Nutritional Supplements in Support of Resistance Exercise to Counter Age-Related Sarcopenia (original) (raw)
Abstract
Age-related sarcopenia, composed of myopenia (a decline in muscle mass) and dynapenia (a decline in muscle strength), can compromise physical function, increase risk of disability, and lower quality of life in older adults. There are no available pharmaceutical treatments for this condition, but evidence shows resistance training (RT) is a viable and relatively low-cost treatment with an exceptionally positive side effect profile. Further evidence suggests that RT-induced increases in muscle mass, strength, and function can be enhanced by certain foods, nutrients, or nutritional supplements. This brief review focuses on adjunctive nutritional strategies, which have a reasonable evidence base, to enhance RT-induced gains in outcomes relevant to sarcopenia and to reducing risk of functional declines.
Keywords: aging, function, dynapenia, protein, creatine, vitamin D, β-HMB, Leu
Introduction
Age-related sarcopenia begins in approximately the fifth decade of life and proceeds, at a population level, at a rate of ∼0.8% annually (1). Declines in skeletal muscle strength with sarcopenia, known as dynapenia, are more precipitous at ∼2–3% annually (2). The reduction in skeletal muscle mass and strength with advancing age is associated with diseased states including type 2 diabetes, cancer, metabolic syndrome (3), and reduced mobility and disability, as well as mortality (4). Current estimates suggest that ~200 million people worldwide will experience sarcopenia to a degree that could affect their health over the next 4 decades (2). Thus, the development of strategies to counteract the negative impact of sarcopenia is warranted.
Current Status of Knowledge
The mass of skeletal muscle is underpinned, to a large extent, by coordinated changes in the rates of muscle protein synthesis (MPS)3 and muscle protein breakdown (MPB) (5, 6). Both protein ingestion and resistance exercise are potent stimuli for MPS; however, when combined there is synergistic interaction between these stimuli that leads to an accrual of skeletal muscle mass (7). There are now data to suggest that aging is characterized by an attenuated response of MPS (and possible MPB) to amino acid ingestion (8) and also to exercise (9). The aim of this review is to examine how exercise and nutritional strategies can counteract the negative impact of sarcopenia in older adults. Because of space limitations it is not possible to discuss all areas relevant to this topic and the interested reader is instead referred to other informative reviews (10, 11).
Resistance Training
There are currently no viable pharmaceutical interventions to slow progression of sarcopenia with the exception of testosterone administration (12). Resistance training (RT) is a highly effective strategy to offset sarcopenia and it has numerous beneficial “spillover” effects. The main RT-induced outcomes relevant to this review are obvious increases in muscle mass, strength, and functional performance in older individuals (13–16). Resistance exercise stimulates MPS through the mammalian (mechanistic) target of the rapamycin complex 1–ribosomal protein of 70-kDa S6 kinase 1 (mTORC1-p70S6K1) pathway (17). Over time, persistent stimulation of this pathway via loaded contractions and combined with adequate protein ingestion leads to lean mass accretion. For example, in a 16-wk training trial involving older adults between the ages of 65 and 75 y, RT increased muscle mass by 1.5 kg and overall strength by 60% (15). Similarly, leg lean mass increased by 3%, strength by just over 40%, and sit-to-stand time decreased by ∼20% after a 24-wk RT program in older men and women (16). Importantly, because type II muscle fiber atrophy and loss predominates in sarcopenia (18), RT also resulted in increased type II muscle fiber area (16). Furthermore, when consumed in close temporal proximity to the resistance exercise session, protein can act synergistically with resistance exercise to further heighten the MPS response (19–21). In fact, a recent meta-analysis of randomized controlled trials found that dietary protein supplementation during RT (>6 wk) resulted in greater gains in lean body mass and strength than RT alone in younger and older adults (7). Whether age-related cell senescence may limit gain, lean mass even with persistent RT is unclear; however, systematic reviews indicate that the elderly >75 y of age are still capable of hypertrophy and strength gains (22).
Contrary to belief and prescriptive guidelines for older adults, it is not necessary to lift heavier loads in order to induce muscle hypertrophy (23). Work from our laboratory has shown similar gains in muscle mass in young adults after 12 wk of either low-load, high-repetition or high-load, low-repetition RT (23) and recent reviews find little evidence for a superiority of heavier weights in terms of inducing hypertrophy (24). In fact, a recent systematic review of RT in older adults found that the only RT variable that influenced outcome was the duration of the training program (25). An important consideration for RT prescription at lower intensities is loads that are lifted to the point of momentary muscle failure or, more simply put, lifted with a high degree of effort (26). Intensity, as it is usually defined in RT, is the percentage of maximal strength or percentage of a single-repetition maximum (1-RM); however, performance of resistance exercise, even with lighter loads, with high effort would also be considered/perceived to be intense. Although muscle gains were similar, training-induced gains in muscle strength in practiced tasks (isotonic maximal strength of exercises performed during the training regimen) were greater in a higher-load, low-repetition group (23). However, strength gains in an unpracticed task (isometric maximal voluntary torque of the knee extensors tested pre- and post-training only) were not different between groups (23), which indicates that the differences in voluntary strength observed were neural in nature. Similar results have been found using low-load RT in older adults (27). Of note, the RT protocol used in this study (27) involved the participants completing the exercises at maximum voluntary velocity, putting emphasis on power (i.e., force ⋅ velocity) generating ability of the muscle. Similarly, Van Roie and colleagues (28) compared 12 wk of high-load low repetitions, low-load high repetitions, and mixed low- and moderate-load high repetitions (all sets being completed to voluntary fatigue) and showed greater increases in strength in the high-load and mixed low- and moderate-load groups than those in the low-load group. However, there were similar gains in muscle volume and knee extensor peak torque in all groups (28), which is similar to our work (23).
RT is effective in terms of promotion of gains or attenuating loses in skeletal muscle as well as promoting gains in strength and functional status. However, it is proposed that RT regimens involving high load (i.e., 80% of 1-RM) are not a requirement to achieve muscle hypertrophy, strength gains, and/or improvements in functional performance in older adults (29). Although recent meta-analyses have found greater strength gains with higher-intensity (i.e., higher percentage of 1-RM) RT (29, 30), it is suggested that such differences are functionally unimportant because they did not translate into any differences in functional performance in older adults.
Protein
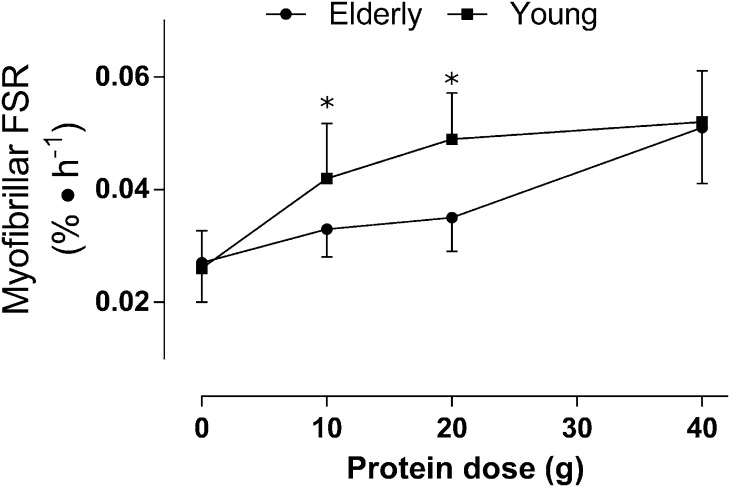
Consumption of protein leading to hyperaminoacidemia can act synergistically with resistance exercise to enhance the MPS response (31). We also know that protein can act independent of exercise to increase rates of MPS; however, the ability of protein to stimulate MPS is blunted in older adults (8). In fact, in older adults the ingestion of 35 and 40 g of protein at rest (32) and after resistance exercises (33), compared with 20 g in young individuals (34), was needed to maximally stimulate MPS. Recently, an attempt to define the protein dose, relative to body mass, required on a per meal basis in young and older individuals was made (8). Briefly, data from 6 previously published studies investigating dose-response effects of protein on MPS in young and older individuals were analyzed using a biphasic model (8). The findings from this study confirmed different protein dose needs in young and older individuals whereby MPS was maximally stimulated by 0.24 g of protein ⋅ kg−1 ⋅ meal−1 and 0.40 g of protein ⋅ kg−1 ⋅ meal−1 in young and older individuals, respectively (8). Importantly, the increased protein dose needed to maximally stimulate MPS was not the result of differences in lean body mass between older and younger individuals because when expressed relative to fat-free mass the protein dose was still greater in the older adults: 0.60 compared with 0.25 g of protein ⋅ kg fat-free mass−1 ⋅ meal−1 (8). This evidence (8) is consistent with protein dose-response studies that have shown an attenuated MPS response in the elderly to low, but not to higher, protein doses (32). Other data show that a low dose (5 g) of essential amino acids (EAAs) (35) was less effective than a higher dose (15 g) of EAA (36) in stimulating MPS in the elderly and, more importantly, that older adults achieved rates of MPS when ingesting 15 g of EAA that were no different than those seen in the young individuals. In fact, when the young individuals and elderly are compared after ingestion of beef (30 g of protein) a similar result was observed (37, 38), which is consistent with the protein dose-response of the elderly with beef ingestion (39). Thus, the “anabolic resistance” of MPS to protein feeding seen in older adults is evident at lower doses of protein (8). Evidence from acute studies (35) would also suggest that a key amino acid in the protein is likely the indispensable amino acid Leu. Thus, it is likely more correct to say that rather than lower doses of protein it is the Leu content of the ingested proteins (35) that is likely critical. Figure 1 illustrates the point that anabolic resistance of protein metabolism in aging is only evident at lower doses of protein and that at higher doses of ingested protein the elderly can overcome this resistance and have a “youthful” MPS response. Viewed collectively, these data (Figure 1) point to a recommendation that, at least insofar as stimulation of MPS to maintain muscle mass is concerned, the elderly have higher protein requirements than the young individuals, which is in agreement with position stands in other studies (40, 41). Of issue is that although younger individuals are exceeding the RDA, consuming ∼1.3 g of protein ⋅ kg−1 ⋅ d−1 (42), one-third of older adults are not meeting these requirements and up to 10% of older women are not even meeting the Estimated Average Requirement for protein currently set by nitrogen balance at 0.66 g of protein ⋅ kg−1 ⋅ d−1 (43, 44). In fact, recent recommendations suggest that older adults should consume 25–30 g of high-quality protein (2.5 g of Leu per meal; see in the next section) at each meal to attenuate age-associated muscle mass loss (40).
FIGURE 1.
Absolute protein dose-responses of skeletal muscle myofibrillar protein synthesis in older (71 ± 1 y; n = 43) and younger (22 ± 4 y; n = 65) men. Data are presented as means ± SEMs and were analyzed with a 2-factor ANOVA, but within-group dose differences are not indicated for the sake of clarity. *Significantly different between groups, P < 0.05. FSR, fractional synthetic rate. Adapted from reference 8 with permission.
Further evidence supporting recommendations for higher protein intakes in older adults comes from studies showing that in older adults higher protein intakes are protective against mass (45) and lean mass loss (43) and are positively associated with lean mass (46, 47). Furthermore, the addition of 15 g of protein at breakfast and lunch, which increased the protein content of these meals to at least 25 g [the minimum recommended amount of protein per meal for older adults (40)], increased strength and physical performance in frail elderly persons (48). As mentioned previously, the combination of RT, which sensitizes the muscle-to-protein intake for a long period of time, should then provide a more potent stimulus for MPS and result in greater hypertrophy or better attenuation of muscle loss. This thesis is supported by findings of no increase in muscle mass when RT was performed without protein supplementation compared with protein supplementation (30 g of protein: 15 g, twice daily) in frail elderly individuals (49).
Leucine
Protein quality is a function of protein amino acid content, digestibility, and bioavailability. Proteins from animal sources such as meat, poultry, fish, dairy, eggs, and isolated soy protein are high-quality proteins given their complete complement of EAA and digestibility. Thus, it is not surprising that ingestion of these proteins provides a robust increase in MPS on consumption. In addition, milk proteins, particularly whey protein, have been shown to be superior to other types of protein at stimulating MPS in older men (50, 51). In particular, despite the equal (albeit because of enforced truncation of scores at 1.0) protein digestibility–corrected amino acid scores (PDCAASs) of milk protein and soy, milk is better able to stimulate MPS (20) and results in greater muscle hypertrophy after RT when consumed postexercise, at least in younger persons (52). However, it has been advocated that PDCAASs be replaced by a newer protein scoring system: the digestible indispensable amino acid score (DIAAS). The main difference between the PDCAAS and DIAAS scoring systems is that DIAAS is not truncated and that the true illeal digestibility (if known) of individual amino acids within proteins is used (53). In fact, the most recent guidelines state that “…dietary amino acids be treated as individual nutrients and that wherever possible data for digestible or bioavailable amino acids be given…,” which could have important implications (53). The recognition placed on individual amino acids as nutrients could explain why isolated whey protein has been found to stimulate MPS to a greater extent than casein (the other main milk protein) in the elderly (50, 54). The differences between whey and casein in terms of stimulating MPS are attributed to whey being digested more rapidly and to having a higher Leu content, resulting in a more rapid and robust hyperaminoacidemia and hyperleucinemia (55).
Although all of the EAA would be needed to allow MPS to occur with protein ingestion (56), Leu is the key amino acid that triggers the stimulation of key regulatory proteins and the initiation of MPS (57) from a state of net negative protein balance. The potency of Leu was shown when subjects consumed a lower dose of protein (6 g), which had previously been shown to be less effective in stimulating MPS (58), with added Leu, which effectively elicited the same MPS response as an optimal dose (25 g) of protein, in young individuals (59). Similarly, after a session of resistance exercise, the addition of Leu to a protein/carbohydrate beverage increased MPS to a greater extent than the protein/carbohydrate beverage alone (60). These findings indicate that proteins with higher Leu content would be more effective than those with lower Leu content at stimulating MPS and this may be particularly true in the elderly in whom it appears there is a reduced sensitivity to Leu (35). As such, higher-quality proteins with high DIAAS scores and a high Leu content would be the best sources with which to supplement older adults.
To date, there have been 2 longer-term trials of Leu supplementation in populations one would predict could benefit from the ingestion of supplemental Leu (61, 62). The studies did not involve resistance exercise but are reviewed here because the results are relevant to how Leu may (or may not) enhance muscle mass in older adults. One study involved 3 mo of ingesting 7.5 g of Leu ⋅ d−1 (3 × 2.5 g ⋅ meal−1) in elderly men (61), and the other study lasted 6 mo with the same dosing regimen in older men with type 2 diabetes (62). No benefit was observed in either study in terms of Leu promoting lean mass or strength gains (61, 62). There are reasons why Leu supplementation may not have resulted in any change in muscle mass: participants may already have been consuming adequate protein (∼1–1.1 g ⋅ kg−1 ⋅ d−1) so supplemental Leu was ineffective; alternatively, provision of Leu alone resulted in a physiologically relevant reduction in systemic Val and Ile concentration because of branched-chain amino acid antagonism (63); and/or despite 3–6 mo of supplementation this was simply too short a period of time to see an effect on the expected change in lean mass. Regarding the last point, if we consider that a 70-y-old man weighing 85 kg and having ∼40–45 kg of skeletal muscle (i.e., ∼60–62 kg of fat- and bone-free mass) would lose muscle at a rate of 0.8% annually (1), there would be a reduction in total muscle mass of ∼160–180 g in a 6-mo period. Leu supplementation may have been able, at best, to offset such a loss but this would be difficult to detect using most commonly available methods (i.e., DXA, MRI, and/or measures of muscle fiber area). This last possibility also underscores recent observations that acute effects of many interventions, e.g., those seen with Leu supplementation (35), on MPS fail to align to changes in muscle lean mass accretion (64). Thus, it is perhaps not surprising that these trials of Leu supplementation (61, 62) have not shown any effect on changes in muscle mass in nonexercising older adults.
Creatine
Creatine is a nitrogenous organic acid that exists naturally in the body, being synthesized in the liver and kidneys from the amino acids Arg, Met, and Gly. Exogenous creatine is obtained in the diet from consumption of meat or creatine supplements, the most common being creatine monohydrate and creatine hydrochloride. Creatine is stored in the muscle and functions as an energy buffer during high-intensity exercise and as part of the creatine-phosphocreatine system, where it is reversibly converted to phosphocreatine by creatine kinase during periods of low muscular activity (65). At the onset of high-intensity exercise phosphocreatine donates a high-energy phosphate to ADP, serving as an anaerobic energy source to support the exercise session; however, it can be rapidly depleted within 15–30 s (65). As such, phosphocreatine is an important energy buffer in transitions from rest to various workloads and is particularly important for short-duration (<30 s), high-intensity activities, such as sprinting and resistance exercise, allowing high-power outputs to be achieved.
The ergogenic effects of creatine are mediated through 1 or more of the following mechanisms: increasing skeletal muscle phosphocreatine stores, speeding up phosphocreatine resynthesis, reducing muscle damage, and/or decreasing the reliance on anaerobic glycolysis, thus, decreasing lactate production [reviewed by Branch (65) and Rawson and Venezia (66)]. Although the exact mechanism of how creatine supplementation may augment exercise performance is unknown, each of the hypothesized mechanisms would allow a greater amount of work to be done during and/or a more rapid recovery after an intense short-duration exercise session.
The benefits of creatine are not confined to athletes because several trials have found an ergogenic effect of taking creatine on its own or in combination with RT in older adults. Some (67–69), but not all (70–72), trials investigating the effects of creatine supplementation alone have found positive effects on strength and functional performance in older adults. A recently completed meta-analysis from our group showed that creatine consumed concurrently with RT had a greater effect than RT alone in improving body composition, strength, and functional performance in older men and women (73). This meta-analysis was based on the findings from 8 randomized, placebo-controlled trials from 10 published reports that included a total of 252 older adults as subjects. Although there was disparity in the results between trials, overall creatine supplementation increased total-body fat-free mass by 1.5 kg (95% CI: 0.92, 2.02), chest press strength by 1.7 kg (95% CI: 0.49, 2.98), and the number of chair stands in 30 s (a measure of functional performance and an important measure of ability to perform activities of daily living) by 2 repetitions (95% CI: 0.19, 3.67) more than with RT alone. The conclusions of this meta-analysis (73) are concordant with similar analyses performed by another group (74). These findings support a role for creatine ingestion (∼5 g ⋅ d−1) paired with RT to attenuate sarcopenia.
As mentioned previously, not all studies showed a greater effect of creatine on its own or when added to RT to improve body composition, strength, and/or performance (70–72, 75–80), indicating some degree of response variability between trials and/or subjects. Both trial and individual factors might influence whether subjects respond to creatine and include whether or not the RT regimen was progressive and resulted in a greater training volume being completed by the creatine group, whether muscle creatine stores increased in response to the creatine supplementation, and whether creatine was consumed with a carbohydrate source. As such, recommended creatine dosing strategies for older adults would be to consume 5 g of creatine ⋅ d−1 with some carbohydrate paired with a progressive RT program, recognizing that those with naturally higher muscle creatine stores before supplementation may not respond to creatine supplementation.
β-Hydroxy-β-Methylbutyrate
The stimulatory impact of the branched-chain amino acid Leu on MPS is well documented (57). This effect is associated with the ability of Leu to activate mTORC1, which subsequently targets downstream signaling protein kinases such as 4E binding protein 1 (4EBP1) and p70S6K1, both of which facilitate translation initiation and stimulation of MPS (81). The discovery that Leu positively influences skeletal muscle metabolism makes it perhaps unsurprising that other molecules related to Leu, such as β-hydroxy-β-methylbutyrate (β-HMB), a Leu metabolite, would also possess anabolic properties capable of influencing skeletal muscle protein turnover (82). Currently, β-HMB is a patent-protected compound with a number of applications but most relevant to this review are US patents: 5,348,979 (83), β-HMB is described as useful for promoting nitrogen retention in humans; and 6,031,000 (84), which, among other claims, is a compound stated as being used, “…to treat disease-associated wasting of an animal….” In addition, other patents list β-HMB (in combination with vitamin D) as being useful in the promotion of muscular function and strength (85). In a recent systematic review of trials involving β-HMB in health and disease, Molfino et al. (82) concluded that a meta-analysis of the effects of β-HMB supplementation in the elderly was not possible mostly because of the heterogeneity of trials and the lack of pure β-HMB being compared with a placebo; nevertheless, a recent review states that, “Essential amino acid (EAA) supplements, including… β-hydroxy β-methylbutyric acid (HMB) supplements, show some effects in improving muscle mass and function parameters (86)” thus, the trials relevant to aging are reviewed here.
β-HMB is a metabolite of Leu and is produced in skeletal muscle when Leu is transaminated to α-ketoisocaproic acid, which is then converted to β-HMB by α-ketoisocaproic acid dioxygenase. Oral supplementation with β-HMB increases both plasma and intramuscular β-HMB concentrations (87) and there are reports that supplementation with β-HMB plus amino acids leads to improvements in both skeletal muscle mass and function (88–90). For example, supplementation with 3 g of β-HMB for 5 d before and during 10 d of bed rest in older adults attenuated losses in skeletal muscle (91). Moreover, supplementing older women with 2 g of β-HMB, 5 g of Arg, and 1.5 g of Lys for 12 wk was shown to enhance muscle strength and function as determined by a “get up and go” test when compared with placebo (90). There also are other reports of improved skeletal muscle functionality associated with β-HMB supplementation when combined with resistance exercise (92, 93). As such, β-HMB supplementation would appear to positively affect skeletal muscle health in a variety of settings and in different populations by as yet undetermined mechanisms.
Although the impact of protein/Leu on MPS is a topic of intense research, there is comparably less information regarding the cellular and molecular processes by which β-HMB influences muscle protein turnover. In a recent study by Wilkinson et al. (87) oral consumption of 3.42 g of the free-acid form of β-HMB increased rates of MPS (∼70%) as well as simultaneously decreasing MPB (∼57%) 150 min after ingestion in young resistance-trained males. β-HMB consumption also resulted in an increase in p70S6K1 and 4EBP1 phosphorylation; however, the suppression of MPB was not congruent with measures of proteolytic activity. Interestingly, in the same study (87), as a positive control, Leu ingestion (3.42 g) resulted in similar effects as β-HMB regarding a stimulation of MPS. Nevertheless, taken together (89–91, 93) what this study shows is that β-HMB ingestion exerts a synergistic impact on MPS in humans; however, Leu is at least equally as potent (on a gram-for-gram basis) in this regard (87). Such findings could have clinical relevance for those individuals who undergo short-term periods of muscle disuse, e.g., hospitalization, when reductions in postprandial MPS and smaller but transient increase in MPB drive skeletal muscle disuse atrophy (10). It is important to acknowledge, however, that changes in MPS in humans in response to β-HMB ingestion are not always detected (91).
Importantly, a relevant question is whether β-HMB is a useful compound in promoting muscle mass gains and/or retention in the elderly. In older persons (>65 y of age), randomized controlled trials with β-HMB are relatively few and highly heterogeneous in the health of the populations studied, the interventions used such as bed rest (91), or combined with RT (94), and the unknown influence of amino acids included with β-HMB [Arg and Lys (89, 90, 95)]. Because EAA and the potentially vasoactive amino acid Arg were given in addition to β-HMB, it is not possible to isolate the effect of β-HMB because the placebo group in these trials received the same amino acids. In the longest β-HMB–Arg–Lys supplementation trial in older adults published to date, Baier et al. (89) reported that older persons receiving an Lys–Arg–β-HMB combination (2 g of β-HMB, 5 g of Arg, and 1.5 g of Lys) showed greater strength gains than those receiving an isonitrogenous (5.6 g of Ala, 0.9 g of glutamate, 3.1 g of Gly, and 2.2 g of Ser) placebo. These authors reported greater gains (after 12 mo of supplementation) in the lysine–Arg–β-HMB–supplemented group in fat-free mass (∼0.75 kg), total cell mass (∼0.45 kg; both by single-frequency bioelectrical impedance analysis), and ∼0.37 kg of fat- and bone-free mass by DXA. Importantly, there were no associated functional gains associated with these differential changes in body composition. In a reanalysis of the same trial (89) only those receiving the Lys–Arg–β-HMB supplement with a clinically deficient (<30 ng ⋅ mL−1) concentration of vitamin D showed greater strength gains; however, the Lys–Arg–β-HMB–supplemented group had a baseline strength that was less than one-half that of the similarly vitamin D–deficient elderly subjects in the placebo group. In studies in which β-HMB (or combinations of β-HMB plus amino acids) has been supplemented in addition to RT, none have reported differential strength or functional gains compared with a control group. Thus, β-HMB does not augment RT-induced gains in muscle strength or function, with a possible exception of those that are starting with very low strength and clinically deficient concentrations of vitamin D. In summary, currently available evidence shows that supplementation with β-HMB would not influence gains in lean mass in older adults or to a trivial degree (i.e., <0.4 kg of lean mass) if there is an effect, and it has no effect on changing muscle function or mobility.
ω-3 PUFAs
ω-3 (n–3) PUFAs are critical components of cell membranes serving as substrates for the production of lipid signaling molecules as well as favorably modulating the biophysical properties of the cell membrane. Classically, n–3 PUFAs, specifically the 2 key FAs EPA (20:5–3) and DHA (22:6n–3), have been linked with improved cardiovascular health largely because of their anti-inflammatory properties (96). Given that sarcopenia has been reported to be associated with chronic low-grade inflammation (97), the use of n–3 PUFA supplementation to counter inflammation and to potentially affect sarcopenic muscle loss means that supplementation with n–3 PUFAs in older persons is receiving more attention. However, to date, very few studies have characterized the impact of n–3 PUFA supplementation on skeletal muscle in older populations or in those who experience muscle disuse atrophy.
Despite the lack of data, there are studies that do show a positive effect of n–3 PUFA supplementation on skeletal muscle. In one such study (98), it was demonstrated that supplementing older women with fish oil, containing 2 g of EPA/DHA, enhanced muscle strength during 90 d of RT. Moreover, 8 wk of n–3 PUFA–containing fish oil supplementation was shown to potentiate MPS in response to a hyperaminoacidemic-hyperinsulinemic clamp in young, middle-aged (99), and older adults (100). Interestingly, in the latter study, the potentiation of MPS was accompanied by enhanced mechanistic target of rapamycin (mTOR)-p70S6K1 phosphorylation. In this regard, there also is evidence that only 4 wk of n–3 PUFA supplementation increases the expression of the mechanically sensitive protein focal adhesion kinase in skeletal muscle (101). However, it is important to acknowledge that in the latter study, and in that of Rodacki et al. (98), no placebo group or measures of changes in skeletal muscle mass were made. In addition, although the potentiation of MPS and anabolic signaling in response to a hyperaminoacidemic-hyperinsulinemic clamp after fish oil supplementation provides excellent proof of concept data, the consumption of amino acids in the real-world setting does not occur via an intravenous infusion. Thus, future studies that identify if fish oil supplementation renders skeletal muscle more anabolically sensitive to hyperaminoacidemia that are accompanied by concomitant assessments of changes in skeletal muscle mass and function, particularly in older adults, would be of interest.
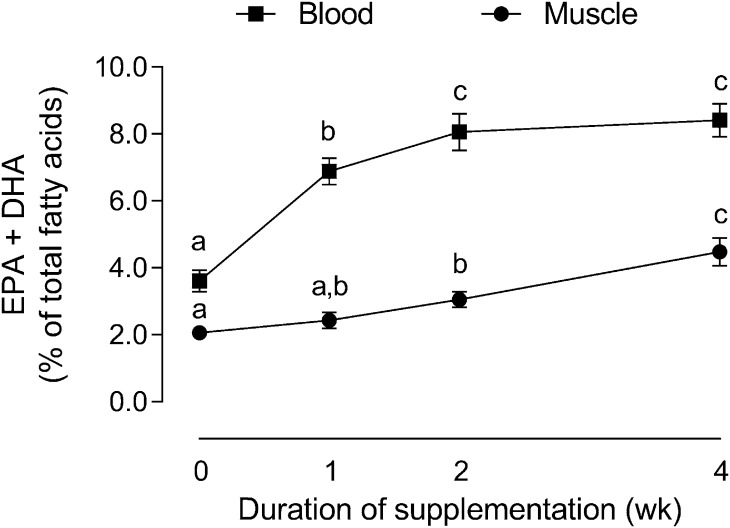
Other important questions also still remain with regard to how n–3 PUFA supplementation impacts skeletal muscle anabolism and function. Although the time course of changes in skeletal muscle EPA and DHA composition with fish oil supplementation have been established in younger, healthy persons (Figure 2) (101), similar time course changes in the skeletal muscle of older adults have not been determined. If n–3 PUFA supplementation is to be prescribed as a viable strategy to counteract the detrimental effects of sarcopenia, then these questions will need to be answered.
FIGURE 2.
Time course changes in skeletal muscle and RBC EPA plus DHA composition during 4 wk of 5 g ⋅ d−1 supplementation with n–3 PUFAs in younger men. Data presented as means ± SEMs and were analyzed using 1-factor ANOVA for both muscle and blood within RBCs or skeletal muscle, means without a common letter differ, P < 0.05. Adapted from reference 101 with permission.
Conclusions
Sarcopenia and dynapenia are serious health issues that increase disease and disability risk in our rapidly aging societies. The age-related decline in skeletal muscle mass is complex and multifaceted; however, it is proposed that by engaging in appropriate nutritional and exercise strategies such as the consumption of high-quality protein and participation in RT, older adults may partially be able to sustain skeletal muscle mass and function and thus enhance their quality of life. In this regard, data reviewed here show that enhancements of RT-induced skeletal muscle mass and function could reasonably be achieved by supplementation with protein and creatine. Emerging data are suggestive that the n–3 class of PUFAs may render skeletal muscle more sensitive to the anabolic effects of resistance exercise and feeding and this is an area that is ripe for research. Given the reduced sensitivity of MPS in older individuals to lower doses of protein intake, increasing the n–3 PUFA content of the diet may be one method by which to combat sarcopenia and associated conditions. At present, it appears that a key amino acid in protein is Leu, but that supplementation with this amino acid alone is not likely to yield benefits. Studies that have used β-HMB with or without other amino acids are heterogeneous but suggestive of effects on muscle mass, with no indication of improvements in muscle function. In addition, increasing both the quality and the amount of daily protein intake, especially when combined with RT, may also be efficacious. More work in the clinical setting is now required to experimentally test these promising adjunctive nutritional and supplement-based strategies to offset sarcopenia.
Acknowledgments
I thank Dr. Chris McGlory and Dr. Michalea Devries for expert technical and editorial assistance, as well as numerous helpful suggestions, in preparing this manuscript, and the National Science and Engineering Research Council (NSERC) of Canada and the Canadian Institutes for Health Research (CIHR). The sole author had responsibility for all parts of the manuscript.
Footnotes
3
Abbreviations used: DIAAS, digestible indispensable amino acid score; EAA, essential amino acid; MPB, muscle protein breakdown; MPS, muscle protein synthesis; mTOR, mechanistic target of rapamycin; mTORC1, mammalian (mechanistic) target of rapamycin complex 1; PDCAAS, protein digestibility–corrected amino acid score; p70S6k1, ribosomal protein of 70-kDa S6 kinase 1; RT, resistance training; 1-RM, single-repetition maximum; 4EBP1, 4E binding protein 1; β-HMB, β-hydroxy-β-methylbutyrate.
References
- 1.Janssen I. Evolution of sarcopenia research. Appl Physiol Nutr Metab 2010;35:707–12. [DOI] [PubMed] [Google Scholar]
- 2.Janssen I. The epidemiology of sarcopenia. Clin Geriatr Med 2011;27:355–63. [DOI] [PubMed] [Google Scholar]
- 3.Gong Z, Muzumdar RH. Pancreatic function, type 2 diabetes, and metabolism in aging. Int J Endocrinol 2012:320482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srikanthan P, Karlamangla AS. Muscle mass index as a predictor of longevity in older adults. Am J Med 2014;127:547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol 2004;66:799–828. [DOI] [PubMed] [Google Scholar]
- 6.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol 1997;273:E99–107. [DOI] [PubMed] [Google Scholar]
- 7.Cermak NM, Res P, de Groot L, Saris H, van Loon L. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr 2012;96:1454–64. [DOI] [PubMed] [Google Scholar]
- 8.Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci 2015;70:57–62. [DOI] [PubMed] [Google Scholar]
- 9.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, et al. . Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 2009;587:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wall BT, Dirks ML, van Loon LJ. Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing Res Rev 2013;12:898–906. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton B. Vitamin D and human skeletal muscle. Scand J Med Sci Sports 2010;20:182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horstman AM, Dillon EL, Urban RJ, Sheffield-Moore M. The role of androgens and estrogens on healthy aging and longevity. J Gerontol A Biol Sci Med Sci 2012;67:1140–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charette SL, McEvoy L, Pyka G, Snow-Harter C, Guido D, Wiswell R, Marcus R. Muscle hypertrophy response to resistance training in older women. J Appl Physiol (1985) 1991;70:1912–6. [DOI] [PubMed] [Google Scholar]
- 14.Campbell WW, Crim M, Young V, Evans W. Increased energy requirements and changes in body composition with resistance training in older adults. Am J Clin Nutr 1994;60:167–75. [DOI] [PubMed] [Google Scholar]
- 15.Yaraskeski KE, Zachwieja JJ, Campbell JA, Bier DM. Effect of growth hormone and resistance exercise on muscle growth and strength in older men. Am J Physiol 1995;268:E268–76. [DOI] [PubMed] [Google Scholar]
- 16.Leenders M, Verdijk L, van der Hoeven L, van Kranenburg J, Nilwik R, van Loon L. Elderly men and women benefit equally from prolonger resistance-type exercise training. J Gerontol A Biol Sci Med Sci 2013;68:769–79. [DOI] [PubMed] [Google Scholar]
- 17.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 2009;587:1535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deschenes MR. Effects of aging on muscle fibre type and size. Sports Med 2004;34:809–24. [DOI] [PubMed] [Google Scholar]
- 19.Tipton KD, Elliot TA, Cree MG, Wolf SE, Sanford AP, Wolfe RR. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med Sci Sports Exerc 2004;36:2073–81. [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson SB, Tarnopolsky MA, MacDonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr 2007;85:1031–40. [DOI] [PubMed] [Google Scholar]
- 21.Tipton KD, Elliot TA, Cree MG, Aarsland AA, Sanford AP, Wolfe RR. Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise. Am J Physiol Endocrinol Metab 2007;292:E71–6. [DOI] [PubMed] [Google Scholar]
- 22.Stewart VH, Saunders DH, Greig CA. Responsiveness of muscle size and strength to physical training in very elderly people: a systematic review. Scand J Med Sci Sports 2014;24:e1–10. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell CJ, Churchward-Venne TA, West DW, Burd NA, Breen L, Baker SK, Phillips SM. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J Appl Physiol 2012;113:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burd NA, Mitchell CJ, Churchward-Venne TA, Phillips SM. Bigger weights may not beget bigger muscles: evidence from acute muscle protein synthetic responses after resistance exercise. Appl Physiol Nutr Metab 2012;37:551–4. [DOI] [PubMed] [Google Scholar]
- 25.Silva NL, Oliveira RB, Fleck SJ, Leon AC, Farinatti P. Influence of strength training variables on strength gains in adults over 55 years-old: a meta-analysis of dose-response relationships. J Sci Med Sport 2014;17:337–44. [DOI] [PubMed] [Google Scholar]
- 26.Phillips SM, Winett RA. Uncomplicated resistance training and health-related outcomes: evidence for a public health mandate. Curr Sports Med Rep 2010;9:208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reid KF, Martin KI, Doros G, Clark DJ, Hau C, Patten C, Phillips EM, Frontera WR, Fielding RA. Comparative effects of light or heavy resistance power training for improving lower extremity power and physical performance in mobility-limited older adults. J Gerontol A Biol Med Sci 2015;70:372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Roie E, Delecluse C, Coudyzer W, Boonen S, Bautmans I. Strength training at high versus low external resistance in older adults: effects on muscle volume, muscle strength, and force-velocity characteristics. Exp Gerontol 2013;48:1351–61. [DOI] [PubMed] [Google Scholar]
- 29.Steib S, Schoene D, Pfeifer K. Dose-response relationship of resistance training in older adults: a meta-analysis. Med Sci Sports Exerc 2010;42:902–14. [DOI] [PubMed] [Google Scholar]
- 30.Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev 2009;3:CD002759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Churchward-Venne TA, Burd NA, Mitchell CJ, West DW, Philp A, Marcotte GR, Baker SK, Baar K, Phillips SM. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol 2012;590:2751–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pennings B, Groen B, de Lange A, Gijsen A, Zorenc A, Senden J, van Loon L. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab 2012;302:E992–9. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Breen L, Burd N, Hector A, Churchward-Venne T, Josse A, Tarnopolsky M, Phillips S. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr 2012;108:1780–8. [DOI] [PubMed] [Google Scholar]
- 34.Witard OC, Jackman SR, Breen L, Smith K, Selby A, Tipton KD. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am J Clin Nutr 2014;99:86–95. [DOI] [PubMed] [Google Scholar]
- 35.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 2006;291:E381–7. [DOI] [PubMed] [Google Scholar]
- 36.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab 2004;286:E321–8. [DOI] [PubMed] [Google Scholar]
- 37.Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr 2007;86:451–6. [DOI] [PubMed] [Google Scholar]
- 38.Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc 2009;109:1582–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson MJ, Burd NA, Breen L, Rerecich T, Yang Y, Hector AJ, Baker SK, Phillips SM. Dose-dependent responses of myofibrillar protein synthesis with beef ingestion are enhanced with resistance exercise in middle-aged men. Appl Physiol Nutr Metab 2013;38:120–5. [DOI] [PubMed] [Google Scholar]
- 40.Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft A, Morley J, Phillips S, Sieber C, Stehle P, Teta D, et al. . Evidence-based recommendation for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc 2013;14:542–59. [DOI] [PubMed] [Google Scholar]
- 41.Wolfe RR, Miller S, Miller K. Optimal protein intake in the elderly. Clin Nutr 2008;27:675–84. [DOI] [PubMed] [Google Scholar]
- 42.Fulgoni VL III. Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003–2004. Am J Clin Nutr 2008;87:1554S–7S. [DOI] [PubMed] [Google Scholar]
- 43.Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB, et al. . Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr 2008;87:150–5. [DOI] [PubMed] [Google Scholar]
- 44.Volpi E, Campbell WW, Dwyer JT, Johnson MA, Jensen GL, Morley JE, Wolfe RR. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J Gerontol A Biol Sci Med Sci 2013;68:677–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gray-Donald K, St-Arnauld-McKenzie D, Gaudreau P, Morais J, Shatenstein B, Payette H. Protein intake protects against weight loss in healthy community-dwelling older adults. J Nutr 2014;144:321–6. [DOI] [PubMed] [Google Scholar]
- 46.Stookey JD, Adair LS, Popkin BM. Do protein and energy intakes explain long-term changes in body composition? J Nutr Health Aging 2005;9:5–17. [PubMed] [Google Scholar]
- 47.Geirsdottir OG, Arnarson A, Ramel A, Jonsson PV, Thorsdottir I. Dietary protein intake is associated with lean body mass in community-dwelling older adults. Nutr Res 2013;33:608–12. [DOI] [PubMed] [Google Scholar]
- 48.Tieland M, van de Rest O, Dirks M, van der Zwaluw N, Mensink M, van Loon L, de Groot L. Protein supplementation improves physical performance in frail elderly people: A randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2012;13:720–6. [DOI] [PubMed] [Google Scholar]
- 49.Tieland M, Dirks M, van der Zwaluw N, Verdijk L, van de Rest O, de Groot L, van Loon L. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2012;13:713–9. [DOI] [PubMed] [Google Scholar]
- 50.Pennings B, Boirie Y, Senden JM, Gijsen AP, Kuipers H, van Loon LJ. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr 2011;93:997–1005. [DOI] [PubMed] [Google Scholar]
- 51.Yang Y, Churchward-Venne TA, Burd N, Breen L, Tarnopolsky MA, Phillips S. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr Metab (Lond) 2012;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr 2007;86:373–81. [DOI] [PubMed] [Google Scholar]
- 53.Food and Agriculture Organization of the United Nations. Dietary protein quality evaluation in human nutrition. Report of an FAO expert consultation; 2011 Mar 31–Apr 2; Auckland, New Zealand. Rome (Italy): Food and Agriculture Organization of the United Nations; 2013.
- 54.Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJ. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr 2011;93:322–31. [DOI] [PubMed] [Google Scholar]
- 55.Burd NA, Yang Y, Moore DR, Tang JE, Tarnopolsky MA, Phillips SM. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br J Nutr 2012;108:958–62. [DOI] [PubMed] [Google Scholar]
- 56.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 2003;78:250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 2000;130:2413–9. [DOI] [PubMed] [Google Scholar]
- 58.Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 2009;89:161–8. [DOI] [PubMed] [Google Scholar]
- 59.Churchward-Venne TA, Breen L, Di Donato DM, Hector AJ, Mitchell CJ, Moore DR, Stellingwerff T, Breuille D, Offord EA, Baker SK, et al. . Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Am J Clin Nutr 2014;99:276–86. [DOI] [PubMed] [Google Scholar]
- 60.Koopman R, Wagenmakers A, Manders R, Zorenc A, Senden J, Gorselink M, Keizer H, van Loon L. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am J Physiol Endocrinol Metab 2005;288:E645–53. [DOI] [PubMed] [Google Scholar]
- 61.Verhoeven S, Vanschoonbeek K, Verdijk LB, Koopman R, Wodzig WK, Dendale P, van Loon LJ. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr 2009;89:1468–75. [DOI] [PubMed] [Google Scholar]
- 62.Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Hartgens F, Wodzig WK, Saris WH, van Loon LJ. Prolonged leucine supplementation does not augment muscle mass or affect glycemic control in elderly type 2 diabetic men. J Nutr 2011;141:1070–6. [DOI] [PubMed] [Google Scholar]
- 63.Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr 1984;4:409–54. [DOI] [PubMed] [Google Scholar]
- 64.Mitchell CJ, Churchward-Venne TA, Cameron-Smith D, Phillips SM. What is the relationship between the acute muscle protein synthesis response and changes in muscle mass? J Appl Physiol 2015;118:495–7. [DOI] [PubMed] [Google Scholar]
- 65.Branch JD. Effect of creatine supplementation on body composition and performance: A meta-analysis. Int J Sport Nutr Exerc Metab 2003;13:198–226. [DOI] [PubMed] [Google Scholar]
- 66.Rawson ES, Venezia AC. Use of creatine in the elderly and evidence for effects on cognitive function in young and old. Amino Acids 2011;40:1349–62. [DOI] [PubMed] [Google Scholar]
- 67.Gotshalk LA, Kraemer WJ, Mendonca MA, Vingren JL, Kenny AM, Spiering BA, Hatfield DL, Fragala MS, Volek JS. Creatine supplementation improves muscular performance in older women. Eur J Appl Physiol 2008;102:223–31. [DOI] [PubMed] [Google Scholar]
- 68.Gotshalk LA, Volek JS, Staron RS, Denegar CR, Hagerman FC, Kraemer WJ. Creatine supplementation improves muscular performance in older men. Med Sci Sports Exerc 2002;34:537–43. [DOI] [PubMed] [Google Scholar]
- 69.Stout JR, Sue Graves B, Cramer JT, Goldstein ER, Costa PB, Smith AE, Walter AA. Effects of creatine supplementation on the onset of neuromuscular fatigue threshold and muscle strength in elderly men and women (64–86 years). J Nutr Health Aging 2007;11:459–64. [PubMed] [Google Scholar]
- 70.Rawson ES, Clarkson PM. Acute creatine supplementation in older men. Int J Sports Med 2000;21:71–5. [DOI] [PubMed] [Google Scholar]
- 71.Jackobi JM, Rice CL, Curtin SV, Marsh GD. Neuromuscular properties and fatigue in older men following acute creatine supplementation. Eur J Appl Physiol 2001;84:321–8. [DOI] [PubMed] [Google Scholar]
- 72.Rawson ES, Wehnert M,L Clarkson PM. Effects of 30 days of creatine ingestion in older men. Eur J Appl Physiol Occup Physiol 1999;80:139–44. [DOI] [PubMed] [Google Scholar]
- 73.Devries MC, Phillips SM. Creatine supplementation during resistance training in older adults—a meta-analysis. Med Sci Sports Exerc 2014;46:1194–203. [DOI] [PubMed] [Google Scholar]
- 74.Candow DG, Chilibeck PD, Forbes SC. Creatine supplementation and aging musculoskeletal health. Endocrine 2014;45:354–61. [DOI] [PubMed] [Google Scholar]
- 75.Bemben MG, Witten MS, Carter JM, Eliot KA, Knehans AW, Bemben DA. The effects of supplementation with creatine and protein on muscle strength following a traditional resistance training program in middle-aged and older men. J Nutr Health Aging 2010;14:155–9. [DOI] [PubMed] [Google Scholar]
- 76.Bermon S, Venembre P, Sachet C, Valour S, Dolisi C. Effects of creatine monohydrate ingestion in sedentary and weight-trained older adults. Acta Physiol Scand 1998;164:147–55. [DOI] [PubMed] [Google Scholar]
- 77.Carter JM, Bemben DA, Knehans AW, Bemben MG, Witten MS. Does nutritional supplementation influence adaptability of muscle to resistance training in men aged 48 to 72 years. J Geriatr Phys Ther 2005;28:40–7. [DOI] [PubMed] [Google Scholar]
- 78.Cornelissen VA, Defoor JG, Stevens A, Schepers D, Hespel P, Decramer M, Mortelmans L, Dobbels F, Vanhaecke J, Fagard RH, et al. . Effect of creatine supplementation as a potential adjuvant therapy to exercise training in cardiac patients: a randomized controlled trial. Clin Rehabil 2010;24:988–99. [DOI] [PubMed] [Google Scholar]
- 79.Eijnde BO, Van Leemputte M, Goris M, Labarque V, Taes Y, Verbessen P, Vanhees L, Ramaekers M, Vanden Eynde B, Van Schuylenbergh R, et al. . Effects of creatine supplementation and exercise training on fitness in men 55–75 yr old. J Appl Physiol 2003;95:818–28. [DOI] [PubMed] [Google Scholar]
- 80.Eliot KA, Knehans AW, Bemben DA, Witten MS, Carter J, Bemben MG. The effects of creatine and whey protein supplementation on body composition in men aged 48 to 72 years during resistance training. J Nutr Health Aging 2008;12:208–12. [DOI] [PubMed] [Google Scholar]
- 81.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 2010;141:290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Molfino A, Gioia G, Rossi Fanelli F, Muscaritoli M. Beta-hydroxy-beta-methylbutyrate supplementation in health and disease: a systematic review of randomized trials. Amino Acids 2013;45:1273–92. [DOI] [PubMed] [Google Scholar]
- 83.Google Patent Search [Internet]. Method of promoting nitrogen retention in humans. Mountain View (CA): Google. [cited 2014 Dec 1]. Available from: https://www.google.com/patents/US5348979.
- 84.Google Patent Search [Internet]. Composition comprising β-hydroxy-β-methylbutyric acid and at least one amino acid and methods of use. Mountain View (CA): Google. [cited 2014 Dec 1]. Available from: https://www.google.com/patents/US6031000.
- 85.Google Patent Search [Internet]. Nutritional intervention for improving muscular function and strength. Mountain View (CA): Google. [cited 2014 Dec 1]. Available from: https://www.google.com/patents/CA2746420A1.
- 86.Cruz-Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, Chen LK, Fielding RA, Martin FC, Michel JP, et al. . Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014;43:748–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, Loughna P, Churchward-Venne TA, Breen L, Phillips SM, et al. . Effects of leucine and its metabolite beta-hydroxy-beta-methylbutyrate on human skeletal muscle protein metabolism. J Physiol 2013;591:2911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Holecek M, Muthny T, Kovarik M, Sispera L. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on protein metabolism in whole body and in selected tissues. Food Chem Toxicol 2009;47:255–9. [DOI] [PubMed] [Google Scholar]
- 89.Baier S, Johannsen D, Abumrad N, Rathmacher JA, Nissen S, Flakoll P. Year-long changes in protein metabolism in elderly men and women supplemented with a nutrition cocktail of beta-hydroxy-beta-methylbutyrate (HMB), L-arginine, and L-lysine. JPEN J Parenter Enteral Nutr 2009;33:71–82. [DOI] [PubMed] [Google Scholar]
- 90.Flakoll P, Sharp R, Baier S, Levenhagen D, Carr C, Nissen S. Effect of beta-hydroxy-beta-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition 2004;20:445–51. [DOI] [PubMed] [Google Scholar]
- 91.Deutz NE, Pereira SL, Hays NP, Oliver JS, Edens NK, Evans CM, Wolfe RR. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin Nutr 2013;32:704–12. [DOI] [PubMed] [Google Scholar]
- 92.Nissen S, Sharp R, Ray M, Rathmacher JA, Rice D, Fuller JC Jr, Connelly AS, Abumrad N. Effect of leucine metabolite beta-hydroxy-beta-methylbutyrate on muscle metabolism during resistance-exercise training. J Appl Physiol 1996;81:2095–104. [DOI] [PubMed] [Google Scholar]
- 93.Wilson GJ, Wilson JM, Manninen AH. Effects of beta-hydroxy-beta-methylbutyrate (HMB) on exercise performance and body composition across varying levels of age, sex, and training experience: a review. Nutr Metab (Lond) 2008;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stout JR, Smith-Ryan AE, Fukuda DH, Kendall KL, Moon JR, Hoffman JR, Wilson JM, Oliver JS, Mustad VA. Effect of calcium beta-hydroxy-beta-methylbutyrate (CaHMB) with and without resistance training in men and women 65+yrs: a randomized, double-blind pilot trial. Exp Gerontol 2013;48:1303–10. [DOI] [PubMed] [Google Scholar]
- 95.May PE, Barber A, D'Olimpio JT, Hourihane A, Abumrad NN. Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am J Surg 2002;183:471–9. [DOI] [PubMed] [Google Scholar]
- 96.Yusof HM, Miles EA, Calder P. Influence of very long-chain n-3 fatty acids on plasma markers of inflammation in middle-aged men. Prostaglandins Leukot Essent Fatty Acids 2008;78:219–28. [DOI] [PubMed] [Google Scholar]
- 97.Beyer I, Mets T, Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr Metab Care 2012;15:12–22. [DOI] [PubMed] [Google Scholar]
- 98.Rodacki CL, Rodacki AL, Pereira G, Naliwaiko K, Coelho I, Pequito D, Fernandes LC. Fish-oil supplementation enhances the effects of strength training in elderly women. Am J Clin Nutr 2012;95:428–36. [DOI] [PubMed] [Google Scholar]
- 99.Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, Mittendorfer B. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin Sci (Lond) 2011;121:267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, Mittendorfer B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr 2011;93:402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McGlory C, Galloway SD, Hamilton DL, McClintock C, Breen L, Dick JR, Bell JG, Tipton KD. Temporal changes in human skeletal muscle and blood lipid composition with fish oil supplementation. Prostaglandins Leukot Essent Fatty Acids 2014;90:199–206. [DOI] [PubMed] [Google Scholar]