Estrogen Receptor β Protein in Human Breast Cancer: Correlation with Clinical Tumor Parameters (original) (raw)
. Author manuscript; available in PMC: 2015 Jun 26.
Published in final edited form as: Cancer Res. 2003 May 15;63(10):2434–2439.
Abstract
The recent discovery of a second estrogen receptor, designated ERβ, raises pressing questions about its role in estrogen regulation of human breast cancer cells, and its significance for the prediction of recurrence and treatment responses in clinical breast cancer. Most of what we know about ERβ expression comes from studies examining a limited number of samples at the RNA level. We have now generated a monoclonal antibody useful for the detection of ERβ at the protein level in archival, formalin-fixed breast tumors, and have examined its expression using immunohistochemistry (IHC) in a pilot series of 242 breast cancer patients. Co-expression of ERβ and ERα was found in the majority of the tumors, with 76% of the tumors expressing ERβ as determined by IHC. ERα, but not ERβ, was strongly associated with progesterone receptor (PR) expression, suggesting that ERα is the predominant regulator of this estrogen-induced gene in breast tumors. Although ERα expression was positively correlated with low tumor grade, diploidy, and low S-phase fraction, all biological parameters of a good prognostic profile, ERβ trended toward an association only with aneuploidy; no association with tumor grade or S-phase fraction was seen for ERβ. We found that ERβ expression does cause false-positive readings for ERα. These results suggest that ERβ expression is not a surrogate for ERα in clinical breast tumors, and as such, could be a useful biomarker in its own right.
Keywords: estrogen receptor β, breast cancer
Introduction
Many recent discoveries in the nuclear receptor field have contributed to our understanding of steroid hormone action, and the mechanisms by which estrogens exert their effects in breast cancer cells. Until recently, estrogen action was thought to be mediated through a single ER, now called ERα (1). However, the identification of a second ER, called ERβ (2), casts uncertainty upon this understanding of estrogen action. ERα expression is a very useful clinical biomarker of breast tumor progression, so that ERα and the progesterone receptor (PR) are now routinely used to estimate patient prognosis and select optimal therapies. Overall, approximately 50% to 60% of women with ER-positive, advanced breast cancer will receive some degree of benefit from standard endocrine treatment with the antiestrogen tamoxifen, while the majority of early ER-positive breast cancers will respond to treatment (3) (4). However, most of the clinical implications of ER expression have been assessed using biochemical ligand-binding methods, such as the dextran-coated charcoal assay. What we do not know is whether the new ERβ subtype will confound our simple interpretation of these clinical ER assays since it has almost the same binding affinity for estradiol as ERα (2).
ERα and ERβ belong to a superfamily of nuclear hormone receptors which function as transcription factors when they are bound to their respective ligands, and they share common structural and functional features. ERα contains 595 amino acids with a central DNA binding domain, along with a carboxy-terminal hormone-binding domain. We have previously shown that human ERβ protein is somewhat shorter than ERα (5), with a predicted size of 530 residues. Based on their structural dissimilarities, especially in the amino-terminal AF-1 region, it has been suggested that ERβ expression may regulate a different set of genes than ERα, a difference that may have important consequences for tumor growth. It is also possible that the biological function of ERβ is dependent on the co-expression of ERα in certain tissues, including the breast. In fact, ERβ can function as an inhibitor of ERα activity under certain circumstances in vitro (6). We believe that the ultimate way to address these questions and ask what is the potential clinical significance of ERβ is to determine its role directly in patients’ samples and compare its expression with ERα.
There are now a number of published studies examining ERβ expression in breast tumors, but the majority of these assessed RNA levels, often using semi-quantitative methods that might not accurately reflect ERβ protein expression. These studies, examining a limited number of tumors, have been contradictory in their conclusions, suggesting that ERβ is either a poor prognostic factor associated with PR-negative, lymph node-positive tumors (7), or conversely, a marker of good prognosis and associated with negative lymph nodes and low proliferative status (8).
Our first goal was to develop an immunohistochemical (IHC) assay to measure ERβ protein in archival breast specimens to resolve these apparent discrepancies. To accomplish this goal, we generated a monoclonal antibody to the amino-terminal region of ERβ and developed an IHC assay useful for formalin-fixed, archival specimens. Since the epitope of this antibody is localized to the amino-terminal region of ERβ, it is capable of detecting both full-length ERβ (called ERβ1) and various carboxy-truncated isoforms of ERβ (5, 9), therefore measuring total ERβ protein in tumors. In the present pilot study of 242 breast tumors, we have determined that ERβ is co-expressed along with ERα in the majority of specimens, and have investigated the relationships between ERβ, ERα, and clinical tumor parameters.
Materials and Methods
Tumor samples
261 human breast tumor specimens in the Baylor Breast Cancer SPORE Tissue Resource were included in this pilot study. Treatment histories and long-term follow-up for disease recurrence and death were not available for these patients. Breast tumor specimens were frozen in liquid nitrogen immediately after excision, and sent to a central laboratory for steroid receptor assays and DNA flow cytometry. One paraffin-embedded, ERβ-positive breast tumor was used to evaluate whole tissue section staining with the ERβ antibody, and different areas of the slide was photographed to examine for inter-tumor heterogeneity of ERβ protein expression.
Steroid receptor assays
Tumor cytosols were prepared for ligand binding assay (LBA) as described (10), using a standard multipoint dextran-coated charcoal assay incorporating 125I-estradiol and 3H-R5020 in a single assay, allowing for the simultaneous determination of both ER and PR status. Tumors with an ER or PR content of ≥3 fmol/mg protein or ≥5 fmol/mg protein, respectively, were considered to be positive for receptor expression. The pulverized tissue that remained after LBA assay was stored at −70°C for future use.
Flow cytometric evaluation of S-phase fraction and DNA ploidy measurments
Flow cytometry was carried out as described previously (11). Briefly, approximately 100 mg of frozen pulverized tumor were homogenized, filtered, and centrifuged. Chicken red cells were added as an internal standard, and the cells were lysed and stained for DNA. DNA-stained nuclei were prepared and run on an Epics V flow cytometer (Coulter Electronics, Hialeah, FL). Approximately 50,000 tumor events were acquired on a single-parameter 256-channel integrated fluorescence histogram. Frequency distributions of cells in G0/G1, S-phase (SPF), and G2/M phases of the cell cycle were evaluated using a modeling program (MODFIT, Verity Software House, Inc., Topsham, ME). Debris was modeled as an exponential, and SPF was modeled as a single trapezoid. Proliferation status as determined with Ki67 staining has been previously described (12).
Tumor histologic grade
Histologic grading of the tumors was performed on complete sections (see below) using the Elston-modified Scarff-Bloom-Richardson (SBR) system (13). This system utilizes a semi-quantitative method to assess degree of differentiation (tubule formation), nuclear pleomorphism, and mitotic activity. Since our tissue sections were prepared from pulverized tissue, it was often not possible to count 10 individual and non-overlapping fields. Therefore mitotic activity was scored based on one field with maximum number of mitotic figures, an approach similar to the original SBR system (14).
Generation of a monoclonal ERβ antibody
RNA from MCF-7 cells was used for RT/PCR of the amino-terminal fragment (amino acid residues 1–146) of ERβ (15). This fragment was then cloned into the pET28a+ mammalian expression vector. GST-fusion recombinant ERβ protein was purified on a Histidine affinity column, and used for immunization of female Balb/c mice. Spleen cells from mice producing immunoreactive antibody were fused to NS-1 myeloma cells by standard hybridoma methods. Specific monoclonal antibody to ERβ as determined by Western blot analysis was purified and concentrated through protein G columns, and utilized for the IHC and Western studies shown. The one monoclonal antibody useful for IHC was named 14C8, and is now commercially available through GeneTex (San Antonio, TX).
For Western blot analysis, MCF-7 total cell lysates were prepared as previously described (16). Briefly, a cell pellet was homogenized in a high salt buffer (20 mM Tris/HCl pH 7.5, 2mM dithithreitol, 0.4 M KCl, and 20% glycerol) containing a cocktail of protease inhibitors (10 μg/ml each of aprotinin, antipain, leupeptin, and pepstatin plus 0.3 mM phenylmethylsulfonyl fluoride) (Sigma). Homogenates were then centrifuged at 100,000 × g for 1 h, and the supernatants stored at −80° C until use. 50 μg of extract protein was separated by electrophoresis on 8% SDS-PAGE, and transferred onto nylon membranes (Schleicher and Schuelle, Keene, NH). The blots were first stained with Ponceau S (Sigma, St. Louis, MO) to confirm uniform transfer of all samples, and then incubated in blocking solution [5% non-fat dry milk in Phosphate-buffered saline-Tween (PBST: 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.05% Tween-20)]. After brief washes with TBST, the filters were then reacted with the 14C8 monoclonal antibody at a dilution of 1:250 or the NCL-ER-6F11 mouse monoclonal antibody against human ERα (Vector Laboratories, Burlingame, CA) at a dilution of 1:100 for 1 hr at room temperature followed by extensive washes with PBST. Blots were then incubated with horseradish peroxidase-conjugated secondary antibody (Amersham, England) for 1 hr, washed with TBST, and developed using the ECL procedure (Amersham, England). As positive controls, 1 μl of in vitro translated extract from two mammalian expression vectors (pSG5) containing the complete open-reading frames of full-length ERβ1 and ERβ2 variant isoform expression cDNA clones (5) were used on the Western blots. As a negative control, in vitro translated ERα (17) was also included.
Preparation of tissue arrays (TAs)
Tissue sections were initially prepared from the pulverized frozen tumor specimens left over from the LBA as previously described (18). TAs were assembled manually as previously described (5). Briefly, an H&E stained slide from a complete tissue section was used as guide to mark the area of maximum tumor cellularity on the corresponding formalin-fixed, paraffin-embedded donor block. Then, using a 3 mm dermal biopsy punch (Miltex Instruements Inc., NY), a 3 mm core of tumor was punched out from the donor block. To create the TA recipient block, an empty paraffin cast was punched out using a stainless steel mold template in a configuration of 6 × 5 (30 samples). Twenty-nine tumor samples and one normal control tissue (used as a marker of orientation) were arranged using a predetermined map into the paraffin cast. This was then annealed at 62°C for 20 minutes to create a TA block.
IHC for ERs α, β, PR and Ki67
4 micron thick sections of TA were used for immunohistochemistry (IHC). Monoclonal antibodies NCL-ER-6F11 (Novocastra, UK) at a 1:200, antibody 1294 (Dako Carpinteria, CA) at 1:600, Mib1 (Dako) at 1:200, and our in house monoclonal antibody 14C8 at a 1:200 dilution, were used for ERα, PR, Ki67, and ERβ IHC, respectively. Heat-induced epitope retrieval was performed using Tris-HCl buffer, pH 9.0, in a pressure cooker for 5 minutes for each of the markers. Slides were blocked for endogenous peroxidase in a 3% H2O2 solution for 5′ followed by the A/B Blocking kit reagents (Vector Laboratories, Burlingame, CA) per manufactuer’s recommendations for endogenous biotin. The linking antibodies step (30′) utilized biotinylated rabbit anti-mouse (#E0345 by Dako) at 1:200 for all antibodies except ERβ where 1:100 was used. The chromagen was applied using DAB + solution (Dako) for 15′, which was intensified with 0.2% osmium tetraoxide for 30s. Slides were counterstained with methyl green and cover slipped with a permanent medium.
Immunostained slides were scored for ERα, ERβ, and PR as previously described (19). In brief, each entire slide was evaluated by light microscopy. First, a proportion score was assigned, which represented the estimated proportion of positive-staining tumor cells (0, none; 1, <1/100; 2, 1/100 to 1/10; 3, 1/10 to 1/3; 4, 1/3 to 2/3; and 5, > 2/3). Next an intensity score was assigned that represented the average intensity of positive tumor cells (0, none; 1, weak; 2, intermediate; and 3, strong). The proportion and intensity scores were then added to obtain a total score, which ranged from 0 to 8. Mib1/Ki67 was scored by directly counting percent positive cells (average denominator = 500 cells) as previously described (12). Slides were scored without knowledge of the LBA results or prognostic factors.
Statistical methods
Associations between continuous variables were analyzed using nonparametric Spearman rank correlation coefficients. Associations between categorical variables were assessed by Fisher’s exact tests. All analyses were performed using SAS (Version 6.12 SAS Institute, Cary, NC) on a Sun SparcStation (Sun Microsystems, Inc., Mountain View, CA).
Results
Specificity of ERα and ERβ antibodies
Although the commercially available ERα NCL-ER-6F11 antibody was generated to recombinant ERα protein, because of the high degree of shared homology between the two ERs, we first had to demonstrate its specificity for the α-receptor subtype. Therefore, in vitro translated ERα and full-length ERβ1 extract, along with total cellular extracts from MCF-7 breast carcinoma cells, were prepared and subjected to Western blot analysis with the 6F11 antibody (Fig. 1, Panel A). This antibody was indeed specific for the α-receptor form, and did not cross-react with ERβ; a band corresponding to the 65–66 kDa ERα subtype was the only form detected in MCF-7 cells with the 6F11 antibody.
Figure 1.
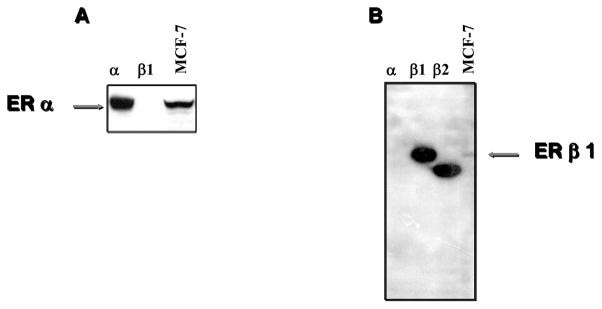
Western blot comparison of ERα NCL-ER-6F11 antibody and our 14C8 ERβ antibody. Lysate (5 microliters) from in vitro translation reactions primed with pSG5 expression plasmid containing either ERβ1 (β1), or ERβ2 (β2), and cell lysate (50–100 micrograms) prepared from the MCF-7 breast carcinoma cell line were electrophoresed on SDS-PAGE gels and transferred to nylon filters. The blots were incubated with the 6F11 antiserum diluted 1:500 (Panel A), or ERβ antiserum diluted 1:200 (Panel B). Binding of primary antibody was detected with horseradish-peroxidase conjugated secondary antibody.
We next analyzed a number of different monoclonal antibodies that we generated to a GST-fusion protein of the amino-terminal region of ERβ. Although all of these antibodies reacted with recombinant ERβ protein on Western blot analyses, only one, the 14C8 ERβ-specific antibody, demonstrated a strong nuclear signal in formalin-fixed material (data not shown). Since we have shown that ERβ variant isoforms exist in breast tumor cell lines (5), we included in vitro translated ERβ1 and the COOH-terminal splicing variant ERβ2, along with ERα and extracts from MCF-7 cells, to examine the specificity of the 14C8 monoclonal ERβ antibody in immunoblot analysis (Fig. 1, panel B). Our antibody reacted specifically with ERβ but not with ERα, and detected full-length ERβ1 of approximately 58–60 kDa molecular weight in MCF-7 cells. Since our antibody was generated to a fusion protein of the amino-terminal region of ERβ, it also detected the carboxy-terminal truncated ERβ2 isoform. This result suggests that our antibody is capable of detecting total ERβ expression in cells, since most of the ERβ variants described arise from alternative splicing at the carboxy-terminus (9).
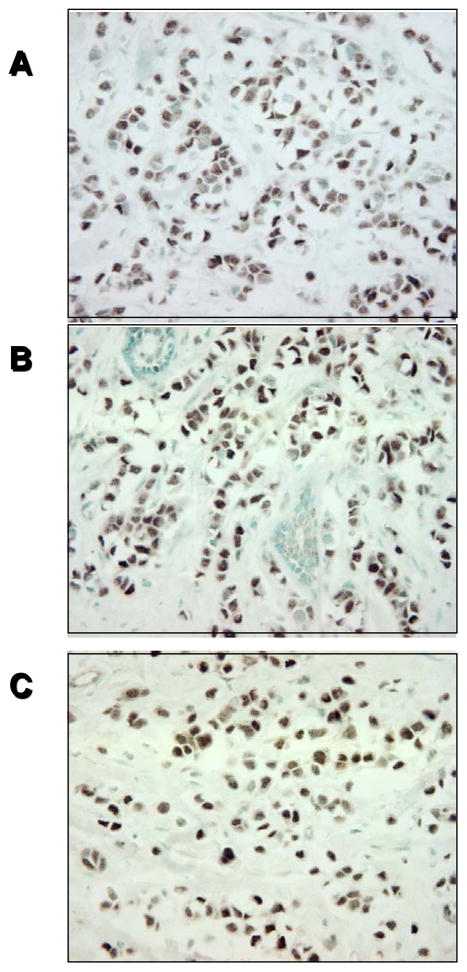
We next performed immunohistochemical analysis on a panel of whole tissue sections from primary breast tumors; representative photographs from one tumor is shown in Fig. 2. To look for heterogeneity of ERβ expression in the same tumor using whole sections, we examined three different areas of the same tumor and found the same pattern of staining throught the tissue (compare Fig. 2, panels A–C). Thus, the expression of ERβ appears to be homogeneous. This finding suggests that the use of tissue arrays would be representative of a given tumor sample, and in our experience, we have not seen significant heterogeneity of ERβ expression within a tumor to be concerned about sampling error with this antibody.
Figure 2.
IHC of a representative breast tumor using a whole tissue section and our ERβ-specific 14C8 antibody. Panels A thru C represent three different areas of the same tumor staining homogenously positive for ERβ protein expression
Validation of tissue arrays
TAs offer the advantage of rapid staining and scoring of a large number of tumors. An obvious question, however, is whether the results obtained from tissue arrays are indeed representative of results obtained from larger specimens as suggested by the homogeneity of staining obtained and shown in Fig. 2. To address this question, we arrayed 261 human breast tumors on 9 tissue blocks and stained for ERα using the NCL-ER-6F11 and ERβ antibodies. Representative IHC staining of four breast tumor specimens for ERα and ERβ is shown in Fig. 3. For comparison, ERα had previously been determined in larger sections from these same tumors using IHC with the 6F11 antibody (19). ERα results were obtained in the TAs from 237 of the 261 tumors (91%). Complete concordance of total IHC scores between tissue array and the previously stained, larger sections was observed in 46% of cases, and concordance within one IHC score was observed in 82% of cases. The Spearman correlation coefficient was 0.76. When results were classified as ER-positive (ER+) or ER-negative (ER−) using our previously defined criteria (total IHC score >2 = ER+), only 12 cases (5%) were discordant (11 ER+ on larger sections, but ER− on tissue array; 1 ER-on larger sections, but ER+ on tissue array).
Figure 3.
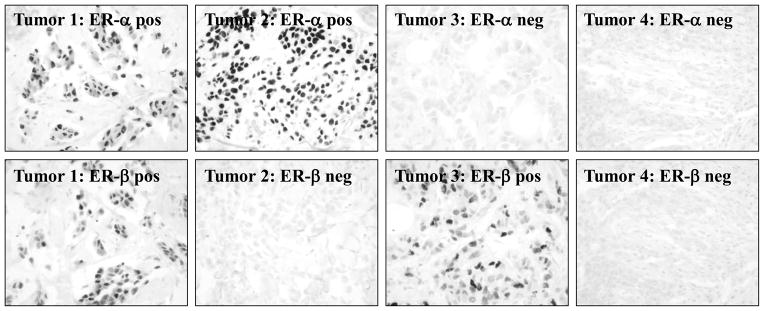
IHC of four representative breast tumors from the tissue array using the ERα 6F11 antibody or our ERβ-specific 14C8 antibody. Tumors representative of the different subgroups are included: tumor 1--ERα-positive/ERβ-positive, tumor 2--ERα-positive/ERβ-negative, tumor 3--ERα-negative/ERβ-positive, and tumor 4--ERα-negative/ERβ-negative.
To put these results in perspective, we examined the inter-rater reliability of scoring the same slides from the larger sections. Complete concordance of total IHC scores from two independent pathologists was observed in 72% of cases, and concordance within one IHC score was observed in 99% of cases. The Spearman correlation coefficient was 0.92. Only two cases had different qualitative outcomes, and in each case the results differed by only one score (IHC = 3 and 2, ER+ and ER−, respectively).
Therefore, we conclude that the scoring system is reproducible between observers, and, although there is a slight loss of sensitivity, tissue arrays yield results comparable to those obtained on larger specimens.
Distribution of ERβ expression
Results for ERβ were obtained from 242 tumors on the tissue arrays. Nuclear staining was observed in 184 (76%) of the cases. Although the rate of staining is very similar to that observed for ERα, the pattern is somewhat different (Table 1), with higher levels of ERα expression detected in the tumors. The Spearman correlation coefficients between ERβ and ERα were relatively modest (rsp = 0.33 and 0.36, respectively, for tissue array and larger samples).
Table 1.
Distribution of ERα and ERβ as determined by IHC in 242 primary breast cancer cases
| ER IHC Score | Patients | |||
|---|---|---|---|---|
| β-positive | α-positive | |||
| No. | % | No. | % | |
| 0 | 58 | 24 | 54 | 23 |
| 2 | 0 | 0 | 4 | 2 |
| 3 | 48 | 20 | 14 | 6 |
| 4 | 39 | 16 | 17 | 7 |
| 5 | 36 | 15 | 18 | 8 |
| 6 | 44 | 18 | 46 | 19 |
| 7 | 14 | 6 | 45 | 19 |
| 8 | 3 | 1 | 39 | 16 |
Relationships between ER expression and other prognostic factors
Spearman correlation coefficients between ERβ and ERα and other prognostic factors are displayed in Table 2. ERβ expression was positively correlated with ERα determined by IHC (rsp = 0.33), but this relationship was not seen with ER LBAs (rsp = 0.09). This result is consistent with the report by Dotzlaw and colleagues (20) showing that expression of ERβ RNA using RT/PCR amplification was not significantly correlated with ER by LBA in breast tumors. Similarly, ERβ expression was positively correlated with PR determined by IHC (rsp = 0.25), but not with PR by LBA (rsp = 0.05). The very strong, positive correlations between ERα by IHC and ER by LBA both in this study and in our previous larger study (19), suggests that the ER status of breast tumors as defined by the standard clinical dextran-coated charcoal assay fails to accurately reflect the levels of ERβ present in breast tumors.
Table 2.
Spearman correlation coefficients between ER expression and other prognostic factors
| Factor | ERβ | ERα |
|---|---|---|
| ERα (IHC) | 0.33 (p=0.0001) | ----- |
| ER (LBA) | 0.09 (p=0.15) | 0.76 (p=0.0001) |
| PR (IHC) | 0.25 (p=0.0001) | 0.58 (p=0.0001) |
| PR (LBA) | 0.04 (p=0.49) | 0.44 (p=0.0001) |
| Tumor grade | −0.01 (p=0.90) | −0.40 (p=0.0001) |
| Ki67 | −0.09 (p=0.16) | −0.36 (p=0.0001) |
| SPF | −0.08 (p=0.25) | −0.34 (p=0.0001) |
| Ploidy | 0.08 (p=0.20) | −0.11 (p=0.10) |
No significant relationships were observed between ERβ expression and tumor grade, proliferation determined by Ki67 staining, S-phase fraction, or DNA ploidy. In contrast, ERα expression was significantly correlated with each of these prognostic factors, as we have previously described for ER by LBA (21), providing additional evidence that the samples in this pilot study are representative of clinical breast cancer. The different results for ERβ and ERα suggest that ERβ expression is not a surrogate for ERα, and is not correlated with the same clinical parameters as ERα. As such, ERβ could predict different biological features of breast tumors, but unfortunately, ERβ-specific functions or signaling pathways that may be clinically important for tumor progression have not yet been elucidated.
ERα expression is the major determinant of PR expression in breast tumors
Although the study was relatively small, we next investigated the biological importance of ERα and ERβ co-expression and interactions with other prognostic factors. We defined tumors to be positive for either ER if its IHC scores were greater than two. This corresponds to our published definition for ERα (19) that was based on correlations with clinical outcome, but is admittedly arbitrary for ERβ. Using these definitions, 9% of the tumors (21/234) were negative for both receptors, 14% (32/234) were positive only for ERα, 15% (36/234) were positive only for ERβ, and 62% (145/234) of the tumors were positive for both receptors. Examples of all four categories are seen in Fig. 1. We found (Table 3) that receptor-negative tumors rarely (5% by LBA and 14% by IHC) expressed the PR. However, PR expression by IHC was more closely associated with ERα expression than ERβ: PR was expressed in 88% of ERα-positive / ERβ-negative tumors, but in only 6% of the ERα-negative / ERβ-positive tumors (p<0.0001). Similar results were found for PR by LBA.
Table 3.
Comparison of combined ER status with standard prognostic factors
| Factor | α−/β− | α−/β+ | α+/β− | α+/β+ | α−/β− vs. α−/β+ | α+/β− vs. α+/β+ | α−/β− vs. α+/β− | α−/β+ vs. α+/β+ | α−/β+ vs. α+/β− | α−/β− vs. α+/β+ |
|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | p-value | p-value | p-value | p-value | p-value | p-value | |
| + by IHC | 1/21 (5%) | 2/36 (6%) | 28/32 (88%) | 134/145 (92%) | 1.00 | 0.48 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| PR+ by LB | 3/21 (14%) | 4/36 (11%) | 25/32 (78%) | 115/145 (79%) | 0.70 | 1.00 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| ER+ by LB | 8/21 (38%) | 11/36 (31%) | 31/32 (97%) | 139/145 (96%) | 0.58 | 1.00 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Grade 3 | 14/21 (67%) | 30/36 (83%) | 9/32 (28%) | 27/145 (19%) | 0.20 | 0.23 | 0.01 | <0.0001 | <0.0001 | <0.0001 |
| Aneuploid | 13/20 (65%) | 27/33 (82%) | 14/32 (44%) | 86/145 (59%) | 0.20 | 0.12 | 0.16 | 0.016 | 0.002 | 0.81 |
| High SPF | 9/15 (60%) | 16/24 (67%) | 8/32 (25%) | 35/145 (24%) | 0.74 | 1.00 | 0.027 | <0.0001 | 0.003 | 0.006 |
ERα expression is the major determinant of some, but not all, breast cancer prognostic factors
High tumor grade and high S-phase fractions were significantly related to ERα expression, and the same strong relationships were observed when patients were further stratified by ERβ status (Table 3). For example, 24% of tumors with both receptors had high SPF, compared to 25% of tumors in the ERα-positive / ERβ-negative subset of tumors; conversely 60% of tumors lacking both receptors had high SPF, compared to 67% of the ERα-negative / ERβ-positive subset. Based on these data, one could speculate that co-expression of ERβ may have little impact on the prognosis of patients with breast tumors, at least as assessed by established factors such as proliferation and tumor grade.
However, the relationships with DNA ploidy appear to be more complex. There was a trend (p = 0.12) for tumors with both receptors to be more aneuploid (59%), compared to ERα-positive / ERβ-negative tumors (44%). Similarly, the rate of aneuploidy was marginally higher (p = 0.20) in ERα-negative / ERβ-positive tumors (82%), compared to tumors that lack both receptors (65%), suggesting that ERβ-positive tumors might be more aggressive biologically, similar in characteristics to the receptor-negative breast tumors. Therefore, the specific measurement of ERβ, combined with the measurement of ERα by IHC, might provide useful clinical information in certain breast cancer patients, a possibility that will require a larger study with clinical follow-up information to validate.
Discussion
ER and PR are measured in breast tumor specimens for prognostication of disease recurrence and prediction of treatment response. In guidelines published by the ASCO Tumor Marker Expert Panel (22), ER and PR were the only biomarkers recommended for routine use in the management of patients with breast cancer. The ER assay is most useful if the tumor is ER-negative; these patients seldom respond to endocrine therapy. However, predicting the probability of response in ER-positive patients is more difficult. Overall, approximately 50% to 60% of women with ER-positive, advanced breast cancer will receive some degree of benefit from endocrine treatment (3). With the discovery of the second ER subtype, ERβ, it was reasonable to hope that an accurate measurement of the two forms might provide additional prognostic or predictive clinical information.
An accurate assessment of ERβ expression requires the availability of specific antibodies useful for routinely-fixed clinical material. Only recently have antibodies to ERβ become available (23) (24), but unfortunately most of these have not proven useful for IHC, or worked only in frozen material (8). Although we had previously developed two antibodies to ERβ that were specific in Western blot analyses (5), neither of these antibodies worked in paraffin-embedded sections. Therefore we developed a third new antibody, now called 14C8, which is capable of recognizing ERβ protein in archival samples. Since the antibody was prepared to the amino-terminal region of the protein, it is capable of recognizing putative carboxy-terminal truncated forms as well as full-length ERβ, thus presumably detecting total translated ERβ protein unlike many of the other commercially available ERβ antibodies (8).
We found that the majority of breast tumors co-expressed both receptors, which is in agreement with a number of other published studies. In a study of 60 tumors, Speirs et al (7) hypothesized that breast tumors co-expressing both receptors, as opposed to those only expressing ERα, were more frequently associated with poor prognostic biomarkers, such as positive axillary nodes and higher tumor grade. This is certainly a viable hypothesis given that the two receptors can form functional heterodimers on DNA (25), and that the heterodimer may be preferentially formed as opposed to homodimers (26). However, our results do not completely agree with this hypothesis. For instance, when comparing high tumor grade and high S-phase fraction with ER status, we found little difference between these two clinical parameters in tumors co-expressing both receptors vs. those only expressing ERα. These results could lead us to hypothesize that ERβ expression might have little clinical impact on ERα function. However, we also found that tumors expressing both receptors tended to be more aneuploid, and tumors expressing only ERβ exhibited even slightly higher rates of aneuploidy. Our observed association between ERβ expression and tumor aneuploidy undoubtedly needs to be validated in a larger data set, but it does suggest an intriguing biological association for ERβ in breast tumors that has not been previously appreciated.
We know very little about the specific function of ERβ in the breast. Studies of ERβ knock-out mice suggest that, although these mice have impaired ovarian function, breast development and function are not compromised (27). It has also been suggested that ERβ may function to inhibit the induction of PR by ERα, at least in the normal rodent mammary gland (28). However, this does not appear to be the case in human breast tumors, where there was no difference between the induction of PR in tumors expressing both receptors compared to those only expressing ERα. Our results strongly support the conclusion that ERα rather than ERβ is the predominant regulator of PR expression in clinical breast cancers, in agreement with reports of an inverse correlation of ERβ RNA expression and levels of the PR (20).
The failure of ERβ IHC measurements to correlate with ER or PR LBA is another relatively surprising finding from our study, especially since ERβ has a similar binding capacity for estradiol as ERα (2). Again, there is controversial data in the literature concerning this question, with one study also reporting no correlation between ERβ RNA and ER LBA (20), and one study reporting a good correlation between ERβ protein (using a carboxy-terminal-specific ERβ antibody), and PR IHC (8). These discordant results highlight the necessity of developing reliable ERβ-specific antibodies useful for clinical studies. In addition, since there are a number of carboxy-terminal truncated forms of ERβ (9), it is unsure what forms may be measured when using these different assays. It is possible that expression of these ERβ variant forms, especially in the ERα-negative / ERβ-positive subgroup of breast tumors, is indicative of a particular “bad” prognosis, equivalent to the truly ER-negative group.
Finally, RNA-based ERβ studies have also raised the possibility that the presence of ERβ in breast tumors may be a marker of endocrine therapy resistance. In a limited but provocative study of 17 breast cancer patients with treatment response follow-up, ERβ was significantly elevated in the tamoxifen-resistant group of tumors (29). This would be consistent with our observed inverse correlation with PR, since PR is a known marker of endocrine responsiveness. We did not have endocrine response data on our pilot study of 242 breast tumors, but a predictive clinical study is currently under way in tumors archived in the Baylor Breast Tumor Bank.
In summary, our results suggest that ERα rather than ERβ expression is correlated with most, but perhaps not all, prognostic factors in breast cancer. Furthermore, we present data suggesting that ERβ is not just a surrogate for ERα in breast cancer prognosis, and may have distinct but as yet unknown functions. Assessment of the ultimate clinical utility of ERβ IHC in breast cancer prognosis, and its possible usefulness for the prediction of treatment response, awaits its examination in large clinical studies.
Acknowledgments
This work was supported in part by NIH grant CA30195 and funding through the Breast Center at Baylor College of Medicine (to SAWF). The authors would like to thank Dawn Allen and Kim Kennedy for excellent secretarial assistance in preparation of this manuscript, and technical assistance from Carlos Gentry.
The abbreviations used are
ER
estrogen receptor
PR
progesterone receptor
IHC
immunohistochemistry
LBA
ligand binding assay
SPF
S-phase fraction
TA
tissue array
References
- 1.Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human oestrogen receptor cDNA; sequence, expression and homology to v-erbA. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 2.Kuiper G, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson J. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 3.Early_Breast_Cancer_Trialists’_Collaborative_Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy: 133 randomized trials involving 31000 recurrences and 24000 deaths among 75000 women. Lancet. 1992;339:1–15. 71–85. [PubMed] [Google Scholar]
- 4.Elledge RM, Fuqua SAW. Estrogen and Progesterone Receptors. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the Breast. Vol. 2. Philadelphia, PA: Lippincott, Williams & Wilkins; 2000. pp. 471–488. [Google Scholar]
- 5.Fuqua SA, Schiff R, Parra I, Friedrichs WE, Su JL, McKee DD, Slentz-Kesler K, Moore LB, Willson TM, Moore JT. Expression of wild-type estrogen receptor β and variant isoforms in human breast cancer. Cancer Res. 1999;59:5425–5428. [PubMed] [Google Scholar]
- 6.Hall JM, McDonnell DP. The estrogen receptor β-isoform (ER β) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 7.Speirs V, Parkes A, Kerin M, Walton D, Carleton P, Fox J, Atkin S. Coexpression of estrogen receptor α and β: poor prognostic factors in human breast cancer? Cancer Research. 1999;59:525–528. [PubMed] [Google Scholar]
- 8.Jarvinen T, Pelto-Huikko M, Holli K, Isola J. Estrogen Receptor β Is Coexpressed with ERα and PR and Associated with Nodal Status, Grade, and Proliferation Rate in Breast Cancer. Am J Pathol. 2000;156:29–35. doi: 10.1016/s0002-9440(10)64702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore J, McKee D, Slentz-Kesler K, Moore L, Jones S, Horne E, Su JL, Kliewer S, Lehmann J, Willson T. Cloning characterization of human estrogen receptor β isoforms. Biochemical and biophysical research communications. 1998;247:75–78. doi: 10.1006/bbrc.1998.8738. [DOI] [PubMed] [Google Scholar]
- 10.McGuire WL, Carbone PP, Vollmer EP, editors. Estrogen Receptors in Human Breast Cancer. New York: Raven Press; 1975. [Google Scholar]
- 11.Wenger CR, Beardslee S, Owens MA, Pounds G, Oldaker T, Vendely P, Pandian MR, Harrington D, Clark GM, McGuire WL. DNA ploidy, S-phase, and steroid receptors in more than 127,000 breast cancer patients. Breast Cancer Research and Treatment. 1993;28:9–20. doi: 10.1007/BF00666351. [DOI] [PubMed] [Google Scholar]
- 12.Brown RW, Allred DC, Clark GM, Osborne CK, Hilsenbeck SG. Prognostic value of Ki67 compared to S phase fraction in axillary node-negative breast cancer. Clinical Cancer Research. 1996;2:585–592. [PubMed] [Google Scholar]
- 13.Elston CE, Ellis IO. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 14.Patey DH, Scarff RW. The position of histology in the prognosis of carcinoma of the breast. Lancet. 1928;1:801–804. [Google Scholar]
- 15.Mosselman S, Polman J, Dijkema R. ER β : identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- 16.Fuqua SAW, Allred DC, Elledge RM, Krieg SL, Benedix MG, Nawaz Z, O’Malley BW, Greene GL, McGuire WL. The ER-positive/PgR-negative breast cancer phenotype is not associated with mutations within the DNA binding domain. Breast Cancer Res Treat. 1993;26:191–202. doi: 10.1007/BF00689692. [DOI] [PubMed] [Google Scholar]
- 17.Fuqua S, Wolf D. Molecular aspects of estrogen receptor variants in breast cancer. Breast Cancer Research and Treatment. 1995;35:233–241. doi: 10.1007/BF00665974. [DOI] [PubMed] [Google Scholar]
- 18.Allred DC, Clark gM, Tandon AK, McGuire WL. Immunohistochemistry on histological sections from small (50 mg) samples of pulverized breast cancer. J Histotechnol. 1993;16:117–120. [Google Scholar]
- 19.Harvey JM. Estrogen receptor status by immunohistochemistry is superior to the igand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. Journal of Clinical Oncology. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 20.Dotzlaw H, Leygue E, Watson P, Murphy L. Estrogen receptor-β RNA expression in human breast tumor biopsies: relationship to steroid receptor status and regulation by progestins. Cancer Research. 1999;59:529–532. [PubMed] [Google Scholar]
- 21.Clark GM. Prognostic and Predictive Factors. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the Breast. Vol. 2. Philadelphia, PA: Lippincott, Williams & Wilkins; 2000. [Google Scholar]
- 22.Ravdin PM. Prognostic factors in breast cancer. American Society of Clinical Onocology Educational Book. 1997:217–227. [Google Scholar]
- 23.Pavao M, Traish AM. Estrogen receptor antibodies: specificity and utility in detection, localization and analyses of estrogen receptor α and β. Steroids. 2001;66:1–16. doi: 10.1016/s0039-128x(00)00143-4. [DOI] [PubMed] [Google Scholar]
- 24.Chio I, Ko C, Park-Sarge OK, Nie R, Hess RA, Graves C, BSK Human estrogen receptor beta-specific antibodies: characterization and use in studies of estrogen receptor beta protein expression in reproductive tissues. Molecular and Cellular Endocrinology. 2001;181:139–150. doi: 10.1016/s0303-7207(01)00492-0. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa S, Inoue S, Watanabe T, Hiroi H, Orimo A, Hosoi T, Ouchi Y, Muramatsu M. The complete primary structure of human estrogen receptor β (hERβ) and its heterodimerization with ER α in vivo and in vitro. Biochemical Biphysical Research Communications. 1998;243:122–126. doi: 10.1006/bbrc.1997.7893. [DOI] [PubMed] [Google Scholar]
- 26.Pettersson K, Grandien K, Kuiper G, Gustafsson JA. Mouse estrogen receptor β forms estrogen response element-binding heterodimers with estrogen receptor α. Molecular Endocrinology. 1997;11:1486–1496. doi: 10.1210/mend.11.10.9989. [DOI] [PubMed] [Google Scholar]
- 27.Krege JH. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. National Academy of Sciences. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saji S, Jensen EV, Nilsson S, Rylander T, Warner M, Gustafsson J-A. Estrogen receptor α and β in the rodent mammary gland. Proceedings of the National Academy of Sciences (USA) 2000;97:337–342. doi: 10.1073/pnas.97.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Speirs V, Malone C, Walton DS, Kerin MJ, Atkin SL. Increased expression of estrogen receptor β mRNA in tamoxifen- resistant breast cancer patients. Cancer Res. 1999;59:5421–5424. [PubMed] [Google Scholar]