Population risk factor estimates for abdominal aortic aneurysm from electronic medical records: a case control study (original) (raw)
Abstract
Background
Using abdominal aortic aneurysm (AAA) as a model, this case–control study used electronic medical record (EMR) data to assess known risk factors and identify new associations.
Methods
The study population consisted of cases with AAA (n =888) and controls (n =10,523) from the Geisinger Health System EMR in Central and Northeastern Pennsylvania. We extracted all clinical and diagnostic data for these patients from January 2004 to December 2009 from the EMR. From this sample set, bootstrap replication procedures were used to randomly generate 2,500 iterations of data sets, each with 500 cases and 2000 controls. Estimates of risk factor effect sizes were obtained by stepwise logistic regression followed by bootstrap aggregation. Variables were ranked using the number of inclusions in iterations and P values.
Results
The benign neoplasm diagnosis was negatively associated with AAA, a novel finding. Similarly, type 2 diabetes, diastolic blood pressure, weight and myelogenous neoplasms were negatively associated with AAA. Peripheral artery disease, smoking, age, coronary stenosis, systolic blood pressure, age, height, male sex, pulmonary disease and hypertension were associated with an increased risk for AAA.
Conclusions
This study utilized EMR data, retrospectively, for risk factor assessment of a complex disease. Known risk factors for AAA were replicated in magnitude and direction. A novel negative association of benign neoplasms was identified. EMRs allow researchers to rapidly and inexpensively use clinical data to expand cohort size and derive better risk estimates for AAA as well as other complex diseases.
Keywords: Aortic Aneurysm, Abdominal, Electronic medical record, Neoplasms, Benign, Risk factors, Blood pressure, Diabetes mellitus, Type 2, Case–control studies
Background
Epidemiological research studies on risk factors are traditionally performed with case–control or cohort studies, requiring a considerable sample size, cost and time investment. Electronic medical records (EMR) contain a wealth of phenotypic information with high potential to replace costly traditional epidemiological methods for purposes such as determining disease risk factors. In this study we utilized an extensive EMR to determine risk factors associated with incident cases of abdominal aortic aneurysm (AAA) in a population-based case–control study from the Geisinger Health System (GHS) serving populations in Central and Northeastern Pennsylvania [1, 2]. Pennsylvania has one of the highest rates of mortality from AAA in the USA [3].
AAA is defined as a dilatation of >3 cm in the infrarenal aorta [4–7]. A leading cause of death in the United States, AAAs often exist undetected until the aneurysm ruptures, with a concomitant fatality rate of up to 90% [5, 8–11]. Rupture can be prevented by endovascular repair or traditional open aortic surgery, which is usually performed after the aneurysm reaches a diameter of ≥5.5 cm. Since AAAs can be detected non-invasively by ultrasonography and can be surgically repaired, AAA is an ideal disease for a screening program. Ultrasonographic screening is noninvasive, relatively inexpensive and has a sensitivity and specificity of ≥99% [9, 12–14]. Currently, the United States Preventative Services Task Force (USPSTF) recommends one AAA ultrasound screening for males, 65 to 75 years of age who smoked ≥100 cigarettes in their lifetime [15, 16]. The USPSTF does not recommend screening for women, although females with AAA have a poorer prognosis and higher mortality rate in the event of a rupture [17–23]. Medicare began covering ultrasonography screening in 2007 for the initial “Welcome to Medicare” enrollment examination for men 65 to 75 years of age who have ever smoked or those with a family history of AAA [24]. According to recent studies fewer than 30% of eligible patients are actually screened [16, 25]. The current guidelines are under-utilized and exclude many at increased risk [26]. Additionally, a recent retrospective analysis indicated that 77% of ruptured AAA patients were unaware of their AAA prior to the rupture, despite a visit to a clinician within the past 5 years [27].
Current risk prediction based only on sex, age and smoking has low sensitivity and specificity, and therefore is used relatively infrequently. Better risk estimates and risk prediction models should improve utility and utilization. Our study established the feasibility of utilizing an EMR to identify novel risk factors and replicate risk factor associations with the incidence of AAA found in the literature. It also demonstrated the utility of EMRs to rapidly expand the available cohort size for identifying risk factors and obtaining refined effect size estimates.
Methods
Study population
GHS provides primary and specialty care to a highly stable population of 2.6 million residents in Central and Northeastern Pennsylvania [2]. Geisinger serves a large catchment area. We restricted the region to those regional divisions (counties) where Geisinger serves more than 10% of the county population. Among these counties Geisinger serves about half of their two million inhabitants. The cases and controls for this study consisted of individuals with AAA and individuals without diagnosed AAA from GHS with clinical records in the EMR from January 2004 to December 2009. Cases (n =888; 686 males and 202 females) were identified by the following criteria: International Classification of Diseases, 9th Revision (ICD-9) codes for AAA (441.3, 441.4), Current Procedural Terminology (CPT) codes (34800, 34802, 34803, 34804, 34805, 34830, 34831, 34832, 35081, 35082, 35091, 35092, 35102, 35103, 35131, 35132), or referral to the GHS Department of Vascular Surgery with an imaging study (such as ultrasound, computed tomography scan or MRI) to confirm an infrarenal aortic diameter >3.0 cm. All AAA cases in GHS are referred to the Department of Vascular Surgery, which diminishes possible ascertainment bias.
Statistical analyses were performed using bootstrap aggregation (see Statistical Analyses section below for details). A control sample was selected to reflect population census demographics from all available patients without known AAA at the start of the study (n =10,523; 4,132 males and 6,391 females) enrolled in the GHS MyCode® biobanking repository [1]. All individuals in the study were Caucasian, which reflects the homogeneous ethnicity of the population in the GHS service area and the demographics of the disease. The MyCode® repository consists of individuals attending primary care sites in the communities served by GHS. Inclusion criteria were: adults >18 years of age, patient at a GHS primary care clinic, and no diagnosis of dementia. The MyCode® participants are representative of the demographic and clinical characteristics of the GHS outpatient population. Individuals gave written informed consent to allow their EMR data to be used for research purposes and to have biological specimens stored in the biobank.
GHS has utilized EMR since 1996, and implemented a data warehouse system for research data mining and analysis in 2008 [1]. This data warehouse includes the outpatient records of the patients seen by primary care and specialty providers. Analysts in the biostatistical core extract and de-identify the data through a data broker system before the investigators receive the dataset. The study was approved by the Institutional Review Board of GHS.
Data source
Demographic and clinical variables of interest were extracted from the Geisinger EMR. Clinical risk factors were selected from the literature or were those of biological interest based on AAA pathobiology [7–9, 11, 28–42]. All diagnoses, laboratory measures and clinical values from primary care and specialty clinic visits (as of the date of the data extraction) were extracted. Age was defined as the age at AAA diagnosis for cases, and age at data extraction for the controls. Individuals >89 years of age were removed to protect potential identification of subjects. The ICD-9 codes and diagnoses used to define these variables are listed in Table 1. Since there were a number of infrequent diagnoses among the 565 distinct ICD-9 codes used for the data extraction, the codes were collapsed into 17 categories to reduce the number of variables for modeling.
Table 1.
ICD-9 diagnostic codes and assigned categories used to identify comorbidities
| Diagnostic category | ICD-9 codes |
|---|---|
| Arterial dissection | 443.21–443.29 |
| Atherosclerosis | 440 |
| Benign neoplasm | 210.00–229.9, 238.8, 238.9 |
| Cerebral thrombosis | 434, 434.01, 434.1, 437.1 |
| Cerebrovascular disease | 434.11, 434.91, 435.8, 435.9, 436, 437, 437.0A, 437.4, 437.6–437.9 |
| Coronary stenosis | 411.81, 414.00–414.9 |
| Cranial artery stenosis | 433–433.9, 434.9, 435–435.3 |
| Hypertension | 401.0–405.99 |
| Intracranial aneurysm | 430, 437.3 |
| Intracranial hemorrhage | 431, 432.1, 432.9 |
| Kidney disease | 585.3–585.9, 586 |
| Malignant neoplasm | 140.00–209.6, 230.00–234.9, 235.1–238.3 |
| Myelogenous neoplasm | 200–208.9, 238.4–238.7, 238.71–238.73, 238.75, 238.76, 238.79, 272.2 |
| Peripheral artery disease | 440.21, 440.22, 440.3, 440.31, 440.32, 443.9 |
| Pulmonary disease | 491.00–492.8, 493.2, 496, 518.1 |
| Type 1 diabetes | 250.01, 250.03, 250.11, 250.13, 250.2, 250.23, 250.41, 250.43, 250.51, 250.53, 250.61, 250.63, 250.71, 250.73, 250.81, 250.83, 250.93 |
| Type 2 diabetes | 250.00, 250.02, 250.1, 250.12, 250.22, 250.4, 250.42, 250.5, 250.52, 250.6, 250.62, 250.7, 250.72, 250.8, 250.82, 250.9, 250.92 |
All variables were examined for consistency and distribution. Extreme, clinically or biologically implausible values were attributed to data entry error and excluded from the analysis. The median was used as a measure of centrality for continuous variables of the cleaned data set.
Statistical analyses
U.S. Census Bureau data for 2010 [43] for all counties within the GHS service area were used to standardize the control sample for population age and sex. Traditional bootstrap methods [44, 45], with replacement, were used to randomly generate 2,500 iterations of data sets of 2,000 controls and 500 AAA cases with complete data (Figure 1). Each set of controls was selected to reflect the census age and sex demographic structure. Younger individuals, especially males, are underrepresented among patients in health care systems. To prevent oversampling of individuals under 35 years of age, census age classes (18,35] were collapsed into a single class. The number of controls was limited to 2,000 to ensure that the sampling of young males was not extreme. Cases were selected at random from the 888 available cases. The 2,500 bootstrap data sets were analyzed using logistic regression with AAA as the outcome variable and 26 explanatory variables. Variable selection was achieved by bidirectional stepwise elimination using Aikaike’s information criterion (AIC) [46] to evaluate model fit. A final model was generated using variables that were consistently retained in most bootstrap iterations. A second set of 2,500 bootstrap data sets were generated and analyzed using logistic regression with the final model of a fixed number of variables (Table 2), i.e., each bootstrap set was analyzed with the same model [47]. Regression estimates were recorded for each iteration and the estimates aggregated using meta-analytic techniques (using random-effects weighting). Variables were ranked by how often they were retained in the model, and by the P value, which was based on the mean z score weighted by the number of iterations the corresponding variable was included in the model. The 14 highest ranked variables were then fixed in a second bootstrap analysis (no stepwise elimination). We considered variables statistically significant at P <0.05, two-sided. Analyses were performed in R v.2.16.2, 64-bit (R Foundation for Statistical Computing; http://www.R-project.org, Vienna, Austria) [48] using the glm and rmeta packages.
Figure 1.
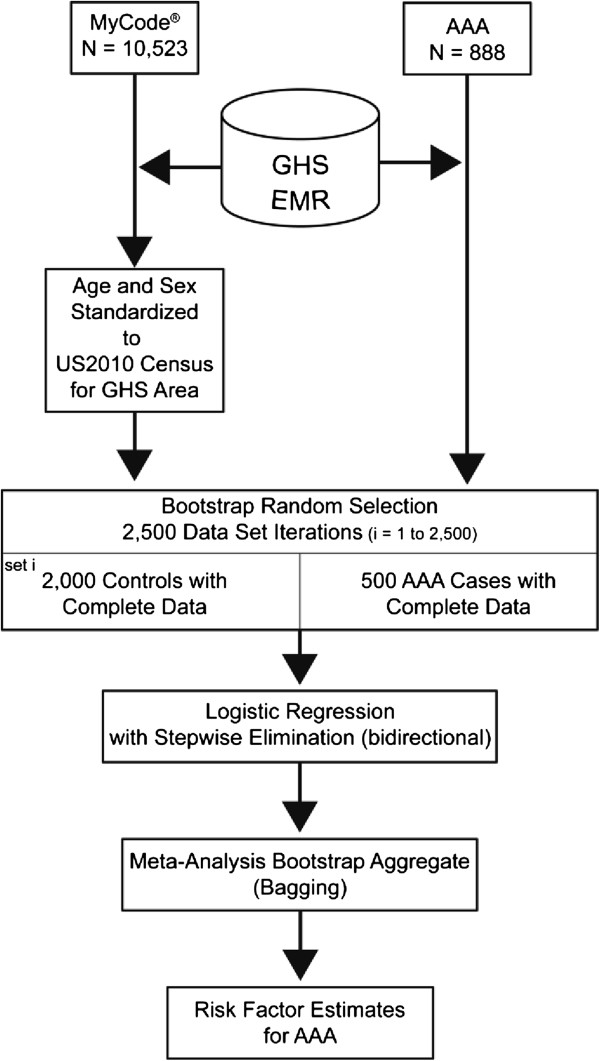
Study design. MyCode® is the biobank at GHS, Geisinger Health System. AAA, abdominal aortic aneurysm; EMR, electronic medical record.
Table 2.
Risk factor estimates for AAA
| Variable | Meta*estimate | Meta*SE | P † | OR | 95% CI |
|---|---|---|---|---|---|
| Peripheral artery disease | 1.49 | 0.0040 | 3.71E-16 | 4.42 | 3.08–6.35 |
| Smoking, ever/never | 1.36 | 0.0036 | 3.92E-15 | 3.91 | 2.77–5.53 |
| Coronary stenosis | 1.07 | 0.0032 | 2.01E-12 | 2.91 | 2.15–3.93 |
| Type 2 diabetes | −0.84 | 0.0043 | 1.69E-05 | 0.43 | 0.29–0.64 |
| Systolic blood pressure | 0.03 | 0.0001 | 2.20E-07 | 1.03 | 1.02–1.05 |
| Diastolic blood pressure | −0.05 | 0.0003 | 1.84E-05 | 0.95 | 0.93–0.98 |
| Age | 0.05 | 0.0001 | 1.63E-15 | 1.05 | 1.04–1.06 |
| Weight | −0.01 | 3.94E-05 | 9.13E-07 | 0.99 | 0.98–0.99 |
| Height | 0.12 | 0.0001 | 2.78E-05 | 1.13 | 1.06–1.20 |
| Sex | 0.65 | 0.0044 | 0.00172 | 1.92 | 1.24–2.97 |
| Benign neoplasm | −0.39 | 0.0029 | 0.00263 | 0.67 | 0.51–0.89 |
| Pulmonary disease | 0.54 | 0.0035 | 0.00061 | 1.72 | 1.24–2.38 |
| Hypertension | 0.62 | 0.0038 | 0.00056 | 1.86 | 1.28–2.72 |
| Myelogenous neoplasm | −0.32 | 0.0032 | 0.02131 | 0.73 | 0.54–0.99 |
Results
We identified 888 AAA cases from the GHS Department of Vascular Surgery. We also identified a pool of 10,523 patients without AAA from the Geisinger EMR who were consented into the MyCode® biobanking project with complete data for the variables of interest (Figure 1). Using 2010 census demographics, we standardized the control sample sets to match the demographics of the population residing in the GHS catchment area. After randomly generating 2,500 iterations of 500 cases and 2,000 controls each, we used meta-analysis and weighted the variables by how often they appeared in the 2,500 iterations and their significance (P value). The highest ranking variables were included in a second bootstrap analysis to obtain unbiased estimates (Table 2). Peripheral artery disease (PAD), smoking, coronary stenosis, systolic blood pressure, age, height (taller stature), male sex, pulmonary disease and hypertension were significantly associated with an increased risk for AAA. Type 2 diabetes mellitus (T2DM), diastolic blood pressure, weight, benign neoplasms and myelogenous neoplasms had a significantly negative association with AAA. Blood pressure remained in the model as diastolic and systolic measurements, as well as the diagnosis of hypertension.
The significant association between AAA and benign neoplasms was a novel finding. We compared all AAA cases with at least one benign neoplasm diagnosis (n = 365) to all controls with a benign neoplasm diagnosis (n = 5,419) (Table 3). Some individuals had more than one type of neoplasm. Benign neoplasm of the skin was the most common subtype in controls (73%), significantly more common than in cases (59%, P <0.001). Benign neoplasm of the digestive system was the most prevalent in cases (61%) as compared to controls (43%) and this difference was also significant (P <0.001). Benign neoplasm of the mouth/throat was only borderline significantly different between cases and controls, the remaining subtypes were not significantly different.
Table 3.
Comparison of subclasses of benign neoplasms between AAA cases and controls
| Type of neoplasm | Cases (n = 365) with neoplasm | Controls (n = 5419) with neoplasm | P † | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Skin | 216 | 59.18 | 3962 | 73.11 | <0.001 |
| Digestive | 226 | 61.92 | 2317 | 42.76 | <0.001 |
| Benign Neoplasm NOS* | 61 | 16.71 | 764 | 14.1 | 0.164 |
| Lipoma | 31 | 8.49 | 632 | 11.66 | 0.074 |
| Hemangioma | 12 | 3.29 | 248 | 4.58 | 0.296 |
| Endocrine gland | 22 | 6.03 | 211 | 3.89 | 0.053 |
| Eye | 10 | 2.74 | 106 | 1.96 | 0.33 |
| Brain/nervous | 7 | 1.92 | 104 | 1.92 | 0.98 |
| Soft tissue | 2 | 0.55 | 88 | 1.62 | 0.126 |
| Mouth/throat | 11 | 3.01 | 82 | 1.51 | 0.048 |
| Respiratory | 6 | 1.64 | 37 | 0.68 | 0.051 |
| Urinary | 2 | 0.55 | 21 | 0.39 | 0.654 |
| Bone | 0 | 0 | 10 | 0.18 | 0.969 |
Discussion
This study demonstrated the feasibility of utilizing EMR data in a retrospective study for risk factor assessment of AAA, a complex disease. Previous studies have identified a number of risk factors for AAA including age, male sex, and smoking [28–38, 49–52] which were confirmed as important risk factors in the current study (Figure 2; Table 2). Age of the patient has also been significantly associated with survival based on repair type [53]. Strong and consistent evidence of an association of smoking with AAA warranted the inclusion of AAA in the Surgeon General’s report on The Heath Consequences of Smoking in 2004 [54]. Smoking also affects AAA expansion and rupture [36, 55, 56]. In addition, PAD [33, 34, 36, 56], coronary stenosis [31, 34, 36–38, 56], systolic blood pressure [32, 34, 41, 42], height [30, 31, 37, 41], pulmonary disease [39, 57], a diagnosis of hypertension [28, 30, 32, 34, 37, 41, 49, 55] and malignant neoplasms [58, 59] were all significantly associated with an increased AAA risk in this population. The negative association with T2DM replicated published AAA epidemiologic studies [23, 30, 34–36, 38, 55, 60]. Diabetes has also been associated with a decrease in growth of AAA [36]. A negative association was also found with weight. Height was found to be significantly associated with AAA, independent of body mass index (BMI), replicating published findings for AAA [30, 31, 36, 41]. We found a negative, but not statistically significant, association of BMI with AAA. In previous studies the association of BMI with AAA has been inconsistent, many studies have found a positive association with AAA [31, 38, 61], while others have found a negative association [36] or no association [32, 37, 41, 62].
Figure 2.
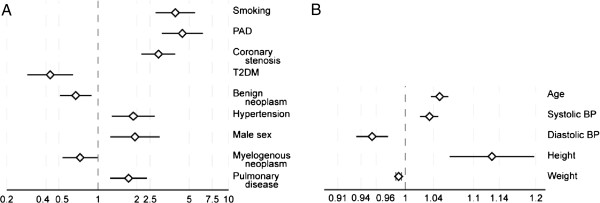
Forest plot of risk factors for AAA identified using EMR data. For scaling purposes, the data are separated into. A, discrete variables; and B, continuous variables. Odds ratios and 95% confidence intervals for the variables associated with AAA are shown. PAD, peripheral artery disease; T2DM, type 2 diabetes mellitus; BP, blood pressure.
We found a negative association of myelogenous (OR =0.73, P =0.021) neoplasms with AAA. An association of AAA and cancer has been reported in two studies when comparing AAA cases to patients with atherosclerotic occlusive disease (AOD) [58, 59]. The sample size in the first study was small, 69 AAA and 61 AOD cases [59]. The second study with a larger group, 298 AAA and 151 AOD patients also found an association of AAA with cancer, but it was not statistically significant when controlling for the confounders of age and smoking [58].
The negative association of benign neoplasms with AAA identified in the current study is intriguing. The most common type of neoplasm was of the skin, significantly more common in controls than cases. Neoplasms of the digestive system were more common in cases. The identified association is biologically plausible since two genes (CDKN2BAS and DABP2) with strong associations to AAA have roles in cell growth [63, 64]. Further research is necessary and may provide a clue to the molecular biology of AAA. Since the current study is cross-sectional, we cannot determine causation, but rather the results reveal correlations between incident AAA and various clinical variables.
The major strength of this study lies in the demonstration that EMR data collected as part of standard clinical care is suitable for retrospective epidemiologic analysis. This has profound implications for future use of EMR data for other risk analyses. The Medicare and Medicaid EHR Incentive Program provides incentives to eligible health care providers, and EMR data should become more readily available to a majority of researchers [2]. A limitation of EMRs, and this study, is that EMRs are designed for clinical utility rather than research purposes. Consequently, data entry errors such as missing observations, inconsistent entries, outliers or improbable values must be resolved prior to analysis. Approaches to using EMR data are being investigated by a number of groups including the NIH-funded electronic Medical Record and Genomics (eMERGE) Network [65, 66] and the Health Maintenance Organization Research Network (HMORN) Virtual Data Warehouse (VDW) project [67]. Despite gaps and inconsistencies in EMR data, the information available for our study was of sufficient quality to identify the major known risk factors for AAA. We focused on identifying risk factors for the incidence of AAA, not AAA progression, which likely has different risk factors.
As Geisinger is the primary health care provider for the population residing in Northeastern Pennsylvania, the EMR contains extensive medical history for all the patients in our study. The EMR allows us to mitigate several issues inherent in epidemiologic research, such as selection bias and unknown confounders. A major advantage of the EMR is that our study had much higher participation rates than traditional epidemiological studies, since data could be extracted from the EMR post facto. The proportion of elderly individuals in Central and Northeastern Pennsylvania is higher than the national average, increasing the number of AAA cases in the current study. A bias inherent to EMRs includes over-representation of sick participants and underrepresentation of the young, who tend to be healthy and less prone to seeking medical care. In our case, MyCode® recruits patients from primary care in addition to tertiary care, and the general health of the participants, therefore, is more representative of the general population. Generalizability of the results to other populations is unknown, although it is encouraging that all the known major risk factors found in previous studies in other populations were detected in our study. Previous studies have indicated that family history is a significant risk factor [30, 39, 51, 68, 69] but family history of AAA was not recorded in the Geisinger EMR.
Conclusions
One of the goals of the study was to identify risk factors for AAA which could then be used to refine the eligibility criteria for AAA ultrasonography screening programs. The current screening guidelines have low sensitivity and specificity, and an improved risk prediction model would be of great public health benefit [38, 70]. Future work on AAA risk prediction models should include genotypes of genetic variants [63, 71–73] along with the recognized demographic and clinical variables. We would also like to study risk factors for progression and growth rates of AAAs. EMRs allow researchers to rapidly and inexpensively use clinical data to expand cohort size to derive better estimates for AAA as well as other complex diseases.
Acknowledgements
Part of this study was presented at the 2012 American Heart Association Scientific Sessions: Cardiovascular Disease Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, San Diego, CA. The work was supported by a grant from the Pennsylvania Commonwealth Universal Research Enhancement program (to D.J.C.); a grant from the Geisinger Clinical Research Fund (to J.R.E.); a Grant-In-Aid from the American Heart Association (to D.J.C.); and a grant from the National Institutes of Health (U01HG006382 to D.J.C.). The Geisinger MyCode® Project was funded in part by a grant from the Ben Franklin Technology Development Fund of Pennsylvania.
Abbreviations
AAA
Abdominal aortic aneurysm
USPSTF
United States Preventative Services Task Force
GHS
Geisinger Health System
EMR
Electronic medical records
eMERGE
Electronic MEdical Records and Genomics
BP
Blood pressure
BMI
Body mass index
HbA1c
Glycosylated hemoglobin
MyCode®
Geisinger system-wide biobanking program of adult primary care patients
PAD
Peripheral artery disease
OR
Odds ratio
CI
Confidence interval
ICD-9
International Classification of Diseases, Ninth Revision
CPT
Current Procedural Terminology.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contribution
DTS and GT participated in the design of the study, coordinated the data collection and performed statistical analyses. DTS, GT and HK drafted the manuscript. HK, JRE and DPF provided clinical expertise and interpretation of data. DJC contributed to the design of the study, interpretation of data and provided resources. All authors contributed to data interpretation and critical revision of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Diane T Smelser, Email: dtsmelser@geisinger.edu.
Gerard Tromp, Email: gctromp@geisinger.edu.
James R Elmore, Email: jelmore@geisinger.edu.
Helena Kuivaniemi, Email: shkuivaniemi@geisinger.edu.
David P Franklin, Email: dfranklin@geisinger.edu.
H Lester Kirchner, Email: hlkirchner@geisinger.edu.
David J Carey, Email: djcarey@geisinger.edu.
References
- 1.Gerhard GS, Carey DJ, Steele GDJ. Electronic health records in genomic medicine. In: Ginsburg GS, Willard HF, editors. Genomic and Personalized Medicine. 2. London: Academic Press; 2013. pp. 287–294. [Google Scholar]
- 2.Steele GD., Jr Re-engineering systems of care: surgical leadership. J Am Coll Surg. 2010;210:1–5. doi: 10.1016/j.jamcollsurg.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 3.CDC Wonder . Underlying Cause of Death. 2010. [Google Scholar]
- 4.Johnston KW, Rutherford RB, Tilson MD, Shah DM, Hollier L, Stanley JC. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg. 1991;13:452–458. doi: 10.1067/mva.1991.26737. [DOI] [PubMed] [Google Scholar]
- 5.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman E, et al. Heart disease and stroke Statistics—2012 update. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47:1239–1312. doi: 10.1016/j.jacc.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8:92–102. doi: 10.1038/nrcardio.2010.180. [DOI] [PubMed] [Google Scholar]
- 8.Brown LC, Powell JT. Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. UK Small Aneurysm Trial Participants. Ann Surg. 1999;230:289–296. doi: 10.1097/00000658-199909000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Upchurch GR, Jr, Schaub TA. Abdominal aortic aneurysm. Am Fam Physician. 2006;73:1198–1204. [PubMed] [Google Scholar]
- 10.Moore CL, Holliday RS, Hwang JQ, Osborne MR. Screening for abdominal aortic aneurysm in asymptomatic at-risk patients using emergency ultrasound. Am J Emerg Med. 2008;26:883–887. doi: 10.1016/j.ajem.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 11.Golledge J, Muller J, Daugherty A, Norman P. Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler Thromb Vasc Biol. 2006;26:2605–2613. doi: 10.1161/01.ATV.0000245819.32762.cb. [DOI] [PubMed] [Google Scholar]
- 12.Lederle FA, Walker JM, Reinke DB. Selective screening for abdominal aortic aneurysms with physical examination and ultrasound. Arch Intern Med. 1988;148:1753–1756. doi: 10.1001/archinte.1988.00380080049015. [DOI] [PubMed] [Google Scholar]
- 13.Lindholt JS, Vammen S, Juul S, Henneberg EW, Fasting H. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472–475. doi: 10.1053/ejvs.1999.0835. [DOI] [PubMed] [Google Scholar]
- 14.Tayal VS, Graf CD, Gibbs MA. Prospective study of accuracy and outcome of emergency ultrasound for abdominal aortic aneurysm over two years. Acad Emerg Med. 2003;10:867–871. doi: 10.1111/j.1553-2712.2003.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 15.Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2005;142:203–211. doi: 10.7326/0003-4819-142-3-200502010-00012. [DOI] [PubMed] [Google Scholar]
- 16.Eaton J, Reed D, Angstman KB, Thomas K, North FK, Stroebel R, Tulledge-Scheitel SM, Chaudhry R. Effect of visit length and a clinical decision support tool on abdominal aortic aneurysm screening rates in a primary care practice. J Eval Clin Pract. 2011;18:593–598. doi: 10.1111/j.1365-2753.2010.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeRubertis BG, Trocciola SM, Ryer EJ, Pieracci FM, McKinsey JF, Faries PL, Kent KC. Abdominal aortic aneurysm in women: prevalence, risk factors, and implications for screening. J Vasc Surg. 2007;46:630–635. doi: 10.1016/j.jvs.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 18.Lederle FA, Larson JC, Margolis KL, Allison MA, Freiberg MS, Cochrane BB, Graettinger WF, Curb JD. Abdominal aortic aneurysm events in the women’s health initiative: cohort study. BMJ. 2008;337:a1724. doi: 10.1136/bmj.a1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lederle FA. Should abdominal aortic aneurysm be managed differently in women? Scand J Surg. 2008;97:125–127. doi: 10.1177/145749690809700209. [DOI] [PubMed] [Google Scholar]
- 20.Norman PE, Powell JT. Abdominal aortic aneurysm: the prognosis in women is worse than in men. Circulation. 2007;115:2865–2869. doi: 10.1161/CIRCULATIONAHA.106.671859. [DOI] [PubMed] [Google Scholar]
- 21.Solberg S, Singh K, Wilsgaard T, Jacobsen BK. Increased growth rate of abdominal aortic aneurysms in women. The Tromsø Study. Eur J Vasc Endovasc Surg. 2005;29:145–149. doi: 10.1016/j.ejvs.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Kent KC, Zwolak RM, Jaff MR, Hollenbeck ST, Thompson RW, Schermerhorn ML, Sicard GA, Riles TS, Cronenwett JL, Society for Vascular Surgery, American Association of Vascular Surgery, Society for Vascular Medicine and Biology Screening for abdominal aortic aneurysm: a consensus statement. J Vasc Surg. 2004;39:267–269. doi: 10.1016/j.jvs.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Villard C, Swedenborg J, Eriksson P, Hultgren R. Reproductive history in women with abdominal aortic aneurysms. J Vasc Surg. 2011;54:341–345. doi: 10.1016/j.jvs.2010.12.069. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Medicare & Medicaid Services . November 17, 2006.Baltimore, MD: Report No.: MLN Matters Number: MM5235, Related Change Request Number: 5235. 2006. Implementation of a one-time only ultrasound screening for abdominal aortic aneurysms (AAA), resulting from a referral from an initial preventive physical examination. [Google Scholar]
- 25.Federman DG, Carbone VG, Kravetz JD, Kancir S, Kirsner RS, Bravata DM. Are screening guidelines for abdominal aortic aneurysms being implemented within a large VA primary health care system? Postgrad Med. 2009;121(1):132–135. doi: 10.3810/pgm.2009.01.1962. [DOI] [PubMed] [Google Scholar]
- 26.Eckroth-Bernard K, Garvin RP, Ryer EJ, Elmore JR, Franklin DP. The SAAAVE act and routine ambulatory medical care fail to diagnose patients with abdominal aortic aneurysms prior to rupture: a single-institution experience. ISRN Vascular Medicine. 2013;2013:Article ID 134019. doi: 10.1155/2013/134019. [DOI] [Google Scholar]
- 27.MacDonald AJ, Faleh O, Welch G, Kettlewell S. Missed opportunities for the detection of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2008;35:698–700. doi: 10.1016/j.ejvs.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: The Tromsø Study, 1994–2001. Circulation. 2009;119:2202–2208. doi: 10.1161/CIRCULATIONAHA.108.817619. [DOI] [PubMed] [Google Scholar]
- 29.Singh K, Bonaa KH, Jacobsen BK, Bjork L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: The Tromsø Study. Am J Epidemiol. 2001;154:236–244. doi: 10.1093/aje/154.3.236. [DOI] [PubMed] [Google Scholar]
- 30.Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, Krupski WC, Barone GW, Acher CW, Ballard DJ. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med. 1997;126:441–449. doi: 10.7326/0003-4819-126-6-199703150-00004. [DOI] [PubMed] [Google Scholar]
- 31.Alcorn HG, Wolfson SK, Jr, Sutton-Tyrrell K, Kuller LH, O’Leary D. Risk factors for abdominal aortic aneurysms in older adults enrolled in The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1996;16:963–970. doi: 10.1161/01.ATV.16.8.963. [DOI] [PubMed] [Google Scholar]
- 32.Palazzuoli A, Gallotta M, Guerrieri G, Quatrini I, Franci B, Campagna MS, Neri E, Benvenuti A, Sassi C, Nuti R. Prevalence of risk factors, coronary and systemic atherosclerosis in abdominal aortic aneurysm: comparison with high cardiovascular risk population. Vasc Health Risk Manag. 2008;4:877–883. doi: 10.2147/vhrm.s1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Badger SA, O’Donnell ME, Sharif MA, Boyd CS, Hannon RJ, Lau LL, Lee B, Soong CV. Risk factors for abdominal aortic aneurysm and the influence of social deprivation. Angiology. 2008;59:559–566. doi: 10.1177/0003319708321586. [DOI] [PubMed] [Google Scholar]
- 34.Baumgartner I, Hirsch AT, Abola MT, Cacoub PP, Poldermans D, Steg PG, Creager MA, Bhatt DL, for the REACH Registry investigators Cardiovascular risk profile and outcome of patients with abdominal aortic aneurysm in out-patients with atherothrombosis: data from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. J Vasc Surg. 2008;48:808–814. doi: 10.1016/j.jvs.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 35.Blanchard JF, Armenian HK, Friesen PP. Risk factors for abdominal aortic aneurysm: results of a case–control study. Am J Epidemiol. 2000;151:575–583. doi: 10.1093/oxfordjournals.aje.a010245. [DOI] [PubMed] [Google Scholar]
- 36.Brady AR, Thompson SG, Fowkes FG, Greenhalgh RM, Powell JT, UK Small Aneurysm Trial Participants Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation. 2004;110:16–21. doi: 10.1161/01.CIR.0000133279.07468.9F. [DOI] [PubMed] [Google Scholar]
- 37.Iribarren C, Darbinian JA, Alan SG, Fireman BH, Lee CD, Grey DP. Traditional and novel risk factors for clinically diagnosed abdominal aortic aneurysm: the Kaiser multiphasic health checkup cohort study. Ann Epidemiol. 2007;17:669–678. doi: 10.1016/j.annepidem.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, Moskowitz AJ, Gelijns AC, Greco G. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539–548. doi: 10.1016/j.jvs.2010.05.090. [DOI] [PubMed] [Google Scholar]
- 39.Larsson E, Granath F, Swedenborg J, Hultgren R. A population-based case–control study of the familial risk of abdominal aortic aneurysm. J Vasc Surg. 2009;49:47–51. doi: 10.1016/j.jvs.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 40.Lederle FA, Nelson DB, Joseph AM. Smokers’ relative risk for aortic aneurysm compared with other smoking-related diseases: a systematic review. J Vasc Surg. 2003;38:329–334. doi: 10.1016/S0741-5214(03)00136-8. [DOI] [PubMed] [Google Scholar]
- 41.Rodin MB, Daviglus ML, Wong GC, Liu K, Garside DB, Greenland P, Stamler J. Middle age cardiovascular risk factors and abdominal aortic aneurysm in older age. Hypertension. 2003;42:61–68. doi: 10.1161/01.HYP.0000078829.02288.98. [DOI] [PubMed] [Google Scholar]
- 42.Shantikumar S, Ajjan R, Porter KE, Scott DJ. Diabetes and the abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2009;39:200–207. doi: 10.1016/j.ejvs.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 43.State demographic profiles [http://www2.census.gov/census_2010/03-Demographic_Profile/]. [updated May 19, 2011; cited March 4, 2013]
- 44.Ludbrook J, Dudley H. Why permutation tests are superior to t and F tests in biomedical research. Am Stat. 1998;52:127–132. [Google Scholar]
- 45.Efron B, Tibshirani RJ. An introduction to the bootstrap. Chapman & Hall/CRC: Boca Raton, FL; 1993. [Google Scholar]
- 46.Austin PC, Tu JV. Bootstrap methods for developing predictive models. Am Stat. 2004;58:131–137. doi: 10.1198/0003130043277. [DOI] [Google Scholar]
- 47.Harrell FE., Jr . Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression and Survival Analysis. New York: Springer-Verlag; 2001. Springer Series in Statistics; pp. 53–85. [Google Scholar]
- 48.R: a language and environment for statistical computinghttp://www.r-project.org/
- 49.Svensjö S, Bjorck M, Gurtelschmid M, Djavani Gidlund K, Hellberg A, Wanhainen A. Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation. 2011;124:1118–1123. doi: 10.1161/CIRCULATIONAHA.111.030379. [DOI] [PubMed] [Google Scholar]
- 50.Pleumeekers HJ, Hoes AW, van der Does E, van Urk H, Hofman A, de Jong PT, Grobbee DE. Aneurysms of the abdominal aorta in older adults. The Rotterdam Study. Am J Epidemiol. 1995;142:1291–1299. doi: 10.1093/oxfordjournals.aje.a117596. [DOI] [PubMed] [Google Scholar]
- 51.Wanhainen A, Bergqvist D, Boman K, Nilsson TK, Rutegard J, Bjorck M. Risk factors associated with abdominal aortic aneurysm: a population-based study with historical and current data. J Vasc Surg. 2005;41:390–396. doi: 10.1016/j.jvs.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 52.Wilmink TBM, Quick CRG, Day NE. The association between cigarette smoking and abdominal aortic aneurysms. J Vasc Surg. 1999;30:1099–1105. doi: 10.1016/S0741-5214(99)70049-2. [DOI] [PubMed] [Google Scholar]
- 53.Lederle FA, Freischlag JA, Kyriakides TC, Matsumura JS, Padberg FT, Jr, Kohler TR, Kougias P, Jean-Claude JM, Cikrit DF, Swanson KM. Long-term comparison of Endovascular and open repair of abdominal aortic aneurysm. N Engl J Med. 2012;367:1988–1997. doi: 10.1056/NEJMoa1207481. [DOI] [PubMed] [Google Scholar]
- 54.CDC Surgeon General’s Reports: Smoking & tobacco use. [http://www.cdc.gov/tobacco/data_statistics/sgr/2004/complete_report/index.htm]; [cited 7/14/2011]
- 55.Sweeting MJ, Thompson SG, Brown LC, Powell JT, RESCAN collaborators Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655–665. doi: 10.1002/bjs.8707. [DOI] [PubMed] [Google Scholar]
- 56.VegadeCéniga M, Gómez R, Estallo L, Rodríguez L, Baquer M, Barba A. Growth rate and associated factors in small abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2006;31:231–236. doi: 10.1016/j.ejvs.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 57.Meijer CA, Kokje VBC, van Tongeren RBM, Hamming JF, van Bockel JH, Möller GM, Lindeman JHN. An association between chronic obstructive pulmonary disease and abdominal aortic aneurysm beyond smoking: results from a case–control study. Eur J Vasc Endovasc Surg. 2012;44:153–157. doi: 10.1016/j.ejvs.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 58.Chan EL, Belem P, Ciocca RG, Madsen D, Cody RP, Mackenzie JW, Boyd CD, Graham AM. Incidence of cancer and abdominal aortic aneurysms: a logistic regression analysis. Ann N Y Acad Sci. 1996;800:68–73. doi: 10.1111/j.1749-6632.1996.tb33299.x. [DOI] [PubMed] [Google Scholar]
- 59.Tilson M, Fieg EL, Harvey M. Malignant neoplasia in patients with abdominal aortic aneurysms. Arch Surg. 1984;119:792–794. doi: 10.1001/archsurg.1984.01390190036008. [DOI] [PubMed] [Google Scholar]
- 60.Le MT, Jamrozik K, Davis TM, Norman PE. Negative association between infra-renal aortic diameter and glycaemia: the Health in Men Study. Eur J Vasc Endovasc Surg. 2007;33:599–604. doi: 10.1016/j.ejvs.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 61.Wong DR, Willett WC, Rimm EB. Smoking, hypertension, alcohol consumption, and risk of abdominal aortic aneurysm in men. Am J Epidemiol. 2007;165:838–845. doi: 10.1093/aje/kwk063. [DOI] [PubMed] [Google Scholar]
- 62.Stackelberg O, Björck M, Sadr-Azodi O, Larsson SC, Orsini N, Wolk A. Obesity and abdominal aortic aneurysm. Br J Surg. 2013;100:360–366. doi: 10.1002/bjs.8983. [DOI] [PubMed] [Google Scholar]
- 63.Helgadottir A, Thorleifsson G, Magnusson KP, Gretarsdottir S, Steinthorsdottir V, Manolescu A, Jones GT, Rinkel GJ, Blankensteijn JD, Ronkainen A, Jääskeläinen JE, Kyo Y, Lenk GM, Sakalihasan N, Kostulas K, Gottsater A, Flex A, Stefansson H, Hansen T, Andersen G, Weinsheimer S, Borch-Johnsen K, Jorgensen T, Shah SH, Quyyumi AA, Granger CB, Reilly MP, Austin H, Levey AI, Vaccarino V, et al. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet. 2008;40:217–224. doi: 10.1038/ng.72. [DOI] [PubMed] [Google Scholar]
- 64.Gretarsdottir S, Baas AF, Thorleifsson G, Holm H, den Heijer M, de Vries JP, Kranendonk SE, Zeebregts CJ, van Sterkenburg SM, Geelkerken RH, van Rij AM, Williams MJ, Boll AP, Kostic JP, Jonasdottir A, Jonasdottir A, Walters GB, Masson G, Sulem P, Saemundsdottir J, Mouy M, Magnusson KP, Tromp G, Elmore JR, Sakalihasan N, Limet R, Defraigne JO, Ferrell RE, Ronkainen A, Ruigrok YM, et al. Genome-wide association study identifies a sequence variant within the DAB2IP gene conferring susceptibility to abdominal aortic aneurysm. Nat Genet. 2010;42:692–697. doi: 10.1038/ng.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kho AN, Pacheco JA, Peissig PL, Rasmussen L, Newton KM, Weston N, Crane PK, Pathak J, Chute CG, Bielinski SJ, Kullo IJ, Li R, Manolio TA, Chisholm RL, Denny JC. Electronic medical records for genetic research: results of the eMERGE consortium. Sci Transl Med. 2011;3:79re1. doi: 10.1126/scitranslmed.3001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ritchie MD, Denny JC, Crawford DC, Ramirez AH, Weiner JB, Pulley JM, Basford MA, Brown-Gentry K, Balser JR, Masys DR, Haines JL, Roden DM. Robust replication of genotype-phenotype associations across multiple diseases in an electronic medical record. Am J Hum Genet. 2010;86:560–572. doi: 10.1016/j.ajhg.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.The virtual data warehouse (VDW) and how to use it HMO Research Network [http://www.hmoresearchnetwork.org/en/Tools%20&%20Materials/VDW/The%20VDW%20an%20How%20to%20Use%20It.pdf]
- 68.Kuivaniemi H, Shibamura H, Arthur C, Berguer R, Cole CW, Juvonen T, Kline RA, Limet R, Mackean G, Norrgård O, Pals G, Powell JT, Rainio P, Sakalihasan N, van Vlijmen-van KC, Verloes A, Tromp G. Familial abdominal aortic aneurysms: collection of 233 multiplex families. J Vasc Surg. 2003;37:340–345. doi: 10.1067/mva.2003.71. [DOI] [PubMed] [Google Scholar]
- 69.Wahlgren CM, Larsson E, Magnusson PKE, Hultgren R, Swedenborg J. Genetic and environmental contributions to abdominal aortic aneurysm development in a twin population. J Vasc Surg. 2010;51:3–7. doi: 10.1016/j.jvs.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 70.Greco G, Egorova NN, Gelijns AC, Moskowitz AJ, Manganaro AJ, Zwolak RM, Riles TS, Kent KC. Development of a novel scoring tool for the identification of large ≥5 cm abdominal aortic aneurysms. Ann Surg. 2010;252:675–682. doi: 10.1097/SLA.0b013e3181f621c8. [DOI] [PubMed] [Google Scholar]
- 71.Karanjia PN, Madden KP, Lobner S. Coexistence of abdominal aortic aneurysm in patients with carotid stenosis. Stroke. 1994;25:627–630. doi: 10.1161/01.STR.25.3.627. [DOI] [PubMed] [Google Scholar]
- 72.Bown MJ, Jones GT, Harrison SC, Wright BJ, Bumpstead S, Baas AF, Gretarsdottir S, Badger SA, Bradley DT, Burnand K, Child AH, Clough RE, Cockerill G, Hafez H, Scott DJ, Futers S, Johnson A, Sohrabi S, Smith A, Thompson MM, van Bockxmeer FM, Waltham M, Matthiasson SE, Thorleifsson G, Thorsteinsdottir U, Blankensteijn JD, Teijink JA, Wijmenga C, de Graaf J, Kiemeney LA, et al. Abdominal aortic aneurysm is associated with a variant in low-density lipoprotein receptor-related protein 1. Am J Hum Genet. 2011;89:619–627. doi: 10.1016/j.ajhg.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hinterseher I, Tromp G, Kuivaniemi H. Genes and abdominal aortic aneurysm. Ann Vasc Surg. 2011;25:388–412. doi: 10.1016/j.avsg.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pre-publication history
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2261/14/174/prepub