TLRs and interferons: a central paradigm in autoimmunity (original) (raw)
. Author manuscript; available in PMC: 2015 Jan 28.
Published in final edited form as: Curr Opin Immunol. 2013 Nov 16;25(6):720–727. doi: 10.1016/j.coi.2013.10.006
Abstract
Investigations into the pathogenesis of lupus have largely focused on abnormalities in components of the adaptive immune system. Despite important advances, however, the question about the origin of the pathogenic process, the primary disease trigger, and the dominance of autoantibodies against nuclear components, remained unanswered. Discoveries in the last decade have provided some resolution to these questions by elucidating the central role of nucleic acid-sensing TLRs and the attendant inflammatory response, particularly the production of type I interferons. These priming events are responsible for initiating the adaptive responses that ultimately mediate the pathogenic process.
Introduction
Discoveries underpinning current understanding of the basic pathophysiology of systemic lupus erythematosus (SLE) have begun to dissect fundamental pathways and branches and provide an explanation for the common presence of antinuclear antibodies (ANAs). This has focused attention on two major innate immune system factors, the type I interferons (IFN-I) and the nucleic acid-sensing Toll-like receptors (NA-TLRs). Here, we will review this area focusing on recent publications.
Type I interferons in SLE
It is now widely accepted that IFN-I are a driving pathogenic force in the majority of SLE patients based on substantial clinical, epidemiologic, and genetic data (reviewed in [1•,2,3,4]) as well as direct evidence from animal models using IFN-I receptor-deficient lupus mice or anti-IFN-α/βR antibody treatment [5,6•]. Additional studies in these models have also documented: (a) the existence of IFN-I-independent lupus in MRL-Faslpr mice due to background genes and not Fas deficiency [7,8]; (b) a requirement for IFN-I in mouse lupus models despite the absence of elevated IFN-α or interferon-stimulated genes (ISGs, so called ‘IFN-I signature’) [5], consistent with the recent finding that IFN-I expression even at very low concentrations modulates immune homeostasis by affecting tonic signaling [9]; (c) IFN-α induction of clinically-significant lupus required genetic susceptibility [10], which could explain the infrequent occurrence of lupus in patients treated with high dose IFN-I; and (d) inhibition of lupus was most effective when IFN-I signaling was blocked in early disease stages, implying IFN-I is mainly important at this innate stage, but not after the pathogenic adaptive autoimmune response has been established [6•].
Production of IFN in lupus
Plasmacytoid dendritic cells (pDCs) are considered the main source of IFN-I in SLE because of their capacity to produce 100–1000-fold greater amounts of IFN-α than other cell types and evidence of pDC activation in SLE patients [1•]. The importance of these cells in disease pathogenesis is supported by the finding that, in lupus mice, significant disease suppression occurred either with IRF8 deficiency, which arrests development of predominantly pDCs, or with the feeble mutation in the endosomal histidine transporter, Slc15a4, which blocks NA-TLR-induced cytokine production, including IFN-I, selectively in pDCs [11•]. Other cell types, such as macrophages and nonhematopoietic cells, have been implicated as the primary source of aberrantly elevated IFN-I in certain monogenic diseases as well as in pristane-induced lupus [12••,13,14•,15].
Cellular mechanisms of IFN-I production by nucleic acid sensors
Nucleic acid pattern recognition receptors (PRRs) that mediate production of IFN-I can be divided into the endosomal TLRs and several types of cytosolic sensors. The NA-TLRs include TLR3, TLR7, TLR8, and TLR9, recognizing dsRNA, ssRNA, and DNA (reviewed in [16]). All, except TLR3, signal through MyD88 and IRAK4 to activate IRF3/7 and IKK pathways leading to IFN-I production or NF-κB activation. TLR3 signaling occurs through TRIF and activation of TBK1 and IKK pathways.
The cytosolic nucleic acid sensors can be grouped by response to RNA or DNA. RNA is recognized by RIG-I-like receptor (RLR) family members, RIG-I and MDA5, that bind 5′ triphosphate RNA or long double-stranded RNA, respectively [17]. They both signal through MAVS (encoded by IFIH1) located on the mitochondrial membrane to mediate IRF-dependent and NF-κB-dependent cell activation and transcription of inflammatory genes including IFN-I. The main sensor for cytosolic self-DNA was recently shown to be the cyclase, cGAS, which, upon encountering DNA, produces a second messenger, cGAMP [18•,19•]. The generated cGAMP then dimerizes and activates the adapter protein STING (encoded by TMEM173), a five-membrane-spanning receptor on the endoplasmic reticulum and outer mitochondria, resulting in phosphorylation of IRF3 and IKK, and activation of the TBK1 and NF-kB pathways, respectively, the former inducing IFN-β synthesis [20•]. Several other DNA-recognizing receptors, some signaling through STING, have also been identified, including DAI that binds zDNA, IFI16 (p204 in mice), RNA polymerase III that transcribes A-T rich dsDNA into uncapped 5′ triphosphate RNA recognized by RIG-I, several DHX helicases, and LRRFIP1 that senses both RNA and DNA, and activates the transcriptional co-factor β-catenin, which enhances the IFN-β gene promoter activity of IRF3 [21,22]. Although these other receptors were shown to detect cytoplasmic DNA, including those from specific pathogens, their role in the recognition of self-DNA is not known.
Mechanisms for increased IFN-I
The cause of the type I IFN signature has been attributed to several possible acquired and genetic factors [4]. The most prominent of these is probably endosomal NA-TLR-mediated activation of pDCs by phagocytized IgG immune complexes that contain nucleic acid cargos. DNA entry into phagosomes is also facilitated by LL37, a cationic antimicrobial peptide that can bind to DNA and inhibit nuclease activity, and by HMGB1, a proinflammatory nuclear protein that, when bound to DNA, can mediate phagocytosis by binding to the RAGE receptor [23,24]. Although their role in lupus is less certain, both LL37 and HMGB1 coat DNA in neutrophil extracellular traps (NETs), an antimicrobial product which recent evidence suggests is a potential source of self-nucleic acids and is prevalent in SLE because of induction by anti-ribonucleoprotein antibodies [25•,26,27]. The significance, however, of NETosis in SLE is controversial [28].
In terms of genetic causes of type I IFN signature in SLE, a wide range of genes and mechanisms, have been implicated that extend throughout the IFN-I pathway (reviewed in [29•]). These include genetic variants that affect response to stimuli (e.g. STAT4, IFIH1) [30], regulation of IFN-I (TRAP) [31], clearance of cytosolic DNA (TREX1, DNaseII, ADAR1) [13,32–34], expression of IRGs (IRF5) [35], clearance of apoptotic material (C1Q), and regulation of pDCs (C1Q) [36,37].
IFN-I enhancement of lupus
IFN-I have pleiotropic effects on the immune system, many of which have the potential to promote lupus. In addition to maintaining immune homeostasis and development of certain immune cell populations, these include upregulation of TLR7 [16], activation of cDCs, induction of T-dependent immune responses [38], and impairment of germinal center selection [39]. Importantly, IFN-I promote a feed-forward amplification process by inducing genes responsible for the production of IFN-I, enhancing the production of interferogenic immune complexes, and increasing expression of TLR7 [23,40].
NA-TLRs are central mediators of ANAs and lupus
Similar to IFN-I, there is substantial evidence, primarily in studies using overexpression or deletion of TLRs in lupus-prone mice, that NA-TLRs are crucial for the development of lupus (reviewed in [23,41–43]). Furthermore, recent studies showed that NA-TLRs are necessary for the production of a broad range of autoantibody specificities, including nuclear antigens, cardiolipin, β2-GPI, myeloperoxidase, and red blood cells, all self-antigens that to varying extents contain or are associated with nucleic acids, but are dispensable for autoantibody specificities not associated with nucleic acids [44••]. Thus the requirement for NA-TLRs appears to tie together the many diverse autoantibodies, manifestations, and genetic heterogeneity that constitute SLE.
Mechanism of TLR-mediated autoimmunity
NA-TLRs can respond to virtually all forms of self-nucleic acids, including non-CpG DNA, as natural DNA with phosphate backbone activates TLR9 regardless of sequence motif, in contrast to synthetic phosphorothioates [45]. To prevent their deleterious activation, several mechanisms have evolved. These include limiting expression to certain cell types (e.g. selective expression of human TLR9 in pDCs and B cells), sequestering NA-TLRs inside endosomes and restricting the active form of TLR7/9 to this compartment, the abundant presence of nucleases, and inhibition of TLR activation when phagocytosis of apoptotic material is mediated by scavenger and complement receptors [16,46,47]. In lupus, these barriers can be overcome in two related ways. For B cells, antigen-receptor engagement leads to transport of antigenic material containing nucleic acids to the endosomal compartment where binding to the relevant NA-TLR is followed by cell activation. For pDCs, DCs, and other cell types, entry of IgG immune complex-containing nucleic acids into the endosome is mediated by FCGR2A (Fcgr3 in mice) [23,48]. IgM immune complexes, on the other hand, lack FcR binding, but instead deposit C3 split products via C1q and mannose-binding lectin, which, by engaging complement receptors, induce an inhibitory signal [46]. In B cells, IgG immune complexes are less stimulatory than antigen alone because the IgG on immune complexes binds to the inhibitory FcγRIIb, the only FcγR expressed in B cells in both humans and mice [49].
TLRs on B cells mediate autoantibody production
Recent studies using different animal models have shown that expression of TLRs in B cells is required for the development of most lupus-associated autoantibodies [44••,50,51••,52]. Lack of NA-TLRs, however, has minimal effect on antibody response to certain foreign antigens [53,54]. Taken together this suggests that NA-TLRs have a specific role in the loss of tolerance to nucleic acid-containing self-antigens or in the expansion of corresponding B cells and plasma cells. On the basis of this conclusion, it was suggested that targeting NA-TLR signaling specifically in the B cell population could potentially result in a largely lupus-specific therapy [44••]. In addition to B cells, TLR signaling in pDCs was also recently shown to play a crucial role in the development of lupus, as discussed above.
TLR7 and TLR9 in lupus
Of the NA-TLRs, TLR7 and TLR9 appear to be the most important based on studies of TLR-deficient lupus-prone mice [55]. Furthermore, consistent with the requirement for TLRs in B cells, mice lacking TLR7 are unable to produce anti-RNA, while loss of TLR9 to varying degrees, depending on the study, impairs the anti-nucleosome response [41,55]. However, although TLR3 was not required for spontaneous lupus [56], administration of poly(I:C), a TLR3 agonist, was reported to induce lupus in MyD88-deficient (lacks TLR7/9 signaling) MRL-Faslpr mice, suggesting that under certain circumstances this sensor can also mediate disease [57]. Accordingly, in human SLE, it is possible that TLR8, which binds ssRNA in contrast to mouse TLR8, which does not, may also play a role [58].
Despite the strong association of SLE with anti-double stranded DNA (dsDNA), several lines of evidence suggest TLR7 may be more important than TLR9. This was first suggested by an early experiment showing that the interferogenic activity of nucleic acid-containing IgG immune complexes (generated by combining SLE sera with apoptotic or necrotic cells) for pDCs was more sensitive to RNase than to DNase [59]. Lupus-prone mice lacking only TLR7 had a substantial reduction in disease, albeit not as great as TLR7/9 double deficiency, whereas absence of TLR9, contrary to expectations, resulted in greater severity [55]. Although an initially perplexing result, subsequent studies have attributed this to the absence of competition from TLR9 for UNC93B1-mediated endoplasmic reticulum to endosome trafficking resulting in increased transport and activation of TLR7 [41,60•]. Similarly, knockout of TLR8, which in mice does not bind nucleic acids but still relies on UNC93B1 for trafficking to the endosome, also leads to the development of systemic autoimmunity [61], presumably by the same mechanism. It should be mentioned that lupus-prone mouse strains produce, in addition to conventional ANAs, species-specific anti-gp70 autoantibodies to circulating RNA-containing endogenous retroviral particles, and this specificity is TLR7-dependent and associated with disease development [62,63]. It is possible that this mouse-specific response may be a factor in the TLR7 predominance in murine lupus.
NA-TLR centric model of SLE autoantibody production
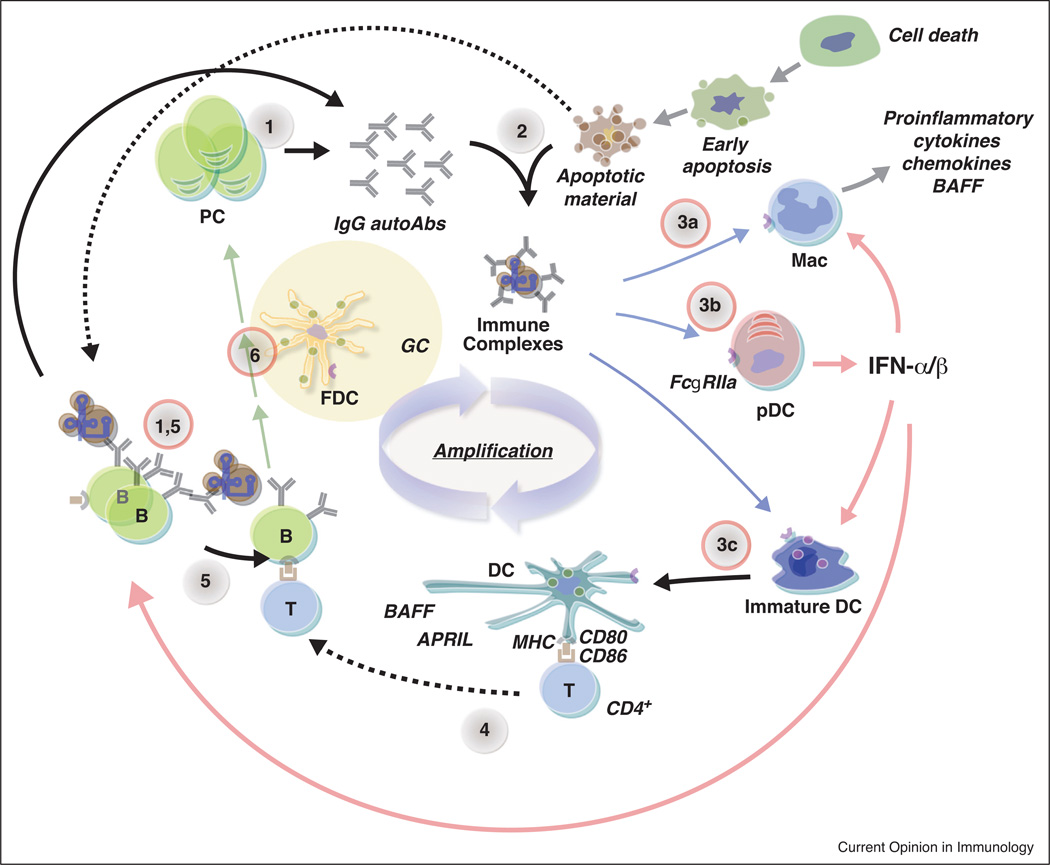
Both IFN-I and NA-TLRs are central to the current model of lupus pathogenesis wherein it is postulated that autoantibody production results from a positive feedback loop (Figure 1). This loop consists of several steps: (a) autoreactive B cells recognizing nucleic acid-containing self-antigens are activated following engagement of their antigen-receptors and NA-TLRs; (b) B cells subsequently produce IgG anti-nuclear antibodies; (c) autoantibodies form nucleic acid-containing immune complexes such as those with apoptotic material; (d) such complexes are transported by the FCGR2A receptor into the endosomal compartments of pDCs, conventional DCs (cDCs), and potentially macrophages and other cell types where release of nucleic acids activates NA-TLRs; (e) activated pDCs produce copious amounts of IFN-I as well as other cytokines; (f) activated cDCs produce proinflammatory cytokines including IFN-I and are potent APCs for T cells; (g) IFN-I in particular have been shown to promote lupus in many ways, including enhancing B cell response and loss of tolerance; (h) these processes then further enhance autoantibody production leading to amplification of the autoimmune state. The sequence of events that lead to the initial production of IgG antinuclear antibodies responsible for triggering the forward feedback loop has not yet been deduced, but evidence from animal models indicate that DCs are not required, thus implying a B cell costimulation of CD4 T cell mechanism [64••].
Figure 1.
Role of nucleic acid TLRs and type I IFN to the pathogenesis of autoantibody production in lupus. Antinuclear autoantibody production in lupus is thought to involve several steps. (1) Secretion of IgG autoantibodies by plasma cells (or B cells). (2) Formation of IgG immune complexes containing nucleic acid material, immune complexes with apoptotic material is depicted as an example. (3) Engulfment of these immune complexes via FCGR2A (FcγRIIa) by macrophages (Mac), pDCs, and, immature DCs (3a–c) into endosomes where released nucleic acids bind to the corresponding TLR resulting in cell activation. Each of these cell types can promote inflammation, immune responses, and autoimmunity by different mechanisms including release of proinflammatory factors and BAFF, and maturation into potent antigen presenting cells. Importantly, pDCs produce copious amounts of IFN-I, which drives multiple processes that enhance the development of autoimmiunity (red arrows, see text for details). (4) Activated DCs can present self-antigen to autoreactive CD4+ T cells. (5) Autoreactive B engage corresponding helper T cells. (6) Activated B cells differentiate into plasma cells or enter germinal centers (GCs) where higher affinity autoreactive plasma cells and memory B cells are generated. Plasma cells then produce more IgG autoantibodies resulting in promulgation and amplification of this process. The nucleic acid-sensing TLR-dependent steps are circled in red.
Altered cytosolic nucleic acid regulation elicits IFN-mediated autoinflammatory and autoimmune diseases
Failure to regulate cytosolic self-nucleic acids has been shown to cause aberrant and destructive responses mediated primarily by IFN-I. Mice deficient in DNase II (endonuclease located in lysosomes) die in utero because of IFN-β-induced apoptosis of erythroid precursors in the liver [13]. Double DNase II/IFN-I deficiency allows survival, but adults develop a TNF-α-mediated TLR9-independent inflammatory arthritis with anticitrullinated peptide antibodies, rheumatoid factor, and low titers of anti-dsDNA [65]. STING reverses all manifestations, including arthritis [66] suggesting that disease is caused by the accumulation of undigested DNA in the cytosol.
Defects in TREX1 (Dnase III) leads to Aicardi-Goutieres syndrome (AGS), chilblain lupus (CLE), and retinal vasculopathy with cerebral leukodystrophy (RVCL) [14•,67]. AGS also occurs with mutations in RNASEH2A, RNASEH2B, RNASH2C, SAMHD1, and ADAR1 [34,14•]. Most cases of AGS are not associated with lupus, although features commonly found in SLE were observed in 60% of patients in one small study [67,68]. Similarly, with CLE, association with SLE does not exist in the few reported familial forms [69]. Nevertheless, TREX1 variants although rare in the population are associated with SLE [14•,67]. Taken together, this suggests that TREX1 defects may act more as a facilitator of SLE, perhaps as a result of overproduction of IFN-I and other cytokines. In mice, deletion of TREX1 leads to a STING-dependent autoinflammatory/autoimmune syndrome, primarily affecting the heart and muscles [33]. This is mediated by overproduction of IFN-I caused by inadequate degradation of single stranded DNA from endogenous retroelements [12••,33] or aberrant replication intermediates [32]. Initial production of IFN-I occurs locally in nonhematopoietic cells, which drives T cell-mediated inflammation and autoantibodies to target tissue antigens [12••]. Deletion of B cells does not reduce tissue inflammation, but significantly extends survival by an undetermined mechanism.
The cytosolic RNA sensing-related adaptor, MAVS, is also associated with enhanced IFN-I production and SLE. IFIH1 is linked to SLE, with loss of function variants linked to resistance and high expression variants to susceptibility [30,70–72]. Transgenic overexpression of MDA5 resulted in chronic elevation of IFN-I associated with IFN signature and resistance to viral infection, but no autoimmunity unless combined with lupus-predisposing FcγR2b deficiency [73].
Treatment
On the basis of considerable evidence supporting crucial roles of both IFN-I and NA-TLRs in SLE pathogenesis, efforts are ongoing to apply this information to the clinic. Anti-IFN-α therapy with broadly reactive monoclonal antibodies, including sifalimumab (MEDI-545, Medimmune) [74], rontalizumab (Genentech) [75], and AGS-009 (Argos Therapeutics) (ClinicalTrials.gov website, EULAR Congress 2012), has advanced to early phase II clinical trials for SLE following acceptable adverse effect profiles, inhibition of IFN signature and, for some, a trend toward therapeutic response. IFNα Kinoid (IFNα-K001, Novacs) therapy, a series of immunizations with an IFN-α-carrier protein to induce self-polyclonal antibodies to this cytokine [76], is currently in phase I/II trials. An antagonist anti-IFN-α monoclonal antibody (MEDI-546, Medimmune) gave promising phase I trial results for systemic sclerosis [77] and could also be used in SLE.
Two TLR antagonists are in early phase clinical trials for SLE, DV1179 (inhibits TLR7/9, Dynavax, Glaxo-SmithKline) and IMO-8400 (inhibits TLR7/8/9, Indera Pharmaceuticals, also Psoriasis in phase 2). These agents inhibited lupus manifestations including anti-nuclear antibodies and glomerulonephritis in BWF1 mice [78–80]. As an additional potential benefit, Dynavax TLR antagonist could reverse the glucocorticoid resistance associated with nucleic acid-TLR activation [81].
Concluding remarks
It has frequently been said that deciphering the pathogenesis of lupus, a complex polygenic disorder with multiple affected systems and organs, would be an exercise in futility, and that studies of organ-specific diseases offered a more fruitful endeavor. Recent advances in the definition of nucleic acid sensors as initiators of both normal and pathogenic immune responses strongly suggest that the Cassandras were wrong and that we now have a good handle on defining the basic processes by which this disease is triggered. Furthermore, these findings provide the catalyst to delineate how disease-initiating endogenous self-products, and in some instances exogenous pathogens, might be recognized in other autoimmune diseases. Overall, it can be postulated that a unified principle can be formulated for most autoimmune conditions that connects disease predisposition to the engagement of pathogen pattern recognition receptors. It follows therefore, that treatment based on these sometimes adversarious sensing processes would be a means to intervene in a broad spectrum of autoimmune diseases.
Acknowledgements
This is publication 25090-IMM from the Department of Immunology and Microbial Science, The Scripps Research Institute. We would like to acknowledge Kat Occhipinti for editorial assistance. This work was supported by National Institutes of Health Research Grants AR53731, AR39555, and ES14847.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Ronnblom L, Eloranta ML. The interferon signature in autoimmune diseases. Curr Opin Rheumatol. 2013;25:248–253. doi: 10.1097/BOR.0b013e32835c7e32. An excellent summary of the evidence linking IFN-I to SLE.
- 2.Ronnblom L, Alm GV. A pivotal role for the natural interferon alpha-producing cells (plasmacytoid dendritic cells) in the pathogenesis of lupus. J Exp Med. 2001;194:F59–F63. doi: 10.1084/jem.194.12.f59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Domizio J, Cao W. Fueling autoimmunity: type I interferon in autoimmune diseases. Expert Rev Clin Immunol. 2013;9:201–210. doi: 10.1586/eci.12.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elkon KB, Wiedeman A. Type I, IFN system in the development and manifestations of SLE. Curr Opin Rheumatol. 2012;24:499–505. doi: 10.1097/BOR.0b013e3283562c3e. [DOI] [PubMed] [Google Scholar]
- 5.Santiago-Raber M, Baccala R, Haraldsson MK, Choubey D, Stewart TA, Kono DH, Theofilopoulos AN. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baccala R, Gonzalez-Quintial R, Schreiber RD, Lawson BR, Kono DH, Theofilopoulos AN. Anti-IFN-alpha/beta receptor antibody treatment ameliorates disease in lupus-predisposed mice. J Immunol. 2012;189:5976–5984. doi: 10.4049/jimmunol.1201477. Anti-IFNα/βR treatment of lupus mice provides insights into the potential benefits and limitations of blocking IFN-I in SLE.
- 7.Hron JD, Peng SL. Type I IFN protects against murine lupus. J Immunol. 2004;173:2134–2142. doi: 10.4049/jimmunol.173.3.2134. [DOI] [PubMed] [Google Scholar]
- 8.Braun D, Geraldes P, Demengeot J. Type I interferon controls the onset and severity of autoimmune manifestations in lpr mice. J Autoimmun. 2003;20:15–25. doi: 10.1016/s0896-8411(02)00109-9. [DOI] [PubMed] [Google Scholar]
- 9.Gough DJ, Messina NL, Clarke CJ, Johnstone RW, Levy DE. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity. 2012;36:166–174. doi: 10.1016/j.immuni.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baccala R, Gonzalez-Quintial R, Blasius AL, Rimann I, Ozato K, Kono DH, Beutler B, Theofilopoulos AN. Essential requirement for IRF8 and SLC15A4 implicates plasmacytoid dendritic cells in the pathogenesis of lupus. Proc Natl Acad Sci U S A. 2013;110:2940–2945. doi: 10.1073/pnas.1222798110. Provides evidence that activation of NA-TLRs in pDCs is required for lupus.
- 12.Gall A, Treuting P, Elkon KB, Loo YM, Gale M, Jr, Barber GN, Stetson DB. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity. 2012;36:120–131. doi: 10.1016/j.immuni.2011.11.018. The pathogenesis of autoimmunity caused by Trex1 deficiency is defined and a new paradigm of IFN-mediated pathology is proposed.
- 13.Yoshida H, Okabe Y, Kawane K, Fukuyama H, Nagata S. Lethal anemia caused by interferon-beta produced in mouse embryos carrying undigested DNA. Nat Immunol. 2005;6:49–56. doi: 10.1038/ni1146. [DOI] [PubMed] [Google Scholar]
- 14.Crow YJ. Type I. interferonopathies: a novel set of inborn errors of immunity. Ann N Y Acad Sci. 2011;1238:91–98. doi: 10.1111/j.1749-6632.2011.06220.x. This review argues for a new category of genetic disorders that have in common pathology induced by over-production of IFN-I.
- 15.Lee PY, Weinstein JS, Nacionales DC, Scumpia PO, Li Y, Butfiloski E, van Rooijen N, Moldawer L, Satoh M, Reeves WH. A novel type I IFN-producing cell subset in murine lupus. J Immunol. 2008;180:5101–5108. doi: 10.4049/jimmunol.180.7.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Loo YM, Gale M., Jr Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Sun L, Chen X, Du F, Shi H, Chen C, Chen ZJ. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. Identification of the cytosolic ligand (second messenger) for STING.
- 19.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. Identification of the main cytosolic DNA sensor.
- 20.O’Neill LA. Immunology. Sensing the dark side of DNA. Science. 2013;339:763–764. doi: 10.1126/science.1234724. Exellent brief review of cytosolic DNA sensing.
- 21.Atianand MK, Fitzgerald KA. Molecular basis of DNA recognition in the immune system. J Immunol. 2013;190:1911–1918. doi: 10.4049/jimmunol.1203162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavlar T, Ablasser A, Hornung V. Induction of type I IFNs by intracellular DNA-sensing pathways. Immunol Cell Biol. 2012;90:474–482. doi: 10.1038/icb.2012.11. [DOI] [PubMed] [Google Scholar]
- 23.Theofilopoulos AN, Kono DH, Beutler B, Baccala R. Intracellular nucleic acid sensors and autoimmunity. J Interferon Cytokine Res. 2011;31:867–886. doi: 10.1089/jir.2011.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanai H, Ban T, Taniguchi T. High-mobility group box family of proteins: ligand and sensor for innate immunity. Trends Immunol. 2012;33:633–640. doi: 10.1016/j.it.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3001180. 73ra19. Evidence for a role for NETs in SLE.
- 26.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3001201. 73ra20. Evidence that NETs contribute to the pathogenesis of type I IFN production in SLE.
- 27.Knight JS, Kaplan MJ. Lupus neutrophils: ‘NET’ gain in understanding lupus pathogenesis. Curr Opin Rheumatol. 2012;24:441–450. doi: 10.1097/BOR.0b013e3283546703. [DOI] [PubMed] [Google Scholar]
- 28.Campbell AM, Kashgarian M, Shlomchik MJ. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3004801. 157ra141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bronson PG, Chaivorapol C, Ortmann W, Behrens TW, Graham RR. The genetics of type I interferon in systemic lupus erythematosus. Curr Opin Immunol. 2012;24:530–537. doi: 10.1016/j.coi.2012.07.008. Summary of IFN-I-related genes identified in SLE genome-wide association studies (GWAS).
- 30.Robinson T, Kariuki SN, Franek BS, Kumabe M, Kumar AA, Badaracco M, Mikolaitis RA, Guerrero G, Utset TO, Drevlow BE, et al. Autoimmune disease risk variant of IFIH1 is associated with increased sensitivity to IFN-alpha and serologic autoimmunity in lupus patients. J Immunol. 2011;187:1298–1303. doi: 10.4049/jimmunol.1100857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Briggs TA, Rice GI, Daly S, Urquhart J, Gornall H, Bader-Meunier B, Baskar K, Baskar S, Baudouin V, Beresford MW, et al. Tartrate-resistant acid phosphatase deficiency causes a bone dysplasia with autoimmunity and a type I interferon expression signature. Nat Genet. 2011;43:127–131. doi: 10.1038/ng.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang YG, Lindahl T, Barnes DE. Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell. 2007;131:873–886. doi: 10.1016/j.cell.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 33.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rice GI, Kasher PR, Forte GM, Mannion NM, Greenwood SM, Szynkiewicz M, Dickerson JE, Bhaskar SS, Zampini M, Briggs TA, et al. Mutations in ADAR1 cause Aicardi-Goutieres syndrome associated with a type I interferon signature. Nat Genet. 2012;44:1243–1248. doi: 10.1038/ng.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cham CM, Ko K, Niewold TB. Interferon regulatory factor 5 in the pathogenesis of systemic lupus erythematosus. Clin Dev Immunol. 2012;2012:780436. doi: 10.1155/2012/780436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lood C, Gullstrand B, Truedsson L, Olin AI, Alm GV, Ronnblom L, Sturfelt G, Eloranta ML, Bengtsson AA. C1q inhibits immune complex-induced interferon-alpha production in plasmacytoid dendritic cells: a novel link between C1q deficiency and systemic lupus erythematosus pathogenesis. Arthritis Rheum. 2009;60:3081–3090. doi: 10.1002/art.24852. [DOI] [PubMed] [Google Scholar]
- 37.Santer DM, Hall BE, George TC, Tangsombatvisit S, Liu CL, Arkwright PD, Elkon KB. C1q deficiency leads to the defective suppression of IFN-alpha in response to nucleoprotein containing immune complexes. J Immunol. 2010;185:4738–4749. doi: 10.4049/jimmunol.1001731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Bon A, Schiavoni G, D’Agostino G, Gresser I, Belardelli F, Tough DF. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–470. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 39.Moisini I, Huang W, Bethunaickan R, Sahu R, Ricketts PG, Akerman M, Marion T, Lesser M, Davidson A. The Yaa locus and IFN-alpha fine-tune germinal center B cell selection in murine systemic lupus erythematosus. J Immunol. 2012;189:4305–4312. doi: 10.4049/jimmunol.1200745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green NM, Laws A, Kiefer K, Busconi L, Kim YM, Brinkmann MM, Trail EH, Yasuda K, Christensen SR, Shlomchik MJ, et al. Murine B cell response to TLR7 ligands depends on an IFN-beta feedback loop. J Immunol. 2009;183:1569–1576. doi: 10.4049/jimmunol.0803899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Theofilopoulos AN, Gonzalez-Quintial R, Lawson BR, Koh YT, Stern ME, Kono DH, Beutler B, Baccala R. Sensors of the innate immune system: their link to rheumatic diseases. Nat Rev Rheumatol. 2010;6:146–156. doi: 10.1038/nrrheum.2009.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marshak-Rothstein A, Rifkin IR. Immunologically active autoantigens: the role of toll-like receptors in the development of chronic inflammatory disease. Annu Rev Immunol. 2007;25:419–441. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- 43.Shlomchik MJ. Activating systemic autoimmunity: B’s, T’s, and tolls. Curr Opin Immunol. 2009;21:626–633. doi: 10.1016/j.coi.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koh YT, Scatizzi JC, Gahan JD, Lawson BR, Baccala R, Pollard KM, Beutler BA, Theofilopoulos AN, Kono DH. Role of nucleic acid-sensing TLRs in diverse autoantibody specificities and anti-nuclear antibody-producing B cells. J Immunol. 2013;190:4982–4990. doi: 10.4049/jimmunol.1202986. Provides evidence linking endosomal TLRs to diverse autoantibody specificities in lupus and that endosomal TLRs expression in B cells is required for generating autoantibody.
- 45.Haas T, Metzger J, Schmitz F, Heit A, Muller T, Latz E, Wagner H. The DNA sugar backbone 2′ deoxyribose determines toll-like receptor 9 activation. Immunity. 2008;28:315–323. doi: 10.1016/j.immuni.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 46.Gronwall C, Chen Y, Vas J, Khanna S, Thiel S, Corr M, Kono DH, Silverman GJ. MAPK phosphatase-1 is required for regulatory natural autoantibody-mediated inhibition of TLR responses. Proc Natl Acad Sci U S A. 2012;109:19745–19750. doi: 10.1073/pnas.1211868109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng Y, Martin DA, Kenkel J, Zhang K, Ogden CA, Elkon KB. Innate and adaptive immune response to apoptotic cells. J Autoimmun. 2007;29:303–309. doi: 10.1016/j.jaut.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bave U, Magnusson M, Eloranta ML, Perers A, Alm GV, Ronnblom L. Fc gamma RIIa is expressed on natural IFN-alpha-producing cells (plasmacytoid dendritic cells) and is required for the IFN-alpha production induced by apoptotic cells combined with lupus IgG. J Immunol. 2003;171:3296–3302. doi: 10.4049/jimmunol.171.6.3296. [DOI] [PubMed] [Google Scholar]
- 49.Avalos AM, Uccellini MB, Lenert P, Viglianti GA, Marshak-Rothstein A. FcgammaRIIB regulation of BCR/TLR-dependent autoreactive B-cell responses. Eur J Immunol. 2010;40:2692–2698. doi: 10.1002/eji.200940184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nickerson KM, Christensen SR, Cullen JL, Meng W, Luning Prak ET, Shlomchik MJ. TLR9 promotes tolerance by restricting survival of anergic anti-DNA B cells, yet is also required for their activation. J Immunol. 2013;190:1447–1456. doi: 10.4049/jimmunol.1202115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teichmann LL, Schenten D, Medzhitov R, Kashgarian M, Shlomchik MJ. Signals via the adaptor MyD88 in B cells and DCs make distinct and synergistic contributions to immune activation and tissue damage in lupus. Immunity. 2013;38:528–540. doi: 10.1016/j.immuni.2012.11.017. Defines the contribution of MyD88 (includes NA-TLRs) in B cells and DCs in the pathogenesis of lupus.
- 52.Walsh ER, Pisitkun P, Voynova E, Deane JA, Scott BL, Caspi RR, Bolland S. Dual signaling by innate and adaptive immune receptors is required for TLR7-induced B-cell-mediated autoimmunity. Proc Natl Acad Sci U S A. 2012;109:16276–16281. doi: 10.1073/pnas.1209372109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kono DH, Haraldsson MK, Lawson BR, Pollard KM, Koh YT, Du X, Arnold CN, Baccala R, Silverman GJ, Beutler BA, et al. Endosomal TLR signaling is required for anti-nucleic acid and rheumatoid factor autoantibodies in lupus. Proc Natl Acad Sci U S A. 2009;106:12061–12066. doi: 10.1073/pnas.0905441106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nickerson KM, Christensen SR, Shupe J, Kashgarian M, Kim D, Elkon K, Shlomchik MJ. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. 2010;184:1840–1848. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sadanaga A, Nakashima H, Akahoshi M, Masutani K, Miyake K, Igawa T, Sugiyama N, Niiro H, Harada M. Protection against autoimmune nephritis in MyD88-deficient MRL/lpr mice. Arthritis Rheum. 2007;56:1618–1628. doi: 10.1002/art.22571. [DOI] [PubMed] [Google Scholar]
- 58.Cervantes JL, Weinerman B, Basole C, Salazar JC. TLR8: the forgotten relative revindicated. Cell Mol Immunol. 2012;9:434–438. doi: 10.1038/cmi.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lovgren T, Eloranta ML, Bave U, Alm GV, Ronnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50:1861–1872. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 60.Sasai M, Iwasaki A. Love triangle between Unc93B1, TLR7, and TLR9 prevents fatal attraction. Immunity. 2011;35:3–5. doi: 10.1016/j.immuni.2011.07.006. Provides evidence for why autoimmunity in lupus mice is exacerbated by TLR9 deficiency.
- 61.Demaria O, Pagni PP, Traub S, de Gassart A, Branzk N, Murphy AJ, Valenzuela DM, Yancopoulos GD, Flavell RA, Alexopoulou L. TLR8 deficiency leads to autoimmunity in mice. J Clin Invest. 2010;120:3651–3662. doi: 10.1172/JCI42081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoshinobu K, Baudino L, Santiago-Raber ML, Morito N, Dunand-Sauthier I, Morley BJ, Evans LH, Izui S. Selective up-regulation of intact, but not defective env RNAs of endogenous modified polytropic retrovirus by the Sgp3 locus of lupus-prone mice. J Immunol. 2009;182:8094–8103. doi: 10.4049/jimmunol.0900263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baudino L, Yoshinobu K, Morito N, Santiago-Raber ML, Izui S. Role of endogenous retroviruses in murine SLE. Autoimmun Rev. 2010;10:27–34. doi: 10.1016/j.autrev.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 64.Teichmann LL, Ols ML, Kashgarian M, Reizis B, Kaplan DH, Shlomchik MJ. Dendritic cells in lupus are not required for activation of T and B cells but promote their expansion, resulting in tissue damage. Immunity. 2010;33:967–978. doi: 10.1016/j.immuni.2010.11.025. Uses targeted deletion of CD11c cells to determine the role of DCs (cDCs and pDCs) in lupus pathogenesis.
- 65.Kawane K, Ohtani M, Miwa K, Kizawa T, Kanbara Y, Yoshioka Y, Yoshikawa H, Nagata S. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature. 2006;443:998–1002. doi: 10.1038/nature05245. [DOI] [PubMed] [Google Scholar]
- 66.Ahn J, Gutman D, Saijo S, Barber GN. STING manifests self DNA-dependent inflammatory disease. Proc Natl Acad Sci U S A. 2012;109:19386–19391. doi: 10.1073/pnas.1215006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramantani G, Kohlhase J, Hertzberg C, Innes AM, Engel K, Hunger S, Borozdin W, Mah JK, Ungerath K, Walkenhorst H, et al. Expanding the phenotypic spectrum of lupus erythematosus in Aicardi-Goutieres syndrome. Arthritis Rheum. 2010;62:1469–1477. doi: 10.1002/art.27367. [DOI] [PubMed] [Google Scholar]
- 68.Rice G, Patrick T, Parmar R, Taylor CF, Aeby A, Aicardi J, Artuch R, Montalto SA, Bacino CA, Barroso B, et al. Clinical and molecular phenotype of Aicardi-Goutieres syndrome. Am J Hum Genet. 2007;81:713–725. doi: 10.1086/521373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hedrich CM, Fiebig B, Hauck FH, Sallmann S, Hahn G, Pfeiffer C, Heubner G, Lee-Kirsch MA, Gahr M. Chilblain lupus erythematosus — a review of literature. Clin Rheumatol. 2008;27:1341. doi: 10.1007/s10067-008-0975-0. [DOI] [PubMed] [Google Scholar]
- 70.Pothlichet J, Niewold TB, Vitour D, Solhonne B, Crow MK, Si-Tahar M. A loss-of-function variant of the antiviral molecule MAVS is associated with a subset of systemic lupus patients. EMBO Mol Med. 2011;3:142–152. doi: 10.1002/emmm.201000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Molineros JE, Maiti AK, Sun C, Looger LL, Han S, Kim-Howard X, Glenn S, Adler A, Kelly JA, Niewold TB, et al. Admixture mapping in lupus identifies multiple functional variants within IFIH1 associated with apoptosis, inflammation, and autoantibody production. PLoS Genet. 2013;9:e1003222. doi: 10.1371/journal.pgen.1003222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gateva V, Sandling JK, Hom G, Taylor KE, Chung SA, Sun X, Ortmann W, Kosoy R, Ferreira RC, Nordmark G, et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat Genet. 2009;41:1228–1233. doi: 10.1038/ng.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crampton SP, Deane JA, Feigenbaum L, Bolland S. Ifih1 gene dose effect reveals MDA5-mediated chronic type I IFN gene signature, viral resistance, and accelerated autoimmunity. J Immunol. 2012;188:1451–1459. doi: 10.4049/jimmunol.1102705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petri M, Wallace DJ, Spindler A, Chindalore V, Kalunian K, Mysler E, Neuwelt CM, Robbie G, White WI, Higgs BW, et al. Sifalimumab, a human anti-interferon-alpha monoclonal antibody, in systemic lupus erythematosus: a phase I randomized, controlled, dose-escalation study. Arthritis Rheum. 2013;65:1011–1021. doi: 10.1002/art.37824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McBride JM, Jiang J, Abbas AR, Morimoto A, Li J, Maciuca R, Townsend M, Wallace DJ, Kennedy WP, Drappa J. Safety and pharmacodynamics of rontalizumab in patients with systemic lupus erythematosus: results of a phase I, placebo-controlled, double-blind, dose-escalation study. Arthritis Rheum. 2012;64:3666–3676. doi: 10.1002/art.34632. [DOI] [PubMed] [Google Scholar]
- 76.Zagury D, Le Buanec H, Mathian A, Larcier P, Burnett R, Amoura Z, Emilie D, Peltre G, Bensussan A, Bizzini B, et al. IFNalpha kinoid vaccine-induced neutralizing antibodies prevent clinical manifestations in a lupus flare murine model. Proc Natl Acad Sci U S A. 2009;106:5294–5299. doi: 10.1073/pnas.0900615106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang B, Higgs BW, Chang L, Vainshtein I, Liu Z, Streicher K, Liang M, White WI, Yoo S, Richman L, et al. Pharmacogenomics and translational simulations to bridge indications for an anti-interferon-alpha receptor antibody. Clin Pharmacol Ther. 2013;93:483–492. doi: 10.1038/clpt.2013.35. [DOI] [PubMed] [Google Scholar]
- 78.Guiducci C, Tripodo C, Gong M, Sangaletti S, Colombo MP, Coffman RL, Barrat FJ. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med. 2010;207:2931–2942. doi: 10.1084/jem.20101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barrat FJ, Meeker T, Chan JH, Guiducci C, Coffman RL. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur J Immunol. 2007;37:3582–3586. doi: 10.1002/eji.200737815. [DOI] [PubMed] [Google Scholar]
- 80.Zhu F, Jiang W, Dong Y, Kandimalla E, La Monica N, Agrawal S. IMO-8400, a novel TLR7, TLR8, and TLR9 antagonist, inhibits disease development in lupus-prone NZBW/F1 mice (abstract) J Immunol. 2012;188:119.112. [Google Scholar]
- 81.Guiducci C, Gong M, Xu Z, Gill M, Chaussabel D, Meeker T, Chan JH, Wright T, Punaro M, Bolland S, et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465:937–941. doi: 10.1038/nature09102. [DOI] [PMC free article] [PubMed] [Google Scholar]