The eukaryotic translation initiation factor eIF4E in the nucleus: taking the road less traveled (original) (raw)
. Author manuscript; available in PMC: 2016 Jan 1.
Published in final edited form as: Immunol Rev. 2015 Jan;263(1):210–223. doi: 10.1111/imr.12240
Summary
The eukaryotic translation initiation factor eIF4E is a potent oncogene. Although eIF4E has traditional roles in translation initiation in the cytoplasm, it is also found in the nucleus, suggesting that it has activities beyond its role in protein synthesis. The road less traveled has been taken to study these nuclear activities and to understand their contribution to the oncogenic potential of eIF4E. The molecular features and biological pathways underpinning eIF4E’s nuclear mRNA export are described. New classes of eIF4E regulators have been identified and their relevance to cancer shown. The studies presented here reveal the molecular, biophysical, and structural bases for eIF4E regulation. Finally, recent clinical work targeting eIF4E in acute myeloid leukemia patients with ribavirin is discussed. In summary, these findings provide a novel paradigm for eIF4E function and the molecular basis for targeting it in leukemia patients.
Keywords: eIF4E, PML, mRNA export, ribavirin, clinical trials
General overview
Normal development, differentiation, and cellular growth rely on post-transcriptional as well as transcriptional control of gene expression. The eukaryotic translation initiation factor, eIF4E, regulates gene expression post-transcriptionally at multiple levels including mRNA translation and mRNA export (1–4). Through these activities, eIF4E promotes cellular proliferation, growth, and survival (1–4). Even moderate overexpression of eIF4E leads to dysregulated cellular proliferation and malignant transformation. eIF4E is dysregulated in an estimated 30% of human cancers.
The traditional view is that eIF4E recruits mRNAs to the ribosome and that when dysregulated, this leads to inappropriate translation leading to transformation (1). This viewpoint further suggests that eIF4E is controlled almost exclusively through the mTOR eIF4E-binding protein (BP) axis (1), in which cytoplasmic eIF4E-mediated protein synthesis is regulated by signaling events upstream of mTOR which leads to eventual phosphorylation of BP1 which releases the BP1-eIF4E interaction leading to activation of eIF4E dependent translation. Although satisfying in its simplicity, this view leaves many unanswered questions and does not explain many key observations in the eIF4E field. For instance, many cell types contain nuclear eIF4E suggesting that eIF4E plays additional roles outside of translation (5–8) (Fig. 1A). Indeed, the localization of eIF4E is dynamic and sensitive to cellular stimuli (9). eIF4E does not modulate the translation of all transcripts equally: some housekeeping transcripts are not affected by eIF4E. In the traditional model, the eIF4E binding proteins, BP1 (and to a lesser extent other BP family members BP2 and BP3) are considered the key eIF4E modulators (1). Loss of BPs does not lead to oncogenic phenotypes in mice (10, 11) and indeed, BP1 is not conserved in yeast or Drosophila (12). BP1 is also found in the nucleus as well as the cytoplasm (13). These observations suggest that eIF4E functions outside of its traditional role in translation and that it can be regulated by a myriad of regulators aside from BPs (4). Thus, paraphrasing the words of Robert Frost, by studying the nuclear functions and novel regulatory networks of eIF4E, we set out on a journey on the road less traveled.
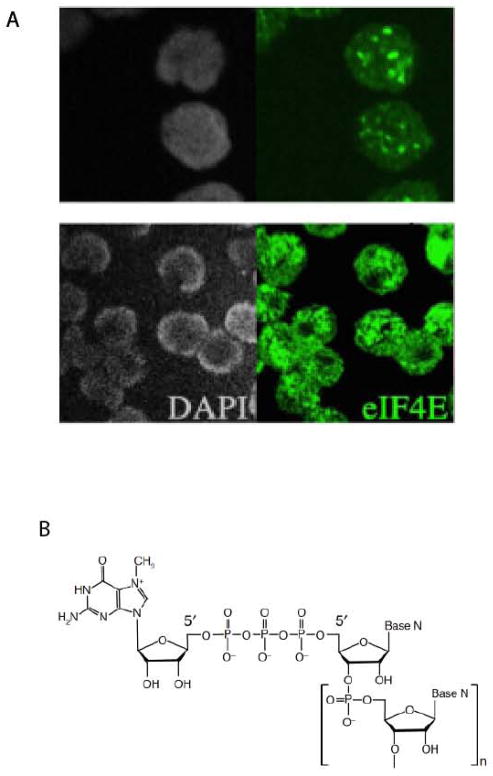
Fig. 1. The location and structure of eIF4E.
(A) eIF4E is found in both the nuclear and cytoplasmic compartments. Immunofluorescence in conjunction with confocal microscopy is used to monitor eIF4E localization in intact normal CD34+ cells or CD34+ cells from a patient with high eIF4E leukemia. DAPI is shown in grey. The micrograph was taken as a single section through the plane of the cell. (B) Structure of the mRNA ‘cap’. The 5′ guanosine is methylated at the N7 position and is linked to the first nucleoside of the mRNA chain via an inverted 5′-5′ triphosphate bridge. The base, N, can be adenine, cytosine, guanine or uracil.
The traditional view
eIF4E was discovered as a cytoplasmic cap binding protein functioning in translation by Witold Filipowicz in 1976 (14, 15). eIF4E associates with the 5′ end of mRNAs via the 7-methyl guanosine dinucleotide ‘cap’ structure, m7GpppN (where N is any nucleotide, see Fig. 1B for the structure) (1–4). The m7G form of guanosine possesses a partial positive charge which allows it to interact with the cap binding site of eiF4E, where it interacalates between two tryptophan residues characterized by negatively charged pi-electron clouds. Most mRNAs require the eIF4E-m7Gcap association for translation. This activity is linked to its biochemical and subsequent growth promoting activities (4, 7). Many mRNAs with highly structured 5′ untranslated regions (UTRs) are sensitive to eIF4E, whereas housekeeping mRNAs, such as GAPDH and actin with short, unstructured 5′UTRs are not sensitive to eIF4E(1–4). Increased complexity of the UTR is linked to slowed translation rates. eIF4E overexpression increases translational efficiency of mRNAs with structured UTRs. These messages are considered eIF4E sensitive (1–4). Translational efficiency is determined using polysomal loading where the presence of mRNAs in heavier polysomes (more ribosomes/transcript) indicates more efficient translation.
The road not taken: nuclear eIF4E
Despite its identification in the mid-1970s as a key factor in translation, there was no report monitoring the localization of eIF4E in intact human cells until the early 1990s (5). These studies, as well as others, indicate that eIF4E is found in the nucleus as well as the cytoplasm (5) (Fig. 2). eIF4E forms nuclear bodies in a variety of organisms e.g. yeast, Drosophila, Xenopus, mouse, and human (5–7, 16). eIF4E’s subcellular distribution is modulated in human cancers, with the M4 and M5 subtypes of AML characterized by the vast majority of eIF4E being nuclear (8, 17–19) (Fig. 1). In the nucleus of normal cells, eIF4E is found in discrete spherical bodies, in the micron range in terms of diameter with approximately 10 bodies per nucleus. eIF4E is also found distributed throughout the nucleus in a diffuse pattern (5, 7). In terms of its nuclear localization, eIF4E is not found in the nucleolus, in splicing speckles or in Cajal bodies (7). eIF4E nuclear bodies are disrupted by addition of the m7GpppG analogue but not GpppG (7), whereas other nuclear structures such as nucleoli and splicing speckles are unaffected by m7Gcap treatment (7). Thus the m7Gcap plays an important role in the integrity of these bodies suggesting that the m7Gcap may play an important role in the nuclear functions of eIF4E.
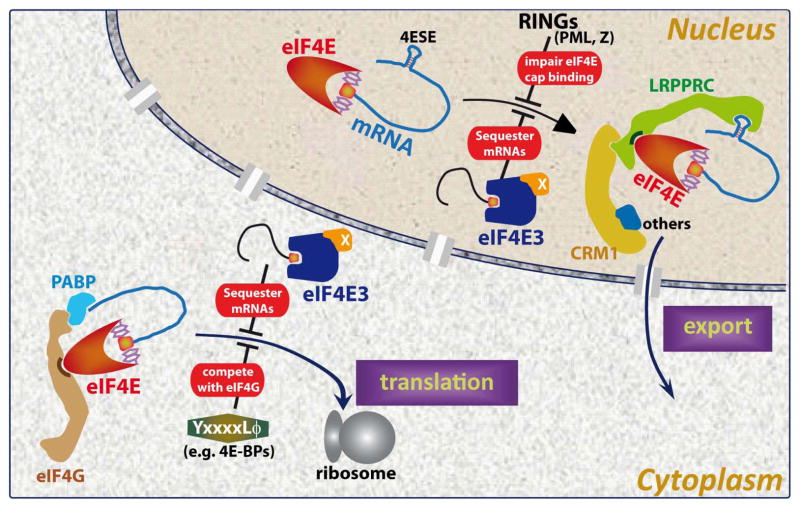
Fig. 2. Cartoon illustrating functions and regulators of eIF4E in both subcellular compartments.
In the nucleus, LRPPRC binds both the 4ESE RNA (depicted as a stem loop) and eIF4E directly (21–23). The two tryptophans central to m7Gcap binding are shown in eIF4E molecule’s m7Gcap binding site. CRM1 (yellow) and other components (shown in blue, indicated by ‘others’) are also part of the RNP (ribonucleoparticle). Which parts of the RNP directly bind CRM1 are not yet known. X by eIF4E3 indicates other factors in the RNP that increase its affinity for the m7Gcap as described in the text (73). In the cytoplasm, ribosomes are depicted by the large gray spheres. PolyA binding protein binds eIF4G as part of translation inhibition and this is blocked by BP1. Not all regulatory modes or mechanistic aspects are depicted in the figure for the sake of simplicity.
eIF4E-dependent mRNA export
Nuclear eIF4E functions in nuclear-cytoplasmic export of selected transcripts (3)(Fig. 2). For instance, eIF4E overexpression increased cyclin D1 mRNA export with no change in its total mRNA levels (20–23). Further, eIF4E does not increase polysomal loading of cyclin D1 mRNAs according to the Sonenberg laboratory, demonstrating that increased cyclin D1 protein levels result from increased nuclear export and not more efficient translation (20). The export of housekeeping mRNAs e.g. GAPDH and actin are not altered by eIF4E (20). Here, export is measured by monitoring the levels of mRNAs in the nuclear and cytoplasmic fractions as a function of eIF4E overexpression.
To understand transcript specificity, we assessed whether eIF4E specifically bound target mRNAs in the nucleus. This would strongly suggest that eIF4E was directly influencing mRNA export and not mediating the expression of other export factors at the translation level. RNA immunoprecipitation (RIP) studies indicated that eIF4E physically associated with specific export target mRNAs, including cyclin D1 and ODC (ornithine decarboxylase) in the nuclear fraction whereas in the cytoplasm it associated with all mRNAs. The association in the nucleus was m7Gcap-dependent as shown by competition with m7Gcap analogues and further with eIF4E mutants in the m7Gcap-binding site which were unable to interact. Fluorescence in situ hybridization (FISH) studies indicated that eIF4E associated with its target transcripts in a subset of its nuclear bodies suggesting that these bodies could be functionally involved in the mRNA export pathway perhaps as a pre-assembly point.
We characterized several important features of this export pathway. We determined this is an m7Gcap-dependent process (21–23). Additionally, we identified a ~50 nucleotide secondary structure element, denoted the eIF4E sensitivity element (4E-SE) (Fig. 2), found in the 3′UTR of sensitive transcripts which is required for eIF4E dependent mRNA export (21–23). Indeed, lacZ-4E-SE chimeras are sensitive to eIF4E-mediated mRNA export whereas lacZ is not. The 4E-SE element forms a specific secondary structure which when disrupted by mutation leads to an inactive export element. Our experiments suggest that the expression of over 700 transcripts is modulated by eIF4E at the mRNA export level including growth-promoting mRNAs such as cyclin D1, ODC, Pim 1 and c-Myc (21–23). eIF4E forms a specific protein RNA complex, known as a ribonucleoparticle (RNP), in the nucleus recruiting the leucine rich pentatricopeptide repeat protein LRPPRC which directly associates with the 4E-SE mRNA through its pentatricopeptide repeat domain and with eIF4E through a consensus eIF4E binding motif, which is defined by YXXXXLΦ (where X is any residue and Φ is any hydrophobic)(24) (Fig. 2). This complex also associates with the RNA helicase UAP56 as well as hnRNPA1, and transits through the nuclear pore complex via the CRM1 export receptor (22–24) (Fig. 2). This pathway is distinct from bulk mRNA export which uses the TAP/NXF1 receptor (22, 23). Indeed, RNAi-mediated knockdown of TAP does not affect eIF4E dependent transcripts but does affect bulk mRNA export. In contrast, the CRM1 inhibitor leptomycin B inhibits eIF4E dependent mRNA export but not bulk export. Thus, eIF4E-dependent mRNA export requires a specific structural element in the RNA and is distinct from bulk mRNA export pathways.
While studying the mRNA export process, we monitored whether eIF4E had any effects on the nuclear pore complex (NPC). The NPC enables macromolecules to be trafficked between the nucleus and cytoplasm (3). Intriguingly, eIF4E reprograms the NPC to promote 4E-SE mRNA export (25). This is correlated with its oncogenic activity (25). Specifically we observed a substantial decrease in the levels of RanBP2 (Nucleoporin 358) which is the major protein comprising the cytoplasmic fibrils of the NPC thereby altering the pore architecture, suggesting a functional reorganization of the NPC and unique release and recycling mechanisms vis-à-vis the eIF4E export RNP. We also observed a concomitant increase in RanBP1 levels and relocalization of Nup214 from the cytoplasmic face of the NPC (3, 25). These effects were specific as eIF4E did not alter other nucleoporins examined. eIF4E also elevated bulk mRNA export factors Gle1 and the RNA helicase DDX19 suggesting there is cross-talk between eIF4E and bulk mRNA export pathways (25). Further, eIF4E may affect the termination steps of translation given the roles DDX19 and Gle1 also play here. We further demonstrated that reduction in RanBP2 levels was central for the oncogenic potential of eIF4E (3, 25). These were the first studies to demonstrate that an oncogene could reprogram the NPC and in this way drive its oncogenic activity.
In summary, eIF4E drives gene expression by increasing the cytoplasmic concentration of specific transcripts (via enhanced mRNA export) and in some cases by then additionally enhancing the translation efficiency of these once in the cytoplasm. We note that for RNAs to be sensitive to eIF4E at the translation level, they require a complex 5′UTR and at the export level, the 4E-SE element (Fig. 2). Thus, only RNAs with both elements will be sensitive to eIF4E at both levels. In this way, altering the subcellular localization of eIF4E can affect specific subsets of gene expression and thus lead to altered physiological responses.
eIF4E’s mRNA export activity contributes to its oncogenic potential
It is well established that eIF4E overexpression leads to loss of contact inhibition as shown by foci formation, increased growth in soft agar, and apoptotic rescue to a variety of stimuli (4, 12). eIF4E mediated transformation was thought to solely involve increased translation of growth promoting mRNAs (1). Our studies demonstrated that eIF4E’s function in mRNA export is critical for its oncogenic activities in several contexts. For instance, a mutant of eIF4E, W73A, which acts in mRNA export but is deficient in promoting translation, increases foci formation and apoptotic rescue as readily as wildtype eIF4E (7, 21–23). Conversely, the S53A eIF4E mutant acts in translation but cannot promote 4E-SE mRNA export, apoptotic rescue or form foci (25). Further, the ability of eIF4E to modulate the nuclear pore to increase mRNA export is central to its oncogenic activity and again the S53A mutant is deficient in this activity (25). Indeed, the S53A mutant can bind RNAs in the cytoplasm and drive translation but in the nucleus, does not even associate with RNAs despite the fact that in vitro, it retains full m7Gcap binding activity.
Additionally, exogenous cyclin D1 constructs that contained the 4ESE element were generated to be active in export but not in translation. These constructs drove eIF4E mediated transformation in cyclin D1−/− cells (22). In this case, only the transcripts containing the 4ESE element could transform cells (22). Furthermore, our studies demonstrated that eIF4E overexpression represses differentiation of U937 cells consistent with the phenotypes observed in high eIF4E leukemia patients (8). Mutational analysis suggests this occurs via this mRNA export activity (8). Further, the nuclear localization and mRNA export activity of eIF4E are highly elevated in M4/M5 AML patients (8).
Studies into the survival function of eIF4E revealed that not only did the mRNA export function contribute to this activity, but that eIF4E acts upstream as well as downstream of mTOR (21). Specifically, we examined the ability of eIF4E to rescue cells upon serum deprivation (21). One of our mRNA export targets, NBS1, is known to play a role not only in DNA repair but also in Akt activation (26). We observed that eIF4E could not rescue Akt1−/− cells from serum deprivation induced apoptosis but could rescue littermate control cells (21). Re-expression of Akt in these cells recovered this eIF4E activity. Knockdown of NBS1 in the control cells also eliminated the ability of eIF4E to rescue cells. Indeed, these studies indicated that eIF4E overexpression led to Akt activation (phosphorylation) via its mRNA export effects on NBS1. Thus, eIF4E is not only a downstream target of mTOR signaling via BP1, but also acts upstream via its effects on NBS1.
Controlling nuclear eIF4E activity-direct regulators
We set out to understand how the nuclear activities of eIF4E are regulated (Fig. 2). In the cytoplasm, control is usually considered to be limited to interactions with the BP proteins (1); however, even these views are being revised with new modes of regulation in these compartments (27). Our studies into the regulation of nuclear eIF4E reveal an intricate control system with new partner proteins, as well as suggesting that regulation of eIF4E’s localization itself is important.
We begin by discussing direct eIF4E regulators. To date, three classes of direct eIF4E-binding proteins have been identified: ones containing conserved eIF4E binding site such as BP1 and eIF4G (1), ones using RING domains such as PML, HHARI and Z (7, 28–32); and the amphipathic helix strategy used by VpG proteins (33, 34). Our studies have revealed a complex regulatory pathway with nuclear specific as well more general proteins directly regulating eIF4E activity. We have expanded the number of consensus binding regulators to more than 200 homeodomain containing proteins which can act as tissue specific regulators of eIF4E’s functions and other factors that directly bind eIF4E and act in the mRNA export process itself like LPRPRC.
The promyelocytic leukemia protein PML
PML was the first nuclear regulator of eIF4E identified (7, 28, 35). Note that PML is almost exclusively a nuclear protein (36). Indeed, a subset of eIF4E nuclear bodies co-localize with PML nuclear bodies (7, 22, 37, 38). Unlike other PML body components, eIF4E does not require PML to be present to form bodies, consistent with the fact that eIF4E is evolutionarily conserved to yeast and PML is not (7). eIF4E nuclear bodies that contain 4E-SE RNAs never contain PML and vice versa (22). This suggests that the eIF4E-PML nuclear bodies are inactive in mRNA export (22). Consistent with this observation, PML potently suppresses the ability of eIF4E to export target mRNAs, transform cells or rescue cells from serum starvation induced apoptosis (7, 21–23, 37) (Fig. 2). How does PML inhibit eIF4E? Our studies show that PML uses its RING domain to directly bind to eIF4E. This association leads to a dramatic decrease in the affinity (100 fold) of eIF4E for the m7Gcap, and thus its mRNA export targets (see structure section) (7, 28, 35). We noted that in PML−/− fibroblasts, mRNA export is highly elevated (7, 37) but PML−/− mice did not get cancer more readily than controls. This suggested to us that there were other, likely tissue specific, inhibitors of eIF4E. This is explored in the next section.
The homeodomain regulators
While studying eIF4E dysregulation in AML and trying to understand the tissue specific nature of eIF4E regulation, we identified a new regulator, the proline rich homeodomain protein (PRH) also known as hematopoietically expressed homeodomain (Hex)(39). PRH expression is tissue restricted but is found in normal myeloid cells. Interestingly, we observed a loss of its expression in M4 and M5 AML patient specimens, where eIF4E levels, nuclear localization and export activity are highly elevated. We found here that PRH suppressed the ability of eIF4E to transform cells and cause proliferation. Unlike PML, PRH used a conserved eIF4E binding site in its extreme N-terminus to associate with eIF4E in a manner similar to the BP1s or eIF4G. Furthermore, the ability of PRH to suppress eIF4E mediated transformation depended on having an intact nuclear localization signal (NLS). PRH did not modulate the translation activity of eIF4E thereby underlying the relevance of its effects on mRNA export to transformation.
Given these findings, we used bioinformatics to determine whether other homeodomain containing proteins may also bind to eIF4E and regulate its activity at either the translation or mRNA export levels. We identified approximately 200 homeodomain containing proteins that also contained putative eIF4E binding sites (17, 39). Of these, we showed that recombinant Hox11, HoxA9 and Bicoid did directly bind eIF4E (17, 39). Further, HoxA9, through its binding site was able to stimulate eIF4E dependent mRNA export and in some, cases translation of specific target RNAs (17). Interestingly, some of these target RNAs were also transcriptional targets of HoxA9 suggesting that stimulatory homeodomains could drive expression of their own target genes at multiple levels in a coordinated fashion. Other groups have extended these studies to show that EN2 (40) and Emx2 (41) also bound eIF4E and modulated its activity suggesting that this mechanism may be widely used. Thus, eIF4E can be regulated by a variety of factors some of which are tissue specific such as in blood cells or neurons, and others are more generally expressed. This suggests that the effects of modulating eIF4E expression could differ between tissue types depending on the complement of regulatory proteins available. Thus the effects of eIF4E on the proteome would be expected to be cell type specific in many cases.
Dysregulation of eIF4E levels
eIF4E is highly elevated in many human cancers (12). However, there are not many studies into the underlying mechanisms for this observation. In some cancers, eIF4E is elevated due to gene amplification (12). Traditionally, eIF4E transcription is considered to be controlled only by c-myc (42); however, serum stimulation still leads to elevated eIF4E levels in c-myc null cells (43). We identified several new factors that regulate eIF4E levels. For instance, eIF4E is a direct NF-κB target which is dysregulated in M4/M5 AML (43). Other groups have shown that eIF4E is also a target of C/EBP (44). Furthermore, eIF4E mRNA stability is elevated by the HuR protein and is destabilized by AUF1 (45). These modes of regulation can overlap. For instance in head and neck cancer, eIF4E RNA stability is increased partly due to increased HuR levels and also, the eIF4E gene is reported to be amplified here (12, 45).
Modulating the nuclear localization of eIF4E
Our studies also suggested a means by which to modulate eIF4E activity would be to modulate its nuclear trafficking. eIF4E does not contain a classical NLS and thus how it enters the nucleus has been a major question. While an initial study purported the eIF4E transporter protein 4ET facilitated nuclear entry, it is now known that this protein plays no role in eIF4E mediated nuclear entry, but rather alters the localization of eIF4E to cytoplasmic processing bodies (P-bodies) where the function of eIF4E is still not completely clear (46). Our recent studies have identified a specific Importin that is responsible for nuclear entry of eIF4E (Ramteke and Borden, unpublished observations). Indeed, this factor is highly elevated in M4/M5 AML and contributes to the massive nuclear localization observed for eIF4E in these patients. Interestingly, treatment of cell lines or patients with m7Gcap analogues, also leads to disruption of eIF4E nuclear bodies (7, 18, 47, 48). The ability to modulate eIF4E localization is key to the molecular responses in AML patients and to eIF4E targeting (see human malignancy section).
We noted that several factors could modulate the subcellular localization of eIF4E and thus regulate its activity. For instance, overexpression of PRH leads to cytoplasmic retention of eIF4E activity correlating with a loss of its mRNA export activity and suppression of transformation (39). PML overexpression leads to more eIF4E nuclear bodies with PML present and thus fewer bodies with RNA present (7, 22, 23). This again corresponds to repressed eIF4E dependent mRNA export and transformation activity (Fig. 2). Expression of the dominant negative inhibitor of NF-κB, IκB-SR, in primary AML patient specimens leads to re-distribution of eIF4E to a more normal phenotype as well as elevation of its negative regulator PRH and decreases in eIF4E levels (8). Stimulators of eIF4E can also modify its distribution. For instance, overexpression of LRPPRC reduces its co-localization with PML in the nucleus and leads to increased mRNA export activity of eIF4E (24).
Biophysics and structural biology: molecular underpinnings of activity and control of eIF4E
The studies above show that eIF4E function is regulated in both the nucleus and cytoplasm by specific interactions with other proteins. The details of eIF4E function have been greatly enhanced by the availability of the three-dimensional atomic resolution structures (49–52). m7Gcap binds eIF4E on a central, concave 8-standed β-sheet via complimentary π-π interactions of two tryptophan residues, W56 and W102 with the partially positively charged π ring system of the m7G (Fig. 3A). While the m7G moiety alone binds to eIF4E, addition of phosphate groups significantly enhances affinity via interactions with positively charged residues (53). On the other side of the central sheet, three helices form a convex surface, known as the dorsal surface. X-ray structures have shown it is at this site, particularly around helices I and II that the modulator proteins with the 4E consensus motif use to bind to eIF4E, such as the 4E BPs and eIF4G (50, 51). The dorsal surface and m7Gcap binding sites are over 25Å away (Fig. 3A), yet allosteric communication between these two sites is critical for eIF4E regulation in the cell. For example eIF4G and the BPs increase eIF4E affinity for m7Gcap (54–56), conversely m7Gcap binding enhances eIF4E affinity for the proteins (51, 56, 57). Thus, the same biochemical activity can lead to different outputs on the eIF4E protein. We note that BP1 also interferes with binding of eIF4G to the dorsal surface and thus does not permit association with the rest of the translation machinery (Fig. 2).
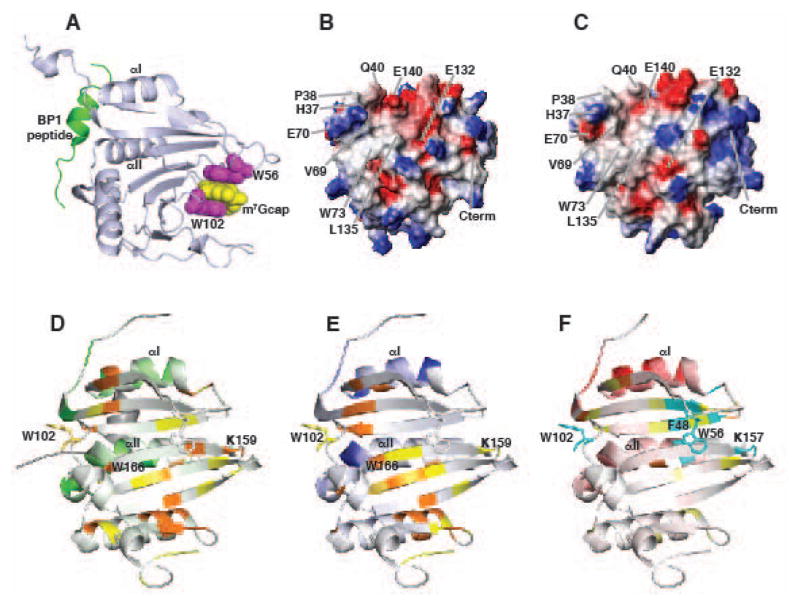
Fig. 3. Structures of eIF4E.
(A) Cartoon representation of eIF4E showing the m7Gcap (yellow) interaction with W102 and W56 (purple), and the binding site for proteins with the 4E binding consensus at the dorsal surface (BP1 peptide is shown in red). The binding sites are over 25Å away. Electrostatic potential map of the dorsal surfaces for (B) apo-eIF4E and (C) m7Gcap-bound eIF4E are altered upon m7Gcap association. (D–F) Summary of 1H-15N amide chemical shift changes for apo eIF4E upon addition of (D) the eIF4G peptide, (E) the BP1 peptide, and (F) the arenavirus Z RING domain are mapped onto the structure of apo-eIF4E. Perturbations at the dorsal surface (binding site) are shown in green, blue and red for eIF4G, BP1, and Z, respectively. Residues away from the dorsal surface that are affected are colored orange and yellow, indicating relatively strong and medium perturbations: residues undergoing conformational exchange are colored cyan. Binding at the dorsal surface induces changes at the cap site through distinct allosteric pathways depending upon the modulator protein.
A major goal in our laboratory has been to use NMR to elucidate a structural basis for the allostery that underlies eIF4E’s physiological function. Although many structures of eIF4E had been solved in the late 1990s and early 2000s a structural mechanism for the allosteric changes observed in eIF4E that occur upon m7Gcap or protein partner binding had not been described. Significantly all the structural studies were on m7Gcap-bound complexes in the presence or absence of 4EBP and eIF4G peptides, which exhibited no change in eIF4E-cap complex structure. We therefore investigated the structure of apo-eIF4E and its interactions with both m7Gcap and partner proteins in order to understand the allosteric affects that underlie eIF4E’s physiological function.
Early biophysical studies using circular dichroism (CD) indicated that apo-eIF4E from yeast was well folded and that addition of the m7Gcap did not substantially alter the global fold of the protein (58). However, it was difficult to find conditions where apo-eIF4E did not aggregate at NMR concentrations which are ~10–100 times greater than those used for CD (59, 60). Thus, we screened conditions and identified that apo human eIF4E was well folded and stable in phosphate buffers (at least over the time period to collect NMR data
We solved the first ever structure of apo eIF4E (61) which revealed m7Gcap binding modulated changes at dorsal surface site. Specifically, structural changes were observed on eIF4E towards the N-terminus (residues 37-40) and the loop region at the end of helix II which make direct contacts with the 4EBP1 or eIF4G peptides. These structural changes manifest in alterations to the electrostatic potential on the dorsal surface (Fig. 3B,C). Given that the m7Gcap-bound dorsal surface of eIF4E is virtually identical to that when bound to the eIF4G or 4EBP peptides, our data suggest that m7Gcap binding causes favourable pre-structuring at the distal dorsal surface, enabling this surface to better associate with modulator proteins. This data also suggests specific proteins might only recognize the apo or the m7Gcap-bound forms of eIF4E.
At the m7Gcap site, we noted two major differences between apo and m7Gcap bound forms: first, increased flexibility at the unoccupied m7Gcap sites, specifically for the W56 and W102 loops, and second, rotation of W102 out of the m7Gcap binding site. The mobile W56 loop retains its secondary structure and moves in a hinge-like motion. Our data suggest eIF4E-m7Gcap recognition involves a concerted hinge-lock mechanism in which the W56 loop moves in a concerted hinge-like manner to lock W56 onto the m7Gcap and the W102 loop rotates into the m7Gcap site, although the relative order of events is unknown. Consistent with our model, all published X-ray structures of eIF4E with m7Gcap and m7Gcap-analogues exhibit virtually identical conformations of W56 whereas the ring for W102 has been reported to flip by 180° for N7-benzylated m7Gcap analogues (62). The unfolded N-terminus (residues 1-38) and the 203-211 loop, which is the binding site for the second nucleotide of the m7Gcap, are also flexible in the apo form. The high degree of motions in the apo-eIF4E are likely responsible for difficulties in obtaining m7Gcap-free eIF4E crystal structures.
Motions at the W102 and W56 sites are abrogated upon binding m7GDP, however our data indicates residues at the N-terminus of the W56 loop, specifically residues 50–52, retain some degree of flexibility. Analysis of X-ray structures of a number of m7Gcap complexes indicates these regions have higher B-factors suggesting that in the solid state these motions are still present. Whether these motions confer an entropic advantage to m7Gcap association or present a mechanism for modulator proteins to alter m7Gcap affinity through dynamic allostery remains to be determined.
Similar but distinct allosteric routes
To address the question of how modulator proteins bind the dorsal surface and differentially regulate eIF4E for m7Gcap, we undertook extensive NMR and X-ray studies on apo-eIF4E in the presence of regulators including active peptides from eIF4G, 4E-BP1 and the RING domain of Z. Indeed, we solved the structure of eIF4E bound to a 4EBP1 peptide in the absence of m7Gcap to address this question (63). However, a major problem understanding ligand-protein interactions using crystallography is the effect of extensive crystal contacts that can distort conformations at a surface (64) and our crystal structure of BP1-eIF4E showed more extensive contacts between neighboring eIF4E proteins at the dorsal surface than for the BP1 peptide.
We therefore analyzed 1H-15N HSQC NMR spectra to monitor changes in backbone amide residues (chemical shift mapping) upon binding of ligands to address this question (Fig. 3D–F). These methods are exquisitely sensitive for not only describing binding sites but also for detecting conformational changes (both steady state and dynamic) for residues not at a distal site. Indeed, changes in chemical shift at regions far removed from a binding site faithfully represent structural changes at that region or changes in a dynamic ensemble of conformations at that site (65).
First we compared the changes in apo eIF4E amide chemical shifts upon addition of functional eIF4G and BP1 peptides that contained the consensus eIF4E binding motif (61, 63). As expected, the largest changes were observed at the dorsal surface consistent with the known binding sites of BP1 and eIF4G on eIF4E (Fig. 3D,E). However, many residues not directly involved in binding are perturbed including those at, or close to, the unoccupied m7Gcap site including W166, K159 and W102, which make direct interactions with m7Gcap (Fig. 3D,E). Interestingly many of the residues affected are common to both eIF4G and BP1 peptides indicating that a similar allosteric track is utilized to promote binding. However, even though these peptides share the consensus-binding motif, notably differences are observed in specific residues and in direction and/or magnitude of chemical shifts indicating their relative conformations at the distal m7Gcap site are differentially perturbed (Fig. 3D,E). These data therefore suggest an allosteric mechanism in which the structure of eIF4E at the unoccupied m7Gcap site is altered depending on the modulator protein bound. It is interesting that although both eIF4G and BP1 increase the affinity of eIF4E for the m7Gcap, one is required for its translation activity and the other is an inhibitor of eIF4E. In one case, eIF4G stimulation of m7Gcap binding increasing translation efficiency of sensitive transcripts is clear. Using the same biochemical strategy, BP1 inhibits eIF4E by not only blocking association with eIF4G by binding the same site, but also through increased m7Gcap affinity sequesters the target RNA, thus dually targeting eIF4E activity (Fig. 2). BP1 is both a nuclear and cytoplasmic protein suggesting that it may use this structural and biochemical strategy in both compartments.
Reduction in m7Gcap binding, a novel strategy to inhibit eIF4E function
Using the same chemical shift mapping methods, the effect of negative regulators of eIF4E’s cap binding activity, the RING domains of PML and Z were investigated (66). Unlike BP1 and eIF4G which increase affinity of eIF4E for m7Gcap, association of PML or Z with eIF4E leads to a 100-fold loss of affinity for m7Gcap (7, 28). PML-eIF4E complexes were poorly behaved at NMR concentrations and thus we pursued the Z-eIF4E complex structure. Our data indicated Z recognized distinct regions at the dorsal surface of eIF4E compared to BP1 or eIF4G (Fig. 3F). This region was centered around the top of helix I on eIF4E and was confirmed by transferred cross saturation NMR methods and mutational experiments. Notably, this unique Z binding site for eIF4E alters residues at the unoccupied m7Gcap binding site in a specific manner compared to eIF4G and 4E-BP1, providing a possible mechanism for the different effects of Z and eIF4G (and 4EBP) on m7Gcap affinity (Fig. 3F). Specifically, Z binding induced line broadening for many of the residues at the unoccupied m7Gcap site including W102, W56, R157, F48 indicating Z binding has increased conformational exchange around the unoccupied the m7Gcap site. In contrast both 4EBP and eIF4G peptides did not visibly alter the dynamics in this region. Taken together, our data suggests Z RING decreases eIF4E affinity for m7Gcap by inducing conformational exchange, which is not observed upon addition of the BP1 and 4G peptides. Examples of dynamically driven allosteric mechanisms are becoming more frequent as experimental methods improve to detect these processes (67–70). The role of motions in eIF4E function has been studied theoretically for binding of a BP2 peptide to m7Gcap-bound eIF4E (51); however, studying experimentally how motions modulate eIF4E function is an exciting avenue of research that is yet to be explored.
The question remained, how did the RING domains of PML and Z recognize eIF4E? We therefore determined the NMR structure of the RING domain of Z, the first structure of any arenavirus protein reported (66). Our data revealed residues on a loop surrounding the first zinc-binding site were important for eIF4E recognition. These residues are highly conserved in Z proteins and moreover the conformation around this site is conserved in the PML RING structure. In contrast, RING proteins known to interact with ubiquitin conjugating enzymes such as BRCA1 and Cbl, exhibit very different structures in this region (66) and at least for BRCA1 do not bind eIF4E (31). This site represents a novel eIF4E recognition site unlike the helical motif used by proteins containing the consensus 4E binding motif.
Novel modes of m7Gcap recognition and eIF4E inhibition
To better understand ligand recognition in the eIF4E family, we studied the third family member, eIF4E3. Alignment studies indicated this family member had only one conserved aromatic residue (W98, equivalent to W102 in eIF4E) available for m7Gcap recognition, whereas the second aromatic site (W56 in eIF4E) was replaced with a cysteine (C52), leading to the hypothesis that eIF4E3 cannot bind the m7Gcap (71, 72).
Our studies surprisingly identified eIF4E3 as a potent negative regulator with potential tumor suppressor activity (73). eIF4E3 repressed expression of a pool of target mRNAs of eIF4E in both translation (cytoplasmic) and export (nuclear) functions. Importantly, overexpression of eIF4E3 in U2OS and NIH-3T3 cells repressed oncogenic transformation as determined by anchorage-dependent foci formation assays (73). Further, eIF4E3 does not bind to eIF4E indicating that its effects are not on eIF4E itself (unpublished findings). Thus we propose a model whereby eIF4E3 competes for the same pool of RNAs as eIF4E, this would suggest a means by which to impede expression of targeted transcripts (Fig. 2).
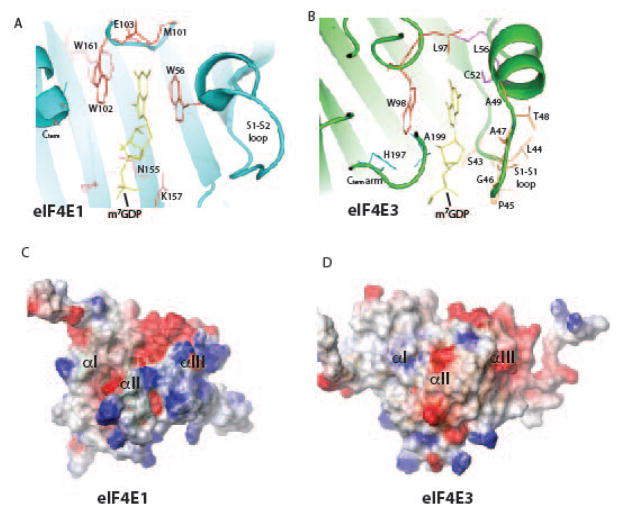
Biophysical techniques indicated eIF4E3 binds m7Gcap and more importantly, could discriminate between m7Gcap and guanosine with at least a 1,000-fold greater affinity for m7Gcap (73). Our NMR solution structures of apo and m7Gcap bound eIF4E3 showed m7Gcap binding is achieved via a novel mode of m7Gcap recognition in which the conserved W98 stacks against the m7G ring in a similar manner to the equivalent W102 in eIF4E (73) (Fig. 4A, B). To accommodate the loss of the second aromatic residue (W56 in eIF4E, equivalent to C52 in eIF4E3), eIF4E3 recruits C52 and residues in a loop preceding C52 to contribute to m7Gcap binding as well as residues towards the C-terminus (Fig. 4A, B). Alanine point mutations of these highly conserved regions in eIF4E3 substantially reduced cap-binding affinity. Together, these residues provide the necessary negative charge to associate with the partially positively charged m7G moiety. This m7Gcap-binding mode is unique and leads to a slightly weaker affinity for m7Gcap relative to eIF4E, but is quite similar to the affinity for the other eIF4E family member, eIF4E2. Interestingly, interactions observed between eIF4E3 and the ribose moiety of the m7Gcap are specific relative to eIF4E (Fig. 4A, B) and represent a possible route for designing specific inhibitors. Indeed researchers developing m7Gcap-based inhibitors for eIF4E have found it difficult to develop molecules which recruit interactions from eIF4E to the ribose moiety (74).
Fig. 4. The m7Gcap binding sites for (A) eIF4E and (B) eIF4E3.
eIF4E3 recruits many more interactions to compensate for loss of W56. The electrostatic potential maps of the dorsal surfaces of (C) eIF4E and (D) eIF4E3.
The overall fold of eIF4E3 closely resembles eIF4E, exhibiting the central sheet and three helices. In the apo form, similar to eIF4E, regions important for m7Gcap recognition are highly mobile and become abrogated upon binding. The C-terminus of eIF4E3 which is ~ 10 residues smaller than eIF4E, is unable the form the final β-sheet (S8) present on eIF4E leaving it in a mobile form and allowing it to fold back on itself and interact with the m7Gcap. The phosphorylation site in eIF4E (S209) is also absent in eIF4E3 and there are no obvious residues close by that can be phosphorylated.
eIF4E forms critical interactions with eIF4G to associate with the ribosome and BP1 to negatively regulate eIF4E function. eIF4E3 is unable interact with either of these proteins in cells and their affinities were reduced by ~ 1000 fold based on ITC studies. Concomitantly, the dorsal surfaces of eIF4E and eIF4E3 differ significantly as indicated by their electrostatic potential surfaces (Fig. 4C, D). Thus eIF4E3 is not expected to actively engage the ribosome or compete for at least one class of inhibitor (4E-BP1).
We suggest that eIF4E3 acts as a tumor suppressor by competing for the same pool of transcripts as eIF4E, negating the ability of eIF4E to promote the expression of these proliferative and survival factors (Fig. 2). Although eIF4E3 binds the m7Gcap more weakly in biophysical experiments than eIF4E, in cells eIF4E3 successfully competes with eIF4E for the m7Gcap. This suggests that other cofactors associate with eIF4E3 to increase its affinity for m7Gcap (Fig. 2). We have yet to investigate the role of additional nucleotides for m7Gcap affinity: although sequence homology with eIF4E suggests the second m7Gcap-binding site for eIF4E is not present in eIF4E3. Experiments with the dinucleotide cap analogue indicated that the second nucleotide in the m7Gcap did not increase affinity for eIF4E3. In summary, our studies reveal not only a novel inhibition strategy for eIF4E (competition for the same pool of RNAs) but also a novel m7Gcap recognition mode that may be used by other proteins as well.
Targeting eIF4E in human malignancy
Overview
Initial studies with eIF4E knockdown and antisense oligonucleotides strongly suggested that targeting both the mRNA export and translation activities of eIF4E would be a worthwhile clinical avenue to explore (12, 75–78). We identified ribavirin, an old antiviral drug and guanosine analogue, as a direct inhibitor of eIF4E function (47, 78–80). For instance, in living cells, 3H ribavirin immunoprecipitates with eIF4E (80). We observed that ribavirin treatment repressed both the mRNA export and translation activities of eIF4E (47, 79, 81). Interestingly, ribavirin treatment, like m7Gcap treatment, leads to redistribution of eIF4E from the nucleus to the cytoplasm (18, 19, 47, 48), again highlighting the relevance of controlling eIF4E activity by controlling its distribution. Ribavirin impaired growth of eIF4E dependent tumours in mice and growth of high eIF4E AML specimens (19, 47). Other groups have since made similar observations and further extended these findings to demonstrate that knockdown of eIF4E eliminated the effects of ribavirin strongly suggesting that in cells ribavirin is acting via eIF4E (82, 83).
Structural studies into eIF4E-ribavirin targeting
Initial interest in ribavirin as a cancer therapeutic agent was triggered from our in vitro biophysical studies (47, 79), which showed ribavirin and its active metabolite in cells, ribavirin triphosphate (RTP), could directly bind to eIF4E. Direct binding of ribavirin and RTP to eIF4E was shown by fluorescence, circular dichroism, mass spectrometry, NMR, 3H ribavirin competition and m7Gcap chromatography (47, 79, 80, 84) with affinities for eIF4E similar to that of m7Gcap. We observed in vitro that this interaction was highly dependent upon solution conditions. Such a dependence is not restricted to RTP-eIF4E recognition but is also observed for the m7Gcap-eIF4E interaction. Using ITC and AUC techniques we showed that at higher eIF4E concentrations, affinity for m7Gcap was reduced by as much as 8-fold over the eIF4E concentration range observable by ITC (1.6 – 12.4 uM)(84). This likely reflects the relatively large variation in affinities of m7Gcap for eIF4E reported in the literature and highlights the importance of working under identical conditions to compare the effect of mutants or different m7Gcap analogues on affinities. 1H-15N chemical shift mapping studies at low eIF4E and RTP concentrations were consistent with high affinity binding previously reported using biophysical techniques (84). Chemical shift perturbations indicated binding was located around the m7Gcap-binding site. This was consistent with our data that RTP competes for m7Gcap binding (47, 79). Further evidence for this is supported by the W56 mutant which reduced RTP affinity for eIF4E by ~ 15-fold similar to the effects for m7Gcap (47, 79, 84); additionally, amide chemical shifts for the W56A eIF4E mutant were not perturbed upon addition of RTP, indicating loss of interaction (84). Similar to the allosteric effects of the m7GTP, RTP binding perturbs peaks at the dorsal surface: although distinct but overlapping residues are perturbed. Whether RTP binding affects association of partner proteins at the dorsal surface differently to m7GTP has to be determined, but these data suggest this is possible. Finally, although our NMR data indicate binding is close to the m7Gcap binding site: it is likely not identical (84). Other binding sites in the eIF4E m7Gcap-binding site have recently been exploited by structure based drug design efforts (62, 74). These indicate that there are sites deeper within the m7Gcap-binding pocket available for binding. Unfortunately efforts to solve the eIF4E-RTP structure are hindered by the concentration dependence in this interaction. Both X-ray and NMR techniques require high eIF4E/RTP concentrations, our NMR data indicate that only at lower concentrations can the high affinity complex be observed.
Targeting eIF4E with ribavirin in the clinic
Given our strong biophysical and pre-clinical data, we used ribavirin as the first therapeutic agent to directly target eIF4E in patients (18). Our initial clinical trial was in M4/M5 AML patients who were refractory, relapsed or unable to undergo standard induction chemotherapy. Of the original 11 patients treated, we observed 1 complete remission, 2 partial remissions, 3 blast responses which was striking given the extremely poor prognosis of this group (18). Remarkably, we observed that eIF4E was relocalized from the nucleus to the cytoplasm upon clinical response and re-entry into the nucleus correlated with clinical relapse (18). In those patients we could directly measure mRNA export, we observed impaired activity by ribavirin treatment in responding patients (18). There was insufficient material to measure translation directly in these specimens. Only patients with clinical responses had molecular responses.
To increase the frequency and duration of responses, we combined ribavirin with low dose cytarabine in a second clinical trial studying the same patient population (48). We saw approximately the same response rate including striking responses, e.g. one patient with AML secondary to her breast cancer therapy achieving a CR who responded for two years. As with the first trial, molecular responses were only seen in clinical responders and were lost at relapse. We noted that cytarabine impaired uptake of oral ribavirin into the plasma and thus may not be the ideal agent to combine with ribavirin.
We observed that all responding patients eventually relapsed and that there were many patients that were resistant up front, despite having elevated eIF4E (18, 48). To understand this, we generated resistant models in the lab and analyzed our patients’ specimens as a function of response (80). This led us to identify two main forms of resistance in this population. One form was characterized by impaired ribavirin uptake either at the drug transporter or pro-drug metabolism step. The second form, which was more prevalent in our patients, was underpinned by elevated levels of the Glioma associate protein 1 (Gli1). Gli1 elevation caused an inducible glucuronidation of ribavirin by elevating the UGT1A family of enzymes. The ribavirin-glucuronide no longer bound eIF4E and thus no longer inhibited its activity. We note that not all glucuronidation leads to elevated drug efflux, consistent with our observation that this form of drug resistant was characterized by normal drug uptake. Further, Gli1 elevation alone was sufficient to drive ribavirin-glucuronidation and resistance. Indeed, it appears that this is a new form of multi-drug resistance as it also led to cytarabine-glucuronidation. Use of FDA approved Gli1 inhibitors reverted these effects and re-sensitized cell lines and primary specimens to both ribavirin and cytarabine treatment. Given these findings, we are scheduled to open a phase I/II clinical trial in M4/M5 AML combining Gli1 inhibitors with ribavirin to determine if we can prolong clinical responses (ClinicalTrials.gov NCT02073838).
Unanswered questions
Many aspects of eIF4E activities and mechanics of these activities remain to be revealed. For instance, it is not yet understood how RNAs with the 4ESEs are directed to eIF4E in the nucleus. At this stage, we know that they associate with the nuclear cap binding protein co-transcriptionally and at some point there is a hand-off between eIF4E and CBC, but how and where this occurs are important questions for future. Similarly, we estimate that approximately 700 mRNAs have 4ESEs from eIF4E RIPs; however, this is a structure based element making global predictions more difficult. Further, it is possible that there will be elements other than the 4ESE that could also be used to guide mRNAs to eIF4E dependent mRNA export. In the cytoplasm, the situation is more complicated where there is no specific element known to lead to increased eIF4E dependent but rather the global attributes of length and complexity are the common feature identified to date. Here, sensitivity is determined experimentally. Thus, an important future area will be to determine the number of RNAs that can be regulated at these levels and how many are co-regulated. Further, given the tissue specific nature of many eIF4E regulators, these mRNAs may vary depending on the cell type interrogated. Many important questions remain relevant to the nuts and bolts of the export process and how these are also dysregulated in cancers like AML. From the structure perspective, the impact of dynamics on both cap binding and regulator binding is an important future area of study.
Perspectives
Our studies have demonstrated that eIF4E functions outside of translation, specifically in mRNA export. Together, these functions contribute to its oncogenic potential. We identified a myriad of new regulators which impact the nuclear and cytoplasmic functions of eIF4E. These studies substantially expand the traditional mode of regulation through BP1. Our structural studies provided the first insights into the allosteric regulation of eIF4E and also aided in the identification of a clinically relevant inhibitor. Our studies culminated in the first clinical trial to ever target eIF4E and furthermore, unveiled a new form of drug resistance. Thus, by studying the nuclear functions of eIF4E, we have elucidated critical functions for eIF4E and identified clinically relevant strategies for targeting its oncogenic activity. Considering the nuclear and cytoplasmic functions of eIF4E as the “two roads diverged in a yellow wood, ” we “took the one less travelled by, and that has made all the difference.”-Robert Frost, The Road Not Taken
Acknowledgments
We thank Laurent Volpon and Biljana Culjkovic Kraljacic for assistance in figure preparation. KLBB is supported by grants from the NIH and LLS. She holds a Canada Research Chair in Molecular Biology of the Cell Nucleus.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Sonenberg N, Gingras AC. The mRNA 5′ cap-binding protein eIF4E and control of cell growth. Current opinion in cell biology. 1998;10:268–275. doi: 10.1016/s0955-0674(98)80150-6. [DOI] [PubMed] [Google Scholar]
- 2.Culjkovic B, Topisirovic I, Borden KL. Controlling gene expression through RNA regulons: the role of the eukaryotic translation initiation factor eIF4E. Cell cycle. 2007;6:65–69. doi: 10.4161/cc.6.1.3688. [DOI] [PubMed] [Google Scholar]
- 3.Culjkovic-Kraljacic B, Borden KL. Aiding and abetting cancer: mRNA export and the nuclear pore. Trends in cell biology. 2013 doi: 10.1016/j.tcb.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carroll M, Borden KL. The oncogene eIF4E: using biochemical insights to target cancer. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2013;33:227–238. doi: 10.1089/jir.2012.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lejbkowicz F, Goyer C, Darveau A, Neron S, Lemieux R, Sonenberg N. A fraction of the mRNA 5′ cap-binding protein, eukaryotic initiation factor 4E, localizes to the nucleus. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:9612–9616. doi: 10.1073/pnas.89.20.9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iborra FJ, Jackson DA, Cook PR. Coupled transcription and translation within nuclei of mammalian cells. Science. 2001;293:1139–1142. doi: 10.1126/science.1061216. [DOI] [PubMed] [Google Scholar]
- 7.Cohen N, Sharma M, Kentsis A, Perez JM, Strudwick S, Borden KL. PML RING suppresses oncogenic transformation by reducing the affinity of eIF4E for mRNA. Embo J. 2001;20:4547–4559. doi: 10.1093/emboj/20.16.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topisirovic I, et al. Aberrant eukaryotic translation initiation factor 4E-dependent mRNA transport impedes hematopoietic differentiation and contributes to leukemogenesis. Molecular and cellular biology. 2003;23:8992–9002. doi: 10.1128/MCB.23.24.8992-9002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strudwick S, Borden KL. The emerging roles of translation factor eIF4E in the nucleus. Differentiation. 2002;70:10–22. doi: 10.1046/j.1432-0436.2002.700102.x. [DOI] [PubMed] [Google Scholar]
- 10.Blackshear PJ, Stumpo DJ, Carballo E, Lawrence JC., Jr Disruption of the gene encoding the mitogen-regulated translational modulator PHAS-I in mice. The Journal of biological chemistry. 1997;272:31510–31514. doi: 10.1074/jbc.272.50.31510. [DOI] [PubMed] [Google Scholar]
- 11.Tsukiyama-Kohara K, et al. Adipose tissue reduction in mice lacking the translational inhibitor 4E-BP1. Nat Med. 2001;7:1128–1132. doi: 10.1038/nm1001-1128. [DOI] [PubMed] [Google Scholar]
- 12.Culjkovic B, Borden KL. Understanding and Targeting the Eukaryotic Translation Initiation Factor eIF4E in Head and Neck Cancer. J Oncol. 2009;2009:981679. doi: 10.1155/2009/981679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rong L, et al. Control of eIF4E cellular localization by eIF4E-binding proteins, 4E-BPs. Rna. 2008;14:1318–1327. doi: 10.1261/rna.950608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filipowicz W, Furuichi Y, Sierra JM, Muthukrishnan S, Shatkin AJ, Ochoa S. A protein binding the methylated 5′-terminal sequence, m7GpppN, of eukaryotic messenger RNA. Proceedings of the National Academy of Sciences of the United States of America. 1976;73:1559–1563. doi: 10.1073/pnas.73.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filipowicz W, Sierra JM, Nombela C, Ochoa S, Merrick WC, Anderson WF. Polypeptide chain initiation in eukaryotes: initiation factor requirements for translation of natural messengers. Proceedings of the National Academy of Sciences of the United States of America. 1976;73:44–48. doi: 10.1073/pnas.73.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang V, Zanchin NI, Lunsdorf H, Tuite M, McCarthy JE. Initiation factor eIF-4E of Saccharomyces cerevisiae. Distribution within the cell, binding to mRNA, and consequences of its overproduction. The Journal of biological chemistry. 1994;269:6117–6123. [PubMed] [Google Scholar]
- 17.Topisirovic I, Kentsis A, Perez JM, Guzman ML, Jordan CT, Borden KL. Eukaryotic translation initiation factor 4E activity is modulated by HOXA9 at multiple levels. Molecular and cellular biology. 2005;25:1100–1112. doi: 10.1128/MCB.25.3.1100-1112.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Assouline S, et al. Molecular targeting of the oncogene eIF4E in acute myeloid leukemia (AML): a proof-of-principle clinical trial with ribavirin. Blood. 2009;114:257–260. doi: 10.1182/blood-2009-02-205153. [DOI] [PubMed] [Google Scholar]
- 19.Kraljacic BC, Arguello M, Amri A, Cormack G, Borden K. Inhibition of eIF4E with ribavirin cooperates with common chemotherapies in primary acute myeloid leukemia specimens. Leukemia. 2011;25:1197–1200. doi: 10.1038/leu.2011.57. [DOI] [PubMed] [Google Scholar]
- 20.Rousseau D, Kaspar R, Rosenwald I, Gehrke L, Sonenberg N. Translation initiation of ornithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:1065–1070. doi: 10.1073/pnas.93.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Culjkovic B, Tan K, Orolicki S, Amri A, Meloche S, Borden KL. The eIF4E RNA regulon promotes the Akt signaling pathway. The Journal of cell biology. 2008;181:51–63. doi: 10.1083/jcb.200707018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KL. eIF4E promotes nuclear export of cyclin D1 mRNAs via an element in the 3′UTR. The Journal of cell biology. 2005;169:245–256. doi: 10.1083/jcb.200501019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KL. eIF4E is a central node of an RNA regulon that governs cellular proliferation. The Journal of cell biology. 2006;175:415–426. doi: 10.1083/jcb.200607020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Topisirovic I, et al. Molecular dissection of the eukaryotic initiation factor 4E (eIF4E) export-competent RNP. EMBO J. 2009 doi: 10.1038/emboj.2009.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Culjkovic-Kraljacic B, Baguet A, Volpon L, Amri A, Borden KLB. The oncogene eIF4E reprograms the nuclear pore complext to promote mRNA export and oncogenic transformation. Cell reports. 2012 doi: 10.1016/j.celrep.2012.07.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen YC, et al. Overexpression of NBS1 contributes to transformation through the activation of phosphatidylinositol 3-kinase/Akt. The Journal of biological chemistry. 2005;280:32505–32511. doi: 10.1074/jbc.M501449200. [DOI] [PubMed] [Google Scholar]
- 27.Cao Q, Padmanabhan K, Richter JD. Pumilio 2 controls translation by competing with eIF4E for 7-methyl guanosine cap recognition. Rna. 2010;16:221–227. doi: 10.1261/rna.1884610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kentsis A, et al. The RING Domains of the Promyelocytic Leukemia Protein PML and the Arenaviral Protein Z Repress Translation by Directly Inhibiting Translation Initiation Factor eIF4E. Journal of molecular biology. 2001;312:609–623. doi: 10.1006/jmbi.2001.5003. [DOI] [PubMed] [Google Scholar]
- 29.Kentsis A, Gordon RE, Borden KL. Self-assembly properties of a model RING domain. Proceedings of the National Academy of Sciences of the United States of America. 2002;15:15. doi: 10.1073/pnas.012317299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kentsis A, Gordon RE, Borden KL. From the Cover: Self-assembly properties of a model RING domain. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:667–672. doi: 10.1073/pnas.012317299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kentsis A, Gordon RE, Borden KL. Control of biochemical reactions through supramolecular RING domain self-assembly. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:15404–15409. doi: 10.1073/pnas.202608799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan NG, Ardley HC, Scott GB, Rose SA, Markham AF, Robinson PA. Human homologue of ariadne promotes the ubiquitylation of translation initiation factor 4E homologous protein, 4EHP. FEBS letters. 2003;554:501–504. doi: 10.1016/s0014-5793(03)01235-3. [DOI] [PubMed] [Google Scholar]
- 33.Michon T, Estevez Y, Walter J, German-Retana S, Le Gall O. The potyviral virus genome-linked protein VPg forms a ternary complex with the eukaryotic initiation factors eIF4E and eIF4G and reduces eIF4E affinity for a mRNA cap analogue. The FEBS journal. 2006;273:1312–1322. doi: 10.1111/j.1742-4658.2006.05156.x. [DOI] [PubMed] [Google Scholar]
- 34.Roudet-Tavert G, Michon T, Walter J, Delaunay T, Redondo E, Le Gall O. Central domain of a potyvirus VPg is involved in the interaction with the host translation initiation factor eIF4E and the viral protein HcPro. The Journal of general virology. 2007;88:1029–1033. doi: 10.1099/vir.0.82501-0. [DOI] [PubMed] [Google Scholar]
- 35.Lai HK, Borden KL. The promyelocytic leukemia (PML) protein suppresses cyclin D1 protein production by altering the nuclear cytoplasmic distribution of cyclin D1 mRNA. Oncogene. 2000;19:1623–1634. doi: 10.1038/sj.onc.1203473. [DOI] [PubMed] [Google Scholar]
- 36.Borden KL. Pondering the promyelocytic leukemia protein (PML) puzzle: possible functions for PML nuclear bodies. Molecular and cellular biology. 2002;22:5259–5269. doi: 10.1128/MCB.22.15.5259-5269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Topisirovic I, Capili AD, Borden KL. Gamma interferon and cadmium treatments modulate eukaryotic initiation factor 4E-dependent mRNA transport of cyclin D1 in a PML-dependent manner. Molecular and cellular biology. 2002;22:6183–6198. doi: 10.1128/MCB.22.17.6183-6198.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seker H, et al. UV-C-induced DNA damage leads to p53-dependent nuclear trafficking of PML. Oncogene. 2003;22:1620–1628. doi: 10.1038/sj.onc.1206140. [DOI] [PubMed] [Google Scholar]
- 39.Topisirovic I, Culjkovic B, Cohen N, Perez JM, Skrabanek L, Borden KL. The proline-rich homeodomain protein, PRH, is a tissue-specific inhibitor of eIF4E-dependent cyclin D1 mRNA transport and growth. Embo J. 2003;22:689–703. doi: 10.1093/emboj/cdg069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brunet I, et al. The transcription factor Engrailed-2 guides retinal axons. Nature. 2005;438:94–98. doi: 10.1038/nature04110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nedelec S, Foucher I, Brunet I, Bouillot C, Prochiantz A, Trembleau A. Emx2 homeodomain transcription factor interacts with eukaryotic translation initiation factor 4E (eIF4E) in the axons of olfactory sensory neurons. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10815–10820. doi: 10.1073/pnas.0403824101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt EV. The role of c-myc in regulation of translation initiation. Oncogene. 2004;23:3217–3221. doi: 10.1038/sj.onc.1207548. [DOI] [PubMed] [Google Scholar]
- 43.Hariri F, et al. The eukaryotic translation initiation factor eIF4E is a direct transcriptional target of NF-kappaB and is aberrantly regulated in acute myeloid leukemia. Leukemia. 2013 doi: 10.1038/leu.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khanna-Gupta A, et al. Up-regulation of translation eukaryotic initiation factor 4E in nucleophosmin 1 haploinsufficient cells results in changes in CCAAT enhancer-binding protein alpha activity: implications in myelodysplastic syndrome and acute myeloid leukemia. The Journal of biological chemistry. 2012;287:32728–32737. doi: 10.1074/jbc.M112.373274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Topisirovic I, et al. Stability of eukaryotic translation initiation factor 4E mRNA is regulated by HuR, and this activity is dysregulated in cancer. Molecular and cellular biology. 2009;29:1152–1162. doi: 10.1128/MCB.01532-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cargnello M, et al. Phosphorylation of the eukaryotic translation initiation factor 4E-transporter (4E-T) by c-Jun N-terminal kinase promotes stress-dependent P-body assembly. Molecular and cellular biology. 2012;32:4572–4584. doi: 10.1128/MCB.00544-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kentsis A, Topisirovic I, Culjkovic B, Shao L, Borden KL. Ribavirin suppresses eIF4E-mediated oncogenic transformation by physical mimicry of the 7-methyl guanosine mRNA cap. Proceedings of the National Academy of Sciences of the United States of America. 2004 doi: 10.1073/pnas.0406927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Assouline S, et al. A Phase I trial of ribavirin and low-dose cytarabine for the treatment of relapsed and refractory acute myeloid leukemia FAB subtypes M4 and M5 or high eIF4E. Haematologica. doi: 10.3324/haematol.2014.111245. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marcotrigiano J, Gingras AC, Sonenberg N, Burley SK. Cocrystal structure of the messenger RNA 5′ cap-binding protein (eIF4E) bound to 7-methyl-GDP. Cell. 1997;89:951–961. doi: 10.1016/s0092-8674(00)80280-9. [DOI] [PubMed] [Google Scholar]
- 50.Marcotrigiano J, Gingras AC, Sonenberg N, Burley SK. Cap-dependent translation initiation in eukaryotes is regulated by a molecular mimic of eIF4G. Molecular cell. 1999;3:707–716. doi: 10.1016/s1097-2765(01)80003-4. [DOI] [PubMed] [Google Scholar]
- 51.Tomoo K, et al. Structural basis for mRNA Cap-Binding regulation of eukaryotic initiation factor 4E by 4E-binding protein, studied by spectroscopic, X-ray crystal structural, and molecular dynamics simulation methods. Biochimica et biophysica acta. 2005;1753:191–208. doi: 10.1016/j.bbapap.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 52.Matsuo H, et al. Structure of translation factor eIF4E bound to m7GDP and interaction with 4E-binding protein. Nature structural biology. 1997;4:717–724. doi: 10.1038/nsb0997-717. [DOI] [PubMed] [Google Scholar]
- 53.Zuberek J, et al. Influence of electric charge variation at residues 209 and 159 on the interaction of eIF4E with the mRNA 5′ terminus. Biochemistry. 2004;43:5370–5379. doi: 10.1021/bi030266t. [DOI] [PubMed] [Google Scholar]
- 54.Haghighat A, Sonenberg N. eIF4G dramatically enhances the binding of eIF4E to the mRNA 5′-cap structure. The Journal of biological chemistry. 1997;272:21677–21680. doi: 10.1074/jbc.272.35.21677. [DOI] [PubMed] [Google Scholar]
- 55.Ptushkina M, von der Haar T, Karim MM, Hughes JM, McCarthy JE. Repressor binding to a dorsal regulatory site traps human eIF4E in a high cap-affinity state. The EMBO journal. 1999;18:4068–4075. doi: 10.1093/emboj/18.14.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.von der Haar T, Gross JD, Wagner G, McCarthy JE. The mRNA cap-binding protein eIF4E in post-transcriptional gene expression. Nature structural & molecular biology. 2004;11:503–511. doi: 10.1038/nsmb779. [DOI] [PubMed] [Google Scholar]
- 57.Shen X, Tomoo K, Uchiyama S, Kobayashi Y, Ishida T. Structural and thermodynamic behavior of eukaryotic initiation factor 4E in supramolecular formation with 4E-binding protein 1 and mRNA cap analogue, studied by spectroscopic methods. Chemical & pharmaceutical bulletin. 2001;49:1299–1303. doi: 10.1248/cpb.49.1299. [DOI] [PubMed] [Google Scholar]
- 58.McCubbin WD, Edery I, Altmann M, Sonenberg N, Kay CM. Circular dichroism and fluorescence studies on protein synthesis initiation factor eIF-4E and two mutant forms from the yeast Saccharomyces cerevisiae. The Journal of biological chemistry. 1988;263:17663–17671. [PubMed] [Google Scholar]
- 59.Niedzwiecka A, Darzynkiewicz E, Stolarski R. Thermodynamics of mRNA 5′ cap binding by eukaryotic translation initiation factor eIF4E. Biochemistry. 2004;43:13305–13317. doi: 10.1021/bi0491651. [DOI] [PubMed] [Google Scholar]
- 60.von der Haar T, et al. Folding transitions during assembly of the eukaryotic mRNA cap-binding complex. Journal of molecular biology. 2006;356:982–992. doi: 10.1016/j.jmb.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 61.Volpon L, Osborne MJ, Topisirovic I, Siddiqui N, Borden KL. Cap-free structure of eIF4E suggests a basis for conformational regulation by its ligands. EMBO J. 2006;25:5138–5149. doi: 10.1038/sj.emboj.7601380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown CJ, McNae I, Fischer PM, Walkinshaw MD. Crystallographic and mass spectrometric characterisation of eIF4E with N7-alkylated cap derivatives. Journal of molecular biology. 2007;372:7–15. doi: 10.1016/j.jmb.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 63.Siddiqui N, et al. Structural insights into the allosteric effects of 4EBP1 on the eukaryotic translation initiation factor eIF4E. Journal of molecular biology. 2012;415:781–792. doi: 10.1016/j.jmb.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sondergaard CR, Garrett AE, Carstensen T, Pollastri G, Nielsen JE. Structural artifacts in protein-ligand X-ray structures: implications for the development of docking scoring functions. Journal of medicinal chemistry. 2009;52:5673–5684. doi: 10.1021/jm8016464. [DOI] [PubMed] [Google Scholar]
- 65.Selvaratnam R, Chowdhury S, VanSchouwen B, Melacini G. Mapping allostery through the covariance analysis of NMR chemical shifts. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6133–6138. doi: 10.1073/pnas.1017311108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Volpon L, Osborne MJ, Capul AA, de la Torre JC, Borden KL. Structural characterization of the Z RING-eIF4E complex reveals a distinct mode of control for eIF4E. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:5441–5446. doi: 10.1073/pnas.0909877107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kern D, Zuiderweg ER. The role of dynamics in allosteric regulation. Current opinion in structural biology. 2003;13:748–757. doi: 10.1016/j.sbi.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 68.Volkman BF, Lipson D, Wemmer DE, Kern D. Two-state allosteric behavior in a single-domain signaling protein. Science. 2001;291:2429–2433. doi: 10.1126/science.291.5512.2429. [DOI] [PubMed] [Google Scholar]
- 69.Henzler-Wildman K, Kern D. Dynamic personalities of proteins. Nature. 2007;450:964–972. doi: 10.1038/nature06522. [DOI] [PubMed] [Google Scholar]
- 70.Shi L, Kay LE. Tracing an allosteric pathway regulating the activity of the HslV protease. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2140–2145. doi: 10.1073/pnas.1318476111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Joshi B, Cameron A, Jagus R. Characterization of mammalian eIF4E-family members. European journal of biochemistry/FEBS. 2004;271:2189–2203. doi: 10.1111/j.1432-1033.2004.04149.x. [DOI] [PubMed] [Google Scholar]
- 72.Joshi B, Lee K, Maeder DL, Jagus R. Phylogenetic analysis of eIF4E-family members. BMC evolutionary biology. 2005;5:48. doi: 10.1186/1471-2148-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Osborne MJ, Volpon L, Kornblatt JA, Culjkovic-Kraljacic B, Baguet A, Borden KL. eIF4E3 acts as a tumor suppressor by utilizing an atypical mode of methyl-7-guanosine cap recognition. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3877–3882. doi: 10.1073/pnas.1216862110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen X, et al. Structure-guided design, synthesis, and evaluation of guanine-derived inhibitors of the eIF4E mRNA-cap interaction. Journal of medicinal chemistry. 2012;55:3837–3851. doi: 10.1021/jm300037x. [DOI] [PubMed] [Google Scholar]
- 75.Graff JR, et al. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. The Journal of clinical investigation. 2007;117:2638–2648. doi: 10.1172/JCI32044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Graff JR, Zimmer SG. Translational control and metastatic progression: enhanced activity of the mRNA cap-binding protein eIF-4E selectively enhances translation of metastasis-related mRNAs. Clin Exp Metastasis. 2003;20:265–273. doi: 10.1023/a:1022943419011. [DOI] [PubMed] [Google Scholar]
- 77.Li BD, et al. Prospective study of eukaryotic initiation factor 4E protein elevation and breast cancer outcome. Ann Surg. 2002;235:732–738. doi: 10.1097/00000658-200205000-00016. discussion 738–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Borden KL, Culjkovic-Kraljacic B. Ribavirin as an anti-cancer therapy: Acute Myeloid Leukemia and beyond? Leukemia and Lymphoma. 2010 doi: 10.3109/10428194.2010.496506. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kentsis A, et al. Further evidence that ribavirin interacts with eIF4E. Rna. 2005;11:1762–1766. doi: 10.1261/rna.2238705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zahreddine HA, et al. The sonic hedgehog factor GLI1 imparts drug resistance through inducible glucuronidation. Nature. 2014;511:90–93. doi: 10.1038/nature13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tan K, Culjkovic B, Amri A, Borden KL. Ribavirin targets eIF4E dependent Akt survival signaling. Biochemical and biophysical research communications. 2008;375:341–345. doi: 10.1016/j.bbrc.2008.07.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pettersson F, et al. Ribavirin treatment effects on breast cancers overexpressing eIF4E, a biomarker with prognostic specificity for luminal B-type breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:2874–2884. doi: 10.1158/1078-0432.CCR-10-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bollmann F, et al. Human inducible nitric oxide synthase (iNOS) expression depends on chromosome region maintenance 1 (CRM1)- and eukaryotic translation initiation factor 4E (elF4E)-mediated nucleocytoplasmic mRNA transport. Nitric oxide : biology and chemistry/official journal of the Nitric Oxide Society. 2013;30:49–59. doi: 10.1016/j.niox.2013.02.083. [DOI] [PubMed] [Google Scholar]
- 84.Volpon L, Osborne MJ, Zahreddine H, Romeo AA, Borden KL. Conformational changes induced in the eukaryotic translation initiation factor eIF4E by a clinically relevant inhibitor, ribavirin triphosphate. Biochemical and biophysical research communications. 2013 doi: 10.1016/j.bbrc.2013.03.125. [DOI] [PMC free article] [PubMed] [Google Scholar]