Systemic agonistic anti-CD40 treatment of tumor bearing mice modulates hepatic myeloid suppressive cells and causes immune-mediated liver damage (original) (raw)
. Author manuscript; available in PMC: 2016 May 1.
Abstract
Immune stimulatory monoclonal antibodies are currently evaluated as anti tumor agents. Although overall toxicity appears to be moderate, liver toxicities have been reported and are not completely understood. We studied the effect of systemic CD40 antibody treatment on myeloid cells in spleen and liver. Naïve and tumor-bearing mice were treated systemically with agonistic anti-CD40 antibody. Immune cell subsets in liver and spleen, serum transaminases and liver histologies were analyzed after antibody administration. Nox2−/−, Cd40−/− as well as bone marrow chimeric mice were used to study the mechanism by which agonistic anti-CD40 mediates its effects in vivo. Suppressor function of murine and human tumor-induced myeloid derived suppressive cells was studied upon CD40 ligation. Agonistic CD40 antibody caused liver damage within 24 hours after injection in two unrelated tumor models and mice strains. Using bone marrow chimeras we demonstrated that CD40 antibody-induced hepatitis in tumor-bearing mice was dependent on the presence of CD40-expressing hematopoietic cells. Agonistic CD40 ligation-dependent liver damage was induced by the generation of reactive oxygen species. Furthermore, agonistic CD40 antibody resulted in increased CD80 and CD40 positive liver CD11b+Gr-1+ immature myeloid cells. CD40 ligation on tumor-induced murine and human CD14+HLA-DRlow PBMC from cancer patients reduced their immune suppressor function. Collectively, agonistic CD40 antibody treatment activated tumor-induced, myeloid cells, caused myeloid dependent hepatotoxicity and ameliorated the suppressor function of murine and human MDSC. Collectively, our data suggests that CD40 may mature immunosuppressive myeloid cells and thereby cause liver damage in mice with an accumulation of tumor-induced hepatic MDSC.
Keywords: Acute hepatitis, CD40, Reactive Oxygen Species, immature myeloid cells, immunosuppression
Introduction
The TNF receptor family member CD40 is a stimulatory molecule constitutively expressed on a large variety of cells, including dendritic cells, B cells, macrophages, and endothelial cells (1–5). CD40 engagement of antigen presenting cells provides the “license” to T cell help and enhances T cell activation (6,7). Agonistic CD40 antibodies were shown to overcome T cell tolerance in tumor-bearing mice and facilitate development of potent cytotoxic T cell responses by enhancing the effects of cancer vaccines (8–12). Recently, immune-modulatory regimens -cytokine therapy (1–5,13–17), radiation (6,7,18–20), chemotherapy (8–12,21–23), kinase inhibitors (24) or monoclonal antibodies (25)- have been shown to synergize with agonistic CD40 antibodies leading to tumor rejection in animal models.
However, systemic administration of immunostimulatory CD40 antibodies has been associated with cytokine release syndrome, lymphopenia and liver toxicity in clinical trials (1,3–5). In preclinical models Fransen and colleagues observed that intravenous delivery of high- or low-dose agonistic CD40 antibody increased liver toxicity in mice bearing virally transformed tumors (6). Agonistic anti-CD40 biodistribution experiments by Sandin and colleagues showed that systemic administration led to higher antibody concentrations in the liver compared with local delivery (9). However, the reason why systemic agonistic CD40 antibody causes liver toxicity remained unknown.
Tumor-induced myeloid derived suppressor cells (MDSC) constitute one of the main players in tumor-induced immune suppression. They are comprised of a heterogeneous population of myeloid cells of diverse differentiation status whose main feature is the suppression of innate and adaptive immune responses (8). Our lab and others have previously described that tumor-induced CD11b+Gr-1+ MDSC accumulate in the liver of mice (13,15,17) and in patients with hepatocellular carcinoma (18,20). In addition, hepatic MDSC have been reported to promote the generation of liver metastasis (21) and CD11b+CCR2+ cells have been detected in liver metastasis from patients with colorectal cancer (26). However, little is known about the biology of tumor-induced hepatic MDSC.
Here, we studied the effect of agonistic anti-CD40 antibody injection on hepatic and splenic MDSC in tumor-bearing mice. While agonistic anti-CD40 treatment led to severe, MDSC-mediated hepatitis in mice, we also provide evidence suggesting that MDSC mature into a pro-inflammatory cell type with less arginase activity. These results are recapitulated in human CD14+HLA-DRlow MDSC, which also lose arginase expression and thereby suppressor function in vitro.
Materials and methods
Mice and cell lines
8- to 10-week-old female BALB/c, C57BL/6-CD45.2 and BL6-CD45.1 were purchased from NCI/Frederick. H-2Kb OVA257-264 TCR transgenic OT-I, Cd40−/− (purchased from Jackson Laboratories, Bar Harbor, USA) and Nox2−/− mice (a kind gift from Robert Mumford, NCI) were bred at NCI/Frederick. Bone marrow chimeric mice were generated as previously described (27). Bone marrow chimerism was confirmed 4 weeks after bone marrow transplant and was above 80%. EL4 and B16 GM-CSF cells were a kind gift of Dr. Drew Pardoll (The Johns Hopkins University, Baltimore, USA) and previously used (27). 4T1 cells were kindly provided by Christopher A. Klebanoff (National Cancer Institute, Bethesda, USA). RIL-175 hepatocellular carcinoma cell line was obtained from Dr. Lars Zender (University Hospital of Tübingen, Germany) and used recently (13,39). All tumor cell lines used were tested negative for mycoplasma using MycoAlert Plus kit (Lonza, USA) routinely. Last test was performed on December 2014. Mice were injected subcutaneously in the flank with 1×106 tumor cells. Tumor size was measured twice a week. Metastatic tumors were established in the liver by intrasplenic injection of 3×105 EL4 cells (28). Mice received antibody treatment 3 weeks after tumor cell inoculation into the spleen. All mice were handled, fed, and housed in accordance with the U.S. Department of Health and Human Services institutional guidelines.
In vivo antibody treatment
Tumor-free littermates or mice bearing subcutaneous tumors between 10 and 15 millimeters maximum diameter were inoculated intra-peritoneally with 100 μg of rat anti-mouse agonist CD40 antibody (clone FGK-45, BioXCell, USA) or irrelevant rat IgG2a (2A3, BioXCell, USA). Mice were sacrificed 24 hours after injection. Alanine/aspartate aminotransferase (ALT/AST) levels were determined in mouse sera by biochemistry analysis in the Department of Laboratory Medicine (NCI). Serum TNF-α levels were quantified by ELISA following manufacturer’s instructions (eBioscience, USA). Hematoxilin-eosin stained liver tissues analyzed by a pathologist (D.K.) in a blinded fashion.
Flow cytometry analysis
Liver mononuclear cells were obtained as previously described (13). Mouse cell samples were stained using antibodies from BD Biosciences and eBioscience (available upon request). When indicated, tumor-induced hepatic myeloid cells were isolated using CD11b beads followed by MACS separation (Miltenyi Biotec, USA). Purity after enrichment was above 90%. Flow cytometry was performed on BD FACS Calibur or LSRII using CellQuest Pro or FACS Diva acquisition software respectively (Becton Dickinson, USA). Data were analyzed using FlowJo software (Tree Star, USA).
Functional assays in vitro
ROS production was determined using Carboxy-H2DCFDA (Invitrogen, USA) as described by Corzo et al. (29). DCFDA expression was quantified on gated mouse CD11b+Gr-1+ cells from liver mononuclear cells 3 hours after injection of 100 μg of either isotype or anti-mouse CD40 antibody. In another setting, DCFDA expression was determined on gated human CD14+HLA-DRhigh and CD14+HLA-DRlow cells after incubation of healthy donor peripheral blood mononuclear cells in the presence or absence of 0.1 μg/ml megaCD40L (Enzo Life Sciences, USA) for 2 hours. For arginase activity and TNF-α determination, hepatic CD11b+ cells were isolated from TB mice and cultured overnight alone or in the presence of 0.1 μg anti-mouse CD40 antibody. Supernatants were collected and TNF-α was quantified by ELISA following manufacturer’s instructions (eBioscience, USA). Arginase activity in cell lysates was determined as described (30). For OVA cross-presentation 1×105 CD11b+ cells were cultured for 24 hours alone or in the presence of 0.1 μg of rat anti-mouse CD40 antibody. Cells were washed twice with PBS, OT-I CD8+ T cells were MACS-sorted using mouse CD8+ T cell isolation kit (Miltenyi Biotec, USA), added to the culture in a 1:1 ratio and stimulated with 0.1 μg/ml OVA-derived SIINFEKL peptide overnight. IFN-γ production by OT-I CD8+ T cells was determined by intracellular staining.
Determination of hepatocyte cytotoxicity by hepatic CD11b+ cells
Luciferase -expressing RIL-175 hepatoma target cells were cultured at a 1:50 (target: effector) ratio with EL4-induced hepatic CD11b+ cells isolated from mice 3 hours after treatment with 100 μg of either IgG or anti-mouse CD40. After 16 hours the number of surviving adherent cells was evaluated using Dual Luciferase Reporter Assay (Promega, Madison, WI, USA). 2 mM H2O2 (Invitrogen, USA) and 100 U/ml catalase (Sigma, USA) were used for apoptosis induction and blocking of ROS release, respectively.
Adoptive cell transfer
Hepatic CD45.1+CD11b+ cells were MACS isolated from B16 GM-CSF TB mice, since GM-CSF expressing tumors have been shown to support the accumulation of large numbers of CD11b+Gr-1+ cells in spleen and liver (13). 5×107 CD11b+ cells were injected into the tail vein of tumor-free CD45.2+Cd40−/− mice. In another set of experiments 5×107 CD11b+ cells from B16 GM-CSF TB wild type or Nox2−/− mice were injected into the tail vein of tumor-free CD45.2+Cd40−/− mice. Mice were subsequently inoculated i.p. either with 100 μg of anti-mouse CD40 or isotype control. Mice were sacrificed 16 hours after antibody injection.
Human MDSC studies
PBMC were obtained from NIH Blood Bank (healthy donors) and patients with GI-related cancer patients (see Supplementary Information). Written consent was obtained from all patients before blood sampling on a research protocol approved by the NCI Institutional Review Board. FACS-sorted CD14+HLA-DRhigh and CD14+HLA-DRlow cells were purified as previously described (31). When indicated, 1×105 or 2×105 sorted cells were cultured in complete RPMI medium with or without 0.1 μg/ml megaCD40L (Enzo Life Sciences, USA) or 5 μg/ml anti-human CD40 antibody (clone 82111, R&D systems, USA) for 24 hours. Then, total RNA was isolated using RNeasy kit (Qiagen, USA). cDNA synthesis was carried out using iScript cDNA synthesis kit according to manufacturer’s instructions (Biorad, USA). Arginase-1 qPCR (primer sequences available upon request) was performed using IQ™ SYBR Green Supermix and thermal cycler (Applied Biosystems, USA). Triplicate reactions were performed for each sample, and expression of tested gene was normalized relative to levels of GAPDH. To assess human MDSC function, CD14+HLA-DRlow cells were isolated from healthy donor PBMC and incubated at different ratios with CD3/CD28 (Miltenyi Biotec, USA)-stimulated CD8+ T cells previously isolated from the remaining CD14− fraction (human CD8+ T cell isolation kit, Miltenyi Biotec, USA) in the presence or absence of 0.1 μg/ml megaCD40L. Proliferation was measured 72 hours later by incorporation of 3H Thymidine (Amersham, USA).
Statistical analysis
Analyses were performed with GraphPad Prism software. Data are provided as mean ± SEM, unless indicated otherwise. For comparisons between two groups, statistical analyses were performed using a Student’s t test and P< 0.05 was considered significant. For comparisons involving 3 groups or more, a one-way ANOVA using the Kruskal-Wallis-Test was performed (p< 0.05 was considered significant). When analyzing the response of two populations to two or more treatments, two-way ANOVA was performed (p< 0.05 was considered significant). Symbols indicating statistical significance are as follows (unless indicated otherwise): n.s., non significant, *, P<0.05.; **, P<0.01; ***, P<0.005.
Results
Systemic agonistic anti-CD40 induces immune-mediated acute hepatitis in tumor-bearing mice
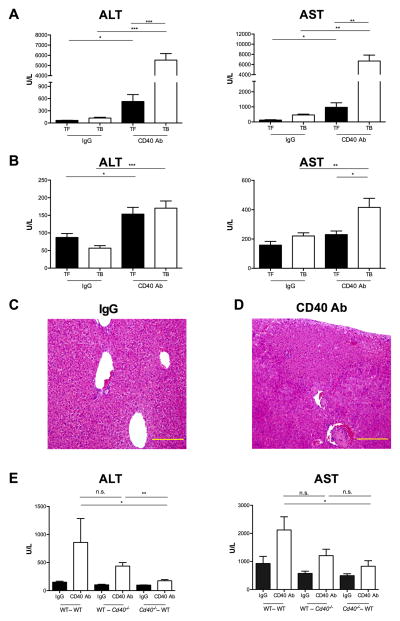
We treated naïve tumor free (TF) and EL4 tumor-bearing (TB) mice with agonistic anti-CD40 or isotype IgG control. Systemic CD40 antibody treatment resulted in a significant increase of serum ALT and AST levels in TB mice (ALT 5529 ± 647 U/L; AST 6687 ± 1166 U/L) compared to TF mice (ALT 528 ± 170 U/L; AST 973 ± 302 U/L) and to TB mice treated with IgG (ALT 122 ± 21 U/L; AST 465 ± 108 U/L) (Figure 1A). Similarly, in BALB/c mice (Figures 1B) transaminases were elevated in 4T1 TB mice treated with agonistic anti-CD40 (ALT 170 ± 20 U/L; AST 415 ± 62 U/L) compared to TB mice treated with isotype control (ALT 57 ± 11 U/L; AST 220 ± 21 U/L). Time course studies demonstrated a peak for ALT and AST serum levels after 24 hours in EL4 TB mice and a return to baseline within 72 hours (Supplementary Figure 1A and B). Histological analysis of liver sections from EL4 TB mice treated with agonistic anti-CD40 antibody showed signs of severe hepatitis with inflammatory cell infiltration and confluent parenchymal necrosis (Figures 1C and 1D). Similar results were obtained in 4T1 TB BALB/c littermates (Supplementary Figure S1B and S1C). Furthermore, in a model of EL4-induced liver metastases (Supplementary Figure S2A) systemic anti-CD40 agonist increased serum ALT and AST levels (ALT 2096 ± 759 U/L; AST 5645 ± 1320 U/L) compared to IgG-treated mice (ALT 414 ± 92 U/L; AST 2542 ± 619 U/L) (Supplementary Figure S2B).
Figure 1. Systemic agonistic anti-CD40 induces immune-mediated liver damage in tumor-bearing mice.
Tumor-free (TF) and tumor-bearing (TB) mice received i.p. either 100 μg agonistic CD40 antibody (CD40 Ab) or control IgG (IgG). (A) Serum ALT and AST levels in C57BL/6 TF and EL4 TB (n=6–8 mice/group) were determined 24 hours after antibody injection. Cumulative data expressed as mean ± SEM, representative of 3 independent experiments. (B) Serum ALT and AST levels in BALB/c TF (n=4 mice/group) and 4T1 TB (n=8 mice/group) were determined 24 hours after antibody injection. Cumulative data of two independent experiments are expressed as mean ± SEM. Representative hematoxilin and eosin staining of liver sections from EL4 TB mice 24 hours after IgG (C) or CD40 Ab injection (D). Images show a 20× magnification and yellow bar = 0.2mm. TB bone marrow chimeric mice (donor→recipient) received i.p. either CD40 Ab or IgG (n=4 mice/group). Serum ALT and AST (E) levels were measured 24 hours after antibody injection. Cumulative data expressed as mean ± SEM, representative of 2 independent experiments. n.s., non significant, *P<0.05, ** P<0.01, ***P<0.005: Student’s t test, (E) one-way ANOVA. TF: Tumor-free; TB: Tumor-bearing; IgG: control IgG: CD40 Ab: agonistic anti-CD40.
CD40 is not only expressed by liver infiltrating immune cells but also by parenchymal cells such as hepatocytes (32). Therefore, we generated Cd40 knockdown bone marrow chimeras to dissociate the relative contribution of the liver immune cell compartment to CD40 antibody-mediated liver damage from direct ligation of CD40 antibody on CD40-expressing hepatocytes. Transaminases were clearly elevated in EL4 TB Cd40−/− mice reconstituted with WT bone marrow upon CD40 ligation (ALT 437 ± 62 U/L; AST 1207 ± 230 U/L) (Figure 1E). In contrast, TB WT mice reconstituted with Cd40−/− bone marrow cells showed lower transaminases (ALT 175 ± 21 U/L; AST 827 ± 199 U/L). TNF-α is a pro-inflammatory cytokine produced upon liver damage (33). Consistently, elevated serum TNF-α levels were only observed in Cd40−/− TB mice after transfer of WT bone marrow cells but not in WT TB mice after transfer of bone marrow from Cd40−/− counterparts (Supplementary Figure S1D).
Agonistic CD40 antibody causes reactive oxygen species-mediated hepatitis in tumor-bearing mice
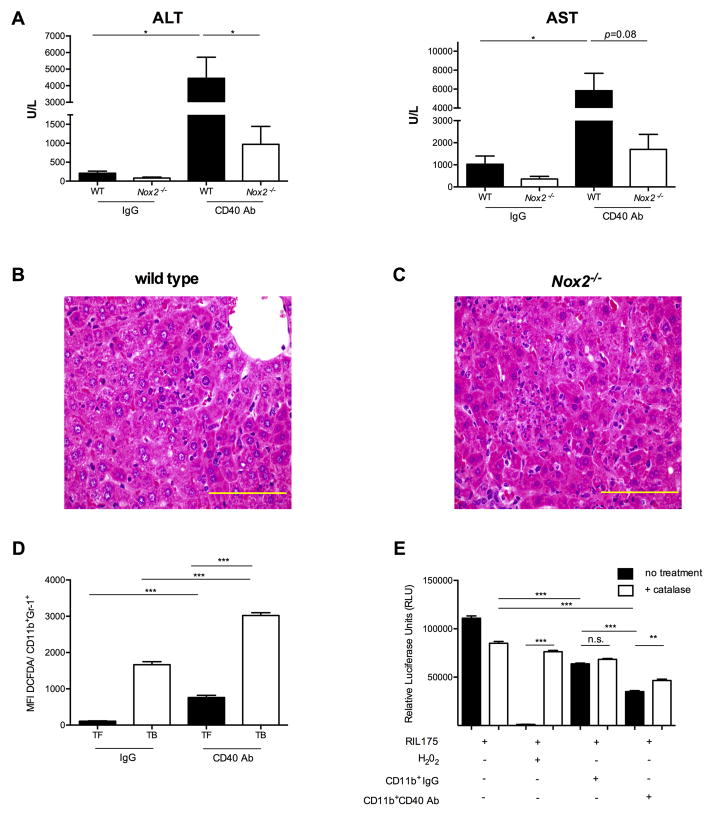
Reactive oxygen species (ROS) produced by mononuclear phagocytes are important mediators of drug-induced hepatotoxicity (34). We studied the role of ROS upon anti-CD40 treatment in TB mice using phagocytic NADPH oxidase 2 (Nox2)- deficient mice. Lower transaminases were observed in EL4 TB Nox2−/− mice (ALT 973 ± 474 U/L; AST 1700 ± 678 U/L) than WT littermate controls upon agonistic anti-CD40 administration (ALT 4452 ± 1266 U/L; AST 5830 ± 1841 U/L) (Figure 2A). As expected, only moderate ALT elevations were found in TB Nox2−/− mice treated with agonistic anti-CD40, possibly due to the direct effect of the antibody on parenchymal cells. In addition, no differences in serum TNF-α levels were observed between WT and Nox2−/− TB mice upon anti-CD40 agonist treatment (data not shown). Histological studies revealed milder immune cell infiltrate, but still evidence of endothelial inflammation and injury in agonistic CD40 antibody-treated Nox2−/− mice (Figures 2B and 2C). Although the pattern of liver injury was similar in wild type and Nox2−/− TB mice, Nox2−/− mice showed less confluent parenchymal necrosis involving only 2–3% of the cross-sectional area. Furthermore less fibrin thrombi were observed in the outflow veins. In line with this finding, systemic CD40 agonist treatment resulted in a significant increase of ROS production by hepatic CD11b+Gr-1+ cells in TB mice compared to TF mice and to TB mice treated with control IgG (Figure 2D). To find out the contribution of hepatic myeloid cells in hepatocyte cell death, we isolated hepatic CD11b+ cells from TB mice 3 hours after treatment either with agonistic anti-CD40 or isotype control and co cultured them with luciferase-expressing hepatoma cells. Using luciferase expression as readout of cell viability, luciferase signal was decreased when hepatoma cells were incubated with hepatic CD11b+ cells from agonistic anti-CD40-treated mice (Figure 2E). CD11b+ mediated cell death was blocked in the presence of the ROS inhibitor catalase, suggesting that CD40 ligation exacerbates ROS-mediated liver cell killing by tumor-induced hepatic myeloid cells. In summary, ROS release plays a role in systemic CD40 agonist-mediated hepatotoxicity.
Figure 2. Agonistic CD40 antibody exacerbates liver inflammation via oxidative stress.
TB WT and Nox2−/− mice (n=4 mice/group) were injected either with CD40 Ab or IgG. Serum ALT and AST levels (A) were measured 24 hours after injection. Cumulative data shown as mean ± SEM are representative of 2 independent experiments. Representative hematoxilin and eosin staining of liver sections from WT TB (B) or Nox2−/− TB (C) 24 hours after systemic CD40 Ab injection. Images show a 60× magnification and yellow bar = 0.1mm. (D) TF and EL4 TB mice received i.p. either 100 μg agonistic CD40 Ab or control IgG (n=2 mice/group). Mean Fluorescence Intensity (MFI) of DCFDA gated on hepatic CD11b+Gr-1+ cells was used to quantify ROS production 3 hours after treatment. Data shown as mean ± SEM are representative of 3 independent experiments. (E) Luminescence intensity by luciferase-expressing RIL-175 cells cultured with or without hepatic CD11b+ cells derived from EL4 TB mice 3 hours after injection of either IgG or anti-CD40 agonist (n=2 mice/group). 100 U/ml catalase was used to block ROS production and 2 mM H2O2 was set as positive control. Data presented here are expressed as mean ± SEM, representative of two independent experiments. n.s. non significant; *P<0.05, ** P<0.01, *** P<0.005; (A,D) Student’s t test, (E) one-way ANOVA.
Agonistic CD40 antibody modulates tumor-induced hepatic CD11b+Gr-1+ cells
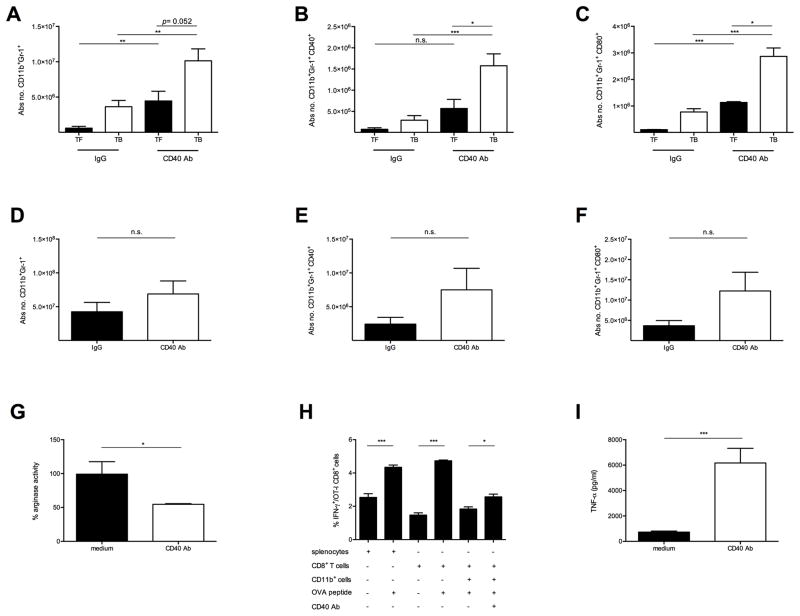
Next, we studied CD11b+Gr-1+ cells after agonistic anti-CD40 treatment. We found that the absolute cell number of hepatic CD11b+Gr-1+ cells increased in EL4 TB mice 24 hours after agonistic anti-CD40 injection (TF 4.4×106±1.4×106 vs. TB 1.1×107±1.9×106, P=0.052) (Figure 3A). This increase was significantly higher in the CD11b+Gr-1low monocytic cell subset (M-MDSC) than in the CD11b+Gr-1high granulocytic subset (G-MDSC) (Supplementary Figure S3A and S3B). Apart from CD11b+Gr-1+ cell changes a moderate decrease of CD3−CD19+ B cells and an increase of CD11c+ dendritic cells were observed upon agonistic CD40 antibody injection (Supplementary Figures S3C–G). Interestingly, agonistic anti-CD40 treatment significantly increased the absolute number of CD40 and CD80 expressing CD11b+Gr-1+ cells (Supplementary Figure S4 and Figures 3B and 3C). Similar results were observed in 4T1 BALB/c TB mice (Supplementary Figures S3I–J). To address whether the effects of agonistic anti-CD40 were restricted to hepatic myeloid cells or whether this was a systemic effect, we also studied splenic CD11b+Gr-1+ cells in our system. An increase in the absolute numbers of CD11b+Gr-1+ cells and more CD40 and CD80 expressing CD11b+Gr-1+ cells were also seen in spleens of TB mice treated with agonistic anti-CD40 (Figures 3D–F). We further analyzed MDSC subsets and found that both hepatic G-MDSC and M-MDSC accumulated over time after anti-CD40 agonist injection (Supplementary Figure S5A and B). However, hepatic G-MDSC produced more ROS than M-MDSC in response to CD40 ligation in vivo (Supplementary Figure S5C).
Figure 3. Systemic anti-CD40 modulates tumor-induced suppressive myeloid cells.
C57BL/6 TF and EL4 TB mice (n=4–8 mice/group) injected i.p. either with CD40 Ab or IgG. (A–C) Absolute number of hepatic CD11b+Gr-1+ cells (A) expressing CD40 (B) and CD80 (C) 24 hours after injection is shown. (D–F) Absolute number of splenic CD11b+Gr-1+ cells (D) expressing CD40 (E) and CD80 (F). (A–F) Data expressed as mean ± SEM, representative of 3 independent experiments. 1×106 EL4- induced liver CD11b+ cells (n=3/mice) were cultured in the presence or absence of 0.1μg CD40 Ab. Arginase activity (G) determined by urea release in the cell lysates was measured with a colorimetric assay 16 hours after incubation. (H) 1×105 EL4- induced liver CD11b+ cells (n=3/mice) were cultured in the presence or absence of 0.1μg CD40 Ab. After 24 hours cells were washed with PBS and incubated overnight with 1×105 sorted CD8+ OT-I OVA-specific T cells in the presence of 0.1 μg/ml OVA. Intracellular IFN-γ production by CD8+ T cells is shown. Data shown as mean ± SEM are representative of 2 independent experiments. 1×106 EL4- induced liver CD11b+ cells (n=3/mice) were cultured in the presence or absence of 0.1 μg CD40 Ab. (I) TNF-α from the cell culture supernatants was determined by ELISA. Data shown as mean ± SEM are representative of 2 independent experiments. n.s. non significant, *P<0.05, ** P<0.01, *** P<0.005: (A–G, I) Student’s t test, (H) one-way ANOVA.
Finally we studied the effects of agonistic anti-CD40 on hepatic CD11b+ cells in vitro. Here, a significant decrease in their arginase activity was seen upon anti-CD40 treatment (Figure 3G). Moreover, anti-CD40 treatment improved their ability to induce antigen-specific IFN-γ release by CD8+ T cells (Figure 3H). Finally, significant levels of TNF-α were detected in cell supernatants after incubation of tumor-induced hepatic myeloid cells with CD40 antibody (Figure 3I). In summary, CD40 ligation on tumor-induced hepatic myeloid cells results in enhanced maturation and activation in vivo and in vitro.
Tumor-induced hepatic CD11b cells mediate liver inflammation upon systemic agonistic CD40 antibody
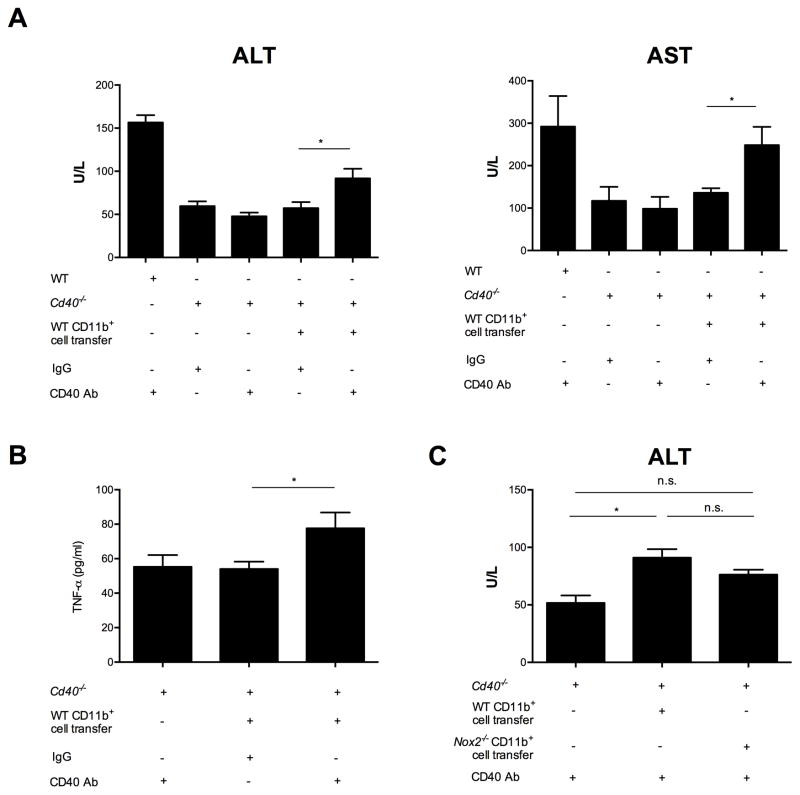
In order to provide a direct link between the presence of hepatic myeloid cells and anti-CD40 mediated liver inflammation, we transferred wild type tumor-induced hepatic CD45.1+CD11b+ cells where 80% were CD11b+Gr-1+ MDSC (Supplementary Figures S6A and S6B) into CD45.2+Cd40−/− naïve recipients followed by systemic injection of the agonistic anti-CD40. While agonistic CD40 antibody did not cause inflammation to regular Cd40−/− naïve mice, transfer of tumor-induced hepatic CD45.1+ myeloid cells and subsequent agonistic anti-CD40 injection to the CD40 knockouts resulted in ALT and AST serum elevation (Figures 4A and B). In addition, an increase in TNF-α serum levels was observed (Figure 4C). Consequently, CD40 ligation on tumor-induced hepatic myeloid cells resulted in enhanced inflammation. To address the role of myeloid-derived ROS in this setting, we transferred either wild type or Nox2−/− tumor-induced hepatic CD11b+ cells into Cd40−/− recipients then challenged them with agonistic anti-CD40. Transfer of wild type tumor-induced hepatic myeloid cells and subsequent agonistic anti-CD40 injection to the CD40 knockouts resulted in higher ALT serum levels compared to Nox2−/− tumor-induced hepatic CD11b+ cell transfer (Figure 4C).
Figure 4. Tumor-induced myeloid cells enhance liver inflammation in vivo upon systemic agonistic anti-CD40 treatment.
5×107 B16 GMCSF-induced WT liver CD45.1+CD11b+ cells (n=3 mice) were injected i.v. into TF CD45.2+ Cd40−/− mice (n=6–8 mice/group). Then either CD40 Ab or IgG were injected i.p. TF WT and Cd40−/− mice received CD40 Ab as control (n= 3mice/group). Serum ALT and AST (A) levels as well as serum TNF-α (B) were measured 16 hours after injection. (A) Cumulative data shown as mean ± SEM are representative of 2 independent experiments. (B) Data shown as mean ± SEM are representative of 2 independent experiments. (C) 5×107 B16 GMCSF-induced WT or Nox2−/− liver CD11b+ cells (n=2 mice/group) were injected i.v. into TF CD45.2+ Cd40−/− mice (n=6–7 mice/group). Then either CD40 Ab or IgG were injected i.p. TF Cd40−/− mice received CD40 Ab as control (n= 2 mice). Serum ALT levels were measured 16 hours after injection. Cumulative data shown as mean ± SEM are representative of 2 independent experiments. n.s. non significant, *P<0.05: one-way ANOVA.
CD40 ligation impairs immunosuppressive function of human CD14+HLA-DRlow MDSC
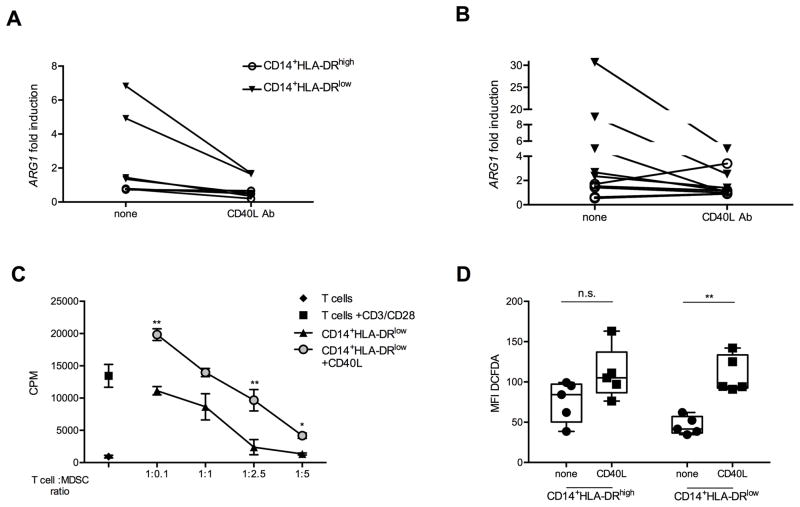
We isolated human CD14+HLA-DRlow MDSC from PBMC of healthy controls (Figure 5A) or patients (Figure 5B) with gastrointestinal cancer (Supplementary Table 1). CD40 engagement using multivalent CD40L reduced Arginase-1 mRNA expression in human CD14+HLA-DRlow MDSC in both healthy controls and patients with cancer. This was not observed in CD14+HLA-DRhigh controls (Figures 5A and B). Similar results were obtained using agonistic anti-human CD40 antibody (Supplementary Figure S7). Next, we isolated CD14+HLA-DRlow cells from PBMC and tested their suppressor function after CD40L treatment. CD40 ligation impaired the suppressor function of human CD14+HLA-DRlow MDSC (Figure 5C). Interestingly, incubation of PBMC in the presence of CD40L resulted in enhanced ROS production by CD14+HLA-DRlow cells (Figure 5D).
Figure 5. CD40 ligation impairs suppressor function in human CD14+HLA-DRlow MDSC.
1×105 sorted CD14+HLA-DRhigh or CD14+HLA-DRlow cells either from healthy (A) or cancer patients (B) were incubated overnight in the presence or absence of human CD40L trimer. Results show the fold change induction in Arginase-1 (ARG1) mRNA expression. Data expressed as mean ± SEM are representative of 2 independent experiments. (C) CD14+HLA-DRlow MDSC were incubated at different ratios with CD8+ T cells isolated from the remaining CD14− fraction, stimulated with anti-CD3/CD28 and in the presence or absence of human CD40L trimer for 48 hours. Proliferation was assessed by thymidine incorporation. Data expressed as mean ± SEM, representative of 2 independent experiments. (D) 1×107 isolated PBMC from healthy donors (n=5) were cultured in complete RPMI medium in the presence or absence of megaCD40L for 2 hours. ROS production was quantified by measuring DCFDA mean fluorescence intensity (MFI) either on CD14+HLA-DRhigh or CD14+HLA-DRlow cells. Cumulative data are representative of two independent experiments. n.s. non significant, *P<0.05, ** P<0.01, *** P<0.005; (A–C) Student’s t test, (D) two-way ANOVA.
Discussion
The approval of ipilimumab by the U. S. Food and Drug Administration in 2011 and very recently pembrolizumab and nivolumab has sparked great interest in immune checkpoint inhibitors in oncology in recent years (35). Similarly, agonistic antibodies to TNF receptor molecules such as activating CD137, OX40 and CD40 antibodies have shown promising results in both preclinical and early clinical settings (5,36,37). Different mechanisms of action have been described for agonistic anti-CD40 antibody therapy in cancer (36). Here we studied the effect of systemic anti-CD40 treatment in TB mice on hepatic and splenic MDSC. We found that agonistic CD40 antibody triggered immune-mediated and ROS-dependent acute liver damage in TB mice by activating hepatic CD11b+Gr-1+ cells. Further studies provide preliminary evidence suggesting that agonistic anti-CD40 treatment causes maturation and loss of suppressor function of hepatic and systemic murine as well as human MDSC.
Various cells in the liver including hepatocytes, Kupffer cells as well as different myeloid cells express CD40 (38). Previously, transaminitis has been reported in patients treated with agonistic anti-CD40 in a phase I trial (1). Similarly, liver toxicity upon agonistic anti-CD40 treatment has been observed in preclinical models upon intravenous administration, but this was reduced when the antibody was injected peri-tumorally (6). This adverse event was initially attributed to a direct effect of agonistic anti-CD40 on CD40-expressing hepatocytes (32). Our findings suggest a potential alternative explanation namely the engagement of hepatic CD11b+Gr-1+ MDSC and ultimately ROS release leading to hepatocyte death upon anti-CD40 treatment. There is a preferential accumulation of MDSC in the liver of TB mice which is in larger numbers than other myeloid populations such as kupffer cells (13,15,17,39). These cells express low levels of CD40 (40–42), which can be further enhanced upon incubation with IFN-γ (43) as well as in a setting of acute inflammation (data not shown). Our data using bone marrow chimeric mice clearly suggest a pivotal role for CD40-expressing myeloid cells in CD40-mediated liver damage in TB mice. First, serum transaminases and TNF-α serum levels were higher in Cd40−/− mice reconstituted with WT bone marrow than in WT mice after reconstitution with Cd40−/− bone marrow. Second, adoptive transfer of WT MDSC into Cd40−/− mice followed by agonistic anti-CD40 treatment resulted in increased transaminases and TNF-α serum levels.
Oxidative stress via ROS release by mononuclear phagocytes plays a pivotal role in inflammatory liver injury (34). Treatment of mice with free radical scavengers decreased ALT in a model of immune-mediated hepatitis (44). Using phagocytic NADPH oxidase 2 (Nox2)- deficient mice to study the potential contribution of reactive oxygen species (29) in agonistic CD40 antibody-mediated liver damage we observed that knockout TB mice demonstrated lower ALT/AST levels than TB littermate controls suggesting a ROS mediated liver cell damage in our model. We provide further biological evidence by showing that tumor-induced hepatic myeloid cells produce ROS upon CD40 ligation, which in turn induces hepatocyte cell death.
Our data show that systemic administration of agonistic anti-CD40 increased the accumulation of CD11b+Gr-1+ cells in the liver of TB mice. Our experiments did not address a possible role for liver infiltrating neutrophils, which accumulate in murine models of immune-mediated hepatitis by margination of neutrophils through sinusoids (45). Inflammatory neutrophils express CD11b and high levels of Gr-1 similar to the granulocytic subset of tumor-induced hepatic MDSC. However, we provide multiple lines of evidence suggesting that tumor-induced hepatic CD11b+Gr-1+ cells and not inflammatory neutrophils were responsible for agonistic anti-CD40 mediated liver toxicity: 1) Transaminase levels were higher in TB mice (in which hepatic MDSC accumulate) than in naïve mice after agonistic anti-CD40 treatment; 2) significant accumulation of CD11b+Gr-1low monocytic-like MDSC rather than CD11b+Gr-1high granulocytic-like MDSC was observed in TB mice upon agonistic CD40 antibody-driven liver toxicity; and 3) transfer of tumor-induced hepatic myeloid cells into Cd40−/− mice caused ALT/AST elevation upon agonistic anti-CD40. In this experiment only tumor-induced MDSC expressed CD40.
Our data suggest that tumor-induced hepatic and -to a lesser extent-splenic CD11b+Gr-1+ cells increase CD40 and CD80 surface marker expression upon agonist CD40 antibody treatment. This study along with others show that tumor-induced CD11b+Gr-1+ MDSC express low levels of CD40 (2,46) and CD80 (7,47). Since CD40 ligation induces up regulation of CD80 (10–12,48), our results suggest that hepatic tumor-induced CD11b+Gr-1+ cells may mature and get activated by CD40 ligation in vivo. However, in the absence of specific markers to clearly separate tumor-induced myeloid cells with suppressor function from other innate immune cells, which migrate to the liver in acute inflammatory settings (14,16,45), we could not formally prove the plasticity of hepatic MDSC.
CD14+HLA-DRlow MDSC accumulate in the peripheral blood and tumors from patients with different types of cancer, including liver cancer (18–20,49,50). While an accumulation of CD11b+CCR2+ cells has been described in sections obtained from patients with colorectal liver metastasis (22,23,26), it is not known so far whether MDSC also accumulate in tumor-free livers of patients with cancer. Therefore, at this point one can only speculate whether hepatic MDSC caused AST/ALT elevations in patients treated with agonistic anti-CD40 and further studies are clearly needed.
Tumor-induced mouse myeloid cells and human CD14+HLA-DRlow MDSC both express high arginase levels and can suppress T cell function through an arginase-dependent mechanism (24,51). Both our murine and human data clearly suggest that agonistic anti-CD40 treatment may impair the suppressor function of MDSC and would therefore represent a potential novel approach to target MDSC as also recently suggested by others (25,52).
Immune-related adverse effects in clinical trials have been associated with the systemic use of immune modulatory drugs (5). Much of anti-CD40 treatment dependent toxicity can be avoided by local delivery in a slow release vehicle, maintaining its anti-tumor effects in animal models (6,9). However, it is not clear whether such treatment would also mature MDSC. Therefore, further studies are need to investigate new administration routes and compounds to stabilize and prolong the effect of immune modulatory compounds with the aim to mitigate immunotherapy-related toxicity and potentially target MDSC.
Overall, our data indicate that liver toxicity caused by systemic CD40 antibody in mice is due to activation of tumor-induced hepatic CD11b+Gr-1+ cells and provides preliminary evidence suggesting a reprogramming of tumor-induced myeloid cells into pro-inflammatory myeloid subsets without suppressor function. Finally, our studies do not only provide a novel potential explanation for anti-CD40 induced hepatotoxicity observed in early clinical trials, but may also open new opportunities for the targeting of immunosuppressive MDSC in patients with cancer.
Supplementary Material
1
Acknowledgments
Financial support: This work was supported by the Intramural Research Program of the NCI, NIH.
Footnotes
Disclosures: The authors declare no conflict of interest
References
- 1.Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, et al. Clinical Activity and Immune Modulation in Cancer Patients Treated With CP-870,893, a Novel CD40 Agonist Monoclonal Antibody. Journal of Clinical Oncology. 2007;25:876–83. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 2.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–35. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 3.Beatty GL, Torigian DA, Chiorean EG, Saboury B, Brothers A, Alavi A, et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013;19:6286–95. doi: 10.1158/1078-0432.CCR-13-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vonderheide RH, Burg JM, Mick R, Trosko JA, Li D, Shaik MN, et al. Phase I study of the CD40 agonist antibody CP-870,893 combined with carboplatin and paclitaxel in patients with advanced solid tumors. Oncoimmunology. 2013;2:e23033. doi: 10.4161/onci.23033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gangadhar TC, Vonderheide RH. Mitigating the toxic effects of anticancer immunotherapy. Nat Rev Clin Oncol. 2014;11:91–9. doi: 10.1038/nrclinonc.2013.245. [DOI] [PubMed] [Google Scholar]
- 6.Fransen MF, Sluijter M, Morreau H, Arens R, Melief CJM. Local Activation of CD8 T Cells and Systemic Tumor Eradication without Toxicity via Slow Release and Local Delivery of Agonistic CD40 Antibody. Clinical Cancer Research. 2011;17:2270–80. doi: 10.1158/1078-0432.CCR-10-2888. [DOI] [PubMed] [Google Scholar]
- 7.Lanzavecchia A. Immunology. Licence to kill. Nature. 1998;393:413–4. doi: 10.1038/30845. [DOI] [PubMed] [Google Scholar]
- 8.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol Nature Publishing Group. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandin LC, Orlova A, Gustafsson E, Ellmark P, Tolmachev V, Tötterman TH, et al. Locally delivered CD40 agonist antibody accumulates in secondary lymphoid organs and eradicates experimental disseminated bladder cancer. Cancer Immunology Research. 2014;2:80–90. doi: 10.1158/2326-6066.CIR-13-0067. [DOI] [PubMed] [Google Scholar]
- 10.French RR, Chan HT, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nature Medicine. 1999;5:548–53. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- 11.Diehl L, Boer den AT, Schoenberger SP, van der Voort EI, Schumacher TN, Melief CJ, et al. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nature Medicine. 1999;5:774–9. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 12.Sotomayor EM, Borrello I, Tubb E, Rattis FM, Bien H, Lu Z, et al. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nature Medicine. 1999;5:780–7. doi: 10.1038/10503. [DOI] [PubMed] [Google Scholar]
- 13.Kapanadze T, Gamrekelashvili J, Ma C, Chan C, Zhao F, Hewitt S, et al. Regulation of accumulation and function of myeloid derived suppressor cells in different murine models of hepatocellular carcinoma. Journal of Hepatology. 2013;59:1007–13. doi: 10.1016/j.jhep.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang M, Yao Z, Dubois S, Ju W, Müller JR, Waldmann TA. Interleukin-15 combined with an anti-CD40 antibody provides enhanced therapeutic efficacy for murine models of colon cancer. Proceedings of the National Academy of Sciences. 2009;106:7513–8. doi: 10.1073/pnas.0902637106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connolly MK, Mallen-St Clair J, Bedrosian AS, Malhotra A, Vera V, Ibrahim J, et al. Distinct populations of metastases-enabling myeloid cells expand in the liver of mice harboring invasive and preinvasive intra-abdominal tumor. Journal of Leukocyte Biology. 2010;87:713–25. doi: 10.1189/jlb.0909607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss JM, Back TC, Wiltrout RH. 11 Successful immunotherapy with IL-2/anti-CD40 induces the chemokine-mediated mitigation of an immunosuppressive tumor microenvironment. Proc Natl Acad Sci USA. 2009;106:19455–60. doi: 10.1073/pnas.0909474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ilkovitch D, Lopez DM. The Liver Is a Site for Tumor-Induced Myeloid-Derived Suppressor Cell Accumulation and Immunosuppression. Cancer Res. 2009;69:5514–21. doi: 10.1158/0008-5472.CAN-08-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krüger C, Manns MP, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–43. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Honeychurch J, Glennie MJ, Johnson PWM, Illidge TM. Anti-CD40 monoclonal antibody therapy in combination with irradiation results in a CD8 T-cell-dependent immunity to B-cell lymphoma. Blood. 2003;102:1449–57. doi: 10.1182/blood-2002-12-3717. [DOI] [PubMed] [Google Scholar]
- 20.Duffy A, Zhao F, Haile L, Gamrekelashvili J, Fioravanti S, Ma C, et al. Comparative analysis of monocytic and granulocytic myeloid-derived suppressor cell subsets in patients with gastrointestinal malignancies. Cancer Immunol Immunother. 2013;62:299–307. doi: 10.1007/s00262-012-1332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van den Eynden GG, Majeed AW, Illemann M, Vermeulen PB, Bird NC, Høyer-Hansen G, et al. The Multifaceted Role of the Microenvironment in Liver Metastasis: Biology and Clinical Implications. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-12-3931. [DOI] [PubMed] [Google Scholar]
- 22.Nowak AK, Robinson BWS, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63:4490–6. [PubMed] [Google Scholar]
- 23.Qu X, Felder MAR, Perez Horta Z, Sondel PM, Rakhmilevich AL. Antitumor effects of anti-CD40/CpG immunotherapy combined with gemcitabine or 5-fluorouracil chemotherapy in the B16 melanoma model. Int Immunopharmacol. 2013;17:1141–7. doi: 10.1016/j.intimp.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang Q, Weiss JM, Back T, Chan T, Ortaldo JR, Guichard S, et al. mTOR kinase inhibitor AZD8055 enhances the immunotherapeutic activity of an agonist CD40 antibody in cancer treatment. Cancer Res. 2011;71:4074–84. doi: 10.1158/0008-5472.CAN-10-3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheidt von B, Leung PSK, Yong MCR, Zhang Y, Towne JE, Smyth MJ, et al. Combined anti-CD40 and anti-IL-23 monoclonal antibody therapy effectively suppresses tumor growth and metastases. Cancer Res. 2014;74:2412–21. doi: 10.1158/0008-5472.CAN-13-1646. [DOI] [PubMed] [Google Scholar]
- 26.Zhao L, Lim SY, Gordon-Weeks AN, Tapmeier TT, Im JH, Cao Y, et al. Recruitment of a myeloid cell subset (CD11b/Gr1 mid) via CCL2/CCR2 promotes the development of colorectal cancer liver metastasis. Hepatology. 2013;57:829–39. doi: 10.1002/hep.26094. [DOI] [PubMed] [Google Scholar]
- 27.Medina-Echeverz J, Haile LA, Zhao F, Gamrekelashvili J, Ma C, Métais J-Y, et al. IFN-γ regulates survival and function of tumor-induced CD11b(+) Gr-1(high) myeloid derived suppressor cells by modulating the anti-apoptotic molecule Bcl2a1. Eur J Immunol. 2014 doi: 10.1002/eji.201444497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu R, Sun X, Tse L-Y, Li H, Chan P-C, Xu S, et al. Long-term expression of angiostatin suppresses metastatic liver cancer in mice. Hepatology. 2003;37:1451–60. doi: 10.1053/jhep.2003.50244. [DOI] [PubMed] [Google Scholar]
- 29.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, et al. Mechanism Regulating Reactive Oxygen Species in Tumor-Induced Myeloid-Derived Suppressor Cells. J Immunol. 2009;182:5693–701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Youn J-I, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. Journal of Leukocyte Biology. 2011 doi: 10.1189/jlb.0311177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoechst B, Gamrekelashvili J, Manns MP, Greten TF, Korangy F. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood. 2011;117:6532–41. doi: 10.1182/blood-2010-11-317321. [DOI] [PubMed] [Google Scholar]
- 32.Afford SC, Randhawa S, Eliopoulos AG, Hubscher SG, Young LS, Adams DH. CD40 activation induces apoptosis in cultured human hepatocytes via induction of cell surface fas ligand expression and amplifies fas-mediated hepatocyte death during allograft rejection. J Exp Med. 1999;189:441–6. doi: 10.1084/jem.189.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwabe RF, Brenner DA. Mechanisms of Liver Injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583–9. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- 34.Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol. 2011;26 (Suppl 1):173–9. doi: 10.1111/j.1440-1746.2010.06592.x. [DOI] [PubMed] [Google Scholar]
- 35.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer Nature Publishing Group. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res. 2013;19:1035–43. doi: 10.1158/1078-0432.CCR-12-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melero I, Hirschhorn-Cymerman D, Morales-Kastresana A, Sanmamed MF, Wolchok JD. Agonist antibodies to TNFR molecules that costimulate T and NK cells. Clin Cancer Res. 2013;19:1044–53. doi: 10.1158/1078-0432.CCR-12-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou F, Ajuebor MN, Beck PL, Le T, Hogaboam CM, Swain MG. CD154-CD40 interactions drive hepatocyte apoptosis in murine fulminant hepatitis. Hepatology. 2005;42:372–80. doi: 10.1002/hep.20802. [DOI] [PubMed] [Google Scholar]
- 39.Eggert T, Medina-Echeverz J, Kapanadze T, Kruhlak MJ, Korangy F, Greten TF. Tumor Induced Hepatic Myeloid Derived Suppressor Cells Can Cause Moderate Liver Damage. PLoS ONE. 2014;9:e112717. doi: 10.1371/journal.pone.0112717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang R, Cai Z, Zhang Y, Yutzy WH, Roby KF, Roden RBS. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res. 2006;66:6807–15. doi: 10.1158/0008-5472.CAN-05-3755. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Zeng B, Zhang Z, Zhang Y, Yang R. B7-H1 on myeloid-derived suppressor cells in immune suppression by a mouse model of ovarian cancer. Clin Immunol. 2008;129:471–81. doi: 10.1016/j.clim.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 42.Ko HJ, Lee JM, Kim YJ, Kim YS, Lee KA, Kang CY. Immunosuppressive myeloid-derived suppressor cells can be converted into immunogenic APCs with the help of activated NKT cells: an alternative cell-based antitumor vaccine. J Immunol. 2009;182:1818–28. doi: 10.4049/jimmunol.0802430. [DOI] [PubMed] [Google Scholar]
- 43.Frasca L, Nasso M, Spensieri F, Fedele G, Palazzo R, Malavasi F, et al. IFN-gamma arms human dendritic cells to perform multiple effector functions. J Immunol. 2008;180:1471–81. doi: 10.4049/jimmunol.180.3.1471. [DOI] [PubMed] [Google Scholar]
- 44.Nakashima H, Kinoshita M, Nakashima M, Habu Y, Shono S, Uchida T, et al. Superoxide produced by Kupffer cells is an essential effector in concanavalin A-induced hepatitis in mice. Hepatology. 2008;48:1979–88. doi: 10.1002/hep.22561. [DOI] [PubMed] [Google Scholar]
- 45.Bonder CS, Ajuebor MN, Zbytnuik LD, Kubes P, Swain MG. Essential role for neutrophil recruitment to the liver in concanavalin A-induced hepatitis. J Immunol. 2004;172:45–53. doi: 10.4049/jimmunol.172.1.45. [DOI] [PubMed] [Google Scholar]
- 46.Pan PY, Ma G, Weber KJ, Ozao-Choy J, Wang G, Yin B, et al. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. (2009) 2010;70:99–108. doi: 10.1158/0008-5472.CAN-09-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Youn J-I, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eshima K, Choi Y, Flavell RA. CD154-CD40-independent up-regulation of B7-2 on splenic antigen-presenting cells and efficient T cell priming by staphylococcal enterotoxin A. International Immunology. 2003;15:817–26. doi: 10.1093/intimm/dxg080. [DOI] [PubMed] [Google Scholar]
- 49.Vasquez-Dunddel D, Pan F, Zeng Q, Gorbounov M, Albesiano E, Fu J, et al. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. Journal of Clinical Investigation. 2013 doi: 10.1172/JCI60083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11:802–7. doi: 10.1016/j.intimp.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vasquez-Dunddel D, Pan F, Zeng Q, Gorbounov M, Albesiano E, Fu J, et al. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J Clin Invest. 2013;123:1580–9. doi: 10.1172/JCI60083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liljenfeldt L, Dieterich LC, Dimberg A, Mangsbo SM, Loskog ASI. CD40L gene therapy tilts the myeloid cell profile and promotes infiltration of activated T lymphocytes. Cancer Gene Ther. 2014;21:95–102. doi: 10.1038/cgt.2014.2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1