Interleukin-34 Restores Blood–Brain Barrier Integrity by Upregulating Tight Junction Proteins in Endothelial Cells (original) (raw)
Abstract
Interleukin-34 (IL-34) is a newly discovered cytokine as an additional ligand for colony stimulating factor-1 receptor (CSF1R), and its functions are expected to overlap with colony stimulating factor-1/macrophage-colony stimulating factor. We have previously shown that the IL-34 is primarily produced by neurons in the central nervous system (CNS) and induces proliferation and neuroprotective properties of microglia which express CSF1R. However, the functions of IL-34 in the CNS are still elucidative. Here we show that CNS capillary endothelial cells also express CSF1R. IL-34 protected blood–brain barrier integrity by restored expression levels of tight junction proteins, which were downregulated by pro-inflammatory cytokines. The novel function of IL-34 on the blood–brain barrier may give us a clue for new therapeutic strategies in neuroinflammatory and neurodegenerative diseases such as multiple sclerosis and Alzheimer's disease.
Introduction
Interleukin-34 (IL-34) has been identified as an additional ligand for colony stimulating factor-1 receptor (CSF1R), and it is broadly expressed in various organs including heart, brain, lung, liver, kidney, spleen, and colon [1]. IL-34 and colony stimulating factor-1/macrophage-colony stimulating factor (CSF-1/M-CSF) bind to the different regions of CSF1R and share no overt sequence homology [2]. Recent studies showed that IL-34 produced by epithelial lineage cells (e.g. keratinocytes, splenic vascular endothelial cells, and neurons) is necessary for the development of tissue macrophage-like cells (e.g. Langerhans cells, osteoclasts, and microglia) [3]–[5]. We also have shown that IL-34 is exclusively produced by neurons in CNS and induces proliferation of microglia [6]. We also showed that IL-34 attenuated the neurotoxic effect of oligomeric amyloid beta (Aβ) in vitro and intracerebroventricular administration of IL-34 ameliorates the impairment of associative learning in an AD mouse model [6]. Another study also demonstrated that IL-34 rescued neuronal damage in mouse models of AD and kinate-induced neurotoxicity [7]. These findings suggest distinct functions of IL-34 in the development of various CNS disorders. However, the precise functions of IL-34 in the CNS still remain to be elucidated.
The blood-brain barrier (BBB) is a tight seal composed of capillary endothelial cells, pericytes, and astrocytes [8]. The BBB contributes to maintenance of CNS homeostasis by limiting the entry of plasma components, erythrocytes, and immune cells from the circulating blood [9]–[11]. Tight junction (TJ) plays an important role in the barrier function of the BBB, which is composed by TJ proteins including claudins, occludin, and zonula occludens-1 (ZO-1) [12]. BBB disruption is frequently associated with synaptic and neuronal dysfunction in various neurological disorders such as multiple sclerosis (MS), AD, Parkinson's disease, and amyotrophic lateral sclerosis [13], [14]. Pro-inflammatory cytokines such as IL-1β, tumor necrosis factor-α (TNF-α), interferon-γ, and IL-17, are thought to downregulate the expression of tight junction proteins and contribute to the transmigration of inflammatory immune cells into the CNS, which exacerbates neuroinflammation in these diseases [14]–[19].
In this study, we found that the CNS capillary endothelial cells as well as microglia express CSF1R. We also showed that IL-34 restored pro-inflammatory cytokine–induced BBB disruption by upregulating the expression levels of tight junction proteins such as claudin-5 and occludin. These findings suggest the presence of neuronal regulation of BBB functions via IL-34, and upregulation of IL-34 in the CNS may be a novel therapeutic strategy against neuroinflammatory and neurodegenerative disorders.
Materials and Methods
Reagents
Recombinant mouse IL-1β, TNF-α, and IL-34 were purchased from R&D Systems (Minneapolis, MN, USA). The c-fms/CSF1R tyrosine kinase inhibitor GW2580 was used as a blocker of CSF1R signaling (Millipore, Bedford, MA, USA). Dylight 594–labeled tomato lectin was used as a capillary endothelial cell marker (Vector Laboratories, Burlingame, CA, USA).
Animals
All protocols were approved by the Animal Experiment Committee of Nagoya University (approved number: 14018). C57BL/6J mice were purchased from Japan SLC (Hamamatsu, Japan).
Cells
Primary neuronal cultures were prepared from the cortices of C57BL/6 mouse embryos at embryonic Day 17 as described previously [20]. Briefly, cortical fragments were dissociated into single cells in dissociation solution (Sumitomo Bakelite, Akita, Japan), and resuspended in neuron culture medium (Sumitomo Bakelite). Neurons were seeded onto 12-mm polyethylenimine-coated glass coverslips (Asahi Techno Glass Corp., Chiba, Japan) at a density of 5.0×104 cells/well in 24-well culture plates and were incubated at 37°C in a humidified atmosphere containing 5% CO2. The purity of the cultures was >95% as determined by NeuN-specific immunostaining. Mouse brain capillary endothelial cell line MBEC4 [21] was maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Confluent monolayer of MBEC4 cells was used as an established BBB model as described previously [22].
BBB Permeability assay
The permeability of MBEC4 cell monolayers was measuring transendothelial electrical resistance (TER) as described previously [22]. Confluent monolayer of MBEC4 cells on the 24-well transwell inserts (3-µm pore size) were incubated with or without 20 ng/ml TNF-α, 20 ng/ml IL-1β, 0–100 ng/ml IL-34, or 1 µmol/L GW2580 for 24 h. TER was measured using a Millicell-ERS (Millipore). Resistances of blank filters were subtracted from those of filters with cells before final resistances (Ω • cm2) were calculated. Assays were carried out in five independent trials.
Immunocytochemistry
Primary neurons and MBEC4 cells were fixed with 4% paraformaldehyde for 10 min, permeabilized using 0.1% Triton X-100 for 5 min, and blocked using 5% normal goat serum in phosphate-buffered saline (PBS) for 1 h at room temperature. Neurons were incubated with rabbit anti-mouse IL-34 polyclonal antibodies (ProSci, Poway, CA, USA), mouse anti-mouse microtubule-associated protein–2 (MAP-2) monoclonal antibody (Chemicon, Temecula, CA, USA) overnight at 4°C followed by a 1-h incubation with Alexa-conjugated secondary antibodies (Invitrogen, Carlsbad, CA, USA). MBEC4 cells were stained using rabbit anti-mouse CSF1R polyclonal antibodies (Abcam, Cambridge, UK) overnight at 4°C followed by a 1-h incubation with Alexa-conjugated secondary antibodies (Invitrogen). Nuclei were counterstained with Hoechst 33342 (Invitrogen). Images were analyzed using a deconvolution fluorescent microscope system (BZ-8000, Keyence, Osaka, Japan).
Immunohistochemistry
Brains and lumbar spinal cords from C57BL/6J mice were fixed with 4% paraformaldehyde overnight, equilibrated in 20% sucrose with PBS for 48 hours, embedded in Tissue Tek O.C.T. compound (Sakura Finetechnical Co., Ltd., Tokyo, Japan), and frozen at −80°C overnight. Coronal brain sections and transverse spinal cord sections (20 µm-thick) were prepared using a cryostat. Sections were permeabilized using 0.3% Triton X-100 after blocking with 5% normal goat serum in PBS for 1 h. Sections were incubated with rabbit anti-mouse IL-34 polyclonal antibodies (ProSci), mouse anti-mouse MAP-2 monoclonal antibody (Chemicon), rabbit anti-mouse CSF1R polyclonal antibodies (Abcam), and Dylight 594–labeled tomato lectin (Vector Laboratories) overnight at 4°C followed by a 1-h incubation with Alexa-conjugated secondary antibodies (Invitrogen). Images were analyzed using a deconvolution fluorescent microscope system (BZ-8000, Keyence).
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR)
MBEC4 cells were cultured at a concentration of 4×105 cells/well in 24-well culture plates and stimulated with 100 ng/ml IL-34 for 24 h. Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA). cDNAs encoding mouse IL-34 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were generated by RT-PCR using SuperScript II (Invitrogen), Blend Taq DNA polymerase (Toyobo, Osaka, Japan), and the following specific primer sets:
CSF1R forward primer: 5′-AAGCAGAAGCCGAAGTACCA-3′
CSF1R reverse primer: 5′-GTCCCTGCGCACATATTTCAT-3′
GAPDH forward primer: 5′-TGTGTCCGTCGTGGATCTGA-3′
GAPDH reverse primer: 5′-CCTGCTTCACCACCTTCTTGA-3′
Western Blotting
MBEC4 Cells were lysed in TNES buffer (50 mM Tris-HCl at pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 2 mM EDTA, and 0.1% SDS) with protease inhibitor mixture (Complete Mini EDTA-free; Roche Diagnostics, Basel, Switzerland). Cell lysate proteins dissolved in Laemmli sample buffer (20 µg/well) were separated on 4–20% SDS-polyacrylamide gels (Mini-Protean TGX; Bio-Rad, Hercules, CA, USA) and transferred to Hybond-P polyvinylidene difluoride membranes (GE Healthcare, Piscataway, NJ, USA) as described previously [23]. The membranes were blocked for 1 h at room temperature with 5% skim milk in Tris-buffered saline containing 0.05% Tween-20, and then incubated overnight at 4°C with rabbit anti-mouse Zonula Occludens–1 (ZO-1) polyclonal antibodies, rabbit anti-mouse occludin polyclonal antibodies, rabbit anti-mouse claudin-5 polyclonal antibodies (Invitrogen), and mouse anti-β-actin monoclonal antibody (Sigma). After an overnight incubation with primary antibodies at 4°C, each blot was probed with horseradish peroxidase-conjugated anti-mouse IgG (GE Healthcare). Blots were then visualized with SuperSignal West Dura Extended Duration Substrate (Thermo Fisher Scientific, Waltham, MA, USA), and quantitated using a CS Analyzer 3.0 system (Atto, Tokyo, Japan). Assays were carried out in five independent trials.
Statistical analysis
Statistical significance was analyzed with one-way analysis of variance followed by post-hoc Tukey's test, using GraphPad Prism version 5.0 (GraphPad Software, La Jolla, CA, USA).
Results
IL-34 is exclusively expressed in CNS neurons
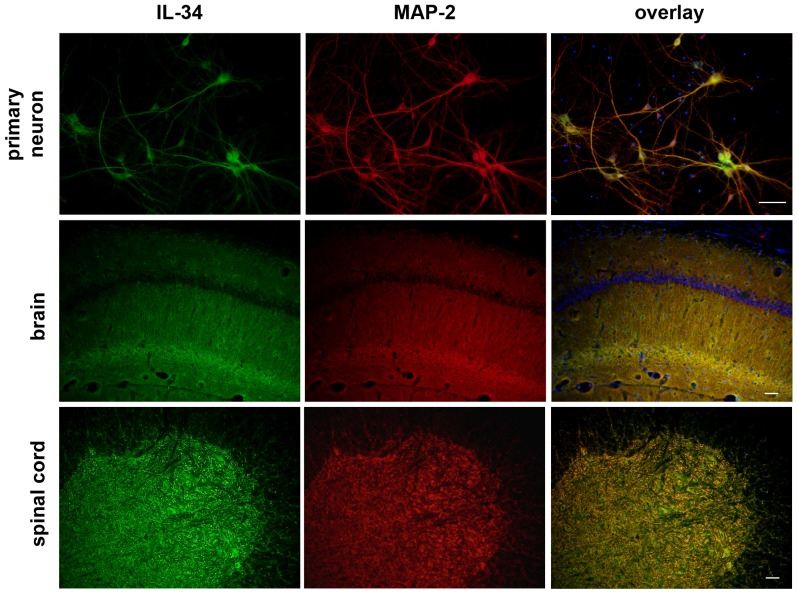
In the previous study, we have reported that IL-34 protein is primarily expressed in neurons whereas IL-34 mRNA expression was detected in neurons and astrocytes [6]. First, we confirmed the expression pattern of IL-34 in the CNS using immunostaining in mouse primary cortical neurons, brains, and spinal cords. As shown in Fig. 1, IL-34 protein was exclusively expressed in neurons in the CNS.
Figure 1. IL-34 is produced by neurons in the CNS.
Immunofluorescence images of primary cortical neurons, brain sections, and lumbar spinal cord sections. Green, IL-34; red, MAP-2; blue, Hoechst nuclear counterstain. Scale bar, 50 µm.
CNS capillary endothelial cells expressed IL-34 receptor CSF1R
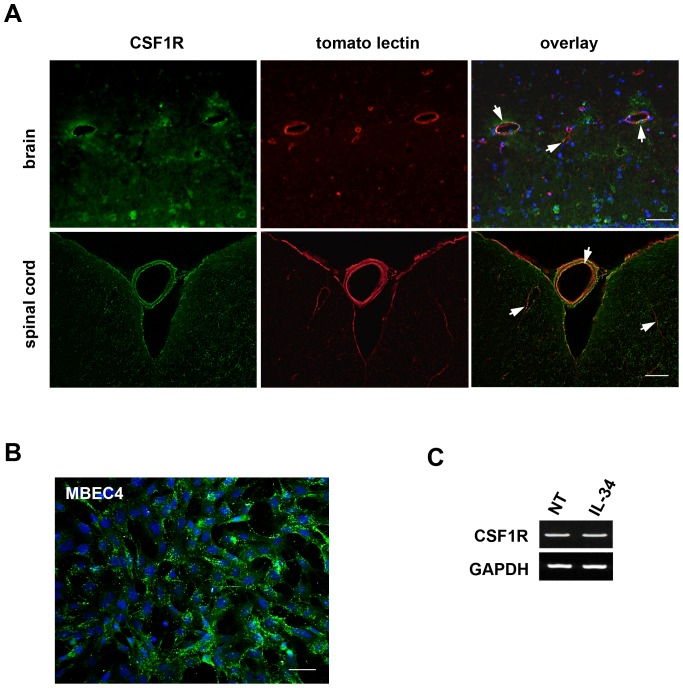
Next, we examined the expression pattern of IL-34 receptor CSF1R protein in the CNS using immunostaining. In addition to microglia, CNS microvessels were also immunopositive for CSF1R (Fig. 2A, green). Its staining pattern in the microvessels was identical to that of tomato lectin (Fig. 2A, red and arrows in the overlap images) which selectively binds to the surface of capillary endothelial cells [24], suggesting that CNS capillary endothelial cells express CSF1R. Meninges and large vessel adventitia were also stained with CSF1R and tomato lectin. Although CSF1R has been detected on fibroblasts and smooth muscle cells which are the main components of meninges and adventitia [1], [7], the meninges and adventitia showed strong non-specific binding of antibodies and lectin. Therefore, the positive staining in meninges and adventitia may be artifact.
Figure 2. CNS endothelial cells express CSF1R.
(A) Immunofluorescence images of brain sections, and lumbar spinal cord sections. Green, CSF1R; red, tomato lectin; blue, Hoechst nuclear counterstain. Arrows indicate CSF1R-immunopositivity in the capillary endothelial cells. Scale bar, 50 µm. (B) Immunofluorescence image of mouse brain capillary endothelial cell line MBEC4. Green, CSF1R; blue, Hoechst nuclear counterstain. Scale bar, 50 µm. (C) RT-PCR data for CSF1R. Stimulation with IL-34 did not alter the expression of CSF1R in MBEC4 cells.
Furthermore, mouse brain capillary endothelial cell line MBEC4 cells strongly express CSF1R protein (Fig. 2B, green). MBEC4 cells constitutively express CSF1R mRNA, and stimulation with IL-34 did not alter CSF1R expression level (Fig. 2C). These data indicate that CNS capillary endothelial cells constitutively express CSF1R and are potential target of IL-34 in the CNS, as well as microglia.
IL-34 restored BBB disruption via CSF1R signaling in endothelial cells
BBB disruption is a common pathological feature of various neurological diseases, and inflammatory cytokines such as IL-1β and TNF-α have been considered as causative factors that damage BBB integrity by downregulating TJ proteins in BBB endothelial cells [14]–[16], [25], [26]. To investigate whether IL-34 affects the BBB integrity, we evaluated BBB permeability by measuring TER in MBEC4 cell monolayer as an in vitro BBB model [22]. IL-34 significantly ameliorated a decrease in TER induced by IL-1β and TNF-α in a dose dependent manner (Fig. 3), whereas treatment with IL-34 alone did not alter untreated BBB integrity (data not shown). Moreover, addition of CSF1R signal inhibitor GW2580 ablated the effect of IL-34 on BBB (Fig. 3). These results indicate that IL-34 restored pro-inflammatory cytokine–mediated BBB disintegrity via CSF1R signaling in endothelial cells.
Figure 3. IL-34 restores damaged BBB integrity.
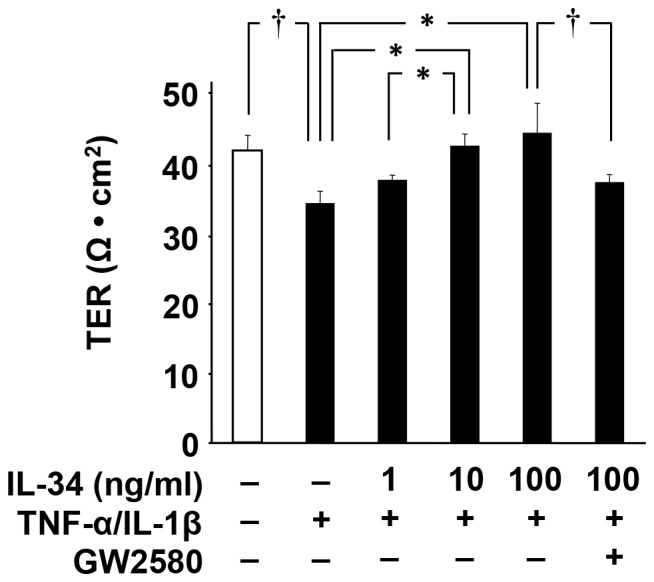
MBEC4 cells were treated with TNF-α (20 ng/ml) and IL-1β (20 ng/ml) in the presence of IL-34 (0–100 ng/ml) and GW2580 (1 µmol/l). TER of MBEC4 cell monolayer was measured after a 24-h incubation. Values are means ± SEM (n = 5). *, p<0.05; †, p<0.01.
IL-34 upregulated TJ proteins in BBB endothelial cells
Next, we assessed whether IL-34 alters the expression levels of TJ proteins that are sensitive to pro-inflammatory cytokines [14]–[16]. Western blotting analysis detected that major TJ proteins such as claudin-5 and occludin were significantly downregulated by treatment with IL-1β and TNF-α (Fig. 4). Addition of IL-34 reversed the expression levels of these TJ proteins (Fig. 4), whereas treatment with IL-34 alone did not alter the expression levels of TJ proteins in untreated MBEC4 cells (data not shown). Addition of GW2580 canceled the effect of IL-34 on the expression of claudin-5 and occludin (Fig. 4). These data suggest that IL-34 rescues pro-inflammatory cytokine–induced BBB disruption via upregulating TJ proteins such as claudin-5 and occludin in BBB endothelial cells.
Figure 4. IL-34 upregulates tight junction proteins in MBEC4 cells.
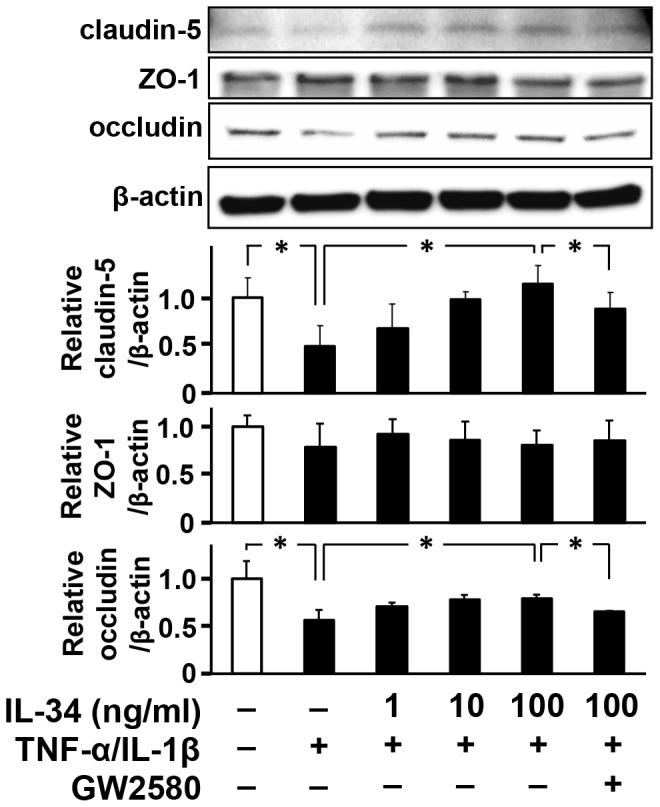
MBEC4 cells were incubated with TNF-α (20 ng/ml) and IL-1β (20 ng/ml) in the presence of IL-34 (0–100 ng/ml) and GW2580 (1 µmol/L) for 24 h. Upper, representative images of Western blots for tight junction proteins. Bottom, quantified expression levels of tight junction proteins relative to those in untreated cells. Values are means ± SEM (n = 5). *, p<0.05.
Discussion
IL-34 is widely expressed in a variety of tissues including brain. Because IL-34 shares the same receptor with CSF-1/M-CSF, it has similar functions on monocyte lineage cells such as induction of proliferation and differentiation of macrophages, Langerhans cells, osteoclasts, and microglia [3]–[5], [27], [28]. In the CNS, IL-34 is mainly released by neurons, especially when they are damaged [6]. Like CSF-1/M-CSF, IL-34 induces microglial proliferation. In addition, IL-34 enhances microglial neuroprotective functions by inducing anti-oxidant enzyme heme oxigenase-1 (HO-1), and amyloid degrading enzyme insulin degrading enzyme (IDE). Moreover, we also found that IL-34 induces microglial production of TGF-β which negatively regulates microglial activation [29]. TGF-β dose-dependently suppressed microglial proliferation by IL-34 but attenuated oligomeric amyloid β–mediated neurotoxicity [29]. The neuroprotective functions of IL-34 was partially suppressed by blockade of TGF-β receptor signaling, suggesting that neuroprotective effect of IL-34 was in part mediated by microglial TGF-β production in response to IL-34. Thus, IL-34 released from damaged neurons acts as a “Help-me” signal which induces microglial neuroprotective effects with subsiding microglial activation (Fig. 5). The expression of CSF1R is reportedly high during early postnatal development, and is very low in adult brain [30]. IL-34 exhibited a broader regional expression than CSF-1/M-CSF, mostly without overlap, suggesting important role of IL-34–CSF1R signaling in regional neurogenesis. A previous study reported that CSF1R expression is increased in microglia of AD brains and microglia overexpressing CSF1R are neuroprotective [31]. Therefore, IL-34 produced by neurons [6] as well as CSF-1/M-CSF produced by astrocytes [32] may be involved in the development of neurodegenerative disesases such as AD via microglial CSF1R signaling.
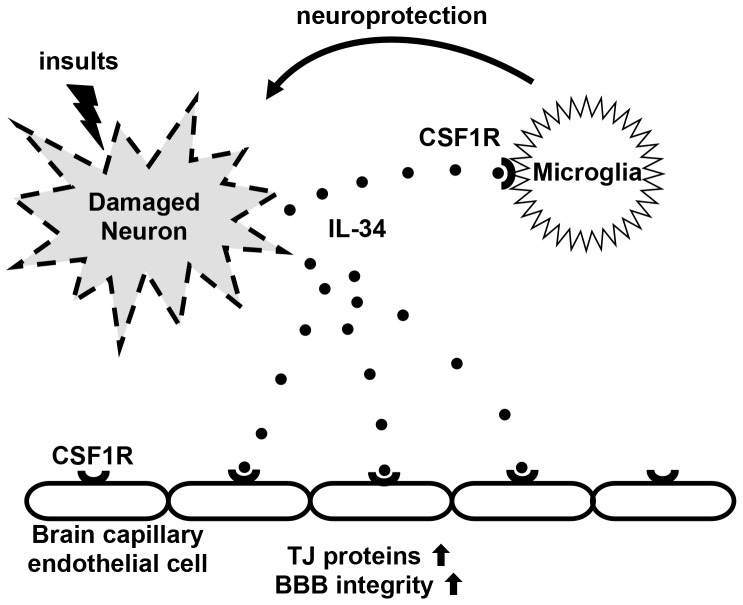
Figure 5. Model of the roles of IL-34 in the CNS.
Damaged neurons secrete IL-34 as a “Help-me” signal. IL-34 binds its receptor CSF1R which is mainly expressed in microglia and BBB endothelial cells. CSF1R signaling enhances neuroprotection in microglia and restores BBB disruption by upregulating TJ proteins in capillary endothelial cells.
In this study, we have shown that BBB endothelial cell expresses CSF1R and is a novel target of IL-34. BBB disruption has been implicated as a pathogenesis of various neurological disorders including MS and AD. A recent study showed that amyloid β suppressed expression of TJ protein ZO-1 in BBB endothelial cells via receptor for advanced glycation end products (RAGE) and claimed that amyloid β-RAGE interaction may be a potential molecular pathway in breakage of BBB integrity [33]. In addition, pro-inflammatory cytokines such as IL-1β, TNF-α, IFN-γ, and IL-17 have been considered as the candidates to increase BBB leakage [14]–[19]. Our findings revealed a novel function of IL-34–CSF1R signaling on the maintenance of BBB integrity via upregulating major TJ proteins claudin-5 and occludin in capillary endothelial cells (Fig. 5). A major downstream target of CSF1R signaling is cAMP responsive element-binding protein (CREB), which modulates the transcription of TJ proteins [34], [35]. Taken together, IL-34 released from damaged neurons may functions as a “Help-me” signal toward restoration of CNS homeostasis via microglia and BBB endothelial cells (Fig. 5). Our study clarified the presence of neuronal regulation of BBB functions via IL-34–CSF1R signaling. IL-34–CSF1R pathway may be novel therapeutic target for neuroinflammatory and neurodegenerative disorders such as MS and AD.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This work was supported in part by a Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan; a grant from the Advanced Research for Medical Products Mining Program of the National Institute of Biomedical Innovation (NIBIO) of Japan; and grants from the Ministry of Health, Labour and Welfare of Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lin H, Lee E, Hestir K, Leo C, Huang M, et al. (2008) Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science 320:807–811. [DOI] [PubMed] [Google Scholar]
- 2.Chihara T, Suzu S, Hassan R, Chutiwitoonchai N, Hiyoshi M, et al. (2010) IL-34 and M-CSF share the receptor Fms but are not identical in biological activity and signal activation. Cell Death Differ 17:1917–1927. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, et al. (2012) IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol 13:753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, et al. (2012) Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity 37:1050–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamichi Y, Mizoguchi T, Arai A, Kobayashi Y, Sato M, et al. (2012) Spleen serves as a reservoir of osteoclast precursors through vitamin D-induced IL-34 expression in osteopetrotic op/op mice. Proc Natl Acad Sci U S A 109:10006–10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizuno T, Doi Y, Mizoguchi H, Jin S, Noda M, et al. (2011) Interleukin-34 selectively enhances the neuroprotective effects of microglia to attenuate oligomeric amyloid-β neurotoxicity. Am J Pathol 179:2016–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo J, Elwood F, Britschgi M, Villeda S, Zhang H, et al. (2013) Colony-stimulating factor 1 receptor (CSF1R) signaling in injured neurons facilitates protection and survival. J Exp Med 210:157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Risau W, Engelhardt B, Wekerle H (1990) Immune function of the blood-brain barrier: incomplete presentation of protein (auto-)antigens by rat brain microvascular endothelium in vitro. J Cell Biol 110:1757–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbott NJ, Romero IA (1996) Transporting therapeutics across the blood-brain barrier. Mol Med Today 2:106–113. [DOI] [PubMed] [Google Scholar]
- 10.Abbott NJ (2002) Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat 200:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Begley DJ (2003) Understanding and circumventing the blood-brain barrier. Acta Paediatr Suppl 92 83–91. [DOI] [PubMed]
- 12.Hawkins BT, Davis TP (2005) The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57:173–185. [DOI] [PubMed] [Google Scholar]
- 13.Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, et al. (2008) ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci 11:420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afonso PV, Ozden S, Prevost MC, Schmitt C, Seilhean D, et al. (2007) Human blood-brain barrier disruption by retroviral-infected lymphocytes: role of myosin light chain kinase in endothelial tight-junction disorganization. J Immunol 179:2576–2583. [DOI] [PubMed] [Google Scholar]
- 15.Fabry Z, Topham DJ, Fee D, Herlein J, Carlino JA, et al. (1995) TGF-beta 2 decreases migration of lymphocytes in vitro and homing of cells into the central nervous system in vivo. J Immunol 155:325–332. [PubMed] [Google Scholar]
- 16.Förster C, Burek M, Romero IA, Weksler B, Couraud PO, et al. (2008) Differential effects of hydrocortisone and TNFalpha on tight junction proteins in an in vitro model of the human blood-brain barrier. J Physiol 586:1937–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, et al. (2007) Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med 13:1173–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minagar A, Alexander JS (2003) Blood-brain barrier disruption in multiple sclerosis. Mult Scler 9:540–549. [DOI] [PubMed] [Google Scholar]
- 19.Minagar A, Long A, Ma T, Jackson T, Kelley R, et al. (2003) Interferon (IFN)-ß1a and IFN-ß1b Block IFN-?-Induced Disintegration of Endothelial Junction Integrity and Barrier. Endothelium 10:299–307. [DOI] [PubMed] [Google Scholar]
- 20.Mizuno T, Zhang G, Takeuchi H, Kawanokuchi J, Wang J, et al. (2008) Interferon-gamma directly induces neurotoxicity through a neuron specific, calcium-permeable complex of IFN-gamma receptor and AMPA GluR1 receptor. FASEB J 22:1797–1806. [DOI] [PubMed] [Google Scholar]
- 21.Tatsuta T, Naito M, Oh-hara T, Sugawara I, Tsuruo T (1992) Functional involvement of P-glycoprotein in blood-brain barrier. J Biol Chem 267:20383–20391. [PubMed] [Google Scholar]
- 22.Sonobe Y, Takeuchi H, Kataoka K, Li H, Jin S, et al. (2009) Interleukin-25 expressed by brain capillary endothelial cells maintains blood-brain barrier function in a protein kinase Cepsilon-dependent manner. J Biol Chem 284:31834–31842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Endong L, Shijie J, Sonobe Y, Di M, Hua L, et al. (2011) The gap-junction inhibitor carbenoxolone suppresses the differentiation of Th17 cells through inhibition of IL-23 expression in antigen presenting cells. J Neuroimmunol 240–241:58–64. [DOI] [PubMed] [Google Scholar]
- 24.Conejo-Garcia JR, Buckanovich RJ, Benencia F, Courreges MC, Rubin SC, et al. (2005) Vascular leukocytes contribute to tumor vascularization. Blood 105:679–681. [DOI] [PubMed] [Google Scholar]
- 25.Blamire AM, Anthony DC, Rajagopalan B, Sibson NR, Perry VH, et al. (2000) Interleukin-1beta -induced changes in blood-brain barrier permeability, apparent diffusion coefficient, and cerebral blood volume in the rat brain: a magnetic resonance study. J Neurosci 20:8153–8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ransohoff RM, Brown MA (2012) Innate immunity in the central nervous system. J Clin Invest 122:1164–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boström EA, Lundberg P (2013) The Newly Discovered Cytokine IL-34 Is Expressed in Gingival Fibroblasts, Shows Enhanced Expression by Pro-Inflammatory Cytokines, and Stimulates Osteoclast Differentiation. PloS one 8:e81665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Colonna M (2014) Interkeukin-34, a cytokine crucial for the differentiation and maintenance of tissue resident macrophages and Langerhans cells. European journal of immunology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma D, Doi Y, Jin S, Li E, Sonobe Y, et al. (2012) TGF-β induced by interleukin-34-stimulated microglia regulates microglial proliferation and attenuates oligomeric amyloid β neurotoxicity. Neuroscience letters 529:86–91. [DOI] [PubMed] [Google Scholar]
- 30.Nandi S, Gokhan S, Dai X-M, Wei S, Enikolopov G, et al. (2012) The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Developmental biology 367:100–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitrasinovic OM, Grattan A, Robinson CC, Lapustea NB, Poon C, et al. (2005) Microglia overexpressing the macrophage colony-stimulating factor receptor are neuroprotective in a microglial-hippocampal organotypic coculture system. The Journal of neuroscience 25:4442–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao C, Guilbert L, Fedoroff S (1990) Production of colony-stimulating factor-1 (CSF-1) by mouse astroglia in vitro. Journal of neuroscience research 27:314–323. [DOI] [PubMed] [Google Scholar]
- 33.Kook S-Y, Hong HS, Moon M, Mook-Jung I (2013) Disruption of blood-brain barrier in Alzheimer disease pathogenesis. Tissue Barriers 1:e23993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong Y, Zhang B, Eum SY, Toborek M (2012) HIV-1 Tat triggers nuclear localization of ZO-1 via Rho signaling and cAMP response element-binding protein activation. J Neurosci 32:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tureci O, Koslowski M, Helftenbein G, Castle J, Rohde C, et al. (2011) Claudin-18 gene structure, regulation, and expression is evolutionary conserved in mammals. Gene 481:83–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.