CaMKII isoforms differ in their specific requirements for nitric oxide regulation (original) (raw)
. Author manuscript; available in PMC: 2015 Dec 20.
Abstract
The Ca2+/calmodulin-dependent protein kinase II (CaMKII) mediates physiological and pathological functions by its Ca2+-independent autonomous activity. Two novel mechanisms for generating CaMKII autonomy include oxidation and S-nitrosylation, the latter requiring both Cys280 and Cys289 amino acid residues of the brain isoform, CaMKIIα. Even though the other CaMKII isoforms have a different amino acid in position 280 [OK?], we show here that nitric oxide (NO)-signaling generated autonomy also for the CaMKIIβ isoform. Furthermore, although oxidation of the Met280/281 residues is sufficient to generate autonomy for most CaMKII isoforms, this autonomy was adversely affected by a Cys289-mutation in the CaMKIIα isoform. Thus, all CaMKII isoforms can be regulated by physiological NO-signaling, but CaMKIIα regulation by oxidation and S-nitrosylation is more stringent.
Keywords: CaMKII, Calmodulin, Nitric oxide, S-nitrosylation, Oxidation, Autonomy, Kinase regulation
1. Introduction
A hallmark feature of CaMKII regulation is the generation of Ca2+-independent “autonomous” kinase activity by T286 autophosphorylation [1–4], a processes considered to be a form of molecular memory and indeed important for induction of long-term changes in synaptic strength [5–7] (for review see [8, 9]). Several additional ways to generate CaMKII autonomy have been described more recently, including by GluN2B binding [10, 11], by O-linked glycosylation [12], by oxidation [13] and by S-nitrosylation [14]. In this study, the two latter mechanisms were examined further. For both oxidation and S-nitrosylation of CaMKII, important pathological functions have been indicated: Oxidation of CaMKIIδ (the dominant isoform in the heart) is involved in important pathological functions in the heart [13], while NO-induced S-nitrosylation of CaMKIIα (the dominant isoform in the brain) appears to contribute to ischemic/excitotoxic neuronal cell death [14]. S-nitrosylation of CaMKII may also contribute to physiological NO-signaling, but such possible functions remain to be elucidated. Like T286 autophosphorylation, autonomous CaMKII activity generated by oxidation or S-nitrosylation requires an initial Ca2+/CaM stimulus [13, 14], likely to make the relevant residues within the regulatory domain accessible for modification (Fig. 1). Three residues are of interest for autonomy induced by oxidation or S-nitrosylation: C280/M281 and C289 in CaMKIIα, which are homologous to M281/M282 and C290 in the other CaMKII isoforms, β, γ, and δ (Fig. 1). Oxidation-induced autonomy of CaMKIIδ was abolished by M281/M282V mutation (and mildly reduced by individual mutation of either site), but was not sensitive to C290V mutation [13]. Oxidation also generated autonomy of CaMKIIα [13, 14] and of a CaMKIIδ mutant with the CaMKIIα regulatory domain sequence (generated by M281C mutation) [13]. The latter results were expected, as both Met and Cys residues can be oxidized. By contrast, S-nitrosylation can occur only at Cys but not at Met residues. While nitrosylation-induced autonomy of CaMKIIα required C289 (homologous to C290 in the other isoforms), it additionally required C280 (which is replaced by M281 in the other isoforms)[14]. This indicates that oxidation could induce autonomy for all CaMKII isoforms, but that S-nitrosylation would induce autonomy only for the CaMKIIα isoform. However, as NO can additionally cause protein oxidation via formation of ONOO−, NO-signaling may also regulate other CaMKII isoforms.
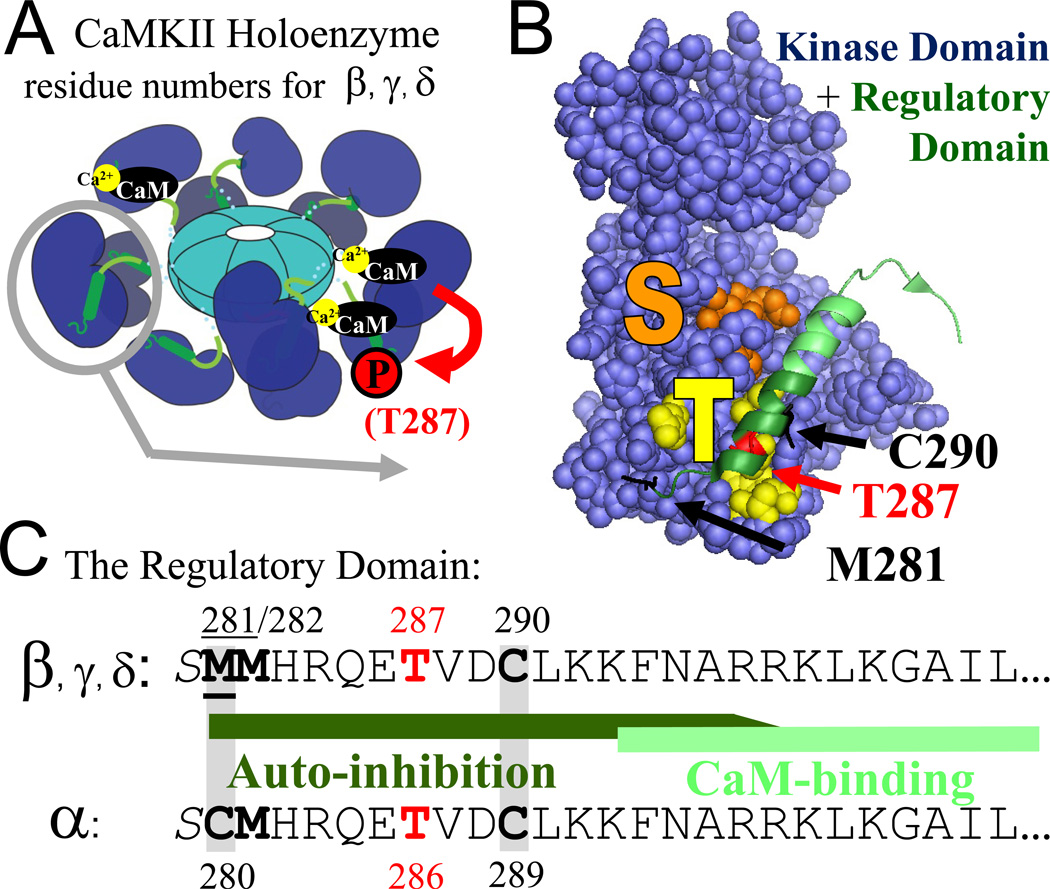
Figure 1.
CaMKII structure and regulation in schematic representation. (A) CaMKII forms 12meric holoenzymes with the N-terminal kinase domains (blue) radiating outward from a central hub formed by the C-terminal association domains (acqua). Each kinase subunit is stimulated separately by Ca2+/CaM binding to the regulatory domain (green), but Ca2+/CaM binding to two neighboring subunits is required to trigger an inter-subunit autophosphorylation at T287 (red; for the α isoform T286) that generates by Ca2+-independent “autonomous” activity. (B) In the basal state, the regulatory domain blocks substrate access to the S-site (orange) and is held in place in part by interaction of the region around T287 with the T-site (yellow). C290 and M281 are homologous to residues that are involved in nitrosylation-or oxidation-induced autonomy of CaMKIIα. The corresponding modifications of the indicated residues likely prevent complete rebinding of the regulatory domain to the kinase domain. (C) The sequence of the N-terminal part of the regulatory domain of the different CaMKII isoforms. The oxidation sites (Met, Cys), nitrosylation sites (Cys), and the T286/287 phosphorylation site are marked in bold; the two Cys residues required for nitrosylation in CaMKIIα and the homologous residues in the other isoforms are marked by grey shading.
This study set out to determine the NO-and oxidation-mediated regulation of CaMKIIβ, the second brain-enriched CaMKII isoform. Notably, CaMKIIβ has several important isoform-specific functions in the brain that are not shared by the α isoform [15–17]; these differential functions have been attributed to the β-specific interaction with F-actin [15, 16, 18]. As expected, we found that NO and oxidation induced autonomy also for CaMKIIβ. However, more surprisingly, CaMKIIβ autonomy was generated by NO even when oxidation was suppressed. Thus, even CaMKII isoforms that contain C290 but lack a Cys in position 281 (i.e. all isoforms except for α) can be directly regulated by nitrosylation. This indicates that all CaMKII isoforms can be regulated not only by pathological oxidation but also by physiological NO-signaling.
2. Materials and methods
2.1. Proteins
CaMKIIα and β wild type isoforms and CaM were purified after baculovirus/Sf9 cell expression or bacterial expression as previously described [4, 19]. For CaMKIIβ purification, the cell extraction buffer was supplemented with 150 mM NaClO4 prior to centrifugation, in order to improve solubility of the cytoskeleton-associated isoform [19]. For comparison of CaMKIIα wild type and mutants, GFP-CaMKII fusion proteins were purified after expression in HEK cells as previously described [14, 20]. For comparison of the CaMKIIβ mutants, GFP-CaMKII fusion proteins in HEK cell extract were used, after adjustments for content of total cellular protein as previously described [14]. Crude HEK cell extracts were spun at 4°C at 20,000×g for 20 min. Then, GFP-CaMKIIβ was solubilized from the pellets using 1.2 M KCl [21].
2.2 CaMKII nitrosylation and oxidation
CaMKII nitrosylation or oxidation was performed as previously described [14]. CaMKII (200–300 nM subunit concentration) was reacted in buffer containing 50 mM PIPES pH 7.1, 1 mM CaCl2, and 1 µM CaM either with 3 mM DEA-NONOate (Cayman Chemicals; for nitrosylation) or with 3 mM H2O2 (Sigma; for oxidation). DEA-NONOate is stable in low pH buffers, but rapidly releases NO when added to the pH 7.1 reaction buffer [14]. When indicated, the nitrosylation reactions also contained the ONOO−-scavengers tryptophan (3 mM; Sigma) or MnTMPyP (0.3 mM; A.G. Scientific). After 5 min at room temperature, the reactions were diluted and Ca2+ was chelated with 5 mM EGTA.
2.3 CaMKII activity assays
CaMKII activity was determined by 32P incorporation into the peptide substrate syntide 2 (Genescript) as previously described [4, 14, 19]. In order to assess basal or autonomous CaMKII activity, reactions contained 2.5 nM CaMKII, 50 mM PIPES pH 7.1, 0.1% bovine serum albumin, 10 mM MgCl2, 100 µM [γ-32P]ATP (~1 mCi/µmol), 75 µM substrate peptide, and 1.5 mM EGTA. In order to determine the relative level of autonomy (the ratio of autonomous over maximally stimulated activity), stimulated reactions were performed in the same buffer, but with EGTA substituted by Ca2+/CaM (1 mM/2 µM). Reactions were performed at 30°C for a duration that ensured that they were within the linear range (1 min for maximally stimulated activity, 5 min for nitrosylation-or oxidation-induced autonomous activity)[4, 14].
For measuring the activity of GFP-CaMKIIβ wild type and mutants, the PIPES in the reaction buffer was replaced with 50 mM Hepes pH 7.4 and 150 mM KCl (as KCl was required for extraction from the cytoskeletal fraction).
3. Results
3.1. NO generated autonomous activity of the C280-lacking CaMKIIβ isoform
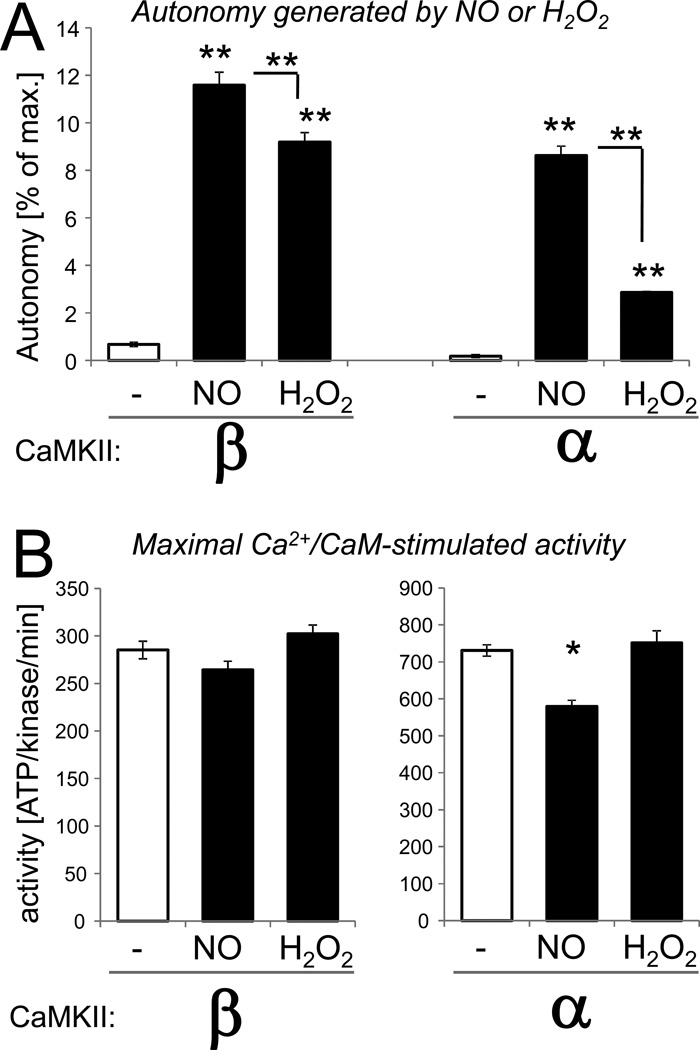
NO induces autonomy of the CaMKIIα isoform via S-nitrosylation at C280 and C289, and autonomy was abolished by mutation of either residue [14]. Like CaMKIIγ and δ, the CaMKIIβ isoform contains M281 at the position homologous to C280 (Fig. 1), a residue that cannot be S-nitrosylated. However, addition of the NO-donor DEA-NONOate to CaMKIIβ (in the presence of Ca2+/CaM) also generated autonomous activity for this C280-lacking isoform, as measured in a kinase activity assay after chelation of Ca2+(Fig. 2). The level of autonomy induced by NO was similar for the CaMKIIα and β isoforms, but slightly higher for CaMKIIβ (Fig. 2A). The maximal Ca2+/CaM-stimulated kinase activity was slightly reduced by the 5 min NO treatment for the CaMKIIα but not the β isoform (Fig. 2B). Note that in a previous study, NO treatment for 5 min did not significantly reduce stimulated CaMKIIα activity either, while NO treatments for 10 min or longer reduced the activity much more substantially [14].
Figure 2.
Both NO and H2O2 induce autonomous CaMKIIβ activity. CaMKIIβ or α was reacted for 5 min in presence of Ca2+/CaM in presence or absence of the NO-donor DEA-NONOate (NO) or H2O2 as indicated. Then, activity was measured by phosphate incorporation into the substrate peptide syntide 2. (A) Autonomous activity was measured after chelation of Ca2+ with EGTA, and is represented here as the percent autonomy compared to the maximal Ca2+/CaM-stimulated activity. Both NO and H2O2 induced significant autonomous activity for both CaMKIIβ and α. For both isoforms, the NO-induced autonomy was significantly greater than the H2O2-induced autonomy. (B) Maximal stimulated activity was measured in presence of Ca2+/CaM. The treatments did not affect stimulated activity of CaMKIIβ, while CaMKIIα activity was mildly but significantly reduced by the NO treatment. Bar graphs indicate mean±sem in all panels. (n=4; *: p<0.01; **: p<0.001; compared to no treatment or as indicated, in ANOVA with Neuman-Keuls posthoc analysis).
In summary, while CaMKIIα requires C280 for S-nitrosylation-induced autonomy [14], the C280-lacking CaMKIIβ does not.
3.2 Oxidation induced autonomous activity of the CaMKIIβ isoform
Oxidation of CaMKIIα or δ (induced by addition H2O2 in the presence of Ca2+/CaM) is another pathway to induce autonomous CaMKII activity [13, 14]. The same oxidizing treatment also induced autonomous activity of the CaMKIIβ isoform (Fig. 2A). This result was expected, as CaMKIIβ has a regulatory domain sequence that is identical to CaMKIIδ (see Fig. 1), including the M281/282 residues that are necessary and sufficient for the oxidation-induced autonomy of CaMKIIδ. As previously observed for CaMKIIα [14], the CaMKIIβ autonomy induced by the H2O2 treatment was lower compared to the NO treatment (Fig. 2A). However, for the CaMKIIβ isoform, this difference appeared to be less pronounced (Fig. 2A). The H2O2 treatment did not affect the maximal Ca2+/CaM-stimulated kinase activity for either isoform (Fig. 2B).
3.3. NO generated CaMKIIβ autonomy even when oxidation was suppressed
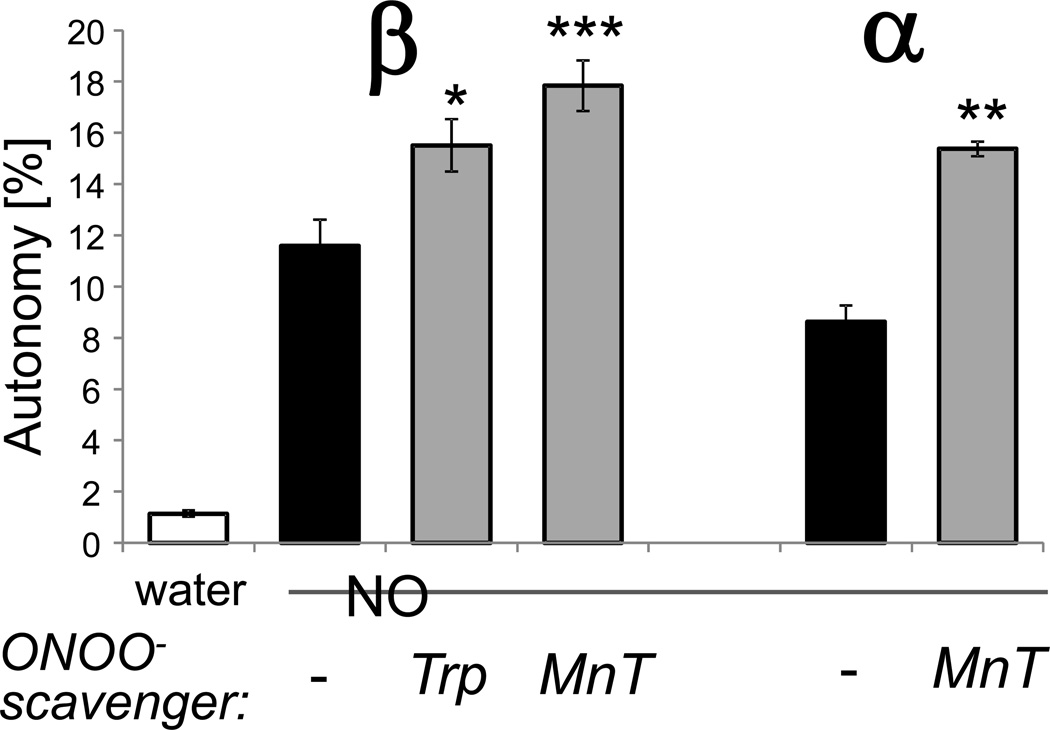
In addition to directly causing S-nitrosylation, NO can also indirectly lead to oxidation via formation of ONOO−. In order to determine if the NO-induced CaMKIIβ autonomy was oxidation-dependent, conditions to suppress oxidation were tested. This was done by adding the NO-donor together with either one of two different ONOO−-scavengers, tryptophane (Trp) or MnTMPyP (MnTP). Note that these scavengers do not directly affect CaMKII activity, i.e. when they are added to the kinase assays after generation of autonomy by NO-donors [14]. As previously described for CaMKIIα [14], scavenger addition during the incubation with NO did not reduce but instead even enhanced NO-induced CaMKIIβ autonomy (Fig. 3). This is consistent with suppression of oxidation of C290 promoting its S-nitrosylation, which generated a higher level of autonomy. Thus, NO-induced CaMKIIβ autonomy does not require ONOO−-mediated oxidation of M281/282. As CaMKIIβ contains only one of the two Cys residues required for NO-induced autonomy of CaMKIIα, this indicates that S-nitrosylation of a single residue, C290, is sufficient for NO-induced CaMKIIβ autonomy.
Figure 3.
NO induces autonomous CaMKIIβ activity even when oxidation is suppressed. CaMKIIβ or α was reacted with NO-donor (NO) as in Fig. 2. However, where indicated, the ONOO− - scavengers tryptophane (Trp) or MnTMPyP (MnT), in order to suppress any oxidation that could be caused by ONOO− -generation. Then autonomous activity was measure after chelation of Ca2+ with EGTA, and the resulting activity was normalized to the autonomy calculated in Fig. 2. For both CaMKII isoforms, the scavengers did not reduce but instead enhanced the autonomous activity generated by NO (n=8 for CaMKIIβ with treatment; n=4 for untreated and CaMKIIα; *: p<0.05; **: p<0.01; ***: p<0.001; compared to NO-treated kinase, in ANOVA with Neuman-Keuls posthoc analysis). Bar graphs indicate mean±sem.
3.4. NO-induced CaMKIIβ autonomy was sensitive to C290-mutation
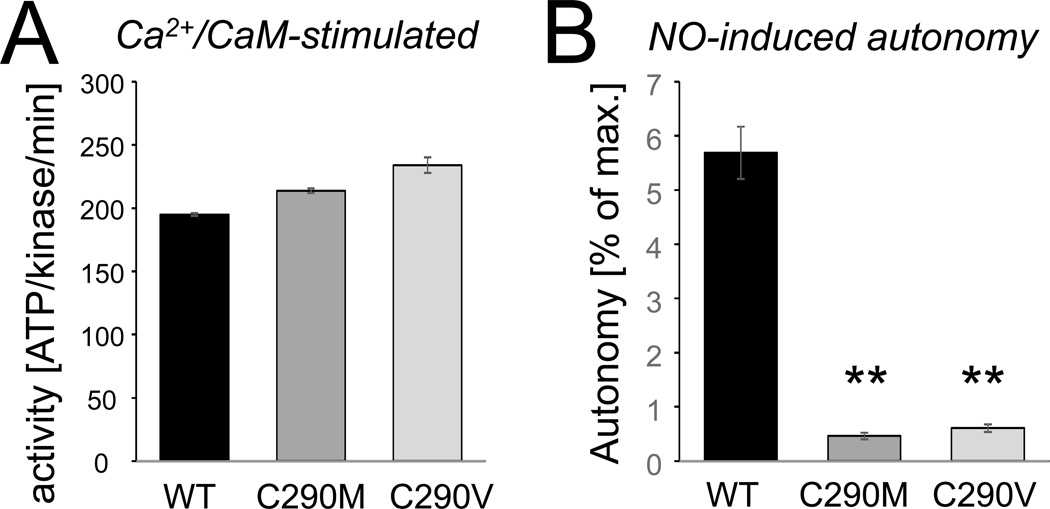
In order to determine the involvement of C290 in NO-induced CaMKIIβ autonomy, we tested the effect of C290 mutation to Val or Met. Both mutations prevent NO-induced S-nitrosylation, but only the Val mutation additionally prevents oxidation. The C290 mutations did not reduce the maximal Ca2+/CaM-stimulated activity of CaMKIIβ (Fig. 4A). If any, stimulated activity of the mutants was slightly higher (a difference that could be due to the individual kinase preparation or could be caused by slightly reduced autoinhibitory interactions). By contrast, both C290V and C290M mutation dramatically reduced the NO-induced autonomous activity (Fig. 4B). These results strongly indicate that NO-induced CaMKIIβ autonomy requires S-nitrosylation at C290.
Figure 4.
NO-induced CaMKIIβ autonomy requires C290. (A) As expected, C290M or C290V mutation does not reduce the maximal Ca2+/CaM-stimulated activity of GFP-CaMKIIβ. If any, stimulated activity was slightly (and statistically significantly) greater for the mutants. (B) C290M and C290V mutation dramatically reduced NO-induced autonomy of GFP-CaMKIIβ (n=4; **: p<0.001; compared to wild type kinase, in ANOVA with Neuman-Keuls posthoc analysis). Bar graphs indicate mean±sem in all panels.
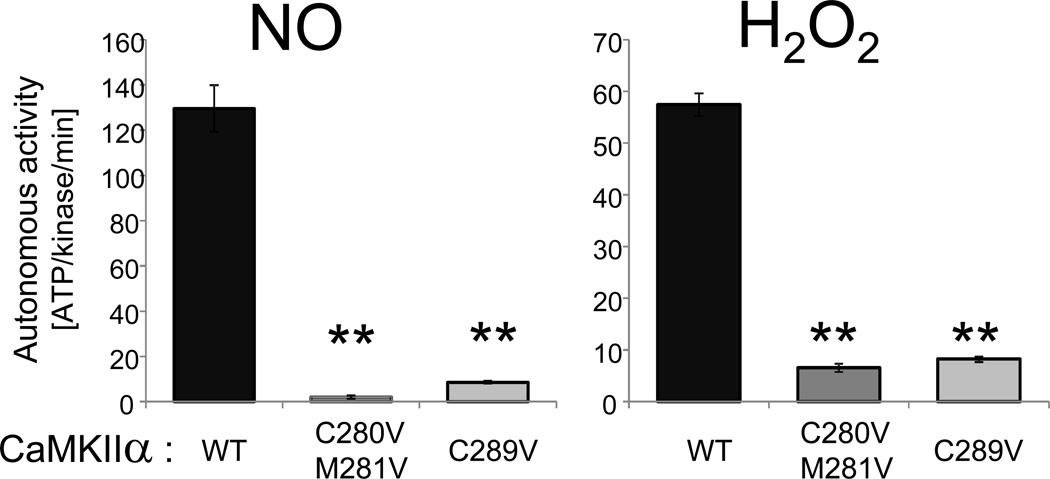
3.5. Oxidation-induced CaMKIIα autonomy was sensitive to C289-mutation
Together with previous results [14], the results described above indicated that the requirements for generation of autonomous activity by S-nitrosylation are more stringent for CaMKIIα (requiring both C280 and C289) than for the other isoforms (which lack C280). Thus, we decided to test whether or not CaMKIIα may also have more stringent requirements than other isoforms for autonomy generation by oxidation. For oxidation-induced CaMKIIδ autonomy, oxidation of M281/282 is sufficient, and oxidation of C290 (the residue homologous to C289 in the α isoform; see Fig. 1) is not required [13]. By contrast, oxidation-induced CaMKIIα autonomy was abolished not only by C280/M281V mutation, but also by C289V mutation (Fig. 4). Thus, while oxidation of M281/282 is sufficient to generate autonomy of other CaMKII isoforms, CaMKIIα requires simultaneous oxidation at both C280/M281 and C289 for oxidation-induced autonomy.
4. Discussion
This study indicates that S-nitrosylation can generate autonomous (Ca2+/CaM-independent) activity for all CaMKII isoforms. This was somewhat surprising, because the brain CaMKIIα isoform required simultaneous S-nitrosylation at both C280 and C289 [14], and the other CaMKII isoforms contain a Methionine instead of C280. However, the results of this study demonstrate that the CaMKIIβ isoform can also be made autonomous by S-nitrosylation. Initially, we hypothesized that this NO-induced autonomy may require oxidation (via NOdependent formation of ONOO−) of the Methionine homologous to C280. However, NO was able to induce even higher autonomy when such oxidation was suppressed. While CaMKIIα expression is restricted to the brain, other CaMKII isoforms have important functions also in other tissues, including in the heart [13, 22, 23]. For heart CaMKII, important pathological functions are mediated by CaMKII autonomy generated by oxidation [13]. This study indicates that all CaMKII isoforms can be made autonomous also by NO-signaling, which can be generated pathologically or physiologically.
Our results indicate that generation of autonomy by S-nitrosylation has a more stringent requirement for the CaMKIIα isoform (requiring simultaneous nitrosylation of C280 and C289) compared to the other isoform (which contain C290 that is homologous to C289 in α, but lack a Cys homologous to C280; see Fig. 1). Thus, while the kinase and regulatory domains of all CaMKII isoforms are highly homologous, the CaMKIIα regulatory domain may have a stronger auto-inhibitory interaction with its kinase domain. We argued that, in this case, CaMKIIα may also have a more stringent requirement for generation of autonomy by oxidation. Indeed, while oxidation of the residues homologous to CaMKIIα C280/M281 is sufficient to generate autonomous activity for heart CaMKIIδ [13], the oxidation-induced autonomy of CaMKIIα was abolished by mutation of either C280/M281 or C289, indicating that simultaneous oxidation of all three residues was required. Thus, the brain-specific CaMKIIα is more tightly controlled than other isoforms in regard to generation of autonomous activity by both S-nitrosylation and oxidation. Pathological functions of oxidation and S-nitrosylation have been described for both heart and brain CaMKII [13, 14]. As NO-induced S-nitrosylation can occur during both pathological and physiological signaling [24, 25], it will be interesting to determine also such physiological functions of CaMKII regulation by NO. For brain CaMKIIα, this may include functions in both potentiation and depression of synaptic strength [7].
Figure 5.
Oxidation-induced CaMKIIα autonomy is sensitive not only to C280/M281 but also C289 mutation. GFP-CaMKIIα wild type or mutants were reacted with NO-donor (NO) or H2O2 as in Fig. 2. Autonomous activity was measure after chelation of Ca2+ with EGTA. As described previously for NO-induced CaMKIIα autonomy, the H2O2-induced autonomy was sensitive not only to C280/M281 mutation, but also to C289 mutation. Thus, both NO-and H2O2-induced autonomy have more stringent requirements in CaMKIIα than in the other isoforms. (n=4 to 5; **: p<0.001; compared to wild type, in ANOVA with Neuman-Keuls posthoc analysis). Bar graphs indicate mean±sem.
Highlights.
- -
NO-induced autonomous CaMKII activity requires C280 in the α but not β isoform. - -
NO-induced CaMKIIβ autonomy requires S-nitrosylation of C290. - -
Oxidation-induced CaMKIIα autonomy requires C289 in addition to C280/M281.
Acknowledgements
This research was supported by National Institutes of Health (NIH) grants R01NS081248 and R01NS080851.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller SG, Kennedy MB. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986;44:861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- 2.Lou LL, Lloyd SJ, Schulman H. Activation of the multifunctional Ca2+/calmodulin-dependent protein kinase by autophosphorylation: ATP modulates production of an autonomous enzyme. Proc Natl Acad Sci U S A. 1986;83:9497–9501. doi: 10.1073/pnas.83.24.9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schworer CM, Colbran RJ, Soderling TR. Reversible generation of a Ca2+-independent form of Ca2+(calmodulin)-dependent protein kinase II by an autophosphorylation mechanism. J Biol Chem. 1986;261:8581–8584. [PubMed] [Google Scholar]
- 4.Coultrap SJ, Buard I, Kulbe JR, Dell'Acqua ML, Bayer KU. CaMKII autonomy is substrate-dependent and further stimulated by Ca2+/calmodulin. J Biol Chem. 2010;285:17930–17937. doi: 10.1074/jbc.M109.069351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- 6.Buard I, Coultrap SJ, Freund RK, Lee YS, Dell'Acqua ML, Silva AJ, Bayer KU. CaMKII "autonomy" is required for initiating but not for maintaining neuronal long-term information storage. J Neurosci. 2010;30:8214–8220. doi: 10.1523/JNEUROSCI.1469-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coultrap SJ, Freund RK, O'Leary H, Sanderson JL, Roche KW, Dell'Acqua ML, Bayer KU. Autonomous CaMKII mediates both LTP and LTD using a mechanism for differential substrate site selection. Cell Reports. 2014;6:431–437. doi: 10.1016/j.celrep.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coultrap SJ, Bayer KU. CaMKII regulation in information processing and storage. Trends Neurosci. 2012;35:607–618. doi: 10.1016/j.tins.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13:169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- 11.O'Leary H, Liu WH, Rorabaugh JM, Coultrap SJ, Bayer KU. Nucleotides and phosphorylation bi-directionally modulate Ca2+/calmodulin-dependent protein kinase II (CaMKII) binding to the N-methyl-D-aspartate (NMDA) receptor subunit GluN2B. J Biol Chem. 2011;286:31272–31281. doi: 10.1074/jbc.M111.233668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, Bers DM. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502:372–376. doi: 10.1038/nature12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coultrap SJ, Bayer KU. Nitric oxide induces Ca2+-independent activity of the Ca2+/calmodulin-dependent protein kinase II (CaMKII) J Biol Chem. 2014;289:19458–19465. doi: 10.1074/jbc.M114.558254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fink CC, Bayer KU, Myers JW, Ferrell JE, Jr, Schulman H, Meyer T. Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron. 2003;39:283–297. doi: 10.1016/s0896-6273(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 16.Okamoto K, Narayanan R, Lee SH, Murata K, Hayashi Y. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc Natl Acad Sci U S A. 2007;104:6418–6423. doi: 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borgesius NZ, van Woerden GM, Buitendijk GH, Keijzer N, Jaarsma D, Hoogenraad CC, Elgersma Y. betaCaMKII plays a nonenzymatic role in hippocampal synaptic plasticity and learning by targeting alphaCaMKII to synapses. J Neurosci. 2011;31:10141–10148. doi: 10.1523/JNEUROSCI.5105-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Leary H, Lasda E, Bayer KU. CaMKIIbeta association with the actin cytoskeleton is regulated by alternative splicing. Mol Biol Cell. 2006;17:4656–4665. doi: 10.1091/mbc.E06-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coultrap SJ, Bayer KU. Ca2+/Calmodulin-Dependent Protein Kinase II (CaMKII) In: Mukai H, editor. Neuromethods: Protein Kinase Technologies. Springer; 2012. pp. 49–72. [Google Scholar]
- 20.Bayer KU, LeBel E, McDonald GL, O'Leary H, Schulman H, De Koninck P. Transition from reversible to persistent binding of CaMKII to postsynaptic sites and NR2B. J Neurosci. 2006;26:1164–1174. doi: 10.1523/JNEUROSCI.3116-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayer KU, Harbers K, Schulman H. alphaKAP is an anchoring protein for a novel CaM kinase II isoform in skeletal muscle. EMBO J. 1998;17:5598–5605. doi: 10.1093/emboj/17.19.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bayer KU, Lohler J, Schulman H, Harbers K. Developmental expression of the CaM kinase II isoforms: ubiquitous gamma-and delta-CaM kinase II are the early isoforms and most abundant in the developing nervous system. Brain Res Mol Brain Res. 1999;70:147–154. doi: 10.1016/s0169-328x(99)00131-x. [DOI] [PubMed] [Google Scholar]
- 23.Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:468–473. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sen N, Snyder SH. Protein modifications involved in neurotransmitter and gasotransmitter signaling. Trends Neurosci. 2010;33:493–502. doi: 10.1016/j.tins.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura T, Tu S, Akhtar MW, Sunico CR, Okamoto S, Lipton SA. Aberrant protein s-nitrosylation in neurodegenerative diseases. Neuron. 2013;78:596–614. doi: 10.1016/j.neuron.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]