Physical activity, motor function, and white matter hyperintensity burden in healthy older adults (original) (raw)
Abstract
Objective:
To test the hypothesis that physical activity modifies the association between white matter hyperintensity (WMH) burden and motor function in healthy older persons without dementia.
Methods:
Total daily activity (exercise and nonexercise physical activity) was measured for up to 11 days with actigraphy (Actical; Philips Respironics, Bend, OR) in 167 older adults without dementia participating in the Rush Memory and Aging Project. Eleven motor performances were summarized into a previously described global motor score. WMH volume was expressed as percent of intracranial volume. Linear regression models, adjusted for age, education, and sex, were performed with total WMH volume as the predictor and global motor score as the outcome. Terms for total daily physical activity and its interaction with WMH volume were then added to the model.
Results:
Higher WMH burden was associated with lower motor function (p = 0.006), and total daily activity was positively associated with motor function (p = 0.002). Total daily activity modified the association between WMH and motor function (p = 0.007). WMH burden was not associated with motor function in persons with high activity (90th percentile). By contrast, higher WMH burden remained associated with lower motor function in persons with average (50th percentile; estimate = −0.304, slope = −0.133) and low (10th percentile; estimate = −1.793, slope = −0.241) activity.
Conclusions:
Higher levels of physical activity may reduce the effect of WMH burden on motor function in healthy older adults.
Late life motor impairment is common in older adults and associated with a wide range of adverse health outcomes.1,2 To mitigate the growing personal and societal burden of motor impairment in our aging population, public health efforts have focused on increasing physical activity level in older adults.3 While many studies have shown that higher levels of physical activity are associated with better motor function in older adults,4 the neurobiologic basis for this beneficial effect remains unclear.
White matter hyperintensities (WMH) on antemortem brain imaging5,6 and postmortem indices of cerebrovascular disease pathologies7,8 are frequent in the brains of older persons and have been linked with level of motor function.9,10 Both animal and human studies11 suggest that the benefit of physical activity on brain health in aging may be at least partially attributable to enhancement of blood flow, angiogenesis, and maintenance of the cerebral vasculature12 and a concomitant reduction of WMH burden.13 These studies suggest that higher levels of physical activity in older adults may provide some degree of protection from the untoward consequences of accumulating brain pathology on motor function in older age.
In this study, we used data from 167 community-dwelling adults without dementia participating in the Rush Memory and Aging Project to test the hypothesis that physical activity would modify the association of WMH burden with motor function in old age. Total daily activity, including both exercise and nonexercise physical activity, was derived from continuous actigraphic recordings for up to 11 days.14 WMH burden, measured as the total volume of WMH corrected for intracranial volume served as a proxy measure of brain pathology. Eleven motor performances that were summarized using a previously described global motor score15 served as the measure of motor function. We previously reported that physical activity was related to change in this measure of motor function.16 We now examine the association of WMH burden with motor function and the extent to which this association is modified by physical activity.
METHODS
Participants.
Participants were subjects from the Rush Memory and Aging Project, an ongoing longitudinal clinical-pathologic study of aging and dementia.17 Although the study began in 1997, actigraphy data collection did not begin until 2005. Neuroimaging was introduced in 2009. At the time of these analyses, 1,545 participants had completed the baseline of the parent project and 1,031 of these participants were enrolled in the actigraphy substudy. Of these 1,031 participants, 867 were enrolled in the MRI component of the parent study, 423 had complete MRI scans, and 393 had MRI scans that were processed for WMH data. Of these 393 participants, 386 were without dementia at all visits up to and including the examination cycle concurrent with the MRI, but 69 did not have actigraphy data from that annual cycle and an additional 154 did not have global motor function from that cycle, leading to a total of 167 subjects with complete data on actigraphy, WMH, and motor function. Compared with those who were alive and eligible for MRI scans when the imaging study was initiated in the parent study, the 167 included subjects had slightly higher level of education and were generally more cognitively healthy (table e-1 on the _Neurology_® Web site at Neurology.org).
Study protocol approvals, registrations, and patient consents.
The institutional review board of Rush University Medical Center approved this study. Written informed consent was obtained from all study participants.
Assessment of clinical and cognitive function.
A composite measure of global cognition was created from 19 individual cognitive tests. The testing was computer-scored and reviewed by a neuropsychologist to diagnose cognitive impairment. Clinical classification of dementia was based on history, neurologic examination, cognitive function testing, and clinical evaluation.18
Assessment of motor function.
A composite measure of motor function was created from 11 individual motor performance tests. Grip and pinch strength were measured using the Jamar hydraulic dynamometer (Lafayette Instrument Company, Lafayette, IN). Motor performances included finger tapping, Purdue Pegboard testing, and 7 tests of lower-extremity function.15 The global score was structured such that a more positive value represented better motor function.
Assessment of physical activity.
Total daily activity including all exercise and nonexercise physical activity was measured 24 h/d for up to 11 days with actigraphs (Actical; Philips Respironics, Bend, OR) worn on the nondominant wrist.14 Total daily activity was the average sum of all daily activity counts recorded. Intensity of activity was calculated by dividing the total daily physical activity counts by the total hours/day of all nonzero epochs (activity counts/h/d). To conceptualize this metric in terms of a familiar physical activity, prior work has estimated that walking at 2.5 mph is associated with about 2,354 activity counts/min.17
Self-reported frequency of participation in physical activities was assessed with questions adapted from the 1985 Health Interview Survey.19 Subjects were asked if they had participated in each of 5 activities (e.g., walking for exercise) during the past 2 weeks and, if so, the number of times and the mean time per occasion. Scores were expressed as hours of activity/wk.
Covariates.
Age was computed from self-reported date of birth to date of interview. Sex and education were recorded at study entry. Body mass index (BMI) was based on measured weight and height. Depressive symptoms were assessed with a 10-item version of the Center for Epidemiologic Studies Depression Scale.20 For vascular covariates, composite measures of vascular disease (myocardial infarction, congestive heart failure, claudication, stroke) and vascular risk factors (hypertension, diabetes, smoking) were used.21 Activities of daily living were assessed using a modified version of the Katz22 scale.
MRI data acquisition.
Brain MRI was conducted on a 1.5T General Electric (Waukesha, WI) MRI scanner. High-resolution T1-weighted anatomical data were obtained using a 3-dimensional magnetization-prepared rapid-acquisition gradient echo (MPRAGE) sequence with the following parameters: echo time = 2.8 milliseconds (ms), repetition time = 6.3 ms, preparation time = 1,000 ms, flip angle = 8°, field of view = 24 × 24 cm, 160 sagittal slices, 1-mm slice thickness, no gap, 224 × 192 acquisition matrix reconstructed to a 256 × 256 image matrix, and 2 repetitions. T2-weighted fluid-attenuated inversion recovery (FLAIR) data were collected using a 2-dimensional fast spin-echo sequence with the following parameters: echo time = 120 ms, repetition time = 8,000 ms, inversion time = 2,000 ms, 42 oblique axial slices, slice thickness = 3 mm, no gap, 256 × 224 acquisition matrix reconstructed to a 256 × 256 image matrix.
MRI processing.
WMH were automatically segmented. The 2 copies of T1-weighted MPRAGE data were first spatially coregistered and averaged, and the result was registered to the T2-weighted FLAIR data using affine registration (FLIRT, FMRIB [Oxford Centre for Functional Magnetic Resonance Imaging of the Brain], University of Oxford, UK).23 The brain was extracted from the coregistered MPRAGE and FLAIR image volumes (BET, FMRIB, University of Oxford).24 WMH lesions were then automatically segmented for each participant using a support vector machine classifier considering both T1-weighted MPRAGE and T2-weighted FLAIR information (WMLS, SBIA [Section of Biomedical Image Analysis], University of Pennsylvania).25 Maps of WMH were generated. The total volume of brain tissue affected by WMH was measured for each participant and then normalized by the corresponding intracranial volume, assessed using FreeSurfer (http://surfer.nmr.mgh.harvard.edu) on the averaged raw T1-weighted MPRAGE data.
Statistical analysis.
First we calculated WMH volume as a percentage of intracranial volume to obtain a measure of total WMH burden. The distribution of WMH burden was negatively skewed, so we applied a logarithmic (log10) transformation. Next we examined the effect of WMH burden and its interaction with total daily activity on the motor function outcome using linear regression controlling for age, education, and sex. Then, to examine whether the association between WMH burden and motor function was modified by other measurements of physical activity or by cognitive function, we replaced total daily activity in the regression model with (1) intensity of daily physical activity, (2) self-report of daily physical activity, and (3) global cognition. Finally, in a series of regression models, BMI, depressive symptoms, vascular disease, vascular risk factors, Katz functional ability, and their interaction with WMH burden were added separately to the core model to examine whether they attenuated the association of WMH burden with motor function. Model validation was performed graphically and analytically and there was appropriate fit. Statistical analyses were programmed using SAS software, version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Characteristics of the study participants.
Participant characteristics and descriptive properties of all measures are given in table 1.
Table 1.
Participant characteristics and descriptive properties of the measures (n = 167)

Association of WMH burden and physical activity with motor function.
We first examined the relationship of total daily activity with WMH burden in a regression model adjusted for age, sex, and education. Total daily activity was not associated with WMH burden (p = 0.169, mixed model).
Consistent with prior reports, we found that a higher level of WMH burden was associated with poorer motor function (table 2, model 2) and a higher level of total daily activity was associated with better motor function (table 2, model 3). When terms for both WMH burden and total daily activity were included in the same model, both showed independent associations with motor function (table 2, model 4). In further analyses, we added an interaction term, which showed that the association of WMH burden and motor function varied with the level of total daily activity (table 2, model 5).
Table 2.
Motor function as a function of total daily activity, WMH burden, and the interaction between WMH burden and total daily activity
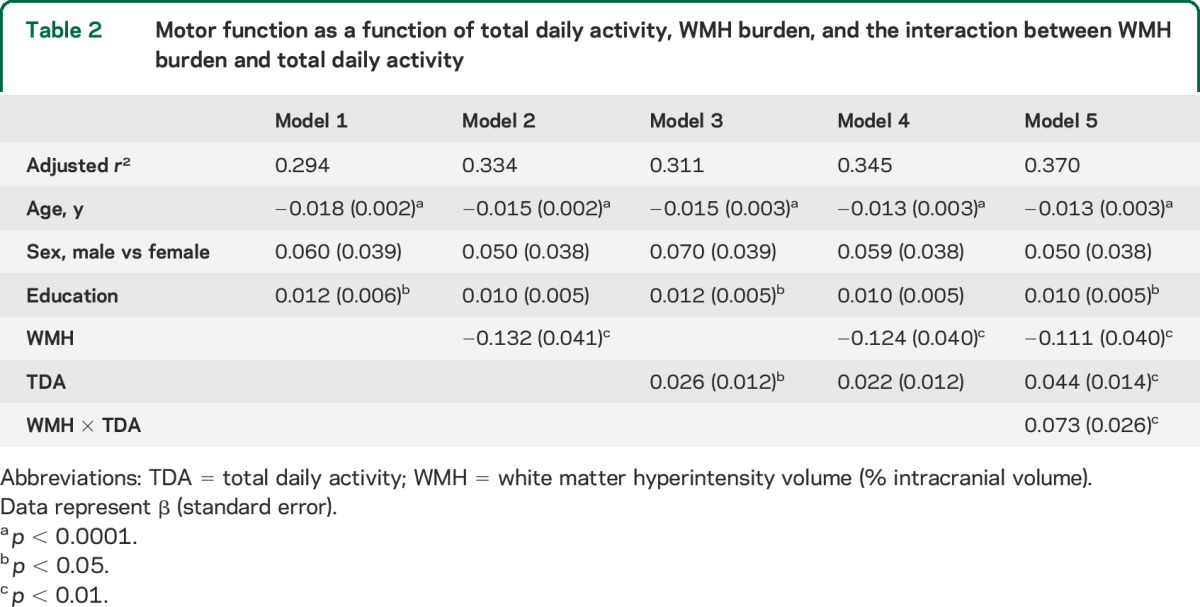
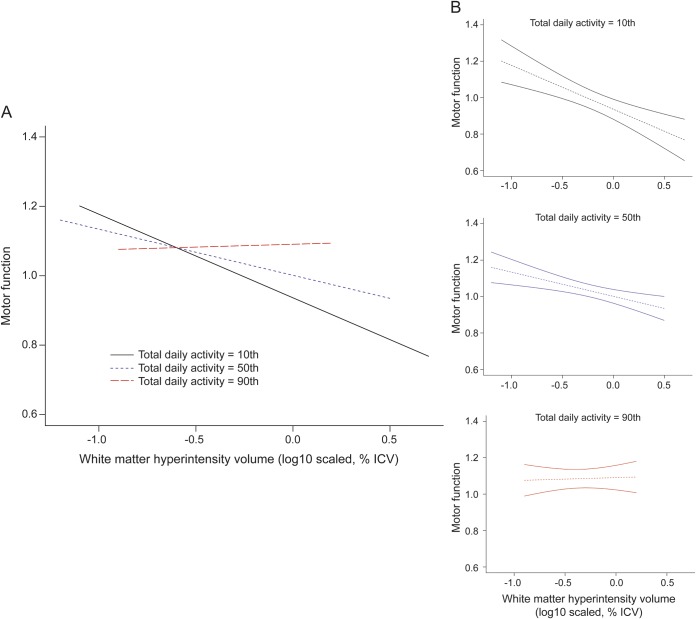
The figure compares 3 participants with 90th, 50th, and 10th percentile of total daily activity to illustrate how increasing level of activity modifies the association of WMH burden with motor function. Based on prior studies,17 the difference in total daily activity between an adult individual at the 90th vs the 50th percentile (4.75 × 105 vs 2.70 × 105 activity counts/d) would be equivalent to the former walking at 2.5 mph for an additional 1.5 hours every day.
Figure. Total daily activity modifies the association of WMH burden to motor function.
The x-axis is the log10 scaled volume of WMH, corrected for ICV, and the y-axis is the motor function based on 11 motor performance tests that were scaled and averaged to obtain a summary measure. (A) The 3 lines represent the estimated means of motor functions for different levels of daily activity, adjusting for age, sex, and education (black: 10th percentile of TDA; blue: 50th percentile of TDA; and red: 90th percentile of TDA). (B) The dotted lines represent the estimated means and the solid lines are the corresponding 95% confidence intervals. ICV = intracranial volume; TDA = total daily activity; WMH = white matter hyperintensity.
Persons with the highest activity (90th percentile, estimated level = 1.750) showed no association between WMH burden and motor function (estimated slope = 0.016). However, level of activity modified the association between WMH burden and motor function (estimated slope = −0.133) for persons at the 50th percentile of activity (estimated level = −0.304), and this effect was stronger (estimated slope = −0.241) for persons at the 10th percentile of activity (estimated level = −1.793). This effect is illustrated in the increasing divergence of the lines for persons at the 10th percentile (solid black) and 50th percentile (dashed blue) as WMH burden increases once a threshold of 1% of intracranial volume is reached.
Next we examined whether other measures of physical activity modified the association of WMH burden and motor function. Intensity of daily activity was highly correlated with total daily physical activity (r = 0.94, p < 0.001) and also modified the association of WMH burden with motor function (estimate for total daily activity × WMH burden = 1.212; p = 0.001, mixed model). We found that physical activity measured by self-report did not modify the association between WMH burden and motor function (estimate for self-reported physical activity × WMH burden = −0.0003; p = 0.976, mixed model).
In further analyses, we examined whether covariates that can potentially affect physical activity and motor function attenuated the interaction between total daily activity and WMH burden and motor function. We repeated model 5 (table 2) adding the following covariate terms separately: BMI, depressive symptoms, vascular disease, vascular risk factors, Katz functional status, and their interactions with WMH burden. The interaction of total daily activity with WMH burden remained significant and was not attenuated by the addition of any of these covariates when added separately or simultaneously to the model (p value range = 0.009–0.028). The interaction also remained significant after controlling for peripheral neurologic disorders including cervical and lumbar radiculopathy and pulmonary function (p value range = 0.007–0.013).
DISCUSSION
This study examined a potential brain mechanism underlying the well-established association between higher levels of physical activity and better motor function in healthy older adults.1,2,16 We found that higher levels of physical activity reduced the effect of WMH burden on motor function in community-dwelling older adults without dementia. Persons with the highest physical activity (90th percentile) showed no effect of WMH burden on motor function. By contrast, persons at the 50th percentile of physical activity showed an effect of WMH burden on motor function and this effect was even stronger for persons at the 10th percentile. This interaction persisted after controlling for various potential confounding variables and was equally robust for the intensity of actigraphic-measured physical activity. Together these findings suggest that higher levels of physical activity may provide reserve against the effects of brain pathology on motor function in older age.
The concept of reserve, or the resiliency of neural networks and behavior in the face of pathology, has been most frequently examined in relation to cognition.26 Studies have shown that older persons with higher levels of education,27,28 social networks,29 and purpose in life30 have better cognitive function despite significant Alzheimer disease pathology burden. One study reported that education reduced the effect of brain infarcts on cognition.31 Thus, higher levels of several lifestyle factors have been shown to protect cognition from brain pathology including brain infarcts. Far fewer studies have examined whether lifestyle factors impart a similar protective effect on motor function. We know of only one study that demonstrated motor reserve by showing that education modified the association between WMH burden and walking speed in older adults.32 Unlike education, physical activity is a modifiable behavior at any age and has been shown to reduce major mobility disability in clinical trials with older adults.4
Physical activity was not associated with WMH burden, suggesting that the benefit on motor function is derived through a neural reserve mechanism rather than through a direct association with WMH. One candidate mechanism is physical activity–induced neurotrophin production. Lower serum level of brain-derived neurotrophic factor (BDNF) is associated with higher WMH burden33 and lower level of physical activity.34 Physical activity increases BDNF and other trophic factors including fibroblast growth factor 2, insulin-like growth factor 1, and vascular endothelial growth factor, which enhance tissue survivability and promote synaptogenesis, neurogenesis, and angiogenesis.11 It is feasible that daily physical activity may provide neural reserve through the production of neuroprotective growth factors such as BDNF, which may offset WMH-associated impairment in motor function. More studies examining other potential neural mechanisms underlying motor reserve are needed.
Physical activity is a complex behavior that requires the integrated action of multiple physiologic systems including cardiopulmonary, metabolic, musculoskeletal, and neurologic. Nonetheless, the CNS is essential for the initiation and execution of all volitional movement and it is therefore likely that physical activity builds reserve on the level of the CNS. Likewise, motor function in older adults can be affected by the accumulation of pathology in one or more sites along the motor pathways that extend from the brain through the brainstem and spinal cord to the peripheral musculoskeletal elements, the final effector of all movement. Thus, the motor reserve provided by physical activity may influence the effect of pathology at multiple sites along the motor pathways. In the current study, our brain proxy measure was limited to WMH burden. Further studies that examine a wider array of CNS pathologies in the brain and other components of the motor pathways will be crucial for defining the neurobiological basis of reserve provided by physical activity.
While current treatments and preventions for some conditions that cause impaired motor function are relatively robust (e.g., Parkinson disease, arthritis), age-related motor impairment is nearly ubiquitous and its underlying biology is poorly understood and untreated in many older adults. Recent brain imaging and autopsy studies suggest an expanded role for cerebrovascular disease pathology, particularly small vessel disease, as an important cause of both chronic late-life motor and cognitive impairments.35,36 Until effective treatments are developed that can target small vessel disease, strategies that lessen the effects of WMH burden are essential to meet the growing public health challenge of late-life motor impairment. The results of this study underscore the importance of efforts to facilitate a more active lifestyle in older adults to prevent late-life motor impairment, improve survivability,37 and maintain independence and well-being.38
The study had important strengths. The large participant group was sampled from a community-dwelling population in which a comprehensive clinical evaluation allowed for careful exclusion of persons with dementia. The measure of motor function included a wide array of both upper- and lower-extremity motor performances. Effect modification was observed with long-term actigraphic recordings of physical activity, which provided objective measures of total daily exercise and nonexercise activities, but was not observed with standard self-reported physical activity measures that are used in many studies.
This study also had some limitations. The subjects were selected, and although we have controlled for many potential confounders, selection bias might exist that could limit the generalization of the findings. WMH burden was used as a proxy measure of brain white matter pathology. WMH are thought to reflect cerebral microvascular disease secondary to ischemic demyelination and gliosis, but recent work suggests they may also be associated with atrophy and Alzheimer disease pathology in older persons.39 More studies are needed that characterize the pathologic basis of WMH seen on MRI. WMH burden was not measured in localized motor networks. However, the purpose of this study was to examine the modifying effects of physical activity on the association between global brain health and motor function. All measures were cross-sectional so we cannot distinguish long-standing from short-term high physical activity and our findings cannot be interpreted as causal. However, our group is acquiring longitudinal scans and we will be able to report on change in WMH burden, motor function, physical activity, and their interactions in the future. A wide range of neurologic as well as nonneurologic conditions contribute to late-life motor impairment and although we tested several potential confounders, it is likely that there are other conditions that we did not measure that may influence the reported association. Although actigraphy is a significant improvement over self-report of physical activity, longer actigraphic recording may have provided important findings that were not captured in this study. Despite these limitations, the results of this study emphasize the importance of a more active lifestyle in protecting motor function from the adverse neurobiological effects of aging.
Supplementary Material
Data Supplement
Accompanying Editorial
ACKNOWLEDGMENT
The authors thank the staff of the Rush Alzheimer's Disease Center, particularly Woojeong Bang, MS, for statistical analyses and Niranjini Rajendran, MS, for image postprocessing. The authors are indebted to the altruism of the participants of the Rush Memory and Aging Project.
GLOSSARY
BDNF
brain-derived neurotrophic factor
BMI
body mass index
FLAIR
fluid-attenuated inversion recovery
MPRAGE
magnetization-prepared rapid-acquisition gradient echo
WMH
white matter hyperintensity
Footnotes
AUTHOR CONTRIBUTIONS
Study concept or design: Dr. Fleischman, Dr. Yang, Dr. Buchman. Analysis or interpretation of the data: Dr. Fleischman, Dr. Yang, Dr. Leurgans, Dr. Buchman. Drafting of the manuscript: Dr. Fleischman, Dr. Yang, Dr. Arfanakis, Dr. Leurgans, Dr. Arvanitakis, Dr. Turner, Dr. Barnes, Dr. Bennett, Dr. Buchman. Statistical analysis: Dr. Yang, Dr. Leurgans. Obtained funding: Dr. Fleischman, Dr. Arfanakis, Dr. Arvanitakis, Dr. Barnes, Dr. Bennett, Dr. Buchman.
STUDY FUNDING
National Institute on Aging grants P30AG10161, R01AG17917, R01AG15819, R01AG036042, R01AG043379, R01NS078009, R01NS084965, R01AG040039, and R21NS076827; National Institute of Minority Health and Health Disparities grant P20MD6886; the Illinois Department of Public Health, and the Rush Translational Science Consortium.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Louis ED, Tang MX, Schupf N, Mayeux R. Functional correlates and prevalence of mild parkinsonian signs in a community population of older people. Arch Neurol 2005;62:297–302. [DOI] [PubMed] [Google Scholar]
- 2.Verghese J, LeValley A, Hall CB, Katz MJ, Ambrose AF, Lipton RB. Epidemiology of gait disorders in community-residing older adults. J Am Geriatr Soc 2006;54:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. The State of Aging and Health in America 2013. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2013. [Google Scholar]
- 4.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE Study randomized clinical trial. JAMA 2014;311:2387–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galluzzi S, Lanni C, Pantoni L, Filippi M, Frisoni GB. White matter lesions in the elderly: pathophysiological hypothesis on the effect on brain plasticity and reserve. J Neurol Sci 2008;273:3–9. [DOI] [PubMed] [Google Scholar]
- 6.Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology 2008;71:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010;9:689–701. [DOI] [PubMed] [Google Scholar]
- 8.Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: the invisible lesions. Lancet Neurol 2012;11:272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray ME, Senjem ML, Petersen RC, et al. Functional impact of white matter hyperintensities in cognitively normal elderly subjects. Arch Neurol 2010;67:1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tseng BY, Gundapuneedi T, Khan MA, et al. White matter integrity in physically fit older adults. Neuroimage 2013;82:510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci 2013;17:525–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nithianantharajah J, Hannan AJ. The neurobiology of brain and cognitive reserve: mental and physical activity as modulators of brain disorders. Prog Neurobiol 2009;89:369–382. [DOI] [PubMed] [Google Scholar]
- 13.Gow AJ, Bastin ME, Munoz Maniega S, et al. Neuroprotective lifestyles and the aging brain: activity, atrophy, and white matter integrity. Neurology 2012;79:1802–1808. [DOI] [PubMed] [Google Scholar]
- 14.Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 2012;78:1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchman AS, Leurgans SE, Boyle PA, Schneider JA, Arnold SE, Bennett DA. Combinations of motor measures more strongly predict adverse health outcomes in old age: the Rush Memory and Aging Project, a community-based cohort study. BMC Med 2011;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchman AS, Boyle PA, Wilson RS, Bienias JL, Bennett DA. Physical activity and motor decline in older persons. Muscle Nerve 2007;35:354–362. [DOI] [PubMed] [Google Scholar]
- 17.Heil DP. Predicting activity energy expenditure using the Actical activity monitor. Res Q Exerc Sport 2006;77:64–80. [DOI] [PubMed] [Google Scholar]
- 18.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Curr Alzheimer Res 2012;9:646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McPhillips JB, Pellettera KM, Barrett-Connor E, Wingard DL, Criqui MH. Exercise patterns in a population of older adults. Am J Prev Med 1989;5:65–72. [PubMed] [Google Scholar]
- 20.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 21.Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology 2006;67:441–445. [DOI] [PubMed] [Google Scholar]
- 22.Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv 1976;6:493–508. [DOI] [PubMed] [Google Scholar]
- 23.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23(suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 24.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002;17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zacharaki EI, Kanterakis S, Bryan RN, Davatzikos C. Measuring brain lesion progression with a supervised tissue classification system. Med Image Comput Comput Assist Interv 2008;11:620–627. [DOI] [PubMed] [Google Scholar]
- 26.Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn Sci 2013;17:502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Education modifies the association of amyloid but not tangles with cognitive function. Neurology 2005;65:953–955. [DOI] [PubMed] [Google Scholar]
- 28.Stern Y, Albert S, Tang MX, Tsai WY. Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology 1999;53:1942–1947. [DOI] [PubMed] [Google Scholar]
- 29.Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol 2006;5:406–412. [DOI] [PubMed] [Google Scholar]
- 30.Boyle PA, Buchman AS, Wilson RS, Yu L, Schneider JA, Bennett DA. Effect of purpose in life on the relation between Alzheimer disease pathologic changes on cognitive function in advanced age. Arch Gen Psychiatry 2012;69:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farfel JM, Nitrini R, Suemoto CK, et al. Very low levels of education and cognitive reserve: a clinicopathologic study. Neurology 2013;81:650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elbaz A, Vicente-Vytopilova P, Tavernier B, et al. Motor function in the elderly: evidence for the reserve hypothesis. Neurology 2013;81:417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pikula A, Beiser AS, Chen TC, et al. Serum brain-derived neurotrophic factor and vascular endothelial growth factor levels are associated with risk of stroke and vascular brain injury: Framingham Study. Stroke 2013;44:2768–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc 2007;39:728–734. [DOI] [PubMed] [Google Scholar]
- 35.Buchman AS, Yu L, Boyle PA, et al. Microvascular brain pathology and late-life motor impairment. Neurology 2013;80:712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke 2011;42:722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchman AS, Yu L, Boyle PA, Shah RC, Bennett DA. Total daily physical activity and longevity in old age. Arch Intern Med 2012;172:444–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah RC, Buchman AS, Leurgans S, Boyle PA, Bennett DA. Association of total daily physical activity with disability in community-dwelling older persons: a prospective cohort study. BMC Geriatr 2012;12:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polvikoski TM, van Straaten EC, Barkhof F, et al. Frontal lobe white matter hyperintensities and neurofibrillary pathology in the oldest old. Neurology 2010;75:2071–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Supplement
Accompanying Editorial