IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo (original) (raw)
Abstract
A novel approach for the treatment of ovarian cancer includes immunotherapy with genetically engineered T cells targeted to ovarian cancer cell antigens. Using retroviral transduction, T cells can be created that express an artificial T cell receptor (TCR) termed a chimeric antigen receptor (CAR). We have generated a CAR, 4H11-28z, specific to MUC-16ecto antigen, which is the over-expressed on a majority of ovarian tumor cells and is the retained portion of MUC-16 after cleavage of CA-125. We previously demonstrated that T cells modified to express the 4H11-28z CAR eradicate orthotopic human ovarian cancer xenografts in SCID-Beige mice. However, despite the ability of CAR T cells to localize to tumors, their activation in the clinical setting can be inhibited by the tumor microenvironment, as is commonly seen for endogenous antitumor immune response. To potentially overcome this limitation, we have recently developed a construct that co-expresses both MUC16ecto CAR and IL-12 (4H11-28z/IL-12). In vitro, 4H11-28z/IL-12 CAR T cells show enhanced proliferation and robust IFNγ secretion compared to 4H11-28z CAR T cells. In SCID-Beige mice with human ovarian cancer xenografts, IL-12 secreting CAR T cells exhibit enhanced antitumor efficacy as determined by increased survival, prolonged persistence of T cells, and higher systemic IFNγ. Furthermore, in anticipation of translating these results into a phase I clinical trial which will be the first to study IL-12 secreting CAR T cells in ovarian cancer, an elimination gene has been included to allow for deletion of CAR T cells in the context of unforeseen or off-tumor on-target toxicity.
Keywords: chimeric antigen receptors, human ovarian cancer, IL-12, MUC16, tumor microenvironment
Abbreviations: AAPCs, artificial antigen presenting cells; ADCC, antibody-dependent cellular cytotoxicity; ALL, acute lymphocytic leukemia; CAR, chimeric antigen receptor; EGFRt, truncated epidermal growth factor; EOC, epithelial ovarian cancer; i.p., intraperitoneal; IL-12, interleukin-12; i.v., intravenous; MDSC, myeloid-derived suppressor cells; PBL, peripheral blood leukocytes; PBMCs, peripheral blood mononuclear cells; scFv, single-chain fragment antibody; TAA, tumor-associated antigen; TCR, T cell receptor; TIL, tumor-infiltrating lymphocytes; Tregs, regulatory T cells.
Introduction
Ovarian cancer is the most common cause of death among women with gynecologic cancer, partly due to the late stage of patient presentation and also the high rate of recurrence despite a multi-modality therapeutic approach that includes cytoreductive surgery and chemotherapy.1-4 Therefore, other therapeutic strategies need to be developed such as the use of genetically engineered T cells targeted to tumor-associated antigens (TAA) on ovarian cancer cells.
T cells may be genetically modified to express CARs specific to any TAA for which a monoclonal antibody can be generated, including proteins, carbohydrates, and glycolipids, and will recognize this TAA in a human leukocyte antigen-independent manner. The first generation CARs are composed of the extra-cellular domain of single chain anti-TAA fused to a transmembrane domain and a cytoplasmic tail most commonly composed of TCR zeta chain, which is required for downstream signaling and T cell activation.5,6 However, CARs that have included an additional signaling domain derived from a co-stimulatory receptor (2nd generation), such as CD28, 4-1BB or OX-40, have been noted to have increased antitumor efficacy.7-9
CAR technology has made great strides in treatment of certain hematological malignancies, particularly acute lymphocytic leukemia (ALL), but has more limited success with solid tumors. A microenvironment generated by the tumor, which includes myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs), immunosuppressive cytokines (IL-10 and TGF-B), as well as ligands for tumor-expressed T cell inhibitory signaling receptors (e.g. PD-1 and CTLA-4) likely impair in vivo persistence and antitumor activity of CAR T cells in solid tumors.10 Therefore, additional modifications need to be made to the second generation CAR to overcome the harsh solid tumor microenvironment and enhance antitumor function.
We have developed a second generation CAR, 4H11-28z, containing a single-chain fragment antibody (scFv) that recognizes MUC16ecto, with promising preclinical results.11 MUC16ecto is the retained extracellular portion of the glycosylated mucin, MUC16. MUC-16 consists of a cytoplasmic tail, a transmembrane domain, and an extracellular portion. The extracellular region contains a cleavage site distal to which includes 16–20 tandem repeats of 156 amino acids, each with potential glycosylation sites.12 Of note, MUC16ecto remains on the cell surface after the distal N-terminus of MUC-16 called CA-125 (a well-known ovarian tumor antigen routinely used for monitoring disease) is cleaved and released.13 About 80% of women with epithelial ovarian cancer (EOC) have elevated levels (> 65U/mL) of serum CA-125, and a majority of these patients have been found to express MUC16ecto on their tumor cells.12,14 MUC16ecto is also expressed on normal tissues including the uterus, fallopian tubes, and ovaries, which are removed initially during surgery as part of treatment, and also at low levels in the trachea.15 Therefore, expression of MUC16ecto in patients with advanced ovarian cancer is likely to be highly specific to the tumor cells, so this antigen offers an excellent target for immunotherapy of EOC.12
We have recently shown that 4H11-28z T cells eradicate orthotopic human ovarian cancer xenografts in SCID-Beige mice in a subset of treated mice.11 Herein, we have further modified CAR T cells to secrete IL-12, a stimulatory cytokine which acts through multiple mechanisms to enhance CD8+ T cell function and potentially overcome the inhibitory microenvironment.16 Previous clinical trials in ovarian cancer have used intraperitoneal (i.p.) injection of recombinant IL-12 with some efficacy, but these studies resulted in various treatment-related toxicities.17 In this report we show that MUC16ecto-specific CAR T cells that secrete IL-12 can enhance antitumor efficacy and as a safety measure, incorporated an “elimination” gene.
Results
Generation of 4H11-28z/IL-12 T cells
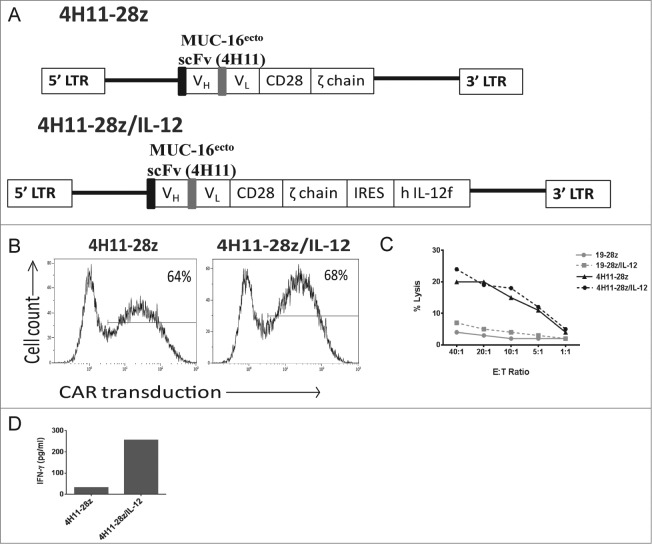
We have previously constructed a SFG retroviral vector encoding a second-generation CAR targeted to MUC-16ecto (4H11-28z) using a monoclonal antibody specific for MUC-16ecto antigen (4H11 hybridoma) (Fig. 1A -top panel).11 The second-generation CAR contains a scFv domain that allows for antibody recognition along with CD28 and CD3ζ signaling domains. We further modified the 4H11-28z construct with an IRES element followed by a gene encoding human IL-12 p35 and p40 subunit fusion connected via a flexi linker (flexi-IL-12 (hIL-12f); Fig. 1A -bottom panel). To assess the function of the 4H11-28z/IL-12 CAR (also referred to as armored CAR), healthy donor T cells isolated from peripheral blood were retrovirally transduced to express either the 2nd generation CAR, armored CAR, or an irrelevant CAR targeted to CD19 (19-28z and 19-28z/IL-12) as controls. Transduction efficiency was determined using flow cytometry and was comparable for all constructs (Figs. 1B and 2B-right panel, p > 0.05).
Figure 1.
Design and production of IL-12 secreting 4H11-28z T cells and in vitro studies of 4H11-28z/IL-12 T cells compared to second-generation 4H11-28z CAR T cells. (A) Schema of 4H11-28z and 4H11-28z/IL-12 retroviral vectors. 4H11 scFv: MUC16-specific scFv derived from heavy (VH) and light (VL) chain variable regions of the mAb 4H11; CD28: human CD28 transmembrane and cytoplasmic signaling domains; ζ chain: human TCRζ chain cytoplasmic signaling domain; flexi human IL-12 (hIL-12f); LTR: 5′ and 3′ long terminal repeat; black box: CD8 leader sequence; gray box: (Gly4Ser)3 linker. (B) Flow cytometric analysis of transduction efficiency using a 4H11-CAR specific antibody. Transduction efficiencies achieved were routinely >50%. Data shown is representative of >7 independent experiments. (C) The ability of CAR T cells to lyse SKOV3 (MUC-16ecto) ovarian cancer cells was assessed using a 4-h 51Cr release assay. CAR T cells targeting tumor cells demonstrated increased killing by 4H11-specific CARs compared to irrelevant non-specific CAR T cells (1928z and 19-28z/IL-12). Data shown is representative of four independent experiments. (D) Peripheral mononuclear cells (PBMCs) were exposed to conditioned media overnight from viral producers and the amount of IFNγ in each sample was assessed using a luminex assay. Elevations in IFNγ was seen in 4H11-28z/IL-12 compared to 4H11-28z conditioned media. Data shown is representative of two independent experiments.
Figure 2.
(See previous page). (G) CD16+ NK-92 cells were used in an ADCC assay to determine the ability of cetuximab or rituximab to mediate clearance of EGFRt/4H11-28z/IL-12 CAR T cells. The samples were analyzed by flow cytometry for the decrease in percentage of CAR T cells with the antibody compared to CAR T cells in the absence of antibody. EGFRt/4H11-28z/IL-12 CAR T cells showed increased ADCC when treated with cetuximab compared to rituximab (p < 0.05). Cetuximab did not induce ADCC on 4H11-28z/IL-12 CAR T cells. Data shown is mean percentage of ADCC (±SEM) from three independent experiments.
In vitro function of 4H11-28z/IL-12 T cells
In order to determine MUC-16ecto-specific cytolytic function of CAR T cells, we performed a standard 51Cr release assay using MUC-16ecto expressing SKOV3 human ovarian tumor cells as targets. The 4H11-28z and 4H11-28z/IL-12 T cells kill SKOV3 (MUC-16ecto) tumor targets equally effectively. 4H11 CAR T cells lyse tumor targets at higher levels compared to control CAR T cells (19-28z and 19-28z/IL-12), which express a CAR that specifically binds an irrelevant antigen, CD19 (Fig. 1C). Given that the lysis assay was brief (4 h), to assess specificity and killing function of T cells against cognate tumor, we would not expect to see a difference between IL-12 secreting and non-IL-12 secreting 4H11-28z T cells.
Validation of biologic activity of the secreted hIL-12f was determined by measuring IFNγ secretion from human peripheral blood mononuclear cells (PBMCs) cultured in supernatant from 293-Glv9 4H11-28z/IL-12 viral-producing cell line compared to control supernatant from 293-Glv9 4H11-28z viral producers. 1 × 106 PBMCs were co-cultured in 1 mL of viral supernatant overnight, and a luminex assay was used to measure the amount of IFNγ secreted from the PBMCs. As expected, only PBMCs cultured with viral supernatant containing flexi human IL-12 gene showed elevated amounts of IFNγ (Fig. 1D). At this point, we concluded from our in vitro studies that 4H11-28z/IL-12 T cells express the CAR, are able to lyse cognate tumor targets, and secrete biologically active IL-12.
Generation and testing of EGFR/4H11-28z/IL-12 T cells in vitro
Given the concern for potential toxicity associated with IL-12, we incorporated an elimination gene into the 4H11-28z/IL-12 construct. The elimination gene, epidermal growth factor receptor (EGFR), is a truncated form of the gene for human EGFR, known as EGFRt. It consists of amino acids 310-646 of human EGFR, incorporating extracellular domains III/IV and the transmembrane domain (bp 1000–2004).18 Because EGFRt lacks extracellular domains I/II and the cytoplasmic tail of EGFR, EGFRt does not bind to EGFR ligands (EGF, TGF-α) or signal through the receptor tyrosine kinase cytoplasmic tail of EGFR. However, it retains the ability to be bound by cetuximab, which induces cell death via multiple mechanisms, including antibody-dependent cellular cytotoxicity (ADCC) and complement-mediated cytotoxicity.18,19
The EGFRt gene is upstream of a P2A element followed by the 4H11-28z CAR, an IRES element and the flexi IL-12 gene. Thus, the gamma-retroviral vector is a tricistronic vector that encodes the CAR specific for MUC16ecto, the IL-12 gene and the truncated EGFR elimination gene (EGFRt/4H11-28z/IL-12, Fig. 2A).
Healthy donor T cells isolated from peripheral blood were retrovirally transduced with EGFRt/4H11-28z/IL-12, with equivalent transduction efficiencies between 19-28z (55% ± 3), 19-28z/IL-12(52% ± 6), 4H11-28z (49% ± 7), 4H11-28z/IL-12 (55% ± 5) and EGFRt/4H11-28z/IL-12 (57% ± 7) T cells (Fig. 2B-right panel, p > 0.05). Furthermore, by flow cytometry, EGFRt/4H11-28z/IL-12 T cells express both the EGFRt and 4H11 CAR on the cell surface (Fig. 2B-left panel). To ensure that addition of the elimination gene did not impede specificity and killing function of CAR T cells against cognate tumors, we performed a 51Cr release assay and found that the transduced T cells lyse tumor targets equally effectively as the “parental” 4H11-28z and 4H11-28z/IL-12 T cells compared to control transduced T cells, 19-28z and 19-28z/IL-12 T cells, which did not show specific killing (Fig. 2C).
Next, we assessed the ability of CAR T cells to secrete IL-12(p70) using a luminex assay. In the absence of antigen stimulation, IL-12 was secreted at low amounts. When CAR T cells were co-cultured with SKOV3 (MUC-16ecto+) tumor cells, IL-12(p70) secretion increased 4-fold in the 4H11-28z/IL-12 and EGFRt/4H11-28z/IL-12 groups compared to 4H11-28 (p < 0.05, Fig. 2D-tumor). The increase in IL-12 secretion from 19-28z/IL-12 CAR T cells when co-cultured with tumor compared to either 19-28z/IL-12 CAR T cells alone or compared to 19-28z co-cultured with tumor was not significant (_p_ > 0.05).
We also assessed the ability of CAR T cells to secrete IFNγ using a luminex assay. In the absence of stimulation, IFNγ secretion was low in all of the groups (Fig. 2E-no tumor). When CAR T cells were co-cultured with tumor cells, high amounts of IFNγ was secreted from CAR T cells specific for tumor targets (4H11-28z, 4H11-28z/IL-12 and EGFRt/4H11-28z/IL-12) (Fig. 2E-tumor). The amount of IFNγ secretion was 27-fold higher in 4H11-28z/IL-12 and 28-fold higher in EGFRt/4H11-28z/IL-12 compared to 4H11-28z CAR T cells co-cultured with tumor (p < 0.05).
We next assessed in vitro expansion of CAR T cells following stimulation with NIH-3T3 (MUC-16ecto/B7.1) artificial antigen presenting cell (AAPC) monolayers. EGFRt/4H11-28z/IL-12 and 4H11-28z/IL-12 CAR T cells initially expanded to a similar degree compared to other CAR T cells at day 3 (p > 0.05 for all groups), but by day 6 and 9 EGFRt/4H11-28z/IL-12 and 4H11-28z/IL-12 CAR T cells demonstrated increased fold expansion compared to 4H11-28z CAR T cells (p < 0.05, Fig. 2F). To confirm the function of the elimination gene, EGFRt, we performed an ADCC assay. CAR T cells (targets) with and without the elimination gene were co-cultured with CD-16+ NK-92 cells (effectors) in the presence or absence of cetuximab (1 μg/mL) or rituximab (10 μg/mL), as demonstrated previously.18 After 4 h, the samples were analyzed by flow cytometry, and the percentage of CAR T cells remaining after the addition of cetuximab or rituximab was expressed as a percentage of ADCC. At the 1:1 ratio of effectors:targets, EGFRt/4H11-28z/IL-12 CAR T cells with cetuximab were killed at 65%, (compared to the 5% and 4%, for 4H11-28z/IL-12 T cells + cetuximab and EGFRt/4H11-28z/IL-12 + rituximab, respectively) verifying the function of EGFRt as an elimination gene (Fig. 2G).
In vivo antitumor activity of EGFR/4H11-28z/IL-12 T cells
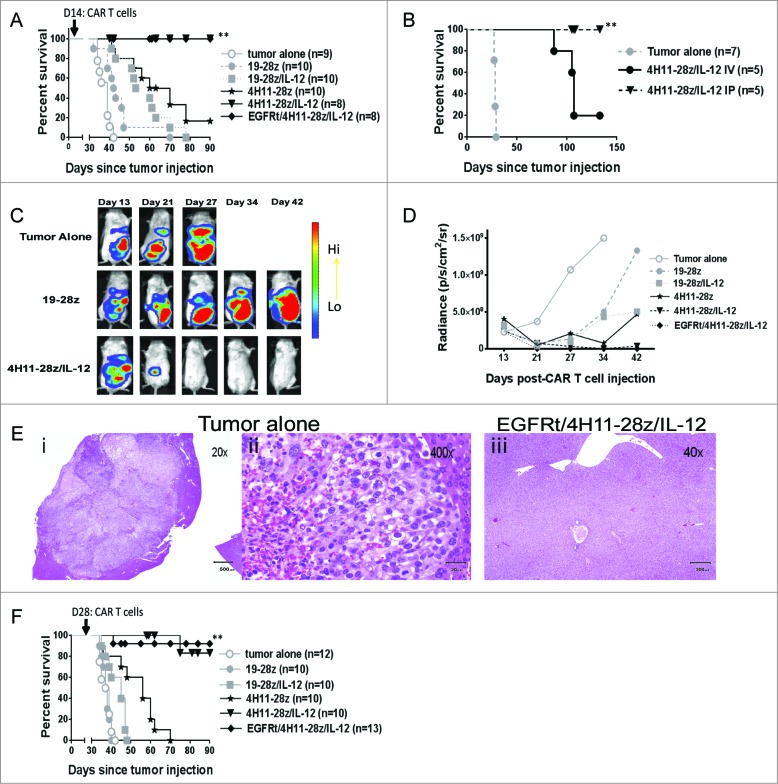
We next assessed CAR T cell function in vivo. To assess in vivo antitumor activity of 4H11-28z/IL-12 T cells, we used an orthotopic xenotransplant ovarian cancer tumor model in SCID-Beige mice. Mice were injected i.p. with 1 × 107 SKOV3 (MUC-16ecto/GFP-FFLuc) ovarian tumor cells. After 5–6 weeks, untreated mice demonstrated increased abdominal girth and were sacrificed. To determine if CAR T cells could treat mice with established ovarian tumors, mice were injected i.p. with CAR T cells 2 weeks post-tumor injection, and tumor progression was monitored. Mice treated with 4H11-28z/IL-12 and EGFRt/4H11-28z/IL-12 CAR T cells showed enhanced survival compared to mice treated with 4H11-28z CAR T cells (p < 0.05, Fig. 3A). Furthermore, 4H11-28z/IL-12 and EGFRt/4H11-28z/IL-12 treated mice showed complete eradication of tumors.
Figure 3.
In vivo antitumor efficacy of EGFRt/4H11-28z/IL-12 CAR T cells. (A) Mice inoculated with 1 × 107 SKOV3 (MUC-16ecto+) tumor cells i.p. were treated with i.p. infusion of 2.5 × 106 CAR T cells 2 weeks later. Survival of SKOV3 tumor bearing mice was significantly enhanced with 4H11-28z/IL-12 and EGFRt/4H11-28z/IL-12 CAR T cells compared to 4H11-28z CAR T cells (**p < 0.05). Data shown is from two independent experiments. (B) Mice inoculated with 1 × 107 SKOV3 (MUC-16ecto+) tumor cells i.p. were treated with i.v. or i.p. infusion of 2.5 × 106 CAR T cells 2 weeks later. Survival of SKOV3 tumor bearing mice was significantly enhanced with i.p. infusion of 4H11-28z/IL-12 CAR T cells compared to i.v. infusion of 4H11-28z/IL-12 CAR T cells (**p < 0.05). Data shown is from one experiment. (C) Representative bioluminescent imaging (BLI) of tumor progression following i.p administration of tumor alone compared to mice treated with 19-28z and 4H11-28z/IL-12 CAR T cells (i.p.) 2 week post-tumor administration. Day 13 was imaged one day prior to CAR T cell treatment. Data shown is representative of five mice from two independent experiments. (D) Mean radiance of tumor progression imaged with BLI in mice bearing SKOV3(MUC-16ecto+) tumors and treated 2 weeks later. Mice treated with 4H11-28z/IL-12 and EGFRt/4H11-28z/IL-12 CAR T cells have less tumor compared to untreated control mice at day 27. Data shown is mean radiance from five mice and representative of two experiments. (E) H&E staining of SKOV3(MUC-16ecto+) tumor in the liver of untreated mice (i and ii) at day 35 and normal liver at day 93 in SKOV3(MUC-16ecto+) tumor bearing mice treated with EGFRt/4H11-28z/IL-12 (iii). Data shown is representative of three mice from two independent experiments. (F) Mice inoculated with 1 × 107 SKOV3 (MUC-16ecto+) tumor cells i.p. were treated with i.p. infusion of 2.5 × 106 CAR T cells 4 weeks later. Survival of SKOV3 tumor bearing mice was significantly enhanced with 4H11-28z/IL-12 and EGFRt/4H11-28z/IL-12 CAR T cells compared to 4H11-28z CAR T cells (**p < 0.05). Data shown is from two independent experiments.
In our orthotopic xenotransplant ovarian cancer tumor model, tumors only engrafted i.p. since MUC-16ecto expressing SKOV3 were injected i.p. We were interested in assessing whether i.v. infusion of CAR T cells could prolong the survival of mice with established i.p. tumors as well as CAR T cells delivered i.p. Both i.p. and i.v. administration of CAR T cells prolong survival of mice compared to tumor alone, but i.p. administration was found to be significantly more effective compared to i.v. administration of CAR T cells (p < 0.05, Fig. 3B).
Bioluminescence imaging (BLI) was used to assess tumor growth after i.p. MUC-16ecto+ SKOV3 (GFP-FFLuc) human ovarian tumor injection. The mice were imaged one day prior to CAR T cell injection (on day 13) and on days 21, 27, 34, and 42 post-tumor injection. BLI demonstrated that while tumor-bearing untreated mice or control CAR T cell treated mice (19–28z) continued to develop tumor by day 27, no tumor can be detected in 4H11-28z/IL-12 treated mice (Fig. 3C). Fig. 3D shows untreated and control (19-28z T cell treated) mice rapidly developed large tumors compared to 19–28z/IL-12 and 4H11-28z T cell treated mice, which developed tumors at a slower rate. On the other hand, both 4H11-28z/IL-12 and EGFRt/4H11-28z/IL-12 T cell-treated mice demonstrated no evidence of tumor at 42 d post-tumor injection. Tumor burden was extremely low as early as day 27 (mean at each time point shown, Fig. 3D). At 92 d, mice from the EGFRt/4H11-28z/IL-12 group were sacrificed for pathology studies along with mice from the tumor alone group at 5 weeks. The tumor alone mice showed disease i.p. in the intestine, liver, pancreas, kidneys, and abdominal wall while the EGFRt/4H11-28z/IL-12 mice demonstrated no evidence of disease in any of these tissues (data not shown). Fig. 3E illustrates representative images of tumor in the liver of an untreated mouse at 20x and 400x magnification (left and middle panels, respectively) compared to the liver of an EGFRt/4H11-28z/IL-12 treated mouse (right panel) with normal liver histology.
To assess the ability of 4H11 CAR T cells to eradicate advanced tumors, we treated mice with CAR T cells 4 weeks post-tumor infusion. Mice treated with 4H11-28z/IL-12 and EGFRt/4H11-28z/IL-12 CAR T cells demonstrate long-term survival that was statistically significant compared to mice treated with 4H11-28z CAR T cells (p < 0.05, Fig 3F). Therefore, even in the delayed treatment model, 4H11-28z/IL-12 and EGFRt/4H11-28z/IL-12 treated mice showed enhanced survival.
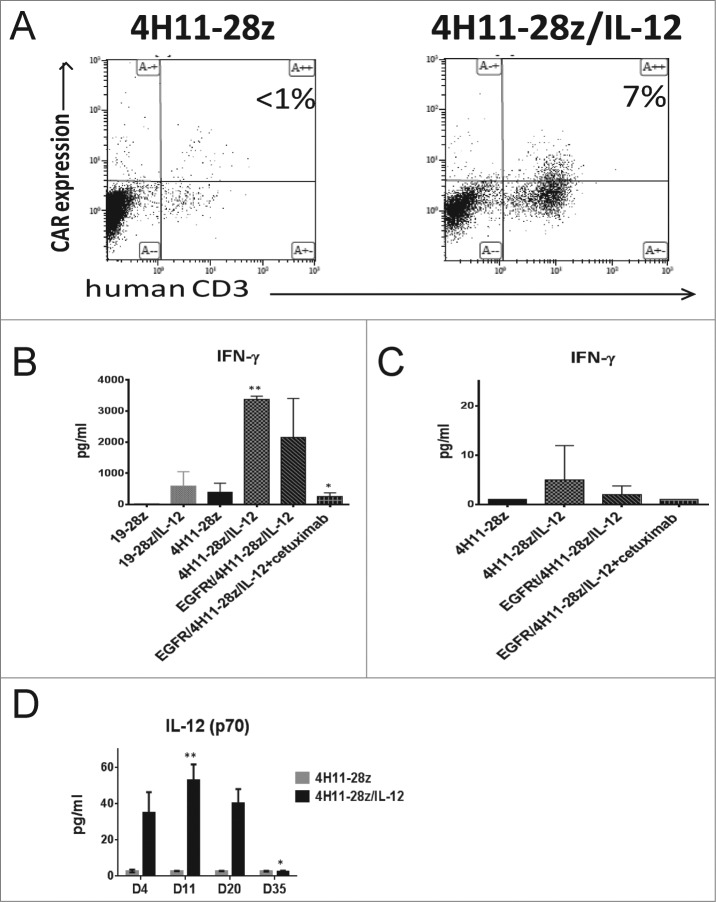
In order to elucidate the mechanism of 4H11-28z/IL-12 CAR T cell antitumor function, peripheral blood samples were obtained from mice treated with IL-12 secreting and the non-IL-12 secreting CAR T cells 6 d post-CAR T cell injection. CAR T cell persistence was determined by flow cytometry. While the 4H11-28z/IL-12 T cell treated mice showed T cell persistence, the 4H11-28z T cell treated mice failed to show any appreciable numbers of CAR T cells remaining (Fig. 4A).
Figure 4.
Increased CAR T cell persistence and serum IL-12 levels in mice treated with EGFRt/4H11-28z/IL-12 CAR T cells. (A) Mice were inoculated with 1 × 107 SKOV3 (MUC-16ecto+) tumor cells i.p. followed 2 weeks later by i.p. infusion of 2.5 × 106 CAR T cells. Peripheral blood (PB) of mice 6 d post-CAR treatment with 4H11-28z/IL-12 T or 4H11-28z CAR T cells was collected and analyzed by flow cytometry for presence of CAR T cells by staining with Ab specific for hCD3 and 4H11-CAR. PB from mice treated with 4H11-28z/IL-12 has increased amounts of CAR T cells compared to mice treated with 4H11-28z CAR T cells. Data shown is representative of four mice from two independent experiments. (B) Serum levels of IFNγ in mice treated with CAR T cells 6 d post-T cell infusion were analyzed by luminex assay. Increased serum IFNγ was shown in mice treated with 4H11-28z/IL-12 and EGFRt/4H11-28z/IL-12 compared to mice treated with 4H11-28z (**p < 0.05). Mice treated with _EGFRt_/4H11-28z/IL-12+cetuixmab shows no difference in serum IL-12 compared to mice treated with 4H11-28z (*_p_ > 0.05). Data shown is mean (±SEM) from four mice, representative of two independent experiments. (C) Serum levels of IFNγ in mice treated with CAR T cells 35 d post-T cell infusion were analyzed by luminex assay. No statistically significant differences were identified between any of the groups (p > 0.05). Data shown is mean (±SEM) from four mice, representative of two independent experiments. (D) Serum levels of IL-12(p70) in mice treated with CAR T cells 4, 11, 20, and 35 d post-T cell infusion were analyzed by luminex assay. Mice treated with 4H11-28z/IL-12 showed increased IL-12(p70) on days 11 and 20 compared to day 35 post-CAR T cell injection (**p < 0.05). Mice treated with 4H11-28z showed no increase in IL-12 (p70) on any of the days post-CAR T cell injection (*_p_ > 0.05). Data shown is mean (±SEM) from four mice, representative of two independent experiments.
Next, we assessed the amount of IFNγ in the sera of tumor bearing mice treated with CAR T cells using a luminex assay 6 d post-CAR T cell injection. IFNγ was elevated to a greater degree in 4H11-28z/IL-12 and EGFRt/4H11-28z/IL-12 T cell treated mice compared to 4H11-28z CAR T cell treated mice (p < 0.05, Fig. 4B). The effectiveness of the CAR elimination gene _in vivo_ was tested. _EGFRt_/4H11-28z/IL-12 T cell treated mice injected with cetuximab i.p. for 6 d showed a decrease in serum IFNγ similar to 4H11-28z CAR T cell treated mice (_p_ > 0.05, Fig. 4B, respectively). At 35 d post-CAR T cell injection, which correlates to the absence of tumor in 4H11-28z/IL-12 and EGFRt/4H11-28z/IL-12 groups, we re-analyzed mice for the presence of CAR T cells and could no longer identify this population (data not shown). Moreover, we re-assessed for serum IFNγ and found that IFNγ was low in all groups with no statistically significant differences between any of the groups (p > 0.05, Fig. 4C). Lastly, we assessed the amount of IL-12 in the serum of SCID-Beige mice with established ovarian tumors treated with 4H11-28z and 4H11-28z/IL-12 CAR T cells at various time points post-CAR injection (days 4, 11, 20, and 35). Mice treated with 4H11-28z/IL-12 CAR T cells showed significantly increased serum IL-12(p70) on days 11 and 20 compared to day 35 post-CAR T cell injection (p < 0.05, Fig. 4D). However, mice treated with 4H11-28z CAR T cells showed no increase in IL-12(p70) on days 4, 11, and 20 compared to day 35 post-CAR T cell injection (_p_ > 0.05).
Discussion
The use of CAR technology has shown great promise in the treatment of hematological malignancies, particularly ALL, but these benefits have not been translated to the solid tumor setting. In order for adoptive CAR T cell therapy to be successful in solid tumors, an appropriate target needs to be chosen. However, while a proper target is necessary, it is not sufficient given that the tumor microenvironment may inhibit CAR T cell function. Therefore, the addition of another factor such as a cytokine can serve as a mechanism to overcome this inhibition in a directed manner.
The current research demonstrates the use of CAR technology in the treatment of human ovarian tumors in an immunocompromised murine model. CAR T cells that are specific for MUC-16ecto and further modified to secrete IL-12 demonstrated enhanced proliferation and IFNγ production compared to 2nd generation anti-ovarian tumor CAR. Furthermore, SCID-Beige mice injected with 4H11-28z/IL-12 T cells showed improved long-term survival, prolonged CAR T cell persistence, and higher amounts of IFNγ and IL-12 compared to the mice injected with 4H11-28z T cells. Although the majority of our mouse experiments were done with i.p. administration of CAR T cells because the tumor was limited to the abdomen, in patients where metastatic disease outside of the peritoneum may be present, both i.v. and i.p. administration will likely to be important to achieve optimal responses. The addition of an elimination gene, EGFRt, to CAR T cells allows a safety mechanism that should facilitate application of this technology to a phase I clinical trial.
Determining an appropriate target is one of the first critical steps in creating a functional CAR T cell. CA-125 is an ovarian cancer marker, but since it is cleaved from the tumor surface, it is not an ideal target for CAR T cells. In our model, the ovarian CAR is specific to a small region of MUC-16ecto, the retained extracellular domain of MUC-16 after CA-125 becomes cleaved. The 4H11 antibody from which the CAR was derived, was studied extensively in terms of reactivity on human high-grade serous ovarian carcinomas as well as normal tissues.12 While the 4H11 antibody bound to high-grade serous ovarian tumors, normal tissues such as adult colon, rectum, ectocervix, small intestine, ovary, liver, pancreatic ducts, spleen, kidney and skin (as well as fetal heart, gallbladder, colon, small intestine, liver, rectum, adrenal, thyroid, spleen, skin, bone, epididymis, brain, lung, muscle, smooth muscle, kidney, eye, umbilical cord, and placenta) were not stained with this antibody.12 However, 4H11 did stain the luminal side of esophageal glands and a few other areas, such as bronchial epithelium, endocervical glands, and gastric glands, but only in the cytoplasm.12 Therefore, given the limited staining of the 4H11 antibody on the extracellular surface of normal cells and the high specificity to serous ovarian tumors, the 4H11 CAR is a promising target for use in a phase I clinical trial.
To date, CAR T cell technology has not yet been applied successfully to the treatment of ovarian cancer. Previously, there has been a phase I study using adoptive immunotherapy of CAR T cells reactive with α-folate receptor.20 Of the 14 patients treated on this trial, none showed a reduction in tumor burden. Moreover, there was a lack of specific localization to the tumor site and modified T cells could only be detected in circulation for the first 2 d after transfer. The authors concluded that though large numbers of gene-modified tumor-reactive T cells can be safely administered to patients, future studies should include strategies to extend T cell persistence.20
The apparent lack of localization and T cell persistence in that phase I study could be partly due to the immunosuppressive effects of the tumor microenvironment. Given that the tumor is surrounded by other suppressive elements including inhibitory cells such as MDSCs and Tregs, cytokines (IL-10 and TGF-B), as well as T cell suppressing ligands (PD-1 and CTLA-4), an ovarian-specific CAR alone is not sufficient for tumor elimination.21-25 Ovarian cancers have endogenous tumor infiltrating lymphocytes (TIL), the extent of which correlates to prolonged patient survival, suggesting that ovarian tumors are in part recognized by the immune system.26,27 However, ovarian cancers have also been found specifically to have immunosuppressive CD4+CD25hi Tregs that can potentially inhibit the function of TIL and are associated with reduced survival. 22,25 Thus, the addition of another element to allow for the CAR T cells to navigate through the harsh tumor microenvironment is needed for a more successful therapy.
CAR T cells can be modified to secrete stimulatory cytokines that promote a productive antitumor immune response. Specifically, IL-12 stimulates T cells (signal 3) and increases secretion of IFNγ thereby allowing for improved cytotoxic capacity.16 More significantly, IL-12 has been shown to modulate the hostile tumor microenvironment through multiple mechanisms, including reactivation of anergic TILs, inhibition of Treg-mediated suppression of effector T cells, recruitment of NK cells to the tumor site, and inhibition of IL-10 and TGF-β secretion by TAMs.28-30 We have shown enhanced in vivo persistence and antitumor activity of these IL-12 secreting CAR T cells, now potentially resistant to inhibition by Tregs.
Despite all the expected benefits, expression of IL-12 by the vector raises safety concerns based on previous studies administering i.p. IL-12. However, the IL-12 levels published in humans trials (> 4000 pg/mL) are 80-fold higher than the values in our murine studies (< 50pg/mL) which is anticipated to reduce deleterious effects.17 A potential alternative approach includes inducible secretion of IL-12 as published by other groups.31,32 In terms of production of IL-12 _in vitro_, the amount of IL-12 secreted by CAR T cells are > 5 fold lower compared to CAR expressing flexi-IL-12 or inducible IL-12 under control of NFAT promoter from a recent publication from the NIH.31 The lower IL-12 observed is likely due to the fact that (1) the IL-12 is located after an IRES element and (2) the construct is tricistronic (both of which led to lower amounts of IL-12 to be expressed).
It remains a possibility that native TCRs present on CAR modified T cells can induce secretion of increased amounts of IL-12. This may further augment the immune response in the patient, and could also further potentiate the cytokine storm. Therefore, the safety features including the addition of the elimination gene become even more critical. Given the breadth and importance of understanding the effects of CAR modified T cells on the endogenous immune system and to determine if the addition of IL-12 can truly overcome a completely intact immune system, we are in the process of utilizing a syngeneic mouse model to address these issues.
Despite the lower amounts of IL-12 shown in our studies compared to the previous studies, there remains a concern for potential on-target off-tumor toxicity associated with this approach. The CAR is specific to human MUC16 antigen, and though there is some homology against mouse MUC16, this model does not study any of the potential on-target off-tumor toxicity. If toxicity is encountered, the addition of an elimination gene allowing expression of a truncated human epidermal growth factor receptor (EGFRt) will permit the removal of genetically-modified T cells in vivo as mentioned above.15 Since it lacks extracellular domains I/II and the cytoplasmic tail of EGFR, EGFRt does not bind to EGFR ligands (EGF, TGF-α) or signal through the receptor tyrosine kinase pathway. However, it retains the ability to bind cetuximab, which rapidly induces cell death via multiple mechanisms, including ADCC and complement-mediated cytotoxicity. Because huEGFR is only expressed on cells of epithelial origin, and thus not on T lymphocytes, administration of cetuximab should not show toxicity to the patient's native T cells. We have demonstrated that the gene for EGFRt can be stably and efficiently expressed in human T cells without impairing phenotype, function or antigen specificity, and confirmed both in vitro and in vivo elimination of EGFRt/4H11-28z/IL-12 T cells after exposure to cetuximab. Given these results, we are implementing a phase I clinical trial with this construct for patients with platinum-resistant advanced ovarian cancer.
Materials and Methods
Cell lines and T cells
The SKOV3 (MUC-16ecto/GFP-FFLuc) tumor cell line were cultured in RPMI 1640 (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Atlanta Biologicals), nonessential amino acids, HEPES (_N_-2-hydroxyethylpiperazin-_N_’-2-ethanesulfonic acid) buffer, pyruvate, L-glutamine, penicillin/streptomycin, and 2-Mercaptoethanol (Invitrogen). Retroviral producer cell lines (293 Glv9) producing either 19-28z, 19-28z/IL-12, 4H11-28z, 4H11-28z/IL-12 or EGFRt/4H11-28z/IL-12 were cultured in DMEM (Invitrogen). Human T cells were isolated from peripheral blood of healthy donors under Institutional Review Board approved protocol 95-054 using BD Vacutainer CPT tubes (Becton Dickinson) as per the manufacturer's instructions or as a leukopak from the New York Blood Center and separated using density gradient centrifugation with Accu-prep (Axis-Shield PoC AS). T cells were cultured in RPMI 1640 supplemented with 100 IU/mL interleukin-2 (Proluekin, Novartis). All media were supplemented with 10% heat inactivated FBS, 2 mmol/L L-glutamine (Invitrogen), 100 units/mL penicillin, and 100 μg/mL streptomycin (Invitrogen).
Generation of retroviral constructs
4H11-28z in SFG retroviral vector backbone was constructed as described previously.11 Briefly, the heavy and light chain variable regions of 4H11 mAb were derived from 4H11 hybridoma cell line by PCR. The VH and VL fragments were ligated to a (Gly4Ser)3 spacer domain generating the 4H11 scFv and fused to the CD28 transmembrane and cytoplasmic signaling domains along with the TCR CD3ζ signaling domain. The fusion gene encoding the complete human IL-12 with a serine-glycine repeat between the p35 and the p40 chain-coding domains (hIL-12f) was generously provided by Alan Houghton and Jedd Wolchok,33 and modified to include a hCD8 leader peptide and an internal ribosome entry sites.34 The truncated epidermal growth factor receptor (EGFRt) gene was generously provided by Michael Jensen18 and a P2A element (from Integrated DNA Technologies) was incorporated at the end of the gene. The resulting CAR construct were subcloned into the modified Moloney murine leukemia virus retroviral vector SFG. All constructs were verified by sequencing at Memorial Sloan-Kettering Core Sequence Facility.
T-cell isolation and retroviral gene transfer
Isolated healthy donor PBMCs were activated with phytohemagglutinin at 2 μg/mL (Sigma) and recombinant human IL-2 (rhIL-2) (100 IU/mL). Activated T cells were retrovirally transduced on retronectin-coated nontissue culture plates as described previously.35 Gene transfer was assessed on day 7 by flow cytometry.
To generate the relevant NIH-3T3 murine fibroblast activated antigen presenting cells (AAPCs), a MUC-16ecto construct encoding the retained extracellular, transmembrane, and cytoplasmic domains of the MUC 16 antigen was initially subcloned into SFG retroviral vector, SFG(MUC-16ecto). AAPCs were generated by retroviral transduction of SFG(MUC-16ecto) into previously established NIH-3T3 (B7.1) fibroblasts.36,37 The NIH-3T3 (MUC-16ecto/B7.1) artificial antigen-presenting cells (AAPC) were cultured in DMEM supplemented with head-inactivated donor calf serum. Enriched cell lines were isolated by FACS.
SKOV3(MUC-16ecto/GFP-FFLuc) cell line was generated by retroviral transduction with SFG (GFP-FFLuc) and SFG (MUC-16ecto) VSV-G-pseudotyped retroviral supernatants derived from gpg29 fibroblasts as described elsewhere.38,39 Resulting tumor cells were sorted by FACS for MUC-16ecto expression.
Flow cytometry analyses
All flow cytometric analyses of cells were performed using a Gallios Flow Cytometer with Kaluza software (Beckman Coulter). T cells were labeled with the following antibodies as per manufacturer's instructions (clone numbers indicated): CAR-specific monoclonal goat anti-mouse phycoerythrin (PE) antibody (Invitrogen) for CD19-targeted CAR, Armenian hamster 4H11 Ab conjugated to Alexa Fluor 647 (Memorial Sloan-Kettering Cancer Center Monoclonal Antibody Facility), CD3-PE (OKT3) (eBiosciences), rituximab (Genentech), and cetuximab (Bristol-Myers Squibb) requiring a secondary antibody anti-human IgG Fc (PE, clone HP6017, BioLegend). MUC-16ecto expression was measured by flow cytometry with mouse polyclonal sera (generously provided by Dr. Spriggs laboratory).
In vitro analyses of proliferation of CAR human T cells
To assess in vitro expansion, 1 × 106 transduced T cells were co-cultured for 7 d after retroviral transduction in six-well tissue culture treated plates (BD Biosciences) on confluent NIH-3T3 AAPCs in the absence of supplemented cytokines. The cell count for each sample was assessed on d0, d3 d6, and d9 by Guava as per the manufacturer's instructions (Millipore) and the percentage of transduced T cells was monitored by flow cytometry. Proliferation assay results were expressed as fold expansion calculated as a ratio (cells on day x/cells on day 0) for each sample.
Analysis of function of truncated EGFR elimination gene
Antibody-dependent cell-mediated cytotoxicity (ADCC) was determined by using NK-16 cells (expressing CD16) as effectors co-cultured with CAR T cells as targets at a ratio of 1:1 with or without cetuximab or rituximab antibody (1 μg/mL, Bristol-Myers Squibb, 10 μg/mL, Genentech, respectively). At 4 h, the percentage ADCC was determined by flow cytometric analysis of the decrease in CAR T cells in the presence of cetuximab or rituximab compared to in the absence of cetuximab or rituximab, specifically ((1-(percentage of CAR T cells in presence of Ab/percentage of CAR T cells in absence of Ab)) × 100) = percentage of ADCC.
Cytokine detection assays
Cytokine assays were performed by overnight co-culture of 1 × 106 CAR T cells with 1 × 106 tumor cells. Supernatant was harvested and analyzed. Cytokine detection assays were completed as per the manufacturer's specifications using a 4 plex Human Cytokine Magnetic Bead Panel and the Luminex FlexMap3D system (Millipore Corporation). Cytokine concentrations were assessed using Luminex Xponent 4.2 (Millipore Corporation).
Cytotoxicity assays
Cytolysis activity of transduced human T cells was determined using a standard 51Cr release assay, as previously described.36 Briefly, 51Cr (PerkinElmer) radiolabeled tumor cells were incubated with transduced T cells at varying CAR effector T cell to target ratios. The amount of 51Cr released in the supernatant was measured using a Perkin Elmer Top Count NXT (Perkin Elmer) and percentage specific lysis was calculated.
In vivo SCID-Beige mouse tumor model
In all in vivo studies, 8–12 week old CB17.Cg-PrkdcscidLystbg-J/Crl mice (SCID-Beige mice; Charles River Laboratories) were used. Experiments involving animals were conducted with the approval of an Institutional Animal Care and Use Committee protocol at Memorial Sloan-Kettering Cancer Center (00-05-065). Mice were injected i.p. with 1 × 107 tumor cells. Subsequently, 2 or 4 weeks later CAR T cells were injected either i.p. or intravenously (i.v.) at the indicated doses and time intervals following tumor injection. Mice were monitored for distress as assessed by increasing abdominal girth, ruffled fur, and decreased response to stimuli. Distressed mice were euthanized. To determine the amount of serum cytokines, peripheral blood was obtained from mice. Samples were centrifuged and the serum was used directly in cytokine detection assay as indicated above.
Bioluminescent imaging of GF-FFLuc+ human ovarian tumor cells in SCID-Beige mice
Bioluminescent imaging was performed using Xenogen IVIS imaging system with Living Image software (Xenogen). Briefly, GFP-FFLuc+ tumor-being mice were injected i.p. with D-luciferin (150 mg/kg; Xenogen) suspended in PBS and imaged under 2% isoflurane anesthesia after 5–10 min. Image acquisition was done on a 25 cm field of view at medium binning level at various exposure times.
Assessment of in vivo T-cell persistence
SCID-Beige mice were infused i.p. with tumor cells (as described in detail above) followed 2 weeks later by i.p. administration of CAR T cells. Subsequently, blood was obtained at the indicated times. RBCs were lysed with ACK lysis buffer (Lonza) and cells were washed with FACS buffer (2% FBS/PBS) and analyzed by flow cytometry for persistence of human CD3+/CAR+ T cells. Samples for serum cytokine detection assays were centrifuged and used for assay (as described above).
Statistical analysis
All analyses were calculated using Graphpad Prism 5.0 software and the survival data were assessed using a log-rank analysis. ANOVA and post-hoc analysis was completed (Tukey's multiple comparisons test) for multiple comparisons in the mean transduction efficiency figure, proliferation assay, and cytokine secretion data. Individual groups comparing tumor vs. no tumor in the cytokine secretion data was assessed by student's _t_-test. p < 0.05 was considered significant. Results from transduction efficiencies, cytokine secretion data, proliferation assay, ADCC assay are expressed as mean ± SEM.
Acknowledgments
The authors thank Drs. Alan Frey and Sarwish Rafiq for their suggestions and helpful discussions as well as Dr. Kathryn Boland from the Laboratory of Comparative Pathology core facility at MSKCC for mouse pathology images.
Authorship
M.K. designed and performed the experiments, analyzed the data and prepared the manuscript. T.J.P. designed and performed the experiments and analyzed the data. D.S. analyzed the data and revised the manuscript. S.K. analyzed the data and revised the manuscript. R.J.B designed the experiments, analyzed the data, and revised the manuscript.
Disclosure of Potential Conflicts of Interest:
No potential conflicts of interest were disclosed.
Funding
M.K. was supported by American Society of Clinical Oncology-Young Investigator Award and Milton Endowed Fellowship.
References
- 1.Bookman MA. Standard treatment in advanced ovarian cancer in 2005: the state of the art. Int J Gynecol Cancer 2005; 15 Suppl 3:212-20; PMID: [DOI] [PubMed] [Google Scholar]
- 2.du Bois A, Luck HJ, Meier W, Adams HP, Mobus V, Costa S, Bauknecht T, Richter B, Warm M, Schröder W, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst 2003; 95:1320-9; PMID:; http://dx.doi.org/ 10.1093/jnci/djg036 [DOI] [PubMed] [Google Scholar]
- 3.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a gynecologic oncology group study. J Clin Oncol 2003; 21:3194-200; PMID:; http://dx.doi.org/ 10.1200/JCO.2003.02.153 [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CACancer J Clin 2014; 64:9-29; PMID:; http://dx.doi.org/10.3322/caac.21208 [DOI] [PubMed] [Google Scholar]
- 5.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A 1993; 90:720-4; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heuser C, Hombach A, Losch C, Manista K, Abken H. T-cell activation by recombinant immunoreceptors: impact of the intracellular signalling domain on the stability of receptor expression and antigen-specific activation of grafted T cells. Gene Therapy 2003; 10:1408-19; PMID:; http://dx.doi.org/ 10.1038/sj.gt.3302023 [DOI] [PubMed] [Google Scholar]
- 7.Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, King PD, Larson S, Weiss M, Rivière I, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med 2003; 9:279-86; PMID:; http://dx.doi.org/ 10.1038/nm827 [DOI] [PubMed] [Google Scholar]
- 8.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, Campana D. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 2004; 18:676-84; PMID: [DOI] [PubMed] [Google Scholar]
- 9.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotech 2002; 20:70-5; PMID:; http://dx.doi.org/ 10.1038/nbt0102-70 [DOI] [PubMed] [Google Scholar]
- 10.Moon EK, Wang LC, Dolfi DV, Wilson CB, Ranganathan R, Sun J, Kapoor V, Scholler J, Puré E, Milone MC, et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res 2014; 20:4262-73; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chekmasova AA, Rao TD, Nikhamin Y, Park KJ, Levine DA, Spriggs DR, Brentjens RJ. Successful eradication of established peritoneal ovarian tumors in SCID-Beige mice following adoptive transfer of T cells genetically targeted to the MUC16 antigen. Clin Cancer Res 2010; 16:3594-606; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-0192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharma Rao T, Park KJ, Smith-Jones P, Iasonos A, Linkov I, Soslow RA, Spriggs DR. Novel monoclonal antibodies against the proximal (carboxy-terminal) portions of MUC16. Appl Immunohistochem Mol Morphol 2010; 18:462-72; PMID:; http://dx.doi.org/ 10.1097/PAI.0b013e3181dbfcd2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Brien TJ, Beard JB, Underwood LJ, Dennis RA, Santin AD, York L. The CA 125 gene: an extracellular superstructure dominated by repeat sequences. Tumour Biol 2001; 22:348-66; PMID:; http://dx.doi.org/ 10.1159/000050638 [DOI] [PubMed] [Google Scholar]
- 14.Cannistra SA. Cancer of the ovary. N England J Med 2004; 351:2519-29; PMID: [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Cheon DJ, Lu Z, Cunningham SL, Chen CM, Luo RZ, Xing D, Orsulic S, Bast RC, Jr, Behringer RR. MUC16 expression during embryogenesis, in adult tissues, and ovarian cancer in the mouse. Differentiation 2008; 76:1081-92; PMID:; http://dx.doi.org/ 10.1111/j.1432-0436.2008.00295.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med 2003; 197:1141-51; PMID:; http://dx.doi.org/ 10.1084/jem.20021910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenzi R, Edwards R, June C, Seiden MV, Garcia ME, Rosenblum M, Freedman RS. Phase II study of intraperitoneal recombinant interleukin-12 (rhIL-12) in patients with peritoneal carcinomatosis (residual disease < 1 cm) associated with ovarian cancer or primary peritoneal carcinoma. J Trans Med 2007; 5:66; PMID:; http://dx.doi.org/ 10.1186/1479-5876-5-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Chang WC, Wong CW, Colcher D, Sherman M, Ostberg JR, Forman SJ, Riddell SR, Jensen MC. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 2011; 118:1255-63; PMID:; http://dx.doi.org/ 10.1182/blood-2011-02-337360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffioen M, van Egmond EH, Kester MG, Willemze R, Falkenburg JH, Heemskerk MH. Retroviral transfer of human CD20 as a suicide gene for adoptive T-cell therapy. Haematologica 2009; 94:1316-20; PMID:; http://dx.doi.org/ 10.3324/haematol.2008.001677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res 2006; 12:6106-15; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnett BG, Ruter J, Kryczek I, Brumlik MJ, Cheng PJ, Daniel BJ, Coukos G, Zou W, Curiel TJ. Regulatory T cells: a new frontier in cancer immunotherapy. Adv Exp Med Biol 2008; 622:255-60; PMID:; http://dx.doi.org/ 10.1007/978-0-387-68969-2_20 [DOI] [PubMed] [Google Scholar]
- 22.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10:942-9; PMID:; http://dx.doi.org/ 10.1038/nm1093 [DOI] [PubMed] [Google Scholar]
- 23.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev 2008; 222:162-79; PMID:; http://dx.doi.org/ 10.1111/j.1600-065X.2008.00602.x [DOI] [PubMed] [Google Scholar]
- 24.Nelson BH. The impact of T-cell immunity on ovarian cancer outcomes. Immunol Rev 2008; 222:101-16; PMID:; http://dx.doi.org/ 10.1111/j.1600-065X.2008.00614.x [DOI] [PubMed] [Google Scholar]
- 25.Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Gastl G, Gunsilius E, Marth C. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res 2005; 11:8326-31; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-1244 [DOI] [PubMed] [Google Scholar]
- 26.Leffers N, Gooden MJ, de Jong RA, Hoogeboom BN, ten Hoor KA, Hollema H, Boezen HM, van der Zee AG, Daemen T, Nijman HW. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol 2009; 58:449-59; PMID:; http://dx.doi.org/ 10.1007/s00262-008-0583-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Nat Acad Sci U S A 2005; 102:18538-43; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broderick L, Brooks SP, Takita H, Baer AN, Bernstein JM, Bankert RB. IL-12 reverses anergy to T cell receptor triggering in human lung tumor-associated memory T cells. Clin Immunol 2006; 118:159-69; PMID:; http://dx.doi.org/ 10.1016/j.clim.2005.09.008 [DOI] [PubMed] [Google Scholar]
- 29.Kilinc MO, Aulakh KS, Nair RE, Jones SA, Alard P, Kosiewicz MM, Egilmez NK. Reversing tumor immune suppression with intratumoral IL-12: activation of tumor-associated T effector/memory cells, induction of T suppressor apoptosis, and infiltration of CD8+ T effectors. J Immunol 2006; 177:6962-73; PMID:; http://dx.doi.org/ 10.4049/jimmunol.177.10.6962 [DOI] [PubMed] [Google Scholar]
- 30.Watkins SK, Egilmez NK, Suttles J, Stout RD. IL-12 rapidly alters the functional profile of tumor-associated and tumor-infiltrating macrophages in vitro and in vivo. J Immunol 2007; 178:1357-62; PMID:; http://dx.doi.org/ 10.4049/jimmunol.178.3.1357 [DOI] [PubMed] [Google Scholar]
- 31.Chinnasamy D, Yu Z, Kerkar SP, Zhang L, Morgan RA, Restifo NP, Rosenberg SA. Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin Cancer Res 2012; 18:1672-83; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res 2011; 71:5697-706; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-0103 [DOI] [PubMed] [Google Scholar]
- 33.Ferrone CR, Perales MA, Goldberg SM, Somberg CJ, Hirschhorn-Cymerman D, Gregor PD, Turk MJ, Ramirez-Montagut T, Gold JS, Houghton AN et al. Adjuvanticity of plasmid DNA encoding cytokines fused to immunoglobulin Fc domains. Clin Cancer Res 2006; 12:5511-9; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-0979. [DOI] [PubMed] [Google Scholar]
- 34.Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, Brentjens RJ. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 2012; 119:4133-41; http://dx.doi.org/10.1182/blood-2011-12-400044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quintas-Cardama A, Yeh RK, Hollyman D, Stefanski J, Taylor C, Nikhamin Y, Imperato G, Sadelain M, Rivière I, Brentjens RJ. Multifactorial optimization of gammaretroviral gene transfer into human T lymphocytes for clinical application. HumGene Ther 2007; 18:1253-60; PMID:; http://dx.doi.org/ 10.1089/hum.2007.088 [DOI] [PubMed] [Google Scholar]
- 36.Gong MC, Latouche JB, Krause A, Heston WD, Bander NH, Sadelain M. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia 1999; 1:123-7; PMID:; http://dx.doi.org/ 10.1038/sj.neo.7900018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Latouche JB, Sadelain M. Induction of human cytotoxic T lymphocytes by artificial antigen-presenting cells. Nat Biotechnol 2000; 18:405-9; PMID:; http://dx.doi.org/ 10.1038/74455 [DOI] [PubMed] [Google Scholar]
- 38.Santos EB, Yeh R, Lee J, Nikhamin Y, Punzalan B, La Perle K, Larson SM, Sadelain M, Brentjens RJ. Sensitive in vivo imaging of T cells using a membrane-bound Gaussia princeps luciferase. Nature Medicine 2009; 15:338-44; PMID:; http://dx.doi.org/ 10.1038/nm.1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riviere I, Brose K, Mulligan RC. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc Natl Acad Sci USA 1995; 92:6733-7; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]