Quaternary structure defines a large class of amyloid-β oligomers neutralized by sequestration (original) (raw)
. Author manuscript; available in PMC: 2016 Jun 23.
Summary
The accumulation of amyloid-β (Aβ) as amyloid fibrils and toxic oligomers is an important step in the development of Alzheimer's disease (AD). However, there are numerous potentially toxic oligomers and little is known about their neurological effects when generated in the living brain. Here, we show that Aβ oligomers can be assigned to one of at least two classes (Type 1 and Type 2) based on their temporal, spatial and structural relationships to amyloid fibrils. The Type 2 oligomers are related to amyloid fibrils and represent the majority of oligomers generated in vivo, but remain confined to the vicinity of amyloid plaques and do not impair cognition at levels relevant to AD. Type 1 oligomers are unrelated to amyloid fibrils and may have greater potential to cause global neural dysfunction in AD because they are dispersed. These results refine our understanding of the pathogenicity of Aβ oligomers in vivo.
Introduction
Alzheimer's disease (AD) is believed to be caused by neurotoxic assemblies of the amyloid-β (Aβ) peptide. Distinct assemblies of Aβ have been found in the human brain, including amyloid fibrils and a few specific oligomers (Lasagna-Reeves et al., 2011; Lesne et al., 2013; Lu et al., 2013; Noguchi et al., 2009; Shankar et al., 2008). It is widely accepted that Aβ oligomers (Aβo) are more potent neurotoxins than amyloid fibrils, and several oligomers have been isolated and studied in detail. These oligomers differ in size and, to varying degrees, in their temporal and spatial patterns of expression, association with other brain proteins, and effects on neuronal function and viability (Lasagna-Reeves et al., 2011; Lesne et al., 2006; Lesne et al., 2013;Noguchi et al., 2009; Shankar et al., 2008). However, there is no general consensus as to which oligomers are the most significant in the pathophysiology of AD nor, indeed, on the total number of types of Aβo produced in the brain (reviewed in (Benilova et al., 2012)). The diversity of form and function among the Aβo studied thus far, and the likely existence of an indeterminate number of other Aβo, make the isolation and functional characterization of all potential forms a laborious, if not impossible, task. In an attempt to resolve this predicament, we asked whether Aβo generated in vivo could be categorized according to their spatiotemporal patterns of expression and structural features as inferred from their reactivity with “conformation-selective” antibodies, and, if so, whether the different classes of oligomers exert different pathological effects on neural function.
Studies using conformation-selective antibodies have identified at least two classes of oligomers that are generated in vitro and in the brains of AD patients and amyloid precursor protein (APP) transgenic mice (Glabe, 2008). The OC and A11 conformation-selective antibodies detect mutually exclusive structural epitopes of amyloid-forming proteins, independent of primary amino acid sequence (Kayed et al., 2007; Wu et al., 2010). OC antibodies recognize Aβ amyloid fibrils as well as Aβ oligomers (Kayed et al., 2007); it has been suggested, but never directly demonstrated, that OC detects in-register parallel β-sheets (Glabe, 2009; Wu et al., 2010). Conversely, A11 antibodies have been shown to recognize out-of-register anti-parallel β-sheet structures (Laganowsky et al., 2012; Liu et al., 2012). Our objective in the current work is to address the following questions related to Aβo generated in vivo: i) are there distinct subtypes of Aβo based on quaternary structural motifs, as defined by their reactivity with OC and A11 antibodies; ii) do OC- and A11-reactive oligomers differ in their spatial and temporal patterns of appearance; and iii) what is the most abundant type of Aβo produced in the brain and how does it affect neurological function?
To address these questions, we employed transgenic mice expressing APP variants linked to AD. The types of Aβo (e.g., Aβ dimers and Aβ*56, a putative dodecamer) and fibrils in mice vary dynamically, over a time scale of weeks to months (for examples in Tg2576, Arc6 and hAPP-J20 mice, see (Cheng et al., 2007; Lesne et al., 2006; Shankar et al., 2009)). In the lines that have been characterized, the sequence of appearance of specific Aβ assemblies is consistent. Here, we studied four lines of transgenic mice. In Tg2576 (Hsiao et al., 1996), hAPP-J20 (Cheng et al., 2007) and TetO-APPSweInd mice (Jankowsky et al., 2005), the A11-reactive oligomer Aβ*56 appears in young mice ~3-6 months prior to dense-core plaques and correlates with spatial memory deficits (Cheng et al., 2007;Fowler et al., 2014; Lesne et al., 2006). In rTg9191 mice (Liu et al., in press), we found dense-core plaques emerging at ~12 months but no Aβ*56 at any age (Liu et al., in press).
Here, we show that OC antibodies selectively recognized quaternary structural motifs found in naturally occurring Aβ amyloid fibrils, namely in-register parallel β-sheets (for review, see (Glabe, 2009; Tycko, 2011)). We also show that OC-immunoreactive Aβo appeared only after plaque formation and that they were highly concentrated around amyloid plaques in the brains of APP transgenic mice. Conversely, A11-immunoreactive oligomers appeared prior to amyloid plaques in multiple lines of APP transgenic mice (here, and see also (Cheng et al., 2007; Fowler et al., 2014; Lesne et al., 2006)) and were dispersed in the brain parenchyma without any relationship to amyloid plaques. We classified the Aβo generated in the brains of these APP transgenic mice into two categories: Type 1, which have no temporal, spatial or structural relationship to amyloid fibrils, and Type 2, which are related to amyloid fibrils temporally, spatially and structurally. We found that in brains bearing dense-core plaques, Type 2 Aβo predominate, consistent with predictions from_in vitro_ studies (Cohen et al., 2013). However, Type 2 Aβo appear to have limited potential to diffuse away from dense-core plaques or to disrupt forebrain neural networks, as assessed by tests of cognition.
Results
OC antibodies selectively detect in-register parallel β-sheet structures
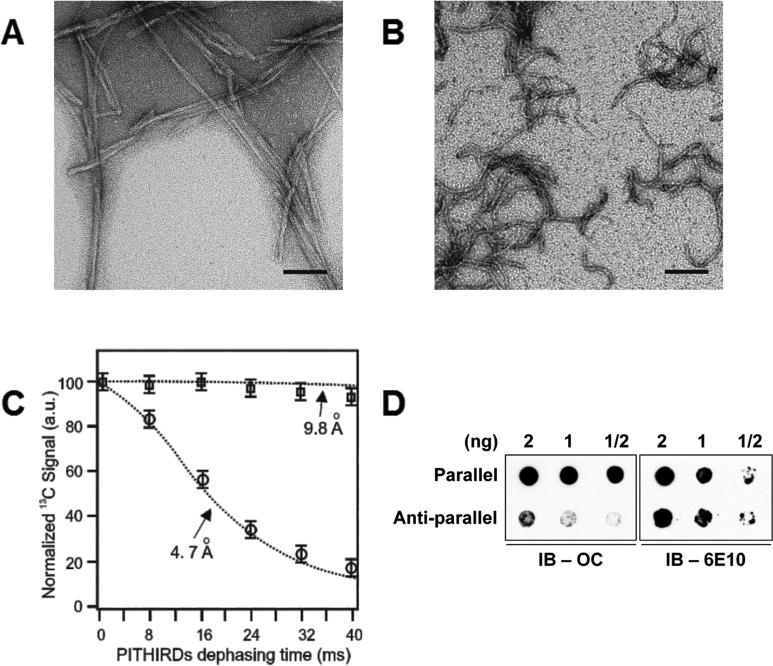
We first sought to more precisely define the structures recognized by OC and A11 antibodies. It was not possible to isolate from the brains of transgenic mice Aβo of sufficient purity or quantity to perform biophysical characterization of their structures, so we turned to synthetically prepared Aβ fibrils with defined quaternary structures. It had been suggested that OC detects in-register parallel β-sheets (Glabe, 2009; Wu et al., 2010), but this hypothesis had not been directly tested. Amyloid fibrils containing in-register parallel β-sheets or anti-parallel β-sheets were prepared from the 40-residue Aβ peptide with the AD-linked “Iowa” mutation (D23N_Aβ40). Transmission electron microscopy and solid state nuclear magnetic spectroscopy confirmed that these fibrils had the morphological features of “parallel” and “anti-parallel” fibrils whose backbone registries in the hydrophobic core regions were defined (Qiang et al., 2012; Sgourakis et al., 2015) (Figures 1A-1C). OC antibodies preferentially recognized parallel, over anti-parallel, fibrils in immunoblots (Figure 1D).
Figure 1. OC antibodies recognize in-register parallel β-sheet structures.
Transmission electron micrographs show D23N_Aβ40 fibrils with (A) in-register parallel β-sheet structure and (B) anti-parallel structure. (C) 13C-PITHIRDs-CT decay curves for parallel and anti-parallel fibrils with 13C labeling at Ala21-13C’. Theoretical decay curves with 4.7 and 9.8 angstrom 13C-13C distances are shown as dotted lines. Experimental data for the parallel and anti-parallel fibrils are indicated by circles and squares, respectively. The error bars were determined from the experimental spectral noise. (D)Left panel, dot blot shows that OC antibodies selectively detect D23N_Aβ40 fibrils with in-register parallel β-sheet structure. Right panel, blot shown at left was stripped and re-probed with monoclonal antibody 6E10 to confirm that ~equal amounts of parallel and anti-parallel fibrils were loaded. Scale bars (A,B): 100 nm. See also Figure S1.
It has also been suggested that A11 antibodies recognize structures containing anti-parallel β-sheets (Glabe, 2009; Wu et al., 2010), and A11 indeed reacts with synthetic oligomers composed of out-of-register anti-parallel β-sheets (Laganowsky et al., 2012; Liu et al., 2012). We first tested whether A11 antibodies prefer anti-parallel over parallel β-sheets, using synthetic Aβ fibrils. A11 antibodies also detected these synthetic fibrils, although with less sensitivity than did OC (Figure S1A). Similarly to OC, A11 preferred parallel fibrils to anti-parallel fibrils. While this result might seem at odds with the notion of two structurally distinct classes of oligomers, a potential resolution may be found in Figure S1B. We compared the A11 signal generated by synthetic Aβ fibrils to the signal generated by Aβo in the brains of 4-month-old hAPP-J20 mice, which lack OC-reactive Aβ species (see below). A much stronger signal was observed from the brain extracts, estimated to contain ~3 pg _total_Aβ, based on semi-quantitative analysis of Aβ in Western blots (data not shown) and previously reported ELISA measurements of Aβ levels in hAPP-J20 mice at this age (Cheng et al., 2007), than from ng quantities of the synthetic Aβ fibrils. We concluded that while A11 can bind amyloid fibrils, it preferentially recognizes some structural feature of the brain-derived Aβo that is not found in fibrils containing in-register β-sheets.
A11 antibodies recognize Aβo prior to plaque formation, while OC antibodies recognize Aβo only after plaques appear
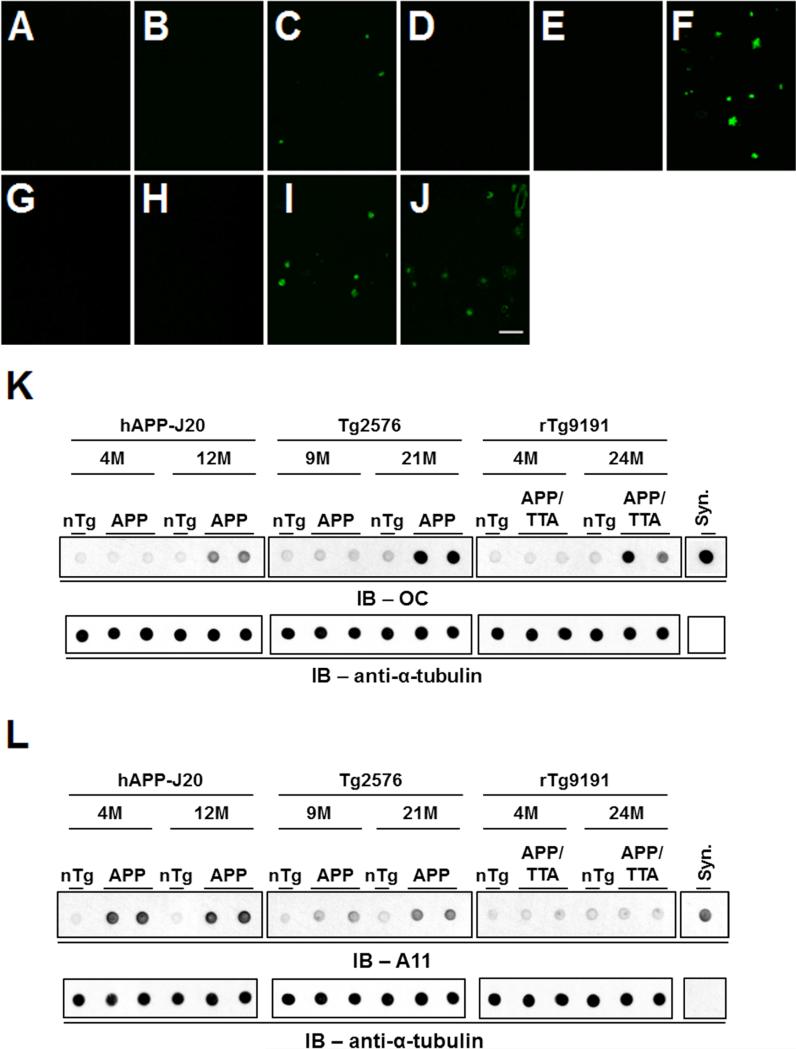
Aqueous extracts were prepared from the brains of Tg2576, hAPP-J20, and rTg9191 APP transgenic mice at ages prior to and following the appearance of dense-core plaques (Figures 2A-2J), and subjected to immunoblotting under non-denaturing conditions. OC immunoreactivity was seen only in extracts from plaque-containing brains in all three lines examined (Figures 2K and 6A; see also (Liu et al., in press)). Conversely, A11 immunoreactivity appeared prior to plaque deposition in Tg2576 and hAPP-J20 mice and remained present in plaque-bearing brains (Figure 2L); no A11 immunoreactivity was observed in rTg9191 brain extracts from any age (Figures 2L, S2A and S2B, and (Liu et al., in press)). A11 and OC immunoreactivity disappeared when extracts were immunodepleted of Aβ using a mixture of Aβ antibodies prior to immunoblotting (Figures S2C and S2D), demonstrating that the oligomers seen by A11 and OC in the brains of APP transgenic mice were indeed composed of Aβ. These results show that A11 and OC antibodies recognize different structures generated in vivo, as there exist brain-derived Aβ assemblies that are A11-positive/OC-negative (in young Tg2576 and hAPP-J20 mice) or OC-positive/A11-negative (in rTg9191 mice), extending the findings that OC and A11 recognize mutually exclusive epitopes on synthetic Aβo generated_in vitro_ (Kayed et al., 2007; Wu et al., 2010).
Figure 2. Age-dependent appearance of A11- and OC-immunoreactive Aβo.
(A-J) Brain sections stained with Thioflavin S to reveal dense-core plaques in cerebral cortex. A-C: hAPP-J20 (A, non-transgenic, 4M; B, hAPP-J20, 4M; C, hAPP-J20, 12M); D-F: Tg2576 (D, non-transgenic, 9M; E, Tg2576, 9M; F, Tg2576, 21M); G-I: rTg9191 (G, non-transgenic, 4M; H, rTg9191, 4M; I, rTg9191, 24M); J: AD brain. Scale bar in (J), 100 μm, applies to (A-J). (K) OC-reactive aggregates are seen after the appearance of dense-core plaques. Upper panels, dot blots showing protein aggregates detected by polyclonal OC antibodies in water-soluble brain extracts from hAPP-J20, Tg2576 and rTg9191 mice prior to (hAPP-J20, 4M; Tg2576, 9M; rTg9191, 4M) and after (hAPP-J20, 12M; Tg2576, 21M; rTg9191, 24M) the appearance of dense-core plaques. Extracts from non-transgenic littermates (nTg) are shown for comparison.
Syn, synthetic soluble Aβ aggregates with in-register parallel β-sheets (2 ng) were used as a positive control. Lower panels, α-tubulin served as the loading control. (L) A11-reactive aggregates are seen prior to the appearance of dense-core plaques.Upper panels, dot blots, prepared as in (K), but probed with A11 antibodies. Syn, synthetic Aβ40 oligomers prepared as in (Kayed et al., 2003). Lower panels, α-tubulin loading control. See also Figures S2 and S7.
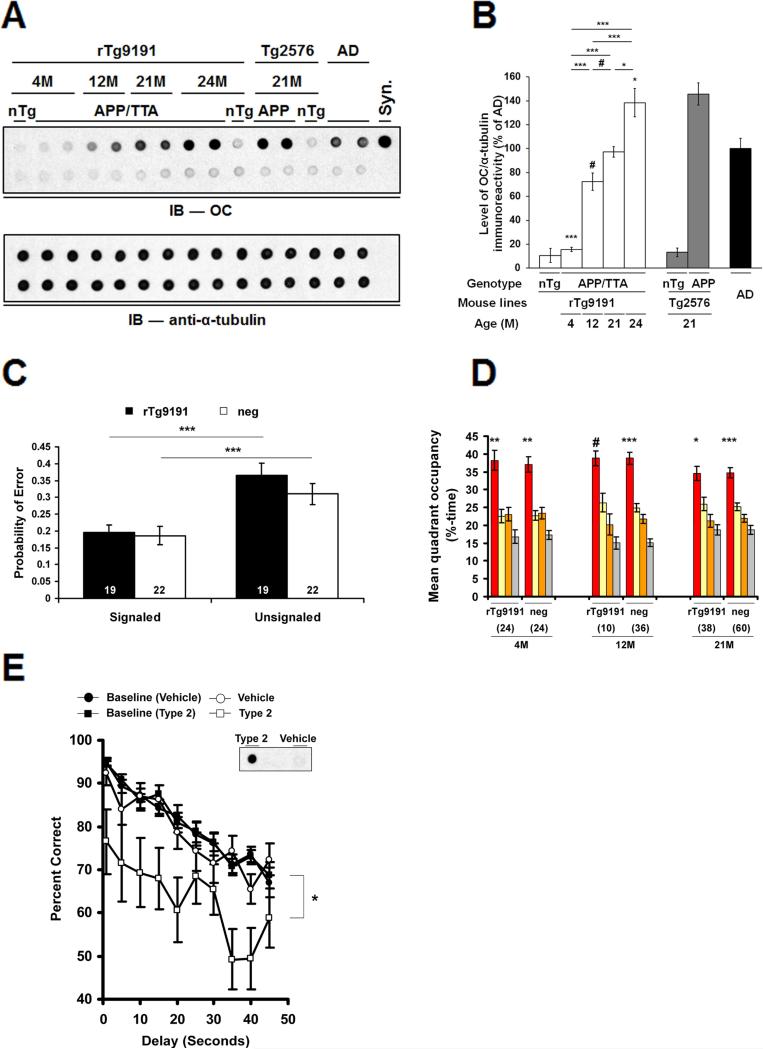
Figure 6. Type 2 Aβo do not disrupt cognition when located in situ in rTg9191 brains, but do impair cognition when dispersed.
(A-D) rTg9191 mice producing levels of Type 2 Aβo comparable to those of AD patients have intact cognition. (A) Upper panel, Dot blot showing the relative levels of soluble OC-reactive aggregates in the brains of rTg9191 mice, non-transgenic littermates (nTg) and AD patients. Ages of mice are shown above the blot. Upper lane, water-soluble brain extracts and synthetic aggregates; lower lane, samples immunodepleted of Aβ. Syn, synthetic soluble Aβ aggregates with in-register parallel β-sheets.Lower panel, α-tubulin served as the loading control for both the untreated (upper lane) and Aβ-immunodepleted extracts (lower lane). (B) Quantification of dot blots. Levels of OC-reactive aggregates in the brains of rTg9191 mice increase with age (# p < 0.05, * p < 0.01, *** p < 0.0001, one-way ANOVA followed by Fisher's post hoc analysis). (C) Cognitive performance in 23-month-old APP- positive (rTg9191) and negative (neg) rTg9191 mice do not differ in the Signaled and Unsignaled components of the fixed consecutive number (FCN-4) test. The probability of a given trial producing an error in the Signaled component is significantly lower than in the Unsignaled component, indicating intact motor and visual function (*** p < 0.0001, paired _t_-test). (D) Spatial reference memory in rTg9191 mice does not differ significantly from that of APP-neg littermates. Young (4M), middle-aged (12M) and old (21M) mice were tested in the water maze; mean %-time in each quadrant (target, red; left of target, yellow; right of target, orange; and opposite the target, grey) is shown. Significant search biases favoring the target quadrant were found in both rTg9191 mice and APP-neg littermates at all three ages. # p < 0.05, * p < 0.01, ** p < 0.001, *** p < 0.0001, percentage time spent in the target compared to that in each of the other three quadrants, repeated measures ANOVA (RMANOVA) followed by Fisher's _post hoc_analysis. Numerals in (C) and (D) represent the number of mice tested. (E) Water-soluble brain extracts of rTg9191 mice containing Type 2 Aβo disrupt cognition when exogenously administered to rats. Rats (N = 18) previously trained on a delayed non-matched to position task were injected intracerebroventricularly with brain extracts of rTg9191 mice containing Type 2 Aβo or with extracts immunodepleted of Aβ (Vehicle). Type 2 Aβo, but not Vehicle, injections impaired performance on this task (* P < 0.01, RMANOVA compared to baseline performance (mean of three contiguous sessions)). Inset: dot blot showing OC-immunoreactivity in Type 2 Aβo-containing (“Type 2”) and immunodepleted (“Vehicle”) injectates. See also Figure S6 and Table S1.
Reducing APP in plaque-bearing mice selectively lowers A11-reactive Aβo
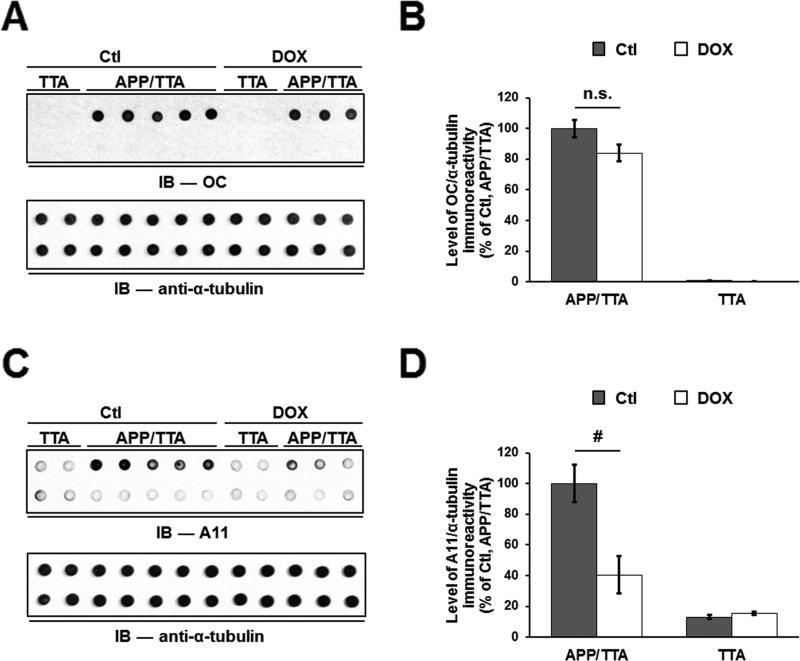
Tg2576, hAPP-J20, TetO-APPSweInd and rTg9191 mice express human APP at levels that vary between lines and contain one or more variants (Swedish in Tg2576; both Swedish and Indiana in hAPP-J20 and TetO-APPSweInd, and both Swedish and London in rTg9191). These alterations result in different levels and amino acid compositions of Aβ, which we hypothesize contribute to the variability in the amount and types of Aβo generated, by analogy with in vitro studies. To determine whether the amount of Aβ expressed does indeed affect the relative levels of A11- and OC-reactive Aβo, we turned to TetO-APPSweInd mice. These mice carry a regulatable APP transgene, allowing us to manipulate levels of APP expression, and, hence, levels of Aβ. When APP expression was suppressed by 90% for five weeks in plaque-bearing TetO-APPSweInd mice, TBS-soluble A11-reactive species decreased 60%, but TBS-soluble OC-reactive species remained stable (Figure 3). These results support the hypothesis that in the presence of amyloid fibrils, the reaction kinetics favor the generation of OC-reactive over A11-reactive Aβo.
Figure 3. Suppression of transgenic APP selectively lowers A11-immunoreactive Aβo in TetO-APPSweInd mice.
(A) Upper panel, protein aggregates detected by OC antibodies in aqueous brain extracts prepared from 8.5-month-old TetO-APPSweIndmice harboring both activator and responder transgenes (APP/TTA) or only the activator transgene (TTA). DOX, mice administered doxycycline to suppress APP expression for the 5 weeks immediately preceding sacrifice; Ctl, untreated mice. Upper lane, brain extracts; lower lane, brain extracts immunodepleted of Aβ. Lower panel, α-tubulin loading control. (B) Quantification of OC immunoreactivity. There is no difference between levels of OC-immunoreactivity, normalized to α-tubulin levels, in extracts from control (Ctl) and DOX-treated mice. (C) Upper panel, protein aggregates detected by A11 in the extracts described in (A). Upper lane, brain extracts; lower lane, brain extracts immunodepleted of Aβ. Lower panel, α-tubulin loading control. (D) Quantification of A11 immunoreactivity. Suppression of transgenic APP expression in TetO-APPSweInd mice resulted in a ~60% reduction in the levels of A11-reactive oligomers. n.s. = not significant, # p < 0.05, twoway ANOVA followed by Fisher's post-hoc analysis. See alsoFigure S7.
Type 2 Aβo are concentrated around dense-core plaques and occupy only a small fraction of the cortex
We went on to use OC antibodies to detect Aβo in the brains of two mouse models: rTg9191 and Tg2576. We found that brains of both rTg9191 and Tg2576 mice showed areas of intense OC-immunoreactivity encircling Congo red-positive dense plaque cores (Figure S3A). In contrast to dense-core plaques, we found no OC-reactivity in diffuse plaques lacking dense cores and plaque-associated cytopathology (Serrano-Pozo et al., 2011).
Since immunohistological methods are not optimally suited to measure finely dispersed molecules, it is possible that we did not detect low concentrations of OC-immunoreactive Aβo located outside the immediate vicinity of dense-core plaques. We therefore performed biochemical analyses on microdissected brain tissue fractions to more accurately define the spatial distribution of OC-immunoreactive Aβo and to discriminate between insoluble and soluble OC-immunoreactive assemblies.
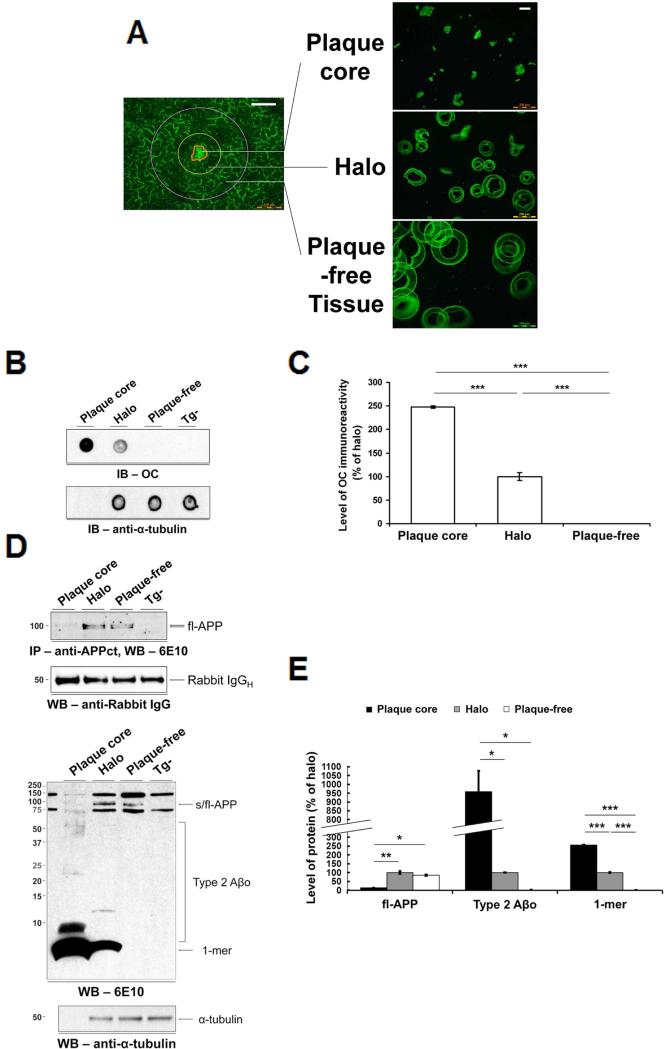
Using laser microdissection, we isolated three issue fractions in rTg9191 mice: i) dense plaque cores containing Aβ fibrils; ii) halos extending 50 μm from the edge of the plaque cores; and iii) plaque-free zones, annuli extending 80-100 μm from the outer edges of the halos (Figure 4A). We opted to use autofluorescence rather than Thioflavin S to identify candidate dense-core plaques, because Thioflavin S artificially generated SDS-stable oligomers (Figure S3B). We performed control studies to verify the identity of autofluorescent structures by Thioflavin S and Aβ immunohistochemistry using 4G8 (Figure S3C). We then measured native Aβ assemblies in each tissue fraction in dot blots using OC antibodies, and found >99.9% of immunoreactivity confined to the cores and halos (Figures 4B and 4C).
Figure 4. Type 2 Aβo are confined to dense-core plaques in rTg9191 mice.
(A) Photomicrographs of microdissected fractions. White scale bars: 100 μm. (B) Upper panel, dot blot, probed with OC antibodies, illustrates the topographical distribution of native Type 2 Aβo; lower panel, above blot stripped and re-probed with anti-α-tubulin, showing that equal amounts of protein were loaded for the halo and plaque-free regions. (C) Quantification of dot blots. OC-reactive aggregates are confined to the dense-core and halo regions. (D) Immunoblotting under denaturing conditions confirms that Type 2 Aβo are restricted to the dense-core and halo regions. Upper two panels, Western blot showing full-length APP (fl-APP) in detergent-soluble extracts of laser microdissected fractions. Fl-APP was immunoprecipitated with a polyclonal antibody directed against a C-terminal epitope in APP (anti-APPct) and detected using 6E10 (top). Blot stripped and re-probed with an antibody directed against rabbit immununoglobulin (IgG) showing that ~equal amounts of capture antibody were immunoprecipitated (bottom).Lower two panels, Western blot probed with monoclonal antibody 6E10 (top). Blot stripped and re-probed with anti-α-tubulin (bottom). (E) Quantification of Western blots. Type 2 Aβo and monomers reside in the dense-core and halo regions, while fl-APP is primarily found in halo and plaque-free regions. * p < 0.01, ** p < 0.001, *** p < 0.0001, one-way ANOVA followed by Fisher's post hoc analysis. Abbreviations: 1-mer, Aβ monomer; s/fl-APP, soluble and full-length amyloid precursor protein; Tg-, nontransgenic littermates of rTg9191 mice; IgGH, immunoglobulin heavy chain. See also Figures S3, S4 and S7.
OC-immunoreactive Aβo thus are spatially, as well as temporally, associated with amyloid fibrils in the brains of APP transgenic mice. Further, OC-immunoreactive Aβo very probably share the in-register parallel β-sheet structure that is characteristic of brain-derived amyloid fibrils (Lu et al., 2013). The spatial and temporal patterns of appearance of OC-immunoreactive oligomers strongly support the hypothesis that these oligomers form via a process of secondary self-assembly dependent upon pre-existing amyloid fibrils (see Discussion and Figure S7). We hereafter refer to OC-positive oligomers that exhibit spatial and temporal relationships to amyloid fibrils as Type 2 Aβo. The laser microdissection results indicate that ~all OC-immunoreactive Aβo in the brains of rTg9191 mice meet the spatial criterion for Type 2 oligomers.
We next asked what fraction of the cortex is occupied by Type 2 oligomers. Based on stereological analyses of Thioflavin S-stained sections and the geometry of dense-core plaques (see Note S1), we estimated the volume of the cortex occupied by Type 2 Aβo in rTg9191 mice to be 11.4%; therefore, >99.9% of the OC-immunoreactive Aβo in rTg9191 mice were confined to 11.4% of the cortex.
To measure other potential soluble Aβ assemblies in rTg9191 brains, we solubilized the Aβ assemblies from each tissue fraction in detergents, fractionated them by SDS-polyacrylamide gel electrophoresis (PAGE) (which denatures the assemblies, as described below), and detected the denatured Aβ assemblies by immunoblotting with two different anti-Aβ-specific antibodies (6E10, Figure 4D; 4G8, Figure S4A). We found an array of Aβ species in the cores and halos, but no Aβ assemblies in the plaque-free fraction. In addition, we found a strong concordance between measurements of the percentage of soluble Aβ assemblies located in the cores and halos obtained from dot blots with OC antibodies (>99.9%, Figure 4C) and measurements obtained from Western blots with the two anti-Aβ antibodies (≥99.6%, Figures 4E and S4B), suggesting that all, or nearly all, of the soluble Aβ oligomers in rTg9191 mice are Type 2 Aβo.
In additional biochemical studies, we found that when extracts of rTg9191 brains containing native Type 2 Aβo were fractionated by size-exclusion chromatography and subsequently denatured by SDS-PAGE, Aβ species appeared in immunoblots as monomers, dimers, and larger oligomers (Figure S4C). Dimers were the most abundant Aβ oligomeric species in the immunoblots and eluted in two distinct sets of fractions; one set of fractions corresponded to the molecular masses of globular proteins ranging from ~500 kDa to ~2000 kDa while the other set of fractions corresponded to ~60 kDa to ~200 kDa proteins. We inferred from these results that the dimers in the immunoblots of denatured rTg9191 brain extracts represent disassembled high-molecular-mass Type 2 Aβo, but we cannot exclude the existence of free non-globular dimers with larger radii of hydration than would be expected for a globular protein with a molecular mass of ~9 kDa, the theoretical molecular mass of dimers.
The Type 1 Aβ oligomer Aβ*56 is distributed throughout the cortex
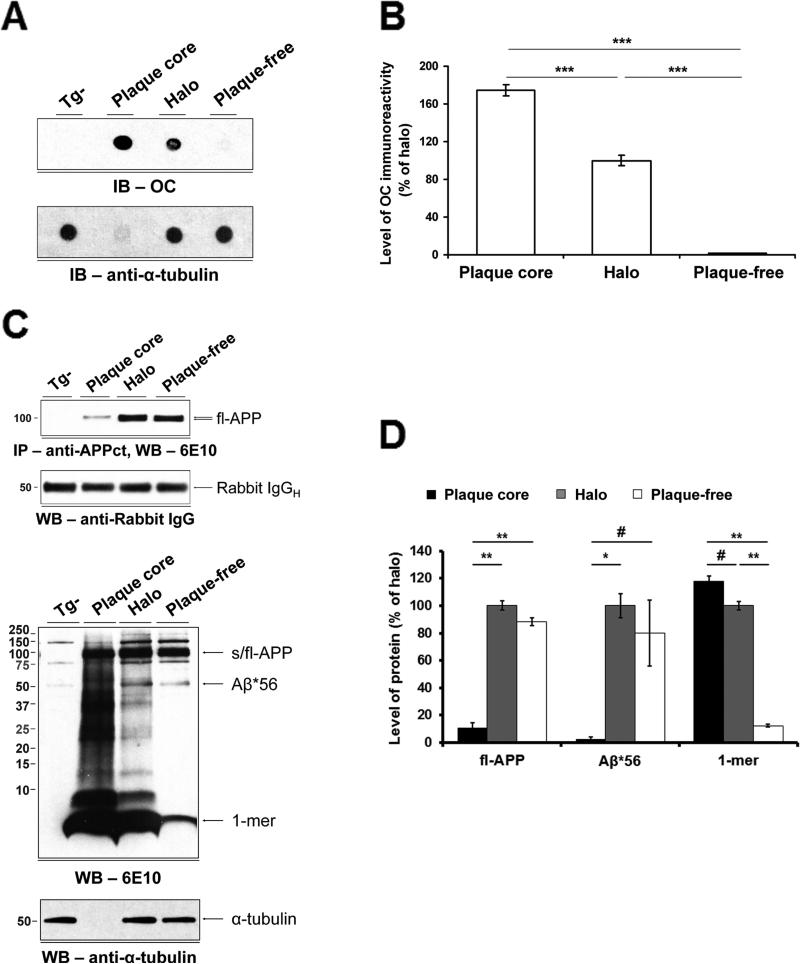
The A11-immunoreactive oligomer Aβ*56 appears months before the emergence of the first detectable dense-core plaques in Tg2576 mice (Kawarabayashi et al., 2001; Lesne et al., 2006) and hAPP-J20 (Cheng et al., 2007; Wright et al., 2013). We used Tg2576 mice, in which Aβ*56 was originally described (Lesne et al., 2006), to compare the spatial distribution of Aβ*56 to that of Type 2 Aβo. We analyzed microdissected tissue fractions by dot blotting and Western blotting (Figures 5A, 5C and S4D). In quantitative analyses, we found a striking divergence in the distribution of OC-immunoreactive Aβo and Aβ*56: 99.5% of OC-immunoreactive Aβo were concentrated within plaque cores and halos (Figure 5B), whereas most of the Aβ*56 (≥98.7%) was distributed outside the core, in the halo and plaque-free zone (Figures 5D and S4E). We estimated that Type 2 Aβo and Aβ*56 occupy 13.9% and 98.4%, respectively, of the volume of the cortex we sampled in aged Tg2576 mice (see Note S1).
Figure 5. Type 2 Aβo and Aβ*56 have different spatial distributions in the brains of Tg2576 mice.
(A) Upper panel, dot blot probed with OC antibodies shows the topographical distributions of Type 2 Aβo; lower panel, α-tubulin blot shows that equal amounts of protein were loaded for the halo and plaque-free regions. (B) Quantification of dot blots. Type 2 Aβo overwhelmingly reside in the dense-core and halo regions. (C) Upper two panels, Western blot showing fl-APP in detergent-soluble extracts of laser microdissected fractions (top). Blot stripped and re-probed with anti-rabbit IgG antibody showing that ~equal amounts of capture antibody were immunoprecipitated (bottom).Lower two panels, Western blot probed with 6E10 antibody shows the topographical distributions of APP, Aβ*56 and monomers (1-mer) (top). Alpha-tubulin blot shows that equivalent amounts of protein were loaded in the halo and plaque-free lanes (bottom). (D) Quantification of Western blots. Aβ*56 and fl-APP are primarily found in halo and plaque-free regions. In contrast, Aβ monomers overwhelmingly reside in the dense-core and halo regions. # p < 0.05, * p < 0.01, ** p < 0.001, *** p < 0.0001, one-way ANOVA followed by Fisher's_post-hoc_ analysis. Tg-, non-transgenic littermates of Tg2576; other abbreviations as in Figure 4. See also Figures S3, S4 and S7.
Thus, in contrast to Type 2 Aβo, Aβ*56 differs from amyloid fibrils in its temporal expression; spatial distribution; and quaternary structure, as inferred from its immunoreactivity with conformation-selective antibodies. We hereafter refer to A11-positive oligomers that appear independently of amyloid fibrils as Type 1 Aβo.
Type 2 Aβo levels greatly exceed those of the Type 1 oligomer Aβ*56 in aqueous brain extracts from Tg2576 mice
To determine the relative abundance of Type 2 Aβo and Aβ*56, we estimated their masses in aqueous brain extracts from 21-month-old Tg2576 mice. To ascertain the Aβ content of Type 2 Aβo, we compared OC-immunoreactivity of extracts to that of synthetically-produced in-register parallel β-sheet Aβ fibrils containing known quantities of Aβ (measured by ELISA using 6E10 antibodies, following denaturation with hexa-fluoro-isopropanol (HFIP)). This method assumes that the relationship between OC immunoreactivity and Aβ content is the same for the synthetic fibrils and brain-derived Type 2 Aβo. Using this method, Type 2 oligomers were found to contain 14,500 ± 1,500 ng Aβ per gram of brain tissue. Levels of Aβ*56 were assessed by comparing the density of the 56-kilodalton band to known quantities of synthetic Aβ monomers on Western blots probed with 6E10. This method assumes that the affinity of the 6E10 antibody was the same for the Aβ peptides within Aβ*56 as for the synthetic Aβ, in blots subjected to antigen retrieval prior to incubation with 6E10. Our estimate of the amount of Aβ*56 depends upon the stoichiometry of antibody binding to the synthetic standards and to Aβ*56. We assume a stoichiometry of antibody:Aβ monomer of 1:1. Each molecule of Aβ*56, a putative Aβ dodecamer, could bind between 1 and 12 antibody molecules. Under the assumption that one molecule of Aβ*56 binds 12 antibodies (i.e., a 1:1 antibody:monomeric sub-unit ratio) , we found that Aβ*56 contains 57 ± 2 ng Aβ per gram of brain tissue. However, this value could be as much as 12 times higher, if only one antibody binds to each molecule of Aβ*56. Finally, the total mass of Aβ (Aβx-38, Aβx-40, and Aβx-42) in the extracts was measured by ELISA using 6E10 antibodies, following denaturation with formic acid. We found that the total Aβ in aqueous extracts from aged Tg2576 mice is comprised of 0.4% - 4.8% Aβ*56, and ~95% Type 2 Aβo (Table 1; Figure S5).
Table 1.
The relative levels and spatial occupancy of Type 1 and Type 2 Aβo in aged Tg2576 mice. See also Figures S5 and S7.
| Type of Aβo | Protein level (ng/gram brain tissue) | % of cortex occupied |
|---|---|---|
| Type 2 | 14,500 ± 1,500a | 13.9%d |
| Aβ*56 (Type 1) | 57 ± 2b | 98.4%d |
| Total Aβ | 15,200 ± 7,000c | NA |
Mice producing only Type 2 Aβo are cognitively intact
Multiple studies have shown a correlation between the Type 1 Aβo Aβ*56 and memory dysfunction in transgenic mice (Billings et al., 2007; Cheng et al., 2007; Lesne et al., 2006). rTg9191 mice provide the opportunity to test the effects of Type 2 Aβo on cognition, as rTg9191 mice produce Type 2 Aβo exclusively. We studied cognitive function in rTg9191 mice that were aged until 21 to 24 months, when the relative quantity of Type 2 Aβo in the brain, as assessed in dot blots using OC antibodies, was comparable to that in AD patients (Figures 6A and 6B). In a cohort of mice tested at 23 months, we found no significant transgene-associated effects on cognitive performance in the fixed consecutive number task, an operant behavioral task sensitive to lesions of the prefrontal cortex (van Haaren et al., 1988) (Figure 6C). Similarly, we found no significant impairment of spatial memory in any of three other cohorts of mice [young (4 months); middle-aged (12 months); old (21 months)] evaluated using a water maze test of spatial reference memory that is sensitive to hippocampal lesions (Morris, 2007) (Figure 6D). When the cohort of old mice was re-tested at 24 months, when levels of Type 2 Aβo were significantly higher than in 21-month-old mice, there was still no decrement in performance (Table S1). Furthermore, there were no significant differences in performances between the old cohort (21-month-old) and the other two cohorts (Figure 6D, Table S1). The slight increase in the proximity index (Table S1) and decrease in target-quadrant occupancy in the old mice appear to be related to aging since the same decrease can be seen in old, non-transgenic littermates (Figure 6D). Based on OC-immunoreactivity in dot blots, the levels of Type 2 Aβo in 24-month-old mice were 25- and 2.1-fold greater (calculated after subtracting background immunoreactivity observed in non-transgenic mice) than those in 4- and 12-month-old mice, respectively (Figure 6B). In summary, within the statistical limits of our measurements, there were no differences in cognitive function between mice with Type 2 Aβo (at levels comparable to those in humans with AD), non-transgenic mice, and young rTg9191 mice with few or no such oligomers.
Type 2 Aβo impair cognition when dispersed and tested in rats
The absence of any impact on cognitive function of Type 2 Aβo produced in situ suggests that Type 2 Aβo sequestered within dense-core plaques do not interfere with global network function, or that compensatory neural reorganization effectively preserves brain function when the oligomers occupy <15% of the cortex. It is possible that the background strain (129FVBF1) of rTg9191 is not permissive for the disruptive effects of Aβ on cognition, but this is unlikely, because Tg2576 mice in the same background strain showed spatial memory deficits (Kotilinek et al., 2008). We also considered the possibility that the Type 2 Aβo produced by rTg9191 mice lack neurotoxicity. To test this hypothesis, we prepared aqueous protein extracts containing Type 2 Aβo from the brains of 26-month-old rTg9191 mice and injected the extracts into the lateral cerebral ventricles of rats previously trained in a delayed non-matching-to-place task, a test of short-term working memory (Paule et al., 1998). Each rat sequentially received brain extracts containing ~0% (Aβ-immunodepletion), ~0.1% (low dose) or ~1% (high dose) of the total amount of Type 2 Aβo in a 26-month-old rTg9191 mouse, and was tested two hours after the injections. Compared to baseline performance, memory was significantly impaired when rats were injected with the high dose of Aβ-containing extracts, but not when rats received the low dose or extracts from which Aβ was depleted (Figures 6E and S6). These results indicate that normal cognition in rTg9191 mice is probably not due to the lack of neurotoxicity of Type 2 Aβo, since extracts containing minute quantities of dispersed Type 2 Aβo disrupted cognition. These findings support the conclusion that spatial localization is a more significant determinant of the neurological effects of Aβo than overall abundance.
Discussion
The data in this paper indicate that brain-derived Aβo can be categorized based upon distinct temporal, spatial and structural relationships to fibrils. Type 2 Aβo are observed only after dense-core plaque formation, are highly concentrated around dense-core plaques, and are recognized by OC antibodies, which we here show selectively bind to structures containing in-register parallel β-sheets. The in-register parallel β-sheet conformation is characteristic of all known naturally occurring Aβ fibrils (for review, see (Glabe, 2009; Tycko, 2011)), so it appears that Type 2 Aβo and Aβ fibrils share a common quaternary structure. By contrast, Type 1 Aβo are present both prior to and following the appearance of dense-core plaques, are dispersed in brain tissue, and are recognized by A11 antibodies. Importantly, these two classes of Aβo are differentially associated with impaired cognition: the Type 1 Aβo Aβ*56 is associated with memory dysfunction in APP transgenic mice (Billings et al., 2007; Cheng et al., 2007; Lesne et al., 2006; Lesne et al., 2008), while mice with levels of Type 2 oligomers similar to those of AD patients have intact cognition. These results are summarized in Table S2. Taken together, these findings refine our understanding of how Aβo are organized and impact global neural network function in the brain.
The results of recently reported behavioral studies in TetO-APPSweInd mice are consistent with our conclusion that Type 2 Aβo are less harmful to neurological function than Type 1 Aβo (Fowler et al., 2014). Using TetO-APPSweInd mice of the identical age and strain background, exposed to the same dose of doxycycline for the same amount of time as the mice used here, Fowler et al. showed that 90% suppression of APP expression led to cognitive improvement, assayed with multiple behavioral tests. Cognitive improvement in these mice was accompanied by significant reductions in the level of A11-immunoreactive species generally, and in the level of Aβ*56 particularly, with no changes in amyloid plaque burden. In the current study, we have confirmed the findings of Fowler et al., showing that suppression of APP leads to reductions in A11-immunoreacitve species, and have extended these findings to show that levels of OC-immunoreactive Aβo are unchanged. To summarize, an experimental manipulation that reduces levels of A11-reactive Aβo, while preserving levels of OC-reactive Aβo, leads to cognitive improvement in TetO-APPSweInd mice.
We hypothesize that the differences in the spatiotemporal distributions of Type 1 and Type 2 Aβo result from distinct modes of biogenesis. Aβo have been shown in vitro to form via one of two processes: primary self-assembly occurring independently of amyloid fibrils, or secondary self-assembly requiring pre-existing fibrils that catalyze the self-assembly of monomers (Cohen et al., 2013) (Figure S7). If these processes also occur in vivo, Aβo formed via secondary self-assembly should be seen only in brains containing amyloid fibrils and should be at highest concentration in the immediate vicinity of foci of Aβ fibrils (i.e., dense-core plaques). We propose that Type 2 Aβo are generated by a secondary self-assembly process, because they appear only after dense-core plaques are present and they are highly concentrated around these plaques. Conversely, Aβo that form via primary self-assembly may be present prior to plaque formation and need not be concentrated around plaques – characteristics of Type 1 Aβo, as defined here.
The two-pathway model of the self-assembly of Aβ (Figure S7) can explain the quantitative differences between Type 1 and Type 2 Aβo reported here.In vitro studies have shown that, in the presence of amyloid fibrils, reaction kinetics favor the generation of oligomers via a secondary self-assembly process (Cohen et al., 2013). After amyloid fibrils form in vitro, Aβ monomers are consumed by the elongation of fibrils and the secondary self-assembly reaction; if the consumption of monomers by these processes exceeds the production of Aβ monomers needed to form Aβo via primary self-assembly, then Aβo formed by primary self-assembly will decline or disappear. This model predicts that Type 2 Aβo should predominate in plaque-bearing brains; indeed, Type 2 Aβo are the most abundant Aβo in aged Tg2576 mice, comprising ~95% of the Aβ species in aqueous brain extracts from Tg2576 mice (with plaque loads slightly higher than those found in AD). The model also anticipates our result_in vivo_ in TetO-APPSweInd mice − when we reduced the pool of monomers by lowering APP expression, levels of Type 1 Aβo fell but levels of Type 2 Aβo remained unchanged. Finally, the presence of Type 1 Aβo in Tg2576 mice and their absence in rTg9191 is also consistent with this model. Tg2576 mice express more total Aβ than rTg9191 mice, but relatively less fibrillogenic Aβ42, because Tg2576 lack the_London_ mutation; therefore, more monomers are available to form Type 1 Aβo in Tg2576 than in rTg9191 mice.
Surprisingly, the prolific Type 2 Aβo were not dispersed throughout brain tissue, but were instead encapsulated around dense-core plaques that in total occupied <15% of the cortex. Within this microenvironment, Type 2 Aβo very likely cause neurodegenerative changes such as dystrophic neurites. It was previously shown that anti-Aβ immunotherapy reverses plaque-associated neuritic abnormalities without affecting plaque burden (Rozkalne et al., 2009), strongly suggesting that soluble Aβo cause cytopathology. Our rTg9191 mice, which generate only Type 2 Aβo, exhibit plaque-associated cytopathology (Liu et al., in press), yet remain cognitively normal. Thus, the encapsulation of Type 2 Aβo in cyst-like dense-core plaques appears to neutralize their potential to disrupt neurological function. These results indicate that the spatial localization of Aβo is critically important, certainly more important than their overall abundance.
To evaluate the relevance of our findings to AD, we estimated the volume of human cortex occupied by Type 2 Aβo in AD, using two different methods. One method depended upon immunohistological data of tissue stained with the Aβ oligomer-specific antibody NAB-61 (Lee et al., 2006), which we inferred selectively recognizes Type 2 Aβo and amyloid fibrils from its almost exclusive staining of neuritic plaques (dense-core plaques associated with neuritic abnormalities (Serrano-Pozo et al., 2011)). The average volume of AD cortex occupied by Aβ assemblies detected using NAB-61 was ~5% (Perez-Nievas et al., 2013). The other method relied upon neuritic plaque densities. We estimated that the upper limit of the volume of cortex occupied by neuritic plaques in AD brain is ~10% (see Note S1). Both estimates are smaller than those in the two mouse model systems studied here, suggesting that in humans, Type 2 Aβo influence a relatively small volume of cortex and, as in the mice, would participate little if at all in disrupting cognition or inducing widespread neurodegeneration. However, we cannot exclude the possibility that Type 2 Aβo might disrupt intrinsic connectivity networks in AD if higher-than-average concentrations of neuritic plaques accumulate in critical “nodes.” Nor can we conclude with certainty that Type 1 Aβ oligomer levels are equivalent across all brain regions; for example, within a given region, local rates of Aβ production may be affected by neural activity (Cirrito et al., 2005; Kamenetz et al., 2003), which might alter the kinetics of self-assembly. A better understanding of the roles of Type 1 and Type 2 Aβo in AD might be gained by comparing their respective levels in brain regions that are differentially vulnerable to neurodegenerative and functional abnormalities.
Although the biological factors involved in maintaining the sequestration of Type 2 Aβo in dense-core plaques are not well understood, neuroinflammation seems to be important. While Type 2 Aβo are smaller and therefore more diffusible than amyloid fibrils, their diffusion range may be circumscribed by microglial cells that may “seal” them inside amyloid plaques (Bolmont et al., 2008), thus potentially limiting their influence on brain function. It is possible that the compartmentalization eventually breaks down in symptomatic AD patients, allowing Type 2 Aβo to leak out and damage a broader population of neurons. If Type 2 Aβo in AD are as neurotoxic as those in rTg9191 mice, our results indicate that small breaches allowing only ~1% to pass through the putative barrier might be sufficient to disrupt global network function. Actual quantification is needed to test the hypothesis directly, but the poor correlation between neuritic plaques, β-amyloidosis, and cognition in humans (Katzman et al., 1988; Terry et al., 1991) argues against this possibility.
The uncertainty about which Aβo contribute to the pathogenesis of AD is a major impediment to progress on disease prevention, treatment and diagnosis. Apparently conflicting results obtained in clinical trials of anti-amyloid therapies highlight the need for a more complete understanding of the types and toxicity of Aβ assemblies that are seen during the progression of AD. Passive immunization with two monoclonal antibodies that recognize Aβ fibrils, Bapineuzumab and Gantenerumab, reduced amyloid burdens but failed to provide cognitive or functional benefits (Alzforum, 2015a, b; Ostrowitzki et al., 2012; Rinne et al., 2010). However, preliminary reports that a third antibody, Aducanumab, practically eliminates plaques and slows cognitive and functional decline in patients with prodromal or mild AD has generated tremendous excitement in the field (Strobel, 2015). Aducanumab reportedly is highly selective for Aβo over Aβ monomers, but further details of the conformational selectivity of the antibody have not been made public. Taken together with the findings in mice reported here, the results of the clinical trials raise several questions: How did the stage at which therapy was administered, the degree of target engagement, and/or the precise identities of Aβ species targeted contribute to the differences in the outcomes of the clinical trials? Does Aducanumab discriminate between Type 1 and Type 2 Aβo, as defined here, or does it recognize an epitope common to both types of Aβo? Does the microlocalization of different structural classes of Aβo in human brain parallel that seen in the brains of APP transgenic mice, reported here, and does this spatial distribution change during the progression of AD from the prodromal phase to dementia? Are there regions of the human brain where Type 2 Aβo reach a high enough concentration to disrupt network function (the critical “nodes” mentioned above)?
In summary, the findings reported here suggest that while most of the soluble Aβ in brains bearing dense-core plaques (e.g., AD brains) are Type 2 Aβo, the bulk of these oligomers are rendered functionally innocuous by their effective containment within plaques. Type 1 Aβo may be more directly pathogenic in many brain regions because they are more finely dispersed than Type 2 Aβo. We hope that our findings will open up new directions of research, particularly in understanding and developing therapies that target the rarer Type 1 Aβo. A better understanding of how Type 1 and Type 2 Aβo impact the pathogenesis of AD may result from the synthesis of future biophysical and biological studies.
Experimental Procedures
Animals
rTg9191 mice were generated using a binary system of responder and activator transgenes (Paulson et al., 2008). Tg2576 mice (Hsiao et al., 1996) in a B6SJLF1 background (Westerman et al., 2002) and 4-month-old hAPP-J20 mice in a C57Bl6 background were from K.H.A.'s colony at the University of Minnesota. TetO-APPSweInd mice in a B6FVB F1 background were from J.L.J.'s laboratory at Baylor College of Medicine. Brains from 12-month-old hAPP-J20 were a gift of Dr. Lennart Mucke of the University of California, San Francisco. All experiments involving mice and rats were conducted in full accordance with the Association for Assessment and Accreditation of Laboratory Animal Care and the Institutional Animal Care and Use Committee guidelines at the University of Minnesota, Minneapolis Veterans Administration Medical Center, or Baylor College of Medicine.
Human brain tissue
De-identified samples of inferior temporal gyrus (Brodmann Area 20) from individuals with a clinical diagnosis of Alzheimer's disease were obtained from the Religious Order Study (Rush Alzheimer's Disease Center, Chicago, IL).
Sample Preparation for parallel and anti-parallel fibrils and synthetic Aβo
Details of preparation procedures are provided in the Supplemental Experimental Procedures.
Protein extraction and immunoblotting
To extract native Aβ aggregates, water-soluble extracts were prepared based on a protocol previously described (Shankar et al., 2008). Briefly, tissue specimens were weighed and transferred to 4 volumes of ice-cold buffer (25 mM Tris-HCl, pH7.4; 140 mM NaCl; 3 mM KCl; 0.1 mM phenylmethylsulfonyl fluoride; 0.2 mM 1,10-phenanthroline monohydrate; protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO); and phosphatase inhibitor cocktails (Sigma-Aldrich)) and homogenized using a Dounce homogenizer. The resulting materials were centrifuged for 90 minutes (16,100 g; 4°C); the supernatant was depleted of endogenous immunoglobulins and stored at -20°C until further use.
Details of the dot blotting method are provided in the Supplemental Experimental Procedures. Briefly, 0.5 μg protein from brain extracts or 0.02-2 ng synthetic Aβ fibrils (Qiang et al., 2012) were spotted onto nitrocellulose membranes, which were then probed with either OC (Millipore, Billerica, MA, AB2286, 1:50,000) or A11 (Invitrogen, Carlsbad, CA, AHB0052, 1:1,000) antibodies.
Detergent-soluble Aβ species were extracted and Western blotting was performed using the method of Liu et al. (Liu et al., 2011).
Micro-dissection
Details of micro-dissection are provided in the Supplemental Experimental Procedures. Native and detergent-soluble proteins were extracted as described above; to compare protein levels in the micro-dissected fractions, extracts from equal volumes of tissue were used in dot blots and Western blots. For negative controls, protein extracts from age-matched, nontransgenic littermates were loaded such that the total protein content equaled that of the plaque-free regions used for each blot.
Behavioral tests
Details of the behavioral tests are provided in the Supplemental Experimental Procedures.
Fixed Consecutive Number (FCN)
Forty-one (19 rTg9191, 22 “neg”) mice completed training under this assay. Mice were 20.5-month-old at the start of training. After training, mice were run under the FCN-4 five days per week for 30 daily sessions.
Water maze
Spatial reference memory was measured in rTg9191 mice using a modified version of a water maze tailored to more rapid learning in the 129S6/FVB background strain and more sensitive to subtle deficits (Westerman et al., 2002). rTg9191 mice (female and male) were tested cross-sectionally at 4 and 12 months of age, and longitudinally from 21 to 24 months. The spatial cues and hidden platform location were changed for the mice tested longitudinally at 24 months of age. Illness and spontaneous deaths were observed at the 21-24 month time point (4/60 “neg” and 6/40 rTg9191 mice, Chi Square p = 0.15). Mice requiring euthanasia were not included in the analysis. The power of our study was sufficient to have an 80% chance of detecting an 8-point drop in mean percent target quadrant occupancy at a confidence level of 0.05.
Delayed Non-Matching to Position
Twenty male Sprague Dawley rats previously trained on a Delayed Non-Matching to Position task were injected intracerebroventricularly with brain extracts through chronically implanted guide cannulae (Reed et al., 2011). Each rat received 10 μL brain extract, prepared using the protocol for native aggregates described above; control extracts were prepared by two rounds of immunodepletion with monoclonal antibody 4G8. Animals were tested 2 hours post-injection. Each animal received injections of both fibril-dependent Aβ oligomer-containing and control extracts, on different days.
The task yields an accuracy-by-delay relationship, with high accuracy (percent of correct responses) at the short delays and near random performance (50% correct) at the longest delays. This relationship is shifted leftwards (working memory deficit) by compounds or conditions that adversely affect cognition. Performance on the day of injection was compared to baseline performance, defined as the mean of the scores at each delay (per cent correct choices) for three days contiguous to the day of injection. Two animals that lost their cannulae were excluded from the analysis.
Histology and immunohistochemistry
Details of histological procedures are provided in the Supplemental Experimental Procedures.
Statistics
Statistics were performed using StatView Version 5.0.1 (SAS Institute Inc., Cary, NC). Data are expressed as mean ± SEM.
Supplementary Material
2
3
Highlights.
Brain-derived amyloid-β oligomers (Aβo) are classified as Type 1 and Type 2.
Type 1 Aβo, A11-immunoreactive, have no spatio-temporal relationship with Aβ plaques.
Type 2 Aβo, OC-immunoreactive, emerge after, and accumulate around, Aβ plaques.
Type 2 Aβo, despite being highly abundant, do not impair cognition in situ.
Acknowledgments
The authors thank Dr. David Bennett, Rush University Medical Center, Chicago, IL for providing human brain specimens, Dr. Sylvain Lesné and Mathew Sherman for providing aqueous extracts of human brains, and Dr. Robert Tycko for valuable advice. This work was supported by the National Institute for Neurological Diseases and Stroke (NS33249) and a gift from B. Grossman (KHA), a grant from the Robert A. and Renee E. Belfer Family Foundation (JLJ), and the NIH intramural research program (WQ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
P.L., K.R.Z. and K.H.A. conceived the project. P.L. planned and performed the biochemistry experiments, including characterization of the rTg9191 mice and laser microdissection, and analyzed the results. M.N.R., L.A.K., P.L., and J.P.C. planned, performed and analyzed the behavioral experiments. M.K.O.G. performed biochemistry experiments, including dot blots and size exclusion chromatography. S.L.S. performed biochemistry experiments, including dot blots. P.L. and C.L.F, planned, performed and analyzed the immunohistochemistry experiments. J.H.R. performed stereology-based quantification of amyloid load in Tg2576 mice. W.Q. prepared synthetic amyloid fibrils and characterized them by electron microscopy. A.C.A.C. treated and prepared tissue from TetO-APPSweInd mice. J.L.J. supplied tissue from TetO-APPSweInd mice and contributed to the interpretation of results. P.L., C.M.W., K.R.Z. and K.H.A. wrote the paper.
References
- Alzforum Bapineuzumab. 2015a [Google Scholar]
- Alzforum Gantenerumab. 2015b [Google Scholar]
- Benilova I, Karran E, De Strooper B. The toxic Abeta oligomer and Alzheimer's disease: an emperor in need of clothes. Nat Neurosci. 2012;15:349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- Billings LM, Green KN, McGaugh JL, LaFerla FM. Learning decreases A beta*56 and tau pathology and ameliorates behavioral decline in 3xTg-AD mice. J Neurosci. 2007;27:751–761. doi: 10.1523/JNEUROSCI.4800-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolmont T, Haiss F, Eicke D, Radde R, Mathis CA, Klunk WE, Kohsaka S, Jucker M, Calhoun ME. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J Neurosci. 2008;28:4283–4292. doi: 10.1523/JNEUROSCI.4814-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng IH, Scearce-Levie K, Legleiter J, Palop JJ, Gerstein H, Bien-Ly N, Puolivali J, Lesne S, Ashe KH, Muchowski PJ, et al. Accelerating amyloid-beta fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J Biol Chem. 2007;282:23818–23828. doi: 10.1074/jbc.M701078200. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Cohen SI, Linse S, Luheshi LM, Hellstrand E, White DA, Rajah L, Otzen DE, Vendruscolo M, Dobson CM, Knowles TP. Proliferation of amyloid-beta42 aggregates occurs through a secondary nucleation mechanism. Proc Natl Acad Sci U S A. 2013;110:9758–9763. doi: 10.1073/pnas.1218402110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler SW, Chiang AC, Savjani RR, Larson ME, Sherman MA, Schuler DR, Cirrito JR, Lesne SE, Jankowsky JL. Genetic modulation of soluble Abeta rescues cognitive and synaptic impairment in a mouse model of Alzheimer's disease. J Neurosci. 2014;34:7871–7885. doi: 10.1523/JNEUROSCI.0572-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glabe C. Amyloid oligomer structures and toxicity. The Open Biology Journal. 2009;2:222–227. [Google Scholar]
- Glabe CG. Structural classification of toxic amyloid oligomers. J Biol Chem. 2008;283:29639–29643. doi: 10.1074/jbc.R800016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Slunt HH, Gonzales V, Savonenko AV, Wen JC, Jenkins NA, Copeland NG, Younkin LH, Lester HA, Younkin SG, et al. Persistent amyloidosis following suppression of Abeta production in a transgenic model of Alzheimer disease. PLoS Med. 2005;2:e355. doi: 10.1371/journal.pmed.0020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, Renbing S, Peck A. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Annals of Neurology. 1988;23:138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid ß protein in the Tg2576 transgenic mouse model of Alzheimer's disease. Journal of Neuroscience. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Head E, Sarsoza F, Saing T, Cotman CW, Necula M, Margol L, Wu J, Breydo L, Thompson JL, et al. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegener. 2007;2:18. doi: 10.1186/1750-1326-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Kotilinek LA, Westerman MA, Wang Q, Panizzon K, Lim GP, Simonyi A, Lesne S, Falinska A, Younkin LH, Younkin SG, et al. Cyclooxygenase-2 inhibition improves amyloid-beta-mediated suppression of memory and synaptic plasticity. Brain. 2008;131:651–664. doi: 10.1093/brain/awn008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laganowsky A, Liu C, Sawaya MR, Whitelegge JP, Park J, Zhao M, Pensalfini A, Soriaga AB, Landau M, Teng PK, et al. Atomic view of a toxic amyloid small oligomer. Science. 2012;335:1228–1231. doi: 10.1126/science.1213151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna-Reeves CA, Glabe CG, Kayed R. Amyloid-beta annular protofibrils evade fibrillar fate in Alzheimer disease brain. J Biol Chem. 2011;286:22122–22130. doi: 10.1074/jbc.M111.236257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EB, Leng LZ, Zhang B, Kwong L, Trojanowski JQ, Abel T, Lee VM. Targeting amyloid-beta peptide (Abeta) oligomers by passive immunization with a conformation-selective monoclonal antibody improves learning and memory in Abeta precursor protein (APP) transgenic mice. J Biol Chem. 2006;281:4292–4299. doi: 10.1074/jbc.M511018200. [DOI] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Lesne S, Kotilinek L, Ashe KH. Plaque-bearing mice with reduced levels of oligomeric amyloid-beta assemblies have intact memory function. Neuroscience. 2008;151:745–749. doi: 10.1016/j.neuroscience.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesne SE, Sherman MA, Grant M, Kuskowski M, Schneider JA, Bennett DA, Ashe KH. Brain amyloid-beta oligomers in ageing and Alzheimer's disease. Brain. 2013;136:1383–1398. doi: 10.1093/brain/awt062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Zhao M, Jiang L, Cheng PN, Park J, Sawaya MR, Pensalfini A, Gou D, Berk AJ, Glabe CG, et al. Out-of-register beta-sheets suggest a pathway to toxic amyloid aggregates. Proc Natl Acad Sci U S A. 2012;109:20913–20918. doi: 10.1073/pnas.1218792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Kemper LJ, Wang J, Zahs KR, Ashe KH, Pasinetti GM. Grape seed polyphenolic extract specifically decreases abeta*56 in the brains of Tg2576 mice. J Alzheimers Dis. 2011;26:657–666. doi: 10.3233/JAD-2011-110383. [DOI] [PubMed] [Google Scholar]
- Liu P, Paulson JB, Forster CL, Shapriro SL, Ashe KH, Zahs KR. Characterization of a novel mouse model of Alzheimer's disease - amyloid pathology and unique β-amyloid oligomer profile. PLoS One. doi: 10.1371/journal.pone.0126317. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JX, Qiang W, Yau WM, Schwieters CD, Meredith SC, Tycko R. Molecular structure of beta-amyloid fibrils in Alzheimer's disease brain tissue. Cell. 2013;154:1257–1268. doi: 10.1016/j.cell.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. Theories of hippocampal function. In: Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J, editors. The Hippocampus Book. Oxford University Press; Oxford: 2007. pp. 581–694. [Google Scholar]
- Noguchi A, Matsumura S, Dezawa M, Tada M, Yanazawa M, Ito A, Akioka M, Kikuchi S, Sato M, Ideno S, et al. Isolation and characterization of patient-derived, toxic, high mass amyloid beta-protein (Abeta) assembly from Alzheimer disease brains. J Biol Chem. 2009;284:32895–32905. doi: 10.1074/jbc.M109.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowitzki S, Deptula D, Thurfjell L, Barkhof F, Bohrmann B, Brooks DJ, Klunk WE, Ashford E, Yoo K, Xu ZX, et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol. 2012;69:198–207. doi: 10.1001/archneurol.2011.1538. [DOI] [PubMed] [Google Scholar]
- Paule MG, Bushnell PJ, Maurissen JP, Wenger GR, Buccafusco JJ, Chelonis JJ, Elliott R. Symposium overview: the use of delayed matching-to-sample procedures in studies of short-term memory in animals and humans. Neurotoxicol Teratol. 1998;20:493–502. doi: 10.1016/s0892-0362(98)00013-0. [DOI] [PubMed] [Google Scholar]
- Paulson JB, Ramsden M, Forster C, Sherman MA, McGowan E, Ashe KH. Amyloid plaque and neurofibrillary tangle pathology in a regulatable mouse model of Alzheimer's disease. Am J Pathol. 2008;173:762–772. doi: 10.2353/ajpath.2008.080175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Nievas BG, Stein TD, Tai HC, Dols-Icardo O, Scotton TC, Barroeta-Espar I, Fernandez-Carballo L, de Munain EL, Perez J, Marquie M, et al. Dissecting phenotypic traits linked to human resilience to Alzheimer's pathology. Brain. 2013;136:2510–2526. doi: 10.1093/brain/awt171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang W, Yau WM, Luo Y, Mattson MP, Tycko R. Antiparallel beta-sheet architecture in Iowa-mutant beta-amyloid fibrils. Proc Natl Acad Sci U S A. 2012;109:4443–4448. doi: 10.1073/pnas.1111305109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MN, Hofmeister JJ, Jungbauer L, Welzel AT, Yu C, Sherman MA, Lesne S, LaDu MJ, Walsh DM, Ashe KH, et al. Cognitive effects of cell-derived and synthetically derived Abeta oligomers. Neurobiol Aging. 2011;32:1784–1794. doi: 10.1016/j.neurobiolaging.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, Mathis CA, Blennow K, Barakos J, Okello AA, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9:363–372. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]
- Rozkalne A, Spires-Jones TL, Stern EA, Hyman BT. A single dose of passive immunotherapy has extended benefits on synapses and neurites in an Alzheimer's disease mouse model. Brain Res. 2009;1280:178–185. doi: 10.1016/j.brainres.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgourakis NG, Yau WM, Qiang W. Modeling an in-register, parallel “iowa” abeta fibril structure using solid-state NMR data from labeled samples with rosetta. Structure. 2015;23:216–227. doi: 10.1016/j.str.2014.10.022. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Leissring MA, Adame A, Sun X, Spooner E, Masliah E, Selkoe DJ, Lemere CA, Walsh DM. Biochemical and immunohistochemical analysis of an Alzheimer's disease mouse model reveals the presence of multiple cerebral Abeta assembly forms throughout life. Neurobiol Dis. 2009;36:293–302. doi: 10.1016/j.nbd.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel G. Alzforum, editor. Biogen antibody buoyed by Phase 1 data and hungry investors. 2015 [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Tycko R. Solid-state NMR studies of amyloid fibril structure. Annu Rev Phys Chem. 2011;62:279–299. doi: 10.1146/annurev-physchem-032210-103539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haaren F, van Zijderveld G, van Hest A, de Bruin JP, van Eden CG, van de Poll NE. Acquisition of conditional associations and operant delayed spatial response alternation: effects of lesions in the medial prefrontal cortex. Behav Neurosci. 1988;102:481–488. doi: 10.1037//0735-7044.102.4.481. [DOI] [PubMed] [Google Scholar]
- Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AL, Zinn R, Hohensinn B, Konen LM, Beynon SB, Tan RP, Clark IA, Abdipranoto A, Vissel B. Neuroinflammation and neuronal loss precede Abeta plaque deposition in the hAPP-J20 mouse model of Alzheimer's disease. PLoS One. 2013;8:e59586. doi: 10.1371/journal.pone.0059586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JW, Breydo L, Isas JM, Lee J, Kuznetsov YG, Langen R, Glabe C. Fibrillar oligomers nucleate the oligomerization of monomeric amyloid beta but do not seed fibril formation. J Biol Chem. 2010;285:6071–6079. doi: 10.1074/jbc.M109.069542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
2
3