Selective Suppression of Endothelial Cell Apoptosis by the High Molecular Weight Form of Adiponectin (original) (raw)
. Author manuscript; available in PMC: 2015 Mar 26.
Abstract
Adiponectin is an adipocyte-derived, antiatherogenic protein that is present in serum as three isoforms. Total adiponectin levels are decreased in obese or diabetic humans or animal models. This study was designed to elucidate the relative isoform distribution of adiponectin in human disease states and identify the active form of adiponectin toward vascular endothelial cells. The percentage of high molecular weight form (HMW) per total adiponectin was significantly lower in patients with coronary artery disease than control subjects, whereas the hexamer form was similar and the trimer form was significantly higher. During weight reduction in obese subjects, the HMW form increased and the trimer and hexamer forms decreased. Recombinant adiponectin dose-dependently suppressed apoptosis and caspase-3 activity in human umbilical vein endothelial cells (HUVECs). Transduction with dominant-negative AMP-activated protein kinase (AMPK) abolished the suppressive effect of adiponectin on HUVECs. Gel filtration chromatography was used to separate the adiponectin isoforms, and the antiapoptotic effect toward HUVECs was only observed with the HMW form. These data suggest that HMW adiponectin specifically confers the vascular-protective activities of this adipocytokine.
Keywords: atherosclerosis, apoptosis, adiponectin, endothelial cells, AMP-activated protein kinase
Adiponectin is abundantly present in human plasma (ranges 3 to 30 µg/mL), and its levels are decreased in patients and animal models with obesity, diabetes, and coronary artery diseases.1–4 Adiponectin has a number of vascular protective activities,5–9 and it can promote angiogenesis by stimulating AMPK and Akt signaling in endothelial cells.10 Collectively, these findings suggest that decreased plasma adiponectin levels during obesity and diabetes may contribute to vascular disease in these patients. Adiponectin is present in serum as a trimer, hexamer, or high molecular weight form.11 The biological activities of these isoforms is controversial. Waki et al12 has reported that the HMW isoform promotes AMP-activated protein kinase in hepatocytes. In contrast, Tsao et al13 recently reported that only trimers activate AMPK in muscle, whereas hexamers and HMW forms activate NF-κB. Differences in the tissue-specific expression patterns of two adiponectin receptors may contribute to these divergent activities.14 Therefore, isoform abundance should be evaluated when examining the role of adiponectin in different disease states.
Endothelial injury is considered to be a critical event in the pathogenesis of atherosclerosis, plaque erosion, and thrombus formation.15 The turnover rates of endothelial cells (ECs) are accelerated in atherosclerosis-prone regions, and local EC apoptosis has been implicated in this process.16 In patients with obesity-related disorders, EC injury could be caused by high glucose, tumor necrosis factor-α, and oxidized low-density lipoprotein.17–19 In contrast, little is known about antiatherogenic factors that could function to protect EC from apoptosis.
In this study, we examined relative adiponectin isoform abundance in patients with coronary artery disease (CAD) and in obese patients who have undergone weight reduction. It is shown that only HMW adiponectin was suppressed in CAD patients and elevated on weight loss. It is also shown that adiponectin suppresses HUVEC apoptosis and that this activity is exhibited by HMW adiponectin. These data suggest that alterations in the level of HMW adiponectin may be most relevant to the vascular complications associated with obesity-associated diseases.
Materials and Methods
Cell Culture
Human umbilical vein endothelial cells (HUVECs) were purchased from Clonetics and used in passages 3 to 6. HUVECs were maintained in HuMedia-EG2 medium. In some experiments, HUVECs were infected with adenoviral constructs encoding dominant-negative AMP-activated protein kinase (AMPK) α2 (addnAMPK)20 or green fluorescence protein (GFP) at a multiplicity of infection (MOI) of 50 for 24 hours.
Materials
Recombinant human vascular endothelial growth factor (VEGF) was purchased from R&D Systems. Phospho-AMPK (Thr172) and pan-α-AMPK were purchased from Cell Signaling Technology. Recombinant adiponectin was prepared as described previously.10
Cell Viability Assays
The CellTiter 96 AQueous kit (Promega) was used to assess cell viability according to the manufacture’s instructions using MTS reagent. Briefly, cells were plated at a density of 1.5×104 cells/well in a 96-well plate and incubated in growth media for 18 hours. Cells were treated with indicated concentrations of recombinant adiponectin in D-MEM containing 1% FCS for 48 hours. The 490-nm absorbance was measured after two more 1-hour incubations with MTS. The percentage of cell death was calculated as the decrease in absorbance relative to cells incubated in 20% FCS. For heat inactivation, adiponectin was treated with proteinase K (0.2 mg/mL) for 90 minutes at 50°C followed by 20 minutes at 99°C. Alternatively, HUVECs were plated at a density of 3.0×105 cells/well in a 6-well plate and incubated in growth media for 18 hours. Cells were treated in the presence of the indicated concentration of adiponectin or 10 ng/mL VEGF in D-MEM containing 1% FCS for 24 hours. The floating cells were collected in a 15-mL tube. The adherent cells were trypsinized and collected to the same tube. Cells were fixed with 1% glutaraldehyde, stained with Hoechst 33342, and viewed under fluorescence microscopy. Over 500 cells were evaluated for pyknotic nuclei by an investigator who was blinded to the nature of the experiment.
Caspase-3 Activity Assay
Caspase-3 activity was measured using CaspACE assay system (Promega) according to the manufacture’s instructions. Briefly, HUVECs were cultured in 10-cm dish and treated with the indicated concentrations of adiponectin for 24 hours. Then cells were collected, resuspended in cell lysis buffer, subjected to two freeze/thaw cycles, centrifuged, and the supernatant was collected. Five micrograms of protein was used for the assay.
Preparation of Trimer, Hexamer, and High Molecular Weight Form
Recombinant adiponectin was fractionated in a HiLoad 16/60 Superdex 200 column (Amersham) and eluted with PBS. The adiponectin in each peak was concentrated with a centricon 100 (amicon) and used for cell viability assays. The separated fractions of adiponectin (10 µg/mL) were cross-linked with 5 mg/mL of bis (sulfosuccinimidyl) suberate (BS3, Pierce) for 30 minutes at room temperature. Reactions were stopped with 50 mmol/L Tris at pH 8.0 and Western blot analysis was performed using anti-adiponectin monoclonal antibody (ANOC9135).
Western Blot Analysis
After 16-hour serum starvation, cells were treated with 10 µg/mL of each isoform of adiponectin or vehicle for 15 minutes. Cell lysates were resolved by SDS-PAGE. The membranes were immunoblotted with the indicated antibodies.
Analysis of Oligometric State of Adiponectin in Human Plasma
Six obese subjects (3 men and 3 women; BMI, 36.8±2.5 kg/m2; age 50±5 years) who were hospitalized in Osaka University Hospital and achieved significant weight reduction (BMI, 32.8±2.0, P<0.01) for 3 months were enrolled in this study. The weight reduction therapy was performed according to the calorie restriction program in Osaka University Hospital. In brief, starting at 2000 kcal/day, total calorie intake was decreased sequentially (−400 kcal/day for 2 weeks) to 800 kcal/day (carbohydrate 50%, fat 25%, and protein 25%), and this calorie level was maintained. The plasma was obtained before and at the end of the weight reduction period (Mean plasma adiponectin 4.0±0.4 and 6.6±0.4 µg/mL, respectively; P<0.01). Eight male coronary artery disease (CAD) patients with hypoadiponectinemia (BMI, 23.8±0.6 kg/m2; age 60±3 years; mean plasma adiponectin 2.5 µg/mL) and 8 male non-CAD subjects with normoadiponectinemia (BMI, 23.8±0.3 kg/m2; age 60±4 years; mean plasma adiponectin 8.1 µg/mL) who received medical check up in Osaka University Hospital were also enrolled in this study. Plasma samples (50 µL each) were fractionated in a HiLoad 16/60 Superdex 200 column (Amersham) and eluted with PBS. The adiponectin concentration in 1-mL each fraction was determined by ELISA as described previously.1 All subjects enrolled in this study were Japanese and given written informed consent. The Ethic Committee of Osaka University approved this study.
Statistics
Data are expressed as mean±SE. Statistical significance was tested using unpaired t tests. Group differences were determined using analysis of variance (ANOVA) with Fisher’s protected least-significant difference (PLSD) test.
Results
Relative Plasma Adiponectin Isoform Levels in Patients With Obesity and Coronary Artery Disease
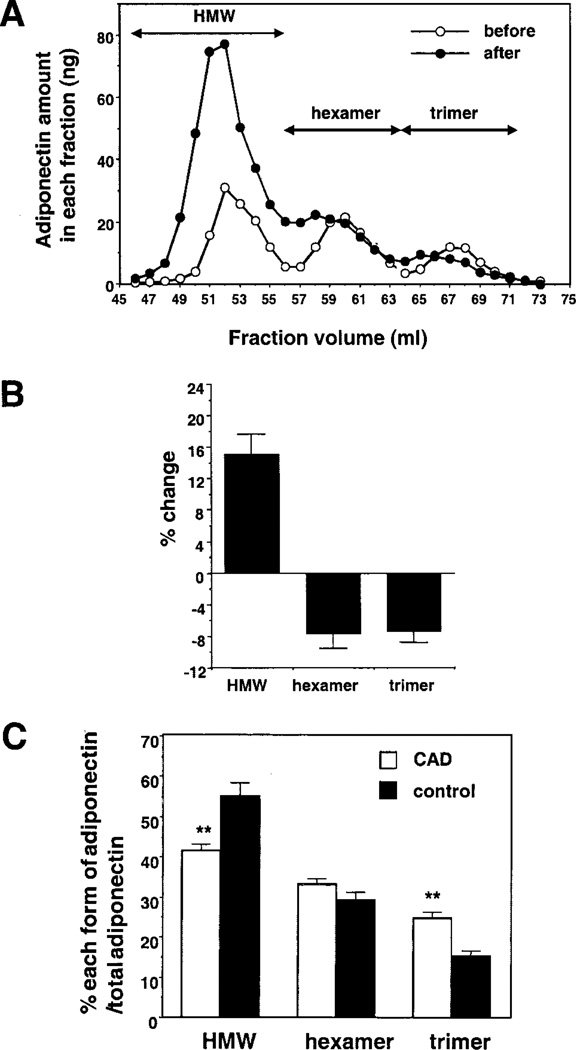
Total plasma adiponectin levels are reduced in patients with obesity and coronary artery disease (CAD) and weight reduction increases adiponectin levels in obese patients.1–4 Therefore, the major adiponectin isoforms were analyzed in obese subjects who achieved significant weight reduction (Figures 1A and 1B). The percentage of HMW per total adiponectin was significantly increased 15.2±2.5% by weight reduction (P<0.001) (Figure 1B). In contrast, levels of trimer and hexamer significantly declined during weight loss (P<0.001). The ratio of three major forms of adiponectin were also assessed in males with CAD and male control subjects (n=8 each). The percentage of HMW per total adiponectin was significantly lower in CAD patients (Figure 1C). In contrast, the relative level of trimer increased under these conditions, although there was no significant change in the level of hexamer. Collectively, these data show that only HMW adiponectin levels are indicative of obesity-related vascular disease.
Figure 1.
Oligometric state of adiponectin in human plasma. A, Representative elution profiles of adiponectin in plasma from the same individual before and after weight reduction. Plasma was fractionated by gel filtration chromatography, and the concentration of adiponectin in each 1-mL fraction was determined by ELISA. B, Average changes of each form of adiponectin per total adiponectin between before and after weight reduction (n=6). C, Percentage of each form of adiponectin per total adiponectin in the patients with CAD or control subjects (n=8 each). Plasma was fractionated by gel filtration chromatography, and the concentration of adiponectin in each fraction was determined by ELISA. **P<0.01.
Adiponectin Protects Endothelial Cells From Apoptosis
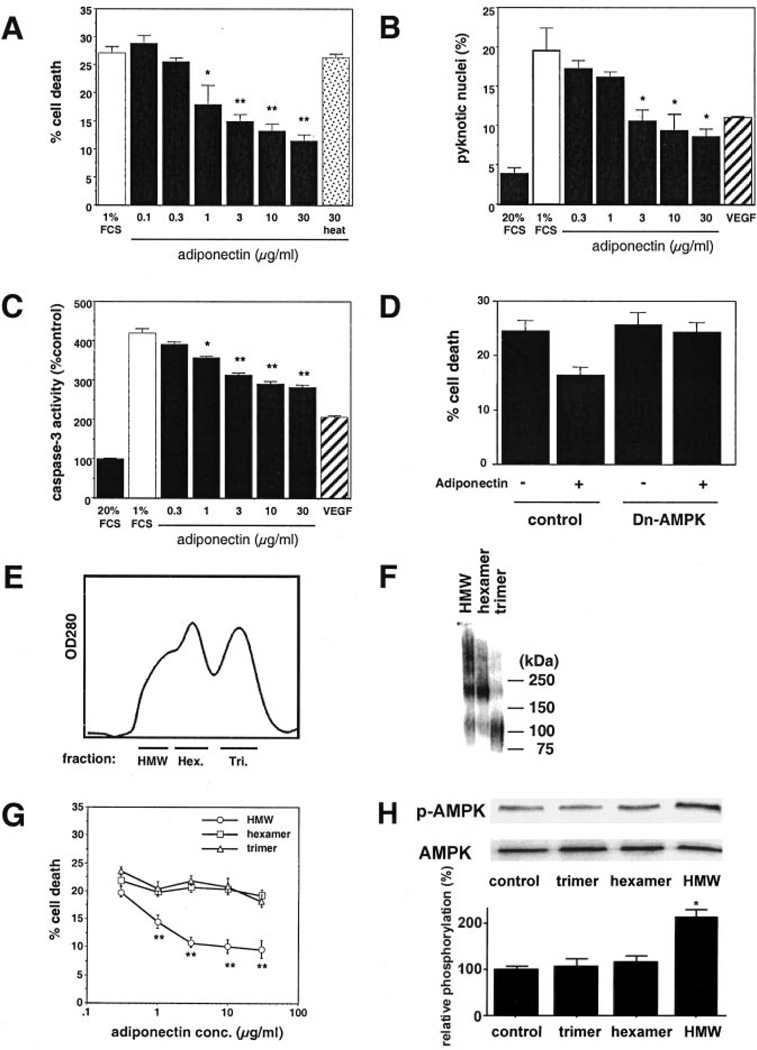
Although a number of studies have examined the role of adiponectin on endothelial cell biology,2,5,10 its effects on EC apoptosis are unknown. Therefore, we examined the effects of nonfractionated, recombinant adiponectin on HUVEC death induced by mitogen-deprivation. An MTS-based assay revealed that adiponectin reduced HUVEC death in a dose-dependent manner (Figure 2A). This effect was lost when adiponectin was treated with proteinase K and heat. Coincubation with anti-adiponectin monoclonal antibody partially reversed the suppression of EC death caused by adiponectin (data not shown). To confirm the antiapoptotic effects of adiponectin, we measured percentage of pyknotic nuclei (Figure 2B) and caspase-3 activity (Figure 2C). Adiponectin decreased both the percentage of apoptotic nuclei and caspase-3 activity induced by serum deprivation. The antiapoptotic effect of adiponectin at 30 µg/mL was similar to that of vascular endothelial growth factor. Recently, it was found that adiponectin stimulates AMPK signaling in endothelial cells.10 Transduction with dominant-negative AMPK abrogated the reduction in EC death caused by adiponectin, but had no effect on basal EC death under these conditions (Figure 2D).
Figure 2.
Effects of adiponectin on HUVEC viability. A, Effect of adiponectin on endothelial cell death induced by serum starvation. Cell viability was quantified with MTS-based assay. Heat indicates heat-digested adiponectin treated with proteinase K. B, Effect of adiponectin on endothelial cell apoptosis induced by serum deprivation. Pyknotic nuclei were quantified. C, Effect of adiponectin on caspase-3 activation induced by serum starvation. D, Role of AMPK in the regulation of adiponectin-induced reduction of endothelial cell death. HUVECs were transduced with an adenoviral vector expressing dominant-negative AMPK (dn-AMPK) or an adenoviral vector expressing GFP (Control). After 24-hour infection, cells were treated with adiponectin (30 µg/mL) or vehicle under serum deprivation conditions. Cell viability was quantified with MTS-based assay. E, Recombinant mouse adiponectin was separated by gel filtration chromatography into trimer (Tri.), hexamer (Hex.), and higher molecular weight form (HMW) fractions. Representative profile is shown. F, Western blot analysis of the adiponectin fractions. G, Effects of 3 different oligometric forms of adiponectin on endothelial cell death induced by serum starvation. Cell viability was quantified by MTS-based assay. Data are mean±SE of 4 experiments. *P<0.05, **P<0.01 vs each control. H, Effects of 3 different oligometric forms of adiponectin on AMPK phosphorylation. Cells were treated with each form of adiponectin (10 µg/mL) or vehicle for 15 minutes. Representative blots are shown. Relative phosphorylation levels of AMPK were quantified using NIH image program. Immunoblots were normalized to total loaded protein. *P<0.01 vs control.
We next investigated which forms of adiponectin exerted the antiapoptotic effect on HUVECs. Recombinant adiponectin was fractionated by gel filtration chromatography (Figure 2E). Aliquots of each fraction were evaluated for trimer, hexamer, and HMW by immunoblot analysis after cross-linking (Figure 2F). The HMW fraction of adiponectin suppressed cell death in serum-deprived HUVEC cultures in a dose-dependent manner (Figure 2G). In contrast, the trimer or hexamer form of adiponectin had little effect on EC survival under these conditions. Finally, we investigated which forms of adiponectin activated AMPK signaling in HUVECs. Among three oligometric forms of adiponectin, only the HMW form of adiponectin stimulated the phosphorylation of AMPK (Figure 2H).
Discussion
Recent data have revealed functional differences among the different adiponectin isoforms.12,13,21 Therefore, we assessed relative adiponectin isoform abundance in patients with CAD. It was found that CAD correlated with a reduction in the HMW isoform, whereas the hexamer and trimer forms were unchanged or increased, respectively. Consistent with these findings, significant weight reduction in patients is associated with an increase in the HMW form of adiponectin. Therefore, these data suggest that the HMW isoform may selectively contribute to the development of vascular diseases that are associated with obesity.
In the present study, we also demonstrated that physiological concentrations of adiponectin promote the survival of macrovascular endothelial cells. Importantly, these effects were specifically mediated by the HMW form of adiponectin. It is well known that the endothelial cell apoptosis can impair endothelial barrier function and increase susceptibility toward coronary thrombosis through the release of apoptotic cell debris into the blood stream, which directly activate coagulation cascade.22–24 Therefore, the prevention of EC apoptosis by HMW adiponectin could be a contributing feature of the antiatherogenic activities of this adipocytokine.9
Finally, our data have shown that AMPK signaling is essential for the antiapoptotic activities of adiponectin on endothelial cells. AMPK is a stress-activated protein kinase that participates in energy regulation and metabolic homeostasis. Recently, we reported that AMPK signaling in endothelial cells is essential for angiogenesis under conditions of hypoxic stress20 and that adiponectin promotes angiogenesis in normoxic EC via activation of AMPK-dependent pathways.10 The identification of an antiapoptotic activity of HMW adiponectin, reported in this study, is consistent with a proangiogenic function for this adipocytokine.
Acknowledgments
This work was supported by the Japanese Ministry of Education and the Japan Society for Promotion of Science-Research for the Future Program and by NIH grants AR40197, HD23681, AG17241, and AG15052 to Dr Walsh. Dr Ouchi was supported by a grant from the Uehara Memorial Foundation.
References
- 1.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 2.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 3.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 4.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 5.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-κB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 6.Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, Hotta K, Muraguchi M, Ohmoto Y, Yamashita S, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 7.Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, Kumada M, Hotta K, Nishida M, Takahashi M, Nakamura T, Shimomura I, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105:2893–2898. doi: 10.1161/01.cir.0000018622.84402.ff. [DOI] [PubMed] [Google Scholar]
- 8.Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, Kumada M, Okamoto Y, Nagaretani H, Nishizawa H, Kishida K, Komuro R, Ouchi N, Kihara S, Nagai R, Funahashi T, Matsuzawa Y. Role of adiponectin in preventing vascular stenosis: the missing link of adipovascular axis. J Biol Chem. 2002;277:37487–37491. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 10.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003;14:561–566. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes: molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 13.Tsao TS, Tomas E, Murrey HE, Hug C, Lee DH, Ruderman NB, Heuser JE, Lodish HF. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity: different oligomers activate different signal transduction pathways. J Biol Chem. 2003;278:50810–50817. doi: 10.1074/jbc.M309469200. [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 15.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 16.Caplan BA, Schwartz CJ. Increased endothelial cell turnover in areas of in vivo Evans Blue uptake in the pig aorta. Atherosclerosis. 1973;17:401–417. doi: 10.1016/0021-9150(73)90031-2. [DOI] [PubMed] [Google Scholar]
- 17.Baumgartner-Parzer SM, Wagner L, Pettermann M, Grillari J, Gessl A, Waldhausl W. High-glucose–triggered apoptosis in cultured endothelial cells. Diabetes. 1995;44:1323–1327. doi: 10.2337/diab.44.11.1323. [DOI] [PubMed] [Google Scholar]
- 18.Karsan A, Yee E, Harlan JM. Endothelial cell death induced by tumor necrosis factor-α is inhibited by the Bcl-2 family member, A1. J Biol Chem. 1996;271:27201–27204. doi: 10.1074/jbc.271.44.27201. [DOI] [PubMed] [Google Scholar]
- 19.Dimmeler S, Haendeler J, Galle J, Zeiher AM. Oxidized low-density lipoprotein induces apoptosis of human endothelial cells by activation of CPP32-like proteases. Circulation. 1997;95:1760–1763. doi: 10.1161/01.cir.95.7.1760. [DOI] [PubMed] [Google Scholar]
- 20.Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem. 2003;278:31000–31006. doi: 10.1074/jbc.M300643200. [DOI] [PubMed] [Google Scholar]
- 21.Tsao TS, Murrey HE, Hug C, Lee DH, Lodish HF. Oligomerization state-dependent activation of NF-κB signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30) J Biol Chem. 2002;277:29359–29362. doi: 10.1074/jbc.C200312200. [DOI] [PubMed] [Google Scholar]
- 22.Bombeli T, Karsan A, Tait JF, Harlan JM. Apoptotic vascular endothelial cells become procoagulant. Blood. 1997;89:2429–2442. [PubMed] [Google Scholar]
- 23.Bombeli T, Schwartz BR, Harlan JM. Endothelial cells undergoing apoptosis become proadhesive for nonactivated platelets. Blood. 1999;93:3831–3838. [PubMed] [Google Scholar]
- 24.Mallat Z, Hugel B, Ohan J, Leseche G, Freyssinet JM, Tedgui A. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: a role for apoptosis in plaque thrombogenicity. Circulation. 1999;99:348–353. doi: 10.1161/01.cir.99.3.348. [DOI] [PubMed] [Google Scholar]