Mechanisms Linking Obesity, Chronic Kidney Disease, and Fatty Liver Disease: The Roles of Fetuin-A, Adiponectin, and AMPK (original) (raw)
Abstract
Obesity is a risk factor for chronic kidney disease (CKD) and nonalcoholic fatty liver disease (NAFLD). Recent studies identify mechanisms common to both diseases linked through an interorgan communication orchestrated by fetuin-A and adiponectin. In liver and kidney, the energy sensor 5′-AMP activated protein kinase (AMPK) is pivotal to directing podocytes and hepatocytes to compensatory and potentially deleterious pathways, leading to inflammatory and profibrotic cascades culminating in end-organ damage. Regulation of these early upstream pathways may provide new therapeutic targets for these increasingly common sequelae of obesity.
The prevalence of obesity in the United States has increased dramatically from approximately 12% in 1991 to over 20% a decade later.1,2 Individuals older than 60 years of age have experienced the most rapid increase in prevalence3; an ominous trend because this age group experiences the greatest burden of chronic kidney disease (CKD), cardiovascular disease, and malignancy on the basis of their age alone, each of which may be exacerbated by obesity. Understanding the mechanisms linking obesity and CKD is important not only because of the societal health burden of both conditions but also because novel insights to underlying mechanisms may lead to new strategies to treat or prevent CKD and its associated comorbidities.
Obesity almost certainly indirectly contributes to CKD because obesity associates with many dominant CKD risk factors such as diabetes, hypertension, and atherosclerosis. However, obesity may also directly lead to CKD. Pathologic studies demonstrate that subjects with severe obesity develop proteinuria with pathologic findings of podocyte hypertrophy, mesangial expansion, glomerular enlargement, and focal segmental glomerular sclerosis in the absence of diabetes and hypertension.4,5 Epidemiologic studies also support a direct effect. Hsu and colleagues evaluated over 300,000 Kaiser Permanente healthcare members, among whom nearly 1500 developed ESRD over approximately 26 years.6,7 There was a graded increase in risk of ESRD for those who were overweight or obese despite adjustment for demographics, smoking, and cardiovascular disease. Even when accounting for blood pressure and diabetes at baseline, the association was only partially attenuated, and individuals with obesity remained at approximately 3-fold greater risk of ESRD. Those with extreme obesity are at even higher risk.6
The liver also frequently develops obesity-related complications. Nonalcoholic fatty liver disease (NAFLD) represents the most common hepatic disorder in western countries8 and is strongly linked with insulin resistance and obesity.9–11 Given these are common risk factors for CKD and NAFLD, it is not surprising that the two conditions are associated with one another.12,13 Intriguingly, mechanisms leading to both diseases may be interlinked through crosstalk between fat, the kidney, and liver through at least two serum proteins—fetuin-A and adiponectin. In response, both tissues exhibit similar local effects mediated through the energy sensor 5′-AMP activated protein kinase (AMPK). Here we review the current understanding of these pathways, highlighting areas that are common to obesity-related CKD and obesity-related NAFLD and that could serve as potential targets for intervention.
Fetuin-A Induces Insulin Resistance and Regulates Adiponectin
In the renal field, fetuin-A has principally been studied as an inhibitor of ectopic calcium deposition,14–16 yet fetuin-A is also an important promoter of insulin resistance. Different from adipocytokines, which are derived from fat cells, fetuin-A is a 64-kDa glycoprotein produced exclusively by the liver and secreted into serum17 where it is found in relatively high concentrations in humans.18 Fetuin-A binds and inhibits the insulin receptor tyrosine kinase in skeletal muscle and hepatocytes, inhibiting insulin signal transduction and resulting in insulin resistance in these target tissues.19–21 Consistent with these in vitro observations, the fetuin-A null mouse is insulin sensitive, has increased skeletal muscle glycogen content, and is resistant to weight gain when challenged with a high-fat diet.22,23 Conversely, treatment of wild-type mice with fetuin-A induces insulin resistance.24 In humans, higher fetuin-A levels associate with obesity and insulin resistance in the general population25–27 and in patients with CKD and ESRD28,29 and associates with future risk of diabetes.30,31 Higher fetuin-A levels also associate with NAFLD, and short-term diet and exercise interventions result in declines in serum fetuin-A levels commensurate with improvement in NAFLD and decline in body weight.27,32 However, as fetuin-A is a liver-secreted protein and also induces insulin resistance, it is uncertain whether fetuin-A directly contributes to development of NAFLD, whether elevated serum levels reflect the presence or severity of NAFLD, or whether other unidentified factors simultaneously influence both.
Fetuin-A and adiponectin may work in concert to regulate insulin resistance. Genes for both proteins are located at 3q27 in the human genome; a diabetes and metabolic syndrome susceptibility locus.33,34 Serum levels of both proteins are consistently associated with key components of the metabolic syndrome, but in opposite directions. For example, higher fetuin-A levels associate with greater body mass index and hypertriglyceridemia,25 whereas lower adiponectin levels associate with the same outcomes.35,36 Treatment with pioglitazone results in a decline in fetuin-A levels37 and an increase in adiponectin levels.38,39 In a cohort of 963 individuals, we found that serum fetuin-A and adiponectin levels were inversely correlated with one another (Figure 1), thus confirming similar findings in a smaller sample by others.24 However, although these correlations are intriguing, evidence for overlapping biology remained uncertain until recently when Hennige and colleagues24 demonstrated that fetuin-A suppresses mRNA encoding adiponectin in cultured human adipocytes, and treatment of wild-type mice with fetuin-A lowered serum adiponectin levels. The effect of fetuin-A on adiponectin is specific because fetuin-A treatment did not affect levels of mRNA encoding leptin and resistin or serum levels. Collectively, these studies suggest the liver-secreted protein fetuin-A inhibits generation of adiponectin in adipose tissue. Higher fetuin-A and lower adiponectin may contribute to obesity-induced insulin resistance and development of diabetes. In turn, adiponectin is a key regulator of end-organ damage in obesity-related CKD and NAFLD (as described in the following section).
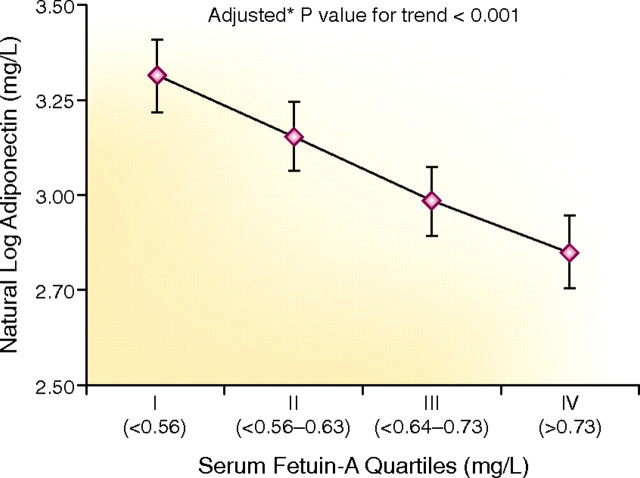
Figure 1.
There is an inverse correlation between serum fetuin-A levels and adiponectin in patients with stable cardiovascular disease. The association was adjusted for age, sex, race, body mass index, and estimated GFR among a population of 963 outpatients (n = 242 or 243 per quartile). Mean estimated GFR = 71 ml/min/1.73 m2 (29% with estimated GFR < 60, none with ESRD). The unadjusted Spearmen correlation (r = −0.27; P < 0.001). Error bars reflect 95% confidence intervals.
Adiponectin Mediates Crosstalk between Adipose, Kidney, and Liver
Adiponectin is a 30-kDa protein secreted from adipose tissue and circulates in multimers ranging from trimers to 12- to 18-mers.40 Adiponectin improves insulin sensitivity and decreases the adverse effects of inflammatory mediators in vascular cells, and the high-molecular-weight multimers may be more potent.40,41 Despite its source from adipose tissue, individuals with obesity consistently have lower serum adiponectin levels.35,36,42 The mechanisms for this paradox are uncertain but may reflect inhibition of gene expression and secretion.43 Adiponectin null mice have increased susceptibility to insulin resistance with high-fat feeding,44 and treatment with adiponectin conversely improves insulin sensitivity.
The best-characterized receptors for adiponectin are the AdipoR1 and AdipoR2 receptors; the former is ubiquitously expressed, whereas the latter is found primarily in hepatocytes.45 Both contain seven transmembrane domains but are structurally and functionally distinct from G-protein-coupled receptors. Unlike G-protein-coupled receptors, the amino (N)-termini of both receptors are intracellular and the C-terminal end is extracellular and binds adiponectin.46 Although the intracellular signaling cascade is not known,47 the function of these receptors was recently elucidated through genetic manipulations in mice. Overexpression of both receptors in liver of db/db mice improves insulin sensitivity.46 AdipoR1 overexpression decreases hepatic enzymes involved in gluconeogenesis,46 whereas AdipoR2 overexpression increases glucose uptake by stimulating glucokinase and peroxisome proliferator-activated receptor-α (PPARα). Genes downstream of PPARα such as acyl-CoA oxidase 1 and uncoupling protein 2 are also stimulated by AdipoR2 overexpression.46 Studies of specific deletions in AdipoR1 or AdipoR2 demonstrate that AdipoR1 predominantly mediates stimulation of AMPK, whereas AdipoR2 mediates stimulation of PPARα.
The potential link between adiponectin and albuminuria was initially raised in a clinical study of men with essential hypertension in which serum adiponectin and albuminuria levels were inversely correlated.48 Because adiponectin levels are secreted from adipocytes and related inversely to the amount of adiposity, these data identify adiponectin as a candidate mediator of adipose and kidney crosstalk. Others observed similar inverse correlations in cross-sectional studies49,50; however, the association between adiponectin levels and kidney disease is complex. There is a direct correlation between adiponectin levels and overt proteinuria,51–53 and studies report conflicting data between adiponectin levels and mortality in patients with CKD or coronary artery disease.36,54–56 Nonetheless, the inverse correlation between adiponectin and low-grade albuminuria prompted us to investigate whether a relative adiponectin deficiency has a causative role in abnormal glomerular function.49
The C57BL/6 adiponectin null mouse exhibits albuminuria, and pathologic evaluation demonstrates foot process effacement of podocytes under baseline conditions.49 Podocytes express the AdipoR1 receptor, and treatment with adiponectin normalizes albuminuria and restores foot process architecture. We hypothesize the renal pathology in these animals may result from oxidative stress because they also have higher urine hydrogen peroxide levels than control mice. When the null mice are treated with exogenous adiponectin, albuminuria decreases to levels similar to wild-type controls. This effect is mediated through adiponectin stimulation of the AMPK pathway, a key regulator of intracellular energy status with potent antiproliferative effects. AMPK suppression of an isoform of NADPH oxidase (Nox4) may account for the improvement in podocyte cytostructure in adiponectin-treated animals. Additional support for the renal protective effects of adiponectin are provided by a recent study using the 5/6 nephrectomy model,57 in which adiponectin also inhibited albuminuria and fibrosis. Collectively, these data suggest adiponectin protects against albuminuria through an AdipoR1 receptor pathway by stimulating AMPK and inhibiting reactive oxygen species. Whether additional renal effects are mediated through AdipoR2 is currently unknown.
NAFLD strongly associates with insulin resistance and obesity9–11 and represents a spectrum of liver pathology ranging from hepatic steatosis to inflammation and fibrosis characteristic of nonalcoholic steatohepatitis and cirrhosis. Although hepatic steatosis may be benign, factors triggering fibrosis and steatohepatitis may also be the consequence of reactive oxygen species by driving peroxidation of hepatic lipids and inducing mitochondrial damage.58–60 Similar to its effect on podocytes, recent studies suggest that adiponectin inhibits this critical transition.
Individuals with NAFLD have lower serum adiponectin levels than healthy subjects,10,61,62 and among individuals with NAFLD adiponectin levels are inversely correlated with the severity of hepatic fibrosis and inflammation.61,63,64 Adachi and colleagues65 evaluated the influence of adiponectin on hepatic stellate cells, a key cell-type promoting liver fibrosis. Adiponectin treatment suppressed the proliferation of hepatic stellate cells in a dose-dependent fashion in vitro. As with podocytes, this effect is mediated through activation of AMPK. Inhibiting AMPK restores proliferation and results in downregulation of the antioxidant enzymes superoxide dismutase 2 and catalase. In independent studies, NAFLD also associates with an increase in NADPH oxidase activity,66 although the specific subtype, Nox2, is not critical to mediating onset of disease.39 To our knowledge, Nox4 has not been evaluated in NAFLD; however, it is stimulated by transforming growth factor-β in hepatocytes,67 thus Nox4 may contribute to the transition from steatohepatitis to fibrosis and inflammation. Therefore, current evidence suggests the fat-derived hormone adiponectin inhibits the transition from hepatic steatosis to fibrosis through an AMPK-dependent pathway, accompanied by suppression of reactive oxygen species, similar to the effect of adiponectin on podocytes.
Inhibition of AMPK Triggers the Onset of Obesity-Related End-Organ Disease
From a teleological prospective, why excess fat would lead to albuminuria and NAFLD is unclear. One explanation may be provided through AMPK. This protein is a serine/threonine kinase that plays a critical role in sensing energy availability at the cellular level. Upon exposure to low glucose or decreased energy stores, AMPK inhibits mRNA translation and protein synthesis of pathways that are nonessential in the short term. In turn, during times when food is plentiful, AMPK activity is inhibited, mRNA translation is up-regulated, and the cells and organism can grow in size. Because most animals do not have continuous access to calories, this function may be critical to evolutionary success. However, what would be the response in the modern situation wherein there is a constant and abundant access to calories? This scenario might result in chronic deactivation of AMPK and promote cellular protein synthesis. The pathways involved in mRNA translation and protein synthesis leading to kidney disease have recently been elegantly reviewed.68,69
Insights into the role of AMPK on kidney function are in their infancy. However, recent studies suggest AMPK suppression leads to cellular hypertrophy, accumulation of matrix molecules, and mesangial expansion that are hallmarks of obesity-related CKD. Activated AMPK is predominant in podocytes under basal conditions in wild-type mice. With adiponectin depletion, AMPK is inactivated in podocytes and associated with foot process effacement.49 Using conditionally differentiated podocytes, inhibition of AMPK dramatically alters podocyte morphology.49 Activation of AMPK with its analogue aminoimidazole carboxamide ribonucleotide restores podocyte morphology in vitro and normalizes albuminuria in vivo in the adiponectin null mouse.
In contrast to its emerging role in kidney disease, the role of AMPK in NAFLD is better studied. AMPK activation plays a major role in mediating the effects of adiponectin in blocking accumulation of liver fat.70,71 Rats fed high sucrose develop NAFLD in association with reduced AMPK.72 Activation of AMPK in the liver leads to fatty acid oxidation, inhibition of glucose production, and inhibition of lipogenesis and protein synthesis. Mice with genetically engineered chronic liver disease and AMPK activation are resistant to weight gain and accumulation of liver fat when fed high-fat diets.73
Intriguingly, therapeutic maneuvers with potential beneficial effects on obesity, the kidney, and liver are related to decreasing fetuin-A levels, increasing adiponectin, and AMPK stimulation. Caloric restriction,74,75 exercise,75 and insulin-sensitizing medications such as pioglitazone76,77 are each associated with declines in levels of serum fetuin-A, increases in adiponectin levels, and stimulation of AMPK. Angiotensin II infusion lowers adiponectin levels, and angiotensin converting enzyme inhibitors and angiotensin receptor blockers raise adiponectin levels,78,79 perhaps by affecting visceral adipose tissue. The sirtuin activator resveratrol also improves organ function of the heart, kidney, and liver80–82 despite high-fat feeding, which may in part be due to stimulation of AMPK.82 Future studies should evaluate whether direct administration of adiponectin or novel agents such as the sirtuin activators have therapeutic potential in patients with obesity and evidence of kidney and liver disease.
In conclusion, excessive caloric intake contributes to adiposity and initiates a cascade that ultimately leads to end-organ dysfunction including obesity-related CKD and NAFLD. Recent studies demonstrate that fetuin-A and adiponectin are key proteins orchestrating organ crosstalk between liver and fat cells and between fat cells and the kidney and liver, respectively. Adiponectin influences changes in end-organ targets, at least in part through AMPK in early stages of disease. These discoveries demonstrate that obesity-related CKD and NAFLD share several similar biologic mechanisms (Figure 2); however, the understanding of these overlapping pathways are presently incomplete. Additional studies elucidating regulatory mechanisms of fetuin-A, adiponectin, and AMPK are required. It is likely that leptin,83–86 resistin,87 free fatty acids,88,89 glucose,90 endothelial dysfunction,91,92 and other factors90 also play important roles in the development of both diseases. Although it may prove challenging to understand this complex biology, analyses from multiorgan integrative studies will provide the insights needed to counter the increasingly prevalent and devastating effects of obesity.
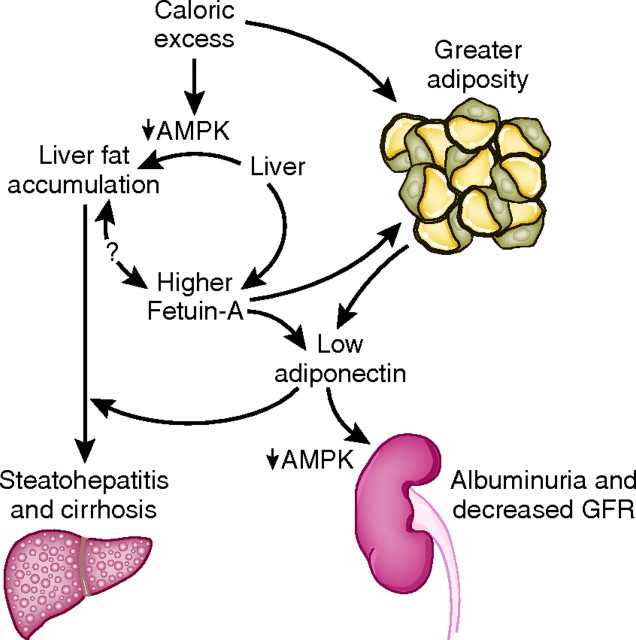
Figure 2.
With caloric excess, there is fatty acid excess and insulin resistance fueling hepatic triacylglycerol synthesis and steatosis. Caloric excess and/or a fatty liver may lead to greater serum fetuin-A levels. Higher fetuin-A levels lead to suppression of adiponectin transcription in adipocytes through direct mechanisms and potentially indirectly through an expansion of adipose tissue. Excess caloric intake and lower adiponectin reduces AMPK activation, promoting hepatic stellate cell proliferation and generation of reactive oxygen species in the liver, leading to conversion from hepatic steatosis to steatohepatitis and ultimately cirrhosis. Through similar pathways, lower adiponectin levels reduce AMPK in podocytes to promote podocyte foot process effacement and albuminuria.
Disclosures
The authors thank Dr. Mary Whooley and the Heart and Soul Study for providing the clinical adiponectin data for this manuscript. These studies were conducted with grants from the American Diabetes Association (1-08-IG-01), American Heart Association (0575021N), and the National Institutes of Health (R01 HL096851) to Dr. Ix and grants from the National Institutes of Health (R01 DK 053867 and U01 DK 060995) to Dr. Sharma.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS: Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289: 76–79, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP: The spread of the obesity epidemic in the United States, 1991–1998. JAMA 282: 1519–1522, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Ogden CL, Johnson CL: Prevalence and trends in obesity among US adults, 1999–2000. JAMA 288: 1723–1727, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Serra A, Romero R, Lopez D, Navarro M, Esteve A, Perez N, Alastrue A, Ariza A: Renal injury in the extremely obese patients with normal renal function. Kidney Int 73: 947–955, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Kambham N, Markowitz G, Valeri A, Lin J, D'Agati V: Obesity-related glomerulopathy: An emerging epidemic. Kidney Int 59: 1498–1509, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS: Body mass index and risk for end-stage renal disease. Ann Intern Med 144: 21–28, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS: Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med 169: 342–350, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chitturi S, Farrell GC, Hashimoto E, Saibara T, Lau GK, Sollano JD: Non-alcoholic fatty liver disease in the Asia-Pacific region: Definitions and overview of proposed guidelines. J Gastroenterol Hepatol 22: 778–787, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Knobler H, Schattner A, Zhornicki T, Malnick SD, Keter D, Sokolovskaya N, Lurie Y, Bass DD: Fatty liver—An additional and treatable feature of the insulin resistance syndrome. QJM 92: 73–79, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Bugianesi E, Pagotto U, Manini R, Vanni E, Gastaldelli A, de Iasio R, Gentilcore E, Natale S, Cassader M, Rizzetto M, Pasquali R, Marchesini G: Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity. J Clin Endocrinol Metab 90: 3498–3504, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Machado M, Cortez-Pinto H: Non-alcoholic fatty liver disease and insulin resistance. Eur J Gastroenterol Hepatol 17: 823–826, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Chang Y, Ryu S, Sung E, Woo HY, Oh E, Cha K, Jung E, Kim WS: Nonalcoholic fatty liver disease predicts chronic kidney disease in nonhypertensive and nondiabetic Korean men. Metabolism 57: 569–576, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Targher G, Chonchol M, Bertolini L, Rodella S, Zenari L, Lippi G, Franchini M, Zoppini G, Muggeo M: Increased risk of CKD among type 2 diabetics with nonalcoholic fatty liver disease. J Am Soc Nephrol 19: 1564–1570, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermans MM, Brandenburg V, Ketteler M, Kooman JP, van der Sande FM, Boeschoten EW, Leunissen KM, Krediet RT, Dekker FW: Association of serum fetuin-A levels with mortality in dialysis patients. Kidney Int 72: 202–207, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Ketteler M, Giachelli C: Novel insights into vascular calcification. Kidney Int Suppl: 105: S5–S9, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Wang AY, Woo J, Lam CW, Wang M, Chan IH, Gao P, Lui SF, Li PK, Sanderson JE: Associations of serum fetuin-A with malnutrition, inflammation, atherosclerosis and valvular calcification syndrome and outcome in peritoneal dialysis patients. Nephrol Dial Transplant 20: 1676–1685, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Swallow C, Partridge E, Macmillan J, Tajirian T, DiGuglielmo G, Hay K, Szweras M, Jahnen-Dechent W, Wrana J, Redston M, Gallinger S, Dennis J: alpha2HS-glycoprotein, an antagonist of transforming growth factor beta in vivo, inhibits intestinal tumor progression. Cancer Res 64: 6402–6409, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Ix JH, Chertow GM, Shlipak MG, Brandenburg VM, Ketteler M, Whooley MA: Fetuin-A and kidney function in persons with coronary artery disease—Data from the Heart and Soul Study. Nephrol Dial Transplant 21: 2144–2151, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auberger P, Falquerho L, Contreres JO, Pages G, Le Cam G, Rossi B, Le Cam A: Characterization of a natural inhibitor of the insulin receptor tyrosine kinase: cDNA cloning, purification, and anti-mitogenic activity. Cell 58: 631–640, 1989 [DOI] [PubMed] [Google Scholar]
- 20.Rauth G, Poschke O, Fink E, Eulitz M, Tippmer S, Kellerer M, Haring HU, Nawratil P, Haasemann M, Jahnen-Dechent W, Muller-Esterl W: The nucleotide and partial amino acid sequences of rat fetuin. Identity with the natural tyrosine kinase inhibitor of the rat insulin receptor. Eur J Biochem 204: 523–529, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Srinivas PR, Wagner AS, Reddy LV, Deutsch DD, Leon MA, Goustin AS, Grunberger G: Serum alpha 2-HS-glycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Mol Endocrinol 7: 1445–1455, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Mathews ST, Rakhade S, Zhou X, Parker GC, Coscina DV, Grunberger G: Fetuin-null mice are protected against obesity and insulin resistance associated with aging. Biochem Biophys Res Commun 350: 437–443, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Mathews ST, Singh GP, Ranalletta M, Cintron VJ, Qiang X, Goustin AS, Jen KL, Charron MJ, Jahnen-Dechent W, Grunberger G: Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes 51: 2450–2458, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Hennige AM, Staiger H, Wicke C, Machicao F, Fritsche A, Haring HU, Stefan N: Fetuin-A induces cytokine expression and suppresses adiponectin production. PLoS ONE 3: e1765, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ix JH, Shlipak MG, Brandenburg VM, Ali S, Ketteler M, Whooley MA: Association between human fetuin-A and the metabolic syndrome: Data from the Heart and Soul Study. Circulation, 113: 1760–1767, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mori K, Emoto M, Yokoyama H, Araki T, Teramura M, Koyama H, Shoji T, Inaba M, Nishizawa Y: Association of serum fetuin-A with insulin resistance in type 2 diabetic and nondiabetic subjects. Diabetes Care 29: 468, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Krober SM, Machicao F, Fritsche A, Haring HU: Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care 29: 853–857, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Collins SP, Peacock WF, Lindsell CJ, Clopton P, Diercks DB, Hiestand B, Hogan C, Kontos MC, Mueller C, Nowak R, Chen WJ, Huang CH, Abraham WT, Amsterdam E, Breidthardt T, Daniels L, Hasan A, Hudson M, McCord J, Naz T, Wagoner LE, Maisel A: S3 detection as a diagnostic and prognostic aid in emergency department patients with acute dyspnea. Ann Emerg Med 53: 748–757, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Axelsson J, Wang X, Ketteler M, Qureshi AR, Heimburger O, Barany P, Lindholm B, Nordfors L, Stenvinkel P: Is fetuin-A/alpha2-Heremans-Schmid glycoprotein associated with the metabolic syndrome in patients with chronic kidney disease? Am J Nephrol 28: 669–676, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Ix JH, Wassel CL, Kanaya AM, Vittinghoff E, Johnson KC, Koster A, Cauley JA, Harris TB, Cummings SR, Shlipak MG: Fetuin-A and incident diabetes mellitus in older persons. JAMA 300: 182–188, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stefan N, Fritsche A, Weikert C, Boeing H, Joost HG, Haring HU, Schulze MB: Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes 57: 2762–2767, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reinehr T, Kiess W, de Sousa G, Stoffel-Wagner B, Wunsch R: Intima media thickness in childhood obesity: Relations to inflammatory marker, glucose metabolism, and blood pressure. Metabolism 55: 113–118, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Sonnenberg GE, Krakower GR, Kissebah AH: A novel pathway to the manifestations of metabolic syndrome. Obesity Res 12: 180–186, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Vionnet N, Hani EH, Dupont S, Gallina S, Francke S, Dotte S, De Matos F, Durand E, Lepretre F, Lecoeur C, Gallina P, Zekiri L, Dina C, Froguel P: Genomewide search for type 2 diabetes-susceptibility genes in French whites: Evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2-diabetes locus on chromosome 1q21–q24. Am J Hum Genet 67: 1470–1480, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanaya AM, Wassel Fyr C, Vittinghoff E, Havel PJ, Cesari M, Nicklas B, Harris T, Newman AB, Satterfield S, Cummings SR: Serum adiponectin and coronary heart disease risk in older Black and White Americans. J Clin Endocrinol Metab 91: 5044–5050, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Laughlin GA, Barrett-Connor E, May S, Langenberg C: Association of adiponectin with coronary heart disease and mortality: The Rancho Bernardo study. Am J Epidemiol 165: 164–174, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mori K, Emoto M, Araki T, Yokoyama H, Lee E, Teramura M, Koyama H, Shoji T, Inaba M, Nishizawa Y: Effects of pioglitazone on serum fetuin-A levels in patients with type 2 diabetes mellitus. Metabolism 57: 1248–1252, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, DeFronzo R, Bannayan GA, Schenker S, Cusi K: A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 355: 2297–2307, 2006 [DOI] [PubMed] [Google Scholar]
- 39.de la Pena A, Leclercq IA, Williams J, Farrell GC: NADPH oxidase is not an essential mediator of oxidative stress or liver injury in murine MCD diet-induced steatohepatitis. J Hepatol 46: 304–313, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Scherer P: Adipose tissue: From lipid storage compartment to endocrine organ. Diabetes 55: 1537–1545, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Goldstein B, Scalia R: Adiponectin: A novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab 89: 2563–2568, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Kizer JR, Barzilay JI, Kuller LH, Gottdiener JS: Adiponectin and risk of coronary heart disease in older men and women. J Clin Endocrinol Metab 93: 3299–3301, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips SA, Ciaraldi TP, Oh DK, Savu MK, Henry RR: Adiponectin secretion and response to pioglitazone is depot dependent in cultured human adipose tissue. Am J Physiol Endocrinol Metab 295: E842–E850, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y: Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 8: 731–737, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Lee MH, Klein RL, El-Shewy HM, Luttrell DK, Luttrell LM: The adiponectin receptors AdipoR1 and AdipoR2 activate ERK1/2 through a Src/Ras-dependent pathway and stimulate cell growth. Biochemistry 47: 11682–11692, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kadowaki T, Yamauchi T, Kubota N: The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett 582: 74–80, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, Liu F, Dong LQ: APPL1 binds to adiponectin receptors and mediates adiponectin signaling and function. Nat Cell Biol 8: 516–523, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Tsioufis C, Dimitriadis K, Chatzis D, Vasiliadou C, Tousoulis D, Papademetriou V, Toutouzas P, Stefanadis C, Kallikazaros I: Relation of microalbuminuria to adiponectin and augmented C-reactive protein levels in men with essential hypertension. Am J Cardiol 96: 946, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, Ouedraogo R, Hough K, McCue P, Chan L, Falkner B, Goldstein BJ: Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 118: 1645–1656, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yano Y, Hoshide S, Ishikawa J, Hashimoto T, Eguchi K, Shimada K, Kario K: Differential impacts of adiponectin on low-grade albuminuria between obese and nonobese persons without diabetes. J Clin Hypertens (Greenwich) 9: 775–782, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Looker HC, Krakoff J, Funahashi T, Matsuzawa Y, Tanaka S, Nelson RG, Knowler WC, Lindsay RS, Hanson RL: Adiponectin concentrations are influenced by renal function and diabetes duration in Pima indians with type 2 diabetes. J Clin Endocrinol Metab 89: 4010–4017, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Menon V, Li L, Wang X, Greene T, Balakrishnan V, Madero M, Pereira AA, Beck GJ, Kusek JW, Collins AJ, Levey AS, Sarnak MJ: Adiponectin and mortality in patients with chronic kidney disease. J Am Soc Nephrol 17: 2599–2606, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Tsuda S, Nakano S, Konishi K, Koya D: A lack of increase in high molecular weight-adiponectin in macroalbuminuric subjects with metabolic syndrome may exert renal and atherosclerotic risks. Diabetes Res Clin Pract 79: 503–509, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Kojima S, Funahashi T, Otsuka F, Maruyoshi H, Yamashita T, Kajiwara I, Shimomura H, Miyao Y, Fujimoto K, Sugiyama S, Sakamoto T, Yoshimura M, Ogawa H: Future adverse cardiac events can be predicted by persistently low plasma adiponectin concentrations in men and marked reductions of adiponectin in women after acute myocardial infarction. Atherosclerosis 194: 204–213, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Ohashi N, Kato A, Misaki T, Sakakima M, Fujigaki Y, Yamamoto T, Hishida A: Association of serum adiponectin levels with all-cause mortality in hemodialysis patients. Intern Med 47: 485–491, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Jorsal A, Tarnow L, Frystyk J, Lajer M, Flyvbjerg A, Parving HH, Vionnet N, Rossing P: Serum adiponectin predicts all-cause mortality and end stage renal disease in patients with type I diabetes and diabetic nephropathy. Kidney Int 74: 649–654, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Ohashi K, Iwatani H, Kihara S, Nakagawa Y, Komura N, Fujita K, Maeda N, Nishida M, Katsube F, Shimomura I, Ito T, Funahashi T: Exacerbation of albuminuria and renal fibrosis in subtotal renal ablation model of adiponectin-knockout mice. Arterioscler Thromb Vasc Biol 27: 1910–1917, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Riley P, O'Donohue J, Crook M: A growing burden: The pathogenesis, investigation and management of non-alcoholic fatty liver disease. J Clin Pathol 60: 1384–1391, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Albano E, Mottaran E, Occhino G, Reale E, Vidali M: Review article: Role of oxidative stress in the progression of non-alcoholic steatosis. Aliment Pharmacol Ther 22[Suppl 2]: 71–73, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Fromenty B, Robin MA, Igoudjil A, Mansouri A, Pessayre D: The ins and outs of mitochondrial dysfunction in NASH. Diabetes Metab 30: 121–138, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J: Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology 40: 46–54, 2004 [DOI] [PubMed] [Google Scholar]
- 62.Musso G, Gambino R, Durazzo M, Biroli G, Carello M, Faga E, Pacini G, De Michieli F, Rabbione L, Premoli A, Cassader M, Pagano G: Adipokines in NASH: Postprandial lipid metabolism as a link between adiponectin and liver disease. Hepatology 42: 1175–1183, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Targher G, Bertolini L, Rodella S, Zoppini G, Scala L, Zenari L, Falezza G: Associations between plasma adiponectin concentrations and liver histology in patients with nonalcoholic fatty liver disease. Clin Endocrinol (Oxf) 64: 679–683, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Musso G, Gambino R, Biroli G, Carello M, Faga E, Pacini G, De Michieli F, Cassader M, Durazzo M, Rizzetto M, Pagano G: Hypoadiponectinemia predicts the severity of hepatic fibrosis and pancreatic Beta-cell dysfunction in nondiabetic nonobese patients with nonalcoholic steatohepatitis. Am J Gastroenterol 100: 2438–2446, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Adachi M, Brenner DA: High molecular weight adiponectin inhibits proliferation of hepatic stellate cells via activation of adenosine monophosphate-activated protein kinase. Hepatology 47: 677–685, 2008 [DOI] [PubMed] [Google Scholar]
- 66.Carmiel-Haggai M, Cederbaum AI, Nieto N: A high-fat diet leads to the progression of non-alcoholic fatty liver disease in obese rats. Faseb J 19: 136–138, 2005 [DOI] [PubMed] [Google Scholar]
- 67.Carmona-Cuenca I, Roncero C, Sancho P, Caja L, Fausto N, Fernandez M, Fabregat I: Upregulation of the NADPH oxidase Nox4 by TGF-beta in hepatocytes is required for its pro-apoptotic activity. J Hepatol 49: 965–976, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Kasinath BS, Feliers D, Sataranatarajan K, Choudhury GG, Lee MJ, Mariappan MM: Regulation of mRNA translation in renal physiology and disease. Am J Physiol Renal Physiol 297: F1153–F1165, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kasinath BS, Mariappan MM, Sataranatarajan K, Lee MJ, Feliers D: mRNA translation: Unexplored territory in renal science. J Am Soc Nephrol 17: 3281–3292, 2006 [DOI] [PubMed] [Google Scholar]
- 70.Viollet B, Foretz M, Guigas B, Horman S, Dentin R, Bertrand L, Hue L, Andreelli F: Activation of AMP-activated protein kinase in the liver: A new strategy for the management of metabolic hepatic disorders. J Physiol 574: 41–53, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Viollet B, Guigas B, Leclerc J, Hebrard S, Lantier L, Mounier R, Andreelli F, Foretz M: AMP-activated protein kinase in the regulation of hepatic energy metabolism: From physiology to therapeutic perspectives. Acta Physiol (Oxf) 196: 81–98, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song Z, Deaciuc I, Zhou Z, Song M, Chen T, Hill D, McClain CJ: Involvement of AMP-activated protein kinase in beneficial effects of betaine on high-sucrose diet-induced hepatic steatosis. Am J Physiol Gastrointest Liver Physiol 293: G894–G902, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang J, Maika S, Craddock L, King JA, Liu ZM: Chronic activation of AMP-activated protein kinase-alpha1 in liver leads to decreased adiposity in mice. Biochem Biophys Res Commun 370: 248–253, 2008 [DOI] [PubMed] [Google Scholar]
- 74.Niemann B, Silber RE, Rohrbach S: Age-specific effects of short- and long-term caloric restriction on the expression of adiponectin and adiponectin receptors: Influence of intensity of food restriction. Exp Gerontol 43: 706–713, 2008 [DOI] [PubMed] [Google Scholar]
- 75.Takekoshi K, Fukuhara M, Quin Z, Nissato S, Isobe K, Kawakami Y, Ohmori H: Long-term exercise stimulates adenosine monophosphate-activated protein kinase activity and subunit expression in rat visceral adipose tissue and liver. Metabolism 55: 1122–1128, 2006 [DOI] [PubMed] [Google Scholar]
- 76.Davis BJ, Xie Z, Viollet B, Zou MH: Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 55: 496–505, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Xie Z, Dong Y, Scholz R, Neumann D, Zou MH: Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation 117: 952–962, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ran J, Hirano T, Fukui T, Saito K, Kageyama H, Okada K, Adachi M: Angiotensin II infusion decreases plasma adiponectin level via its type 1 receptor in rats: An implication for hypertension-related insulin resistance. Metabolism 55: 478–488, 2006 [DOI] [PubMed] [Google Scholar]
- 79.Yenicesu M, Yilmaz MI, Caglar K, Sonmez A, Eyileten T, Acikel C, Kilic S, Bingol N, Bingol S, Vural A: Blockade of the renin-angiotensin system increases plasma adiponectin levels in type-2 diabetic patients with proteinuria. Nephron Clin Pract 99: c115–c121, 2005 [DOI] [PubMed] [Google Scholar]
- 80.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R: Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8: 157–168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ajmo JM, Liang X, Rogers CQ, Pennock B, You M: Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol 295: G833–G842, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan AY, Dolinsky VW, Soltys CL, Viollet B, Baksh S, Light PE, Dyck JR: Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt. J Biol Chem 283: 24194–24201, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Han DC, Isono M, Chen S, Casaretto A, Hong SW, Wolf G, Ziyadeh FN: Leptin stimulates type I collagen production in db/db mesangial cells: Glucose uptake and TGF-beta type II receptor expression. Kidney Int 59: 1315–1323, 2001 [DOI] [PubMed] [Google Scholar]
- 84.Wolf G, Ziyadeh FN: Leptin and renal fibrosis. Contrib Nephrol 151: 175–183, 2006 [DOI] [PubMed] [Google Scholar]
- 85.Asilmaz E, Cohen P, Miyazaki M, Dobrzyn P, Ueki K, Fayzikhodjaeva G, Soukas AA, Kahn CR, Ntambi JM, Socci ND, Friedman JM: Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. J Clin Invest 113: 414–424, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Angulo P, Alba LM, Petrovic LM, Adams LA, Lindor KD, Jensen MD: Leptin, insulin resistance, and liver fibrosis in human nonalcoholic fatty liver disease. J Hepatol 41: 943–949, 2004 [DOI] [PubMed] [Google Scholar]
- 87.Ellington AA, Malik AR, Klee GG, Turner ST, Rule AD, Mosley TH, Jr, Kullo IJ: Association of plasma resistin with glomerular filtration rate and albuminuria in hypertensive adults. Hypertension 50: 708–714, 2007 [DOI] [PubMed] [Google Scholar]
- 88.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM: A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 282: 35279–35292, 2007 [DOI] [PubMed] [Google Scholar]
- 89.Susztak K, Ciccone E, McCue P, Sharma K, Bottinger EP: Multiple metabolic hits converge on CD36 as novel mediator of tubular epithelial apoptosis in diabetic nephropathy. PLoS Med 2: e45, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kataoka H, Sharma K: Renal handling of adipokines. Contrib Nephrol 151: 91–105, 2006 [DOI] [PubMed] [Google Scholar]
- 91.Persson F, Rossing P, Hovind P, Stehouwer CD, Schalkwijk CG, Tarnow L, Parving HH: Endothelial dysfunction and inflammation predict development of diabetic nephropathy in the Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria (IRMA 2) study. Scand J Clin Lab Invest: 68: 731–738, 2008 [DOI] [PubMed] [Google Scholar]
- 92.Lee IT, Lee WJ, Huang CN, H-H Sheu W: The association of low-grade inflammation, urinary albumin, and insulin resistance with metabolic syndrome in nondiabetic Taiwanese. Metabolism 56: 1708–1713, 2007 [DOI] [PubMed] [Google Scholar]