SGLT-2 inhibitors and cardiovascular risk: Proposed pathways and review of ongoing outcome trials (original) (raw)
Abstract
Given the multi-faceted pathogenesis of atherosclerosis in type 2 diabetes mellitus (T2DM), it is likely that interventions to mitigate this risk must address cardiovascular (CV) risk factors beyond glucose itself. Sodium glucose cotransporter-2 (SGLT-2) inhibitors are newer antihyperglycaemic agents with apparent multiple effects. Inherent in their mode of action to decrease glucose reabsorption by the kidneys by increasing urinary glucose excretion, these agents improve glycaemic control independent of insulin secretion with a low risk of hypoglycaemia. In this review, we outline those CV risk factors that this class appears to influence and provide the design features and trial characteristics of six ongoing outcome trials involving more than 41,000 individuals with T2DM. Those risk factors beyond glucose that can potentially be modulated positively with SGLT-2 inhibitors include blood pressure, weight, visceral adiposity, hyperinsulinaemia, arterial stiffness, albuminuria, circulating uric acid levels and oxidative stress. On the other hand, small increases in low-density lipoprotein (LDL)-cholesterol levels have also been observed for the class, which theoretically might offset some of these benefits. The potential translational impact of these effects is being tested with outcome trials, also reviewed in this article, powered to assess both macrovascular as well as certain microvascular outcomes in T2DM. These are expected to begin to report in late 2015.
Keywords: Type 2 diabetes, cardiovascular complications, review, macrovascular, sodium glucose cotransporter-2 inhibitors
Introduction
Type 2 diabetes mellitus (T2DM) is associated with a substantially increased cardiovascular (CV) risk,1,2 and several international guidelines statements addressing the management of T2DM3,4 underscore the need to prevent and reduce CV complications.
Although it is conceivable that glycaemic control plays an important role in this process, as suggested by epidemiological studies, there remains great controversy concerning the impact of glucose lowering on CV outcomes from intensive glycaemic control trials.5 Thus, and in light of the multiple CV risk factors beyond hyperglycaemia that exist in most patients with T2DM,6 a multifactorial approach to addressing CV risk has been emphasized. This includes, in addition to glucose lowering, the control of blood pressure (BP) and lipids, weight management, smoking cessation and, when indicated, anti-platelet therapy.3,4 Despite these recommendations, it is known to be difficult for most patients in clinical practice to reach their therapeutic goals.7 Explanations include prevailing patient factors and clinician factors superimposed upon the progressive nature of the disease, as well as the inherent limitations of our current pharmaceutical armamentarium. In light of the multi-faceted pathogenesis of CV disease in diabetes, it would be viewed as an advantage if a specific intervention could attenuate atherosclerosis risk in a multi-dimensional fashion, and beyond glycaemic control alone.
The potential effect of such interventions on CV risk might ultimately be dependent on the mode of action of the drug in terms of which CV pathway(s) were being modulated. However, to date, the potential effects of specific glucose-lowering agents, that is, sulphonylurea (SU), glinides, metformin, thiazolidinediones, insulin, glucagon-like peptide-1 receptor analogues or dipeptidyl-peptidase-4 (DPP-4) inhibitors, on CV events in patients with T2DM remain uncertain.8 This was recently illustrated with the neutral effect for the composite CV death, myocardial infarction (MI) or stroke from the first two placebo-controlled trials involving the DPP-4 inhibitors saxagliptin (i.e. SAVOR-TIMI53)9 and alogliptin (i.e. EXAMINE),10 a class associated with beneficial effects on several factors and biological processes linked to atherogenesis in mechanistic and preclinical studies.11 It should be noted that both SAVOR-TIMI 53 and EXAMINE were relatively short in duration (median follow-up 2.2 and 1.5 years, respectively) and included patients predominantly, or exclusively, with overt CV disease – two important considerations when assessing the potential CV risk modulation of any compound, although this is also contingent on the mode of action of the therapy being studied. A sufficient duration of treatment might be important since macrovascular (as well as microvascular) disease may be a relatively late complication of a complex and progressive pathogenic process that spans years.12 In addition, in T2DM patients with established CV complications, who are often targeted by these studies, it may be more difficult to further reduce the residual CV risk beyond that which standard of care can offer.13
Sodium glucose cotransporter-2 inhibitors – beyond glucose lowering?
Sodium glucose cotransporter-2 (SGLT-2) inhibitors are a new class of glucose-lowering agents that reduce hyperglycaemia in patients with T2DM by reducing renal glucose reabsorption; as a result, they increase urinary glucose excretion (UGE).14 SGLTs are found in the proximal tubule as SGLT-1 and SGLT-2. SGLT-1 is a low-capacity, high-affinity transporter present in parts of the tubule (segment 3), but that also is expressed in the small intestine (to a greater extent than in the kidney) and in the heart. In contradistinction, SGLT-2, a high-capacity, low-affinity transporter, is present in segment 1 of the tubule and normally accounts for ~90% of the glucose reuptake.15
There are currently three drugs of this class that have been approved by US Food and Drug Administration (FDA) and European Medicines Agency (EMA): canagliflozin, dapagliflozin and empagliflozin (Table 1),16–25 with several others in global or regional development (e.g. ipragliflozin, ertugliflozin, remogliflozin, luseogliflozin, tofogliflozin and sotagliflozin). In placebo-controlled phase III trials in patients with T2DM, these agents, as monotherapy or in combination with other glucose-lowering drugs, improve glycaemic control with a low risk of hypoglycaemia. They also reduce body weight and BP without compensatory increases in heart rate and have some effects on plasma lipids (increase in high-density lipoprotein-cholesterol (HDL-C), increase in low-density lipoprotein-cholesterol (LDL-C), with no change in HDL-C/LDL-C).26 The class has also been reported to increase the incidence of urinary and genitourinary tract infections modestly, in particular in females.26 Common for all labels of the class are also potential volume depletion-related adverse events.16–21,26
Table 1.
Overview of approved SGLT-2 inhibitors by FDA and EMA per 2014.
| Canagliflozin | Dapagliflozin | Empagliflozin | |
|---|---|---|---|
| FDA approval | 29 March 2013 | 8 January 2014 | 1 August 2014 |
| EMA approval | 15 November 2013 | 12 November 2012 | 22 May 2014 |
| SGLT-2 selectivity over SGLT-1 | 1:414 | 1:1200 | >1:2500 |
| Posology | Tablets, 100 and 300 mg | Tablets, 5 and 10 mg, | Tablets, 10 and 25 mg |
| Half-life | 12–15 h | 17 h | 10–19 h |
| Absorption | Peak levels 2.8–4.0 h after dosing | Peak levels 1.5 h after dosing | Peak levels 1.5 h after dosing |
This review provides an overview on the potential for SGLT-2 inhibitors to reduce CV risk beyond glucose-lowering effects and presents an overview of the similarities and differences of the currently six ongoing outcome trials from this class, currently involving more than 41,000 patients.
Potential for modulation of non-glycaemic CV risk factors with SGLT-2 inhibitors
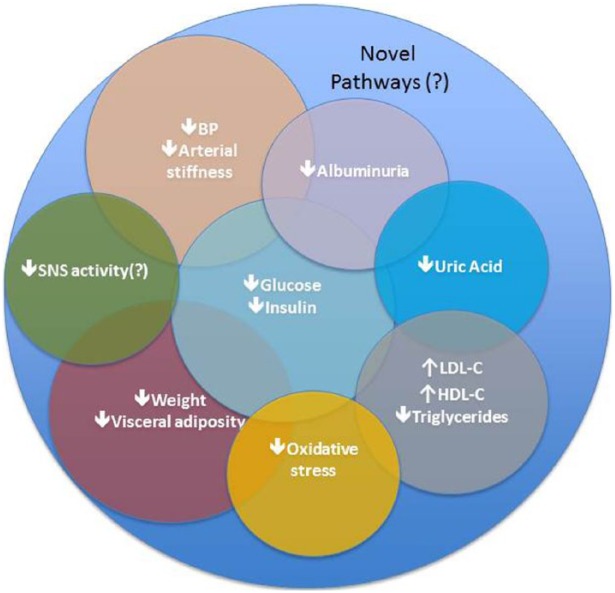
The kidneys play an important role in the modulation of systemic glucose levels, filtering and reabsorbing glucose back into the circulatory system.14 Since the mode of action of SGLT-2 inhibitors is independent of insulin secretion, these agents are associated with a low risk of hypoglycaemia, which has been linked to increased CV events.27 In addition, they have been demonstrated to ameliorate a variety of CV risk factors and potential pathways as described below (Figure 1).
Figure 1.
Identified potential and novel pathways associated with CV effects of SGLT-2 inhibitors based on clinical and mechanistic studies.
Improvement in glucose perturbations and in insulin sensitivity
One potential advantage of SGLT-2 inhibition compared with other classes of glucose-lowering therapies relates to the low potential of this class to induce hypoglycaemia,26 unless combined with insulin or an insulin secretagogue.26 This is attributed to several factors beyond the classes’ insulin-independent mechanism of action. These include a diminished effect of SGLT-2 in the nephron owing to a physiological decline in glomerular filtration rate (GFR) during sympathetic nervous system activation from hypoglycaemia,28 as well as a putative compensatory increase in hepatic gluconeogenesis.29 With the potential for the class to also correct post-prandial glucose levels,22–24 one could at this point also speculate whether such reductions in glucose variability could have beneficial CV effects. Both post-prandial hyperglycaemia and glucose variability have been linked by some investigators to increased CV risk.30
Recent studies have also suggested that enhancing glucosuria with SGLT-2 inhibitors improves insulin sensitivity as measured by peripheral glucose uptake.29,31 One investigation found that insulin-mediated tissue glucose disposal increased by approximately 18% after 2 weeks with dapagliflozin,31 although such changes are likely secondary to reductions in glucose toxicity, which improves beta cell function, as also has been demonstrated with this drug class. Insulin resistance and hyperinsulinaemia have been associated with increased atherosclerosis risk,32 although proof that improving insulin sensitivity (and/or reducing plasma insulin concentrations) leads to CV benefit remains elusive. Moreover, the CV relevance from any indirect changes in insulin sensitivity as a result of SGLT-2 inhibition is entirely unknown.
Reduction in BP and arterial stiffness
In most phase III pivotal trials with SGLT-2 inhibitors, a reduction in systolic BP in the magnitude of ~3–5 mmHg and diastolic BP of ~2 mmHg has been documented.26 Importantly, this has been observed without a compensatory increase in heart rate.
Dedicated 24-h ambulatory BP measurement studies33,34 have confirmed these data, and interestingly, the BP reduction seems to be of the same magnitude irrespective of background therapy, that is, number or type of antihypertensive agents.35
The reason for the observed BP reduction with SGLT-2 inhibitors is not fully understood but likely involves several pathways including a modest diuretic effect,34 weight reduction and potentially some sodium depletion.26 Interestingly, data from an 8-week mechanistic trial demonstrated that empagliflozin reduced arterial stiffness in patients with type 1 diabetes mellitus (T1DM);36 thus, a direct vascular effect might also contribute the BP changes. SGLT-2 inhibitors may also improve endothelial function or the vascular architecture, that is, collagen, elastin, advanced glycation end-products and other components of connective tissue that participate in the process of arterial stiffening.37
The findings of no increased heart rate in the setting of BP reduction are also of note since it may be interpreted as a relative reduction in the sympathetic nervous system tone, although modulation of other neurohormonal factors also could play a role.36
Reduction in body fat and fat mass
With selective SGLT-2 inhibition, glucose reabsorption in the kidneys is decreased and UGE increased, resulting in negative energy balance and weight reduction.14 The glucosuria induced by the agents is typically associated with a net calorie loss of approximately 200–300 kilocalories per day38 leading to weight reductions in studies of ~2–3 kg over 24–52 weeks. This is a consistent finding in the pivotal programmes for the class.26 With SGLT-2 inhibition, as circulating glucose levels are reduced, glucosuria decreases but remains elevated even in those who achieve near-normal glucose control.22–25 The reason for a lesser weight loss than expected from UGE and a plateauing of weight loss after ~3–6 months of therapy is not known, but it has been suggested that this likely could be related to a compensatory increased energy intake39 since these drugs do not affect either resting or meal-induced energy expenditure.29
Of potentially greater interest from a CV risk factor reduction perspective is how they change visceral fat mass. Visceral adiposity is associated with increased risk of T2DM, CV complications and overall mortality, primarily related to abnormal adipocyte biology, with altered production of adipocytokines, leading to modulation of CV pathways that could promote atherosclerosis.40,41 In dedicated body composition studies comparing canagliflozin with glimepiride for 52 weeks,42 dapagliflozin with placebo over 104 weeks43 or empagliflozin with glimepiride44 over 104 weeks and assessing changes in visceral adiposity (VA) mass by dual-energy X-ray absorptiometry (DXA), computer tomography imaging (CTI) or magnetic resonance imaging (MRI), it was demonstrated that the majority of weight loss associated with SGLT-2 inhibition was due to reduction in visceral fat or subcutaneous (SC) fat (Table 2). Interestingly, even in shorter term studies, assessing changes in indirect markers of visceral adiposity, that is, visceral adiposity index,45 waist circumference (WC) or index of central obesity,46 significant reductions have also been observed.47 Data on effects on circulating adipokines are currently sparse. After 24 weeks of therapy, dapagliflozin did not significantly alter either adiponectin or leptin levels in one study.48 Whether reductions in visceral adiposity with SGLT-2 inhibition contribute to CV risk reduction remains to be demonstrated, but it is an interesting characteristic of this class of agents given the discordant impact on visceral fat with other classes of glucose-lowering agents, for example, thiazolidinediones reduce visceral fat whereas SU does not.49
Table 2.
Longer term body composition studies comparing SGLT-2 inhibitors with glimepiride or placebo on a background of metformin (indirect comparisons).
| Study | Ridderstråle et al.44 – 2-year head-to-head study versus glimepiride | Bolinder et al.43 – 2-year placebo-controlled study” | Cefalu et al.42 – 1-year head-to-head study versus glimepiride | ||||
|---|---|---|---|---|---|---|---|
| Intervention | EMPA 25 mg | Glimepiride | DAPA 10 mg | Placebo | CANA 100 mg | CANA 300 mg | Glimepiride |
| Key/baseline characteristics | |||||||
| Study length (weeks) | 104 | 104 | 104 | 104 | 52 | 52 | 52 |
| n | 765 | 780 | 89 | 91 | 483 | 485 | 482 |
| Age (years) | 56 | 56 | 61 | 61 | 56 | 56 | 56 |
| Weight (kg) | 82.5 | 83.0 | 92.1 | 90.9 | 86.9 | 86.6 | 86.5 |
| WC (cm) | 101.9 | 101.6 | 105.6 | 104.5 | NS | NS | NS |
| HbA1c (%) | 7.9 | 7.9 | 7.2 | 7.2 | 7.8 | 7.8 | 7.8 |
| Δ weight (kg) | −3.1 | +1.3 | −4.5 | −2.1 | −3.7 | −4.0 | +0.7 |
| Δ WC (cm) | −2.1 | +1.1 | −5.0 | −2.9 | NS | NS | NS |
| Baseline DXA characteristics | |||||||
| n | 46 | 38 | 89 | 91 | 69 | 71 | 68 |
| Total fat mass | 38.1% | 38.7% | 33.7 kg | 33.4 kg | 28.2 kg | 29.3 kg | 26.3 kg |
| Total lean fat mass | 51.5 kg | 50.4 kg | 55.3? | 56.0? | 47.7 kg | 44.6 kg | 46.6 kg |
| Δ total fat | −1.9% | +0.4% | −2.8 kg | −1.5 kg | −2.9% | −2.5% | +1.0% |
| Δ lean mass | −0.4 kg | +0.5 kg | −1.3 kg | −0.9 kg | −0.9 kg | −1.1 kg | 1.1 kg |
| Baseline VA/SC tissue characteristics | |||||||
| Methodology | MRI | MRI | MRI | MRI | CTI | CTI | CTI |
| n | 39 | 34 | 22 | 24 | 70 | 75 | 72 |
| Total VA | 156.7 cm2 | 174 cm2 | 3309.5 cm3 | 2805 cm3 | 25,506 pixels | 25,090 pixels | 26,269 pixels |
| Total SC | 319.7 cm2 | 337 cm2 | 4613.7 cm3 | 4732.8 cm3 | 31,208 pixels | 32,877 pixels | 29,830 pixels |
| Δ VA tissue | −11.0 | +11.2 | −214.9 | −22.3 | −7.3% | −8.1% | +1.8% |
| Δ SC tissue | −22.3 | +17.7 | −498.0 | −256.3 | −5.4% | −5.6% | +1.8% |
Effects on proteinuria and kidney function
SGLT-2 inhibitors are associated with reductions in urinary albumin excretion as described in dedicated renal studies with canagliflozin,50 dapagliflozin51 or empagliflozin52 or in pooled analysis of phase III studies.53 To what extent such a reduction translates into potential CV benefits remains to be clarified; but from a vascular perspective, this is interesting in light of the haemodynamic renal changes associated with diabetes. At the onset of diabetes mellitus, hyperglycaemia causes increases in proximal tubular reabsorption due to an upregulation of the SGLT-2 expression54,55 leading to secondary increases in sodium/glucose cotransport. The increase in proximal reabsorption leads to a decrease in solute load to the macula densa, alteration of the tubuloglomerular feedback and an increase in GFR. In a renal mechanistic study in T1DM, it was observed that renal hyperfiltration was reduced with SGLT-2 inhibition,56 attributable to increasing afferent arteriolar resistance without altering efferent arteriolar resistance,57 thereby leading to a partial correction of abnormally high baseline glomerular hydrostatic pressure in the context of hyperfiltration that characterizes early diabetic nephropathy.58 These data suggest that SGLT-2 inhibition increases distal tubular sodium delivery, which in turn leads to altered tubuloglomerular feedback and attenuated intraglomerular hypertension. This is also interesting from the single-nephron theory perspective since it could in part explain why a slight reduction in GFR is observed when treatment with SGLT-2 inhibitors is started (i.e. as a consequence of reducing hyperfiltration in functional nephrons). It has been observed that this GFR reduction, in patients with stage 2–4 chronic kidney disease, is reversible when the medication is stopped.52
Reduction in levels of uric acid
Uric acid is the end product of purine metabolism in humans, and hyperuricaemia is a diagnostic marker for gout. Increased uric acid levels have, however, also been associated with chronic kidney disease,59 CV complications60 and congestive heart failure,61 although a cause and effect relationship of uric acid and CV outcomes is far from proven.62 Reduction in levels of uric acid has consistently been seen with SGLT-2 inhibitors,63–65 presumably mediated by the actions of solute carrier family 2, facilitated glucose transporter member 9 (SLC2A9), also called GLUT9, an urate transporter which secretes urate back into the urine in exchange for glucose.66 It is entirely unclear whether these uric acid effects might translate into long-term beneficial outcomes on either renal function or macrovascular complications.
Effect on lipid parameters
SGLT-2 inhibitors are associated with a small increase in HDL-C as well as an increase in LDL-C with concomitant reductions in triglyceride levels63,67–69 (Table 3). Whether these small lipid changes are clinically relevant and whether they could potentially offset any potential CV benefit with SGLT-2 inhibitors will need further clarification.
Table 3.
Changes in LDL-cholesterol, HDL-cholesterol and triglycerides (TG) with SGLT-2 inhibition as reported in pooled analysis.63,68,69.
| Dapagliflozin68 | Empagliflozin63 | Canagliflozin69 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n/no. of trials | 3731/12 | 2700/4 | 2613/4 | |||||||||
| Study duration | Up to 24 weeks | 24 weeks | 26 weeks | |||||||||
| Age | Not stated | Mean: 55.6 years | <65 years (mean: 52.8 years) | ⩾65 years (mean 69.3 years) | ||||||||
| Study arms | 5 mg | 10 mg | Placebo | 10 mg | 25 mg | Placebo | 100 mg | 300 mg | Placebo | 100 mg | 300 mg | Placebo |
| LDL-cholesterol at baseline | 2.93 mmol/L | 2.95 mmol/L | 2.97 mmol/L | 2.57 mmol/L | 2.57 mmol/L | 2.62 mmol/L | 2.8 mmol/L | 2.7 mmol/L | 2.9 mmol/L | 2.6 mmol/L | 2.7 mmol/L | 2.7 mmol/L |
| Change from baseline | +0.6% | +2.7% | −0.4% | +0.08 mmol/L | +0.10 mmol/L | +0.02 mmol/L | +0.06 mmol/L | +0.17 mmol/L | −0.05 mmol/L | +0.04 mmol/L | +0.07 mmol/L | −0.07 mmol/L |
| HDL-cholesterol at baseline | 1.16 mmol/L | 1.17 mmol/L | 1.15 mmol/L | 1.26 mmol/L | 1.27 mmol/L | 1.26 mmol/L | 1.2 mmol/L | 1.2 mmol/L | 1.2 mmol/L | 1.3 mmol/L | 1.3 mmol/L | 1.2 mmol/L |
| Change from baseline | +6.5% | +5.5% | +3.8% | +0.07 mmol/L | +0.07 mmol/L | 0.00 mmol/L | +0.09 mmol/L | +0.09 mmol/L | +0.03 mmol/L | +0.12 mmol/L | +0.12 mmol/L | +0.03 mmol/L |
| TG at baseline | 2.15 mmol/L | 2.19 mmol/L | 2.12 mmol/L | 1.95 mmol/L | 1.96 mmol/L | 1.86 mmol/L | 2.1 mmol/L | 2.1 mmol/L | 2.2 mmol/L | 1.9 mmol/L | 1.7 mmol/L | 1.8 mmol/L |
| Change from baseline | −3.2% | −5.4% | −0.7% | −0.11 mmol/L | −0.02 mmol/L | +0.03 mmol/L | +0.01 mmol/L | −0.10 mmol/L | −0.23 mmol/L | −0.14 mmol/L | −0.16 mmol/L | −0.05 mmol/L |
Effects on other CV risk pathways
In animal and mechanistic models, SGLT-2 inhibitors have been shown to reduce leuokocytosis induced by hyperglycaemia70 and to reduce inflammation and oxidative stress,71–73 which are processes involved in the pathophysiology of atherosclerosis.
Since inhibition of SGLT-2 leads to glucosuria with an accompanying diuresis, weight and BP reductions, all of which are theoretically beneficial in patients with heart failure, it is also conceivable that impaired ventricular function and remodelling could be improved with such an intervention. Although clinical data are yet to be reported, an animal study suggested that SGLT-2 inhibition could attenuate the increase in left ventricle mass and left ventricle end diastolic diameter in a rat model of progressive heart failure.74
Clinical CV outcomes with the use of SGLT-2 inhibitors in T2DM
Pooled analyses of completed phase II/III trials and interim results from ongoing CV outcome trials
While anticipating the results of the ongoing outcome trials, several analyses with pooled data from shorter term trials have been conducted to explore the CV safety profiles of the SGLT-2 inhibitors. One of these was a meta-analysis of 25 canagliflozin and dapagliflozin trials compared with placebo or an active comparator, involving a total of 17,181 patients and 283 4-point major adverse cardiac events (MACE) (CV death, MI, stroke, hospitalized unstable angina). The hazard ratio (HR) was 0.89 (95% confidence interval (CI): 0.70, 1.14) for the combined SGLT-2 inhibitor group.26 This result is consistent with data reported individually from the dapagliflozin trial programme in conjunction with the FDA approval in 2014.75 That analysis included 21 phase 2b/3 studies that combined 2.5–10 mg dose, of which two trials enrolled high CV risk patients, also using a prespecified 4-point MACE definition. MACE events were adjudicated by an independent blinded clinical expert committee (CEC) in 19 of the 21 trials. In this analysis, 178 events occurred in 9339 patient analysis. The HR was 0.81 (0.59, 1.09) in favour of the group randomized to dapagliflozin. A similar pooled analysis from canagliflozin trials, combining 100 and 300 mg doses, was reported in conjunction with its 2013 FDA approval. 4-point MACE were accrued from one phase 2 and seven phase 3 trials between 12 and 104 weeks in duration (including five 52-week and two 104-week trials) and one interim analysis of the on-going Canagliflozin Cardiovascular Assessment Study (CANVAS) trial.76 In this analysis, 201 4-point MACE events (all adjudicated by an independent CEC) occurred in 9632 patients with an overall HR of 0.91 (95% CI: 0.68, 1.22), while the HR for the interim results of CANVAS, which contributed 80% of the overall number of events (n = 161), was 1.00 (95% CI: 0.72, 1.39).
Another approach to assess potential impact on CV risk stems from in silico analysis. One study used the Archimedes Model to predict 20-year outcomes. This programme simulates a projected disease trajectory in a T2DM population with characteristics observed in T2DM subjects in the National Health and Nutrition Examination Survey with HbA1c 7.0%–10.0% and treated with a single oral glucose-lowering agent. The 20-year simulated outcome assumed that patients either received standard of care or dapagliflozin (10 mg) on top of standard of care. The model showed that patients receiving dapagliflozin were likely to experience reductions in the incidence of MI, stroke, CV death and all-cause death by 13.8%, 9.1%, 9.6% and 5.0%, respectively. In addition, there would be relative reductions in the incidence of end-stage renal disease, foot amputation and diabetic retinopathy of 18.7%, 13.0% and 9.8%, respectively, when compared with the current standard of care.77
Collectively, the results of these analyses suggest that these drugs do not appear to increase CV risk. The limitation of these analyses is that the pooled data of limited number of CV events was from heterogeneous, short-term follow-up studies that were neither adequately powered nor designed to address CV outcomes.
Outcome trials in progress
Given the relationship between CV risk and T2DM, and the uncertainty surrounding the CV risk of some glucose-lowering therapies, the US FDA and EMA require evaluation of CV risk for new compounds being developed as therapies for T2DM.78,79 To comply with this, several large clinical trials assessing the CV safety of SGLT-2 inhibitors are ongoing.
There are several common features of these trials (Table 4). They are all double-blinded and placebo-controlled, with patients recruited all having T2DM and being at increased CV risk. In addition, study patients are all receiving standard of care according to recommended guidelines. The trials differ in size and, to some degree, in their target population spanning from higher to lower CV risk, with some studying only people with established CV disease (i.e. the highest CV risk population) and others a mixed population consisting of those with CV risk factors and/or previous macrovascular complications. Primary outcomes also vary. Common to the four trials with a primary CV focus is a primary endpoint definition of a classical 3-point MACE composite (and not 4-point MACE). In contrast, two trials remain focused primarily on renal outcomes.
Table 4.
Contrasting ongoing outcome trials with SGLT-2 inhibitors.
| EMPA-REG Outcome™ | CANVAS | CANVAS-R | CREDENCE | DECLARE-TIMI 58 | Ertugliflozin CVOT | |
|---|---|---|---|---|---|---|
| Clinicaltrials.gov | NCT01131676 | NCT01032629 | NCT01989754 | NCT02065791 | NCT01730534 | NCT01986881 |
| Interventions (rand) | Empagliflozin/Placebo (2:1) | Canagliflozin/Placebo (2:1) | Canagliflozin/Placebo (1:1) | Canagliflozin/Placebo (1:1) | Dapagliflozin/Placebo (1:1) | Ertugliflozin/Placebo (2:1) |
| n | 7034 | 4339 | 5700 | 3627 | 17,150 | 3900 |
| Key inclusion criteria | Established vascular complications, HbA1c 7.0%–10.0%, age ⩾ 18 yearsa | Established vascular complications (age > 30) or ⩾2 CV risk factors (age > 50 years), HbA1c 7.0%–10.5% | Established vascular complications or ⩾2 CV risk factors, HbA1c 7.0%–10.5%, age > 30 years | Stage 2 or 3 CKD and macroalbuminuria and on ACE-i/ARB, HbA1c 6.5%–10.5%, age > 30 years | High risk for CV events, Hba1c TBD, age ⩾ 40 years | Established vascular complications, HbA1c 7.0%–10.5%, age ⩾ 40 years |
| Baseline characteristics | TBD | TBD | TBD | TBD | ||
| Age (years) | 63.1 | 62.4 | ||||
| BMI (kg/m2) | 30.6 | 32.1 | ||||
| HbA1c (%) | 8.1 | 8.2 | ||||
| Vascular complications | 100% | 62.7% | ||||
| Statin use | 77% | 72% | ||||
| Primary endpoint | CV death, non-fatal MI, non-fatal stroke | CV death, non-fatal MI, non-fatal stroke | Progression of albuminuria | ESKD, S-creatinine doubling, renal/CV death | CV death, non-fatal MI, non-fatal ischaemic stroke | CV death, non-fatal MI, non-fatal stroke |
| Target number of events | 691 | ⩾420 | TBD | TBD | 1390 | TBD |
| Primary endpoint powered for superiority/power | Yes, 80% for a 20% RRR | No | Yes/TBD | Yes/TBD | Yes/TBD | No |
| Important secondary endpoint | Hosp. for heart failure, Macroalbuminuria, doubling of serum creatinine and GFR ⩽ 45, renal replacement therapy, renal death | Progression of albuminuria | Regression of albuminuria, change in GFR | CV death, non-fatal MI, non-fatal stroke, hospitalized UAP and hospitalized CHF | Progression/regression of albuminuria, hosp. CHF heart failure | CV death, non-fatal MI, non-fatal stroke and hospitalized UAP |
| FPI | July 2010 | December 2009 | November 2013 | February 2014 | April 2013 | November 2013 |
| Estimated reporting | 2015 | 2017/2018 | 2017 | 2019 | 2019 | 2021 |
| Estimated median follow-up | ~3 years | 6–7 years | 3 years | ~4 years | 4–5 years | 5–7 years |
In each of the trials, CV events are being adjudicated by an independent Clinical Endpoints Committee (CEC), as also requested in the FDA safety requirements and EMA guidance.
The first outcome trial expected to report will be EMPA-REG Outcome™ in 2015.80 This trial is investigating the CV profile of empagliflozin versus placebo superimposed upon prevailing standard of care. There are several other secondary and tertiary outcomes preplanned (e.g. hospitalization for heart failure and several renal endpoints).80 With a minimum of 691 3-point MACE captured, it will allow for both non-inferiority assessment of CV safety as well as CV superiority assessment. The trial commenced in July 2010 and completed recruitment in April 2012 with 7034 patients (mean age 63.1 years, HbA1c 8.1%, body mass index (BMI) 30.6 kg/m2, women/men 28%/72%) with T2DM and established CV manifestations (100%).
CANVAS,81 for which interim results have already been disclosed as part of canagliflozins’ FDA submission,76 will continue until 420 3-point MACE events have been accrued, resulting in relatively limited power due to its relatively smaller number of events being accrued, for evaluation of the potential for CV benefits. This trial commenced in December 2009 and completed recruitment in March 2011 of 4339 patients (mean age 62.4 years, HbA1c 8.2%, BMI 32.1 kg/m2, women/men 34%/66%) with T2DM and established CV disease (62.7%) or CV risk factors. It is expected to report in 2017.82
The much larger DECLARE-TIMI58, a study that currently enrols participants with elevated CV risk, plans to include 17,150 individuals with T2DM and has indicated a target of 1390 3-point MACE.83 It is expected to report in 201882 and is currently the largest outcome trial ongoing involving SGLT-2 inhibitors and will be well powered to answer the question as to whether dapagliflozin could offer CV benefits, as well as addressing other safety-related questions.
Of interest, the SGLT-2 inhibitor outcome trial landscape also includes two trials primarily designed to assess impact of canagliflozin on renal outcomes, one being the CANVAS-R,84 planning to recruit 5700 individuals with T2DM with change in albuminuria as the primary outcome, and CREDENCE85 that targets a renal composite outcome consisting of end-stage kidney disease, doubling of serum creatinine and CV/renal death in 3627 individuals with T2DM and established renal impairment/ complications.
Discussion
Given the increasing prevalence of T2DM in populations worldwide and suboptimal control of glycaemia and other CV risk factors achieved with currently available agents, the need for therapies with novel modes of action remains an important clinical priority. Of the large number of antihyperglycaemic drug classes now available for patients with T2DM, none is recognized unequivocally to reduce CV events over and above any modest effects of glucose lowering itself. Metformin, which is widely viewed as having CV benefits, has actually never been studied in a large properly designed randomized clinical trial powered to answer this specific question.
SGLT-2 inhibitors are novel oral glucose-lowering agents that offer the potential to improve glycaemic control with a low risk of hypoglycaemia, independent of insulin secretion, offering a modest reduction in BP and body weight. Their mode of action, which is independent of endogenous insulin secretion, enables their use in any stage of T2DM. However, while the potential for CV benefits from the SGLT-2 inhibitors is alluring, an actual effect on CV outcomes remains to be proven.
Preliminary attempts have been made to quantify long-term effects of SGLT-2 inhibitors on macrovascular events. These have included pooled analyses, showing beneficial trends, from smaller randomized trials being performed primarily to demonstrate glucose-lowering efficacy and an in silico analysis of uncertain value. Given the frequent discordance between the suspected and actual effects of antihyperglycaemic agents on CV disease, it is important to await the results of properly powered outcome trials specifically designed to assess CV safety and/or benefits.86 Greater knowledge concerning the CV effects of our glucose-lowering therapies is essential for improved evidence-based management of patients with T2DM. As for the class of SGLT-2 inhibitors, we anticipate first reports from ongoing CV outcome trials in 2015.
Footnotes
Declaration of conflicting interests: S.E.I. has served as a consultant to Merck, Boehringer Ingelheim, Bristol-Meyers Squibb, Novo-Nordisk, Eisai, and Lexicon. He serves on data monitoring committees for Novo Nordisk and Intarcia. B.Z. has served as a consultant for Astra Zeneca, Boehringer Ingelheim, Eli Lilly,Janssen, Merck, Novo Nordisk, Takeda and Sanofi. He has received grant support from Merck, Novo Nordisk and Boehringer Ingelheim. C.W. has served as a consultant to Boehringer Ingelheim. R.F. has received honorarium for steering committee membership, consulting, speaking, and support for travel to study meetings, from Servier. He has served as a consultant to Abbott, Amgen, Boehringer-Ingelheim, Novartis, Merck Serono and Irbtech and he is a stockholder in Medical Trials Analyis. D.F. has served as a consultant to Merck, Boehringer-Ingelheim, Bristol-Meyers Squibb, and Amgen. He serves on data monitoring committees for Novo Nordisk. S.H., R.-M.E., H.J.W., U.C.B. and O.E.J. are employees of BI, the developer of empagliflozin.
Funding: No funding was provided for writing this paper. EMPA-REG Outcome™ is funded by Boehringer Ingelheim.
References
- 1.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 2006; 332: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sloan FA, Bethel MA, Ruiz D, Jr, et al. The growing burden of diabetes mellitus in the US elderly population. Arch Intern Med 2008; 168: 192–199. [DOI] [PubMed] [Google Scholar]
- 3.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35: 1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryden L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 2013; 34: 3035–3087. [DOI] [PubMed] [Google Scholar]
- 5.Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycaemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009; 32: 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansen OE. Cardiovascular disease and type 2 diabetes mellitus: a multifaceted symbiosis. Scand J Clin Lab Invest 2007; 67: 786–800. [DOI] [PubMed] [Google Scholar]
- 7.Stark Casagrande S, Fradkin JE, Saydah SH, et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care 2013; 36: 2271–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med 2011; 154: 602–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369: 1317–1326. [DOI] [PubMed] [Google Scholar]
- 10.White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369: 1327–1335. [DOI] [PubMed] [Google Scholar]
- 11.Fadini GP, Avogaro A. Cardiovascular effects of DPP-4 inhibition: beyond GLP-1. Vascul Pharmacol 2011; 55: 10–16. [DOI] [PubMed] [Google Scholar]
- 12.Dzau V, Braunwald E. Resolved and unresolved issues in the prevention and treatment of coronary artery disease: a workshop consensus statement. Am Heart J 1991; 121: 1244–1263. [DOI] [PubMed] [Google Scholar]
- 13.Lee SJ, Leipzig RM, Walter LC. Incorporating lag time to benefit into prevention decisions for older adults. JAMA 2013; 310: 2609–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marsenic O. Glucose control by the kidney: an emerging target in diabetes. Am J Kidney Dis 2009; 53: 875–883. [DOI] [PubMed] [Google Scholar]
- 15.Kanai Y, Lee WS, You G, et al. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest 1994; 93: 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002677/human_med_001764.jsp&mid=WC0b01ac058001d124 (accessed 29 August 2014)
- 17.http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002322/human_med_001546.jsp&mid=WC0b01ac058001d124 (accessed 29 August 2014)
- 18.http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002649/human_med_001707.jsp&mid=WC0b01ac058001d124 (accessed 29 August 2014)
- 19.http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204629s000lbl.pdf (accessed 20 September 2014)
- 20.http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204042s002lbl.pdf (accessed 20 September 2014)
- 21.http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/202293s003lbl.pdf (accessed 20 September 2014)
- 22.Komoroski B, Vachharajani N, Feng Y, et al. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther 2009; 85: 513–519. [DOI] [PubMed] [Google Scholar]
- 23.Obermeier MT, Yao M, Khanna A, et al. In vitro characterization and pharmacokinetics of dapagliflozin (BMS-512148), a potent sodium-glucose cotransporter type II inhibitor, in animals and humans. Drug Metab Dis 2010; 38: 405–414. [DOI] [PubMed] [Google Scholar]
- 24.Heise T, Seman L, Macha S, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple rising doses of empagliflozin in patients with type 2 diabetes mellitus. Diabetes Ther 2013; 4: 331–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sha S, Devineni D, Ghosh A, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab 2011; 13: 669–672. [DOI] [PubMed] [Google Scholar]
- 26.Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159: 262–274. [DOI] [PubMed] [Google Scholar]
- 27.ORIGIN Trial Investigators, Mellbin LG, Rydén L, et al. Does hypoglycaemia increase the risk of cardiovascular events? A report from the ORIGIN trial. Eur Heart J 2013; 34: 3137–3144. [DOI] [PubMed] [Google Scholar]
- 28.Patrick AW, Hepburn DA, Swainson CP, et al. Changes in renal function during acute insulin-induced hypoglycaemia in patients with type 1 diabetes. Diabet Med 1992; 9: 150–155. [DOI] [PubMed] [Google Scholar]
- 29.Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014; 124: 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Standl E, Schnell O, Ceriello A. Postprandial hyperglycemia and glycemic variability: should we care? Diabetes Care 2011; 34(Suppl. 2): S120–S127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014; 124: 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howard G, O’Leary DH, Zaccaro D, et al. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation 1996; 93: 1809–1817. [DOI] [PubMed] [Google Scholar]
- 33.Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes (T2DM) and hypertension. Diabetes Care. Epub ahead of print 30 September 2014. DOI: 10.2337/dc14-1096. [DOI] [PubMed] [Google Scholar]
- 34.Lambers Heerspink HJ, de Zeeuw D, Wie L, et al. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 2013; 15: 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mancia G, Cannon CP, Tikkanen I, et al. BP reduction with the sodium glucose co-transporter 2 inhibitor empagliflozin in type 2 diabetes is similar in treatment naïve as in those on one or ⩾2 antihypertensive agents – further insights from a Dedicated 24h ABPM Study. Circulation 2014; 129: A2343. [Google Scholar]
- 36.Cherney DZ, Perkins BA, Soleymanlou N, et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol 2014; 13: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimlichman R. Treatment of hypertension and metabolic syndrome: lowering blood pressure is not enough for organ protection, new approach-arterial destiffening. Curr Hypertens Rep 2014; 16: 479. [DOI] [PubMed] [Google Scholar]
- 38.MacEwan A, McKay GA, Fisher. M. Drugs for diabetes: part 8 SGLT2 inhibitors. Br J Cardiol 2012; 19: 26–29. [Google Scholar]
- 39.Ferrannini G, Hach T, Crowe S, et al. Energy balance following sodium-glucose co-transporter-2 (SGLT2) inhibition. Diabetologia 2014; OP#3 (OP 01 SGLT2 inhibitors). [Google Scholar]
- 40.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006; 444: 881–887. [DOI] [PubMed] [Google Scholar]
- 41.Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med 2013; 34: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 2013; 382: 941–950. [DOI] [PubMed] [Google Scholar]
- 43.Bolinder J, Ljunggren Ø, Johansson L, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diab Obes Metab 2014; 16: 159–169. [DOI] [PubMed] [Google Scholar]
- 44.Ridderstråle M, Andersen KR, Zeller C, et al. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol 2014; 2: 691–700. [DOI] [PubMed] [Google Scholar]
- 45.Amato MC, Giordano C, Galia M, et al. Visceral adiposity index. A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010; 33: 920–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo W, Guo Z, Wu M, et al. Index of central obesity as a parameter to replace waist circumference for the definition of metabolic syndrome in predicting cardiovascular disease. J Cardiovasc Med 2014; 15: 738–744. [DOI] [PubMed] [Google Scholar]
- 47.Neeland IJ, McGuire DK, Chilton B, et al. The Sodium Glucose Co-transporter 2 Inhibitor (SGLT2i) Empagliflozin reduces weight and markers of visceral adiposity (VA) in type 2 diabetes (T2D) in short- and intermediate term. Circulation 2014; 129: A2340. [Google Scholar]
- 48.Bolinder J, Ljunggren Ö, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus on inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012; 97: 1020–1031. [DOI] [PubMed] [Google Scholar]
- 49.Bays HE. Adiposopathy, diabetes mellitus, and primary prevention of atherosclerotic coronary artery disease: treating ‘sick fat’ through improving fat function with antidiabetes therapies. Am J Cardiol 2012; 110(Suppl.): 4B–12B. [DOI] [PubMed] [Google Scholar]
- 50.Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diab Obes Metab 2013; 15: 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kohan DE, Fioretto P, Tang W, et al. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int 2014; 85: 962–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised double-blind placebo-controlled trial. Lancet Diabetes Endocrinol 2014; 2: 369–384. [DOI] [PubMed] [Google Scholar]
- 53.Cherney D, von Eynatten M, Lund S, et al. Sodium glucose cotransporter 2 (SGLT2) inhibition with empagliflozin (EMPA) reduces microalbuminuria in patients with type 2 diabetes (T2D). Diabetes 2014; 63(Suppl. 1): A293 (1125-P). [DOI] [PubMed] [Google Scholar]
- 54.Mogensen CE. Maximum tubular reabsorption capacity for glucose and renal hemodynamics during rapid hypertonic glucose infusion in normal and diabetic subjects. Scand J Clin Lab Invest 1971; 28: 101–109. [DOI] [PubMed] [Google Scholar]
- 55.Rahmoune H, Thompson PW, Ward JM, et al. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 2005; 54: 3427–3434. [DOI] [PubMed] [Google Scholar]
- 56.Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014; 129: 587–597. [DOI] [PubMed] [Google Scholar]
- 57.Škrtić M, Yang GK, Perkins BA, et al. Characterization of glomerular hemodynamic responses to SGLT2 inhibition in patients with type 1 diabetes and renal hyperfiltration. Diabetologia 2014; 57: 2599–2602. [DOI] [PubMed] [Google Scholar]
- 58.Gilbert RE. Sodium-glucose linked transporter-2 inhibitors: potential for renoprotection beyond blood glucose lowering? Kidney Int 2014; 86: 693–700. [DOI] [PubMed] [Google Scholar]
- 59.Madero M, Sarnak MJ, Wang X, et al. Uric acid and long-term outcomes in CKD. Am J Kidney Dis 2009; 53: 796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ndrepepa G, Braun S, King L, et al. Association of uric acid with mortality in patients with stable coronary artery disease. Metabolism 2012; 61: 1780–1786. [DOI] [PubMed] [Google Scholar]
- 61.Huang H, Huang B, Li Y, et al. Uric acid and risk of heart failure: a systematic review and meta-analysis. Eur J Heart Fail 2014; 16: 15–24. [DOI] [PubMed] [Google Scholar]
- 62.Zand S, Shafiee A, Boroumand M, et al. Serum uric acid is not an independent risk factor for premature coronary artery disease. Cardiorenal Med 2013; 4: 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hach T, Gerich J, Salsali A, et al. Empagliflozin improves glycaemic parameters and cardiovascular risk factors in patients with type 2 diabetes: pooled data from four pivotal phase III trials. Diabetes 2013; 62(Suppl. 1): LB19 [69-LB] (abstract no 94). [Google Scholar]
- 64.Bailey CJ, Gross JL, Pieters A, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomized, double-blind, placebo-controlled trial. Lancet 2010; 375: 2223–2233. [DOI] [PubMed] [Google Scholar]
- 65.Rosenstock J, Aggarwal N, Polidori D, et al. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care 2012; 35: 1232–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheesman C. Solute carrier family 2, member 9 and uric acid homeostasis. Curr Opin Nephrol Hypertens 2009; 18: 428–432. [DOI] [PubMed] [Google Scholar]
- 67.Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2014; 16: 457–466. [DOI] [PubMed] [Google Scholar]
- 68.Hardy E, Ptanszynska A, de Bruin TWA, et al. Changes in lipid profiles of patients with type 2 diabetes mellitus on dapagliflozin therapy. Diabetologia 2013; Suppl. #947, 61. [Google Scholar]
- 69.Sinclair A, Bode B, Harris S, et al. Efficacy and safety of canagliflozin compared with placebo in older patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. BMC Endocr Disord 2014; 14: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nagareddy PR, Murphy AJ, Stirzaker RA, et al. Hyperglycaemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metabolism 2013; 17: 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tahara A, Kurosaki E, Yokono M, et al. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J of Pharmacol 2013; 715: 246–255. [DOI] [PubMed] [Google Scholar]
- 72.Panchapakesan U, Pegg K, Gross S, et al. Effects of SGLT2 inhibition in human kidney proximal tubular cells–renoprotection in diabetic nephropathy? PLoS ONE 2013; 8: e54442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Osorio H, Coronel I, Arellano A, et al. Sodium-glucose cotransporter inhibition prevents oxidative stress in the kidney of diabetic rats. Oxid Med Cell Longev 2012; 2012: 542042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Younis FM, Hollander K, Mayoux EW, et al. Effect of prophylactic treatment with empagliflozin on cardiac function and diabetes in CRDH rats. Diabetes 2014; 63(Suppl. 1): A273 (1056-P). [Google Scholar]
- 75.FDA Briefing Document NDA 202293. Dapagliflozin tablets 5 and 10 mg, http://www.fda.gov/DOWNLOADS/ADVISORYCOMMITTEES/COMMITTEESMEETINGMATERIALS/DRUGS/ENDOCRINOLOGICANDMETA-BOLICDRUGSADVISORYCOMMITTEE/UCM262994.PDF (accessed 29 August 2014).
- 76.FDA briefing material, http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM334550.pdf (accessed 20 September 2014).
- 77.Dziuba J, Alperin P, Racketa J, et al. Modeling effects of SGLT-2 inhibitor dapagliflozin treatment vs. standard diabetes therapy on cardiovascular and microvascular outcomes. Diabetes Obes Metab 2014; 16: 628–635. [DOI] [PubMed] [Google Scholar]
- 78.Food and Drug Administration (Center for Drug Evaluation and Research). Guidance for industry: diabetes mellitus – evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes; 2008, http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627.pdf (accessed 20 September 2014). [Google Scholar]
- 79.European Medicines Agency. Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus, http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129256.pdf (accessed 17 October 2014).
- 80.Zinman B, Inzucchi SE, Lachin JM, et al. Rationale, design, and baseline characteristics of a randomized, placebo-controlled cardiovascular outcome trial of empagliflozin (EMPA-REG OUTCOME™). Cardiovasc Diabetol 2014; 13: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neal B, Perkovic V, de Zeeuw D, et al. Rationale, design, and baseline characteristics of the Canagliflozin Cardiovascular Assessment Study (CANVAS) – a randomized placebo-controlled trial. Am Heart J 2013; 166: 217–223. [DOI] [PubMed] [Google Scholar]
- 82.CANVAS (clinicaltrials.gov identifier: NCT01032629).
- 83.DECLARE-TIMI58 (clinicaltrials.gov identifier: NCT01730534).
- 84.Clintrials.gov. CANVAS-R, http://www.clinicaltrials.gov/ct2/show/NCT01989754?term=canvas-r&rank=1
- 85.Clin.trials.gov. CREDENCE, http://www.clinicaltrials.gov/ct2/show/NCT02065791?term=CREDENCE&rank=2
- 86.Hiatt WR, Kaul S, Smith RJ. The cardiovascular safety of diabetes drugs – insights from the rosiglitazone experience. N Engl J Med 2013; 369: 1285–1287. [DOI] [PubMed] [Google Scholar]