Characterization of kinases involved in the phosphorylation of aggregated α-synuclein (original) (raw)
. Author manuscript; available in PMC: 2015 Jun 29.
Published in final edited form as: J Neurosci Res. 2010 Dec 8;89(2):231–247. doi: 10.1002/jnr.22537
Abstract
α-Synuclein (α-syn) is the major component of pathological inclusions characteristic of several neurodegenerative disorders, such as Parkinson disease. The major post-translational modification of α-syn is phosphorylation at S129, and previous studies estimate that approximately 90% of α-syn in proteinaceous, pathological inclusions is phosphorylated at this site. α-Syn can be phosphorylated by polo-like kinases (PLKs) 1-3 and casein kinases (CK) 1 and 2; however the kinases associated with the hyperphosphorylation of aggregated α-syn is still under debate. Using a high-efficiency cellular model of α-syn aggregate formation, we found that selective inhibitors for CK2 and PLKs each partially inhibited S129 phosphorylation of soluble (non-aggregated) α-syn, but only PLK inhibitors modestly attenuated the phosphorylation of aggregated α-syn. In addition, none of kinase inhibitors used had a substantial effect on the propensity of α-syn to aggregate. Overexpression of PLKs each promoted robust phosphorylation of soluble α-syn, but none altered the propensity of α-syn to aggregate. Overexpression of only PLK2 increased phosphorylation of aggregated α-syn at S129, which is likely due to increased phosphorylation of soluble α-syn, which then incorporated into aggregates. Overexpression of PLK1 and treatment with BI2536 resulted in a significant reduction of phosphorylated, aggregated α-syn protein, beyond that of BI2536 treatment alone. These studies suggest that phosphorylation of α-syn is independent of α-syn aggregate formation, that PLK1 is involved in the phosphorylation of aggregated α-syn at S129 in this system, and that mechanisms resulting in hyperphosphorylation of aggregated α-syn appear independent from those responsible for the phosphorylation of soluble α-syn.
Keywords: α-synuclein, Parkinson disease, polo-like kinase, phosphorylation, aggregation
INTRODUCTION
Parkinson's disease in the second most prevalent neurodegenerative disease and the most prevalent movement disorder. PD is characterized by bradykinesia, resting tremor, cogwheel rigidity and postural instability (Gelb et al., 1999; Simuni and Hurtig, 2000), which are associated with the loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (Damier et al., 1999; Pakkenberg et al., 1991). Pathological analysis reveals intracytoplasmic, perikaryal inclusions, known as Lewy bodies in some remaining DA neurons, and inclusions in neuronal processes, termed Lewy neurites (Goedert, 2001; Lee and Trojanowski, 2006; von Bohlen Und, 2004). The major component of Lewy pathology is α-synuclein (α-syn), which is normally soluble and localized to presynaptic terminals (Goedert, 2001). α-Syn is also the major component of pathological inclusions in other neurodegenerative diseases, including dementia with Lewy bodies (DLB), Lewy body variant of Alzheimer's disease (LBVAD), and multiple systems atrophy (MSA), collectively termed α-synucleinopathies (Duda et al., 2000; Forman et al., 2005; Goedert, 2001; Lippa et al., 1999; Spillantini et al., 1997; Spillantini et al., 1998; Tu et al., 1998).
The major protein modification of α-syn is phosphorylation at residue S129. α-Syn is phosphorylated at low levels in the absence of pathology (Anderson et al., 2006; Fujiwara et al., 2002; Kahle et al., 2002; Waxman and Giasson, 2008); however, α-syn is hyperphosphorylated at S129 in pathological inclusions in post-mortem brain samples (Anderson et al., 2006; Fujiwara et al., 2002; Kahle et al., 2002; Neumann et al., 2002; Nishie et al., 2004; Waxman and Giasson, 2008). S129 phosphorylation slows fibril formation in vitro, which could be a cellular mechanism used to reduce inclusion formation (Paleologou et al., 2008; Waxman and Giasson, 2008). However, α-syn phosphorylation may be toxic in cell models and in vivo (Chau et al., 2009; Chen and Feany, 2005; Gorbatyuk et al., 2008; Kragh et al., 2009; Sugeno et al., 2008).
α-Syn serves as an in vitro substrate for many kinases: G-protein coupled receptor kinases (GRKs), casein kinase I (CK1) and II (CK2), and, most recently identified, polo-like kinases (PLKs) (Anderson et al., 2006; Fujiwara et al., 2002; Inglis et al., 2009; Mbefo et al., 2010; Okochi et al., 2000; Pronin et al., 2000; Waxman and Giasson, 2008). Cellular models show that activation or overexpression of particularly CK2 and PLKs can robustly increase the phosphorylation S129 of α-syn in a manner that can be blocked with specific inhibitors (Inglis et al., 2009; Mbefo et al., 2010; Waxman and Giasson, 2008). However, PLK2 and PLK3 phosphorylate α-syn in vitro, far more efficiently than CK2 (Inglis et al., 2009), and α-syn phosphorylation at S129 is largely reduced in PLK2−/− transgenic mice, supporting PLK involvement in α-syn phosphorylation in vivo (Inglis et al., 2009).
In vitro studies have shown that both soluble and fibrillized α-syn can be a substrate for CK1, CK2, PLK1, PLK2, and PLK3 at S129 (Mbefo et al., 2010; Paleologou et al., 2010; Waxman and Giasson, 2008). These studies have thoroughly investigated the abilities of these kinases in vitro, which suggest that they may robustly phosphorylate α-syn after aggregation. However, the ability of specific kinases to phosphorylate aggregated protein in vivo has yet to be determined. The current study utilizes a high-efficiency cellular model of fibrillar α-syn amyloid inclusion formation (Waxman and Giasson, 2010) to examine the involvement of specific kinases in the phosphorylation of α-syn aggregates that share similar properties to those observed in the pathological state. Using this system, we identified CK2 and PLKs as responsible for the phosphorylation of soluble α-syn at S129, but the data indicate that only PLKs and a potential uncharacterized kinase are involved with the phosphorylation of aggregated α-syn.
MATERIALS AND METHODS
Expression and purification of recombinant α-syn
The human α-syn cDNA was cloned into the Nde I and Hind III restriction sites of the bacterial expression vector pRK172. The pRK172 DNA construct expressing N-terminal truncated 21-140 α-syn (with a Met codon added before amino acid 21) was generously provided by Dr. Virginia Lee (University of Pennsylvania, Philadelphia, PA). α-Syn proteins were expressed in E. coli BL21 (DE3) and purified as previously described (Giasson et al., 2001; Greenbaum et al., 2005). Briefly, bacterial pellets harvested by centrifugation were re-suspended in high-salt buffer (0.75 M NaCl, 50 mM Tris, pH 7.4, 1 mM EDTA) containing a cocktail of protease inhibitors, heated to 100°C for 10 min and centrifuged at 70,000 × g for 30 min. α-Syn proteins were purified by size-exclusion chromatography followed by ion exchange chromatography. Supernatants were dialyzed into 100 mM NaCl, 20 mM Tris, pH 7.5 and applied onto a Superdex 200 gel filtration column (GE Healthcare, Piscataway, NJ) and separated by size exclusion chromatography. The fractions were assayed for the presence of the α-syn proteins by SDS-polyacrylamide gel electrophoresis (PAGE) followed by Coomassie Blue R-250 staining. All α-syn proteins were concentrated using Centriprep-10 units (Millipore Corp., Bedford, MA), dialyzed against 10 mM Tris, pH 7.5, applied to a Mono Q column (GE Healthcare) and eluted with a 0-0.5 M NaCl gradient.
Fibril preparation of recombinant α-Syn
For cellular experiments, recombinant generated 21-140 α-syn protein was assembled into filaments by incubation at 37°C at concentrations greater than 5 mg/ml in sterile phosphate buffered saline (PBS, Invitrogen) with continuous agitation. Experimentation was planned so that α-syn would be visibly assembled (by filamentous clusters observed in the solution) by the day of cellular experimentation. α-Syn fibrils were diluted to 1-3 mg/ml in sterile PBS and treated by water bath sonication for a minimum of 2 hours. Cells were treated with a final concentration of 1 μM of recombinant 21-140 α-syn fibril mix.
Cell culture and transfection
QBI293 cells were maintained using Dulbecco's Modified Eagle's Medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/ streptomycin. The mammalian-expression vector pcDNA3.1 cloned with WT human α-syn cDNA was previously described (Paxinou et al., 2001). Mammalian-expression plasmids containing cDNA for human PLK1, PLK2, and PLK3 were obtained from Origene (Rockville, MD). Cells were plated onto poly-D-lysined 6-well plates and transfected at approximately 40% confluency, using calcium phosphate precipitation, as previously described (Waxman and Giasson, 2010). Experimentation with multiple cDNA plasmids was performed with an equal proportion of each plasmid. Four hours after transfection, 1 μM (final concentration) of sonicated α-syn fibrils was added dropwise to media, after which cells were incubated over night at 37°C, 5% CO2.
Approximately 16 hours after transfection, calcium phosphate was washed two-times with PBS, and media was replaced with warm DMEM containing 3% FBS and 1% penicillin/ streptomycin (reduced serum). For the purpose of experimentation, the time of the media change was considered “Time 0.” Cells were harvested 24 hours after Time 0 by biochemical fractionation (method below), or by total cellular lysate retrieval in 1.5X Laemmli sample buffer (75 mM Tris-HCl, pH 6.8, 3% SDS, 15% glycerol, 3.75 mM EDTA, pH 7.4) and boiled. Protein concentration was determined using BCA protein assay reagent (Pierce Thermo Scientific). Equal amounts of protein (10 μg) were resolved by SDS-PAGE for analysis of total cellular lysate samples. 10 μg of triton-soluble samples were loaded with an equal volume of the equivalent condition of triton-insoluble protein, to allow for the determination of percent α-syn in the triton-insoluble fraction.
Drug treatments, where indicate by experimentation, were added directly to cell cultures 8 hours after removal of calcium phosphate and were matched by a 0.1% DMSO control. The following drugs treatments were performed for 16 hours: CK1 inhibitor D4476 [4-(4-(2,3-dihydrobenzo(1,4)dioxin-6-yl)-5-pyridin-2-yl-1H-imidazol-2-yl)benzamide; 10 μM; EMD Biosciences], CK2 inhibitors DMAT [a derivative of TBB; 2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole; 25 μM; Sigma-Aldrich, St. Louis, MO; (Pagano et al., 2004b; Pagano et al., 2004a)] and TBCA [(E)-3-(2,3,4,5-tetrabiomophenl)acrylic acid; 20 μM; EMD Biosciences; (Pagano et al., 2007)], and PLK inhibitors BI2536 [1 μM; Axon Medchem, Netherlands; (Inglis et al., 2009; Steegmaier et al., 2007)] and GW843682X [(5-(5,6-Dimethoxy-1H-benzimidazol-1-yl)-3-{[2-(trifluoromethyl)-benzyl]oxy}thiophene-2-carboxamide; 1 μM; Tocris, Ellisville, MO) (Johnson et al., 2007)], and MG132 [Z-Leu-Leu-Leu-al; 10 μM; Sigma-Aldrich].
Biochemical cellular fractionation
Cells were washed one time in ice-cold PBS, and samples were harvested in 25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 20 mM NaF, and a cocktail of protease inhibitors containing 1 mM phenylmethylsulfonyl and 1 mg/ml each of pepstatin, leupeptin, N-tosyl-L-phenylalanyl chloromethyl ketone, N-tosyl-lysine chloromethyl ketone and soybean trypsin inhibitor. Samples were sedimented at 100,000 × g for 30 min at 4°C. Supernatants were removed and pellets were sonicated in 1.5X Laemmli sample buffer. Sonication in Laemmli buffer dissociated higher molecular weight, triton-insoluble α-syn to primarily monomeric species for resolution by SDS-PAGE. Minimal SDS-resistent α-syn was observed. 5X SDS sample buffer was added and samples were heated to 100°C for 5 min prior to Western blot analysis. Equal proportions of supernatant (triton-soluble protein) and pellet (triton-insoluble protein) were loaded and analyzed as percent pelleted.
Antibodies
SNL4 is a polyclonal rabbit antibody raised against a synthetic peptide corresponding to amino acids 2-12 of α-syn (Giasson et al., 2000). SNL4 recognizes all LBs and GCIs in cases of PD, DLB, and MSA (Duda et al., 2000; Giasson et al., 2000). Syn514 is a mouse monoclonal antibody, which preferentially recognizes pathological α-syn (Duda et al., 2002; Waxman et al., 2008). pSer129 is a mouse monoclonal antibody that specifically recognizes α-syn phosphorylated at S129 (Waxman and Giasson, 2008). Additional antibodies include monoclonal anti-PLK1 (Santa Cruz Biotechnology, Santa Cruz, CA) – used for Western blot analysis, rabbit mAb anti-PLK1 (Cell Signaling Technology, Danvers, MA) – used for double-immunofluorescence, anti-PLK2 (Santa Cruz Biotechnology), and anti-PLK3 (Santa Cruz Biotechnology).
Western blot analysis
Protein samples were resolved by SDS-PAGE on 16% polyacrylamide gels for α-syn or 8% gels for PLKs, followed by electrophoretic transfer onto nitrocellulose membranes. Membranes were blocked in Tris buffered saline (TBS) with 5% dry milk, and incubated overnight with SNL-4 in TBS/ 5% dry milk or pSer129, PLK1, PLK2, or PLK3 in TBS/ 5% bovine serum albumin (BSA). Each incubation was followed by goat anti-mouse conjugated horseradish peroxidase (HRP) (Amersham Biosciences, Piscataway, NJ), goat anti-rabbit HRP (Cell Signaling Technology), or rabbit anti-goat HRP (Santa Cruz Biotechnology), and immunoreactivity was detected using chemiluminescent reagent (NEN, Boston, MA) followed by exposure onto X-ray film.
Cell culture immunofluorescence
Double-immunofluorescence of transfected cells was completed as previously described (Mazzulli et al., 2006; Waxman et al., 2009). Cells were fixed at −20°C with 100% MeOH for 30 min, followed by 50% MeOH and 50% acetone for 5 min. Following washes with PBS, coverslips were blocked with PBS containing 3% bovine serum albumin (BSA), 1% dry milk, and 1% fish gelatin. Primary antibodies were diluted into blocking solution for 1-2 h at room temperature. Following PBS washes, coverslips were incubated in secondary antibodies conjugated to Alexa 488 or Alexa 594 diluted in PBS with 5% BSA for 1 h. Nuclei were counterstained with Hoechst trihydrochloride trihydrate 33342 (Invitrogen), and coverslips were mounted using Fluoromount-G (Southern Biotech, Birmingham, AL).
Fluorescence microscopy
Immunofluorescence was captured on an Olympus BX51 fluorescence microscope mounted with a DP71 digital camera (Olympus, Center Valley, PA). Cellular co-localization images were captured on a Zeiss Axiovert 200M inverted confocal microscope mounted with a Zeiss LSM510 META NLO digital camera utilizing Zeiss LSM510 META V3.2 confocal microscope software (Zeiss, Thornwood, NY). Confocal images were captured with 63× oil optics, and all representative images were of one Z-plane of <0.7 μm.
In vitro kinase assays
Recombinant generated α-syn (8 μM) was incubated at 30°C for 5 min, 1 h, or 16 h in the presence or absence of commercially available recombinant PLK1, PLK2, or PLK3 (Invitrogen) kinase (100 nM) in a buffer containing 25 mM HEPES, pH 7.5, 50 mM NaCl, 2 mM dithiothreitol, 0.05 mg/ml bovine serum albumin, 200 μM ATP, 20 mM MgCl2. Reactions were performed in the absence or presence of kinase inhibitors, BI2536 (1 μM) or GW843682X (1 μM). Reactions were stopped with the addition of SDS sample buffer and heating to 100°C for 5 min. Relative levels of α-syn phosphorylation were assessed by Western blot analysis and by performing kinase reaction in the presence of [γ-32P]ATP. For amount of P-32 incorporation, samples were resolved onto a 16% SDS-polyacrylamide gel and exposed to a phosphoimaging screen (Molecular Dynamics, Piscataway, NJ), after which Coomassie blue-stained bands were excised and quantified by scintillation counts. Phosphate incorporation was calculated based on specific activity of [γ-32P]ATP, the molar ratio [γ-32P]ATP to cold ATP, the moles of α-syn resolved, and the counts per minute factoring in decay.
Quantitative analysis
Western blot data were quantified by ImageJ software (NIH, Bethesda, MD). Ratio of phosphorylated protein was determined by relative immunoreactivity of pSer129 divided by immunoreactivity of SNL4, and in some instances due to variability between exposures, calculated as a percent of that observed under control conditions. Data were also analyzed as a change of percent triton-insoluble, calculated as [triton-insoluble/(soluble+triton-insoluble)]*100. Comparisons between two groups were completed by two-way, parametric t-tests or by paired t-tests using GraphPad InStat software (San Diego, CA). Comparisons between multiple groups were performed by Kruskal-Wallis non-parametric ANOVA. Comparisons of groups standardized to control conditions were analyzed by one-sample t-tests. Each experiment was performed a minimum of three independent times.
RESULTS
Pharmacological inhibition of phospho-S129 in a cellular model of α-syn aggregation
To date, in situ or in vivo models have identified kinases involved in the phosphorylation of soluble, cytosolic α-syn at S129, and in vitro studies have thoroughly examined the ability of kinases to phosphorylate soluble and aggregated α-syn in isolated environments (Anderson et al., 2006; Fujiwara et al., 2002; Inglis et al., 2009; Mbefo et al., 2010; Okochi et al., 2000; Paleologou et al., 2010; Pronin et al., 2000; Waxman and Giasson, 2008). However, the kinases involved in the phosphorylation of aggregated α-syn at S129, in situ or in vivo, are still under debate. We therefore examined the efficacy of pharmacological inhibitors for CK1, CK2, and PLKs in a cellular model for α-syn aggregation (Waxman and Giasson, 2010).
QBI293 cells were transfected with wild-type α-syn and treated with recombinant 21-140 α-syn fibrils to induce the formation of intracellular α-syn aggregates, as previously characterized (Waxman and Giasson, 2010). 21-140 α-syn recombinant fibrils were used to assess, specifically, the incorporation of intracellularly expressed α-syn protein into cellular aggregates. It is not recognized by antibody SNL4, which recognizes an N-terminal epitope (Giasson et al., 2000), and it would migrate at a lower molecular weight than full-length α-syn. Therefore, full-length α-syn that is expressed in QBI293 cells could be easily differentiated by Western blot analysis and immunofluorescence from recombinant, fibrillar 21-140 α-syn that is exogenously added to the cells. Eight hours after the removal of calcium phosphate used for transfection, cultures were treated with inhibitors for CK2 [DMAT (25 μM) or TBCA (20 μM)], PLKs [BI2536 (1 μM) or GW843682X (GWX; 1 μM)], or CK1 [D4476 (10 μM)]. Cells were also treated with MG132 (10 μM), which robustly increases phosphorylation of α-syn at S129 through activation of CK2 (Waxman and Giasson, 2008). After 16 hours of treatment, cells were harvested, biochemical cellular fractionation was performed, and results were examined by Western blot analysis.
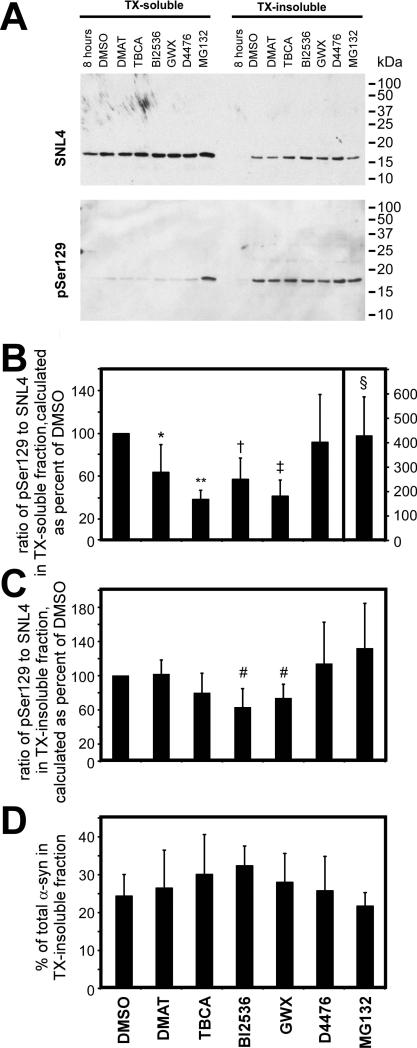
Relative phosphorylation of α-syn at S129 was determined by the ratio of an antibody specific to phospho-S129 [pSer129 (Waxman and Giasson, 2008)] to SNL4, an anti-α-syn antibody specific to the N-terminal region, and compared to this ratio to that observed under control conditions. Changes in phosphorylation were independently evaluated for the triton-soluble and triton-insoluble fractions of α-syn. At the start of drug treatment (8 hours), no significant triton-insoluble α-syn was observed (first lane, Fig. 1A). During the next 16 hours, substantial amounts of triton-insoluble α-syn accumulated due to the formation of intracellular, cytoplasmic amyloid aggregates (Waxman and Giasson, 2010). Treatment during this period (16 hours) with each of the inhibitors specific for CK2 or PLKs significantly reduced, while MG132 significantly increased, S129 phosphorylation of triton-soluble α-syn (Fig. 1A,B). Inhibition of CK1 had no effect on phosphorylation at S129. However, only PLK inhibitor treatment caused a significant, yet modest, reduction in phosphorylation of triton-insoluble α-syn (Fig. 1C). Therefore, of the kinases that were pharmacologically challenged, only PLKs contributed to S129 phosphorylation of triton-insoluble α-syn. None of the tested inhibitors resulted in large alterations in the percent of total α-syn observed in the triton-insoluble fraction (Fig. 1D). Double-immunofluorescence between pSer129 and Syn514 or SNL4 did not reveal any differences in immunoreactivity from control conditions, even with apoptotic nuclei observed with PLK inhibitor and MG132 treatments (data not shown).
Figure 1.
Examination of kinases that affect the phosphorylation of aggregated α-syn by treating cells with inhibitors after cellular seeding of α-syn. QBI293 cells were transfected with a plasmid for the expression of α-syn and then treated with recombinant 21-140 α-syn fibrils. The following drug treatments were initiated at 8 hours of calcium phosphate removal, as indicated in “Materials and Methods”: DMAT (25 μM) or TBCA (20 μM) for CK2 inhibition, BI2536 (1 μM) or GW843682X (GWX; 1 μM) for PLK inhibition, D4476 (10 μM) for CK1 inhibition, and MG132 (10 μM) for proteasome inhibition. Cells were harvested 16 hours after treatment. (A) Representative immunoblots of biochemical cellular fractionation of QBI293, separating triton X-100 (TX)-soluble α-syn protein from TX-insoluble protein. Phosphorylation of α-syn was assessed using antibody pSer129, and total α-syn was assessed with SNL4. (B) Quantitative analyses of the amount of pSer129 immunoreactivity in the TX-soluble fraction. Analysis was performed using a ratio of pSer129 to SNL4 immunoreactivity, and calculated as a percent of that observed in the DMSO control condition. Quantitation of the MG132 condition is indicated with the y-axis values at the far right. CK2 and PLK inhibitors significantly decreased, and MG132 significantly increased relative phosphorylation. p = 0.0006 by Kruskal-Wallis non-parametric ANOVA. Post-tests were performed by one-sample t-test since all conditions were standardized to DMSO control (*, p=0.04; **, p=<0.0001; †, p=0.009; ‡, p=0.001; §, p = 0.03; n=5). (C) Quantitative analyses of the amount of pSer129 immunoreactivity in the TX-insoluble fraction. Analysis was performed using a ratio of pSer129 to SNL4 immunoreactivity, and calculated as a percent of that observed in the DMSO control condition. Statistically significant inhibition was observed with BI2536 or GWX treatment p = 0.0175 by Kruskal-Wallis non-parametric ANOVA. Post-tests were performed by one-sample t-test since all conditions were standardized to DMSO control (#, p=0.02; n=5). (D) Quantitative analyses of the percent of α-syn observed in the triton-insoluble fraction (n=5). Drug treatments for all graphs are indicated at the bottom. All data represent average ± SD.
Co-transfection of PLKs increase phosphorylation at S129
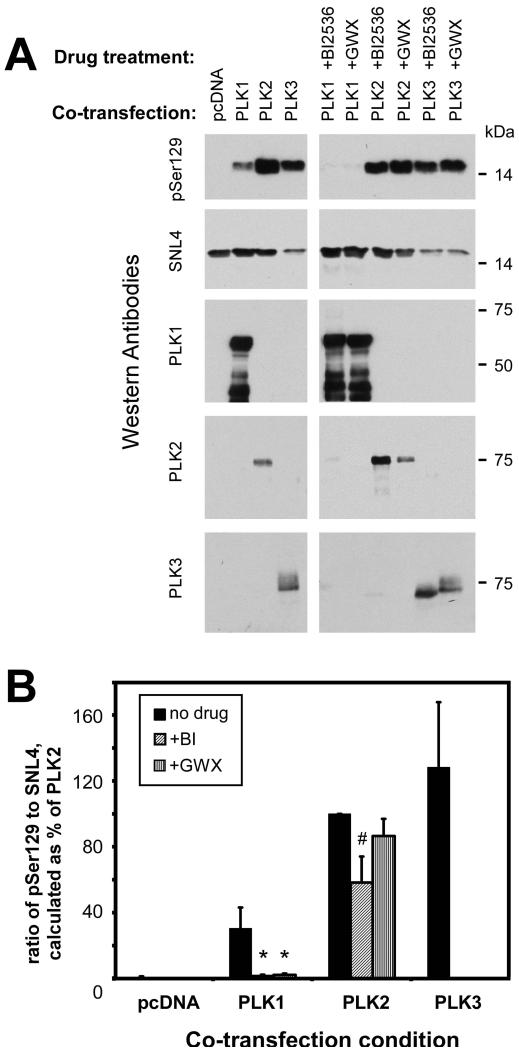
PLK1 and PLK3 mRNA transcripts have been identified in 293 cells (Tategu et al., 2008); however, of the PLKs, only PLK1 endogenous expression was identified by Western blot analysis (data not shown). PLK protein expression may be short-lived as a result of proteasomal degradation [for review, see (Lowery et al., 2005; Winkles and Alberts, 2005)]. We therefore co-transfected plasmids for the expression of PLK1, PLK2, or PLK3 with α-syn to examine the effects of these kinases on α-syn phosphorylation and aggregate formation. Co-expression of each PLK lead to significant increases in the phosphorylation of α-syn at S129 in the total cell lysate (Fig. 2), similar to that observed by others (Inglis et al., 2009; Mbefo et al., 2010). However, toxicity was observed with PLK3 co-expression, leading to a substantial reduction in α-syn expression. Quantitative assessment of the effects of PLK3 was highly variable, and subsequent Western blot analyses with PLK3 co-expression were therefore not informative. Inhibitors BI2536 and GWX significantly reduced S129 phosphorylation resulting from PLK1 overexpression by over 90%. However, BI2536 reduced S129 phosphorylation resulting from PLK2 overexpression by only ~40%, and GWX did not significantly reduce PLK2-induced phosphorylation. The combination of PLK3 and PLK inhibitors was highly toxic and did not result in any observed inhibition of phosphorylation (relative phosphorylation was not quantified with PLK3, but can be observed in Fig. 2A).
Figure 2.
Relative changes in phosphorylation of α-syn observed with co-expression of PLKs. Representative immunoblots (A) and quantitation (B) of QBI293 cells that were co-transfected with plasmids for the expression of α-syn and PLK1, PLK2, PLK3, or control (pcDNA). Total cellular lysates were harvested, and phosphorylation of α-syn was assessed as the ratio between antibodies pSer129 and SNL4, and standardized as a percent of phosphorylation observed in the PLK2 condition. Anti-PLK1, 2 or 3 antibodies were used to confirm overexpression of each kinase. Conditions were accompanied by BI2536 or GWX treatment for 16 hours prior to harvesting of cells. Each PLK significantly increased the phosphorylation of α-syn; however, PLK3 overexpression resulted in a reduction of α-syn expression. Treatment with BI2536 or GWX inhibited PLK1-induced phosphorylation (*, p=0.004 by t-way t-test; n=4). PLK2-induced phosphorylation of α-syn was only modestly inhibited by BI2536 (#, p=0.01 by one-sample t-test; n=4), and GWX did not significantly inhibit (p=0.08 by one-sample t-test). The combination of PLK3 overexpression and PLK inhibitors resulted in largely variable results due to toxicity and were therefore not quantified. Data represent average ± SD.
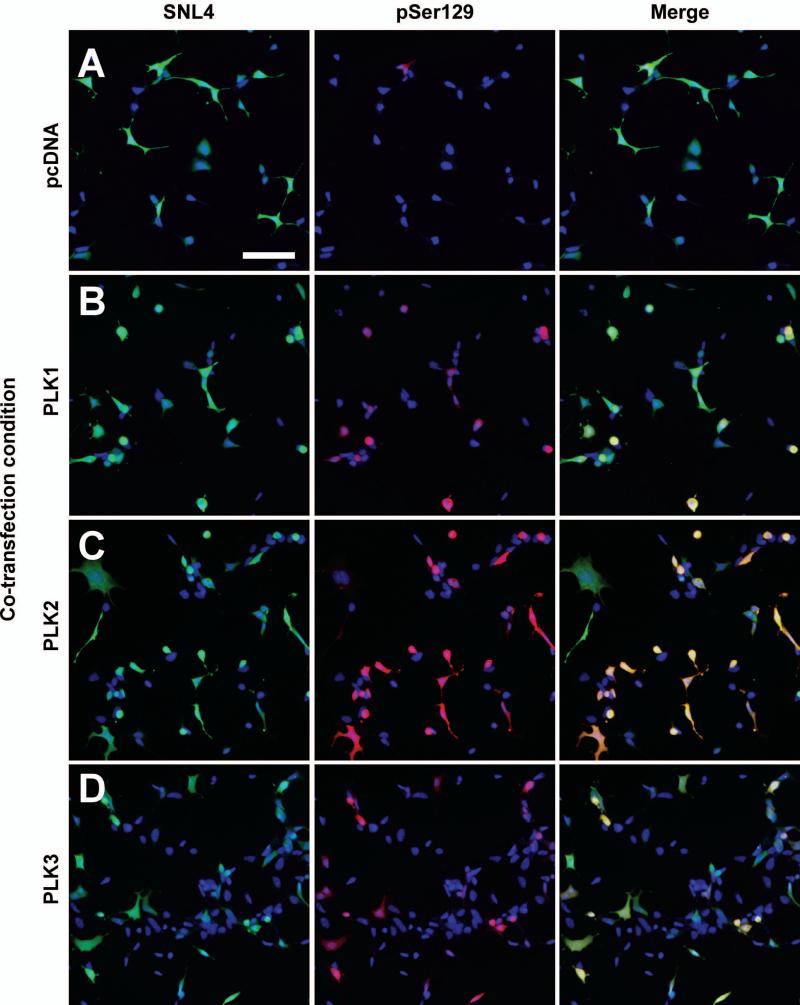
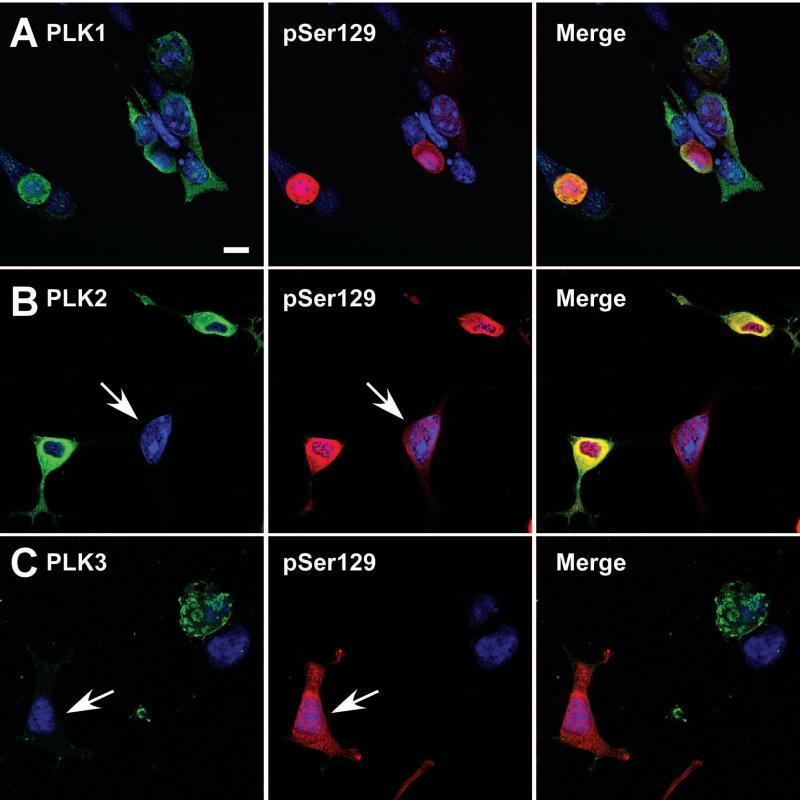
Double immunofluorescence performed between SNL4 and pSer129 antibodies for each of the co-expression conditions exhibited increased α-syn phosphorylation beyond that of vector control (Fig. 3). Many of the robustly pSer129-positive cells were also observed as rounded, particularly in PLK1 and PLK3 overexpression conditions. Overall SNL4 immunoreactivity was also reduced in the PLK3 overexpression condition. Co-localization between pSer129 and PLK1 or PLK2 was also observed by confocal microscopy (Fig.4). However, some cells exhibited greater than basal levels of pSer129 immunoreactivity, but with minimal anti-PLK2 or anti-PLK3 immunoreactivity (arrows), suggesting that PLK expression in these cells may be below the threshold of sensitivity afforded by the antibodies utilized. Nuclear pSer129 immunoreactivity was also noted in all conditions, as previously reported (Mbefo et al., 2010).
Figure 3.
Double-immunofluorescence with SNL4 (green) and pSer129 (red) on QBI293 cells transfected with plasmids for the expression of α-syn and (A) control vector (pcDNA), (B) PLK1, (C) PLK2, or (D) PLK3. Expression of each PLK resulted in increased pSer129 immunoreactivity. Many cells with robust pSer129 immunoreactivity were rounded in morphology, particularly in PLK1 and PLK3 co-expression conditions. Bar scale = 100 μm.
Figure 4.
Confocal microscopy of double immunofluorescence between anti-PLK1, anti-PLK2, or anti-PLK3 antibodies (green) and pSer129 (red). QBI293 cells were transfected with plasmids for the co-expression of α-syn and PLK1 (A), PLK2 (B), or PLK3 (C). (A) PLK1 overexpressing cells exhibited both normal and rounded morphology, and the robust pSer129 positive cells were typically of rounded morphology. In comparison, (B) PLK2 overexpressing cells presented robust pSer129 immunoreactivity in all cells, and (C) high expressing PLK3 cells exhibited rounded morphology. Some cells presented with increased pSer129 immunoreactivity, but with negligible anti-PLK immunoreactivity (arrows). Representative images are of a single Z-plane of <0.7 μm. Bar scale = 10 μm.
PLK1 expression and inhibition reduces the phosphorylation of triton-insoluble α-syn
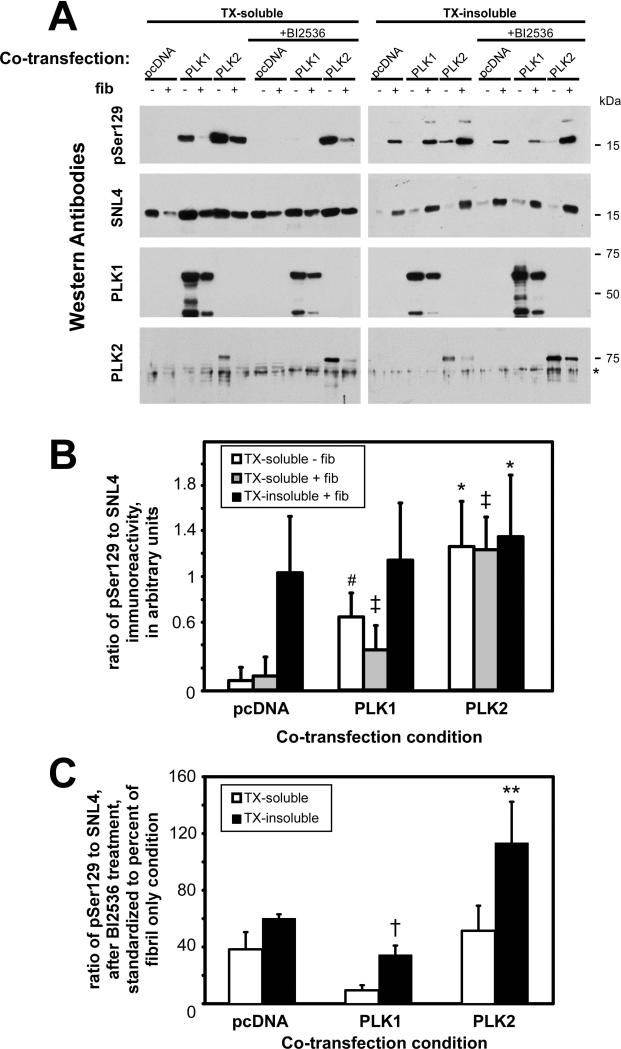
We then examined the effects of PLK co-expression on S129 phosphorylation of aggregated α-syn and the ability of α-syn to aggregate in situ. QBI293 cells were co-transfected with plasmids for the expression of α-syn and either pcDNA3.1 (vector control), PLK1, PLK2, or PLK3, and then treated with 21-140 recombinant α-syn fibrils. Using biochemical cellular fractionation followed by Western blot analyses, the triton-soluble α-syn increased in relative pSer129 immunoreactivity with either PLK1 or PLK2 overexpression in both the absence and presence of fibril treatment (Fig. 5A,B), similar to results observed with total cellular lysates (Fig. 2). However, only with PLK2 was there a significant increase (~30%) in relatively pSer129 immunoreactivity in the triton-insoluble fraction when compared to vector control.
Figure 5.
Biochemical cellular fractionation of QBI293 cells co-transfected with plasmids for α-syn and PLK1 or PLK2 expression, and then treated with recombinant α-syn fibrils. (A) Representative immunoblots of the triton X-100 (TX)-soluble and TX-insoluble fractions of cells in the absence (−) or presence (+) of recombinant fibril treatment (fib). Conditions were matched by cultures treated for 16 hours with BI2536 (1 μM). Antibodies pSer129 and SNL4 were used to evaluate the relative amount of α-syn phosphorylation in each sample. anti-PLK1 and anti-PLK2 immunoreactivities were also evaluated. * non-specific cross-reacting immunoreactivity. (B) Quantitative analyses of the ratio of pSer129 to SNL4 immunoreactivity. Data are provided as the relative ratio in arbitrary quantitative units. The amount of phosphorylation was independently analyzed for the samples in the TX-soluble fraction in the absence of fibrils, for the TX-soluble fraction in the presence of fibrils, and the TX-insoluble fraction in the presence of fibrils. With PLK1 or PLK2 overexpression, increased pSer129 immunoreactivity was observed in all TX-soluble fractions (white and grey bars). However, only PLK2 expression resulted in an increase of phosphorylation with fibrils in the TX-insoluble fraction. *, p=0.01; #, p=0.005; ‡, p=0.003 by paired t-test, compared to equivalent condition of pcDNA co-transfection; n=4. (C) Quantitative analyses of the percent of phosphorylation observed with BI2536 after recombinant fibril treatment, as standardized to the equivalent fibril treatment condition in the absence of drug treatment. Analyzing the TX-soluble and the TX-insoluble fractions independently, reductions in relative phosphorylation were observed in all conditions with the exception of the TX-insoluble fraction of the PLK2 co-transfection. When comparing the relative change in phosphorylation for each co-transfection condition, PLK1 overexpression and BI2536 treatment resulted in a significant decrease in the overall phosphorylation in the TX-insoluble fraction (†, p=0.0003 by two-way t-test compared to pcDNA + BI2536 + fibrils in TX-insoluble; n=4), and PLK2 overexpression and BI2536 treatment resulted in a significantly greater amount of phosphorylation (**, p=0.01 by two-way t-test compared to pcDNA + BI2536 + fibrils in TX-insoluble; n=4). Data represent average ± SD.
Cultures were also treated with the PLK inhibitor BI2536 for 16 hours prior to harvesting. In the presence of BI2536, relative pSer129 immunoreactivity was significantly reduced under all triton-soluble conditions with the greatest relative inhibition observed with PLK1 co-transfection (Fig. 5A,C). However, BI2536 only inhibited relative pSer129 immunoreactivity of triton-insoluble α-syn with fibril treatment in vector control and PLK1 vector co-transfection conditions (p<0.0001 for pcDNA; p=0.0002 for PLK1; by one-sample t-test; n=4). Surprisingly, BI2536 treatment significantly reduced the phosphorylation of triton-insoluble α-syn with PLK1 overexpression beyond that observed with vector control and BI2536 (64 ± 9% [SD] of pSer129:SNL4 ratio to vector control; p=0.04 by paired t-test; n=4). In these studies, neither the transfection conditions nor BI2536 treatment significantly altered the propensity of α-syn to aggregate.
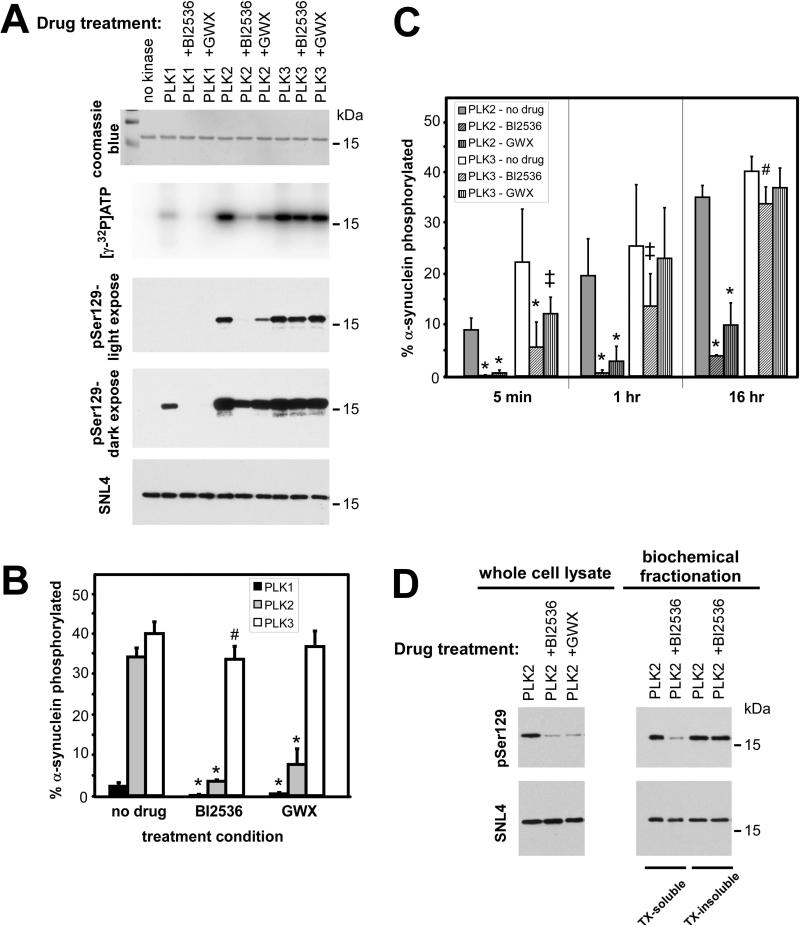
Previous studies indicate that BI2536 and GWX can inhibit PLK1, PLK2, and PLK3 (Johnson et al., 2007), and a previous study showed effective inhibition of PLK2-mediated S129 phosphorylation of α-syn (Inglis et al., 2009). We therefore examined the ability of these drugs to effectively inhibit these kinases in vitro. Recombinant, commercially available PLK1, PLK2, or PLK3 was incubated with recombinant-generated α-syn under kinase assay conditions, as described in “Materials and Methods.” Phosphorylation of α-syn was assessed after 16 hours in the presence or absence of BI2536 (1 μM) or GWX (1 μM), the same conditions utilized in situ. Quantitative assessment of [γ-32P]ATP incorporation by band excision followed by scintillation counts, determined approximately 15-fold greater phosphorylation by PLK2 and PLK3 than by PLK1 in the absence of inhibitors (Fig. 6A,B), similar to the relative activities of these kinases observed by others (Inglis et al., 2009; Mbefo et al., 2010). BI2536 and GWX significantly reduced phosphate incorporation by 97 ± 6% [SD] and 80 ± 12% [SD] for PLK1, respectively, and 90 ± 1% [SD] and 78 ± 11% [SD] for PLK2, respectively. However, due to the robust PLK2-mediated phosphorylation of α-syn, relative to PLK1, PLK2 + inhibitor yielded greater phosphorylation than PLK1 alone. PLK3 activity, however, was only modestly attenuated by BI2536 (15 ± 11% [SD]) and no significant reduction was observed with GWX.
Figure 6.
Inhibition of PLK-mediated phosphorylation of α-synuclein by BI2536 or GW843682X (GWX), in vitro. (A,B) α-Synuclein (8 μM) was incubated with PLK1, PLK2, or PLK 3 (100 nM) in the absence or presence of BI2536 or GWX (1 μM) for 16 h under conditions described in “Materials and Methods.” Representative experiment shows Coomassie blue staining of α-syn, [γ-32P]ATP phospho-imaging, and pSer129 and SNL4 immunoreactivity. Both high and low exposures with antibody pSer129 are shown. Percent phosphorylation of α-syn was determined by [γ-32P]ATP incorporation and quantitated in (B). BI2536 and GWX significantly inhibited PLK1 and PLK2 phosphorylation of α-syn, but only BI2536 significantly decreased PLK3-mediated phosphorylation (p<0.0001 by one-way ANOVA; *, p<0.001; #, p<0.05 by post-test with Bonferroni correction; n=6). (C) Timecourse of BI2536 or GWX inhibition of PLK2 or PLK3 phosphorylation of α-syn in vitro. Phosphate incorporation was assessed at 5 min, 1 hr, and 16 hr. BI2536 and GWX inhibited PLK2 at all timepoints, but they decreased in their effectiveness in inhibiting PLK3 over the course of time (*, p<0.001; ‡, p<0.01; #, p<0.05 by post-test with Bonferroni correction; n=9 for 5min and 1 hr). All data represent mean ± SD. (D) Representative immunoblots of re-analysis of samples transfected with PLK2 and treated with BI2536 or GWX. 1/10th of the protein was resolved for Western blot analysis, and levels of phosphorylation (pSer129) were determined relatively to SNL4 immunoreactivity. Phosphorylation in whole cell lysates of cells expressing PLK2 (left) were reduced to 12±3% [SD] with BI2536 and 22±20% [SD] with GWX (p=0.0004 and p=0.04, respectively, by one-sample t-test). Triton (TX)-soluble and TX-insoluble fractions of PLK2-expressing cells treated with prefibrillized, recombinant 21-140 α-syn were differentially affected by drug treatment. Phosphorylation of the TX-soluble fraction was reduced by BI2536 to 20±7% [SD] (p=0.003 by one-sample t-test); however, no change was observed with BI2536 in the TX-insoluble fraction (94±10% [SD]; n=3).
Since previous studies show robust phosphorylation by PLK3 after shorter timepoints (Mbefo et al., 2010), we examined comparative abilities of PLK2 and PLK3 to be inhibited by BI2536 or GWX at 5 min and at 1 hour in vitro (Fig. 6C). After 5 min BI2536 and GWX were capable of significantly inhibiting PLK2 (by 99.1 ± 0.1% [SD] and 91.4 ± 0.6% [SD], respectively) and PLK3 (by 76 ± 12% [SD] and 38 ± 23%, respectively). While inhibition of PLK2 was maintained at 1hr (97 ± 1% [SD] and 81 ± 21% [SD], respectively), inhibition of PLK3 was reduced to 47 ± 23% [SD] with BI2536 and 10 ± 9% [SD] with GWX. These data show that the relative inhibition of PLK3 is not maintained over the course of 16 hours.
Although BI2536 and GWX can significantly inhibit PLK2 in vitro, a more modest apparent reduction was observed in situ (Fig. 2). pSer129 immunoreactivity by Western blot analysis, depending on the exposure, identified disparities in relative phosphorylation in vitro (Fig. 6A). More apparent and significant inhibition by BI2536 and GWX was observed for PLK2 under low exposure conditions. High exposures allowing concurrent evaluation of PLK1-mediated phosphorylation significantly altered the observed immunoreactivity in PLK2-containing samples. We therefore re-examined whole lysate and biochemical fractionation samples (from Figs. 2 and 5) of cells expressing PLK2, such that one-tenth of the volume was resolved by SDS-PAGE, thereby promoting reduced pSer129 and SNL4 immunoreactivities for quantitation by densitometry (Fig. 6D). PLK2-expressing cells (whole cell lysate, left) exhibited pSer129 immunoreactivity that was significantly reduced to 12 ± 3% [SD] with BI2536 and 22 ± 20% [SD] with GWX, when compared against pSer129 immunoreactivity in the absence of drug. When PLK2-expressing cells were treated with recombinant, fibrillized 21-140 α-syn and then analyzed by biochemical cellular fractionation (right), BI2536 significantly reduced the phosphorylation in the triton-soluble fraction (to 18 ± 7% [SD]), but not in the triton-insoluble fraction (94 ± 10% [SD], when compared against PLK2-expressing cells in the absence of drug).
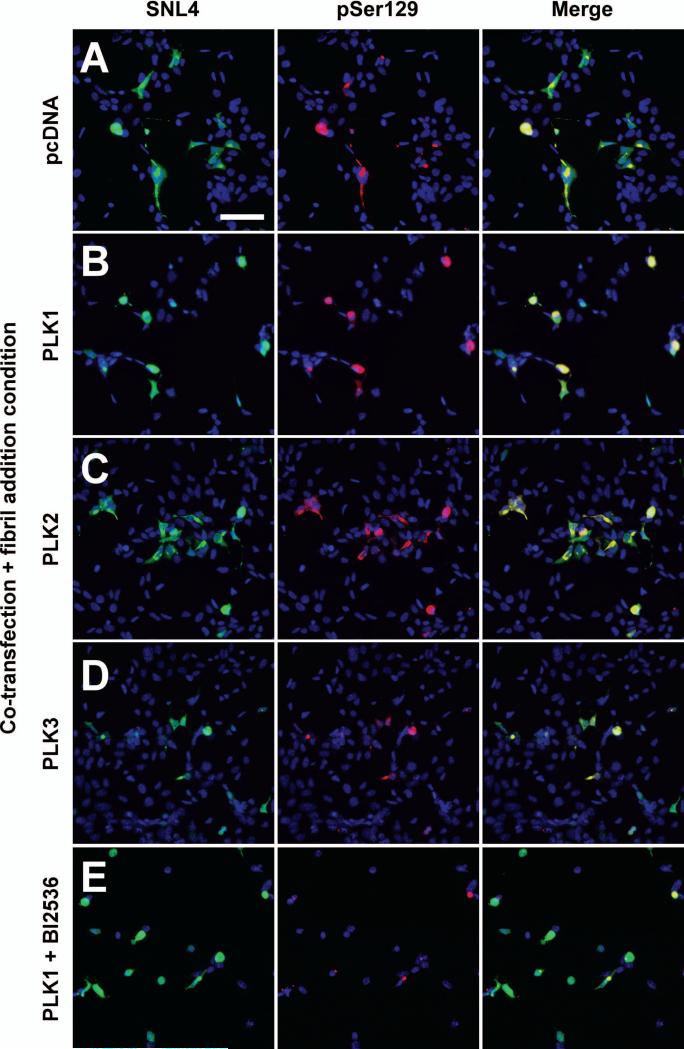
Double-immunofluorescence between SNL4 and pSer129 was performed to examine pSer129-positive aggregates with each transfection condition (Fig. 7). While pSer129 immunoreactivity appeared greater in aggregated form, pSer129 immunoreactivity of soluble α-syn was also observed with PLK1 and PLK2 overexpression (Fig. 7B,C). PLK3 overexpression with fibril treatment resulted in fewer α-syn expressing cells, but these cells also exhibited pSer129 positive aggregates (Fig. 7D). The combination of PLK1 overexpression and BI2536 treatment did not prevent α-syn expressing cells from developing pSer129 positive aggregates, despite the toxicity associated with BI2536 treatment (Fig. 7E). However, these aggregates displayed reduced pSer129 immunoreactivity.
Figure 7.
Double-immunofluorescence with SNL4 (green) and pSer129 (red) on QBI293 cells transfected with plasmids for the expression of α-syn and (A) control (pcDNA), (B) PLK1, (C) PLK2, or (D) PLK3, and then treated with recombinant 21-140 α-syn fibrils. pSer129-positive aggregates were observed in all conditions. Although diffuse, cytosolic pSer129-positive α-syn was observable in PLK1 and PLK2 overexpression conditions, pSer129 immunoreactivity of the aggregates was more robust. PLK3 overexpression and fibrils treatment resulted in fewer α-syn expressing cells, but these cells also presented with pSer129 positive aggregates. pSer129 positive aggregates were also observed with overexpression of PLK1 and BI2536 treatment (E); however pSer129 immunoreactivity was substantially reduced. Bar scale = 100 μm.
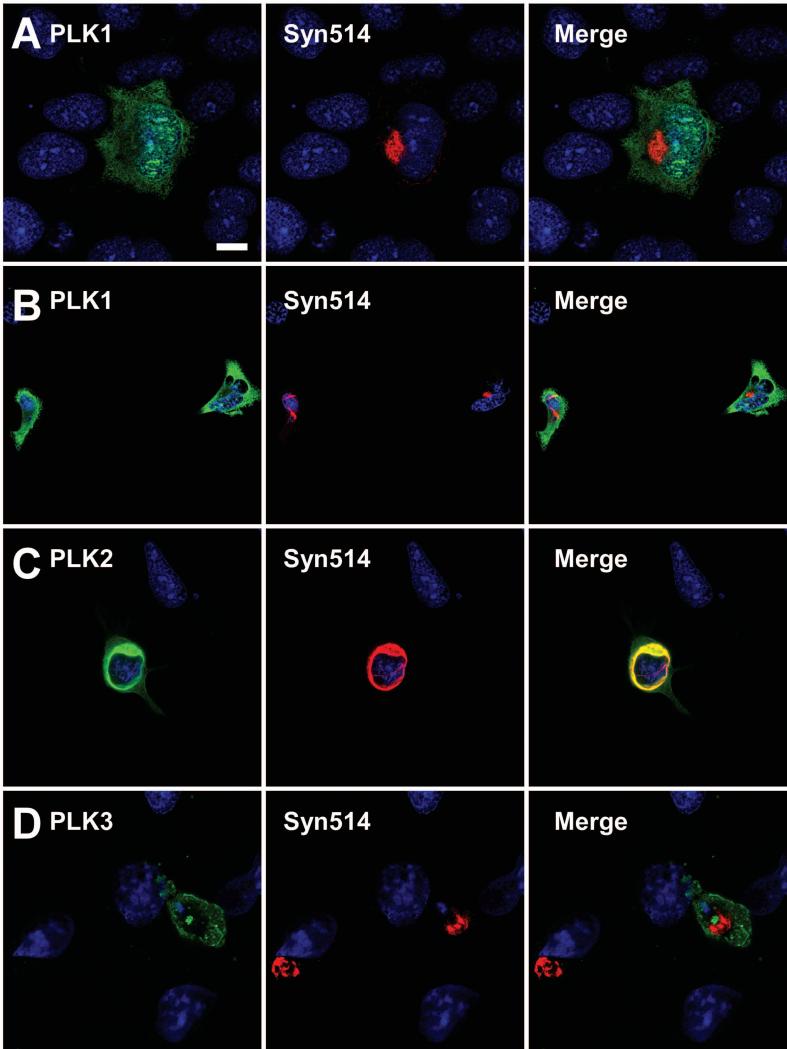
To examine potential associations between PLKs and aggregated α-syn in this system, confocal microscopy was performed using double-immunofluorescence between PLK antibodies and antibody Syn514, which preferentially recognizes pathological α-syn (Duda et al., 2002; Waxman et al., 2008). Anti-PLK1 immunoreactivity was observed to be generally diffuse, both in the cytosol and in the nucleus (Fig. 8A). Syn514 immunoreactivity appeared in the same vicinity as PLK1, but this co-localization appeared to be incidental in nature. No alterations in Syn514 or PLK1 localization were observed in the presence of BI2536 (Fig. 8B). Robust anti-PLK2 immunoreactivity was observed co-localizing with Syn514-positive aggregates (Fig. 8C). In contrast, anti-PLK3 immunoreactivity did not co-localize with Syn514 (Fig. 8D).
Figure 8.
Confocal microscopy of double immunofluorescence between anti-PLK1, anti-PLK2, or anti-PLK3 antibodies (green) and Syn514 (red) on QBI293 cells transfected with plasmids for the expression of α-syn and (A B) PLK1, (C) PLK2, or (D) PLK3, and then treated with recombinant 21-140 α-syn fibrils. PLK1 expression appeared diffuse both in the absence (A) and presence (B) of BI2536 treatment, and although scantly overlapping with the Syn514-positive aggregate, this was incidental and not specific. (C) PLK2 expression co-localized with Syn514 immunoreactivity. (D) PLK3 expression did not co-localize with Syn514. Representative images are of a single Z-plane of <0.7 μm. Bar scale = 10 μm for A,C,D; 17 μm for B.
DISCUSSION
The present study takes advantage of a high-efficiency cellular model of α-syn fibrillar inclusion formation to investigate a) kinases that may be involved in the phosphorylation of aggregated α-syn, b) effects associated with overexpression of PLKs, c) co-localization of PLKs and α-syn aggregates, and d) potential alterations in the propensity of α-syn to aggregate through mechanisms that either decrease or increase S129 phosphorylation of α-syn. This system offers distinct advantages, including direct pharmacological challenge, the ability to overexpress proteins, and the ability to quantitatively analyze differences by Western blot analysis. This examination of the phosphorylation of aggregated α-syn provides the cellular milieu that has been absent from similar studies, in vitro. Recent studies support membrane translocation and recycling of α-syn (Ahn et al., 2006; Liu et al., 2009) and suggest that fibrillized α-syn may be transferred between neurons (Desplats et al., 2009; Lee et al., 2008). This work is the first to examine the involvement of these specific kinases in the development of large, filamentous, hyperphosphorylated α-syn aggregates with characteristics similar to that observed in vivo.
Many kinases have been implicated in the phosphorylation of α-syn, including CK1, CK2, GRKs and PLKs (Anderson et al., 2006; Fujiwara et al., 2002; Inglis et al., 2009; Mbefo et al., 2010; Okochi et al., 2000; Pronin et al., 2000; Waxman and Giasson, 2008). Our initial aim was to identify which of these kinases may be involved in S129 phosphorylation of the aggregated, pathological form of α-syn. Pharmacological inhibition of CK2 and PLKs reduced S129 phosphorylation of soluble α-syn, but only PLK inhibitors affected phosphorylation of triton-insoluble α-syn. In addition, proteasomal inhibition, which increases the phosphorylation of α-syn through increasing CK2 activity (Chau et al., 2009; Waxman and Giasson, 2008), significantly increased the phosphorylation of soluble α-syn without significantly affecting the phosphorylation of triton-insoluble α-syn. These data suggest that several kinases are involve in α-syn phosphorylation in vivo and mechanisms that modify cytosolic verses aggregated α-syn may be independently regulated.
Recent studies support a mechanism in which S129 phosphorylation of α-syn may inhibit fibril formation (Paleologou et al., 2008; Waxman and Giasson, 2008), while fibrillized α-syn is a substrate for kinases (Mbefo et al., 2010; Paleologou et al., 2010; Waxman and Giasson, 2008). In these in vitro studies PLK1, PLK2, PLK3, CK1, and CK2 have been shown to phosphorylate both monomeric and fibrillar α-syn in vitro, which would account for the highly phosphorylated pathological α-syn observed in vivo. However, here we show that experimentation that increased the S129 phosphorylation of soluble α-syn, including proteasome inhibition and overexpression of PLKs, did not alter the propensity of α-syn to aggregate. We previously found that this cellular model is more efficient at promoting amyloid formation than similar studies in vitro (Waxman and Giasson, 2010), which may explain the lack of inhibition observed with induced hyperphosphorylation prior to aggregate formation. Animal models also suggest that phosphorylation neither promotes nor inhibits aggregate formation (Azeredo da Silveira et al., 2009). While the current study suggests that PLKs, and not CKs, may be at least partially involved in the S129 phosphorylation of aggregated α-syn, basal expression of PLKs in the nervous system is minimal (Kauselmann et al., 1999; Tategu et al., 2008; Winkles and Alberts, 2005). However, PLK1 neuronal expression is increased in Alzheimer's disease (AD) (Harris et al., 2000), PLK2 expression is increased in AD and DLB brain (Mbefo et al., 2010), and PLK2 and PLK3 expression increases in animal models after seizure induction (Kauselmann et al., 1999; Pak and Sheng, 2003). Therefore, expression of PLKs in the brain may be elevated in disease states.
By overexpressing PLKs in our model system, we aimed to directly examine the effects of PLKs directly on α-syn phosphorylation. BI2536 inhibited S129 phosphorylation of aggregated α-syn under control conditions by ~40%; however, when PLK1 was overexpressed, BI2536 inhibited this hyperphosphorylation by over 60%. These data support the ability of PLK1 to phosphorylate triton-insoluble α-syn. Further, PLK1 is also likely competing with another endogenous kinase that is responsible for the phosphorylation of aggregated α-syn at S129. This is the first study to provide a mechanism by which phosphorylation of aggregated α-syn may be significantly inhibited.
Only overexpression of PLK2 resulted in a significant increase of phosphorylation of aggregated α-syn at S129. However, while BI2536 significantly inhibited PLK2-mediated phosphorylation of α-syn in triton-soluble fractions, BI2536 did not alter phosphorylation of aggregated α-syn in PLK2-overexpressing cells. These data suggest that PLK2 phosphorylates cytosolic α-syn, and this phosphorylated form may then incorporate into the cellular aggregate, where it can be resistant to dephosphorylation (Waxman and Giasson, 2008), thereby contributing to the phosphorylation of triton-insoluble α-syn. Alternatively, PLK2 may be binding to the α-syn aggregate, promoting its phosphorylation, but in a manner that would prevent inhibition by BI2536. Nevertheless, given the abilities of BI2536 to inhibit PLK1-mediated phosphorylation of aggregated α-syn, PLK2 likely only interacts with soluble α-syn.
Investigations into the inhibitory properties of BI2536 and GWX in vitro revealed potent inhibition of PLK1 and PLK2. However, there was a time-dependent reduction in the inhibition of PLK3. The IC50s for these drugs on each PLK using other substrates is reportedly relatively similar (Johnson et al., 2007). However, PLK3-mediated phosphorylation of α-syn is more robust than PLK1 or PLK2 (Mbefo et al., 2010). In our assays, PLK3 activity was immediate, resulting in robust phosphorylation after 5 min. It appears that these drugs were not capable of completely inhibiting PLK3-mediated α-syn phosphorylation in vitro, and the partially inhibited PLK3 continues to phosphorylate α-syn over time until the maximum was reached. Our data suggest that α-syn phosphorylation by PLK3 also may not be effectively inhibited in situ by these drugs. Given these findings and the toxicity associated with overexpressing PLK3, it is difficult to experimentally assess the involvement of PLK3 in mediating phosphorylation of aggregated α-syn. It is possible that other kinases, in addition to PLKs, may be involved in the S129 phosphorylation of aggregated α-syn. Currently no specific inhibitors for GRKs are available. However, preliminary studies performed with Ro 32-0432, a PKC inhibitor that also blocks GRK activity (Aiyar et al., 2000), also did not reduce the phosphorylation of α-syn in the triton-insoluble pool (n=2; data not shown).
Of the PLKs, only PLK2 exhibited substantial co-localization with the α-syn aggregates when overexpressed. However, recent studies did not identify co-localization PLK2 or PLK3 with α-syn brain pathological inclusions (Mbefo et al., 2010). Further, PLK1, which the current study shows is involved with phosphorylation of α-syn aggregates in our model, did not co-localize with the aggregates. Therefore, kinases that may be involved in the phosphorylation of aggregated protein may not co-localize with aggregates. While it would be ideal to link a functional association with cellular co-localization, this may not be possible as kinases involved in α-syn phosphorylation may not be detectable or present in pathological aggregates.
Phosphorylation of α-syn at S129 is its major post-translational modification (Anderson et al., 2006; Fujiwara et al., 2002; Okochi et al., 2000), and approximately 90% of α-syn in pathological aggregates is phosphorylated at this residue (Fujiwara et al., 2002). While the normal function of this phosphorylation is presently under debate, hyperphosphorylation of α-syn has been linked to increased toxicity (Chau et al., 2009; Chen and Feany, 2005; Gorbatyuk et al., 2008; Kragh et al., 2009; Sugeno et al., 2008). Although kinases involved in the brain may be different than those presented in our model system, the current study presents the first cellular model providing mechanisms of the phosphorylation of aggregated α-syn. Future studies characterizing these kinases and examining their roles in vivo and in situ can provide valuable insights for the generation of therapeutics for disease.
ACKNOWLEDGEMENTS
We thank the Biochemical Imaging Core Facility supported by Abramson Cancer Institute at the University of Pennsylvania for assistance in confocal microscopy studies.
This work was funded by grants from the National Institute on Aging (AG09215) and the National Institute of Neurological Disorders and Stroke (NS053488). E.A.W. was supported by a training grant (T32 AG00255) from the National Institute on Aging.
REFERENCES
- Ahn KJ, Paik SR, Chung KC, Kim J. Amino acid sequence motifs and mechanistic features of the membrane translocation of alpha-synuclein. J Neurochem. 2006;97:265–279. doi: 10.1111/j.1471-4159.2006.03731.x. [DOI] [PubMed] [Google Scholar]
- Aiyar N, Disa J, Dang K, Pronin AN, Benovic JL, Nambi P. Involvement of G protein-coupled receptor kinase-6 in desensitization of CGRP receptors. European Journal of Pharmacology. 2000;403:1–7. doi: 10.1016/s0014-2999(00)00419-2. [DOI] [PubMed] [Google Scholar]
- Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, Barbour R, Huang J, Kling K, Lee M, Diep L, Keim PS, Shen X, Chataway T, Schlossmacher MG, Seubert P, Schenk D, Sinha S, Gai WP, Chilcote TJ. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- Azeredo da Silveira SZ, Schneider BL, Cifuentes-Diaz C, Sage D, bbas-Terki T, Iwatsubo T, Unser M, Aebischer P. Phosphorylation does not prompt, nor prevent, the formation of alpha-synuclein toxic species in a rat model of Parkinson's disease. Hum Mol Genet. 2009;18:872–887. doi: 10.1093/hmg/ddn417. [DOI] [PubMed] [Google Scholar]
- Chau KY, Ching HL, Schapira AH, Cooper JM. Relationship between alpha synuclein phosphorylation, proteasomal inhibition and cell death: relevance to Parkinson's disease pathogenesis. J Neurochem. 2009;110:1005–1013. doi: 10.1111/j.1471-4159.2009.06191.x. [DOI] [PubMed] [Google Scholar]
- Chen L, Feany MB. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat Neurosci. 2005;8:657–663. doi: 10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 1999;122(Pt 8):1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda JE, Giasson BI, Gur TL, Montine TJ, Robertson D, Biaggioni I, Hurtig HI, Stern MB, Gollomp SM, Grossman M, Lee VMY, Trojanowski JQ. Immunohistochemical and biochemical studies demonstrate a distinct profile of alpha-synuclein permutations in multiple system atrophy. J Neuropathol Exp Neurol. 2000;59:830–841. doi: 10.1093/jnen/59.9.830. [DOI] [PubMed] [Google Scholar]
- Duda JE, Giasson BI, Mabon ME, Lee VMY, Trojanoswki JQ. Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann Neurol. 2002;52:205–210. doi: 10.1002/ana.10279. [DOI] [PubMed] [Google Scholar]
- Forman MS, Lee VM, Trojanowski JQ. Nosology of Parkinson's disease: looking for the way out of a quackmire. Neuron. 2005;47:479–482. doi: 10.1016/j.neuron.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. α-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Jakes R, Goedert M, Duda JE, Leight S, Trojanowski JQ, Lee VMY. A panel of epitope-specific antibodies detects protein domains distributed throughout human alpha-synuclein in Lewy bodies of Parkinson's disease. J Neurosci Res. 2000;59:528–533. doi: 10.1002/(SICI)1097-4547(20000215)59:4<528::AID-JNR8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Murray IVJ, Trojanowski JQ, Lee VMY. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem. 2001;276:2380–2386. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- Gorbatyuk OS, Li S, Sullivan LF, Chen W, Kondrikova G, Manfredsson FP, Mandel RJ, Muzyczka N. The phosphorylation state of Ser-129 in human alpha-synuclein determines neurodegeneration in a rat model of Parkinson disease. Proc Natl Acad Sci U S A. 2008;105:763–768. doi: 10.1073/pnas.0711053105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum EA, Graves CL, Mishizen-Eberz AJ, Lupoli MA, Lynch DR, Englander SW, Axelsen PH, Giasson BI. The E46K mutation in alpha -synuclein increases amyloid fibril formation. J Biol Chem. 2005;280:7800–7807. doi: 10.1074/jbc.M411638200. [DOI] [PubMed] [Google Scholar]
- Harris PL, Zhu X, Pamies C, Rottkamp CA, Ghanbari HA, McShea A, Feng Y, Ferris DK, Smith MA. Neuronal polo-like kinase in Alzheimer disease indicates cell cycle changes. Neurobiol Aging. 2000;21:837–841. doi: 10.1016/s0197-4580(00)00218-9. [DOI] [PubMed] [Google Scholar]
- Inglis KJ, Chereau D, Brigham EF, Chiou SS, Schobel S, Frigon NL, Yu M, Caccavello RJ, Nelson S, Motter R, Wright S, Chian D, Santiago P, Soriano F, Ramos C, Powell K, Goldstein JM, Babcock M, Yednock T, Bard F, Basi GS, Sham H, Chilcote TJ, McConlogue L, Griswold-Prenner I, Anderson JP. Polo-like kinase 2 (PLK2) phosphorylates alpha-synuclein at serine 129 in central nervous system. J Biol Chem. 2009;284:2598–2602. doi: 10.1074/jbc.C800206200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EF, Stewart KD, Woods KW, Giranda VL, Luo Y. Pharmacological and Functional Comparison of the Polo-like Kinase Family: Insight into Inhibitor and Substrate Specificity. Biochemistry. 2007;46:9551–9563. doi: 10.1021/bi7008745. [DOI] [PubMed] [Google Scholar]
- Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Spooren W, Fuss B, Mallon B, Macklin WB, Fujiwara H, Hasegawa M, Iwatsubo T, Kretzschmar HA, Haass C. Hyperphosphorylation and insolubility of alpha-synuclein in transgenic mouse oligodendrocytes. EMBO Rep. 2002;3:583–588. doi: 10.1093/embo-reports/kvf109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauselmann G, Weiler M, Wulff P, Jessberger S, Konietzko U, Scafidi J, Staubli U, Bereiter-Hahn J, Strebhardt K, Kuhl D. The polo-like protein kinases Fnk and Snk associate with a Ca(2+)- and integrin-binding protein and are regulated dynamically with synaptic plasticity. EMBO J. 1999;18:5528–5539. doi: 10.1093/emboj/18.20.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragh CL, Lund LB, Febbraro F, Hansen HD, Gai WP, El-Agnaf O, Richter-Landsberg C, Jensen PH. +¦-Synuclein Aggregation and Ser-129 Phosphorylation-dependent Cell Death in Oligodendroglial Cells. J Biol Chem. 2009;284:10211–10222. doi: 10.1074/jbc.M809671200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Bae EJ, Lee JH, Paik SR, Lee SJ. Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol. 2008;40:1835–1849. doi: 10.1016/j.biocel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Lee VM, Trojanowski JQ. Mechanisms of Parkinson's disease linked to pathological alpha-synuclein: new targets for drug discovery. Neuron. 2006;52:33–38. doi: 10.1016/j.neuron.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Lippa CF, Schmidt ML, Lee VM, Trojanowski JQ. Dementia with Lewy bodies. Neurology. 1999;52:893. doi: 10.1212/wnl.52.4.893. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang JP, Shi M, Quinn T, Bradner J, Beyer R, Chen S, Zhang J. Rab11a and HSP90 regulate recycling of extracellular alpha-synuclein. J Neurosci. 2009;29:1480–1485. doi: 10.1523/JNEUROSCI.6202-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery DM, Lim D, Yaffe MB. Structure and function of Polo-like kinases. Oncogene. 2005;24:248–259. doi: 10.1038/sj.onc.1208280. [DOI] [PubMed] [Google Scholar]
- Mazzulli JR, Mishizen AJ, Giasson BI, Lynch DR, Thomas SA, Nakashima A, Nagatsu T, Ota A, Ischiropoulos H. Cytosolic catechols inhibit alpha-synuclein aggregation and facilitate the formation of intracellular soluble oligomeric intermediates. J Neurosci. 2006;26:10068–10078. doi: 10.1523/JNEUROSCI.0896-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbefo MK, Paleologou KE, Boucharaba A, Oueslati A, Schell H, Fournier M, Olschewski D, Yin G, Zweckstetter M, Masliah E, Kahle PJ, Hirling H, Lashuel HA. Phosphorylation of synucleins by members of the Polo-like kinase family. J Biol Chem. 2010;285:2807–2822. doi: 10.1074/jbc.M109.081950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Kahle PJ, Giasson BI, Ozmen L, Borroni E, Spooren W, Muller V, Odoy S, Fujiwara H, Hasegawa M, Iwatsubo T, Trojanowski JQ, Kretzschmar HA, Haass C. Misfolded proteinase K-resistant hyperphosphorylated alpha-synuclein in aged transgenic mice with locomotor deterioration and in human alpha-synucleinopathies. J Clin Invest. 2002;110:1429–1439. doi: 10.1172/JCI15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishie M, Mori F, Fujiwara H, Hasegawa M, Yoshimoto M, Iwatsubo T, Takahashi H, Wakabayashi K. Accumulation of phosphorylated alpha-synuclein in the brain and peripheral ganglia of patients with multiple system atrophy. Acta Neuropathol (Berl) 2004;107:292–298. doi: 10.1007/s00401-003-0811-1. [DOI] [PubMed] [Google Scholar]
- Okochi M, Walter J, Koyama A, Nakajo S, Baba M, Iwatsubo T, Meijer L, Kahle PJ, Haass C. Constitutive phosphorylation of the Parkinson's disease associated alpha-synuclein. J Biol Chem. 2000;275:390–397. doi: 10.1074/jbc.275.1.390. [DOI] [PubMed] [Google Scholar]
- Pagano MA, Andrzejewska M, Ruzzene M, Sarno S, Cesaro L, Bain J, Elliott M, Meggio F, Kazimierczuk Z, Pinna LA. Optimization of protein kinase CK2 inhibitors derived from 4,5,6,7-tetrabromobenzimidazole. J Med Chem. 2004a;47:6239–6247. doi: 10.1021/jm049854a. [DOI] [PubMed] [Google Scholar]
- Pagano MA, Meggio F, Ruzzene M, Andrzejewska M, Kazimierczuk Z, Pinna LA. 2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole: a novel powerful and selective inhibitor of protein kinase CK2. Biochem Biophys Res Commun. 2004b;321:1040–1044. doi: 10.1016/j.bbrc.2004.07.067. [DOI] [PubMed] [Google Scholar]
- Pagano MA, Poletto G, DiMaira G, Cozza G, Ruzzene M, Sarno S, Bain J, Elliott M, Moro S, Zagotto G, Meggio F, Pinna LA. Tetrabromocinnamic acid (TBCA) and related compounds represent a new class of specific protein kinase CK2 inhibitors. Chembiochem. 2007;8:129–139. doi: 10.1002/cbic.200600293. [DOI] [PubMed] [Google Scholar]
- Pak DTS, Sheng M. Targeted Protein Degradation and Synapse Remodeling by an Inducible Protein Kinase. Science. 2003;302:1368–1373. doi: 10.1126/science.1082475. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Moller A, Gundersen HJ, Mouritzen DA, Pakkenberg H. The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson's disease estimated with an unbiased stereological method. J Neurol Neurosurg Psychiatry. 1991;54:30–33. doi: 10.1136/jnnp.54.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paleologou KE, Oueslati A, Shakked G, Rospigliosi CC, Kim HY, Lamberto GR, Fernandez CO, Schmid A, Chegini F, Gai WP, Chiappe D, Moniatte M, Schneider BL, Aebischer P, Eliezer D, Zweckstetter M, Masliah E, Lashuel HA. Phosphorylation at S87 is enhanced in synucleinopathies, inhibits alpha-synuclein oligomerization, and influences synuclein-membrane interactions. J Neurosci. 2010;30:3184–3198. doi: 10.1523/JNEUROSCI.5922-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paleologou KE, Schmid AW, Rospigliosi CC, Kim HY, Lamberto GR, Fredenburg RA, Lansbury PT, Jr., Fernandez CO, Eliezer D, Zweckstetter M, Lashuel HA. Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein. J Biol Chem. 2008;283:16895–16905. doi: 10.1074/jbc.M800747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinou E, Chen Q, Weisse M, Giasson BI, Norris EH, Rueter SM, Trojanowski JQ, Lee VMY, Ischiropoulos H. Induction of alpha-synuclein aggregation by intracellular nitrative insult. J Neurosci. 2001;21:8053–8061. doi: 10.1523/JNEUROSCI.21-20-08053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronin AN, Morris AJ, Surguchov A, Benovic JL. Synucleins are a novel class of substrates for G protein-coupled receptor kinases. J Biol Chem. 2000;275:26515–26522. doi: 10.1074/jbc.M003542200. [DOI] [PubMed] [Google Scholar]
- Simuni T, Hurtig HI. Parkinson's disease: the clinical picture. In: Clark CM, Trojanoswki JQ, editors. Neurodegenerative dementias. McGraw-Hill; New York: 2000. pp. 193–203. [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson's disease and dementia with Lewy bodies. Neurosci Lett. 1998;251:205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Steegmaier M, Hoffmann M, Baum A, Lenart P, Petronczki M, Krssak M, Gnrtler U, Garin-Chesa P, Lieb S, Quant J, Grauert M, Adolf GR, Kraut N, Peters JM, Rettig WJ. BI 2536, a Potent and Selective Inhibitor of Polo-like Kinase 1, Inhibits Tumor Growth In Vivo. Current Biology. 2007;17:316–322. doi: 10.1016/j.cub.2006.12.037. [DOI] [PubMed] [Google Scholar]
- Sugeno N, Takeda A, Hasegawa T, Kobayashi M, Kikuchi A, Mori F, Wakabayashi K, Itoyama Y. Serine 129 phosphorylation of alpha-synuclein induces unfolded protein response-mediated cell death. J Biol Chem. 2008;283:23179–23188. doi: 10.1074/jbc.M802223200. [DOI] [PubMed] [Google Scholar]
- Tategu M, Nakagawa H, Sasaki K, Yamauchi R, Sekimachi S, Suita Y, Watanabe N, Yoshid K. Transcriptional regulation of human polo-like kinases and early mitotic inhibitor. J Genet Genomics. 2008;35:215–224. doi: 10.1016/S1673-8527(08)60030-2. [DOI] [PubMed] [Google Scholar]
- Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, Nakajo S, Iwatsubo T, Trojanowski JQ, Lee VMY. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44:415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- von Bohlen Und HO. Synucleins and their relationship to Parkinson's disease. Cell Tissue Res. 2004;318:163–174. doi: 10.1007/s00441-004-0921-7. [DOI] [PubMed] [Google Scholar]
- Waxman EA, Covy JP, Bukh I, Li X, Dawson TM, Giasson BI. Leucine-rich repeat kinase 2 expression leads to aggresome formation that is not associated with alpha-synuclein inclusions. J Neuropathol Exp Neurol. 2009;68:785–796. doi: 10.1097/NEN.0b013e3181aaf4fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman EA, Duda JE, Giasson BI. Characterization of antibodies that selectively detect alpha-synuclein in pathological inclusions. Acta Neuropathol. 2008;116:37–46. doi: 10.1007/s00401-008-0375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman EA, Giasson BI. Specificity and regulation of casein kinase-mediated phosphorylation of alpha-synuclein. J Neuropathol Exp Neurol. 2008;67:402–416. doi: 10.1097/NEN.0b013e3186fc995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman EA, Giasson BI. A novel, high-efficiency cellular model of fibrillar alpha-synuclein inclusions and the examination of mutations that inhibit amyloid formation. J Neurochem. 2010;113:374–388. doi: 10.1111/j.1471-4159.2010.06592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkles JA, Alberts GF. Differential regulation of polo-like kinase 1, 2, 3, and 4 gene expression in mammalian cells and tissues. Oncogene. 2005;24:260–266. doi: 10.1038/sj.onc.1208219. [DOI] [PubMed] [Google Scholar]