Global epidemiology of type 1 diabetes in young adults and adults: a systematic review (original) (raw)
Abstract
Background
Although type 1 diabetes (T1D) can affect patients of all ages, most epidemiological studies of T1D focus on disease forms with clinical diagnosis during childhood and adolescence. Clinically, adult T1D is difficult to discriminate from certain forms of Type 2 Diabetes (T2D) and from Latent Autoimmune Diabetes in Adults (LADA).
We searched the information available worldwide on the incidence of T1D among individuals over 15 years of age, and which diagnostic criteria should be used use to qualify T1D in adults. We then studied the variation of T1D incidence with age in adults, and compared it to the incidence in the <15 years-old.
Methods
A systematic review of the literature was performed to retrieve original papers in English, French and Spanish published up to November 6, 2014, reporting the incidence of T1D among individuals aged over 15 years. The study was carried out according to the PRISMA recommendations.
Results
We retrieved information reporting incidence of T1D among individuals aged more than 15 years in 35 countries, and published in 70 articles between 1982 and 2014. Specific anti-beta-cell proteins or C-peptide detection were performed in 14 of 70 articles (20%). The most frequent diagnostic criteria used were clinical symptoms and immediate insulin therapy. Country-to-country variations of incidence in those aged >15 years paralleled those of children in all age groups. T1D incidence was larger in males than in females in 44 of the 54 (81%) studies reporting incidence by sex in people >15 years of age. The overall mean male-to-female ratio in the review was 1.47 (95% CI = 1.33-1.60, SD = 0.49, n = 54, p = <0.0001). Overall, T1D incidence decreased in adulthood, after the age of 14 years.
Conclusions
Few studies on epidemiology of T1D in adults are available worldwide, as compared to those reporting on children with T1D. The geographical variations of T1D incidence in adults parallel those reported in children. As opposed to what is known in children, the incidence is generally larger in males than in females. There is an unmet need to evaluate the incidence of autoimmune T1D in adults, using specific autoantibody detection, and to better analyze epidemiological specificities – if any – of adult T1D.
PROSPERO registration number
Electronic supplementary material
The online version of this article (doi:10.1186/s12889-015-1591-y) contains supplementary material, which is available to authorized users.
Keywords: Type 1 diabetes, Systematic review, Adults, Incidence, Epidemiology
Background
The worldwide epidemiology of childhood Type 1 diabetes (T1D) was extensively described in the 6th edition of the International Diabetes Federation (IDF) [1]. Data were retrieved in approximately 45% of the countries [1-4]. In contrast, we are unaware of a similar review on the worldwide epidemiology of adult T1D diabetes, although T1D is known to occur even late in adults [5-7]. A major limitation of the epidemiology of T1D in adults is certainly the difficulty there is to distinguish it from Type 2 diabetes (T2D) requiring insulin treatment or from Latent Autoimmune Diabetes in Adults (LADA), when specific markers of autoimmunity are not searched.
Here, our primary objective was to describe – through a systematic review of the literature – the available published information on adult T1D incidence, and the diagnostic criteria used for case definition. A secondary objective was to study how the variations of T1D incidence in adults mirrored those in children.
Methods
Literature review
A systematic review was conducted according to the PRISMA recommendations to retrieve original papers published in English, French and Spanish up to November 6th, 2014, in peer-reviewed journals reporting the incidence of T1D among individuals aged more than 15 years, in population-based studies (i.e. collected in a defined geographic area [8]) and reporting the diagnostic criteria used to define T1D.
The databases used for the literature search were Medline (PubMed), Google Scholar and Thomson Reuters (Web of Knowledge). The protocol of the search was registered in the International Prospective Register of Systematic Reviews (PROSPERO) and is available on http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42012002369 (Registration number: 2012:CRD42012002369). Figure 1 presents the flow diagram of the bibliographic search, Additional file 1 for the full electronic search strategy, and Additional file 2 for the PRISMA checklist.
Figure 1.
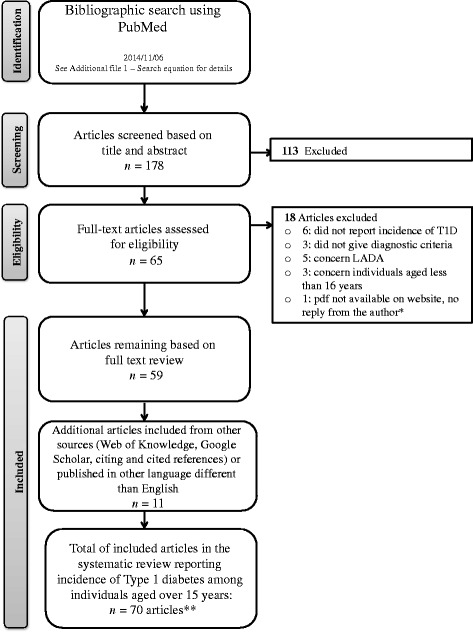
PRISMA Flow diagram bibliographic search strategies. * Kumar P, et al. Indian Med Assoc. 2008;106(11):708–711. ** The article: Radosevic B, et al. Pediatr Diabetes. 2013;14(4):273–4 gives information from two countries: 1) Bosnia and Herzegovina: Republic of Srpska and 2) Slovenia, Nationwide.
Data collection
For each study, the following information was extracted:
- the identification of the study: authors, title, journal, publication year,
- the period and country of study. The country was categorized by its World Health Organization (WHO) region and economic level: high-income (HIGH) or low- and middle-income (LMIC) [9],
- the geographic coverage of the study: nationwide (when the study was performed in the entire nation) and local (when it was restricted to a given region, city, or a geographically defined population),
- the diagnostic criteria used to define T1D in adults: detection of autoantibodies against beta-cells (such as: islet cell antibody (ICA), insulin autoantibody (IAA), islet antigen-2 autoantibody (IA-2), anti-glutamic acid decarboxylase antibodies (GAD)), measurement of the fasting C-peptide level [7], need for permanent insulin therapy, time when the administration of insulin therapy was started, and clinical signals of T1D diabetes such as ketosis, ketonuria and weight loss,
- the sources of data/registers reporting T1D incidence in the studies, defined according to LaPorte et al. [10] as: primary source of information: a “well-established system of standardized registries for identifying new cases”, for example national or regional registers, secondary source of information: other different sources of cases “that would provide a check on the degree of ascertainment”, for example medical records or hospital discharges, and tertiary source of information: a third approach for identifying cases, for example, through surveillance system or death certificates,
- the reported percentage of completeness/ascertainment between sources of information reporting incidence [10],
- the incidence rates reported in the text, tables or graph (expressed as new cases per 100.000 persons/year) by sex and age classes,
- additional information such as those concerning rural/urban, or ethnic differences.
Data analyses
The country distribution of the T1D incidence information and the analysis of the diagnostic criteria used were performed on the entire set of articles retrieved. For the few papers for which the results were presented by ethnic origin, we estimated the mean value of the incidence for the given period in the countries/regions concerned.
Correlation between adult and children T1D incidences
In the geographical correlation analyses between children and adult incidences, we considered for each country the more recent nationwide study published, or if not available, the last published set of local studies retrieved from a given area in the country; in addition, we included all published papers reporting auto-antibodies against beta-cells or C-peptide. To obtain an estimate of the incidence of T1D in children in the countries for which the adult incidence was available, we used the data provided by the same adult paper, when available. The incidence of T1D in children was not available in 9 of these papers included in the geographical correlation analyses. In this case, it was estimated through a separate systematic review focused on the corresponding countries and periods (see Additional file 3).
Statistics
Data were extracted from graphs using GraphClick [11].
The country-to-country co-variation of children and adult incidences was quantified by the Spearman correlation and a linear regression.
The R software (version 3.0.1) was used for statistical and graphic analyses [12].
Results
Description of the information obtained from the systematic review on adult T1D
Seventy articles reporting incidence of T1D in young adults and adults aged over than 15 years concerned one country, and one article concerning two countries were retrieved in this systematic review, resulting in a total of 71 studies covering 35 countries (Table 1). Twenty-four of the 71 studies were nationwide; 43 papers provided information on the T1D incidence in the age class 15–29 years, 26 in the age class 30–59 years, and 6 in the persons aged >60 years.
Table 1.
Systematic review of T1D in adults, diagnostic criteria and sources of information
| Study information | T1D diagnosis criteria in adults and young adults | Source of information and validation of ascertainment between sources | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country, area reported in the article | First author, publication year | Ref | Age range | Period | Detect. AA/C-Peptide | Need of insulin therapy | Administration insulin therapy | Clinical impression | Ketosis/ ketonuria | Weight loss | Primary | Secondary | Tertiary | % of ascertainment |
| African Region, LMIC | ||||||||||||||
| Mauritius: NW | Tuomileht J., 1993† | [13] | 0-19 | 1986-1990 | No | Yes | From diagnosis | Yes | NA | NA | Medical reports | Medical statistics | NA | 95.0 |
| United Republic of Tanzania: Dar es Salaam | Swai A. B., 1993† | [14] | 0-19 | 1982-1991 | No | Yes | From diagnosis | Yes | NA | NA | Medical reports | Hospital records | NA | NA |
| Eastern Mediterranean Region, LMIC | ||||||||||||||
| Iran (Islamic Republic of): Fars | Pishdad G. R., 2005 † | [15] | 0-29 | 1990-1994 | Yes (a) | Yes | From diagnosis | Yes | Yes | Yes | Medical reports from endocrinologists | Medical records | NA | 100 |
| Libyan Arab Jamahiriya: Benghazi | Kadiki O. A., 1996† | [16] | 0-34 | 1981-1990 | No | Yes | From diagnosis | NA | Yes | NA | National Diabetes Program | Hospital registers | NA | 95.0 |
| Tunisia: Beja, Monastir, Gafsa | Ben Khalifa F., 1998† | [17] | 0-19 | 1990-1994 | No | Yes | From diagnosis | Yes | NA | NA | Hospital records | School health centers | NA | 96.0 |
| European Region, LMIC | ||||||||||||||
| Croatia: Zagreb | Roglic G., 1995† | [18] | 0- > 55 | 1988-1992 | No | Yes | Within 1 week of diagnosis | Yes | Yes | NA | National Diabetes Program | Death certificates | Diabetes association | 96.2 |
| Estonia: NW | Kalits I., 1990† | [19] | 0- > 50 | 1988-1988 | No | Yes | From diagnosis | Yes | Yes | Yes | NA | NA | NA | NA |
| Lithuania: NW | Ostrauskas R., 2011† | [20] | 15-34 | 1991-2008 | No | Yes | Within 2 weeks of diagnosis | Yes | Yes | Yes | National Diabetes Program | Regional endocrinologist | Notes of patient insurance | 86.8 |
| Lithuania: NW | Pundziute-Lycka A., 2003 | [21] | 0-39 | 1991-2000 | No | Yes | Within 2 weeks of diagnosis | Yes | Yes | NA | National Diabetes Program | Pediatrician and endocrinologist reports | Death certificates | 91.2 |
| Lithuania: NW | Ostrauskas R., 2000 | [22] | 15-39 | 1991-1997 | No | Yes | Within 2 weeks of diagnosis | Yes | Yes | NA | National Diabetes Program | Pediatrician and endocrinologist reports | Death certificates | 91.2 |
| Poland: Bialystok | Kretowski A., 2001† | [23] | 0-29 | 1994-1998 | No | Yes | From diagnosis | Yes | Yes | Yes | Pediatric and Internal medicine records | Hospital discharge registers | NA | 98.5 |
| Poland: Province of Rzeszow | Sobel-Maruniak A., 2006† | [24] | 0-29 | 1980-1999 | No | Yes | From diagnosis | Yes | NA | NA | Pediatric and Internal medicine records | Others health care registers | NA | 99.0 |
| Poland: Province of Rzeszow | Grzywa M. A., 1995 | [25] | 0-29 | 1980-1992 | No | Yes | From diagnosis | Yes | NA | NA | Pediatric and Internal medicine records | Others health care registers | NA | 99.0 |
| Poland: Warsaw | Wysock M. J., 1992† | [26] | 0-29 | 1983-1988 | No | Yes | From diagnosis | Yes | NA | NA | Medical records from diabetic clinics | General practitioners and diabetologist registers | Death certificates | NA |
| Romania: Bucharest | Ionescu-Tirgoviste C., 1994† | [27] | 0- ≥ 85 | 1981-1991 | No | Yes | From diagnosis | Yes | Yes | NA | Bucharest Diabetes Registry | NA | NA | NA |
| Slovakia: NW | Kyvik K O, 2004† | [28] | 15-29 | 1996-1997 | No | Yes | From diagnosis | Yes | NA | NA | Pediatrician and endocrinologist reports | Other health care registers | NA | 80.0 |
| European Region, HIGH | ||||||||||||||
| Austria: Upper | Rami B., 2001† | [29] | 0-29 | 1994-1996 | No | Yes | From diagnosis | Yes | NA | NA | Pediatricians and endocrinologists reports | Austrian Diabetes Association | NA | 87.0 |
| Belgium: Antwerp | Weets I., 2007 † | [30] | 0-39 | 1989-2003 | Yes | Yes | From diagnosis | Yes | NA | NA | Pediatricians and endocrinologists reports | General practitioners and diabetes nurses reports | Diabetes associations and self-reporting | 97.0 |
| Belgium: Antwerp | Weets I., 2002 † | [31] | 0-39 | 1989-2000 | Yes | Yes | From diagnosis | NA | NA | NA | Pediatrician and endocrinologist reports | General practitioner and diabetes nurse reports | Diabetes associations and self-reporting | 93 |
| Belgium: Antwerp | Vandewalle C., 1997 † | [32] | 0-39 | 1989-1995 | Yes | Yes | From diagnosis | Yes | Yes | Yes | Pediatrician and endocrinologist reports | General practitioner and diabetes nurse reports | Diabetes associations and self-reporting | 85 |
| Bosnia and Herzegovina: Republic of Srpska | Radosevic B., 2013† | [33] | 0-18 | 1998-2010 | No | Yes | From diagnosis | Yes | NA | NA | Hospital records | Insulin prescription registers | NA | 100 |
| Denmark: Copenhagen and Frederiksborg | Molbak A. G., 1994 † | [34] | 30-95 | 1973-1977 | Yes (b) | Yes | From diagnosis | Yes | Yes | Yes | Hospital discharges | General practitioners and diabetologist registers and death certificates | Missing coding of T1D diagnosis in hospital admissions | 99.0 |
| Finland: NW | Lammi N., 2007 † | [35] | 15-39 | 1992-1996 | Yes | Yes | From diagnosis | Yes | NA | NA | National Diabetes Program | Hospital discharge registers | Drug reimbursement registers | 88.0 |
| France: Aquitaine, Lorraine, Basse Normandie, Haute Normandie | Charkaluk M. L, 2002† | [36] | 0-19 | 1988-1997 | No | Yes | None declared | NA | NA | NA | Prospective registers | French Social Security registers | NA | 96.0 |
| France: Aquitaine, Lorraine, Basse Normandie, Haute Normandie | Levy-Marchal, C., 1998 | [37] | 0-19 | 1988-1995 | No | Yes | None declared | NA | NA | NA | Prospective registers | French Social Security registers | NA | 96.0 |
| Israel: NW | Blumenfeld O., 2014† | [38] | 0-17 | 1997-2010 | No | Yes | From diagnosis | Yes | NA | NA | Israel juvenile diabetes register | Israel Center for Disease Control | NA | NA |
| Israel: NW | Sella T., 2011 | [39] | 0-17 | 2000-2008 | No | Yes | None declared | Yes | NA | NA | Israel juvenile diabetes register | Israel Center for Disease Control | NA | NA |
| Israel: NW | Koton S., 2007 | [40] | 0-17 | 1997-2003 | No | Yes | From diagnosis | Yes | NA | NA | Israel juvenile diabetes register | NA | NA | NA |
| Italy: Lombardie | Garancini, P., 1991† | [41] | 0-34 | 1981-1982 | No | Yes | None declared | NA | NA | NA | Hospital discharge records | Hospital admission records | NA | 85.7 |
| Italy: Pavia | Tenconi M. T., 1995† | [42] | 0-29 | 1988-1992 | No | Yes | From diagnosis | Yes | NA | NA | Hospital records | Drug registers | NA | 100 |
| Italy: Sardinia | Muntoni S, 1992† | [43] | 0-29 | 1989-1990 | No | Yes | From diagnosis | Yes | NA | NA | Hospital records | Diabetes association | NA | 92.8 |
| Italy: Sardinia (Oristano) | Frongia O., 1997† | [44] | 0-29 | 1993-1996 | No | Yes | From diagnosis | Yes | NA | NA | Hospital records | Drug registers | NA | 100 |
| Italy: Turin | Bruno G., 2009 † | [45] | 15-29 | 2000-2004 | Yes | Yes | Within 6 months of diagnosis | NA | NA | NA | Hospital records | Drug registers | NA | NA |
| Italy: Turin | Bruno G., 2005 † | [46] | 30-49 | 1999-2001 | Yes | Yes | Within 6 months of diagnosis | NA | Yes | NA | Diabetes clinics | Drug registers | NA | 99.0 |
| Italy: Turin | Bruno G., 1993 | [47] | 0-29 | 1984-1988 | No | Yes | From diagnosis | NA | Yes | NA | Diabetic clinics records | Hospital discharge records | NA | 97.0 |
| Luxembourg: NW | De Beaufort C. E., 1988† | [48] | 0-19 | 1977-1986 | No | Yes | None declared | NA | NA | NA | Pediatric and Internal medicine records | Dutch Diabetes Association | NA | 100 |
| Malta: NW | Schranz A. G., 1989† | [49] | 0-24 | 1980-1987 | No | Yes | Within 3 moths of diagnosis | Yes | Yes | Yes | Medical reports | Diabetic clinic records | NA | NA |
| Netherlands: NW | Ruwaard D., 1994† | [50] | 0-19 | 1988-1990 | No | Yes | None declared | NA | NA | NA | Pediatric and Internal medicine records | NA | NA | 81.0 |
| Norway: NW | Joner G., 1991† | [51] | 15-29 | 1978-1982 | No | Yes | From diagnosis | NA | NA | NA | Pediatricians and endocrinologists reports | Hospital records | NA | 90.0 |
| Slovenia: NW | Radosevic B., 2013† | [33] | 0-18 | 1998-2010 | No | Yes | From diagnosis | Yes | NA | NA | Slovenian National Registry of Childhood diabetes | Insulin prescription registers | NA | 100 |
| Spain: Badajoz | Morales-Perez F. M., 2000† | [52] | 0-29 | 1992-1996 | No | Yes | From diagnosis | Yes | Yes | NA | Pediatricians and endocrinologists reports | Diabetic clinic records | NA | 95.0 |
| Spain: Canarias Islands | Carrillo Dominguez, A., 2000† | [53] | 0-30 | 1995-1996 | No | Yes | None declared | Yes | NA | Yes | Hospital records and Endocrinologist reports | Diabetes association reports and sales on blood glucose monitors | NA | 90.1 |
| Spain: Catalonia | Abellana R., 2009 † | [54] | 0-29 | 1989-1998 | Yes (c) | Yes | From diagnosis | Yes | Yes | NA | Catalan Registry of Type 1 Diabetes | Summer camps, associations, and prescription data | NA | 90.0 |
| Spain: Catalonia | Goday A., 1992 | [55] | 0-29 | 1987-1990 | No | Yes | From diagnosis | Yes | NA | NA | Catalan Registry of Type 1 Diabetes | Summer camps, patient associations, and prescription data | NA | 90.1 |
| Spain: Navarra | Forga L., 2014 † | [56] | 0- > 45 | 2009-2012 | Yes | Yes | Within 6 months of diagnosis | Yes | Yes | NA | Hospital records | Electronic medical records, diabetes associations | NA | 98.4 |
| Spain: Navarra | Forga L., 2013 † | [57] | 0-79 | 2009-2011 | Yes | Yes | Within 6 months of diagnosis | Yes | Yes | NA | Hospital records | Electronic medical records, diabetes associations | NA | 98.4 |
| Sweden: NW | Dahlquist G. G., 2011† | [58] | 0-34 | 1983-2007 | No | Yes | From diagnosis | Yes | Yes | Yes | National Diabetes Program | Pediatricians and endocrinologist reports | NA | 96.0 |
| Sweden: NW | Östman J., 2008 | [59] | 15-34 | 1983-2002 | No | Yes | From diagnosis | Yes | NA | NA | National Diabetes Program | Pediatrician and endocrinologist reports | Computer-based patient administrative register | 82 |
| Sweden: NW | Pundziute-Lycka A., 2002 | [60] | 0-34 | 1983-1998 | No | Yes | From diagnosis | Yes | Yes | Yes | National Diabetes Program | Pediatrician and endocrinologist reports | Computer-based patient administrative register | 91.2 |
| Sweden: NW | Nyström L., 1992 | [61] | 0-34 | 1983-1987 | No | Yes | None declared | NA | NA | NA | National Diabetes Program | Hospital admission and discharge registers | NA | 89 |
| Sweden: NW | Blohme G., 1992 | [62] | 15-34 | 1983-1987 | No | Yes | From diagnosis | Yes | Yes | Yes | National Diabetes Program | Hospital admission and discharge registers | NA | NA |
| Sweden: Kronoberg | Thunander M., 2008 † | [63] | 0-100 | 1998-2001 | Yes | Yes | Within 4 weeks of diagnosis | Yes | Yes | NA | Opportunistic screening of all adult patients in contact with the medical care system | Departments of ophthalmology | NA | 98.0 |
| United Kingdom: NW | Imkampe A. K., 2011† | [64] | 0-34 | 1991-2008 | No | Yes | Within 3 moths of diagnosis | Yes | NA | NA | National Diabetes Program | Pediatricians and endocrinologist reports | NA | NA |
| United Kingdom: Oxford region | Bingley P. J., 1989 | [65] | 0-21 | 1985-1986 | No | Yes | From diagnosis | Yes | NA | NA | Medical reports from general practioners and pediatricians | Regional hospital records | NA | 95.0 |
| Region of the Americas, LMIC | ||||||||||||||
| Barbados: NW | Jordan O. W., 1994† | [66] | 0-29 | 1982-1991 | No | Yes | From diagnosis | Yes | NA | NA | Hospital records | Others health care registers | NA | 94.0 |
| Region of the Americas, HIGH | ||||||||||||||
| Canada: Quebec | Legault L., 2006† | [67] | 0-18 | 2000 | No | Yes | None declared | NA | NA | NA | Departmental program: Régie des Rentes du Québec program | NA | NA | NA |
| United States of America: Alabama (Jefferson County) | Wagenknecht L. E., 1991† | [68] | 0-19 | 1979-1988 | No | Yes | None declared | NA | NA | NA | Hospital records | Summer camps, patient associations, and prescription data | NA | NA |
| United States of America: Alabama (Jefferson County) | Wagenknecht L. E.,1989 | [69] | 0-19 | 1979-1985 | No | Yes | From diagnosis | Yes | NA | NA | Hospital records | Association registers | NA | 95.0 |
| United States of America: Colorado | Vehik K., 2007† | [70] | 0-17 | 2000-2004 | No | Yes | Within 2 weeks of diagnosis | Yes | NA | NA | Pediatricians and endocrinologists reports | Other health care registers | The SEARCH Study | 96.5 |
| United States of America: Colorado | Kostraba J. N., 1992 | [71] | 0-17 | 1978-1988 | No | Yes | Within 2 weeks of diagnosis | Yes | NA | NA | Pediatricians and endocrinologists reports | Hospital registers | NA | 93.3 |
| United States of America: Pennsylvania (Allegheny) | Libman I. M., 1998† | [72] | 0-19 | 1990-1994 | No | Yes | From diagnosis | Yes | NA | NA | Medical reports | General practitioners and diabetes nurses reports | NA | 97.7 |
| United States of America: Rhode Island | Fishbein H. A., 1982† | [73] | 0-29 | 1979-1980 | No | Yes | None declared | NA | NA | NA | Medical reports | Insulin prescription registers | NA | NA |
| United States of America: five areas § | Bell R., 2009 † | [74] | 0-19 | 2002-2005 | Yes | Yes | From diagnosis | Yes | NA | NA | Medical reports | Other health care registers | The SEARCH Study | NA |
| United States of America: Wisconsin | Allen C., 1986† | [75] | 0-29 | 1970-1979 | No | Yes | From diagnosis | Yes | NA | NA | Hospital discharges | Pediatricians and endocrinologist reports | NA | 90.0 |
| United States of America: The United States Navy | Gorham C., 1993 | [76] | 17-34 | 1974-1988 | No | NA* | None declared | Yes | NA | NA | Hospital discharges | NA | NA | NA |
| Western Pacific Region, HIGH | ||||||||||||||
| Australia: New South Wales | Tran F., 2014† | [77] | 10-18 | 2001-2008 | No | Yes | NA | Yes | NA | Yes | Endocrine group diabetes register | National diabetes register | NA | 96.0 |
| Australia: Sydney (Southern Metropolitan Health Region) | Sutton L., 1989† | [78] | 0-19 | 1984-1987 | No | Yes | From diagnosis | Yes | NA | NA | Medical reports from general practioners and pediatricians | Schools in the area | Syringe register | NA |
| Japan: Osaka | Sasaki A., 1992† | [79] | 0-18 | 1978-1988 | No | Yes | None declared | Yes | Yes | NA | Medical benefits system | NA | NA | NA |
| New Zealand: Canterbury | Scott, R. S., 1991† | [80] | 0- ≥ 80 | 1981-1986 | No | Yes | Within 1 year of diagnosis | Yes | NA | Yes | Community-based surveys administrated in pharmacies where diabetic patients acquired their insulin supplies | Hospital admission and discharge registers and diabetologist | NA | 95.0 |
| Other Regions currently non WHO | ||||||||||||||
| Taiwan: NW | Lin W.-H., 2013 † | [81] | 0- ≥ 60 | 1999-2010 | Yes | Yes | None declared | Yes | Yes | NA | National Health Insure register and Illness certificates | Random sample of a database used to reimbursements | NA | 98.3 |
| US Virgin Islands: NW | Washington R. E., 2013† | [82] | 0-19 | 2001-2010 | No | Yes | From diagnosis | Yes | Yes | Yes | Medical reports | Medical providers | NA | 98.7 |
A primary source of information was reported in 99% (70 of 71) of the studies: among these reported sources, 60% (42 of 70) were from medical/hospital records, 36% (25 of 70) from national or regional registers, and 4% (3 of 70) from other sources, such as community-based surveys; a secondary source of information was reported in 90% (64 of 71) of the studies: among these reported sources, 58% (37 of 64) were from medical/hospital records, 16% (10 of 64) from associations of patients, 14% (9 of 64) from drug or supplies prescription registers, 8% (5 of 64) from national or regional registers, and 5% (3 of 64) from death certificates and schools registers; finally, a tertiary source of information was reported in 21% (15 of 71) of the studies: among these reported sources, 27% (4 of 15) were from national or regional registers, 27% (4 of 15) from associations of patients, 20% (3 of 15) from death certificates, 20% (3 of 15) from drug or supplies prescription registers, and 7% (1 of 15) from medical registers; see details in Table 1. Percentage of ascertainment (completeness) between sources of information was evaluated in 53 of 71 (75%) studies. The mean percentage of ascertainment of these 53 studies was 94% (Table 1).
In the group of young adults (15–19), the lowest incidence of T1D was reported in Mauritius, (1.1/100.000 persons/year) [13], and the highest in Estonia (39.9/100.000 persons/year) [19]. In the 70–79 year age group, the lowest incidence was reported in Navarra, Spain (0.8/100.000 persons/year) [57] and the highest in Kronoberg, Sweden (55/100.000 persons /year) [63]. The details of all retrieved incidence by study and age classes are in Additional file 4: Table S1.
Diagnostic criteria used to define T1D in adults reported in 71 epidemiological studies
Autoantibodies against beta-cell antigens or the C-peptide were included in the T1D diagnostic criteria in 14 studies [15,30-32,34,35,45,46,54,56,57,63,74,81], detection of ICAs was reported in 9 studies [15,30-32,34,45,46,54,63], IAA in 4 studies [30-32,54], IA2 in 5 studies [30-32,56,57], and GAD in 11 studies [30-32,35,45,46,56,57,63,74,81]. The C-peptide was measured in 7 studies. In one paper difference of auto-antibodies by age group (0–19) was explored but no significant differences were detected [74]. The other reported diagnostic criteria for T1D were the need for insulin therapy (reported in 70 of 71 studies), clinical symptoms of diabetes (reported in 56 of 71 studies), low or normal body weight (14 of 71 studies), and ketosis or ketonuria (26 of 71 studies). The details are shown in Table 1.
Comparison of adult and children T1D incidences
The variations of incidence of T1D in adults with country and age were studied in each area for which we retrieved information on a geographically defined population. This concerned 35 countries.
Variation of T1D incidence with age in adults
In 23 out of 35 (66%) countries (55 of 71 studies), the incidence of T1D was higher in the age range of 0–14 compared with 15–19 years. When restricted to the 14 reports for which the criteria of diagnosis of T1D were auto-antibodies against beta-cells or C-peptide detection, the variation of adult incidence with age showed a consistent decrease after the age of 14 years (Figure 2 and Additional file 4: Table S1).
Figure 2.
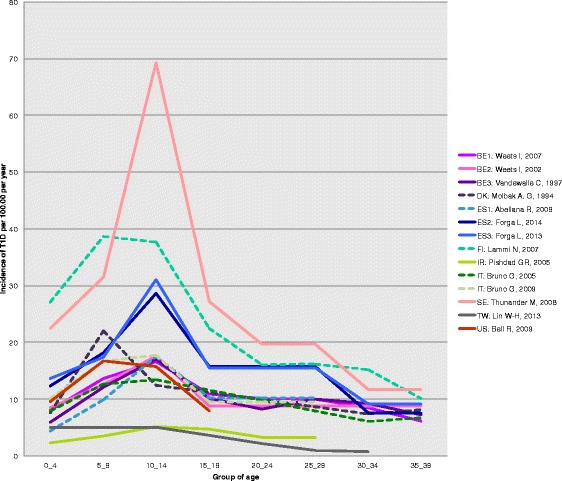
Age variation of incidence from childhood to adult age. On this figure, the adult estimates of incidence were taken from the 14 reports of the systematic review using the autoantibodies/C-peptide as diagnostic criteria. Full lines correspond to articles from which both child as well as adult information could be retrieved. The dotted lines are those for which the child information was searched in the same country as in the adult paper, but was from a different paper (see Additional file 3 for details on this literature search). The corresponding countries are shown as: BE1: Belgium (2007) [30]; BE2: Belgium (2002) [31]; BE3: Belgium (1997) [32]; DK: Denmark [34]; ES1: Spain, Catalonia [54]; ES2: Spain, Navarra (2014) [56]; ES3: Spain, Navarra (2013) [57]; FI: Finland [35]; IR: Iran (Islamic Republic of) [15]; IT: Italy [45,46]; SE: Sweden [63], TW: Taiwan [81]; US: United States of America [74].
Geographical correlation of adult and child T1D incidence
A significant geographical correlation, as measured by the Spearman correlation coefficient, was found between adult T1D incidence and 0–14 incidence in the age classes 15–19 years, 20–24 years, 25–29 years, 30–34 years and overall in the entire 15–60 group (r = 0.75, _p_-value: 5.7 × 10−10). The correlation was not significant in the oldest class where sparse data were available, but the relation was similar (Figure 3).
Figure 3.
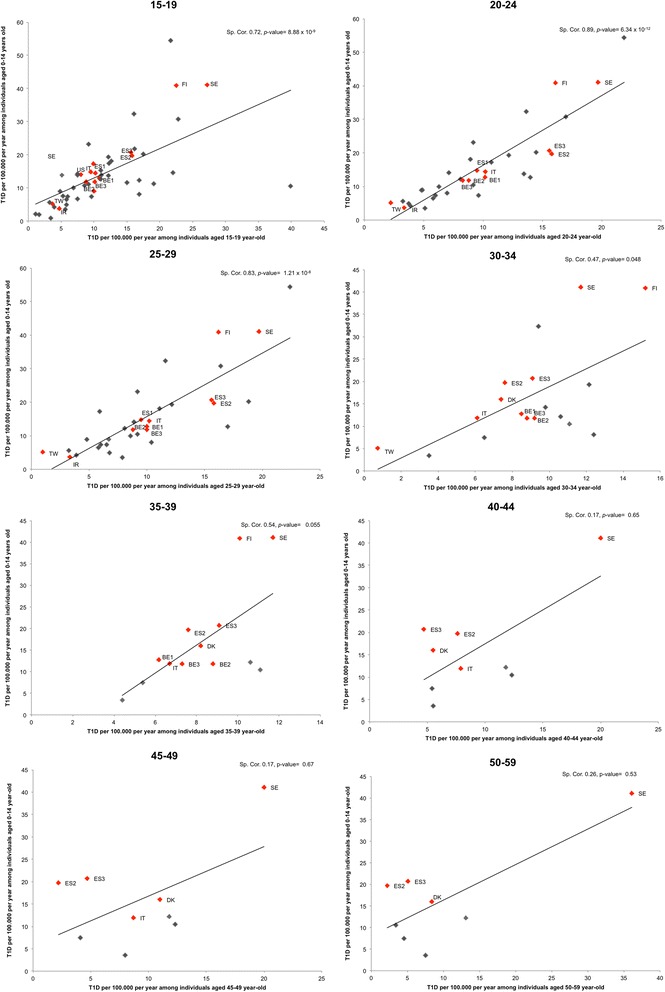
Geographical correlation of T1D incidence between individuals aged 0–14 years and adults. Studies using autoantibodies/C-Peptide for T1D case definition are identified by Red diamonds. The corresponding countries are shown as: BE1: Belgium (2007) [30]; BE2: Belgium (2002) [31]; BE3: Belgium (1997) [32]; DK: Denmark [34]; ES1: Spain, Catalonia [54]; ES2: Spain, Navarra (2014) [56]; ES3: Spain, Navarra (2013) [57]; FI: Finland [35]; IR: Iran (Islamic Republic of) [15]; IT: Italy [45,46]; SE: Sweden [63], TW: Taiwan [81]; US: United States of America [74]. Sp. Cor: Spearman correlation.
Comparison of male and female T1D adult incidences
T1D incidence was larger in males aged 15 to 39 years than in females in 44 (81%) of the 54 studies reporting incidence by sex (Additional file 5: Table S2). The mean male-to-female ratio in our review was 1.47 (95% CI for mean 1.33-1.60, SD = 0.49, n = 54, p = < 0.0001).
Discussion
A first result of this systematic review is the paucity of data available on adult incidence of T1D as compared to those concerning children. The 71 studies retrieved provided information on adult T1D in only 35 countries, 40% of the 88 countries with primary childhood T1D incidence information in the 6th IDF atlas [1].
A second result is that only a small proportion (n = 14) of the 71 studies used detection of specific autoantibodies and/or dosage of C-peptide [83] as diagnostic criteria of adult T1D.
A third result was that in a majority of the retrieved studies, adult T1D incidence was greater in men than in women, which contrasts with incidence of T1D in children where sex ratio is around one [2,84]. Using comparative data, Karvonen et al. also described a male excess among young adults in the 15–39 years of age [85]. Sex differences in exposure to possible environmental triggers of T1D, in hormonal/genetic susceptibility, in lifestyle have been proposed as possible explanations for this difference [62].
A last striking observation of the current analysis is the strong geographical correlation of the incidences in adults and children. This correlation may be explained by the fact that adults with T1D share the gene alleles known to be associated to incidence of T1D in children, [86,87], and/or some predisposing environmental causes [4]. For example, in a previous study on incidence of T1D in children, a significant positive correlation was detected between the percentage of urban population and the incidence of T1D in children (r = 0.41 _p-_value: < 0.0001) [4]; in this review a significantly higher urban proportion of T1D incidence among adults was found in 4 of the 7 studies reporting differences between rural vs urban areas [15,21,42,75].
There was an overall decrease of incidence with age in adults and young adults after the age of 14. A second peak of T1D around the age of 50, as described by Krolewski et al. [88], was only reported in 7% (4 of 58) of the studies [18,63,80,89].
The paucity of data made it impossible to document an increase in adult T1D incidence that would parallel the dramatic increase observed in children [2,3,90]. Indeed, successive studies in the same region over different periods reporting incidence in people aged >30 years of age were only found for Belgium [30-32], Lithuania [20-22] and Sweden [58-62]. Similarly, this review did not dispose of sufficient data to document differences in the clinical presentation of T1D of adults and children as suggested elsewhere [32,40]; indeed only two of the 71 studies describe differences in clinical presentation of T1D between adults and children [89,91].
Improving the quantity and quality of information on adult T1D is not only useful to better understand the epidemiology and natural history of T1D, but can have practical consequences, as delay of T1D diagnosis may mean retardation in insulin treatment, lost opportunities for potential prevention of acute and chronic complications, and even death [92]: in Croatia [18], 14% of the incident cases were identified solely through death certificates, and high mortality was found in the newly-diagnosed T1D aged over 50.
Conclusions
Overall, the results of this systematic review should encourage the launching of epidemiological studies of adult T1D with specific diagnostic criteria.
Availability of supporting data
All the supporting data are included as additional files.
Acknowledgements
We thank Anne-Lise Haenni of the Institut Jacques Monod, CNRS - Paris-Diderot University, for critically reading and reviewing the English of this manuscript.
Funding
This study was supported by grants from the Programme Hospitalier de Recherche Clinique, and from Colciencias, the Administrative Department of Science, Technology and Innovation for Colombia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional files
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PAD-V conducted the data collection and analyses. PAD-V, PB and AJV, contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Paula A Diaz-Valencia, Email: paula.diaz@inserm.fr.
Pierre Bougnères, Email: pierre.bougneres@inserm.fr.
Alain-Jacques Valleron, Email: alain-jacques.valleron@inserm.fr.
References
- 1.Patterson C, Guariguata L, Dahlquist G, Soltesz G, Ogle G, Silink M. Diabetes in the young - a global view and worldwide estimates of numbers of children with type 1 diabetes. Diabetes Res Clin Pract. 2013;103(2):161–75. doi: 10.1016/j.diabres.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 2.The DIAMOND Project Group Incidence and trends of childhood Type 1 diabetes worldwide 1990–1999. The DIAMOND project Group. Diabet Med. 2006;23(8):857–66. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 3.Patterson CC, Gyurus E, Rosenbauer J, Cinek O, Neu A, Schober E, et al. Trends in childhood type 1 diabetes incidence in Europe during 1989–2008: evidence of non-uniformity over time in rates of increase. Diabetologia. 2012;55(8):2142–7. doi: 10.1007/s00125-012-2571-8. [DOI] [PubMed] [Google Scholar]
- 4.Diaz-Valencia PA, Bougneres P, Valleron AJ. Covariation of the incidence of type 1 diabetes with country characteristics available in public databases. PloS one. 2015;10(2):e0118298. doi: 10.1371/journal.pone.0118298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borg H, Arnqvist HJ, Bjork E, Bolinder J, Eriksson JW, Nystrom L, et al. Evaluation of the new ADA and WHO criteria for classification of diabetes mellitus in young adult people (15–34 yrs) in the Diabetes Incidence Study in Sweden (DISS) Diabetologia. 2003;46(2):173–81. doi: 10.1007/s00125-002-1021-4. [DOI] [PubMed] [Google Scholar]
- 6.Tuomi T, Groop LC, Zimmet PZ, Rowley MJ, Knowles W, Mackay IR. Antibodies to glutamic acid decarboxylase reveal latent autoimmune diabetes mellitus in adults with a non-insulin-dependent onset of disease. Diabetes. 1993;42(2):359–62. doi: 10.2337/diab.42.2.359. [DOI] [PubMed] [Google Scholar]
- 7.Zimmet PZ. Diabetes epidemiology as a tool to trigger diabetes research and care. Diabetologia. 1999;42(5):499–518. doi: 10.1007/s001250051188. [DOI] [PubMed] [Google Scholar]
- 8.LaPorte RE, Tajima N, Akerblom HK, Berlin N, Brosseau J, Christy M, et al. Geographic differences in the risk of insulin-dependent diabetes mellitus: the importance of registries. Diabetes Care. 1985;8(Suppl 1):101–7. doi: 10.2337/diacare.8.1.S101. [DOI] [PubMed] [Google Scholar]
- 9.Health Statistics and health information systems. [http://www.who.int/healthinfo/global_burden_disease/definition_regions/en/]
- 10.LaPorte RE, McCarty D, Bruno G, Tajima N, Baba S. Counting diabetes in the next millennium. Application of capture-recapture technology. Diabetes Care. 1993;16(2):528–34. doi: 10.2337/diacare.16.2.528. [DOI] [PubMed] [Google Scholar]
- 11.GraphClick. In_._, 3.0 edn: Arizona Software; 2008. Available in the website: http://www.arizona-software.ch/graphclick/ [last accesed: 12 January, 2012].
- 12.R Development Core Team: R: A language and environment for statistical computing. R Foundation for Statistical Computing. In_._, R version 3.0.1 (2013-05-16). http://www.R-project.org/. Vienna, Austria, 2013.
- 13.Tuomilehto J, Dabee J, Karvonen M, Dowse GK, Gareeboo H, Virtala E, et al. Incidence of IDDM in Mauritian children and adolescents from 1986 to 1990. Diabetes Care. 1993;16(12):1588–91. doi: 10.2337/diacare.16.12.1588. [DOI] [PubMed] [Google Scholar]
- 14.Swai AB, Lutale JL, McLarty DG. Prospective study of incidence of juvenile diabetes mellitus over 10 years in Dar es Salaam, Tanzania. BMJ. 1993;306(6892):1570–2. doi: 10.1136/bmj.306.6892.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pishdad GR. Low incidence of type 1 diabetes in Iran. Diabetes Care. 2005;28(4):927–8. doi: 10.2337/diacare.28.4.927. [DOI] [PubMed] [Google Scholar]
- 16.Kadiki OA, Reddy MR, Marzouk AA. Incidence of insulin-dependent diabetes (IDDM) and non-insulin-dependent diabetes (NIDDM) (0-34 years at onset) in Benghazi, Libya. Diabetes Res Clin Pract. 1996;32(3):165–73. doi: 10.1016/0168-8227(96)01262-4. [DOI] [PubMed] [Google Scholar]
- 17.Ben Khalifa F, Mekaouar A, Taktak S, Hamhoum M, Jebara H, Kodia A, et al. A five-year study of the incidence of insulin-dependent diabetes mellitus in young Tunisians (preliminary results) Diabetes Metab. 1997;23(5):395–401. [PubMed] [Google Scholar]
- 18.Roglic G, Pavlic-Renar I, Sestan-Crnek S, Prasek M, Kadrnka-Lovrencic M, Radica A, et al. Incidence of IDDM during 1988-1992 in Zagreb, Croatia. Diabetologia. 1995;38(5):550–4. doi: 10.1007/BF00400723. [DOI] [PubMed] [Google Scholar]
- 19.Kalits I, Podar T. Incidence and prevalence of type 1 (insulin-dependent) diabetes in Estonia in 1988. Diabetologia. 1990;33(6):346–9. doi: 10.1007/BF00404638. [DOI] [PubMed] [Google Scholar]
- 20.Ostrauskas R, Zalinkevicius R, Jurgeviciene N, Radzeviciene L, Lasaite L. The incidence of type 1 diabetes mellitus among 15-34 years aged Lithuanian population: 18-year incidence study based on prospective databases. BMC Public Health. 2011;11:813. doi: 10.1186/1471-2458-11-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pundziute-Lycka A, Urbonaite B, Ostrauskas R, Zalinkevicius R, Dahlquist GG. Incidence of type 1 diabetes in Lithuanians aged 0-39 years varies by the urban-rural setting, and the time change differs for men and women during 1991-2000. Diabetes Care. 2003;26(3):671–6. doi: 10.2337/diacare.26.3.671. [DOI] [PubMed] [Google Scholar]
- 22.Ostrauskas R, Zalinkevicius R. Incidence in young adulthood-onset Type 1 diabetes mellitus in Lithuania during 1991-1997. Lithuanian Epidemiology Diabetes Study Group. Diabetes Nutr Metab. 2000;13(2):68–74. [PubMed] [Google Scholar]
- 23.Kretowski A, Kowalska I, Peczynska J, Urban M, Green A, Kinalska I. The large increase in incidence of Type I diabetes mellitus in Poland. Diabetologia. 2001;44(Suppl 3):B48–50. doi: 10.1007/PL00002954. [DOI] [PubMed] [Google Scholar]
- 24.Sobel-Maruniak A, Grzywa M, Orlowska-Florek R, Staniszewski A. The rising incidence of type 1 diabetes in south-eastern Poland. A study of the 0-29 year-old age group, 1980–1999. Endokrynol Pol. 2006;57(2):127–30. [PubMed] [Google Scholar]
- 25.Grzywa MA, Sobel AK. Incidence of IDDM in the province of Rzeszow, Poland, 0- to 29-year-old age-group, 1980-1992. Diabetes Care. 1995;18(4):542–4. doi: 10.2337/diacare.18.4.542. [DOI] [PubMed] [Google Scholar]
- 26.Wysocki MJ, Chanska M, Bak M, Czyzyk AS. Incidence of insulin-dependent diabetes mellitus in Warsaw, Poland, in children and young adults, 1983-1988. World Health Stat Q. 1992;45(4):315–20. [PubMed] [Google Scholar]
- 27.Ionescu-Tirgoviste C, Paterache E, Cheta D, Farcasiu E, Serafinceanu C, Mincu I. Epidemiology of diabetes in Bucharest. Diabet Med. 1994;11(4):413–7. doi: 10.1111/j.1464-5491.1994.tb00296.x. [DOI] [PubMed] [Google Scholar]
- 28.Kyvik KO, Nystrom L, Gorus F, Songini M, Oestman J, Castell C, et al. The epidemiology of Type 1 diabetes mellitus is not the same in young adults as in children. Diabetologia. 2004;47(3):377–84. doi: 10.1007/s00125-004-1331-9. [DOI] [PubMed] [Google Scholar]
- 29.Rami B, Waldhor T, Schober E. Incidence of Type I diabetes mellitus in children and young adults in the province of Upper Austria, 1994-1996. Diabetologia. 2001;44(Suppl 3):B45–7. doi: 10.1007/PL00002953. [DOI] [PubMed] [Google Scholar]
- 30.Weets I, Rooman R, Coeckelberghs M, De Block C, Van Gaal L, Kaufman JM, et al. The age at diagnosis of type 1 diabetes continues to decrease in Belgian boys but not in girls: a 15-year survey. Diabetes Metab Res Rev. 2007;23(8):637–43. doi: 10.1002/dmrr.758. [DOI] [PubMed] [Google Scholar]
- 31.Weets I, De Leeuw IH, Du Caju MV, Rooman R, Keymeulen B, Mathieu C, et al. The incidence of type 1 diabetes in the age group 0-39 years has not increased in Antwerp (Belgium) between 1989 and 2000: evidence for earlier disease manifestation. Diabetes Care. 2002;25(5):840–6. doi: 10.2337/diacare.25.5.840. [DOI] [PubMed] [Google Scholar]
- 32.Vandewalle CL, Coeckelberghs MI, De Leeuw IH, Du Caju MV, Schuit FC, Pipeleers DG, et al. Epidemiology, clinical aspects, and biology of IDDM patients under age 40 years. Comparison of data from Antwerp with complete ascertainment with data from Belgium with 40% ascertainment. The Belgian Diabetes Registry. Diabetes Care. 1997;20(10):1556–61. doi: 10.2337/diacare.20.10.1556. [DOI] [PubMed] [Google Scholar]
- 33.Radosevic B, Bukara-Radujkovic G, Miljkovic V, Pejicic S, Bratina N, Battelino T. The incidence of type 1 diabetes in Republic of Srpska (Bosnia and Herzegovina) and Slovenia in the period 1998-2010. Pediatr Diabetes. 2013;14(4):273–9. doi: 10.1111/j.1399-5448.2012.00898.x. [DOI] [PubMed] [Google Scholar]
- 34.Molbak AG, Christau B, Marner B, Borch-Johnsen K, Nerup J. Incidence of insulin-dependent diabetes mellitus in age groups over 30 years in Denmark. Diabet Med. 1994;11(7):650–5. doi: 10.1111/j.1464-5491.1994.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 35.Lammi N, Taskinen O, Moltchanova E, Notkola IL, Eriksson JG, Tuomilehto J, et al. A high incidence of type 1 diabetes and an alarming increase in the incidence of type 2 diabetes among young adults in Finland between 1992 and 1996. Diabetologia. 2007;50(7):1393–400. doi: 10.1007/s00125-007-0690-4. [DOI] [PubMed] [Google Scholar]
- 36.Charkaluk ML, Czernichow P, Levy-Marchal C. Incidence data of childhood-onset type I diabetes in France during 1988-1997: the case for a shift toward younger age at onset. Pediatr Res. 2002;52(6):859–62. doi: 10.1203/00006450-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Levy-Marchal C. Evolution of the incidence of IDDM in childhood in France. Rev Epidemiol Sante Publique. 1998;46(3):157–63. [PubMed] [Google Scholar]
- 38.Blumenfeld O, Dichtiar R, Shohat T, Israel IRSG. Trends in the incidence of type 1 diabetes among Jews and Arabs in Israel. Pediatr Diabetes. 2014;15(6):422–7. doi: 10.1111/pedi.12101. [DOI] [PubMed] [Google Scholar]
- 39.Sella T, Shoshan A, Goren I, Shalev V, Blumenfeld O, Laron Z, et al. A retrospective study of the incidence of diagnosed Type 1 diabetes among children and adolescents in a large health organization in Israel, 2000-2008. Diabet Med. 2011;28(1):48–53. doi: 10.1111/j.1464-5491.2010.03174.x. [DOI] [PubMed] [Google Scholar]
- 40.Koton S. Incidence of type 1 diabetes mellitus in the 0- to 17-yr-old Israel population, 1997-2003. Pediatr Diabetes. 2007;8(2):60–6. doi: 10.1111/j.1399-5448.2007.00230.x. [DOI] [PubMed] [Google Scholar]
- 41.Garancini P, Gallus G, Calori G, Formigaro F, Micossi P. Incidence and prevalence rates of diabetes mellitus in Italy from routine data: a methodological assessment. Eur J Epidemiol. 1991;7(1):55–63. doi: 10.1007/BF00221342. [DOI] [PubMed] [Google Scholar]
- 42.Tenconi MT, Devoti G, Albani I, Lorini R, Martinetti M, Fratino P, et al. IDDM in the province of Pavia, Italy, from a population-based registry. A descriptive study. Diabetes Care. 1995;18(7):1017–9. doi: 10.2337/diacare.18.7.1017. [DOI] [PubMed] [Google Scholar]
- 43.Muntoni S, Songini M. High incidence rate of IDDM in Sardinia. Sardinian Collaborative Group for Epidemiology of IDDM. Diabetes Care. 1992;15(10):1317–22. doi: 10.2337/diacare.15.10.1317. [DOI] [PubMed] [Google Scholar]
- 44.Frongia O, Mastinu F, Sechi GM. Prevalence and 4-year incidence of insulin-dependent diabetes mellitus in the province of Oristano (Sardinia, Italy) Acta Diabetol. 1997;34(3):199–205. doi: 10.1007/s005920050074. [DOI] [PubMed] [Google Scholar]
- 45.Bruno G, Novelli G, Panero F, Perotto M, Monasterolo F, Bona G, et al. The incidence of type 1 diabetes is increasing in both children and young adults in Northern Italy: 1984–2004 temporal trends. Diabetologia. 2009;52(12):2531–5. doi: 10.1007/s00125-009-1538-x. [DOI] [PubMed] [Google Scholar]
- 46.Bruno G, Runzo C, Cavallo-Perin P, Merletti F, Rivetti M, Pinach S, et al. Incidence of type 1 and type 2 diabetes in adults aged 30-49 years: the population-based registry in the province of Turin, Italy. Diabetes Care. 2005;28(11):2613–9. doi: 10.2337/diacare.28.11.2613. [DOI] [PubMed] [Google Scholar]
- 47.Bruno G, Merletti F, Vuolo A, Pisu E, Giorio M, Pagano G. Sex differences in incidence of IDDM in age-group 15-29 yr. Higher risk in males in Province of Turin, Italy. Diabetes Care. 1993;16(1):133–6. doi: 10.2337/diacare.16.1.133. [DOI] [PubMed] [Google Scholar]
- 48.de Beaufort CE, Michel G, Glaesener G. The incidence of type 1 (insulin-dependent) diabetes mellitus in subjects aged 0-19 years in Luxembourg: a retrospective study from 1977 to 1986. Diabetologia. 1988;31(10):758–61. doi: 10.1007/BF00274779. [DOI] [PubMed] [Google Scholar]
- 49.Schranz AG, Prikatsky V. Type 1 diabetes in the Maltese Islands. Diabet Med. 1989;6(3):228–31. doi: 10.1111/j.1464-5491.1989.tb01152.x. [DOI] [PubMed] [Google Scholar]
- 50.Ruwaard D, Hirasing RA, Reeser HM, van Buuren S, Bakker K, Heine RJ, et al. Increasing incidence of type I diabetes in The Netherlands. The second nationwide study among children under 20 years of age. Diabetes Care. 1994;17(6):599–601. doi: 10.2337/diacare.17.6.599. [DOI] [PubMed] [Google Scholar]
- 51.Joner G, Sovik O. The incidence of type 1 (insulin-dependent) diabetes mellitus 15-29 years in Norway 1978-1982. Diabetologia. 1991;34(4):271–4. doi: 10.1007/BF00405087. [DOI] [PubMed] [Google Scholar]
- 52.Morales-Perez FM, Barquero-Romero J, Perez-Miranda M. Incidence of type I diabetes among children and young adults (0-29 years) in the province of Badajoz, Spain during 1992 to 1996. Acta Paediatr. 2000;89(1):101–4. doi: 10.1080/080352500750029158. [DOI] [PubMed] [Google Scholar]
- 53.Carrillo Dominguez A. Incidence of type 1 diabetes mellitus in the Canary Islands (1995-1996). Epidemiologic Group of the Canary Society of Endocrinology and Nutrition. Rev Clin Esp. 2000;200(5):257–60. doi: 10.1016/S0014-2565(00)70625-4. [DOI] [PubMed] [Google Scholar]
- 54.Abellana R, Ascaso C, Carrasco JL, Castell C, Tresserras R. Geographical variability of the incidence of Type 1 diabetes in subjects younger than 30 years in Catalonia, Spain. Med Clin (Barc) 2009;132(12):454–8. doi: 10.1016/j.medcli.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 55.Goday A, Castell C, Tresserras R, Canela J, Taberner JL, Lloveras G. Incidence of type 1 (insulin-dependent) diabetes mellitus in Catalonia, Spain. The Catalan Epidemiology Diabetes Study Group. Diabetologia. 1992;35(3):267–71. doi: 10.1007/BF00400928. [DOI] [PubMed] [Google Scholar]
- 56.Forga L, Goni MJ, Ibanez B, Cambra K, Mozas D, Chueca M. Incidence of type 1 diabetes in Navarre, 2009-2012. An Sist Sanit Navar. 2014;37(2):241–7. doi: 10.4321/S1137-66272014000200007. [DOI] [PubMed] [Google Scholar]
- 57.Forga L, Goni MJ, Cambra K, Ibanez B, Mozas D, Chueca M. En Representacion del Grupo de Estudio de Diabetes tipo 1 de N: [Differences by age and gender in the incidence of type 1 diabetes in Navarre, Spain (2009-2011)] Gac Sanit/SESPAS. 2013;27(6):537–40. doi: 10.1016/j.gaceta.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 58.Dahlquist GG, Nystrom L, Patterson CC. Incidence of type 1 diabetes in Sweden among individuals aged 0-34 years, 1983-2007: an analysis of time trends. Diabetes Care. 2011;34(8):1754–9. doi: 10.2337/dc11-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ostman J, Lonnberg G, Arnqvist HJ, Blohme G, Bolinder J, Ekbom Schnell A, et al. Gender differences and temporal variation in the incidence of type 1 diabetes: results of 8012 cases in the nationwide Diabetes Incidence Study in Sweden 1983-2002. J Intern Med. 2008;263(4):386–94. doi: 10.1111/j.1365-2796.2007.01896.x. [DOI] [PubMed] [Google Scholar]
- 60.Pundziute-Lycka A, Dahlquist G, Nystrom L, Arnqvist H, Bjork E, Blohme G, et al. The incidence of Type I diabetes has not increased but shifted to a younger age at diagnosis in the 0-34 years group in Sweden 1983-1998. Diabetologia. 2002;45(6):783–91. doi: 10.1007/s00125-002-0845-2. [DOI] [PubMed] [Google Scholar]
- 61.Nystrom L, Dahlquist G, Ostman J, Wall S, Arnqvist H, Blohme G, et al. Risk of developing insulin-dependent diabetes mellitus (IDDM) before 35 years of age: indications of climatological determinants for age at onset. Int J Epidemiol. 1992;21(2):352–8. doi: 10.1093/ije/21.2.352. [DOI] [PubMed] [Google Scholar]
- 62.Blohme G, Nystrom L, Arnqvist HJ, Lithner F, Littorin B, Olsson PO, et al. Male predominance of type 1 (insulin-dependent) diabetes mellitus in young adults: results from a 5-year prospective nationwide study of the 15-34-year age group in Sweden. Diabetologia. 1992;35(1):56–62. doi: 10.1007/BF00400852. [DOI] [PubMed] [Google Scholar]
- 63.Thunander M, Petersson C, Jonzon K, Fornander J, Ossiansson B, Torn C, et al. Incidence of type 1 and type 2 diabetes in adults and children in Kronoberg, Sweden. Diabetes Res Clin Pract. 2008;82(2):247–55. doi: 10.1016/j.diabres.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 64.Imkampe AK, Gulliford MC. Trends in Type 1 diabetes incidence in the UK in 0- to 14-year-olds and in 15- to 34-year-olds, 1991-2008. Diabet Med. 2011;28(7):811–4. doi: 10.1111/j.1464-5491.2011.03288.x. [DOI] [PubMed] [Google Scholar]
- 65.Bingley PJ, Gale EA. Incidence of insulin dependent diabetes in England: a study in the Oxford region, 1985-6. BMJ. 1989;298(6673):558–60. doi: 10.1136/bmj.298.6673.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jordan OW, Lipton RB, Stupnicka E, Cruickshank JK, Fraser HS. Incidence of type I diabetes in people under 30 years of age in Barbados, West Indies, 1982-1991. Diabetes Care. 1994;17(5):428–31. doi: 10.2337/diacare.17.5.428. [DOI] [PubMed] [Google Scholar]
- 67.Legault L, Polychronakos C. Annual incidence of type 1 diabetes in Quebec between 1989-2000 in children. Clin Invest Med. 2006;29(1):10–3. [PubMed] [Google Scholar]
- 68.Wagenknecht LE, Roseman JM, Herman WH. Increased incidence of insulin-dependent diabetes mellitus following an epidemic of Coxsackievirus B5. Am J Epidemiol. 1991;133(10):1024–31. doi: 10.1093/oxfordjournals.aje.a115811. [DOI] [PubMed] [Google Scholar]
- 69.Wagenknecht LE, Roseman JM, Alexander WJ. Epidemiology of IDDM in black and white children in Jefferson County, Alabama, 1979-1985. Diabetes. 1989;38(5):629–33. doi: 10.2337/diab.38.5.629. [DOI] [PubMed] [Google Scholar]
- 70.Vehik K, Hamman RF, Lezotte D, Norris JM, Klingensmith G, Bloch C, et al. Increasing Incidence of Type 1 Diabetes in 0- to 17-Year-Old Colorado Youth. Diabetes Care. 2007;30(3):503–9. doi: 10.2337/dc06-1837. [DOI] [PubMed] [Google Scholar]
- 71.Kostraba JN, Gay EC, Cai Y, Cruickshanks KJ, Rewers MJ, Klingensmith GJ, et al. Incidence of insulin-dependent diabetes mellitus in Colorado. Epidemiology. 1992;3(3):232–8. doi: 10.1097/00001648-199205000-00008. [DOI] [PubMed] [Google Scholar]
- 72.Libman IM, LaPorte RE, Becker D, Dorman JS, Drash AL, Kuller L. Was there an epidemic of diabetes in nonwhite adolescents in Allegheny County, Pennsylvania? Diabetes Care. 1998;21(8):1278–81. doi: 10.2337/diacare.21.8.1278. [DOI] [PubMed] [Google Scholar]
- 73.Fishbein HA, Faich GA, Ellis SE. Incidence and hospitalization patterns of insulin-dependent diabetes mellitus. Diabetes Care. 1982;5(6):630–3. doi: 10.2337/diacare.5.6.630. [DOI] [PubMed] [Google Scholar]
- 74.Bell RA, Mayer-Davis EJ, Beyer JW, D'Agostino RB, Jr, Lawrence JM, Linder B, et al. Diabetes in non-Hispanic white youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(Suppl 2):S102–11. doi: 10.2337/dc09-S202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Allen C, Palta M, D'Alessio DJ. Incidence and differences in urban-rural seasonal variation of type 1 (insulin-dependent) diabetes in Wisconsin. Diabetologia. 1986;29(9):629–33. doi: 10.1007/BF00869261. [DOI] [PubMed] [Google Scholar]
- 76.Gorham ED, Garland FC, Barrett-Connor E, Garland CF, Wingard DL, Pugh WM. Incidence of insulin-dependent diabetes mellitus in young adults: experience of 1,587,630 US Navy enlisted personnel. Am J Epidemiol. 1993;138(11):984–7. doi: 10.1093/oxfordjournals.aje.a116818. [DOI] [PubMed] [Google Scholar]
- 77.Tran F, Stone M, Huang CY, Lloyd M, Woodhead HJ, Elliott KD, et al. Population-based incidence of diabetes in Australian youth aged 10-18 yr: increase in type 1 diabetes but not type 2 diabetes. Pediatr Diabetes. 2014;15(8):585–90. doi: 10.1111/pedi.12131. [DOI] [PubMed] [Google Scholar]
- 78.Sutton DL, Lyle DM, Pierce JP. Incidence and prevalence of insulin-dependent diabetes mellitus in the zero- to 19-years' age-group in Sydney. Med J Aust. 1989;151(3):140–1. doi: 10.5694/j.1326-5377.1989.tb139598.x. [DOI] [PubMed] [Google Scholar]
- 79.Sasaki A, Okamoto N. Epidemiology of childhood diabetes in Osaka District, Japan, using the documents from the medical benefits system specific for childhood diabetes. Diabetes Res Clin Pract. 1992;18(3):191–6. doi: 10.1016/0168-8227(92)90145-H. [DOI] [PubMed] [Google Scholar]
- 80.Scott RS, Brown LJ. Prevalence and incidence of insulin-treated diabetes mellitus in adults in Canterbury, New Zealand. Diabet Med. 1991;8(5):443–7. doi: 10.1111/j.1464-5491.1991.tb01629.x. [DOI] [PubMed] [Google Scholar]
- 81.Lin WH, Wang MC, Wang WM, Yang DC, Lam CF, Roan JN, et al. Incidence of and mortality from Type I diabetes in Taiwan from 1999 through 2010: a nationwide cohort study. PloS one. 2014;9(1):e86172. doi: 10.1371/journal.pone.0086172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Washington RE, Orchard TJ, Arena VC, Laporte RE, Tull ES. Incidence of type 1 and type 2 diabetes in youth in the U.S. Virgin Islands, 2001-2010. Pediatr Diabetes. 2013;14(4):280–7. doi: 10.1111/j.1399-5448.2012.00912.x. [DOI] [PubMed] [Google Scholar]
- 83.Bingley PJ, Bonifacio E, Ziegler AG, Schatz DA, Atkinson MA, Eisenbarth GS. Proposed guidelines on screening for risk of type 1 diabetes. Diabetes Care. 2001;24(2):398. doi: 10.2337/diacare.24.2.398. [DOI] [PubMed] [Google Scholar]
- 84.Soltesz G, Patterson CC, Dahlquist G. Worldwide childhood type 1 diabetes incidence–what can we learn from epidemiology? Pediatr Diabetes. 2007;8(Suppl 6):6–14. doi: 10.1111/j.1399-5448.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 85.Karvonen M, Pitkaniemi M, Pitkaniemi J, Kohtamaki K, Tajima N, Tuomilehto J. Sex difference in the incidence of insulin-dependent diabetes mellitus: an analysis of the recent epidemiological data. World Health Organization DIAMOND Project Group. Diabetes Metab Rev. 1997;13(4):275–91. doi: 10.1002/(SICI)1099-0895(199712)13:4<275::AID-DMR197>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 86.Todd JA. Etiology of type 1 diabetes. Immunity. 2010;32(4):457–67. doi: 10.1016/j.immuni.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 87.Caillat-Zucman S, Garchon HJ, Timsit J, Assan R, Boitard C, Djilali-Saiah I, et al. Age-dependent HLA genetic heterogeneity of type 1 insulin-dependent diabetes mellitus. J Clin Invest. 1992;90(6):2242–50. doi: 10.1172/JCI116110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krolewski AS, Warram JH, Rand LI, Kahn CR. Epidemiologic approach to the etiology of type I diabetes mellitus and its complications. N Engl J Med. 1987;317(22):1390–8. doi: 10.1056/NEJM198711263172206. [DOI] [PubMed] [Google Scholar]
- 89.Karjalainen J, Salmela P, Ilonen J, Surcel HM, Knip M. A comparison of childhood and adult type I diabetes mellitus. N Engl J Med. 1989;320(14):881–6. doi: 10.1056/NEJM198904063201401. [DOI] [PubMed] [Google Scholar]
- 90.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027–33. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 91.Sabbah E, Savola K, Ebeling T, Kulmala P, Vahasalo P, Ilonen J, et al. Genetic, autoimmune, and clinical characteristics of childhood- and adult-onset type 1 diabetes. Diabetes Care. 2000;23(9):1326–32. doi: 10.2337/diacare.23.9.1326. [DOI] [PubMed] [Google Scholar]
- 92.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358(9277):221–9. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]