Very Early Administration of Progesterone for Acute Traumatic Brain Injury (original) (raw)
. Author manuscript; available in PMC: 2015 Jun 25.
Published in final edited form as: N Engl J Med. 2014 Dec 10;371(26):2457–2466. doi: 10.1056/NEJMoa1404304
Abstract
BACKGROUND
Traumatic brain injury (TBI) is a major cause of death and disability worldwide. Progesterone has been shown to improve neurologic outcome in multiple experimental models and two early-phase trials involving patients with TBI.
METHODS
We conducted a double-blind, multicenter clinical trial in which patients with severe, moderate-to-severe, or moderate acute TBI (Glasgow Coma Scale score of 4 to 12, on a scale from 3 to 15, with lower scores indicating a lower level of consciousness) were randomly assigned to intravenous progesterone or placebo, with the study treatment initiated within 4 hours after injury and administered for a total of 96 hours. Efficacy was defined as an increase of 10 percentage points in the proportion of patients with a favorable outcome, as determined with the use of the stratified dichotomy of the Extended Glasgow Outcome Scale score at 6 months after injury. Secondary outcomes included mortality and the Disability Rating Scale score.
RESULTS
A total of 882 of the planned sample of 1140 patients underwent randomization before the trial was stopped for futility with respect to the primary outcome. The study groups were similar with regard to baseline characteristics; the median age of the patients was 35 years, 73.7% were men, 15.2% were black, and the mean Injury Severity Score was 24.4 (on a scale from 0 to 75, with higher scores indicating greater severity). The most frequent mechanism of injury was a motor vehicle accident. There was no significant difference between the progesterone group and the placebo group in the proportion of patients with a favorable outcome (relative benefit of progesterone, 0.95; 95% confidence interval [CI], 0.85 to 1.06; P = 0.35). Phlebitis or thrombophlebitis was more frequent in the progesterone group than in the placebo group (relative risk, 3.03; CI, 1.96 to 4.66). There were no significant differences in the other prespecified safety outcomes.
CONCLUSIONS
This clinical trial did not show a benefit of progesterone over placebo in the improvement of outcomes in patients with acute TBI. (Funded by the National Institute of Neurological Disorders and Stroke and others; PROTECT III ClinicalTrials.gov number, NCT00822900.)
More than 2.4 million emergency department visits, hospitalizations, or deaths are related to traumatic brain injury (TBI) annually, and approximately 5.3 million Americans are living with disability from TBI. The aggregate annual cost of TBI in the United States now approaches $76.5 billion.1 Survivors of severe TBI typically require 5 to 10 years of intensive therapy and are often left with substantial disability.2 Despite decades of research, no pharmacologic agent has been shown to improve outcomes after TBI.
Progesterone is a potent neurosteroid synthesized in the central nervous system. Preclinical studies in laboratory animals indicated that the early administration of progesterone after experimental TBI reduced cerebral edema, neuronal loss, and behavioral deficits.3,4 Enthusiasm for progesterone as a treatment for TBI was further stimulated by two single-center clinical trials showing decreased mortality and improved functional outcomes with progesterone as compared with placebo.5,6 We performed a large, controlled, multicenter trial to determine the efficacy of early administration of progesterone for the treatment of severe, moderate-to-severe, or moderate TBI.
METHODS
STUDY DESIGN
The Progesterone for Traumatic Brain Injury, Experimental Clinical Treatment (PROTECT III) trial was a phase 3, randomized, double-blind, placebo-controlled clinical trial designed to determine the efficacy of early intravenous administration of progesterone versus placebo for treating patients with acute nonpenetrating TBI caused by a blunt mechanism. The trial was funded by the National Institute of Neurological Disorders and Stroke (NINDS) and was conducted through the NINDS-funded Neurological Emergencies Treatment Trials (NETT) network. The NETT network is organized into 22 academic medical centers that operate as clinical hubs, each of which has one or more study sites. The investigators were responsible for all the elements of the trial, including the design, data collection, analysis, and interpretation. All the authors wrote the manuscript and vouch for the data and analysis. The trial was conducted under Investigational New Drug application 104,188 with the Food and Drug Administration (FDA). The study was conducted in accordance with the protocol, available with the full text of this article at NEJM.org.
The PROTECT III trial was conducted at 49 trauma centers in the United States. Rigorous training and certification of the investigators, coordinators, and outcomes assessors were performed initially and updated throughout the study. In addition to strict compliance with the study protocol, critical elements of TBI management were standardized across the study sites to minimize the effects of practice variability and secular trends. Adherence to both the study protocol and the TBI management guidelines of the trial were centrally monitored daily. Failures of adherence to the study protocol were identified as protocol deviations, and failures of adherence to standardized care guidelines were defined as clinical transgressions. Both required a prompt response and corrective action.
The trial met the exception from informed-consent requirements for emergency research under FDA code of regulations 21 CFR 50.24.7 As specified by the federal regulations, the institutional review board at each site reviewed and approved local community consultation and public disclosure activities. When a legally authorized representative was available, written informed consent was obtained before enrollment of the patient. For patients enrolled under the exemption from informed consent, patients or their legally authorized representatives were notified about enrollment by the study team as soon as possible and were asked to provide written informed consent to continue in the study. Safety oversight was provided by an NINDS-appointed data and safety monitoring board and two independent medical safety monitors.
STUDY PATIENTS
Eligible patients were adults who had severe, moderate-to-severe, or moderate TBI due to a blunt mechanism, with a Glasgow Coma Scale8 (GCS) score of 4 to 12 (on a scale of 3 to 15, with lower scores indicating a lower level of consciousness). Patients were enrolled if the study treatment could be initiated within 4 hours after injury.
Patients were excluded if, before enrollment, the treatment team determined clinically that the injury sustained was nonsurvivable; the patient had bilateral dilated, unresponsive pupils; cardio-pulmonary resuscitation was performed; or the patient had physiological findings of hypoxemia, hypotension, spinal cord injury, or status epilepticus. Additional exclusion criteria were pregnancy, status as a prisoner or ward of the state, severe intoxication (ethanol level, >249 mg per deciliter), and a known history of reproductive cancer, allergy to progesterone or a fat-emulsion vehicle, or a blood-clotting disorder. Patients with active myocardial infarction, ischemic stroke, pulmonary embolism, or deep-vein thrombosis were also excluded. In addition, patients were excluded if they were wearing an opt-out bracelet or were listed in a registry of persons preemptively requesting not to participate in this trial.
STUDY INTERVENTION
Immediately after enrollment, patients were randomly assigned to receive an infusion containing either progesterone or placebo. Randomization was performed with the use of a combination of minimization and biased-coin algorithms to avoid imbalances in initial injury severity, sex, age, or enrollment site.
Study-drug kits containing four vials of progesterone in ethanol (active treatment) or ethanol alone (placebo) were prepared by the Emory Investigational Drug Service. Drug kits and their contents were identical in appearance, and study assignments remained concealed from all site pharmacists and study teams. Site pharmacists prepared the coded kit assigned by the randomization algorithm by mixing a weight-based dose (0.05 mg of progesterone per kilogram of body weight per milliliter of infusate) from the provided vials and a 250-ml bag of fat-emulsion vehicle (Intralipid 20%, Fresenius Kabi) every 24 hours. The study treatment was initiated within 4 hours after injury and consisted of a 1-hour loading dose, 71 hours of maintenance infusion, and a 24-hour infusion taper. The study drug was infused continuously through a dedicated intravenous catheter at a dose of 14.3 ml per hour for 1 hour and then at 10 ml per hour for 71 hours; the dose was then tapered by 2.5 ml per hour every 8 hours, for a total treatment duration of 96 hours.
Local study teams followed the patients closely. Data on serious adverse events were collected throughout the duration of the study (6 months), and data on all adverse events were collected during the first week. Data on clinical transgressions were collected and reported daily during hospitalization.
STUDY OUTCOMES
The primary outcome was functional recovery as determined with the use of the Extended Glasgow Outcome Scale9,10 (GOS-E) at 6 months (±30 days) after randomization. A GOS-E score of 1 indicates death, 2 indicates a vegetative state, 3 or 4 indicates severe disability, 5 or 6 indicates moderate disability, and 7 or 8 indicates good recovery. Consistent scoring was ensured by means of rigorous training and quality assessment.
A favorable outcome was defined with the use of a stratified dichotomy of the GOS-E scores in which the definition of favorable depended on the severity of the initial injury. The index GCS score, the highest reliable GCS score documented before randomization, determined the initial injury severity. (If the patient was intubated, the index GCS motor score was used to assess severity; scores on the motor component of the GCS range from 1 to 6, with lower scores indicating a lower level of consciousness.) Patients with a less severe initial injury had to have a better recovery than those with a more severe injury in order to have a favorable outcome. Patients with a severe initial injury (an index GCS score of 4 to 5 or, if the patient was intubated, an index GCS motor score of 2 to 3) were considered to have a favorable outcome if the 6-month GOS-E score was 3 or higher. Patients with a moderate-to-severe initial injury (an index GCS score of 6 to 8 or, if the patient was intubated, an index GCS motor score of 4 to 5) were considered to have a favorable outcome if the 6-month GOS-E score was 5 or higher, and those with a moderate initial injury (an index GCS score of 9 to 12) were considered to have a favorable outcome if the 6-month GOS-E score was 7 or higher.
Secondary outcome measures included mortality, the Disability Rating Scale score,11 the rates of nine prespecified adverse events that were considered to be potentially associated with treatment, and the rates of all reported adverse events and serious adverse events. Data on cognitive, psychological, and neurologic outcomes were also collected but are not reported here.
STATISTICAL ANALYSIS
The primary objective was to determine whether progesterone was associated with an absolute increase of 10 percentage points, as compared with placebo, in the proportion of patients with a favorable outcome. We estimated that a total sample of 1140 patients was required in order for the study to detect that effect with 85% power, assuming that 50% of the patients in the placebo group would have a favorable outcome and assuming a two-sided type I error probability of 0.05. This calculation included inflation for a 10% non-adherence rate (owing to withdrawal of consent, loss to follow-up, or treatment crossover) and two equally spaced interim analyses for efficacy and futility with the use of O'Brien and Fleming stopping boundaries.12
After randomization, patients were included in the primary analysis under the intention-to-treat principle. The primary efficacy hypothesis was tested with the use of a generalized linear model relating the probability of a favorable outcome to the study treatment, with adjustment for index GCS score strata, sex, and age. Standard multiple-imputation methods13 were used to impute outcomes for patients without the primary outcome or with the primary outcome obtained outside the specified time window. A complete case sensitivity analysis was also performed. Prespecified covariates were evaluated for an interaction effect with the study treatment. Subgroup analyses were performed for sex, race, ethnic group, and index GCS score strata, regardless of interaction effect. Other subgroups were considered only if the interaction was statistically significant (alpha level of 0.20), was clinically significant, and involved a sufficiently large sample (>100 patients).
RESULTS
ENROLLMENT AND CHARACTERISTICS OF THE PATIENTS
Of 17,681 persons screened, 882 patients underwent randomization between April 5, 2010, and October 30, 2013 (Fig. S1 in the Supplementary Appendix, available at NEJM.org). The study groups were well balanced with respect to demographic and baseline clinical characteristics (Table 1). Initial injury severity was similar in the two groups (as determined with the use of the index GCS score, the total Injury Severity Score, the head component score of the Abbreviated Injury Scale, and the Rotterdam class i fication on the basis of computed tomographic results14). Most patients (53.5%) had moderate-to-severe injury (index GCS score, 6 to 8). The study treatment was initiated an average of 218.1 minutes after injury.
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Progesterone (N = 442) | Placebo (N = 440) | Overall (N = 882) |
|---|---|---|---|
| Age — yr | |||
| Median | 36 | 34 | 35 |
| Range | 17–94 | 17–93 | 17–94 |
| Male sex — no. (%) | 324 (73.3) | 326 (74.1) | 650 (73.7) |
| Black race — no. (%)† | 70 (15.8) | 64 (14.5) | 134 (15.2) |
| Hispanic ethnic group — no. (%)† | 61 (13.8) | 64 (14.5) | 125 (14.2) |
| Cause of injury — no. (%) | |||
| Motor vehicle accident | 159 (36.0) | 163 (37.0) | 322 (36.5) |
| Motorcycle, scooter, or ATV accident | 78 (17.6) | 91 (20.7) | 169 (19.2) |
| Pedestrian struck by moving vehicle | 60 (13.6) | 55 (12.5) | 115 (13.0) |
| Other‡ | 145 (32.8) | 131 (29.8) | 276 (31.3) |
| Index GCS score at randomization — no. (%)§ | |||
| Moderate | 129 (29.2) | 125 (28.4) | 254 (28.8) |
| Moderate to severe | 234 (52.9) | 238 (54.1) | 472 (53.5) |
| Severe | 79 (17.9) | 77 (17.5) | 156 (17.7) |
| Injury Severity Score¶ | 24.7±11.7 | 24.1±11.1 | 24.4±11.4 |
| AIS head score indicating no injury — no. (%) | 12 (2.7) | 19 (4.3) | 31 (3.5) |
| Rotterdam CT classification — no. (%)∥ | |||
| 1 | 8 (1.8) | 7 (1.6) | 15 (1.7) |
| 2 | 155 (35.1) | 157 (35.7) | 312 (35.4) |
| 3 | 200 (45.2) | 193 (43.9) | 393 (44.6) |
| 4 | 41 (9.3) | 39 (8.9) | 80 (9.1) |
| 5 | 31 (7.0) | 37 (8.4) | 68 (7.7) |
| 6 | 7 (1.6) | 6 (1.4) | 13 (1.5) |
| Data missing | 0 | 1 (0.2) | 1 (0.1) |
| Minutes from injury | |||
| To arrival at emergency department | 53.4±30.3 | 54.2±27.2 | 53.8±28.8 |
| To randomization | 173.2±37.5 | 173.0±37.1 | 173.1±37.3 |
| To study-treatment initiation | 219.9±39.4 | 216.4±34.7 | 218.1±37.2 |
PRIMARY OUTCOME
After the second interim analysis, the trial was stopped because of futility. For the primary hypothesis comparing progesterone with placebo, favorable outcomes occurred in 51.0% of patients assigned to progesterone and in 55.5% of those assigned to placebo (Table 2); the model estimated a relative benefit of 0.95 (95% confidence interval [CI], 0.85 to 1.06; P = 0.35), with a relative benefit of less than 1.00 indicating fewer favorable outcomes in the progesterone group than in the placebo group. Additional adjustment for clinical hub yielded a similar result. The results of the complete case analysis were similar.
Table 2.
Outcomes at 6 Months.*
| Outcome | Progesterone (N = 442) | Placebo (N = 440) | Overall (N = 882) | Unadjusted Difference (95% CI) percentage points |
|---|---|---|---|---|
| Primary outcome — no. (%) | ||||
| Favorable outcome | 213 (48.2) | 232 (52.7) | 445 (50.5) | –4.5 (–11.1 to 2.1) |
| Missing data | 28 (6.3) | 24 (5.5) | 52 (5.9) | — |
| According to initial injury severity — no./total no. (%) | ||||
| Moderate injury | ||||
| Favorable | 35/129 (27.1) | 45/125 (36.0) | 80/254 (31.5) | –8.9 (–20.3 to 2.5) |
| Missing data | 10/129 (7.8) | 11/125 (8.8) | 21/254 (8.3) | — |
| Moderate-to-severe injury | ||||
| Favorable | 133/234 (56.8) | 133/238 (55.9) | 266/472 (56.4) | 1.0 (–8.0 to 9.9) |
| Missing data | 13/234 (5.6) | 9/238 (3.8) | 22/472 (4.7) | — |
| Severe injury | ||||
| Favorable | 45/79 (57.0) | 54/77 (70.1) | 99/156 (63.5) | –13.2 (–28.1 to 1.8) |
| Missing data | 5/79 (6.3) | 4/77 (5.2) | 9/156 (5.8) | — |
| Death — no. (%) | 83 (18.8) | 69 (15.7) | 152 (17.2) | — |
| Cause of death — no./total no. (%) | — | |||
| Neurologic | 53/83 (63.9) | 49/69 (71.0) | 102/152 (67.1) | — |
| Not neurologic | 28/83 (33.7) | 20/69 (29.0) | 48/152 (31.6) | — |
| Unknown | 2/83 (2.4) | 0 | 2/152 (1.3) | |
| According to initial injury severity — no./total no. (%) | — | |||
| Moderate | 19/129 (14.7) | 14/125 (11.2) | 33/254 (13.0) | — |
| Moderate to severe | 37/234 (15.8) | 39/238 (16.4) | 76/472 (16.1) | — |
| Severe | 27/79 (34.2) | 16/77 (20.8) | 43/156 (27.6) | — |
| Disability Rating Scale score† | 2.9±4.6 | 3.3±5.1 | 3.1±4.9 | — |
A secondary analysis of the GOS-E score with the use of a fixed dichotomy (in which a score ≥5 was considered to indicate a favorable outcome, regardless of the severity of the initial injury) was concordant (relative benefit, 0.99; 95% CI, 0.91 to 1.08). An additional secondary analysis of the target population, excluding 332 patients who had an eligibility deviation or did not receive a complete course of the study drug, was also concordant with the primary analysis (relative benefit, 0.96; 95% CI, 0.83 to 1.10).
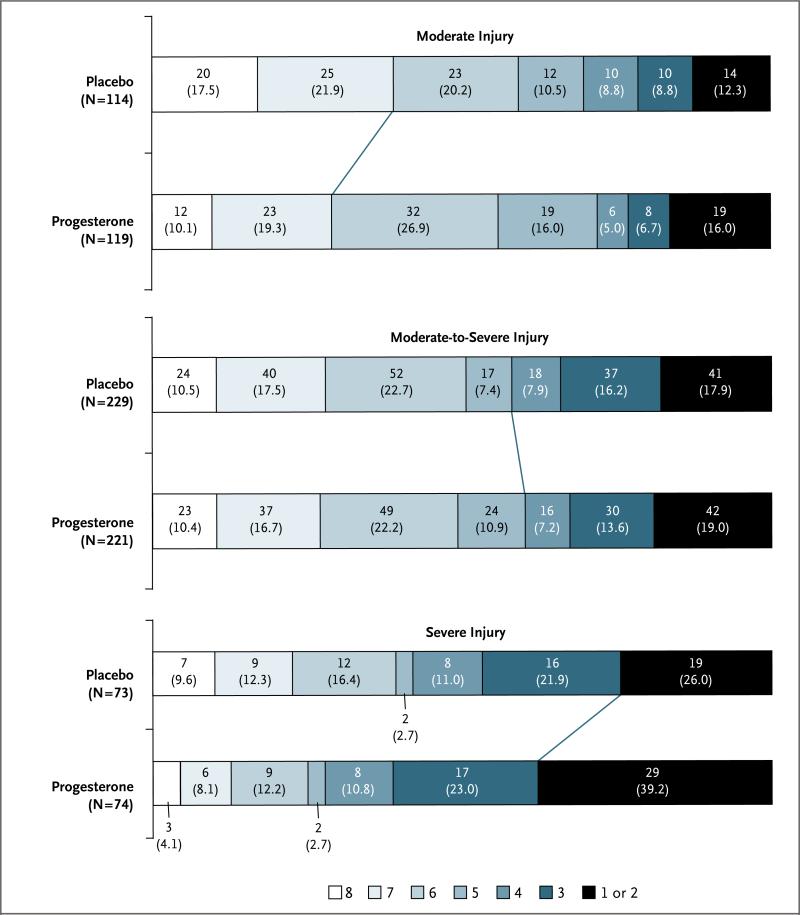
The distribution of the GOS-E score stratified according to initial injury severity, and the different criteria for a favorable outcome in each stratum, are shown in Figure 1. Overall, approximately half the patients had favorable outcomes as determined according to the stratified dichotomy, with a lower proportion meeting the criteria in the moderate-injury group as compared with the severe-injury group (relative benefit, 0.53; 95% CI, 0.43 to 0.64).
Figure 1. Distribution of Extended Glasgow Outcome Scale (GOS-E) Scores, Stratified According to Initial Injury Severity.
The GOS-E is an ordinal scale on which each increment represents a better quality of recovery. A GOS-E score of 1 indicates death, 2 a vegetative state, 3 or 4 severe disability, 5 or 6 moderate disability, and 7 or 8 good recovery. Each cell corresponds to a score on the GOS-E; the width of the cell indicates the proportion of patients with equivalent scores, and the number and percentage of patients are shown within the cell. The diagonal line between the two study groups indicates the favorable-outcome dichotomization in each severity stratum.
SAFETY OUTCOMES
The 6-month mortality in the study population was 17.2%, ranging from 13.0% in the moderate-injury group to 27.6% in the severe-injury group. There was no significant difference in mortality between the progesterone group and the placebo group (hazard ratio for death, 1.19; 95% CI, 0.86 to 1.63). The cause of death was neurologic in 67.1% of the patients who died.
Progesterone was associated with an acceptable safety profile. Eight prospectively defined adverse events that were deemed to be potentially associated with the study drug were similar in frequency in the two groups (Table 3). However, phlebitis or thrombophlebitis was significantly more frequent in the progesterone group than in the placebo group (relative risk, 3.03; 95% CI, 1.96 to 4.66). Episodes of phlebitis were frequently categorized as not serious and were self-limited. The rates of other serious and nonserious adverse events were also similar in the two study groups (Table S2 in the Supplementary Appendix).
Table 3.
Adverse Events Potentially Associated with the Study Drug.
| Event | Progesterone (N = 442) | Placebo (N = 440) | Overall (N = 882) | Relative Risk (95% CI) |
|---|---|---|---|---|
| number of patients (percent) | ||||
| Myocardial infarction | 5 (1.1) | 5 (1.1) | 10 (1.1) | 1.00 (0.29–3.41) |
| Pulmonary embolism | 10 (2.3) | 13 (3.0) | 23 (2.6) | 0.77 (0.34–1.73) |
| Acute ischemic stroke | 6 (1.4) | 13 (3.0) | 19 (2.2) | 0.46 (0.18–1.20) |
| Deep venous thrombosis | 50 (11.3) | 40 (9.1) | 90 (10.2) | 1.24 (0.84–1.85) |
| Unexplained increased liver-enzyme level | 18 (4.1) | 14 (3.2) | 32 (3.6) | 1.28 (0.64–2.54) |
| Sepsis | 9 (2.0) | 9 (2.0) | 18 (2.0) | 1.00 (0.40–2.48) |
| Pneumonia | 142 (32.1) | 140 (31.8) | 282 (32.0) | 1.01 (0.83–1.22) |
| Central nervous system infection | 5 (1.1) | 3 (0.7) | 8 (0.9) | 1.66 (0.40–6.90) |
| Phlebitis or thrombophlebitis | 76 (17.2) | 25 (5.7) | 101 (11.5) | 3.03 (1.96–4.66) |
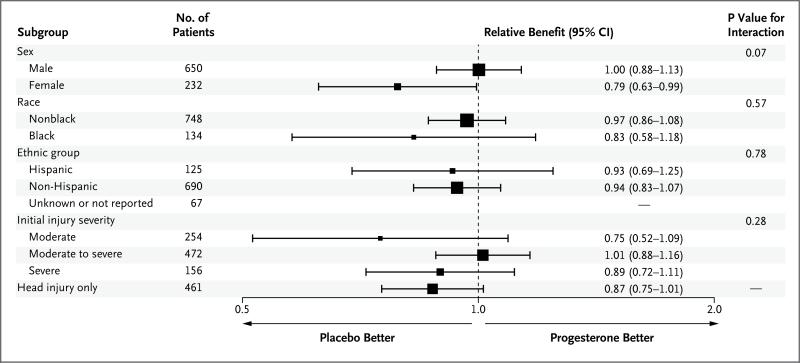
SUBGROUP ANALYSES
The results of the subgroup analyses are shown in the forest plot in Figure 2. Interactions with the study treatment were suggested only for sex and for isolated head injury.
Figure 2. Adjusted Relative Benefit in Predefined Subgroups, as Assessed According to the Stratified Dichotomy of the GOS-E Score.
Models were adjusted for initial injury severity, sex, and age, as specified for the primary analysis. The significance of the interaction effect between study assignment and the corresponding subgroup is provided. Other prespecified subgroups that were considered according to the statistical analysis plan were based on the Rotterdam computed tomographic classification, score on the Injury Severity Scale, score on the Abbreviated Injury Scale head-injury component, time from injury to infusion, pupillary response, and medical histories (neurologic, cardiovascular, pulmonary, gastrointestinal, metabolic, renal, reproductive, psychiatric, social, hematologic, oncologic, and related to the eyes, ears, nose, and throat). The statistical analysis plan limited the reporting of these additional subgroups to those with both clinical and statistical significance.
DISCUSSION
Despite extensive preclinical data and two promising single-center trials,4-6 progesterone was not associated with any benefit over placebo, as measured by the GOS-E score at 6 months, in this large, multicenter clinical trial. The groups were well balanced for injury severity, and both the intention-to-treat analysis and the a priori planned analysis of the target population were congruent in showing no treatment effect.
The PROTECT III trial joins a growing list of negative or inconclusive trials in the arduous search for a treatment for TBI. To date, more than 30 clinical trials have investigated various compounds for the treatment of acute TBI, yet no treatment has succeeded at the confirmatory trial stage.15 Many reasons for the disappointing record of translating promising agents from the laboratory to the clinic have been postulated, including limited preclinical development work, poor drug penetration into the brain, delayed initiation of treatment, heterogeneity of injuries, variability in routine patient care across sites, and insensitive outcome measures.16
In the design of the PROTECT III trial, we attempted to mitigate many of the barriers to translation identified in prior trials. Preclinical data supporting the treatment strategy were robust and met all of the Stroke Therapy Academic Industry Roundtable recommendations for moving from preclinical to clinical studies, with the exception of testing in a nonhuman primate.17 Progesterone has been shown to penetrate the brain rapidly in high concentrations.18 The window for treatment in our study was limited to 4 hours after injury, with a 2-hour target (average injury-to-enrollment time, 2.9 hours; average injury-to-treatment time, 3.6 hours). The early administration of therapy is a difficult task for a trial involving patients with acute TBI, and one that required exception from informed consent. Treatment variability across sites was reduced with a standardized management protocol, multi-disciplinary commitment at each site, meticulous monitoring of patients in real time, and immediate feedback to sites about clinical transgressions and noncompliance. To address the concern that the GOS was insufficiently sensitive, we used the higher-fidelity extended scale (GOS-E) as our primary outcome measure and designed a stratified dichotomy analysis rather than using a single threshold for all patients regardless of injury severity.
Despite these design strategies and extensive efforts, the trial did not confirm the efficacy of progesterone in patients with acute TBI. It is possible that the heterogeneity of the injury, confounding preexisting conditions, and characteristics of individual patients (e.g., resilience), which can be well controlled in animal models, play too large a role to overcome in human disease. Approaches are needed to reduce heterogeneity, but they come at the cost of more homogeneous pathological findings and decreased generaliz-ability of the results. Success at translating from bench to bedside may require new paradigms, including innovative clinical-trial methods (e.g., adaptive designs and profiling of patients who have a response) in early-phase clinical trials to identify effective drug doses and timing (e.g., prehospital administration), the use of targeted outcomes based on the mechanism of injury, and rigorous preclinical multicenter trials in animals that better simulate subsequent human trials and make more accurate predictions regarding results.
TBI is a leading cause of death and disability worldwide. Despite promising preclinical data and supporting preliminary evidence, progesterone did not improve the outcome of patients with acute TBI in the PROTECT III trial.
Supplementary Material
Supplement1
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily represent the official views of the National Institutes of Health or other supporting entities.
Supported by grants from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (NS062778, 5U10NS059032, and U01NS056975) and the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR000454) and by the Emory Emergency Neurosciences Laboratory in the Department of Emergency Medicine, Emory School of Medicine, and Grady Memorial Hospital.
Dr. Wright reports receiving royalties from a patent related to progesterone for the treatment of traumatic brain injury (U.S. patents 7,473,687, 7,915,244, and 8,455,468), which is licensed to BHR Pharma. No other potential conflict of interest relevant to this article was reported.
We thank the staff of the Emory University Investigational Drug Service for drug compounding and preparation of the drug kits; the members of the data and safety monitoring board (T. Bleck [chair], G. Anderson, J. Collins, J. Chamberlain, J. Saver, and L. Gutmann); the independent data safety monitors (C. Robertson and D. Gress); the study coordinators, research assistants, and local site staff; R. Conwit and P. Gilbert (National Institutes of Health); and the patients who participated in this study and the family members who entrusted us with their care.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.CDC grand rounds: reducing severe traumatic brain injury in the United States. MMWR Morb Mortal Wkly Rep. 2013;62:549–52. [PMC free article] [PubMed] [Google Scholar]
- 2.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375–8. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Stein DG, Wright DW. Progesterone in the clinical treatment of acute traumatic brain injury. Expert Opin Investig Drugs. 2010;19:847–57. doi: 10.1517/13543784.2010.489549. [DOI] [PubMed] [Google Scholar]
- 4.Deutsch ER, Espinoza TR, Atif F, Woodall E, Kaylor J, Wright DW. Progesterone's role in neuroprotection, a review of the evidence. Brain Res. 2013;1530:82–105. doi: 10.1016/j.brainres.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Wright DW, Kellermann AL, Hertz- berg VS, et al. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007;49:391–402. doi: 10.1016/j.annemergmed.2006.07.932. [DOI] [PubMed] [Google Scholar]
- 6.Xiao G, Wei J, Yan W, Wang W, Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit Care. 2008;12:R61. doi: 10.1186/cc6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Code of Federal Regulations: exception from informed consent requirements for emergency research. Government Printing Office; Washington, DC: 2013. [Google Scholar]
- 8.Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2:81–4. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 9.Jennett B, Bond M. Assessment of out come after severe brain damage. Lancet. 1975;1:480–4. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 10.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15:573–85. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 11.Rappaport M, Hall KM, Hopkins K, Belleza T, Cope DN. Disability rating scale for severe head trauma: coma to community. Arch Phys Med Rehabil. 1982;63:118–23. [PubMed] [Google Scholar]
- 12.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–56. [PubMed] [Google Scholar]
- 13.Dmitrienko A, Molenberghs G, Chuang-Stein C, Offen W. Analysis of clinical trials using SAS: a practical guide. SAS; Cary, NC: 2005. [Google Scholar]
- 14.Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57:1173–82. doi: 10.1227/01.neu.0000186013.63046.6b. [DOI] [PubMed] [Google Scholar]
- 15.Loane DJ, Faden AI. Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci. 2010;31:596–604. doi: 10.1016/j.tips.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narayan RK, Michel ME, Ansell B, et al. Clinical trials in head injury. J Neuro-trauma. 2002;19:503–57. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher M, Feuerstein G, Howells DW, et al. Update of the Stroke Therapy Academic Industry Roundtable preclinical recommendations. Stroke. 2009;40:2244–50. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ducharme N, Banks WA, Morley JE, et al. Brain distribution and behavioral effects of progesterone and pregnenolone after intranasal or intravenous administration. Eur J Pharmacol. 2010;641:128–34. doi: 10.1016/j.ejphar.2010.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement1