Association Between Early-Onset Parkinson Disease and 22q11.2 Deletion Syndrome: Identification of a Novel Genetic Form of Parkinson Disease and Its Clinical Implications (original) (raw)
. Author manuscript; available in PMC: 2015 Jun 13.
Abstract
IMPORTANCE
Clinical case reports of parkinsonism co-occurring with hemizygous 22q11.2 deletions and the associated multisystem syndrome, 22q11.2 deletion syndrome (22q11.2DS), suggest that 22q11.2 deletions may lead to increased risk of early-onset Parkinson disease (PD). The frequency of PD and its neuropathological presentation remain unknown in this common genetic condition.
OBJECTIVE
To evaluate a possible association between 22q11.2 deletions and PD.
DESIGN, SETTING, AND PARTICIPANTS
An observational study of the occurrence of PD in the world’s largest cohort of well-characterized adults with a molecularly confirmed diagnosis of 22q11.2DS (n = 159 [6 with postmortem tissue]; age range, 18.1–68.6 years) was conducted in Toronto, Ontario, Canada. Rare postmortem brain tissue from individuals with 22q11.2DS and a clinical history of PD was investigated for neurodegenerative changes and compared with that from individuals with no history of a movement disorder.
MAIN OUTCOMES AND MEASURES
A clinical diagnosis of PD made by a neurologist and neuropathological features of PD.
RESULTS
Adults with 22q11.2DS had a significantly elevated occurrence of PD compared with standard population estimates (standardized morbidity ratio = 69.7; 95% CI, 19.0–178.5). All cases showed early onset and typical PD symptom pattern, treatment response, and course. All were negative for family history of PD and known pathogenic PD-related mutations. The common use of antipsychotics in patients with 22q11.2DS to manage associated psychiatric symptoms delayed diagnosis of PD by up to 10 years. Postmortem brain tissue revealed classic loss of midbrain dopaminergic neurons in all 3 postmortem 22q11.2DS-PD cases. Typical α-synuclein–positive Lewy bodies were present in the expected distribution in 2 cases but absent in another.
CONCLUSIONS AND RELEVANCE
These findings suggest that 22q11.2 deletions represent a novel genetic risk factor for early-onset PD with variable neuropathological presentation reminiscent of _LRRK2_-associated PD neuropathology. Individuals with early-onset PD and classic features of 22q11.2DS should be considered for genetic testing, and those with a known 22q11.2 deletion should be monitored for the development of parkinsonian symptoms. Molecular studies of the implicated genes, including DGCR8, may help shed light on the underlying pathophysiology of PD in 22q11.2DS and idiopathic PD.
Parkinson disease (PD) is a progressive neurodegenerative disorder associated with motor, cognitive, and autonomic dysfunction. It is one of the most common neurological disorders worldwide, affecting approximately 1% of individuals older than 65 years.1,2 Early onset of the disease (age <50 years) is far less common1,2 and is associated with mutations in genes including LRRK2, PARK2, SNCA, PARK7, and PINK1.3 Known genetic mutations account for 4% to 16% of early-onset PD cases.4,5
Previous clinical case reports of 4 individuals with parkinsonism and a hemizygous deletion of chromosome 22q11.2 have suggested that this genetic anomaly may also confer an increased risk of early-onset PD.6–8 Three of these cases were reported to be treated with dopamine replacement therapy (L-dopa), and presynaptic dopamine imaging in the other case indicated degeneration of the nigrostriatal dopamine system.6–8 The 22q11.2 deletion affects at least 1 in 4000 live births9,10 and occurs as a spontaneous mutation in approximately 90% of identified cases.11 The associated 22q11.2 deletion syndrome (22q11.2DS) (OMIM #192430, #188400) shows variable expression including congenital, neurodevelopmental, and later-onset features, eg, schizophrenia.10 Classic manifestations of 22q11.2DS include learning difficulties, palatal anomalies such as velopharyngeal insufficiency causing hypernasal speech, congenital heart defects, hypocalcemia, and subtle facial dysmorphic features.10,12,13 The effect of the 22q11.2 deletion on aging and neurodegenerative processes is less clear. Antipsychotic medication used to manage psychosis may obfuscate diagnosis of PD in patients with 22q11.2DS.8 Neuropathological confirmation of PD, including midbrain dopaminergic cell loss and the presence of Lewy bodies,14,15 remains essential in establishing a 22q11.2 deletion as a risk factor for PD.
To investigate the proposed association between 22q11.2 deletions and PD, we assessed the occurrence of a clinical diagnosis of PD in a well-characterized cohort of adults with 22q11.2DS. We examined available brain tissue from 3 individuals with 22q11.2DS and an antemortem diagnosis of PD and compared results with those with a 22q11.2 deletion and no history of parkinsonism as well as with sex- and age-matched controls with no history of either condition. We also assessed a clinical series of individuals with early-onset PD for 22q11.2 deletions. The results of this study provide the first prevalence estimate and neuropathological confirmation of PD in 22q11.2DS, supporting the 22q11.2 deletion as a novel genetic risk factor for developing early-onset PD.
Methods
Subjects
We conducted an observational study in Toronto, Ontario, Canada, of a large, well-characterized cohort of 159 adults (aged ≥18 years) clinically diagnosed as having 22q11.2DS and molecularly confirmed to have a chromosome 22q11.2 deletion using standard methods.11,12 Most subjects were ascertained through adult congenital cardiac, psychiatric, and genetic services using active screening and/or clinical referrals13,16 with no known bias to ascertaining individuals with a neurodegenerative condition. All but 5 of these Canadian subjects were from eastern Canada, mostly from Ontario. Comprehensive neuropsychiatric phenotypic data and other clinical and demographic information were available from direct assessments and lifetime medical records.12,17 We recorded all clinical diagnoses of PD made by a neurologist as of March 2013 that included documentation of standard treatment(s) for PD. We also recorded family history of neurodegenerative disease in first-and second-degree relatives. The diagnosis of PD was made using the UK Parkinson’s Disease Society Brain Bank criteria.18 We did not include cases of suspected PD lacking strong confirmatory evidence. Eighteen of the 159 patients had died (median age at death, 44.7 years; range, 18.1–68.6 years). Written informed consent was obtained for all subjects and from next of kin as necessary for autopsy including use of tissue for research purposes. The study was approved by the research ethics boards of the University of Toronto, Centre for Addiction and Mental Health, and University Health Network.
Statistical Analysis
The observed number of PD cases in our 22q11.2DS cohort was compared with the expected number of PD cases using an age-and sex-adjusted standardized morbidity ratio. The expected number was calculated using Canadian population norms.1 Given the small numbers, we also calculated the standardized morbidity ratio for the main age group examined (ages 35–64 years). Analyses were performed with SAS version 9.3 statistical software (SAS Institute, Inc), with statistical significance defined as P < .05.
Neuropathological Investigation of PD
Immunohistochemistry
Brain tissue from 3 individuals with 22q11.2 deletions and a clinical diagnosis of PD (eTable in Supplement) was fixed in 10% buffered formalin for 2 weeks. Standard blocks were taken from the left hemisphere for light microscopy (5 μm; hematoxylin-eosin and Luxol fast blue–hematoxylin-eosin). Immunohistochemistry using an avidin-biotin–complex, peroxidase-based method was performed for tyrosine hydroxylase (TH) (1:1000, Sigma) and for proteins commonly found to aggregate in PD, including α-synuclein (1:400, Zymed; 1:3000, Santa Cruz Biotechnology) and ubiquitin (1:400, Dako). Markers of other neurodegenerative disorders were also used (β-amyloid, 1:10, Dako; PHF-tau, 1:400, Innogenetics; and TAR DNA-binding protein 43, 1:6000, Cosmobio Co). To investigate the presence of gliosis and microglial activation, tissue was double labeled with glial fibrillary acidic protein (1:1000, Millipore) and Iba-1 (1:200, Millipore) in a sequential manner. The chromogens used were diaminobenzidine and fast red, respectively.
The severity of overall neuronal loss in subcortical and cortical areas of interest was classified semiquantitatively by examining the hematoxylin-eosin–stained sections: 0 indicated none (no apparent neuronal loss); 1, mild (mild gliosis with some free pigments); and 2, extensive (neuronal loss involving >70% of the structure). Dopaminergic cell loss in the substantia nigra pars compacta was further examined by TH immunoreactivity using the same classification system. Sections immunostained against α-synuclein were used to qualitatively evaluate the visible presence or absence of Lewy bodies and Lewy neurites.
Comparable brain tissue obtained from 3 individuals with 22q11.2 deletions but no history of parkinsonism19 and 10 age-and sex-matched subjects with unremarkable brain autopsy findings with a negative clinical history for diagnoses or features of 22q11.2DS or PD was prepared and examined using the methods described earlier. Additional tissue control experiments included known positive material from patients with PD, diffuse Lewy body disease, and Alzheimer disease. No reactions were observed following the omission of primary antibodies.
Genetic Investigations
We used available genomic DNA to conduct genetic analysis of LRRK2, PARK2, PARK7, PINK1, and SNCA20–24 in individuals with a 22q11.2 deletion and a history of PD. In addition, we performed real-time polymerase chain reaction to investigate the presence of copy number variations for each exon of PARK2 and SNCA using SYBR Green reagent (Takara Mirus Bio) on an ABI7500 system (Applied Biosystems).
To ascertain the occurrence of 22q11.2 deletions in early-onset PD, we performed a quantitative polymerase chain reaction study using DNA samples from a clinical series of patients ascertained from the Movement Disorders Centre, Toronto Western Hospital (n = 225; age at onset, ≤50 years). Nearly half (n = 106) of these unrelated individuals had a positive family history of PD; most were white. Copy number variations in two 22q11.2 genes (TBX1 and SNAP29) were examined on a ViiA 7 real-time polymerase chain reaction system (Life Technologies), performed by The Centre for Applied Genomics, The Hospital for Sick Children, Toronto. This study was approved by University Health Network, and written informed consent was obtained from all individuals in this study.
Results
Occurrence of PD and Genetic Findings
The occurrence of PD in adults with a hemizygous 22q11.2 deletion was significantly elevated compared with Canadian population norms1 (standardized morbidity ratio = 69.7; 95% CI, 19.0–178.5). Subgroup analyses showed no subjects aged 18 to 34 years (n = 90) diagnosed as having PD. Four of 68 subjects (5.9%) aged 35 to 64 years with 22q11.2 deletions had been diagnosed as having PD (standardized morbidity ratio = 91.7; 95% CI, 25.0–234.8). The 1 subject older than 65 years had a stroke and showed no signs of PD.
Age at onset of motor symptoms ranged from 39 to 48 years (Table 1; see eAppendix in Supplement for case histories). The use of antipsychotic (and other) medications often delayed or complicated the diagnosis of PD. However, the early onset of typical motor symptoms and progressive course, despite reduction or discontinuation of antipsychotics and/or the use of atypical agents (eg, clozapine), together with a characteristic response to L-dopa supported the consideration of PD in all cases. All had typical 22q11.2 deletions, 3 with the most common deletion of approximately 3 megabases and 1 with the proximal nested 1.5-megabase deletion (Table 1).11 All were negative for the tested PD risk mutations and had no family history of neurodegenerative disease or parental consanguinity.
Table 1.
Demographic and Clinical Features of Individuals With Parkinson Disease and a Hemizygous 22q11.2 Deletion
| Characteristic | Case | Zaleski et al7,a | Booij et al8 | Krahn et al6 | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||
| Sex | F | M | M | M | M | M | M |
| 22q11.2 deletion size, Mb | 1.5 | 3 | 3 | 3 | NRb | NRb | NRb |
| Age, y | |||||||
| Motor symptom onset | 45 | 48 | 43 | 39 | ~42 | ~42 | <27 |
| PD diagnosis | 55 | 54 | 44 | 48 | 42 | 52 | Parkinsonism at <27 |
| Death | 56 | 58 | 61 | NAc | NAc | NAc | NAc |
| Motor symptom | |||||||
| Tremor | Yesd | Yes | Yesd | Yes | No | NR | Yesd |
| Bradykinesia | Yes | Yes | Yesd | Yesd | Yesd | NR | Yes |
| Postural instability | Yes | Yesd | Yesd | Yes | Yes | NR | No |
| Rigidity | Yesd | Yes | Yes | Yesd | Yesd | NR | Yesd |
| Laterality of onset | Yes | Yes | No | Yes | Yes | NR | NR |
| Nonmotor symptom | |||||||
| Incontinence | Yes | Yese | Yes | Yes | No | NR | NR |
| Mood disturbances | No | No | Yes | Yes | Yes | NR | NR |
| Cognitive decline | No | Yes | Yes | Yes | No | NR | NR |
| L-dopa responsive | Yes | Yes | Yes | Yes | Yes | NR | Uncertainf |
| History of antipsychotic use before PD onset | Yes | Yes | No | Yes | No | Yes | Yes |
| Family history of PD | No | No | No | No | No | NR | No |
In contrast to the general population estimate of 1 in 4000 (0.025%),9,10 we found 1 of 225 individuals (0.4%) in the early-onset PD series to have a 22q11.2 deletion. Closer examination of records revealed that this individual was our case 3. Long before this study of PD in 22q11.2DS began, he was recruited into the early-onset PD cohort following a clinical referral to the Movement Disorders Centre by one of us (E.W.C.C.). His 22q11.2 deletion was known at the time but not considered to have contributed to his PD when he entered the PD cohort.
Neuropathology
Neuropathological examination showed similar neurodegenerative pathology in cases 1 and 2. Classic PD synucleinopathy, including the subcortical and cortical presence of α-synuclein–positive Lewy bodies and Lewy neurites, corresponding to Braak stages V and VI,14 respectively, was observed (Table 2). There was extensive degeneration of TH-positive cells in the substantia nigra and depletion of TH in the striatum (Figure 1). Neuronal loss was also visible in the locus ceruleus and the dorsal motor nucleus of the 10th cranial nerve (Table 2).
Table 2.
Staging of Pathological Features in 3 Autopsy Cases With Parkinson Disease and 22q11.2 Deletion Syndrome
| Brain Region | Severity of Pathological Featuresa | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Case 2 | Case 3 | |||||||
| Lewy Bodies | Lewy Neurites | Neuronal Loss | Lewy Bodies | Lewy Neurites | Neuronal Loss | Lewy Bodies | Lewy Neurites | Neuronal Loss | |
| Olfactory bulbs and tracts | 1 | 1 | NA | 1 | 1 | NA | 0 | 0 | NA |
| Dorsal motor nucleus of the vagus | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 2 |
| Locus ceruleus | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 |
| Substantia nigra | 1 | 1 | 2 | 1 | 1 | 2 | 0 | 0 | 2 |
| Thalamus | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 |
| Nucleus basalis of Meynert | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 |
| Anterior cingulate cortex | 1 | 1 | 0–1 | 1 | 1 | 0–1 | 0 | 0 | 0–1 |
| Temporal insular cortex | 1 | 1 | 0–1 | 1 | 1 | 0–1 | 0 | 0 | 0–1 |
| Allocortex | 1 | 1 | 0–1 | 1 | 1 | 0–1 | 0 | 0 | 0–1 |
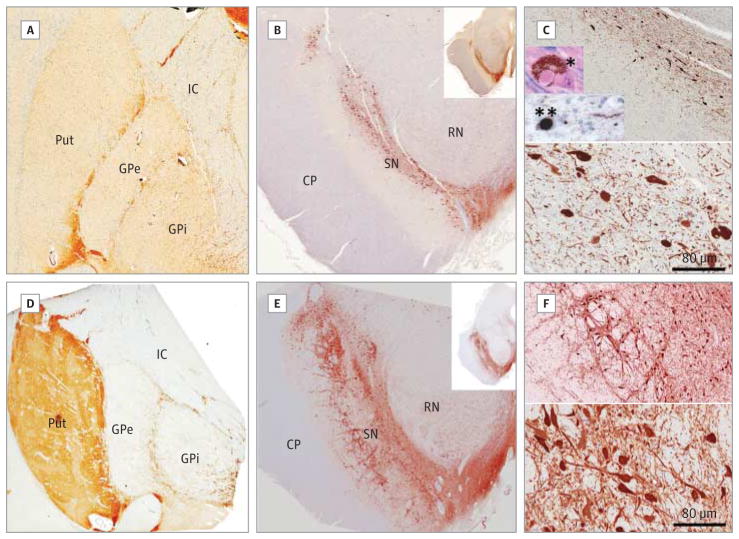
Figure 1. Cases 1 and 2 With Hemizygous 22q11.2 Deletions and Diagnosed Parkinson Disease Show Loss of Dopamine Cells and α-Synuclein Pathology.
Results of immunohistochemical studies for tyrosine hydroxylase in the striatum (A and D) and substantia nigra (B, C, E, and F) are shown from a representative case of 22q11.2 deletion syndrome with Parkinson disease (case 1; A–C) and a case of 22q11.2 deletion syndrome without Parkinson disease (D–F). C and F, Density of tyrosine hydroxylase–positive neurons in the substantia nigra at low power (upper panels) and high power (lower panels). Insets in C, Example of a Lewy body visualized with hematoxylin-eosin staining (*) and an α-synuclein–positive Lewy body and neurites (**) in the substantia nigra pars compacta. CP indicates cerebral peduncle; GPe, external segment of globus pallidus; GPi, internal segment of globus pallidus; IC, internal capsule; Put, putamen; RN, red nucleus; and SN, substantia nigra (original magnification ×1 in A, B, D, E, and insets in B and E; ×10 in upper panels of C and F; ×25 in lower panels of C and F; and ×40 in insets in C).
Case 3 showed no Lewy body or Lewy neurite pathology but, like the first 2 cases, showed extensive nigral degeneration and loss of TH immunoreactivity in the striatum. Investigations for abnormal neuronal inclusions or aggregates, including immunohistochemisty for α-synuclein, tau, TAR DNA-binding protein 43, and ubiquitin, were all negative. Neuronal loss was observed in the expected pattern, with prominent cell loss in the ventrolateral aspect of the substantia nigra pars compacta with extensive gliosis and free pigments (Figure 2A–D). There was neuronal loss in the locus ceruleus and dorsal motor nucleus of the 10th cranial nerve as well as some gliosis reminiscent of neuronal loss in the following structures: pedunculopontine nucleus, amygdala, and thalamus, all areas usually described to be affected in PD (Figure 2E and F, Table 2). Like cases 1 and 2, TH staining revealed extensive degeneration of TH-positive cells in the substantia nigra pars compacta and depletion of TH in the motor territory of the striatum (dorsolateral putamen) (Figure 2G–I).
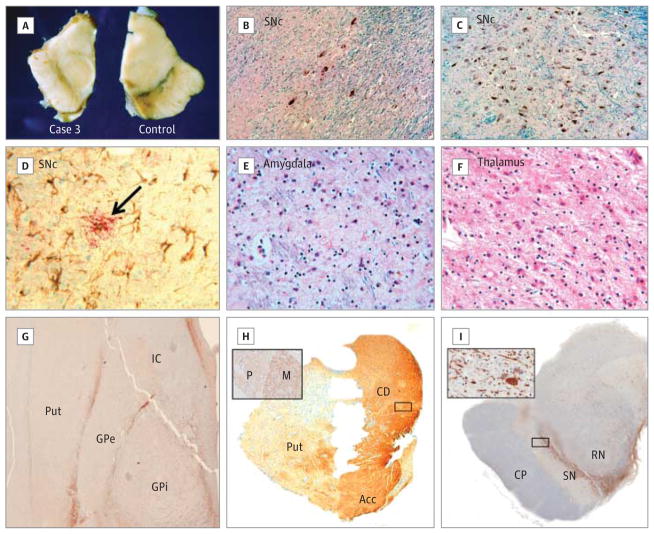
Figure 2. Details of Pathological Changes in Case 3.
A, Case 3 showed a smaller midbrain with gross depigmentation of the substantia nigra (left) compared with a control case without either 22q11.2 deletion syndrome or parkinsonism (right). Hematoxylin-eosin–Luxol fast blue staining of the substantia nigra pars compacta (SNc) revealed decreased density of pigmented cells in case 3 (B) compared with a control (C). D, Extensive gliosis and microglial activation in the substantia nigra as visualized with glial fibrillary acidic protein (brown chromogen) and Iba-1 (red chromogen, arrow), respectively. The amygdala (E) and thalamus (F) also showed gliosis and neuronal loss (hematoxylin-eosin–Luxol fast blue). Severe depletion of tyrosine hydroxylase–positive axons was visible in the dorsal putamen (Put) (G) and the dorsolateral aspect of the rostral putamen (H). Acc indicates accumbens; CD, caudate; GPe, external segment of globus pallidus; GPi, internal segment of globus pallidus; and IC, internal capsule. Tyrosine hydroxylase immunoreactivity was preserved in the CD and Acc (H) and was more abundant in the matrix (M) compared with the patches (P) (inset in H). I, Tyrosine hydroxylase–positive neurons were severely depleted in the ventrolateral aspect of the substantia nigra (SN) (inset). CP indicates cerebral peduncle; RN, red nucleus (original magnification ×25 in B–F; ×1 in G–I; and ×25 in insets in H and I).
There were no neuropathological features of PD, including midbrain dopaminergic cell loss or Lewy body pathology, in individuals with 22q11.2DS who had no history of parkinsonism (Figure 1D–F) or in the matched controls (eg, Figure 2A and C). Alzheimer-type pathology was limited to β-amyloid–positive diffuse plaques that were rare and restricted to the cortex in case 2 but more widespread and with moderate neuritic plaques and cortical neurofibrillary tangles in case 1 (eFigure in Supplement). There was no evidence of AD-type pathology in any other 22q11.2DS case or in any of the matched controls (data not shown). All cases and controls were immunonegative for TAR DNA-binding protein 43 aggregates.
Discussion
The results of this study provide evidence of a significantly increased occurrence of PD with early-onset and typical symptom pattern, treatment response, and disease course in adults with 22q11.2 deletions. Neuropathological data confirmed the antemortem clinical diagnoses, providing, for the first time to our knowledge, details of the neurodegenerative pathology of PD associated with this genetic anomaly. Together with previous reports of unrelated patients6–8 (Table 1), the findings suggest the 22q11.2 deletion as a mutation increasing risk for early-onset PD. The development of early-onset PD in this population is likely related to the effects of the hemizygous 22q11.2 deletion and is likely to involve 1 or more dosage-sensitive genes in the 22q11.2 region.
Two of our 3 pathologically confirmed 22q11.2DS cases had typical neuropathological features of PD with prominent Lewy body and Lewy neurite formation in a classic distribution. Our third case had extensive neuronal loss in the substantia nigra pars compacta along with neuronal loss in the locus ceruleus and the dorsal motor nucleus of the 10th cranial nerve. Gliosis was also observed in the midline nuclei and the anterior and posterior group of the intralaminar nuclei but in the absence of α-synuclein pathology. This variable presence of α-synuclein aggregation is identical to the situation found in PD associated with LRRK2 mutations, the most common known genetic cause of PD. Lewy body pathology is most frequent in LRRK2; however, some cases, even in the same family as Lewy body cases, have a “bland” nigral degeneration as seen in our case 3, while others have tau-related pathology.3,25 _PARK2_-associated PD typically lacks α-synuclein pathology, but some cases with Lewy bodies have been reported (although these may represent examples of incidental Lewy body disease coincident with PARK2 mutations).3,26
Candidate Genetic Pathways in 22q11.2DS-Associated PD
The proximal 22q11.2 deletion region shared by the individuals with PD involves approximately 30 genes, none of which overlap any known PD loci (OMIM #168600). However, this region does contain plausible candidate genes implicated in PD-related pathways. These include microRNA miR-185, predicted to target LRRK2,27 and DGCR8, a key gene in the biogenesis of brain microRNA.28 Disruption of microRNA-mediated posttranscriptional regulation of gene expression in 22q11.2DS could directly or indirectly affect the expression of PD risk genes elsewhere in the genome (without the necessity of associated mutations).29 Other possible candidate genes in this 22q11.2 deletion region include SEPT5, encoding a protein that functionally interacts with the product of PARK2,30 COMT, essential to dopamine level regulation, and 6 mitochondrial genes.31 Of these genes, all have shown brain expression and all examined have shown gene dosage effects in mouse models of the 22q11.2 deletion.31–34 However, to our knowledge, there are no studies of these models at older ages that could help inform potential changes relevant to PD.
Advantages and Limitations
Our ascertainment strategies ensured that sample selection was unbiased with respect to recruiting adults with 22q11.2 deletions who had PD, minimizing the risk of artificially inflating the observed frequency of their co-occurrence. Importantly, we had access to exceedingly rare postmortem tissue from adults with 22q11.2 deletions who had been extensively genetically and phenotypically characterized. The pathological signs of PD found in the 22q11.2DS cases with and without schizophrenia strongly suggest that the parkinsonian clinical features were not due to adverse effects from antipsychotics used to manage schizophrenia. A recent epidemiological study has proposed that antipsychotic use may be associated with risk of developing PD.35 However, neither the extensive nigrostriatal dopaminergic cell loss nor Lewy body pathology typical of PD has been observed in neuropathogical studies of antipsychotic medication effects36 or of individuals with schizophrenia treated with antipsychotics.37 A recent meta-analysis of neuroimaging studies examining striatal presynaptic dopamine in patients with schizophrenia found no evidence of altered density of dopamine terminals and no significant effect of antipsychotic medication.38 The absence of dopaminergic cell loss in our 3 cases with 22q11.2DS and treated schizophrenia without PD corresponds with these findings. However, further studies on the possible effect of antipsychotic drugs on dopamine cell degeneration are needed.
There were some limitations of our study. The occurrence of PD in the cohort with 22q11.2DS is a minimum figure. Most individuals in our cohort were younger than 50 years and there may be as yet undiagnosed adults with 22q11.2DS and PD, including those in the early stages of the disease. For example, we know of 2 other individuals (aged 36 and 54 years) who were noted as exhibiting parkinsonian symptoms possibly indicative of early-onset PD but had not received a definitive PD diagnosis. Additional studies including functional imaging of the nigrostriatal dopamine system may help to further clarify this. Small numbers, likely related to the early mortality associated with the 22q11.2 deletion,17 prevented assessment of the occurrence of PD over age 65 years in patients with 22q11.2DS. Individuals with schizophrenia and congenital heart defects were overrepresented in our 22q11.2DS sample, given our ascertainment strategies. This could affect the generalizability of the findings to adults with milder manifestations of 22q11.2DS, many of whom remain unrecognized.10
Replication in an independent sample of adults with 22q11.2 deletions would be desirable. However, to our knowledge, our cohort remains the largest available in this age range with extensive phenotypic data of all participants.10 Although we ruled out known PD mutations elsewhere in the genome and family history of PD, other very rare or as yet unknown PD risk variants are possible, as for any individual with PD. Our relatively small early-onset PD clinical series precluded a formal analysis of the occurrence of 22q11.2 deletions. The series was also biased toward individuals with a positive family history. We note, however, that if the sample was restricted to patients with no family history of PD, the occurrence of 22q11.2DS would have been 1 in 119. Evaluation of larger cohorts of individuals with early-onset PD will be needed to determine the relative contribution of 22q11.2 deletions to this disease.4,5 The few published studies of genome-wide copy number variation in PD have not reported any 22q11.2 deletions.39–42 However, these have used ascertainment strategies that would bias against inclusion of individuals with 22q11.2 deletions, eg, restricting to patients with a positive family history of PD,42 restricting to older age and/or later onset of PD,40 and/or excluding individuals with a history of antipsychotic exposure.39,41 In contrast, most 22q11.2 deletions are new mutations; therefore, family history of 22q11.2DS, let alone 22q11.2DS with PD, is unlikely, there is premature mortality in patients with 22q11.2DS, and 1 in 4 individuals with a 22q11.2 deletion develops schizophrenia requiring antipsychotic treatment.10
Clinical Implications
The results of this study may help inform best practices for individuals with early-onset PD and for individuals with 22q11.2DS. Patients with PD who have features associated with a 22q11.2 deletion, such as learning difficulties, congenital palatal, cardiac, or other birth defects, and/or schizophrenia,10 should be considered for clinical genetic referral and/or testing. Management changes for PD with a diagnosis of 22q11.2DS, such as anticipatory care recommendations and attention to associated multisystem conditions, would be essential.10 For example, hypocalcemia, a common treatable feature of 22q11.2DS that can induce or aggravate tremors, should be monitored carefully, especially when considering dosage changes in dopaminergic replacement therapy.10 Neurological assessment for signs of parkinsonism in younger adults with 22q11.2DS should become part of standard practice; when these occur in patients taking antipsychotic medications for schizophrenia, consideration should be given to functional imaging (single-photon emission computed tomography or positron emission tomography) of the presynaptic nigrostriatal dopamine system8,43 where available.
Importantly, the results of our study indicate that although individuals with PD and the 22q11.2 deletion associated with 22q11.2DS present with classic motor symptoms, diagnosis of PD may be delayed or obscured by antipsychotic treatment for schizophrenia. Individuals with 22q11.2DS without psychosis were diagnosed within a year of motor symptoms onset, compared with 6 to 10 years in individuals with schizophrenia (Table 1). Identifying PD in adults with 22q11.2 deletions and schizophrenia presents a unique challenge in clinical practice. As indicated, future studies will need to include careful evaluation and monitoring of signs of PD, perhaps aided by functional neuroimaging.8,43 Management in these patients will entail careful balancing of antiparkinsonian medication with antipsychotic treatments. Based on studies of the management of psychosis in PD, one would expect good tolerance to quetiapine fumarate and clozapine but better efficacy with clozapine.44 Other atypical antipsychotics are generally much less tolerated by patients with PD, and the development of parkinsonism in individuals with schizophrenia and a 22q11.2 deletion receiving previously well-tolerated doses of these medications might be an important indicator of underlying progressive PD. We cannot rule out the possibility that antipsychotic use may have prompted earlier detection of PD in our 3 cases with a long history of antipsychotic use. However, we note the similar age at onset of parkinsonian motor symptoms with individuals with 22q11.2DS who had no history of antipsychotic use. The possible effects of antipsychotic medications on either accelerating or unmasking clinical PD merit further study.
Conclusions
Hemizygous 22q11.2 deletions may constitute a new genetic risk factor for early-onset PD with important clinical implications. Additional studies are needed to replicate these results and further establish the prevalence of 22q11.2 deletions in adults with early-onset PD, especially for those with no family history of PD. Further postmortem studies in similar patients are critical to advancing our knowledge of this disorder. Our initial findings suggest that, as with LRRK2 mutations, the pathogenesis of PD associated with 22q11.2 deletions may or may not be accompanied by α-synuclein aggregates in Lewy bodies and Lewy neurites. Understanding how these genetic disorders are capable of causing nigral degeneration both with and without α-synuclein aggregation will be critical to advances in understanding of sporadic PD and developing effective disease-modifying therapies. Although 22q11.2DS is underrecognized in adults and there is a degree of premature mortality associated with the condition,17 these patients could represent an identifiable, high-risk group for future studies of PD. Studies of 22q11.2 deletions and other genetic forms of PD promise to add to the global understanding of the genetic architecture of this common neurodegenerative disease.
Supplementary Material
Supplemental content
Acknowledgments
Funding/Support: This work was supported by the Canadian Institutes of Health Research (Butcher and Bassett), the Canada Research Chairs Program (Bassett), the Ontario Research Fund (Rogaeva), and the National Parkinson Foundation (Lang).
Footnotes
Conflict of Interest Disclosures: None reported.
Role of the Sponsors: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Author Contributions: Butcher and Kiehl contributed equally to this work. Bassett had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Butcher, Kiehl, Bassett.
Acquisition of data: All authors.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: Butcher, Bassett.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Butcher, Chow.
Obtained funding: Butcher, Rogaeva, Lang, Bassett.
Administrative, technical, or material support: Butcher, Kiehl, Hazrati, Rogaeva.
Study supervision: Bassett.
Additional Contributions: We thank the patients’ families who made this study possible, the pathologists who performed the general autopsies, and our colleagues for referring patients.
References
- 1.Lai BC, Schulzer M, Marion S, Teschke K, Tsui JK. The prevalence of Parkinson’s disease in British Columbia, Canada, estimated by using drug tracer methodology. Parkinsonism Relat Disord. 2003;9(4):233–238. doi: 10.1016/s1353-8020(02)00093-7. [DOI] [PubMed] [Google Scholar]
- 2.Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol. 2011;26(suppl 1):S1–S58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- 3.Houlden H, Singleton AB. The genetics and neuropathology of Parkinson’s disease. Acta Neuropathol. 2012;124(3):325–338. doi: 10.1007/s00401-012-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macedo MG, Verbaan D, Fang Y, et al. Genotypic and phenotypic characteristics of Dutch patients with early onset Parkinson’s disease. Mov Disord. 2009;24(2):196–203. doi: 10.1002/mds.22287. [DOI] [PubMed] [Google Scholar]
- 5.Alcalay RN, Caccappolo E, Mejia-Santana H, et al. Frequency of known mutations in early-onset Parkinson disease: implication for genetic counseling: the consortium on risk for early onset Parkinson disease study. Arch Neurol. 2010;67(9):1116–1122. doi: 10.1001/archneurol.2010.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krahn LE, Maraganore DM, Michels VV. Childhood-onset schizophrenia associated with parkinsonism in a patient with a microdeletion of chromosome 22. Mayo Clin Proc. 1998;73(10):956–959. doi: 10.4065/73.10.956. [DOI] [PubMed] [Google Scholar]
- 7.Zaleski C, Bassett AS, Tam K, Shugar AL, Chow EW, McPherson E. The co-occurrence of early onset Parkinson disease and 22q11.2 deletion syndrome. Am J Med Genet A. 2009;149A(3):525–528. doi: 10.1002/ajmg.a.32650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Booij J, van Amelsvoort T, Boot E. Co-occurrence of early-onset Parkinson disease and 22q11.2 deletion syndrome: potential role for dopamine transporter imaging. Am J Med Genet A. 2010;152A(11):2937–2938. doi: 10.1002/ajmg.a.33665. [DOI] [PubMed] [Google Scholar]
- 9.Goodship J, Cross I, LiLing J, Wren C. A population study of chromosome 22q11 deletions in infancy. Arch Dis Child. 1998;79(4):348–351. doi: 10.1136/adc.79.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassett AS, McDonald-McGinn DM, Devriendt K, et al. International 22q11.2 Deletion Syndrome Consortium. Practical guidelines for managing patients with 22q11.2 deletion syndrome. J Pediatr. 2011;159(2):332–339. e1. doi: 10.1016/j.jpeds.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassett AS, Marshall CR, Lionel AC, Chow EW, Scherer SW. Copy number variations and risk for schizophrenia in 22q11.2 deletion syndrome. Hum Mol Genet. 2008;17(24):4045–4053. doi: 10.1093/hmg/ddn307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bassett AS, Chow EW, Husted J, et al. Clinical features of 78 adults with 22q11 deletion syndrome. Am J Med Genet A. 2005;138(4):307–313. doi: 10.1002/ajmg.a.30984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fung WL, Chow EW, Webb GD, Gatzoulis MA, Bassett AS. Extracardiac features predicting 22q11.2 deletion syndrome in adult congenital heart disease. Int J Cardiol. 2008;131(1):51–58. doi: 10.1016/j.ijcard.2007.08.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 15.Milber JM, Noorigian JV, Morley JF, et al. Lewy pathology is not the first sign of degeneration in vulnerable neurons in Parkinson disease. Neurology. 2012;79(24):2307–2314. doi: 10.1212/WNL.0b013e318278fe32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bassett AS, Costain G, Fung WLA, et al. Clinically detectable copy number variations in a Canadian catchment population of schizophrenia. J Psychiatr Res. 2010;44(15):1005–1009. doi: 10.1016/j.jpsychires.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassett AS, Chow EW, Husted J, et al. Premature death in adults with 22q11.2 deletion syndrome. J Med Genet. 2009;46(5):324–330. doi: 10.1136/jmg.2008.063800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiehl TR, Chow EWC, Mikulis DJ, George SR, Bassett AS. Neuropathologic features in adults with 22q11.2 deletion syndrome. Cereb Cortex. 2009;19(1):153–164. doi: 10.1093/cercor/bhn066. [DOI] [PubMed] [Google Scholar]
- 20.Bonifati V, Rizzu P, van Baren MJ, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299(5604):256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 21.Hattori N, Kitada T, Matsumine H, et al. Molecular genetic analysis of a novel Parkin gene in Japanese families with autosomal recessive juvenile parkinsonism: evidence for variable homozygous deletions in the Parkin gene in affected individuals. Ann Neurol. 1998;44(6):935–941. doi: 10.1002/ana.410440612. [DOI] [PubMed] [Google Scholar]
- 22.Rogaeva E, Johnson J, Lang AE, et al. Analysis of the PINK1 gene in a large cohort of cases with Parkinson disease. Arch Neurol. 2004;61(12):1898–1904. doi: 10.1001/archneur.61.12.1898. [DOI] [PubMed] [Google Scholar]
- 23.Singleton AB, Farrer M, Johnson J, et al. Alpha-synuclein locus triplication causes Parkinson’s disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 24.Paisán-Ruíz C, Lang AE, Kawarai T, et al. LRRK2 gene in Parkinson disease: mutation analysis and case control association study. Neurology. 2005;65(5):696–700. doi: 10.1212/01.wnl.0000167552.79769.b3. [DOI] [PubMed] [Google Scholar]
- 25.Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44(4):601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Pramstaller PP, Schlossmacher MG, Jacques TS, et al. Lewy body Parkinson’s disease in a large pedigree with 77 Parkin mutation carriers. Ann Neurol. 2005;58(3):411–422. doi: 10.1002/ana.20587. [DOI] [PubMed] [Google Scholar]
- 27.Dweep H, Sticht C, Pandey P, Gretz N. miRWalk—database: prediction of possible miRNA binding sites by “walking” the genes of three genomes. J Biomed Inform. 2011;44(5):839–847. doi: 10.1016/j.jbi.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Stark KL, Xu B, Bagchi A, et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008;40(6):751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- 29.Brzustowicz LM, Bassett AS. miRNA-mediated risk for schizophrenia in 22q11.2 deletion syndrome. Front Genet. 2012;3:291. doi: 10.3389/fgene.2012.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Gao J, Chung KK, Huang H, Dawson VL, Dawson TM. Parkin functions as an E2-dependent ubiquitin-protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci U S A. 2000;97(24):13354–13359. doi: 10.1073/pnas.240347797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maynard TM, Meechan DW, Dudevoir ML, et al. Mitochondrial localization and function of a subset of 22q11 deletion syndrome candidate genes. Mol Cell Neurosci. 2008;39(3):439–451. doi: 10.1016/j.mcn.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maynard TM, Haskell GT, Peters AZ, Sikich L, Lieberman JA, LaMantia AS. A comprehensive analysis of 22q11 gene expression in the developing and adult brain. Proc Natl Acad Sci U S A. 2003;100(24):14433–14438. doi: 10.1073/pnas.2235651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meechan DW, Tucker ES, Maynard TM, LaMantia AS. Diminished dosage of 22q11 genes disrupts neurogenesis and cortical development in a mouse model of 22q11 deletion/DiGeorge syndrome. Proc Natl Acad Sci U S A. 2009;106(38):16434–16445. doi: 10.1073/pnas.0905696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Earls LR, Fricke RG, Yu J, Berry RB, Baldwin LT, Zakharenko SS. Age-dependent microRNA control of synaptic plasticity in 22q11 deletion syndrome and schizophrenia. J Neurosci. 2012;32(41):14132–14144. doi: 10.1523/JNEUROSCI.1312-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foubert-Samier A, Helmer C, Perez F, et al. Past exposure to neuroleptic drugs and risk of Parkinson disease in an elderly cohort. Neurology. 2012;79(15):1615–1621. doi: 10.1212/WNL.0b013e31826e25ce. [DOI] [PubMed] [Google Scholar]
- 36.Harrison PJ. The neuropathological effects of antipsychotic drugs. Schizophr Res. 1999;40(2):87–99. doi: 10.1016/s0920-9964(99)00065-1. [DOI] [PubMed] [Google Scholar]
- 37.Harrison PJ. The neuropathology of schizophrenia: a critical review of the data and their interpretation. Brain. 1999;122(pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 38.Fusar-Poli P, Meyer-Lindenberg A. Striatal presynaptic dopamine in schizophrenia, part I: meta-analysis of dopamine active transporter (DAT) density. Schizophr Bull. 2013;39(1):22–32. doi: 10.1093/schbul/sbr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim JS, Yoo JY, Lee KS, et al. Comparative genome hybridization array analysis for sporadic Parkinson’s disease. Int J Neurosci. 2008;118(9):1331–1345. doi: 10.1080/00207450802174522. [DOI] [PubMed] [Google Scholar]
- 40.Simon-Sanchez J, Scholz S, del Matarin MM, et al. Genomewide SNP assay reveals mutations underlying Parkinson disease. Hum Mutat. 2008;29(2):315–322. doi: 10.1002/humu.20626. [DOI] [PubMed] [Google Scholar]
- 41.Bademci G, Edwards TL, Torres AL, et al. A rare novel deletion of the tyrosine hydroxylase gene in Parkinson disease. Hum Mutat. 2010;31(10):1767–1771. doi: 10.1002/humu.21351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pankratz N, Dumitriu A, Hetrick KN, et al. PSG-PROGENI and GenePD Investigators, Coordinators, and Molecular Genetic Laboratories. Copy number variation in familial Parkinson disease. PLoS One. 2011;6(8):e20988. doi: 10.1371/journal.pone.0020988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cummings JL, Henchcliffe C, Schaier S, Simuni T, Waxman A, Kemp P. The role of dopaminergic imaging in patients with symptoms of dopaminergic system neurodegeneration. Brain. 2011;134(pt 11):3146–3166. doi: 10.1093/brain/awr177. [DOI] [PubMed] [Google Scholar]
- 44.Goldman JG, Vaughan CL, Goetz CG. An update expert opinion on management and research strategies in Parkinson’s disease psychosis. Expert Opin Pharmacother. 2011;12(13):2009–2024. doi: 10.1517/14656566.2011.587122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental content