31, 143–146 [DOI] [PubMed] [Google Scholar]
- 44.Feldmann H. M., Golozoubova V., Cannon B., Nedergaard J. (2009) UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 9, 203–209 [DOI] [PubMed] [Google Scholar]
- 45.Vosselman M. J., Brans B., van der Lans A. A., Wierts R., van Baak M. A., Mottaghy F. M., Schrauwen P., van Marken Lichtenbelt W. D. (2013) Brown adipose tissue activity after a high-calorie meal in humans. Am. J. Clin. Nutr. 98, 57–64 [DOI] [PubMed] [Google Scholar]