Cross-Sectional Analysis of Selected Genital Tract Immunological Markers and Molecular Vaginal Microbiota in Sub-Saharan African Women, with Relevance to HIV Risk and Prevention (original) (raw)
Abstract
Data on immune mediators in the genital tract and the factors that modulate them in sub-Saharan women are limited. Cervicovaginal lavage (CVL) samples from 430 sexually active women from Kenya, South Africa, and Rwanda were analyzed for 12 soluble immune mediators using Bio-Plex and Meso Scale Discovery multiplex platforms, as well as single enzyme-linked immunosorbent assays. Ten bacterial species were quantified in vaginal swab samples. Bacterial vaginosis (BV) was defined by Nugent scoring. CVL samples from HIV-infected women showed a clear-cut proinflammatory profile. Pregnant women, adolescents, and women engaging in traditional vaginal practices differed in specific soluble markers compared to reference groups of adult HIV-negative women. Cervical mucus, cervical ectopy, abnormal vaginal discharge, and having multiple sex partners were each associated with an increase in inflammatory mediators. The levels of interleukin-1α (IL-1α), IL-1β, IL-6, IL-12(p70), and IL-8 were elevated, whereas the IL-1RA/IL-1(α+β) ratio decreased in women with BV. The level of gamma interferon-induced protein 10 was lower in BV-positive than in BV-negative women, suggesting its suppression as a potential immune evasion mechanism by BV-associated bacteria. Lactobacillus crispatus and Lactobacillus vaginalis were associated with decreased proinflammatory cytokines and each BV-associated species with increased proinflammatory cytokines. Remarkably, the in vitro anti-HIV activity of CVL samples from BV-positive women was stronger than that of BV-negative women. In conclusion, we found significant associations of factors, including vaginal microbiota, which can influence immune mediators in the vaginal environment in sexually active women. These factors need to be considered when establishing normative levels or pathogenic cutoffs of biomarkers of inflammation and associated risks in African women.
INTRODUCTION
The majority of HIV transmission in sub-Saharan Africa (SSA) is through heterosexual contact, and young women have very high HIV incidence rates (1). Immune activation in the female genital tract (FGT) is associated with the secretion of proinflammatory cytokines and chemokines by mucosal cells. Concomitant attraction of cells expressing the HIV receptor and coreceptors to the FGT mucosa enhances susceptibility to infection (2). Indeed, increased levels of soluble markers of inflammation were observed in the FGTs of South African women prior to acquiring HIV in the CAPRISA 004 vaginal microbicide trial (3).
There is a paucity of data on the clinical and epidemiological factors associated with immunological markers and consequently risk of HIV acquisition in various groups of women from SSA. Hormonal variation during the menstrual cycle is accompanied by a transient immune suppression that is necessary to ensure successful fertilization and embryo implantation in the uterus (4). Hormonal differences may result in different mucosal immunological profiles in pregnant women (5, 6) and adolescent girls compared to nonpregnant adult women. Differential exposure to mucosal infections (7) and other behavioral factors that alter HIV acquisition risk such as traditional vaginal practices and having multiple sexual partners might also have an impact on mucosal immunology in the FGT. SSA has the highest prevalence of bacterial vaginosis (BV) (8), which has been associated with greater susceptibility to HIV infection (9) and increased female-to-male HIV-1 transmission (10). All of these factors work in concert and are best studied together for a holistic view of mucosal immunology in the FGT.
To address the research gap described above, we set out to characterize 12 soluble immune markers in the FGTs of groups of women at different risk for HIV infection from three SSA countries differentially affected by the HIV pandemic. In the present study, we present cross-sectional data of the immune markers and their correlations with epidemiological, physiological, behavioral, and clinical factors. The levels of the mucosal immune markers in a reference group of adult HIV-negative heterosexual women at average risk of HIV infection were compared to levels in subgroups of HIV-negative pregnant women, adolescents, women engaging in intravaginal practices, sex workers and a group of HIV-positive women on combination antiretroviral therapy. We then studied the associations between levels of markers with proximate local factors, e.g., recent sexual exposure, including a semen biomarker, BV, as measured by Nugent scoring, quantitative PCR (qPCR) data of the vaginal microbiota, specifically a selection of protective Lactobacillus species and BV-associated species, and more distal underlying factors, e.g., study site and group. Because BV was shown to have a particularly strong proinflammatory influence and is known to increase susceptibility to HIV infection, we evaluated the in vitro anti-HIV activity of cervicovaginal lavage (CVL) samples from BV-positive women compared to BV-negative women.
(The abstract was presented as a poster at the HIV Research for Prevention Conference held in Cape Town, South Africa, 28 to 31 October 2014.)
MATERIALS AND METHODS
Study participants.
A total of 430 sexually active women were enrolled in 2010 and 2011 and followed up for 8 months at the International Centre of Reproductive Health Kenya (ICRHK), Mombasa, Kenya; the Wits Reproductive Health and HIV Institute (Wits RHI), Johannesburg, South Africa, and Rinda Ubuzima (RU), Kigali, Rwanda. This cohort was composed of 400 HIV-negative women and 30 nonpregnant HIV-positive women (22 to 35 years), the latter recruited from public HIV treatment clinics in Kigali, Rwanda. The HIV-negative women included the following subgroups: 219 adult women (18 to 35 years) who were at average risk for HIV acquisition, did not engage in traditional vaginal practices, and were not pregnant, recruited in Kenya and South Africa, henceforth referred to as the reference group; 60 pregnant women (18 to 40 years); 60 nonpregnant adolescents (16 to 17 years), recruited from youth-friendly family planning services (Mombasa and Johannesburg); 31 nonpregnant women (19 to 33 years) engaging in traditional vaginal practices, i.e., they used substances (cloth/lemon juice/detergents) other than water and/or fingers to clean, dry, or tighten the vagina on a regular basis, recruited from health centers in inner-city Johannesburg and the surrounding communities; and 30 nonpregnant women (22 to 33 years) at high risk for HIV acquisition, recruited from the sex worker community in Kigali, Rwanda, using community mobilizers. The women in the reference group and the pregnant women were recruited from family planning clinics, “women's groups,” and antenatal clinics in Mombasa County and Johannesburg.
Written informed consent was sought at screening, eligibility was assessed, and women were tested for HIV infection, herpes simplex virus 2 (HSV-2), reproductive tract infections (RTIs) (i.e., Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, syphilis, and vaginal candidiasis infections), BV by Amsel criteria, pregnancy by human chorionic gonadotropin (hCG) test on urine, urinary tract infection (UTI) by dipstick, and cervical dysplasia by Pap smear. Symptomatic women with positive microscopy results for trichomoniasis, BV (by Amsel criteria) and candidiasis were treated at the screening visit and women testing positive for syphilis, trichomoniasis, N. gonorrhoeae and/or C. trachomatis received treatment at the result visit. STI/RTI testing was not routinely repeated at enrollment (when CVL samples for cytokine analysis were collected) but was available for women presenting with symptoms. Women who were eligible and gave consent were enrolled (visit 1) soon after the last day of their menstrual period (a maximum of 2 months after the screening visit). At this visit, women were interviewed about their sexual activity, vaginal hygiene practices, condom use, and sexual partners. A physical and vaginal speculum examination was carried out. After this first visit, women were scheduled to visit the clinic on day 23 (visits 2 and 4) and on day 9 (visits 3, 5, 6, and 7) of their menstrual cycle. The present study focuses on the data collected at the enrollment visit (visit 1), i.e., baseline data of the study participants.
Participants were eligible for inclusion if they were (i) in good physical and mental health and able and willing to participate in the study as required by the protocol, (ii) able and willing to give informed consent (and assent for minors) according to national guidelines, including parental consent for adolescents, and (iii) HIV negative at screening, as confirmed by rapid HIV testing unless confirmed HIV-positive for inclusion in the “HIV-positive women” group. Pregnant women were only included in the study in the “pregnant women” group if they were ≤14 weeks pregnant, as determined by abdominal ultrasonography. HIV-positive women were included if they had been on combination antiretroviral therapy (cART) for at least 6 months, were currently asymptomatic, and had a CD4 count of more than 350 cells/μl.
The exclusion criteria for participation in the study included meeting one or more of the following criteria: (i) a history of hysterectomy and other genital tract surgery in the 3 months prior to the screening visit or never having had penetrative vaginal intercourse; (ii) enrollment in HIV prevention trials involving investigational products; (iii) confirmed internal and/or external genital warts at screening and/or enrollment; and (iv) currently being breast-feeding and <6 months postpartum at the time of enrollment and being pregnant (unless for inclusion in the “pregnant women” group). Women with STIs were not excluded from the study.
Ethical approval.
All women provided their written informed consent or assent for minors and consent of legal representatives. The study protocol was approved by the Ethical Review Committee, Kenyatta National Hospital, Kenya; the Human Research Ethics Committee (Medical), University of the Witwatersrand, South Africa; the Rwanda National Ethics Committee, Rwanda; the Institutional Review Board of the Institute of Tropical Medicine (ITM), Belgium; and the ethics committees of the Ghent University Hospital in Ghent and the Antwerp University Hospital in Antwerp, Belgium. In addition, the study was approved by the National Council of Science and Technology, Kenya, and the National AIDS Control Commission, Rwanda.
Clinic visits and procedures.
The rapid HIV antibody tests, wet mount microscopy for candidiasis, BV by Amsel criteria, a urine dipstick test for UTI, and a urine hCG test for pregnancy were performed on site during the screening visit according to local or national guidelines, and their results were made available immediately. A physical and pelvic exam was also carried out. HIV and RTI risk reduction counseling and testing were performed according to local standard operational procedures based on national guidelines, and women were encouraged to bring their partner for couple counseling. Women newly diagnosed with HIV or found to be pregnant were referred for appropriate care in public clinics. Condoms were provided free of charge at this and all subsequent visits.
The results of the remaining diagnostic tests (for C. trachomatis, N. gonorrhoeae, T. vaginalis, syphilis, and HSV-2 infections) were reviewed 2 weeks later (results visit) and the Pap smear results as soon as they were available. Women received treatment for RTIs/UTIs as needed, according to national guidelines. A reassessment of eligibility was also performed.
Enrollment visit sample collection.
A vaginal smear sample for BV-testing was collected during the enrollment visit (visit 1). The vaginal pH was determined by the study physician using pH-Fix 3.6-6.1 color-fixed indicator strips (Macherey-Nagel GmbH & Co. KG, Duren, Germany). Two high vaginal swab specimens were collected using flocked synthetic swabs (COPAN Innovation, Italy). The swab specimens were used for quantification of vaginal bacterial species by quantitative PCR (qPCR) and for prostate-specific antigen (PSA) testing. The swabs were stored in a cool box with ice at 2 to 8°C before transport to the laboratory where they were stored dry at −80°C until analysis. For CVL samples, 10 ml of normal saline at room temperature was flushed over the cervix and the lateral vaginal walls. This fluid was aspirated from the posterior fornix using the same pipette and collected in a 15-ml Falcon tube that was then put in a cool box with ice (2 to 8°C) and immediately transported to the laboratory for processing. A midstream urine sample was collected for testing for UTIs and pregnancy as in the screening visit.
Laboratory procedures.
CVL sample processing was started within a maximum of 1 h after sample collection. CVL samples were centrifuged at 1,000 × g for 10 min at 4°C, and the supernatant (∼9 ml) was divided into three aliquot fractions: two of ∼4 ml each and one of 1 ml. The aliquots were stored at −80°C at each of the study sites. CVL samples and vaginal swabs were shipped in batches using a temperature-monitored dry shipper to the central laboratory at the Institute of Tropical Medicine (ITM) in Antwerp, Belgium, where they were stored at −80°C before analysis for soluble markers of inflammation or use in the CVL antiviral assay. The concentrations of the cytokines interleukin-1α (IL-1α), IL-1β, IL-6, and IL-12(p70), the CC chemokine MIP-1β, the CXC chemokines gamma interferon (IFN-γ)-induced protein (IP-10) and IL-8, and the growth factors granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) in CVL samples were analyzed at the ITM in Antwerp, Belgium, using the Bio-Plex human cytokine assay kit (Bio-Rad Laboratories NV-SA, Nazareth, Belgium) as previously described (11). Fluorescence data for nine soluble immune mediators were collected using a Bio-Plex array reader, and Bio-Plex Manager 5.0 software was used to calculate cytokine concentrations with a weighted five-parameter logistic curve-fitting method on the 4-fold dilution series of the standard provided with the kit at the ITM. The assay linearity ranges, the lower limit of detection (LLD), and the interassay coefficient of variation (CV% = 100·SD/mean) based on a quality control sample assessment on each assay plate are provided in Table S3 in the supplemental material.
Elafin, SLPI, IL-1RA, and total protein concentrations in CVL samples were measured at the Laboratory of Genital Tract Biology, Brigham and Women's Hospital, Boston, MA. Elafin and SLPI were quantified by using enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems (Minneapolis, MN) according to the manufacturers' instructions. IL-1RA was measured using a Meso Scale Discovery (MSD) multiplex platform and Sector Imager 2400 (MSD, Gaithersburg, MD). MSD Discovery Workbench software was used to convert relative luminescent units into protein concentrations (pg/ml) using interpolation from several log calibrator curves. Total protein measurement in CVL samples was done by a bicinchoninic acid (BCA) assay (Thermo Scientific, Rockford, IL) using the Victor2 counter. The optical densities were read at 450 nm with a second reference filter of 570 nm using a Victor2 multilabel reader and WorkOut software (Perkin-Elmer, Waltham, MA). Samples were first screened at one dilution (1:2,500 for elafin, 1:500 for SLPI, and 1:50 for IL-1RA), followed by a repeated measurement with dilution adjusted to fit the assay linearity. Samples were tested undiluted for the total protein BCA assay. Samples with initial values below or above the assay detection ranges for all of these analytes were repeatedly tested at lower or higher dilutions to obtain accurate protein measurements. The assay linearity ranges, LLD and interassay CV based on a quality control CVL pool assessment on each plate are provided in Table S3 in the supplemental material. The CV% (means ± the standard deviations) assessed for duplicate measurements of all study samples was 18.2 × 10.8% for elafin, 17.6% ± 11.6% for SLPI, and 8.4% ± 5.0% for IL-1RA.
DNA extraction from two vaginal swab specimens (per woman) was performed by thawing the swabs at room temperature for 30 min. After thawing, 1,200 μl of diluted phosphate-buffered saline (PBS; 1 part PBS and 9 parts saline) at pH 7.4 was added to each swab and gently vortexed for 15 s. One milliliter of each swab suspension was pooled into a final volume of 2 ml. An aliquot of 250 μl was used for DNA extraction using an Abbott _m_24sp automated extraction platform (Abbott, Maidenhead, United Kingdom) according to the manufacturer's instructions. Then, 200 μl of eluted DNA was stored at −80°C until use in the qPCR assays.
The total Lactobacillus species, L. crispatus, L. iners, L. jensenii, L. gasseri, and L. vaginalis, the BV related species Atopobium vaginae, Gardnerella vaginalis, and Prevotella bivia, and Escherichia coli were measured and expressed as genome equivalents (geq)/ml using qPCR (12, 71). For total Lactobacillus species, L. crispatus, L. iners, L. jensenii, L. gasseri, and L. vaginalis, the 25-μl PCR mixture contained 12.5 μl of Rotor-Gene SYBR green RT-PCR Master mix (Rotor-Gene SYBR green PCR kit; Qiagen, Venlo, Netherlands), 5 μl of DNA extract, 0.5 to 1.0 μM concentrations of their respective primers (Integrated DNA Technologies, Leuven, Belgium), and the RNase-free water provided with the Rotor-Gene SYBR green PCR kit. The amplification reactions were performed using the Rotor Gene Q MDx 5 Plex (Qiagen). The qPCRs for A. vaginae, G. vaginalis, P. bivia, and E. coli were performed in a final volume of 10 μl, containing 5 μl of LightCycler 480 SYBR green I Master (Roche Applied Science, Basel, Switzerland), 0.2 to 1.25 μM concentrations of their respective primers (Eurogentec, Liège, Belgium) and 2 μl of DNA extract. Amplification was carried out with the LightCycler480 platform and the LightCycler 480 software version 1.5 (Roche).
For each of the organisms, standard curves were constructed. A total of six standards were prepared by a 10-fold dilution of the DNA stock in high-pressure liquid chromatography grade water. The DNA of the lactobacilli were extracted from cultures of L. crispatus LMG 9479T, L. gasseri LMG 9203T, L. iners LMG 18914T, L. jensenii LMG 6414T, and L. vaginalis LMG 12891T grown at 35 ± 2°C on Columbia agar base (BBL/Becton Dickinson, Erembodegem, Belgium) plus 5% horse blood under anaerobic conditions (Anaerocult A; Merck/VWR International, Leuven, Belgium). The DNA was extracted from cultures of A. vaginae CCUG 38953T, G. vaginalis ATCC 14018T, and E. coli ACM1803T grown on TSA plus 5% sheep blood (Becton Dickinson) and P. bivia ATCC 29303T grown on Columbia agar (Becton Dickinson) at 37 ± 2°C under anaerobic conditions (BugBox; LedTechno, Heusden-Zolder, Belgium). After extraction, the DNA concentrations were determined using NanoDrop (Thermo Fisher Scientific, Erembodegem, Belgium). The genomic concentrations were calculated using the described genomic sizes of the type strains. Both the standard curve and the samples were run in duplicate. The number of bacteria was expressed as geq/ml.
Vaginal Gram-stained smears were scored at the ITM using the Nugent method (Nugent scores 7 to 10, positive for BV; Nugent scores 4 to 6, intermediate; Nugent scores 0 to 3, normal microbiota) (13). Vaginal swab material was eluted and tested for the presence of PSA, as a marker of recent unprotected sexual intercourse (14, 15), using the Seratec PSA semiquant assay (Seratec Diagnostica, Göttingen, Germany). A volume of 150 μl of the eluted swab suspension was centrifuged for 10 min at 13,000 × g. After centrifugation, 120 μl of supernatant was used for testing according to the manufacturer's instructions.
To determine the antiviral activity of cervicovaginal fluid, 100 μl of each CVL sample (at a 1:4 dilution) was preincubated for 1 h (37°C/7% CO2) with 104 TZM-bl cells in 50 μl of medium supplemented with 30 μg of DEAE-dextran/ml per well in a 96-well flat-bottom tissue culture plate. Subsequently, 50 μl of virus (subtype B HIV-1BaL or subtype C HIV-1VI829) at 200 50% tissue culture infective doses was added to each well for a final CVL dilution of 1:8 (a dilution previously determined to be nontoxic to TZM-bl cells). After 48 h (37°C, 7% CO2), 125 μl of the culture supernatant was removed, 75 μl of Steadylite HTS (Perkin-Elmer/Life Sciences, Zaventem, Belgium) was added, and the luciferase activity was measured using a TriStar LB941 luminometer (Berthold Technologies GmbH & Co. KG, Bad Wildbad, Germany). The antiviral activity was expressed as a percentage of growth in the positive-control wells containing cells, virus, and control buffer (normal saline with 200 μg of bovine serum albumin/ml). All conditions were tested in triplicate. We also compared the concentrations of the soluble immune markers in the CVL samples of these BV-negative versus BV-positive women.
The potential toxicity of CVL samples and the control buffer was tested using a water-soluble tetrazolium 1 proliferation assay (Roche Diagnostics GmbH, Germany) as previously described (16). TZM-bl cells (104 cells/well) were plated in a 96-well flat-bottom plate, and CVL samples, or control buffer at the same dilution as in the CVL antiviral assay, was added. Cell proliferation reagent was added after 48 h, and cell viability was measured compared to untreated control cultures.
Data analysis.
Data analysis was performed using SAS 9.4 and R 3.0.1 according to a data analysis plan that was prepared prior to data analysis. For this cross-sectional analysis, each participant contributed a single data point for each analysis. The data were used from the enrollment visit, except for the RTIs (C. trachomatis, N. gonorrhoeae, T. vaginalis, HSV-2, and syphilis), which were not retested at enrollment, and the data for the number of sex partners in the past 3 months that were collected at screening. To ensure comparability across plates, we assigned to values below (or above) the quantification limit half (or twice) of the lowest (or highest) average accepted value of the standard series across plates. Women with missing data on Nugent scores and/or immune mediators were excluded from relevant analyses. All soluble marker data were log transformed before analysis to obtain a normal distribution. In addition to the individual soluble marker concentrations, we analyzed the IL-1RA/IL-1(α+β) ratio. Distributions of soluble immune markers were examined overall and by group. Comparisons among different study groups with respect to mean levels of soluble markers were performed using Student t tests. In this analysis, pregnant, adolescent, and adult women engaging in vaginal practices were each compared to the reference groups from their respective countries. Women in the sex worker and HIV-positive groups from Rwanda were compared to the combined reference groups from both Kenya and South Africa because we did not have a reference group recruited in Rwanda.
Determination of the factors that influenced the concentrations of the soluble immune mediators was conducted in three steps. First, we performed a principal component (PC) analysis (17) to describe the associations among the soluble immune mediators. SLPI was excluded from the PC analysis because it was only measured in a subsection of the women for budgetary reasons. Second, bivariate associations between the a priori selected possible predictors and the three main PCs as well as each of the soluble immune mediators and the IL-1RA/IL-1(α+β) ratio were determined using simple linear regression analysis (see Table S2 in the supplemental material). Lastly, all determinants that were significantly (P ≤ 0.05) associated with the three main PCs in the bivariate analysis (see Table S2 in the supplemental material) were then assessed in a multiple linear regression model. The model was simplified using stepwise deletion to determine the main factors associated with relevant soluble markers in the FGT. The final multiple regression model (see Table 2) retained all independent, influential variables associated with any of the main principal components and was subsequently applied to each of the soluble markers, the PC scores and the IL-1RA/IL-1(α+β) ratio (for all women). If two determinants were strongly correlated (e.g., PSA and recent vaginal sex), the most influential variable was retained in the model. This final model was used to describe the effects of each of the determinants, while correcting for possible confounders. RTIs were not included in the final analysis mainly because they were assessed (and treated) at a different time point (screening visit), whereas the soluble markers were assessed at baseline (enrollment visit).
TABLE 2.
Multiple regression analysis of determinants of soluble immunological markers
| Marker | Pa | |||||
|---|---|---|---|---|---|---|
| Bacterial vaginosis | Cervical ectopy | Cervical mucus | Cervical epithelial abnormalities | Abnormal vaginal discharge | Vaginal washing | No. of sex partners |
| IL-1α | ≤0.001 (+)* | ≤0.100 (+) | ≤0.050 (a) | ≤0.050 (+) | ||
| IL-1β | ≤0.001 (+)* | ≤0.001 (+)* | ≤0.010 (+)* | ≤0.010 (a)* | ||
| IL-6 | ≤0.010 (+) | ≤0.001 (+)* | ≤0.050 (+) | ≤0.100 (+) | ≤0.010 (+) | |
| IL-12γ | ≤0.001 (+) | ≤0.001 (+)* | ≤0.100 (+) | |||
| IL-1RA | ≤0.100 (+) | ≤0.050 (–) | ≤0.100 (–) | |||
| MIP-1β | ≤0.001 (+)* | ≤0.050 (+) | ||||
| IP-10 | ≤0.001 (–)* | ≤0.001 (+)* | ≤0.100 (+) | |||
| IL-8 | ≤0.010 (+)* | ≤0.001 (+)* | ≤0.010 (+)* | ≤0.100 (+) | ||
| GM-CSF | ||||||
| G-CSF | ≤0.100 (+) | ≤0.001 (+)* | ≤0.050 (+) | ≤0.100 (+) | ≤0.010 (+)* | |
| Elafin | ≤0.100 (–) | |||||
| SLPI | ≤0.100 (–) | |||||
| Total protein | ≤0.010 (–)* | ≤0.050 (+) | ≤0.050 (+) | ≤0.050 (a) | ||
| IL1-RA/IL-1(α+β) | ≤0.001 (–)* |
Associations between the presence or absence of vaginal microbiota species and mean soluble marker concentrations were assessed by using Student t tests. Concentrations of soluble markers of inflammation in samples used for the CVL antiviral activity tests were analyzed using the Wilcoxon rank sum test.
RESULTS
The median age of the study groups ranged from 25 to 26 years, except for the adolescents, who had a median age of 16 years (by design), and the HIV-positive women, who had a median age of 30 years. In all groups, except the sex workers, the majority (>80%) of women reported having a single sex partner in the 3 months preceding the first study visit. A comprehensive description of the sociodemographic, behavioral, and clinical characteristics of the study population by group is published elsewhere (18). The average baseline prevalence of BV by Nugent score was 33% in the reference groups, and it was similar in pregnant women and adolescents (both 30%, on average), 37% in women engaging in vaginal practices, 68% in sex workers, and 48% in HIV-positive women (18). The median time between the screening and enrollment visits was 25 days (interquartile range, 14 to 39 days). Systemic antibiotics, excluding cotrimoxazole prophylaxis for the prevention of HIV-associated opportunistic infections (26 women), were used by 62 women (14%) during the last 14 days before the enrollment visit. The last day of antibiotic use was on average 7 days (median, 7 days) prior to the enrollment visit. Antibiotics were prescribed for 17 cases of BV (by Amsel criteria), 12 cases of C. trachomatis, 10 pelvic inflammatory disease cases, six cases of trichomoniasis, four cases of N. gonorrhoeae infection, two syphilis infections, one ulcer disease infection, five respiratory infections, two urinary tract infections, and three other indications. HSV-2 infection prevalence rates at screening ranged from 3% in South African adolescents to 83% in HIV-positive women. The T. vaginalis and C. trachomatis prevalence rates were highest in the vaginal practices group (13 and 26%, respectively). N. gonorrhoeae and syphilis were not common except in sex workers (7 and 7%, respectively) and HIV-positive women (13 and 20%, respectively).
Distribution of soluble markers of inflammation.
Nine proteins were quantified by using a Bio-Plex multiplex assay, and for these, 1% (IL-1α), 3% (IL-1β), 3% (IL-6), 14% [IL-12(p70)], 4% (MIP-1β), 2% (IP-10), 7% (GM-CSF), and 1% (G-CSF) of the samples assayed were below the lower limit of detection (LLD) for undiluted CVL samples. For IL-8, 0.5% of the samples were above the upper limit of detection (ULD) of the multiplex immunoassay. IL-1RA was originally included in the Bio-Plex multiplex panel, but 70% of the undiluted CVL samples had concentrations above the ULD, and the samples were reanalyzed using a single-spot Meso Scale Discovery electrochemiluminescence assay with appropriate sample dilutions. The same sample dilution approach was used for the quantification of the antimicrobial proteins elafin and SLPI and total protein in the CVL samples. The range in concentrations of all proteins measured varied widely between women (Fig. 1).
FIG 1.
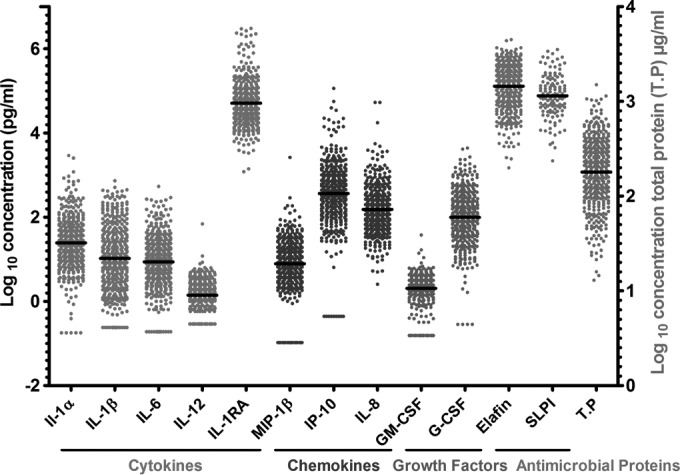
Distribution of the concentrations of soluble immunological markers in cervicovaginal lavage samples collected on visit 1 from women enrolled in the EDCTP Vaginal Biomarkers Study. Quantification of the soluble markers was done using the Bio-Plex multiplex assay except for IL-1RA, elafin, and SLPI quantified using single ELISAs and total protein quantified using the BCA assay. Each data point represents an individual sample from a single woman with the median concentration represented by the black line across the data points. IL, interleukin; IL-12, IL-12(p70); IL-1RA, IL-1 receptor antagonist; MIP-1β, macrophage inflammatory protein 1β; IP-10, gamma interferon-induced protein 10; GM-CSF, granulocyte-macrophage colony-stimulating factor; G-CSF, granulocyte colony-stimulating factor, SLPI, secretory leukocyte protease-inhibitor; T.P, total protein.
Differences of immune mediators between the study groups.
From Table 1, it is evident that HIV-positive women had the most distinct immunological profile with half of the immune mediators measured [IL-1β, IL-6, IL-12(p70), IL-1RA, MIP-1β, and IL-8] significantly higher than those in the reference group. Other groups showed a limited number of differences with the reference group. Because BV was present in a variable number of women in the different groups and was associated with clear immune activation, we focused here on the analysis in women without BV (highlighted in bold in Table 1). IL-1α for pregnant women in South Africa and IL-6 and MIP-1β for the vaginal practices group were elevated compared to their respective reference groups. Pregnant women from Kenya had significantly lower levels of elafin compared to their reference group. Adolescents from South Africa had significantly lower levels of IL-12(p70) and MIP-1β. No differences were seen between BV-negative sex workers and the reference groups.
TABLE 1.
Log10 concentrations and analysis of expression of soluble immunological markers between study groups and sitesa
| Markerb | Mean log10 concn in pg/ml (standard deviation)c | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference groups | Pregnant women | Adolescents | Vaginal practicesd | Sex workers | HIV-positive women | ||||
| Kenya (n = 110) | South Africa (n = 109) | Kenya (n = 30) | South Africa (n = 30) | Kenya (n = 30) | South Africa (n = 30) | South Africa (n = 31) | Rwanda (n = 30) | Rwanda (n = 30) | |
| Cytokines | |||||||||
| IL-1α | 1.21 (0.60) | 1.43 (0.54) | 1.48 (0.81)* | 1.76 (0.69)** | 1.22 (0.58) | 1.35 (0.55) | 1.44 (0.60) | 1.48 (0.62) | 1.46 (0.63) |
| IL-1β | 0.94 (0.82) | 1.00 (0.71) | 0.92 (0.70) | 1.09 (0.65) | 0.83 (0.65) | 0.87 (0.75) | 1.32 (0.79)* | 1.16 (0.81) | 1.31 (0.73)* |
| IL-6 | 0.83 (0.72) | 0.93 (0.57) | 0.86 (0.54) | 0.88 (0.49) | 0.90 (0.52) | 0.78 (0.64) | 1.24 (0.57)** | 1.09 (0.67) | 1.18 (0.52)* |
| IL-12(p70) | 0.06 (0.40) | 0.22 (0.30) | 0.04 (0.36) | 0.09 (0.36) | −0.04 (0.38) | –0.01 (0.36)***†‡ | 0.33 (0.33) | 0.26 (0.34) | 0.38 (0.25)***‡ |
| IL-1RA | 4.59 (0.62) | 4.74 (0.43) | 4.70 (0.68) | 4.94 (0.50)* | 4.48 (0.50) | 4.58 (0.41) | 4.79 (0.51) | 4.83 (0.39) | 4.90 (0.49)* |
| Chemokines | |||||||||
| MIP-1β | 0.88 (0.75) | 0.91 (0.45) | 0.69 (0.68) | 0.78 (0.51) | 0.65 (0.63) | 0.63 (0.45)**† | 1.18 (0.42)** | 1.18 (0.53)* | 1.16 (0.42)* |
| IP-10 | 2.38 (0.71) | 2.69 (0.64) | 2.333 (0.69) | 2.86 (0.52) | 2.47 (0.70) | 2.37 (0.70)*† | 2.85 (0.62) | 2.65 (0.72) | 2.81 (0.97) |
| IL-8 | 2.15 (0.67) | 2.19 (0.46) | 2.10 (0.58) | 2.21 (0.45) | 2.01 (0.48) | 1.93 (0.54)**† | 2.34 (0.58) | 2.40 (0.73)* | 2.42 (0.42)* |
| Growth factors | |||||||||
| GM-CSF | 0.29 (0.46) | 0.31 (0.46) | 0.29 (0.37) | 0.44 (0.22) | 0.20 (0.43) | 0.32 (0.22) | 0.45 (0.30) | 0.32 (0.23) | 0.15 (0.48) |
| G-CSF | 1.94 (0.70) | 2.01 (0.62) | 1.91 (0.59) | 1.93 (0.61) | 1.94 (0.52) | 1.90 (0.55) | 2.29 (0.54)* | 1.91 (0.77) | 2.21 (0.59) |
| Antimicrobial proteins | |||||||||
| Elafin | 5.06 (0.48) | 5.19 (0.54) | 4.79 (0.59)**† | 5.21 (0.49) | 5.08 (0.41) | 5.03 (0.66) | 5.31 (0.37) | 5.12 (0.48) | 5.13 (0.63) |
| SLPI | 4.79 (0.61) | 4.92 (0.44) | 4.83 (0.65) | 5.13 (0.64) | 4.89 (0.49) | 5.10 (0.24) | 4.85 (0.45) | 4.86 (0.26) | ND |
| Total protein | 8.09 (0.40) | 8.38 (0.27) | 8.12 (0.28) | 8.44 (0.29) | 8.09 (0.33) | 8.28 (0.31) | 8.29 (0.33) | 8.31 (0.31) | 8.41 (0.35)* |
Association of proximate factors with proinflammatory immune mediators.
Three main PCs each accounted for at least 10% of the variability (see Table S1 in the supplemental material and Fig. 2) and were therefore further analyzed to identify important associations with candidate determinants to be selected for further regression analysis. Women with BV were shown to have higher levels of the proinflammatory cytokines IL-1α, IL-1β, IL-6, IL-12(p70), and IL-8 compared to BV-negative women (Table 2). Conversely, the IP-10 level, the IL-1RA/IL-1(α+β) ratio, and the total protein levels were lower in women with BV. The presence of cervical ectopy was strongly associated with increased IL-1β, IL-6, IL-12(p70), MIP-1β, IL-8, and G-CSF, as well as the total protein levels. The presence of cervical mucus on a speculum exam was associated with an increase in proinflammatory IL-6, MIP-1β, and G-CSF but a decrease in anti-inflammatory IL-1RA. Abnormal vaginal discharge on speculum exam was associated with increased levels of IL-1β, IP-10, IL-8, G-CSF, and total protein. There was a “mixed” association between IL-1α, IL-1β, and total protein levels and vaginal washing during bathing—there was a trend toward an increase in women with a history of vaginal washing, but the levels of these markers were decreased when the washing was reported to be recent. An increase in the number of sexual partners in the previous 3 months was associated with increased IL-1α and IL-6. Even though detection of PSA was associated with an increase in IL-1α and IL-12(p70) in the bivariate analysis (see Table S2 in the supplemental material), these associations were not significant in the final regression model.
FIG 2.
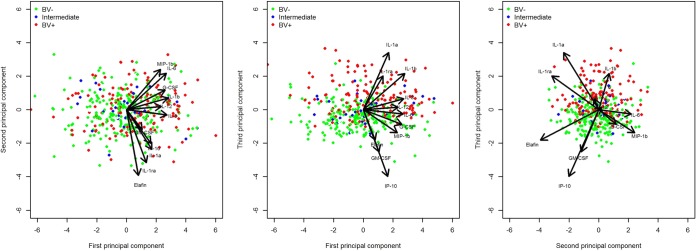
Scatter plot of the first three principal components from the principal component analysis of the soluble immunological markers quantified in cervicovaginal lavage samples collected during visit 1 from all women enrolled in the EDCTP Vaginal Biomarkers Study. The colors of the individual dots show the bacterial vaginosis (BV) status based on the Nugent score for each corresponding vaginal swab sample. The arrows show the factor loadings of the soluble markers on the three principal components that cumulatively explain 66% of the variability of the data.
Unique associations of individual bacterial species with soluble immune mediators.
In view of the strong associations between BV and markers of immune activation, we set out to analyze the relation between various bacterial species and immune markers in more detail in all women enrolled in the study. As can be seen in Table 3, the presence of L. crispatus and L. vaginalis but not of the other lactobacilli species was associated with lower levels of proinflammatory cytokines, whereas the BV-associated bacteria A. vaginae, G. vaginalis, and P. bivia and E. coli clearly skewed the proinflammatory balance upwards. Of note, the IFN-γ-induced chemokine IP-10 was significantly increased in the presence of all of the Lactobacillus species except L. gasseri but was significantly lower in the CVL samples of women who had A. vaginae and G. vaginalis present in their vaginal swab samples. The presence of L. gasseri was also associated with increased IL-1RA, IL-12(p70), and GM-CSF, and the presence of L. jensenii was associated with increased GM-CSF concentrations. The concentrations of the protective antimicrobial proteins SLPI and elafin were elevated in women with specific Lactobacillus species: L. vaginalis and L. jensenii for SLPI and L. iners for elafin.
TABLE 3.
Analysis of associations between soluble immunological markers in cervicovaginal lavage and the presence of vaginal bacterial species in vaginal swab samples
| Bacterial species | Marker(s) (P)a | |
|---|---|---|
| Lower levels | Higher levels | |
| Lactobacilli | ||
| Lactobacillus crispatus | IL-8 (≤0.050)*, IL-1α (≤0.010), IL-1β (≤0.010), GM-CSF (≤0.050)*, IL-12(p70) (≤0.001) | IP-10 (≤0.050) |
| Lactobacillus vaginalis | IL-1α (≤0.050), MIP-1β (≤0.050)*, IL-12(p70) (≤0.010), IL-1β (≤0.001) | SLPI (≤0.050)*, IP-10 (≤0.001) |
| Lactobacillus iners | IP-10 (≤0.050)*, elafin (≤0.050)* | |
| Lactobacillus jensenii | GM-CSF (≤0.050)*, SLPI (≤0.010), IP-10 (≤0.001) | |
| Lactobacillus gasseri | IL-1RA (≤0.050)*, IL-12(p70) (≤0.050)*, GM-CSF (≤0.010) | |
| BV-associated bacteria | ||
| Gardnerella vaginalis | IP-10 (≤0.001) | IL-8 (≤0.050), IL-12(p70) (≤0.010), IL-1α (≤0.001), IL-1β (≤0.001) |
| Atopobium vaginae | IP-10 (≤0.001) | IL-8 (≤0.050)*, IL-12(p70) (≤0.010), IL-1α (≤0.001), IL-1β (≤0.001) |
| Prevotella bivia | IL-1β (≤0.010), IL-8 (≤0.010) | |
| Other | ||
| Escherichia coli | IL-1RA (≤0.050)*, GM-CSF (≤0.050)*, IL-12(p70) (≤0.010), IL-1β (≤0.001), IL-6 (≤0.001), IL-8 (≤0.001), G-CSF (≤0.001), IP-10 (≤0.001), MIP-1β (≤0.001) |
Anti-HIV activity of CVL samples in vitro and associations with the presence or absence of BV.
BV has previously been associated with an increased risk of HIV acquisition. We hypothesized that CVL samples from women with BV would have decreased anti-HIV activity compared to CVL samples from women without BV. To test this hypothesis, we selected from all women enrolled, 15 HIV-negative women with a Nugent score of 0 (BV negative) and 14 HIV-negative women with a Nugent score of 7 to 10 (BV positive) and compared the CVL antiviral activity of these two groups. In contrast to our expectations, the CVL-antiviral activity against HIVBaL was slightly but significantly higher in the BV-positive women than in the BV-negative women, and the same trend was observed with HIVVI829, but the difference was not statistically significant (Fig. 3A). CVL samples from BV-positive women had significantly higher levels of IL-1α, IL-1β, IL-6, and IL-8 but lower levels of IP-10 and SLPI (Fig. 3B).
FIG 3.
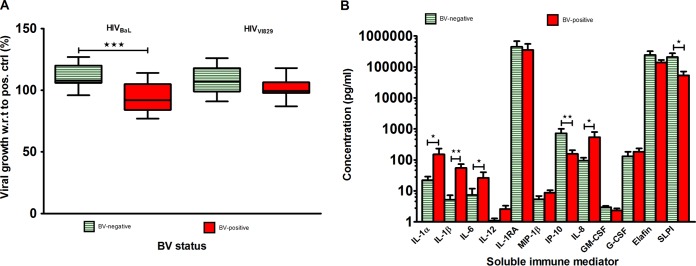
Cervicovaginal lavage (CVL) antiviral activity and corresponding soluble marker concentrations of selected CVL samples from women with or without bacterial vaginosis (BV). Women without BV with the highest bacterial counts of L. crispatus were selected versus women with BV with the highest bacterial counts of A. vaginae. Full bacterial counts for women in both groups are given in Table 4. (A) Percent infection of TZM-bl cells by HIVBaL (subtype B) and HIVVI829 (subtype C) in the presence of CVL at a 1:8 final dilution. The indicated viral growth concentration is compared to control wells, with a 1:8 final dilution of the same control buffer (normal saline) used for CVL collection. The box plots depict the median (bold line), 25th and 75th percentiles (box), and range (whiskers). All CVL samples were assayed in triplicate. (B) Concentrations of soluble immune mediators in corresponding aliquots of CVL samples used in CVL antiviral assay. *, P ≤ 0.050; **, P ≤ 0.010; ***, P ≤ 0.001.
The BV-negative women selected had high counts of L. crispatus and L. vaginalis, as well as lower levels of other lactobacilli, but no or low counts of G. vaginalis, A. vaginae, and Prevotella spp. (Table 4). The BV-positive women had high counts of G. vaginalis, A. vaginae, and Prevotella spp. and high counts of L. iners (higher than the BV-negative women) but no or low counts for all other lactobacilli. The E. coli counts were similar in both groups (Table 4).
TABLE 4.
Nugent scores and bacterial genome equivalent concentrations at visit 1 for women used in the CVL antiviral activity assay
| Nugent score | Bacterial geq/mla | ||||
|---|---|---|---|---|---|
| Lactobacillus spp. | L. crispatus | L. vaginalis | G. vaginalis | A. vaginae | |
| BV-negative samples | |||||
| 0 | 16,383,840 | 22,575,520 | 10 | 0 | 0 |
| 0 | 18,779,360 | 22,568,320 | 0 | 0 | 0 |
| 0 | 308,705,280 | 343,613,600 | 64,960 | 25,640 | 0 |
| 0 | 2,702,560 | 3,606,240 | 0 | 0 | 0 |
| 0 | 197,018,400 | 269,372,640 | 153,600 | 0 | 0 |
| 0 | 1,864,051,840 | 1,173,857,920 | 1,833,440 | 0 | 0 |
| 0 | 209,579,520 | 239,378,560 | 243,520 | 0 | 0 |
| 0 | 1,542,049,600 | 1,776,754,720 | 10 | 0 | 0 |
| 0 | 800,430,240 | 665,880,000 | 265,600 | 0 | 0 |
| 0 | 2,644,447,200 | 3,538,097,280 | 2,853,600 | 0 | 0 |
| 0 | 596,383,520 | 707,406,560 | 516,640 | 0 | 0 |
| 0 | 111,943,520 | 18,580,480 | 1,252,320 | 0 | 0 |
| 0 | 577,958,720 | 656,584,480 | 0 | 0 | 0 |
| 0 | 523,155,680 | 587,784,160 | 10 | 0 | 0 |
| 0 | 226,582,080 | 163,470,400 | 450,880 | 0 | 0 |
| BV-positive samples | |||||
| 7 | 39,149,120 | 0 | 0 | 9,220,000 | 162,400,000 |
| 8 | 1,351,360 | 0 | 0 | 868,000 | 116,800,000 |
| 8 | 1,502,880 | 0 | 0 | 3,784,000 | 80,800,000 |
| 8 | 945,280 | 0 | 0 | 1,84,800 | 22,240,000 |
| 9 | 46,783,680 | 0 | 0 | 1,968,000 | 53,400,000 |
| 8 | 12,671,680 | 0 | 0 | 59,000,000 | 24,080,000,000 |
| 8 | 15,869,920 | 0 | 0 | 8,140,000 | 916,000,000 |
| 8 | 10 | 0 | 0 | 1,880,000 | 440,400,000 |
| 10 | 7,381,120 | 0 | 0 | 27,400,000 | 1,312,000,000 |
| 10 | 791,360 | 0 | 0 | 2,468,000 | 240,400,000 |
| 8 | 3,871,200 | 0 | 0 | 23,320,000 | 1,264,000,000 |
| 8 | 24,632,000 | 0 | 0 | 18,360,000 | 467,600,000 |
| 8 | 2,440,320 | 0 | 0 | 23,480,000 | 1,880,000,000 |
| 7 | 235,892,320 | 0 | 10,020,640 | 35,840,000 | 36,560,000 |
DISCUSSION
We extensively characterized the ranges of vaginal soluble immune mediators and their physiological and behavioral determinants in various groups of women from three SSA countries. All of the CVL immune markers studied showed a wide variability between the women. Across all groups, BV was strongly positively associated with proinflammatory cytokines/chemokines and negatively associated with protective antimicrobial proteins SLPI and elafin and the chemokine IP-10. In BV-negative women, those with HIV infections showed high levels of proinflammatory markers, as expected, but at-risk BV-negative sex workers had remarkably “quiet” profiles. Some discrete differences between pregnant, adolescent, and adult women using vaginal practices on the one hand and the reference healthy adult population on the other were noted. Pregnant women in South Africa had higher levels of IL-1α, whereas those in Kenya showed lower elafin levels compared to the reference groups. Adolescents from South Africa showed lower IL-12(p70) and MIP-1β levels compared to reference adult women. Women who used vaginal practices had higher levels of IL-6 and MIP-1β. Lastly, our study demonstrated that the in vitro anti-HIV activity of cervicovaginal fluid tends to be increased in BV-positive samples and varies depending on the bacterial load of specific species of these women.
Pregnant women (up to 14 weeks gestational age) were found to have increased levels of the proinflammatory cytokine IL-1α compared to the reference group of low-risk HIV-negative women. A recent study of Ugandan and Zimbabwean women found higher cervical levels of IL-1β, IL-6, IL-8, VEGF, SLPI, and IL-1RA and lower IL-1RA/IL-1β ratios in pregnant versus nonpregnant HIV negative women (19). Kenyan pregnant women in our study showed lower levels of elafin. Elafin has been shown to be expressed in the genital tract of pregnant women (20), and its anti-HIV activity was previously described (21, 22), making it a probable correlate of immunity against HIV infection. In another relatively small study of predominantly white U.S. women (23), the levels of IL-1α and IL-1RA were relatively higher in the CVL samples of pregnant women with a mean gestational age of 23.5 weeks. Elafin levels were also lower in the CVL samples of pregnant women in the present study, but only when controlled for total protein. A different study (24) among predominantly white U.S. women near term (35 to 37 weeks gestational age) also noted higher levels of IL-1α, IL-1β, IL-8, and IL-1RA but lower levels of human beta defensin 2 in pregnant versus nonpregnant women. These studies, performed in geographically diverse populations, seem to point toward a state of elevated inflammatory cytokines and decreased antimicrobial/anti-inflammatory protein levels in the FGT at various gestational periods, which may partially explain the increased risk of HIV acquisition during pregnancy (25). Clearly then, pregnant women differ from their nonpregnant peers with regard to cervicovaginal immune mediators, but these differences may vary by country or cohort (5), limiting their extrapolation.
Young women in South Africa have been shown to have a high HIV incidence (1); they may be highly susceptible to HIV infection from both a biological and a behavioral perspective (26, 27). The observation of lower levels of IL-12(p70) and MIP-1β in adolescents compared to nonpregnant adults in South Africa may therefore seem counterintuitive. However, it may indicate an immature state of the immune barrier. The lack of higher levels of IL-8 and IL-1β, which are considered the hallmark of vaginal inflammation (28), in these adolescents suggests that increased biologic risk of HIV infection in South African adolescents cannot be fully explained by our panel of soluble immune mediators.
Martin-Hilber et al. (29) describe vaginal practices as tools used by girls and women to navigate their reproductive life and marital roles. Some of these practices may directly impact HIV susceptibility (30). Certain over-the-counter feminine hygiene products also negatively affect vaginal microbiota, resulting in increased IL-8 production (31). In our study, women in South Africa who reported using substances other than water to clean, dry, or tighten their vaginas were found to have higher levels of IL-6 and MIP-1β in the subanalyses of BV-negative women. Our results on the effect of washing the vagina during bathing suggest that, in the long term, it may result in an inflamed FGT. Vaginal washing has previously been associated with a lower likelihood of Lactobacillus isolation from a cohort of HIV-1 seronegative female sex workers from Kenya (32). Our study therefore supports the hypothesis that specific vaginal practices can be potential risk factors for HIV acquisition (33), probably as a result of mucosal inflammation. Of importance is the fact that when women reported having recently washed their vaginas, there was a corresponding decrease in the amounts of cytokines. Vaginal washing before sample collection may therefore interfere with the measurement of immunological markers.
HIV-positive women were found to have a generally more inflamed vaginal profile compared to HIV-negative women, even after controlling for BV. These data agree with previous studies, showing increased soluble proinflammatory markers in the genital tracts of HIV-infected individuals (34, 35). It is important to note that the women in our study were on a successful cART regime, which means that treatment suppressed viral load but did not fully suppress inflammation in the FGT. This is probably part of the well-known overall HIV-associated immune activation that persists even in the presence of antiretroviral therapy (36).
Increased cervical mucus and abnormal vaginal discharge on speculum examination could both be clinical signs of infection in the FGT. Since study participants were not retested during visit 1, we cannot definitively attribute these signs to infection. Their association with proinflammatory cytokines, however, means that they may increase susceptibility to HIV. Cervical ectopy is characterized by an extension of the columnar epithelium typically found in the endocervix to cover a proportion of the ectocervix that is normally lined by multilayered squamous epithelial cells (37). The strong association between the presence of cervical ectopy and increased CVL concentrations of IL-1β, IL-6, MIP-1β, IL-8, IL-12(p-70), and G-CSF in this African population agrees perfectly with our previous observations in a cohort of healthy Caucasian women (11) and that of others (38). It may be explained by previous in vitro observations that columnar epithelial cells of endocervical origin produce higher levels of proinflammatory mediators than isogenic stratified squamous epithelial cells of ectocervical or vaginal origin (39–41). An enhanced proinflammatory environment in the lower FGT could potentially increase susceptibility to HIV acquisition and may account for reported associations (42, 43) of cervical ectopy with HIV infection.
BV by Nugent score in our study was associated with a significant increase in a number of proinflammatory cytokines/chemokines [IL-1α, IL-1β, IL-6, IL-12(p70), and IL-8], extending the findings of other studies (40, 44, 45). Further, in a study on HIV-1-infected female sex workers in Kenya, treatment and elimination of BV by Nugent score was associated with reduced genital tract levels of IL-1β, IL-8, and RANTES (46). In addition, we found an association of a lower IL-1RA/IL-1(α+β) ratio with BV, a finding which is indicative of excessive production of IL-1α and IL-1β in the vaginal mucosa (47). Clearly, epithelial and immune cells of the FGT mount an innate immune response after stimulation with bacterial products from the diverse bacteria (reviewed in reference 48) that are characteristic of BV. This subclinical FGT inflammation may partially explain the increased risk of HIV acquisition associated with BV.
To our knowledge, only one previous Swedish study correlated immunological markers with vaginal microbiota characterized to the individual species level using molecular methods (49), but this has not been investigated in women from SSA. The presence of lactobacilli (specifically L. crispatus and L. vaginalis) was associated with lower levels of proinflammatory cytokines in our study. This is consistent with previous observations about the noninflammatory nature of specific Lactobacillus species (50, 51). These species were also associated with higher levels of the protective antimicrobial proteins SLPI and elafin in our study. SLPI is constitutively expressed in the FGT, but it is also induced by NF-kβ activation and reduced in association with BV signs in adolescent African American women (52).
BV is not often associated with overt clinical inflammatory symptoms. Our analysis of associations between vaginal microbiota and soluble markers of immunity revealed an interesting result that we think may hint at one of the possible explanations for this dampened inflammation: the presence of BV-associated G. vaginalis and A. vaginae was in both cases strongly associated with lower levels of IP-10. This chemokine (also known as CXCL10) is one of the ligands for the CXCR3 receptor (53); it is induced by both type II IFN-γ and type I IFN-α/β and also TNF-α (54, 55) and functions as a chemoattractant for different immune cells to the site of infection or inflammation. Suppression of IP-10 production or function by pathogenic bacteria has been described before (56–58). Fichorova et al. recently reported that although IP-10 expression was upregulated by the FGT pathogens P. bivia and T. vaginalis individually, their combination completely suppressed IP-10 expression in culture (59). Even though the presence of G. vaginalis and A. vaginae was individually associated with lower levels of IP-10 in our study, these two pathogens, considered individually, did not suppress IP-10 in vitro (59). In vivo IP-10 suppression may therefore be due to an association of these bacteria with one or a combination of many other bacteria in the FGT of women with BV. This may explain recent observations of lower genital IP-10 concentrations in HIV-uninfected women with BV in Durban, South Africa (60), but also in Pittsburgh, PA (61). It is interesting that previous reports exist on sialidase and prolidase activity in the genital fluids of some women with BV, an observation that is correlated with the degradation of anti-vaginolysin immunoglobulin A (IgA) and a low IL-8 immune response (45, 62). The degradation of anti-vaginolysin IgA is achieved by removal of sialic acids from the secretory IgA molecules that makes them more sensitive to proteolytic cleavage (63). Taken together, these results hint at the existence of processes through which BV-associated pathogens influence certain innate and adaptive immune responses. Further research is needed to explore interactions between microbial virulence factors in BV and host immune mediators and their implications on BV pathogenicity.
CVL anti-HIV activity has been previously reported in both HIV+ and HIV− women, and this activity has been attributed mainly to the cationic fraction of CVL samples, including correlation with specific antimicrobial peptides (64, 65). Although surprising to us, the observation of modest but significantly higher anti-HIVBaL activity in CVL samples from BV-positive women is similar to previous results in slightly different contexts (66, 67). In our study, increased anti-HIV activity in CVL samples from BV-positive women cannot be attributed to the two peptides with known anti-HIV properties (elafin and SLPI) since the concentrations of these were lower than in the CVL samples from healthy women. SLPI and elafin did not correlate with anti-HIV-1 activity in the study with CVL samples from HIV+ women (64). It is possible that other antimicrobial proteins that we did not quantify in our CVL samples were responsible for the observed anti-HIVBaL activity, especially those, like HBD2, reported to be increased in genital fluids in the context of BV (68, 69). HBD2 correlated positively with in vitro HIV inhibition in a study by Keller et al. but also in a different study of CVL samples from HIV-positive women in which MIP-3α was also implicated in HIV inhibition (64, 70). Caution should always be applied in the interpretation of these results since most vaginal lavage samples constitute a significant dilution of the proteins found in vivo and physiological concentrations needed for activity in vivo might not be reached in vitro. What is clear from our study is that there might be an unidentified substance(s) in BV-positive CVL samples that influences its ability to inhibit HIV infection of TZM-bl cells in vitro. Differences seen with what was expected in the context of natural BV-HIV dynamics also highlight the limitation of assays using cell lines as opposed to in vivo whole-organ responses with possibilities of susceptible cell recruitment and infection. These experiments with BV-positive CVL samples should be repeated with a larger sample size than what we used in our study to confirm their validity and possible implications.
In the present study, a wide variation in the concentrations of the different soluble markers in CVL samples was observed. Although multiplex technologies are useful where there is a limitation in sample volumes, natural variations in soluble marker concentrations in FGT samples limit the combination of markers that can be assayed in a single multiplex assay, as was observed with IL-1RA in our assays. This limitation can be overcome by grouping high- or low-concentration markers together in premixed multiplex panels, by optimizing the multiplex immune assays to fit broad linearity ranges of multiple markers, or by using single-target immunoassays in which multiple aliquots of individual samples can be diluted appropriately for accurate quantification of high-concentration proteins.
We encountered several limitations in the study. An important weakness was that study participants were asked about the number of sex partners in the past 3 months and tested for RTIs (C. trachomatis, N. gonorrhoeae, T. vaginalis, HSV-2, and syphilis) during screening, but they were not retested for the same during the enrollment visit when we collected data on soluble immune mediators unless the women presented with symptoms. It important to note that those who tested positive for any of the RTIs mentioned above were treated during the results visit (2 weeks after screening) before enrollment. Those who were positive for trichomoniasis, BV, and candidiasis were treated during the screening visit. This, however, does not completely exclude the probability of asymptomatic, residual, and/or new infections that could possibly confound our analysis of soluble immune markers during enrollment due to known effects of some STIs on genital inflammation.
Another methodological limitation is that the interpretation of single associations between possible predictors and the different markers is complicated by multiple testing. Second, colinearity between predictors was present, e.g., age and parity, were correlated (r = 0.65), complicating the interpretation of independent effects. Finally, not all study groups were recruited in all countries. Inclusion of both the sex workers and the HIV-positive group from Rwanda was mainly meant to provide a perspective to the values, found in the various low-risk groups, which are the most important focus of the present study.
In conclusion, BV and HIV create a strong proinflammatory cytokine/chemokine environment in the vagina of sub-Saharan women. Soluble immune markers in sexually active women show a wide concentration distribution, with some discrete differences between adolescent, pregnant women engaging in vaginal practices, sex workers, and the reference groups. Physiopathological factors such as cervical ectopy, mucus, epithelial abnormalities, and vaginal discharge could explain part of the variation in particular soluble immune markers. In addition, we identified IP-10 suppression as a potential mechanism of immune evasion by BV-associated bacteria and described the noninflammatory nature of L. crispatus and L. vaginalis. These data constitute a solid basis for future prevention intervention studies, which may target these factors to decrease susceptibility to HIV infection in African women.
ACKNOWLEDGMENTS
This study was supported by the European and Developing Countries Clinical Trials Partnership (EDCTP) as part of a grant titled “Characterization of Novel Microbicide Safety Biomarkers in East and South Africa.” The views expressed here are those of the authors and do not necessarily represent the views of EDCTP.
We thank the following study participants and the Vaginal Biomarkers Study Group: Mombasa, Kenya (ICRHK)—Kishor Mandaliya (overall project manager), Lou Dierick (overall study administrator), Mary Mwaura (site principal investigator), Walter Jaoko (site coinvestigator), Eunice Irungu (site study coordinator), Christine Katingima (study clinician), Mercy Maina and Jane Wanjiru Mazera (study nurses), Josephine Gichuru and Grace Aketch Onuki (counselors), Mary Kiambi (community health worker), Mary Thiong'o (data manager), Salome Wanjiku and Patricia Nduku (data capturers), Carol Njeru and Bernard Mbogho (research assistants), Sammy Wambua (lab manager), Rachel Sidi Baya, Emmanuel Moffat Onduko, Patrick Katana Kombo, Simon Chengo Masha, and Mary Ndinda John (laboratory technologists), Kevin Odeyo and Dora Ngala (intern-laboratory technologists), and Collins Odero (quality assurance monitor); Johannesburg, South-Africa (Wits RHI)—Sinead Delany-Moretlwe (site principal investigator), Vinodh Aroon Edward (site coinvestigator), Krishnaveni Reddy (site study coordinator), Nina Von Knorring and Ishania Mahabeer (study clinicians), Johannah Nkoleleng Mashilo, and Ntombifuthi Mnyandu (study nurses), Keneuoe Mokoatle (clinical quality improvement mentor), Siyabulela Nani (data manager), Gugu Tshabalala and Thembisile Hope Mngwevu (data capturers), Noxolo Mtabane, Puseletso Maria Masalesa, and Zodidi Kumase (community health workers), Sefora Dipolelo Mohale (community liaison officer), Mavis Mantshitseng Madi (administrator), Mandla Mlotshwa (medical scientist/lab manager), Pholo Wilson Maenetje (medical scientist), Nishanee Arjun (quality assurance monitor), and Debra De Assis Rosa (research operations manager); Kigali, Rwanda (PRU)—Gilles F. Ndayisaba (site principal investigator), Evelyne Kestelyn (coinvestigator), Ammiel Gasarabwe (site study coordinator), Servaas Van Eeckhoudt and Stephen Agaba (study clinicians), Rosette Busasa (study nurses), Deogratias Nshimuyimana and Grace Umutoni (data capturers), Vincent Karangwa (administrator), Claire Bukuru (administrative research assistant), Alice Fiat (lab manager), Lambert Mwambarangwe and Viateur Musengamana (laboratory technicians), and Jeanine Nyinawabega (quality assurance monitor); Mwanza, Tanzania (MITU)—Saidi Kapiga (Director of MITU), Aura Andreasen (laboratory), John Changalucha (Head of NIMR and head of the lab during the time of the study), Kaballa Maganja (study coordinator), and Clemens Masesa (data manager); Biomarker Study Group supporting institutions: Antwerp, Belgium (ITM)—Vicky Jespers (coordinating investigator), Liselotte Hardy (study management coordinator), Tania Crucitti (laboratory management coordinator), Joris Menten (statistics coordinator), Céline Schurmans (monitoring coordinator), Harry van Loen (data management coordinator), Anne Buvé (epidemiologist), Jordan K. Kyongo (Ph.D. student), Kevin Ariën and Guido Vanham (virologists), Said Abdellati and Vicky Cuylaerts (laboratory technicians), Wendy Thys and An Ielegems (administrators), and Lieve Casier (shipment logistics); Ghent, Belgium (UGhent/ICRH)—Hans Verstraelen, Marleen Temmerman, and Mario Vaneechoutte (coinvestigators), Rita Verhelst (laboratory coordinator), Piet Cools (Ph.D. student), and Bart Saerens (laboratory technician); Amsterdam, Netherlands (AMC-CPCD)—Janneke van de Wijgert (coinvestigator) and Friso Janssen (administrator); London, United Kingdom (MRC CTU)—Sheena McCormack (coinvestigator) and Sarah Joseph (laboratory coordinator); and London, United Kingdom (LSHTM)—Richard Hayes and Suzanna Francis (coinvestigators) and Kathy Baisley (epidemiologist).
Footnotes
REFERENCES
- 1.UNAIDS. 2004. 2004 Report on the Global AIDS Epidemic Joint United Nations Programme on HIV/AIDS. UNAIDS, Geneva, Switzerland. [Google Scholar]
- 2.Fichorova RN, Tucker LD, Anderson DJ. 2001. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J Infect Dis 184:418–428. doi: 10.1086/322047. [DOI] [PubMed] [Google Scholar]
- 3.Roberts L, Passmore JA, Williamson C, Little F, Naranbhai V, Sibeko S, Walzl G, Abdool Karim Q, Abdool Karim SS. 2011. Genital tract inflammation in women participating in the CAPRISA 004 tenofovir microbicide trial who became infected with HIV: a mechanism for breakthrough infection? Conference on Retroviruses and Opportunistic Infections, Boston, MA. [Google Scholar]
- 4.Wira CR, Fahey JV. 2008. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS 22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donders GG, Vereecken A, Bosmans E, Spitz B. 2003. Vaginal cytokines in normal pregnancy. Am J Obstet Gynecol 189:1433–1438. doi: 10.1067/S0002-9378(03)00653-7. [DOI] [PubMed] [Google Scholar]
- 6.Kutteh WH, Franklin RD. 2001. Quantification of immunoglobulins and cytokines in human cervical mucus during each trimester of pregnancy. Am J Obstet Gynecol 184:865–872. doi: 10.1067/mob.2001.113853. [DOI] [PubMed] [Google Scholar]
- 7.Pala P, Gomez-Roman VR, Gilmour J, Kaleebu P. 2009. An African perspective on mucosal immunity and HIV-1. Mucosal Immunol 2:300–314. doi: 10.1038/mi.2009.23. [DOI] [PubMed] [Google Scholar]
- 8.Kenyon C, Colebunders R, Crucitti T. 2013. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol 209:505–523. doi: 10.1016/j.ajog.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. 2008. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS 22:1493–1501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen CR, Lingappa JR, Baeten JM, Ngayo MO, Spiegel CA, Hong T, Donnell D, Celum C, Kapiga S, Delany S, Bukusi EA. 2012. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African couples. PLoS Med 9:e1001251. doi: 10.1371/journal.pmed.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyongo JK, Jespers V, Goovaerts O, Michiels J, Menten J, Fichorova RN, Crucitti T, Vanham G, Arien KK. 2012. Searching for lower female genital tract soluble and cellular biomarkers: defining levels and predictors in a cohort of healthy Caucasian women. PLoS One 7:e43951. doi: 10.1371/journal.pone.0043951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jespers V, Menten J, Smet H, Poradosu S, Abdellati S, Verhelst R, Hardy L, Buve A, Crucitti T. 2012. Quantification of bacterial species of the vaginal microbiome in different groups of women, using nucleic acid amplification tests. BMC Microbiol 12:83–83. doi: 10.1186/1471-2180-12-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nugent RP, Krohn MA, Hillier SL. 1991. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol 29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallo MF, Behets FM, Steiner MJ, Hobbs MM, Hoke TH, Van Damme K, Ralimamonjy L, Raharimalala L, Cohen MS. 2006. Prostate-specific antigen to ascertain reliability of self-reported coital exposure to semen. Sex Transm Dis 33:476–479. doi: 10.1097/01.olq.0000231960.92850.75. [DOI] [PubMed] [Google Scholar]
- 15.Mauck CK, Doncel GF, Biomarkers of Semen Exposure Clinical Working G. 2007. Biomarkers of semen in the vagina: applications in clinical trials of contraception and prevention of sexually transmitted pathogens including HIV. Contraception 75:407–419. doi: 10.1016/j.contraception.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Arien KK, Venkatraj M, Michiels J, Joossens J, Vereecken K, Van der Veken P, Abdellati S, Cuylaerts V, Crucitti T, Heyndrickx L, Heeres J, Augustyns K, Lewi PJ, Vanham G. 2013. Diaryltriazine non-nucleoside reverse transcriptase inhibitors are potent candidates for pre-exposure prophylaxis in the prevention of sexual HIV transmission. J Antimicrob Chemother 68:2038–2047. doi: 10.1093/jac/dkt166. [DOI] [PubMed] [Google Scholar]
- 17.Genser B, Cooper PJ, Yazdanbakhsh M, Barreto ML, Rodrigues LC. 2007. A guide to modern statistical analysis of immunological data. BMC Immunol 8:27. doi: 10.1186/1471-2172-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jespers V, Crucitti T, Menten J, Verhelst R, Mwaura M, Mandaliya K, Ndayisaba GF, Delany-Moretlwe S, Verstraelen H, Hardy L, Buve A, van de Wijgert J, Vaginal Biomarkers Study Group. 2014. Prevalence and correlates of bacterial vaginosis in different sub-populations of women in sub-Saharan Africa: a cross-sectional study. PLoS One 9:e109670. doi: 10.1371/journal.pone.0109670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison C, Fichorova R, Mauck C, Chen P, Kwok C, Chipato T, Salata R, Doncel GF. 2014. Cervical inflammation and immunity associated with hormonal contraception, pregnancy and HIV-1 seroconversion. J Acquir Immune Defic Syndr 66:109–117. doi: 10.1097/QAI.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 20.Stock SJ, Duthie L, Tremaine T, Calder AA, Kelly RW, Riley SC. 2009. Elafin (SKALP/trappin-2/proteinase inhibitor-3) is produced by the cervix in pregnancy and cervicovaginal levels are diminished in bacterial vaginosis. Reprod Sci 16:1125–1134. doi: 10.1177/1933719109341998. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh M, Shen Z, Fahey JV, Cu-Uvin S, Mayer K, Wira CR. 2010. Trappin-2/Elafin: a novel innate anti-human immunodeficiency virus-1 molecule of the human female reproductive tract. Immunology 129:207–219. doi: 10.1111/j.1365-2567.2009.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drannik AG, Nag K, Yao XD, Henrick BM, Jain S, Ball TB, Plummer FA, Wachihi C, Kimani J, Rosenthal KL. 2012. Anti-HIV-1 activity of elafin is more potent than its precursor's, trappin-2, in genital epithelial cells. J Virol 86:4599–4610. doi: 10.1128/JVI.06561-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson BL, Ghosh M, Raker C, Fahey J, Song Y, Rouse DJ, Wira CR, Cu-Uvin S. 2012. In vitro anti-HIV-1 activity in cervicovaginal secretions from pregnant and nonpregnant women. Am J Obstet Gynecol 207:65.e61-10. doi: 10.1016/j.ajog.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghartey JP, Carpenter C, Gialanella P, Rising C, McAndrew TC, Mhatre M, Tugetman J, Einstein MH, Chazotte C, Herold BC. 2012. Association of bactericidal activity of genital tract secretions with Escherichia coli colonization in pregnancy. Am J Obstet Gynecol 207:297.e1-8. doi: 10.1016/j.ajog.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray RH, Li X, Kigozi G, Serwadda D, Brahmbhatt H, Wabwire-Mangen F, Nalugoda F, Kiddugavu M, Sewankambo N, Quinn TC, Reynolds SJ, Wawer MJ. 2005. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet 366:1182–1188. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- 26.Pettifor AE, Rees HV, Kleinschmidt I, Steffenson AE, MacPhail C, Hlongwa-Madikizela L, Vermaak K, Padian NS. 2005. Young people's sexual health in South Africa: HIV prevalence and sexual behaviors from a nationally representative household survey. AIDS 19:1525–1534. doi: 10.1097/01.aids.0000183129.16830.06. [DOI] [PubMed] [Google Scholar]
- 27.Abdool Karim Q, Abdool Karim SS, Singh B, Short R, Ngxongo S. 1992. Seroprevalence of HIV infection in rural South Africa. AIDS 6:1535–1539. doi: 10.1097/00002030-199212000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Fichorova RN. 2004. Guiding the vaginal microbicide trials with biomarkers of inflammation. J Acquir Immune Defic Syndr 37(Suppl 3):S184–S193. [PMC free article] [PubMed] [Google Scholar]
- 29.Martin-Hilber A, Kenter E, Redmond S, Merten S, Bagnol B, Low N, Garside R. 2012. Vaginal practices as women's agency in sub-Saharan Africa: a synthesis of meaning and motivation through meta-ethnography. Social Sci Med 74:1311–1323. doi: 10.1016/j.socscimed.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 30.Kilmarx PH, Limpakarnjanarat K, Supawitkul S, Korattana S, Young NL, Parekh BS, Respess RA, Mastro TD, St. Louis ME. 1998. Mucosal disruption due to use of a widely distributed commercial vaginal product: potential to facilitate HIV transmission. AIDS 12:767–773. doi: 10.1097/00002030-199807000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Fashemi B, Delaney ML, Onderdonk AB, Fichorova RN. 2013. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis doi: 10.3402/mehd.v24i0.19703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baeten JM, Hassan WM, Chohan V, Richardson BA, Mandaliya K, Ndinya-Achola JO, Jaoko W, McClelland RS. 2009. Prospective study of correlates of vaginal Lactobacillus colonization among high-risk HIV-1 seronegative women. Sex Transm Infect 85:348–353. doi: 10.1136/sti.2008.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClelland RS, Lavreys L, Hassan WM, Mandaliya K, Ndinya-Achola JO, Baeten JM. 2006. Vaginal washing and increased risk of HIV-1 acquisition among African women: a 10-year prospective study. AIDS 20:269–273. doi: 10.1097/01.aids.0000196165.48518.7b. [DOI] [PubMed] [Google Scholar]
- 34.Bebell LM, Passmore J-A, Williamson C, Mlisana K, Iriogbe I, van Loggerenberg F, Karim QA, Karim SA. 2008. Relationship between levels of inflammatory cytokines in the genital tract and CD4+ cell counts in women with acute HIV-1 infection. J Infect Dis 198:710–714. doi: 10.1086/590503. [DOI] [PubMed] [Google Scholar]
- 35.Behbahani H, Walther-Jallow L, Klareskog E, Baum L, French AL, Patterson BK, Garcia P, Spetz AL, Landay A, Andersson J. 2007. Proinflammatory and type 1 cytokine expression in cervical mucosa during HIV-1 and human papillomavirus infection. J Acquir Immune Defic Syndr 45:9–19. doi: 10.1097/QAI.0b013e3180415da7. [DOI] [PubMed] [Google Scholar]
- 36.Klatt NR, Chomont N, Douek DC, Deeks SG. 2013. Immune activation and HIV persistence: implications for curative approaches to HIV infection. Immunol Rev 254:326–342. doi: 10.1111/imr.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobson DL, Peralta L, Graham NM, Zenilman J. 2000. Histologic development of cervical ectopy: relationship to reproductive hormones. Sex Transm Dis 27:252–258. doi: 10.1097/00007435-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Hwang LY, Scott ME, Ma Y, Moscicki A-B. 2011. Higher levels of cervicovaginal inflammatory and regulatory cytokines and chemokines in healthy young women with immature cervical epithelium. J Reprod Immunol 88:66–71. doi: 10.1016/j.jri.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fichorova RN, Anderson DJ. 1999. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol Reprod 60:508–514. doi: 10.1095/biolreprod60.2.508. [DOI] [PubMed] [Google Scholar]
- 40.Eade CR, Diaz C, Wood MP, Anastos K, Patterson BK, Gupta P, Cole AL, Cole AM. 2012. Identification and characterization of bacterial vaginosis-associated pathogens using a comprehensive cervical-vaginal epithelial coculture assay. PLoS One 7:e50106. doi: 10.1371/journal.pone.0050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fichorova RN, Desai PJ, Gibson FC III, Genco CA. 2001. Distinct proinflammatory host responses to Neisseria gonorrhoeae infection in immortalized human cervical and vaginal epithelial cells. Infect Immun 69:5840–5848. doi: 10.1128/IAI.69.9.5840-5848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myer L, Wright TC, Denny L, Kuhn L. 2006. Nested case-control study of cervical mucosal lesions, ectopy, and incident HIV infection among women in Cape Town, South Africa. Sex Transm Dis 33:683–687. doi: 10.1097/01.olq.0000216026.67352.f9. [DOI] [PubMed] [Google Scholar]
- 43.Venkatesh KK, Cu-Uvin S. 2013. Assessing the relationship between cervical ectopy and HIV susceptibility: implications for HIV prevention in women. Am J Reprod Immunol 69(Suppl 1):S68–S73. [DOI] [PubMed] [Google Scholar]
- 44.Balkus J, Agnew K, Lawler R, Mitchell C, Hitti J. 2010. Effects of pregnancy and bacterial vaginosis on proinflammatory cytokine and secretory leukocyte protease inhibitor concentrations in vaginal secretions. J Pregnancy 2010:385981. doi: 10.1155/2010/385981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cauci S, Driussi S, Guaschino S, Isola M, Quadrifoglio F. 2002. Correlation of local interleukin-1beta levels with specific IgA response against Gardnerella vaginalis cytolysin in women with bacterial vaginosis. Am J Reprod Immunol 47:257–264. doi: 10.1034/j.1600-0897.2002.01096.x. [DOI] [PubMed] [Google Scholar]
- 46.Rebbapragada A, Howe K, Wachihi C, Pettengell C, Sunderji S, Huibner S, Ball TB, Plummer FA, Jaoko W, Kaul R. 2008. Bacterial vaginosis in HIV-infected women induces reversible alterations in the cervical immune environment. J Acquir Immune Defic Syndr 49:520–522. doi: 10.1097/QAI.0b013e318189a7ca. [DOI] [PubMed] [Google Scholar]
- 47.Fichorova RN, Lai JJ, Schwartz JL, Weiner DH, Mauck CK, Callahan MM. 2011. Baseline variation and associations between subject characteristics and five cytokine biomarkers of vaginal safety among healthy nonpregnant women in microbicide trials. Cytokine 55:134–140. doi: 10.1016/j.cyto.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 48.Brotman RM, Ravel J, Bavoil PM, Gravitt PE, Ghanem KG. 2013. Microbiome, sex hormones, and immune responses in the reproductive tract: challenges for vaccine development against sexually transmitted infections. Vaccine 32:1543–1552. doi: 10.1016/j.vaccine.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nikolaitchouk N, Andersch B, Falsen E, Strombeck L, Mattsby-Baltzer I. 2008. The lower genital tract microbiota in relation to cytokine, SLPI, and endotoxin levels: application of checkerboard DNA-DNA hybridization (CDH). Acta Pathol Microbiol Immunol Scand 116:263–277. doi: 10.1111/j.1600-0463.2008.00808.x. [DOI] [PubMed] [Google Scholar]
- 50.Fichorova RN, Yamamoto HS, Delaney ML, Onderdonk AB, Doncel GF. 2011. Novel vaginal microflora colonization model providing new insight into microbicide mechanism of action. mBio 2:e00168-11. doi: 10.1128/mBio.00168-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamamoto HS, Xu Q, Fichorova RN. 2013. Homeostatic properties of Lactobacillus jensenii engineered as a live vaginal anti-HIV microbicide. BMC Microbiol 13:4. doi: 10.1186/1471-2180-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huppert JS, Huang B, Chen C, Dawood HY, Fichorova RN. 2013. Clinical evidence for the role of Trichomonas vaginalis in regulation of secretory leukocyte protease inhibitor in the female genital tract. J Infect Dis 207:1462–1470. doi: 10.1093/infdis/jit039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Groom JR, Luster AD. 2011. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol 89:207–215. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farber JM. 1997. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol 61:246–257. [PubMed] [Google Scholar]
- 55.Ohmori Y, Wyner L, Narumi S, Armstrong D, Stoler M, Hamilton TA. 1993. Tumor necrosis factor-alpha induces cell type and tissue-specific expression of chemoattractant cytokines in vivo. Am J Pathol 142:861–870. [PMC free article] [PubMed] [Google Scholar]
- 56.Egesten A, Eliasson M, Johansson HM, Olin AI, Morgelin M, Mueller A, Pease JE, Frick IM, Bjorck L. 2007. The CXC chemokine MIG/CXCL9 is important in innate immunity against Streptococcus pyogenes. J Infect Dis 195:684–693. doi: 10.1086/510857. [DOI] [PubMed] [Google Scholar]
- 57.Jauregui CE, Wang Q, Wright CJ, Takeuchi H, Uriarte SM, Lamont RJ. 2013. Suppression of T-cell chemokines by Porphyromonas gingivalis. Infect Immun 81:2288–2295. doi: 10.1128/IAI.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoermannsperger G, Clavel T, Hoffmann M, Reiff C, Kelly D, Loh G, Blaut M, Holzlwimmer G, Laschinger M, Haller D. 2009. Post-translational inhibition of IP-10 secretion in IEC by probiotic bacteria: impact on chronic inflammation. PLoS One 4:e4365. doi: 10.1371/journal.pone.0004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fichorova RN, Buck OR, Yamamoto HS, Fashemi T, Dawood HY, Fashemi B, Hayes GR, Beach DH, Takagi Y, Delaney ML, Nibert ML, Singh BN, Onderdonk AB. 2013. The villain team-up or how Trichomonas vaginalis and bacterial vaginosis alter innate immunity in concert. Sex Transm Infect 89:460–466. doi: 10.1136/sextrans-2013-051052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Masson L, Mlisana K, Little F, Werner L, Mkhize NN, Ronacher K, Gamieldien H, Williamson C, McKinnon LR, Walzl G, Abdool Karim Q, Abdool Karim SS, Passmore JA. 2014. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: a cross-sectional study. Sex Transm Infect 90:580–587. doi: 10.1136/sextrans-2014-051601. [DOI] [PubMed] [Google Scholar]
- 61.Ryckman KK, Williams SM, Krohn MA, Simhan HN. 2008. Racial differences in cervical cytokine concentrations between pregnant women with and without bacterial vaginosis. J Reprod Immunol 78:166–171. doi: 10.1016/j.jri.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cauci S, Guaschino S, Driussi S, De Santo D, Lanzafame P, Quadrifoglio F. 2002. Correlation of local interleukin-8 with immunoglobulin A against Gardnerella vaginalis hemolysin and with prolidase and sialidase levels in women with bacterial vaginosis. J Infect Dis 185:1614–1620. doi: 10.1086/340417. [DOI] [PubMed] [Google Scholar]
- 63.Lewis WG, Robinson LS, Perry J, Bick JL, Peipert JF, Allsworth JE, Lewis AL. 2012. Hydrolysis of secreted sialoglycoprotein immunoglobulin A (IgA) in ex vivo and biochemical models of bacterial vaginosis. J Biol Chem 287:2079–2089. doi: 10.1074/jbc.M111.278135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghosh M, Fahey JV, Shen Z, Lahey T, Cu-Uvin S, Wu Z, Mayer K, Wright PF, Kappes JC, Ochsenbauer C, Wira CR. 2010. Anti-HIV activity in cervical-vaginal secretions from HIV-positive and -negative women correlate with innate antimicrobial levels and IgG antibodies. PLoS One 5:e11366. doi: 10.1371/journal.pone.0011366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Venkataraman N, Cole AL, Svoboda P, Pohl J, Cole AM. 2005. Cationic polypeptides are required for anti-HIV-1 activity of human vaginal fluid. J Immunol 175:7560–7567. doi: 10.4049/jimmunol.175.11.7560. [DOI] [PubMed] [Google Scholar]
- 66.St John EP, Zariffard MR, Martinson JA, Simoes JA, Landay AL, Spear GT. 2009. Effect of mucosal fluid from women with bacterial vaginosis on HIV trans-infection mediated by dendritic cells. Virology 385:22–27. doi: 10.1016/j.virol.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keller MJ, Madan RP, Shust G, Carpenter CA, Torres NM, Cho S, Khine H, Huang ML, Corey L, Kim M, Herold BC. 2012. Changes in the soluble mucosal immune environment during genital herpes outbreaks. J Acquir Immune Defic Syndr 61:194–202. doi: 10.1097/QAI.0b013e31826867ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fan SR, Liu XP, Liao QP. 2008. Human defensins and cytokines in vaginal lavage fluid of women with bacterial vaginosis. Int J Gynecol Obstet 103:50–54. doi: 10.1016/j.ijgo.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 69.Doerflinger SY, Throop AL, Herbst-Kralovetz MM. 2014. Vaginal microbiota alter the innate immune and barrier properties of the human vaginal epithelia in a species-specific manner. J Infect Dis 209:1989–1999. doi: 10.1093/infdis/jiu004. [DOI] [PubMed] [Google Scholar]
- 70.Quinones-Mateu ME, Lederman MM, Feng Z, Chakraborty B, Weber J, Rangel HR, Marotta ML, Mirza M, Jiang B, Kiser P, Medvik K, Sieg SF, Weinberg A. 2003. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS 17:F39–F48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- 71.Jespers V, van de Wijgert J, Cools P, Verhelst R, Verstraelen H, Delaney-Moretlwe S, Mwaura M, Ndayisaba GF, Mandaliya K, Menten J, Hardy L, Crucitti T, for the Vaginal Biomarkers Study Group. 2015. The significance of Lactobacillus crispatus and L. vaginalis for vaginal health and the negative effect of recent sex: a cross-sectional descriptive study across groups of African women. BMC Infect Dis 15:115. doi: 10.1186/s12879-015-0825-z. [DOI] [PMC free article] [PubMed] [Google Scholar]