Interleukin-10 Receptor Signaling in Innate Immune Cells Regulates Mucosal Immune Tolerance and Anti-Inflammatory Macrophage Function (original) (raw)
. Author manuscript; available in PMC: 2015 Jul 24.
Summary
Intact interkeulin-10 receptor (IL-10R) signaling on effector and regulatory T (Treg) cells are each independently required to maintain immune tolerance. Here we show that IL-10 sensing by innate immune cells, independent of its effects on T cells, was critical for regulating mucosal homeostasis. Following wild-type CD4+ T cell transfer, Rag2−/−Il10rb−/− mice developed severe colitis in association with profound defects in generation and function of Treg cells. Moreover, loss of IL-10R signaling impaired the generation and function of anti-inflammatory intestinal and bone marrow-derived macrophages, and their ability to secrete IL-10. Importantly, transfer of wild-type but not Il10rb−/− anti-inflammatory macrophages ameliorated colitis induction by wild-type CD4+ T cells in Rag2−/−Il10rb−/− mice. Similar alterations in the generation and function of anti-inflammatory macrophages were observed in IL-10R-deficient patients with very early-onset inflammatory bowel disease. Collectively, our studies define innate immune IL-10R signaling as a key factor regulating mucosal immune homeostasis in mice and humans.
Introduction
Interleukin-10 (IL-10) is a key immunosuppressive cytokine that is produced by a wide range of leukocytes as well as non-hematopoietic cells (Shouval et al., 2014). Polymorphisms in the IL-10 locus confer risk for ulcerative colitis and Crohn’s disease (Franke et al., 2008; Franke et al., 2010), and mice and humans deficient in either IL-10 or IL-10 receptor (IL-10R) exhibit severe intestinal inflammation and marked pro-inflammatory cytokines secretion (Begue et al., 2011; Glocker et al., 2010; Glocker et al., 2009; Kotlarz et al., 2012; Kuhn et al., 1993; Moran et al., 2013; Spencer et al., 1998). Thus, IL-10 has a central role in regulation of intestinal mucosal homeostasis and prevention of inflammatory bowel disease (IBD).
IL-10 mediates its anti-inflammatory effects through IL-10R-dependent signals emanating from the cell surface. The IL-10R is a hetero-tetramer that consists of two subunits of IL-10Rα and two subunits of IL-10Rβ (Moore et al., 2001). While the IL-10Rα subunit is unique to IL-10 signaling, the IL-10Rβ subunit is shared by other cytokine receptors, including IL-22, IL-26 and IFN-λ (Moore et al., 2001). IL-10 downstream signaling through the IL-10R inhibits the induction of pro-inflammatory cytokines by blocking NF-κB-dependent signals (Saraiva and O’Garra, 2010).
Although the development of IBD is well established in mice and in humans with IL-10R deficiency, the precise mechanisms of IL-10R-dependent control of immune tolerance and intestinal mucosal homeostasis are not well defined. In mice, intact IL-10R signaling is important in T regulatory (Treg) cells for their suppressive function including prevention of colitis, and in T effector cells for preventing exaggerated T helper-17 (Th17) cell responses in mucosal compartments (Chaudhry et al., 2011; Huber et al., 2011; Kamanaka et al., 2011; Murai et al., 2009). While innate immune cell production of IL-10 is critical for maintaining mucosal homeostasis (Liu et al., 2011; Murai et al., 2009), a role for innate immune IL-10R signaling in the regulation of intestinal immune tolerance has not been explored. Several groups have demonstrated that IL-10 sensing by innate immune cells is required for suppression of pro-inflammatory cytokines secretion (Gu et al., 2008; Pils et al., 2010). Moreover, IL-10R-deficient dendritic cells (DCs) secrete high quantities of pro-inflammatory cytokines after LPS stimulation (Girard-Madoux et al., 2012). We hypothesized that innate immune IL-10R signaling is required for maintenance of intestinal immune tolerance and prevention of IBD.
Here we demonstrate that IL-10R signaling in innate immune cells was critical for regulating mucosal homeostasis and prevention of colitis. Loss of IL-10R-dependent signaling rendered wild-type (WT) CD4+ T cells colitogenic and was associated with markedly aberrant Treg cells generation and function. Importantly, we show that IL-10R-dependent signals modulated the differentiation and function of bone marrow derived macrophages (BMDM) and intestinal macrophages into either pro-inflammatory macrophages or functionally competent anti-inflammatory macrophages. Similarly, monocyte-derived macrophages from very early-onset IBD patients harboring loss of function mutations in IL10RA and IL10RB also exhibited impaired differentiation and function of pro- and anti-inflammatory macrophages. These results define a unique and non-redundant role for IL-10R signaling in innate immune cell control of intestinal mucosal homeostasis.
Results
IL-10 regulates intestinal inflammation independent of T cell specific IL-10R signaling
We have recently reported that aberrant interactions between innate immune cells devoid of the cytoskeletal regulator Wiskott-Aldrich syndrome protein (WASP) and WT CD4+ T cells lead to colitis development (Nguyen et al., 2012a). In this model, _Was_−/−_Rag2_−/− mice develop severe intestinal inflammation following WT CD4+ T cell transfer, characterized by reduced production of IL-10; colitis development can be prevented by exogenous administration of IL-10Ig. To elucidate whether IL-10 acts on innate or adaptive immune cells in this model, we transferred Il10rb−/− CD4+ T cells into _Was_−/−_Rag2_−/− mice, which resulted in severe colitis in less than 2 weeks. We then assessed the effects of exogenous IL-10 in preventing disease, and as depicted in Figure S1, colitis was readily abrogated by exogenous IL-10Ig administration, indicating that IL-10 can prevent intestinal inflammation independent of its function on either regulatory or effector CD4+ T cells. These data are consistent with aberrant function of IL-10R signaling in innate immune cells in the setting of WASP-deficiency.
Colitis development in _Il10rb_−/− mice requires an adaptive immune system
To assess directly the role of IL-10R-dependent signals in innate immune cells in the control of mucosal homeostasis, we first analyzed _Il10rb_−/− mice. Consistent with prior observations (Spencer et al., 1998), _Il10rb_−/− mice (on the 129SvEv background) developed spontaneous colitis starting at 3-4 months of age, characterized by extensive bowel wall thickening, lamina propria (LP) lymphoid cell infiltration, and presence of crypt abscesses, in association with increased IFN-γ+–and IL-17A+–producing CD4+ T cells in the LP and mesenteric lymph node (MLN) (Figure S2). In order to assess whether lymphocytes are required for colitis development in _Il10rb_−/− mice we generated Rag2−/− Il10rb −/− mice, which lack mature B and T lymphocytes. Importantly, these mice are viable and do not develop clinical, endoscopic, or microscopic signs of colitis (data not shown). These data indicate that lymphocytes are essential for colitis development in _Il10rb_−/− mice.
_Il10rb_−/− innate immune cells render WT CD4+ T cells colitogenic
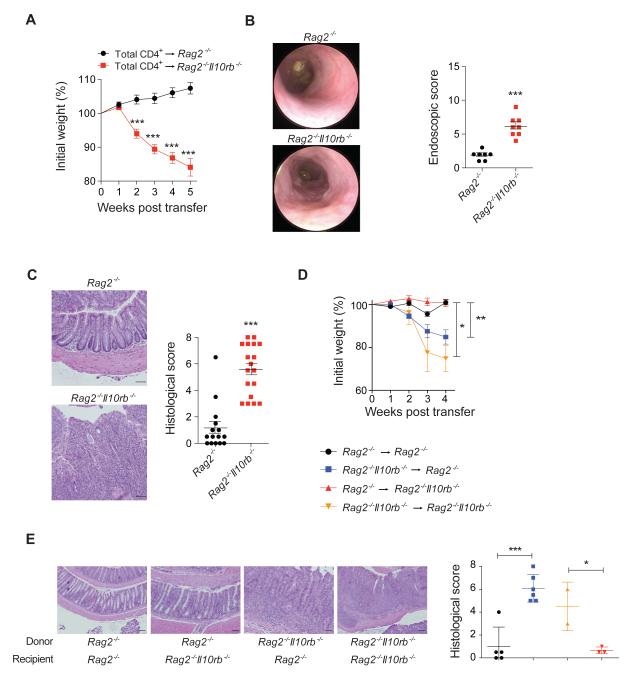
We next hypothesized that colitis development in _Il10rb_−/− mice, although lymphocyte-dependent, is initiated by defects in the innate immune compartment. To assess whether Il10rb −/− deficient innate immune cells cause WT CD4+ T cells to become colitogenic we introduced unfractionated WT CD4+ T cells by intraperitoneal (i.p.) injection into _Rag2_−/− and Rag2 −/− Il10rb −/− recipient mice. Rag2 −/− Il10rb −/− mice developed severe colitis following WT CD4+ T cell transfer within 3-4 weeks (Figure 1A-B). Hematoxylin and eosin (H&E) stained colonic sections demonstrated significant hyperplasia and immune cell infiltration of the LP, as well as occasional crypt abscesses (Figure 1C).
Figure 1. Transfer of WT CD4+ T cells into Rag2−/−Il10rb−/− mice induces severe colitis.
_Rag2_−/− and Rag2 −/− Il10rb−/− mice were injected i.p. with 1×106 WT CD4+ T cells. (A) Mean % initial body weights ± SEM following transfer (n = 30 for each group). (B) Representative endoscopic images and scores ± SEM of _Rag2_−/− and Rag2 −/− Il10rb−/− mice 5 weeks post-transfer. (C) Representative H&E section images (20X) and histological score ± SEM of _Rag2_−/− and Rag2 −/− Il10rb−/− mice following transfer. (D) BM chimeras were generated by transferring _Rag2_−/− or Rag2−/− Il10rb−/− BM cells into lethally irradiated _Rag2_−/− or Rag2 −/− Il10rb−/− recipients, and after 7 weeks WT CD4+ T cells were transferred into these mice. Mean weights ± SEM following T cells transfer displayed in (D) and images of representative H&E stained colonic sections (20X) and mean histological colitis scores ± SEM are displayed in (E). Scale bar = 200 μm. The data is representative of 2 or more independent experiments. Figures S1-S3 and S6 accompany.
Since IL-10Rβ is also expressed on non-hematopoietic cells (Moore et al., 2001), we assessed whether loss of IL-10Rβ signaling in innate immune cells was sufficient to drive intestinal inflammation by generating bone marrow (BM) chimeric animals. BM cells were isolated from either _Rag2_−/− or Rag2 −/− Il10rb −/− mice and transferred into lethally irradiated _Rag2_−/− or Rag2 −/− Il10rb−/− recipient mice, which after reconstitution received unfractionated WT CD4+ T cells. Upon T cell transfer, _Rag2_−/− mice reconstituted with Rag2 −/− Il10rb−/− BM developed colitis within several weeks (Figure 1D-E). In contrast, transfer of WT T cells into Rag2 −/− Il10rb −/− mice reconstituted with _Rag2_−/− BM did not lead to intestinal inflammation. Overall, these findings demonstrate that _Il10rb_−/− innate immune cells transmit a colitogenic signal to WT CD4+ T cells.
Exaggerated pro-inflammatory cytokine responses in _Rag2_−/−_Il10rb_−/− mice following WT CD4+ T cell transfer
We next assessed the effects of innate immune IL-10R deficiency on cytokine expression by analyzing _Rag2_−/− and Rag2 −/− Il10rb−/− mice following WT CD4+ T cell transfer. Prior to transfer, inflammatory cytokines were not elevated in the LP of either _Rag2_−/− or Rag2 −/− Il10rb−/− mice (data not shown). Following WT CD4+ T cell transfer, the T helper-1 (Th1) cell-associated cytokines TNF, IFN-γ, IL-6 and IL-12, but not IL-17A, were elevated in colonic explants and tissue extracts from Rag2 −/− Il10rb−/− compared to _Rag2_−/− recipient mice (Figure S3A-B). Comparable frequencies of IL-17A+ and IFN-γ+ CD4+ T cells were detected by flow cytometry in the LP of both _Rag2_−/− and Rag2 −/− Il10rb−/− mice following WT T cell transfer; however, the absolute numbers of CD4+ IFN-γ+ T cells were significantly increased in LP of Rag2 −/− Il10rb−/− compared to _Rag2_−/− mice (Figure S3C-D). Enhanced Th1 cell activity was reported in mice with a conditional deletion in macrophages and granulocytes of STAT3, a transcription factor down-stream of IL-10 (Takeda et al., 1999). Overall, our data also suggests that loss of IL-10R signaling on innate immune cells is associated with exaggerated pro-inflammatory cytokine responses.
Loss of innate immune IL-10Rβ signaling impairs the function and generation of WT Treg cells in vivo
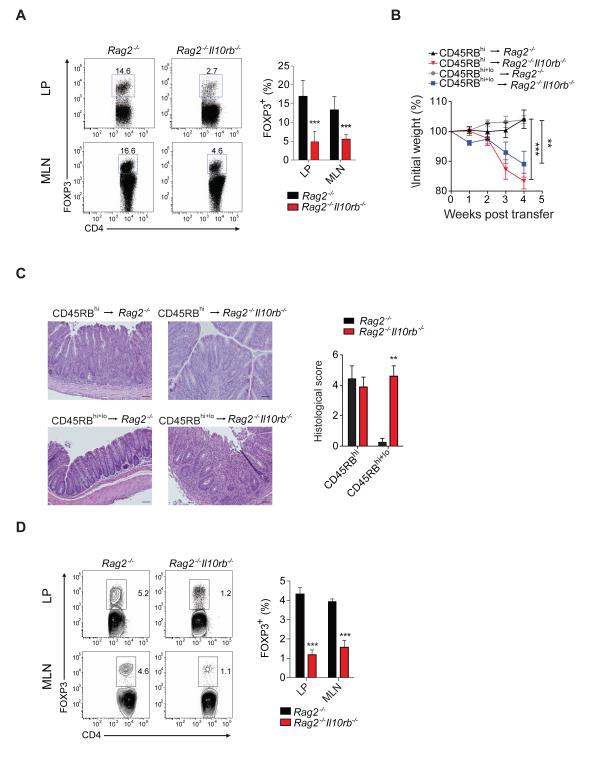
We next hypothesized that colitis development in Rag2 −/− Il10rb −/− mice following T cell transfer results from IL-10Rβ deficiency in innate immune cells affecting the function of either effector and/or regulatory T cell populations. Following transfer of unfractionated WT CD4+ T cells the frequency of FOXP3+ Treg cells was significantly reduced in the LP and MLN of Rag2 −/− Il10rb −/− mice vs. Rag2 −/− mice (Figure 2A). Transfer of WT T naïve cells (CD4+CD25−CD45RBhi) elicited colitis in both _Rag2_−/− and Rag2 −/− Il10rb −/− recipient mice; however, Rag2 −/− Il10rb −/− recipient mice lost significantly more weight compared with _Rag2_−/− control group (Figure 2B-C). We then assessed whether co-transfer of WT Treg cells (CD4+CD25+CD45RBlo) with WT T naïve cells at a ratio of 1:1 (standard ratio used in the T cell transfer model is 1:4) was protective against colitis development in Rag2 −/− Il10rb −/− mice. Despite the marked increase in the fraction of Treg cells, only _Rag2_−/− recipient mice, but not Rag2 −/− Il10rb−/− recipients, were protected from colitis development (Figure 2B-C), suggesting that IL-10Rβ signaling on innate immune cells regulates the suppressive function of WT Treg cells. Upon transfer of WT CD4+ T naïve cells, the generation of inducible Treg cells was also severely impaired in the LP and MLN of Rag2 −/− Il10rb −/− recipient mice (Figure 2D).
Figure 2. Il10rb−/− innate immune cells impair WT Treg cells suppression and generation in vivo.
(A) Frequency of Treg cells in LP and MLN of _Rag2_−/− and Rag2 −/− Il10rb−/− mice that were transferred with unfractionated WT CD4+ T cell transfer. Representative flow cytometry plots of FOXP3+ cells among CD4+ T cells are followed by cumulative data in LP and MLN. (B) Mean % initial body weights ± SEM following transfer of WT T naïve (CD4+CD25−CD45RBhi) cells alone or in combination with Treg cells (CD4+CD25+CD45RBlo) at a 1:1 ratio. (C) Representative H&E images (20X) of _Rag2_−/− and Rag2 −/− Il10rb−/− recipient mice following transfer and mean histological colitis scores ± SEM. Scale bar = 200 μm. (D) Representative flow cytometry plots of the generation of inducible Treg cells in vivo assessed by FOXP3+ expression among CD4+ T cells in LP and MLN, 4 weeks after CD45RBhi transfer, followed by cumulative data. Results pooled from 2 independent experiments. Figure S4 and S6 accompany.
To facilitate tracking of specific cell populations, additional transfer experiments were performed utilizing Rag1 −/− Il10rb −/− recipient mice on the C57BL/6 background. Similar to Rag2 −/− Il10rb −/− mice on the 129SvEv background, these mice rapidly lost weight following transfer of unfractionated WT CD4+ T cells (Figure S4A-B). Moreover, transfer of sorted CD4+CD45RBhiFOXP3neg cells into Rag1 −/− Il10rb −/− mice led to severe colitis, and, similar to Rag2−/− Il10rb −/− recipient mice on the 129SvEv background, was accompanied by a marked reduction in the generation of inducible FOXP3+ Treg cells in the LP (Figure S4C-E). To assess further Treg cell maintenance, CD4+CD45RBloFOXP3pos T cells were transferred into either Rag1 −/− Il10rb −/− or Rag1 −/− mice. Treg cell transfer did not, as expected, induce colitis in either Rag1 −/− Il10rb −/− or Rag1 −/− mice (data not shown); in addition, the frequency of Treg cells isolated from the LP and MLN was comparable between both recipient groups (Figure S4F). Collectively, our data suggest that loss of innate immune IL-10Rβ signaling impairs the generation and function of WT Treg cells in vivo.
IL-10Rβ-dependent signals regulate intestinal macrophage differentiation
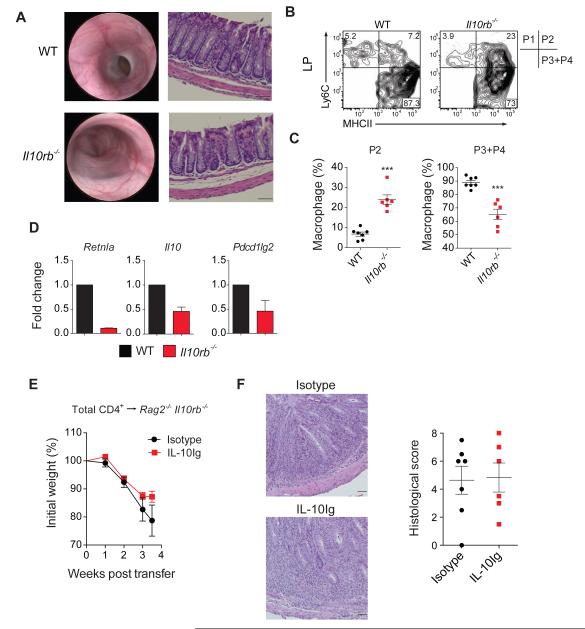
We next sought to investigate whether sensing of IL-10 by intestinal macrophages is important for controlling mucosal homeostasis. Nomenclature for intestinal macrophage subsets is evolving rapidly (Bain et al., 2013; Rivollier et al., 2012; Tamoutounour et al., 2012; Zigmond et al., 2012); for simplicity we have followed the nomenclature described by Tamoutounour et al who showed that circulating monocytes migrate into the LP and undergo a multi-step differentiation process that progresses through four stages of development, including the pro-inflammatory P2 stage, and the anti-inflammatory P3 and P4 stages. Throughout this manuscript, we refer to the P3 and P4 LP macrophage subsets in mice as anti-inflammatory macrophages. To evaluate whether IL-10Rβ-dependent signals regulate this differentiation process we evaluated _Il10rb_−/− mice at 5 weeks of age that lacked any clinical (not shown), endoscopic or histologic signs of intestinal inflammation, (Figure 3A). Initial evaluation by flow cytometry of pre-colitic mice minimized identifying non-specific effects that might be attributable to inflammation alone. LP cell analysis of pre-colitic _Il10rb_−/− mice demonstrated a significant increase in pro-inflammatory macrophages and a concomitant decrease in anti-inflammatory macrophages (Figure 3B-C). Moreover, expression of Retnla (Fizz1), a classical marker of anti-inflammatory macrophages and also identified in CX3CR1hi intestinal (presumably P4) macrophages (Zigmond et al., 2012), was decreased in the anti-inflammatory macrophages population of Il10rb −/− mice compared to WT (Figure 3D). Il10rb −/− anti-inflammatory macrophages also expressed less Il10 and Pdcd1l2 (programmed cell death 1 ligand 2, PD-L2) (Figure 3D). Importantly, similar results, demonstrating a reduction of anti-inflammatory macrophages were observed in the LP of colitic _Il10rb_−/− mice and Rag2 −/− Il10rb −/− mice following T cell transfer (Figure S5). Collectively, these results implicate a critical role for IL-10Rβ signaling in the differentiation of intestinal macrophages.
Figure 3. Reduction in anti-inflammatory intestinal macrophages of pre-colitic ll10rb−/− mice.
(A) Endoscopic and histological (10X) images of WT and Il10rb −/− mice at 5 weeks of age. (B-C) Representative flow cytometry plots of macrophage subsets in LP of 5 week old WT and Il10rb −/− mice, followed by quantification of the pro- and anti-inflammatory populations. Pro-inflammatory population was defined as Ly6C+MHCII+ cells and anti-inflammatory as Ly6CMHCII+. (D) LP anti-inflammatory macrophages were sorted from WT (n = 20) and Il10rb −/− (n = 14) 5 week old mice and qRT-PCR was performed to quantify expression of various anti-inflammatory transcripts. Results representative of two independent experiments. (E) Rag2 −/− Il10rb−/− were injected with 1 μg of IL-10Ig or isotype one day prior to WT CD4+ T cell transfer, and then twice weekly. Mean weights ± SEM shown in (E) and representative H&E sections images (20X) and histological scores ± SEM of both groups shown in (F). Scale bar = 200 μm. Results pooled from 2 independent experiments. Figures S5 and S6 accompany.
Exogenous IL-10 fails to prevent colitis in _Rag2_−/−_Il10rb_−/− following T cell transfer
Since Il10rb −/− anti-inflammatory intestinal macrophages produce less IL-10, we assessed whether reduced IL-10 concentrations may be responsible for colitis development in Rag2 −/− Il10rb −/− by treating recipient mice with exogenous IL-10 following WT CD4+ T cell transfer. Rag2−/− Il10rb −/− mice that received IL-10Ig treatment exhibited weight loss and signs of intestinal inflammation, similar to isotype control treated mice (Figure 3E-F), suggesting that IL-10 deficiency is not solely responsible for the colitis development. Moreover, as the CD4+ T cells in these experiments express an intact IL-10R, this indicates that IL-10R signaling on CD4+ T cells is insufficient to prevent colitis development in this model and suggests a non-redundant role for innate immune IL-10R signaling in regulating mucosal homeostasis.
_Il10rb_−/− M1 BMDM produce elevated quantities of pro-inflammatory cytokines and promote proliferation of WT CD4+ T cells
We next assessed whether BMDM, like their intestinal counterparts, were also dependent on IL-10R signaling for their differentiation and function. Stimulation of BMDM in vitro with LPS and IFN-γ generates M1 pro-inflammatory macrophages, while varying combinations of IL-4, IL-13, transforming growth factor-β (TGF-β and IL-10 generate M2 tolerogenic macrophages (Martinez et al., 2008). Parsa and colleagues recently reported that stimulation of BMDM with IL-4, TGF-β and IL-10 yields macrophages with increased tolerogenic properties that were defined as M2r macrophages (Parsa et al., 2012). These M2r macrophages highly express programmed death-ligand 1 (PD-L1) and PD-L2, secrete IL-10 and TGF-β and, when transferred into NOD mice, prevent diabetes (Parsa et al., 2012).
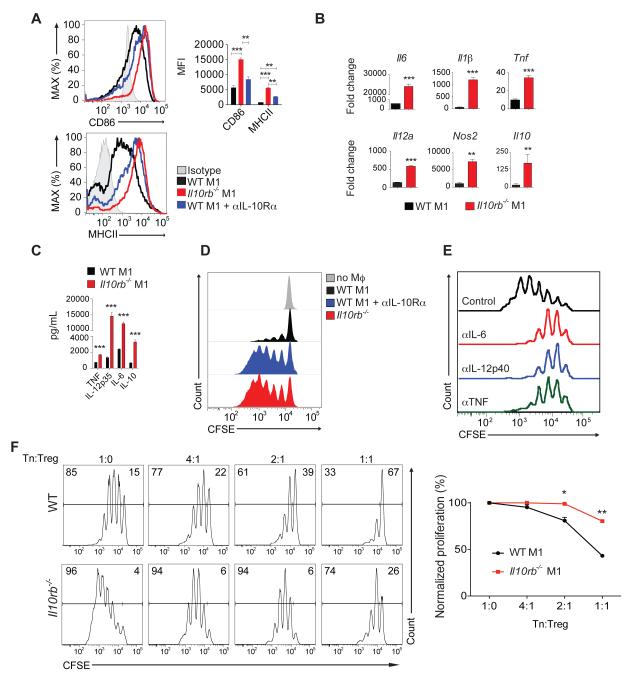
We observed comparable expression of pro- and anti-inflammatory cytokines and costimulatory molecules between WT and Il10rb −/− unstimulated (M0) BMDM (data not shown). However, major histocompatibility complex class II (MHCII) glycoproteins, CD86 and pro-inflammatory mediators were highly expressed in Il10rb −/− BMDM cultured in M1 conditions, when compared to WT M1 BMDM (Figure 4A-C). Similarly, culture of WT BMDM in M1 conditions with a blocking IL-10Rα antibody also led to a significant increase in expression of CD86 and MHCII (Figure 4A). Il10rb −/− M1 BMDM produced significantly more IL-10 (Figure 4BC), suggesting that IL-10Rβ-dependent signaling in pro-inflammatory macrophages inhibits IL-10 production. In addition, in a co-culture system with WT CD4+CD25− T naïve cells and M1 BMDM serving as antigen presenting cells, when compared to WT M1 BMDM, Il10rb −/− M1 BMDM or WT BMDM cultured with anti IL-10Rα antibody under M1 conditions promoted increased proliferation of WT T naïve cells (Figure 4D). Addition of neutralizing antibodies in this in vitro co-culture system against IL-6, IL-12p40 or TNF decreased the degree of T cell proliferation generated by Il10rb −/− M1 BMDM (Figure 4E), suggesting that excessive T cell proliferation is not caused by an excess of a single pro-inflammatory cytokine. Moreover, Il10rb −/− M1 BMDM impaired the ability of WT Treg cells to suppress proliferation of T effector cells (Figure 4F). To rule out the possibility that defective signaling through cytokine receptors which also utilize IL-10Rβ (i.e., IL-22, IL-26 and IFN-λ) might contribute to the observed phenotypes of Il10rb −/− M1 BMDM, we performed additional experiments with BM obtained from Il10ra −/− mice which lack only defective IL-10R signaling. Like Il10rb −/− M1 BMDM, Il10ra −/− M1 BMDM when compared with WT M1 BMDM, expressed higher amounts of CD86 and MHCII and, when cultured with WT CD4+CD25− T naïve cells, promoted increased T cell proliferation (data not shown). Collectively, our data indicate that IL-10R signaling regulates the function of inflammatory macrophages, which in turn can modulate T cell responses.
Figure 4. Il10rb−/− M1 BMDM exhibit a pronounced pro-inflammatory phenotype.
(A) Mean Fluorescence Intensity (MFI) of MHCII and CD86 expression on M1 WT and Il10rb −/− BMDM or WT BMDM cultured with an IL-10Rα blocking antibody in M1 conditions. (B) Cytokine mRNA expression determined by qRT-PCR of BMDM cultured for 24 hours in M1 conditions; fold change is relative to unstimulated (M0) WT BMDM. (C) Cytokines concentrations determined by ELISA in supernatants of BMDM cultured for 48 hours under M1 conditions. (D) Representative flow cytometry plots of CFSE-labeled WT CD4+CD25− T naïve cells proliferation cultured without macrophages or in the presence of WT M1 BMDM, Il10rb −/− M1 BMDM or WT M1 BMDM cultured with an IL-10Rα blocking antibody. (E) Il10rb −/− M1 BMDM were cultured with sorted T naïve cells, in the presence of neutralizing antibodies to IL-6, IL-12p40 or TNF. (F) WT T naïve cells were cultured with WT or Il10rb −/− M1 BMDM in the presence of varying concentrations of WT Treg cells. Representative flow cytometry plot presented following cumulative data showing degree of proliferation normalized to conditions without Treg cells. All data are representative of two or more independent experiments. Figure S6 accompanies.
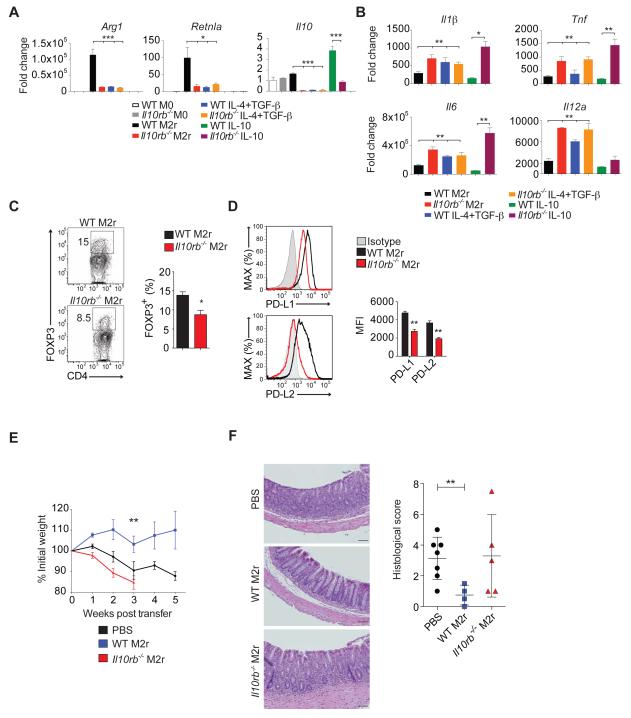
IL-10R signaling promotes tolerogenic properties of anti-inflammatory BMDM
Under M2r conditions, Il10rb −/− BMDM expressed significantly less Arg1 and Retnla (Figure 5A), which are classical markers of M2 anti-inflammatory macrophages (Martinez et al., 2008). Similarly, WT BMDM incubated with IL-4 and TGF-β, but not including IL-10, also resulted in reduced Arg1 and Retnla expression when compared to M2r conditions (Figure 5A), implying that IL-10 is required for the induction of the anti-inflammatory program in BMDM. Sensing of IL-10 by WT BMDM increased production of IL-10 (Figure 5A), indicating that IL-10R-dependent signals positively regulate IL-10 production by M2 macrophages. Baseline secretion of pro-inflammatory cytokines was low and comparable between WT and Il10rb −/− M2r BMDM (data not shown). However, re-stimulation with LPS of established Il10rb −/− M2r cells, or WT BMDM cultured with IL-4 and TGFβ, but not IL-10, led to a significant increase in the expression pro-inflammatory cytokines compared to WT BMDM cultured under M2r conditions (Figure 5B). These data suggest that IL-10R signaling in macrophages is required to inhibit TLR4-dependent pro-inflammatory responses. In addition, Il10rb −/− M2r BMDM, compared to WT M2r BMDM, promoted less Treg cells generation when co-cultured with WT CD4+CD25− T naïve cells (Figure 5C). This correlated with lower expression on Il10rb −/− M2r BMDM of PD-L1 and PD-L2 molecules known to promote Treg cells generation (Francisco et al., 2009; Zhang et al., 2006) (Figure 5D). Finally, we assessed whether transfer of WT M2r BMDM would inhibit the T-cell transfer-induced colitis in Rag2 −/− Il10rb −/− mice. Administration of WT M2r BMDM i.p. one day prior to WT CD4+ T cell transfer protected Rag2 −/− Il10rb −/− mice from intestinal inflammation, while transfer of Il10rb −/− M2r BMDM was associated with rapid weight loss and increased mortality among transferred mice within 2-3 weeks (Figure 5E-F). Overall, our data suggests that loss of IL-10Rβ signaling impairs the generation and function of anti-inflammatory macrophages and that restoration of aberrant macrophages function can ameliorate colitis in Rag2 −/− Il10rb −/− mice.
Figure 5. Loss of IL-10Rβ signaling impairs the generation and function of anti-inflammatory M2r BMDM.
(A) qRT-PCR analysis of Arg1, Retnla (Fizz1) and Il10 transcripts produced by WT or Il10rb −/− BMDM cultured for 24 hours under different conditions. (B) Pro-inflammatory cytokines mRNA expression by WT and Il10rb −/− BMDM cultured in different conditions for 24 hours and then re-stimulated for 4 hours with LPS (100 ng/mL). (C) Representative flow cytometry plots and cumulative data of in vitro generation of FOXP3+ Treg cells among CD4+ T cells in the presence of WT or Il10rb −/− M2r macrophages. (D) Representative flow cyometry plots and cumulative MFI of PD-L1 and PD-L2 surface expression on WT and Il10rb −/− M2r BMDM. (E) 1×106 WT or Il10rb −/− M2r BMDM or PBS were injected i.p. into Rag2 −/− Il10rb−/− mice one day prior to WT CD4+ T cell transfer. Figure depicts mean % initial body weights ± SEM following transfer. (F) Representative H&E stained sections (20X) followed by histological scores ± SEM for treated groups. Scale bar = 200 μm. Results pooled from 2 or more independent experiments. Figure S6 accompanies.
Aberrant generation and function of monocyte-derived macrophages from IL10R-deficient patients
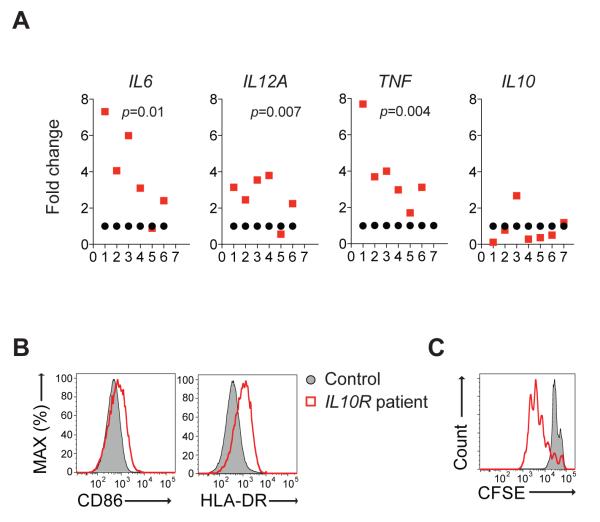
We next sought to investigate whether patients with null mutations in IL-10R genes also exhibit alterations in the generation and function of macrophage subsets. Through our interNational Early Onset Pediatric IBD Cohort Study (NEOPICS; www.neopics.org), we obtained blood samples from seven rare patients with loss of function mutations in IL10RA and IL10RB genes, all diagnosed with severe infantile IBD (Table S1). In humans, stimulation of CD14+ blood monocytes with granulocyte macrophage-colony stimulating factor (GM-CSF) for 8 days generates M1 pro-inflammatory macrophages (Rey-Giraud et al., 2012), while M-CSF treatment for 7 days followed by 24 hours culture with IL-4 generates M2 macrophages (Hedl and Abraham, 2012). Similar to murine Il10rb −/− M1 BMDM, human IL-10R-deficient M1 macrophages expressed higher concentrations of pro-inflammatory cytokines vs. controls (Figure 6A), while IL10 expression among patients was variable (Figure 6B). Human IL-10R-deficient M1 macrophages also expressed elevated concentrations of CD86 and HLA-DR (Figure 6C) and augmented proliferation of CD4+CD25− T naïve cells from allogeneic control subjects (Figure 6D), data that is consistent with the results observed in murine IL-10R deficient M1 BMDM.
Figure 6. Increased pro-inflammatory cytokine production and CD4+ T cell proliferation by human IL-10R-deficient pro-inflammatory macrophages.
(A) qRT-PCR analysis of pro-inflammatory cytokines among seven patients with loss-of-function mutations in IL10R genes vs. healthy controls. Each red circle represents a unique patient, while each black rectangle represents an individual healthy control subject in the same experiment. Cytokine expression is normalized to corresponding healthy controls. (B) Flow cytometry plots demonstrating high CD86 and HLA-DR expression on M1 macrophages from an IL-10R-deficient patient, compared to healthy control. (C) Proliferation of CFSE-labeled CD4+CD25− T naïve cells obtained from an allogeneic healthy donor in the presence of IL-10R-deficient M1 macrophages from a patient compared to M1 macrophages obtained from a healthy control. Surface marker expression and proliferation data representative of 5 patients. Table S1 and Figure S6 accompany.
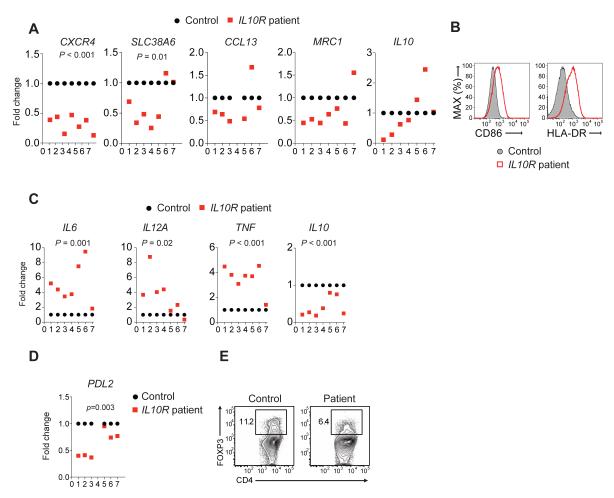
The generation and function of M2 macrophages was also impaired in IL10R-deficient patients, with lower expression of several human M2 markers, while IL10 expression was variable (Figure 7A). In addition, expression of CD86 and HLA-DR was higher in IL-10R-deficient M2 macrophages (Figure 7B), and when re-stimulated with LPS, these cells secreted significantly more pro-inflammatory cytokines (Figure 7C), similar to findings in murine Il10rb −/− anti-inflammatory BMDM. IL10 expression was significantly reduced in human _IL-10R_-deficient M2 macrophages following secondary LPS stimulation (Figure 7C), suggesting that in human anti-inflammatory macrophages IL-10R signaling is required for IL-10 production after TLR-4 stimulation. Finally, as observed in mice, human IL-10R-deficient M2 macrophages expressed lower concentration of PDL2 (Figure 7D) and promoted less generation of Treg cells in vitro (Figure 7E). This human data and our murine data described above indicate that IL-10R signaling modulates the generation and function of pro- and anti-inflammatory macrophages across species. Collectively, based on our findings, we propose a model depicting the role of IL-10R signaling on macrophages in the regulation of intestinal immune homeostasis (Figure S6).
Figure 7. Impaired generation and function of anti-inflammatory macrophages in patients with defective IL-10R chains.
(A) qRT-PCR analysis of M2 markers expressed in IL-4 stimulated monocyte-derived macrophages from IL-10R deficient patients vs. healthy subjects. (B) Surface expression of CD86 and HLA-DR by M2 macrophages generated from an IL-10R-deficient patient compared to healthy control. (C) qRT-PCR analysis of various cytokines expressed by M2 macrophages following re-stimulation with LPS. (D) PDL2 expression by M2 macrophages detected by qRT-PCR. (E) Flow cytometry plot illustrating in vitro Treg cells generation from CD4+ T cells in the presence of M2 macrophages from an IL-10R-deficieint patient compared to healthy control. Treg cell generation and flow cytometry data representative of 2 patients. Table S1 and Figure S6 accompany.
Discussion
Numerous murine studies have established a role for IL-10 and downstream IL-10R signaling as major regulators of immune tolerance in mucosal compartments (Shouval et al., 2014). Recent studies in humans have identified causal loss of function mutations of IL10 or either IL10RA or IL10RB in rare patients presenting with very early-onset IBD and have identified hematopoietic cells broadly as the responsible cells required for such signals (Engelhardt et al., 2013; Glocker et al., 2010; Glocker et al., 2009; Kotlarz et al., 2012; Moran et al., 2013). More mechanistic studies exploring cell types dependent on IL-10R signaling have been limited to murine studies and have concentrated largely on the regulation of mucosal T cell responses (Chaudhry et al., 2011; Huber et al., 2011; Kamanaka et al., 2011; Murai et al., 2009). Although IL-10 is known to control anti-inflammatory responses in DCs and macrophages in peripheral compartments (Bhattacharyya et al., 2004; Fiorentino et al., 1991; Steinbrink et al., 1997), the role of IL-10R-dependent signals in the intestine has not been explored. Here, we demonstrated that loss of IL-10R signaling on innate cells impairs their crosstalk with T cells, leading to defective mucosal immune regulation and severe intestinal inflammation.
Our data show that IL-10R signaling coordinates the differentiation and function of proand anti-inflammatory macrophages in both intestinal as well as peripheral immune compartments. IL-10R-dependent signals suppress the generation of pro-inflammatory LP P2 macrophages, facilitate the generation of tolerogenic intestinal macrophages and enhance their ability to secrete IL-10. IL-10R-dependent signals also suppress pro-inflammatory M1 macrophages derived from BM by inhibiting the secretion of pro-inflammatory cytokines and the ability of these cells to drive CD4+ T naïve cell proliferation. Moreover, the differentiation and function of anti-inflammatory BMDM also requires IL-10-dependent signals, since the expression of M2 markers and the ability of M2r macrophages to both suppress TLR-4-mediated pro-inflammatory cytokine secretion and to generate inducible Treg cells is reduced in IL-10R_−_deficient macrophages. Importantly, mirroring our findings in the murine system, we observed aberrant differentiation and function of pro- and anti-inflammatory macrophages in seven IL-10R-deficient patients who presented with infantile IBD, hence identifying IL-10R signaling as a critical modulator of the development and function of pathogenic and tolerogenic macrophages in mice and humans.
Amelioration of disease by the transfer of WT M2r BMDM, but not Il10rb −/− M2r BMDM, in mice lacking IL-10R in innate immune cells further suggests that IL-10R signaling on macrophages plays a key role in driving intestinal inflammation. Medina-Contreras and colleagues have reported that transfer of WT BMDM can ameliorate DSS-induced colitis in CX3CR1-deficient mice (Medina-Contreras et al., 2011). Similarly and consistent with our findings, Kayama and colleagues have recently reported that transfer of sorted intestinal CX3CR1high macrophages alleviates colitis in _Rag1_−/− mice transferred with CD45RBhi cells (Kayama et al., 2012). However, transfer of CX3CR1hi macrophages obtained from mice with conditional deletion of STAT3 in macrophages failed to rescue disease (Kayama et al., 2012). These findings are also consistent with recent data by Zigmond et al showing that IL-10Rα deficiency in CX3CR1+ macrophages results in spontaneous colitis (manuscript co-submitted).
Several aberrant macrophage–dependent immunoregulatory mechanisms resulting from IL-10R-deficiency may promote intestinal inflammation. Among anti-inflammatory cells, our data indicate that Il10rb −/− mice exhibit a decrease in generation of anti-inflammatory macrophage subsets and a decrease in Il10 and Pdcd1l2 expression, which, in turn, may result in decreased Treg cell generation observed in vitro and in vivo. Diminished generation and function of M2r BMDM in Il10rb −/− mice, with reduced PD-L1 and PD-L2 surface expression, IL-10 production, and Treg cell generation, further support the intestinal findings. Murai and colleagues have reported that IL-10 production by intestinal CD11b+ innate immune cells, likely macrophages, is required for Treg cell maintenance (Murai et al., 2009). In addition, CX3CR1+ macrophages promote the generation and expansion of Treg cells (Denning et al., 2007; Hadis et al., 2011). Our data from seven very early-onset IBD patients harboring causal mutations of IL10RA and IL10RB show aberrant generation of M2 macrophages, diminished Il10 expression, and decreased generation of inducible Treg cells, and hence further validate and add greater relevance to our findings in the murine system. Colitis development in Rag2 −/− Il10rb −/− mice cannot be attributed solely to diminished IL-10 production by IL-10Rβ-deficient innate immune cells, since exogenous administration of IL-10Ig did not protect these mice from intestinal inflammation.
Elevated pro-inflammatory cytokine production and augmentation of CD4+ T cells proliferation in vitro in culture with Il10rb −/− M1 BMDM support the hypothesis that loss of IL-10R signaling may, independent of its role on anti-inflammatory macrophages function, lead to exaggerated intestinal inflammation. Our work is consistent with studies employing _LyzM_-_cre_- or _Itgax-cre_-mediated deletion of IL-10Rα predominantly in macrophages or DCs, respectively, that were associated with elevated LPS-induced pro-inflammatory cytokines and effector T cell responses in the skin (Girard-Madoux et al., 2012; Pils et al., 2010). Moreover, recent studies have demonstrated that peritoneal monocytes lacking IL-10Rα differentiate into a pro-inflammatory MHCIIhigh macrophages subset (Nguyen et al., 2012b). Finally, IL-10-mediated signaling is known to suppress IL-1β secretion (Guarda et al., 2011), and in turn, IL-1β-dependent signals drive effector T cell responses and colitis development (Coccia et al., 2012).
One limitation of _Il10rb_−/− mice as a model for studying the IL-10 pathway is that signaling by IL-22, IL-26 and IFN-λ also use the IL-10Rβ chain as a co-receptor. Nonetheless, we speculate that the contribution of these later cytokines to colitis development in Il10rb −/− and Rag2 −/− Il10rb−/− is minimal, since they are almost exclusively expressed on non-hematopoietic cells (Lasfar et al., 2011; Sabat, 2010). Moreover, in vitro experiments utilizing _Il10ra_−/− M1 BMDM or administration of neutralizing IL-10Rα antibodies mimicked the phenotype observed in _Il10rb_−/− BMDM studies. Finally, to date, the clinical presentation of patients with mutations in either the IL10RA or IL10RB genes appear indistinguishable (Shouval et al., 2014) and in vitro studies with macrophages from _IL10RA_- and _IL10RB_-deficient patients appear similar. Nonetheless, a role for IL-10Rβ signals downstream of other cytokines cannot be excluded; since cytokines such as IL-22 are known to contribute to mucosal homeostasis (Zenewicz et al., 2013); more specific approaches targeting IL-10Rα in specific innate immune cells are warranted. Indeed, the study by Zigmond et al, employing _Cx3cr1-cre_-mediated targeting of IL-10Rα, suggests that defective IL-10Rα-signaling largely limited to this anti-inflammatory macrophages subset results in spontaneous colitis (manuscript co-submitted).
In conclusion, our data defines a critical role for IL-10R signaling in innate immune populations in maintaining mucosal immune tolerance and preventing IBD. Our murine studies indicate that IL-10R-dependent signals suppress pro-inflammatory macrophages function as well as enhance tolerogenic macrophages properties, both in peripheral compartments and in the intestine. Data from several very early-onset IBD patients harboring mutations in IL-10R genes also strengthens these findings and define IL-10R as a key regulator of macrophages differentiation and function in humans as well. Targeted therapies delivering IL-10 to innate immune cells or modulating IL-10R-dependent signals in these cells may provide a future direction of drug development for carefully selected IBD patients.
Experimental Procedures
Mice
WT, Il10rb −/− (obtained from Genentech, San Francisco, CA), _Rag2_−/−, Rag2 −/− Il10rb −/− and _Was_−/−_Rag2_−/− mice, all on 129 SvEv background, as well as WT, _Il10ra_−/−, _Il10rb_−/− (courtesy of Thaddeus Stappenbeck, Washington University), _Rag1_−/−, Rag1 −/− Il10rb −/− and FOXP3-GFP on the C57BL/6 background were maintained in specific pathogen-free animal facility at Boston Children’s Hospital. Experiments were conducted after approval from the Animal Resources at Children’s Hospital and according to regulations of the Institutional Animal Care and Use Committees (IACUC).
Induction of colitis in transfer experiments
In unfractionated CD4+ T-cell transfer experiments, cells from peripheral lymph nodes, MLNs, and spleens from WT mice were enriched for CD4+ cells using a negative selection kit (Miltenyi Biotec, Auburn, CA). The purity of CD4+ cells was > 95%. _Rag2_−/− and Rag2 −/− Il10rb−/−, or _Rag1_−/− and Rag1 −/− Il10rb −/− mice were adoptively transferred with 1×106 WT CD4+ T cells by i.p. injection. In some experiments Il10rb −/− CD4+ T cells were isolated and transferred to _Was_−/−_Rag2_−/− mice. For Tnaïve and Treg cells adoptive transfer experiments, WT CD4+ cells were enriched by negative selection as described above and further sorted by BD FACSAria II SORP (BD Biosciences, San Jose, CA). T naïve cells were defined as CD4+CD25−CD45RBhi and Treg cells as CD4+CD25+CD45RBlo. Post-sort purity was typically > 98%. Age-matched _Rag2_−/− mice or Rag2 −/− Il10rb−/− mice were injected i.p, with 1-2×105 WT T naïve cells with or without Treg cells at a 1:1 ratio. Similarly, CD4+CD45RBhiFOXP3neg or CD4+CD45RBloFOXP3pos cells were obtained from FOXP3-GFP reporter mice and used for adoptive transfer experiments into _Rag1_−/− and Rag1 −/− Il10rb−/− mice.
Isolation of LP cells
Colons underwent epithelial layer stripping with agitation in 10 mM EDTA at 37°C twice before digestion in collagenase VIII. Following that specimens were enriched with a 40% and 90% Percoll (GE Healthcare, Pittsburgh, PA) gradient to remove epithelial cells. In some experiments LP macrophages were sorted. Gating strategy was based on Bain et al. who showed that distinct macrophages subsets can be isolated without using CX3CR1-GFP reporter mice (Bain et al., 2013). We performed some modifications to this method: following initial gating on live CD45+ cells we gated on CD11b+CD64+CD103− cells, then based on SSC and FSC (Bain et al., 2013) and finally gated on Ly6C and MHCII.
Generation of BMDM
BM was flushed from femur and tibia bones and cultured with DMEM, 20% FBS, penicillin 100 IU/ml, streptomycin 100 μg/ml and 30% L cell-conditioned medium, at 37°C in 5% CO2. Media was supplemented every 2-3 days. Following 6-7 days non-adherent cells were aspirated and adherent macrophages were removed by washing plate with ice-cold PBS and scraping. For generation of M1 macrophages, BMDM were stimulated for 24 hours with 100 ng/mL of LPS (Sigma-Aldrich, Natick, MA) and 20 ng/mL IFN-γ (Peprotech, Rocky Hill, NJ). To generate M2r macrophages, BMDM were cultured for 24 hours with 20 ng/mL IL-4, 20 ng/mL, human TGF-β1 and 20 ng/mL IL-10 (all from peprotech). In some experiments WT M1 BMDM were cultured with 10 μg/mL of anti IL-10Rα blocking antibody (Biolegend, San Diego, CA).
Generation of human monocyte-derived macrophages
Blood was collected in EDTA tubes from patients with loss-of-function IL-10R mutations and control subjects (either a healthy parent or an unrelated healthy donor) following protocol approval and in accordance with the Declaration of Helsinki. Blood samples were shipped at room temperature overnight to our lab at Boston Children’s Hospital and upon arrival PBMCs were isolated by Ficoll-Paque PLUS (GE Healthcare, Pittsburgh, PA) gradient, according to manufacturer’s instructions. Monocytes were sorted using CD14 positive selection kit (Miltenyi Biotec) and cultured in RPMI 1640 supplemented with 20% FCS and antibiotics. To generate M1 macrophages media was supplemented with 100 ng/mL GM-CSF for 8 days (Rey-Giraud et al., 2012), and for M2 macrophages media was supplemented with 50 ng/mL of M-CSF for 7 days and an additional day with 20 ng/mL of IL-4 (Hedl and Abraham, 2012).
Quantitative RT-PCR
RNA was extracted from whole colons or from cells using TRIzol® reagent (Invitrogen, Grand Island, NY) according to the manufacturer’s instructions. Complementary DNA was reverse transcribed from 1 μg total RNA using iScript Select cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Analyses of transcripts were performed using iQ SYBR Green on a CFX96 Real-Time System (Bio-Rad). Cytokine transcripts were normalized against hypoxanthine-guanine phosphoribosyltransferase (HPRT), and normalized fold change was calculated using the ΔΔCt method against mean control ΔCt (_Rag2_−/− for Rag2 −/− Il10rb−/−; WT for Il10rb −/− in BMDM experiments, or macrophages from a healthy paired subject in experiments using monocytes derived macrophages from _IL-10R_-deficient patients). For human M1 and M2 macrophages generation experiments, genes associated with each lineage were chosen as reported by Martinez et al (Martinez et al., 2006).
In vitro CD4+ T naïve proliferation and Treg generation
To assess proliferation 2 μM CFSE-labeled 1×105 WT CD4+CD25− T naïve cells were cultured with 2 μg/mL soluble αCD3 and either 2.5×104 WT or Il10rb −/− M1 BMDM, for 4 days. Proliferation was determined by percent of CFSE dilution. For Treg cells generation assays 1×105 WT CD4+CD25− sorted T naïve cells were cultured with 2 μg/mL soluble αCD3 (ebioscience, San Diego, CA), 2 ng/mL human TGF-β1 (Peprotech) and either 2.5×104 WT or Il10rb −/− M2r BMDM, for 5 days. Similar experiments were performed with human M1 or M2 macrophages from IL-10R-deficient patients vs. healthy controls. In these experiments CD4+ T naïve cells were isolated from an unrelated healthy subject. In some proliferation experiments blocking antibodies against IL-6, IL-12p40 and TNF (Biolegend, 10 μg/mL) were added to the culture on day 0 and day 2.
Sequencing of IL10R genes
Patients 1-3 were sequenced as reported elsewhere, while sequencing of patients 4-7 was performed at Muise Lab in Hospital for Sick Children, Toronto. Genomic DNA was purified from whole blood using the Puregene Blood Kit (Qiagen, USA). IL10RA and IL10RB were amplified using intronic primers flanking each exon. Purified PCR products were sequenced with the ABI3730 DNA analyzer (Applied Biosystems, Melbourne, Australia). IL10RA variant is numbered according to GeneBank accession number NM_001588. IL10RB variant is numbered according to GeneBank accession number NM_00628. Numbering of amino acid residues in IL10RA and IL10RB refers to their position in the immature protein that includes the signal peptide.
In some cases RNA was isolated from whole blood by the PAXgene Blood RNA kit (Qiagen, USA) according to the manufacture instructions. cDNA was synthesized using SuperScript III Reverse Transcriptase (Life Technologies, Carlsbad, California, USA). Primers for full-length IL10RA (For: TCA GTC CCA GCC CAA GGG TA; Rev: TGC AGG TCC AAG TTC TTC AGC TCT) and full-length I_L10RB_ (For: TCG TGT GCT TGG AGG AAG CC; Rev: TAA GTC CAG GGT CTG GGA GTT CTA) were designed and synthesized at The Centre for Applied Genomics, Toronto. PCR was performed according to standard protocol and sequenced by ABI 3730 DNA analyzer (Applied Biosystems, Melbourne, Australia).
Statistical analysis
Differences between groups were determined by unpaired two-tailed t-test using GraphPad. Significance was defined if p value was less than 0.05 as following: * p < 0.05; ** p < 0.01; *** p < 0.001.
Supplementary Material
01
Highlights.
IL-10R-deficient innate immune cells render WT CD4+ T cells colitogenic
Loss of innate IL-10R signaling impairs regulatory T cell generation and function
IL-10R signaling regulates murine and human macrophage differentiation and function
Acknowledgments
D.S.S is a recipient of a Research Fellowship Award Grant from the Crohn’s and Colitis Foundation of America (RFA 3784). S.B.S is supported by NIH Grants HL59561, DK034854, and AI50950, the Helmsley Charitable Trust, and the Wolpow Family Chair in IBD Treatment and Research. We would like to thank Thaddeus Stappenbeck for providing us Il10rb −/− mice on the C57BL/6 background.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, Mowat AM. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal immunology. 2013;6:498–510. doi: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begue B, Verdier J, Rieux-Laucat F, Goulet O, Morali A, Canioni D, Hugot JP, Daussy C, Verkarre V, Pigneur B, et al. Defective IL10 signaling defining a subgroup of patients with inflammatory bowel disease. The American journal of gastroenterology. 2011;106:1544–1555. doi: 10.1038/ajg.2011.112. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Sen P, Wallet M, Long B, Baldwin AS, Jr., Tisch R. Immunoregulation of dendritic cells by IL-10 is mediated through suppression of the PI3K/Akt pathway and of IkappaB kinase activity. Blood. 2004;104:1100–1109. doi: 10.1182/blood-2003-12-4302. [DOI] [PubMed] [Google Scholar]
- Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Bruning JC, Muller W, Rudensky AY. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M, Harrison OJ, Schiering C, Asquith MJ, Becher B, Powrie F, Maloy KJ. IL-1beta mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. The Journal of experimental medicine. 2012;209:1595–1609. doi: 10.1084/jem.20111453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nature immunology. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- Engelhardt KR, Shah N, Faizura-Yeop I, Kocacik Uygun DF, Frede N, Muise AM, Shteyer E, Filiz S, Chee R, Elawad M, et al. Clinical outcome in IL-10- and IL-10 receptor-deficient patients with or without hematopoietic stem cell transplantation. The Journal of allergy and clinical immunology. 2013;131:825–830. doi: 10.1016/j.jaci.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. The Journal of experimental medicine. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A, Balschun T, Karlsen TH, Sventoraityte J, Nikolaus S, Mayr G, Domingues FS, Albrecht M, Nothnagel M, Ellinghaus D, et al. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nature genetics. 2008;40:1319–1323. doi: 10.1038/ng.221. [DOI] [PubMed] [Google Scholar]
- Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nature genetics. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard-Madoux MJ, Kel JM, Reizis B, Clausen BE. IL-10 controls dendritic cell-induced T-cell reactivation in the skin to limit contact hypersensitivity. The Journal of allergy and clinical immunology. 2012;129:143–150. doi: 10.1016/j.jaci.2011.08.032. [DOI] [PubMed] [Google Scholar]
- Glocker EO, Frede N, Perro M, Sebire N, Elawad M, Shah N, Grimbacher B. Infant colitis--it’s in the genes. Lancet. 2010;376:1272. doi: 10.1016/S0140-6736(10)61008-2. [DOI] [PubMed] [Google Scholar]
- Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. The New England journal of medicine. 2009;361:2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Yang J, Ouyang X, Liu W, Li H, Bromberg J, Chen SH, Mayer L, Unkeless JC, Xiong H. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. European journal of immunology. 2008;38:1807–1813. doi: 10.1002/eji.200838331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Forster I, Farlik M, Decker T, Du Pasquier RA, Romero P, Tschopp J. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–223. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Muller W, Sparwasser T, Forster R, Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Hedl M, Abraham C. Nod2-induced autocrine interleukin-1 alters signaling by ERK and p38 to differentially regulate secretion of inflammatory cytokines. Gastroenterology. 2012;143:1530–1543. doi: 10.1053/j.gastro.2012.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S, Gagliani N, Esplugues E, O’Connor W, Jr., Huber FJ, Chaudhry A, Kamanaka M, Kobayashi Y, Booth CJ, Rudensky AY, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(-) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamanaka M, Huber S, Zenewicz LA, Gagliani N, Rathinam C, O’Connor W, Jr., Wan YY, Nakae S, Iwakura Y, Hao L, Flavell RA. Memory/effector (CD45RB(lo)) CD4 T cells are controlled directly by IL-10 and cause IL-22-dependent intestinal pathology. The Journal of experimental medicine. 2011;208:1027–1040. doi: 10.1084/jem.20102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayama H, Ueda Y, Sawa Y, Jeon SG, Ma JS, Okumura R, Kubo A, Ishii M, Okazaki T, Murakami M, et al. Intestinal CX3C chemokine receptor 1(high) (CX3CR1(high)) myeloid cells prevent T-cell-dependent colitis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5010–5015. doi: 10.1073/pnas.1114931109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlarz D, Beier R, Murugan D, Diestelhorst J, Jensen O, Boztug K, Pfeifer D, Kreipe H, Pfister ED, Baumann U, et al. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: implications for diagnosis and therapy. Gastroenterology. 2012;143:347–355. doi: 10.1053/j.gastro.2012.04.045. [DOI] [PubMed] [Google Scholar]
- Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Lasfar A, Abushahba W, Balan M, Cohen-Solal KA. Interferon lambda: a new sword in cancer immunotherapy. Clin Dev Immunol. 2011;2011:349575. doi: 10.1155/2011/349575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Tonkonogy SL, Sartor RB. Antigen-presenting cell production of IL-10 inhibits T-helper 1 and 17 cell responses and suppresses colitis in mice. Gastroenterology. 2011;141:653–662. 662, e651–654. doi: 10.1053/j.gastro.2011.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Frontiers in bioscience : a journal and virtual library. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- Medina-Contreras O, Geem D, Laur O, Williams IR, Lira SA, Nusrat A, Parkos CA, Denning TL. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. The Journal of clinical investigation. 2011;121:4787–4795. doi: 10.1172/JCI59150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annual review of immunology. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Moran CJ, Walters TD, Guo CH, Kugathasan S, Klein C, Turner D, Wolters VM, Bandsma RH, Mouzaki M, Zachos M, et al. IL-10R polymorphisms are associated with very-early-onset ulcerative colitis. Inflammatory bowel diseases. 2013;19:115–123. doi: 10.1002/ibd.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nature immunology. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DD, Wurbel MA, Goettel JA, Eston MA, Ahmed OS, Marin R, Boden EK, Villablanca EJ, Paidassi H, Ahuja V, et al. Wiskott-Aldrich syndrome protein deficiency in innate immune cells leads to mucosal immune dysregulation and colitis in mice. Gastroenterology. 2012a;143:719–729. e711–712. doi: 10.1053/j.gastro.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HH, Tran BT, Muller W, Jack RS. IL-10 acts as a developmental switch guiding monocyte differentiation to macrophages during a murine peritoneal infection. J Immunol. 2012b;189:3112–3120. doi: 10.4049/jimmunol.1200360. [DOI] [PubMed] [Google Scholar]
- Parsa R, Andresen P, Gillett A, Mia S, Zhang XM, Mayans S, Holmberg D, Harris RA. Adoptive transfer of immunomodulatory M2 macrophages prevents type 1 diabetes in NOD mice. Diabetes. 2012;61:2881–2892. doi: 10.2337/db11-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pils MC, Pisano F, Fasnacht N, Heinrich JM, Groebe L, Schippers A, Rozell B, Jack RS, Muller W. Monocytes/macrophages and/or neutrophils are the target of IL-10 in the LPS endotoxemia model. European journal of immunology. 2010;40:443–448. doi: 10.1002/eji.200939592. [DOI] [PubMed] [Google Scholar]
- Rey-Giraud F, Hafner M, Ries CH. In vitro generation of monocyte-derived macrophages under serum-free conditions improves their tumor promoting functions. PloS one. 2012;7:e42656. doi: 10.1371/journal.pone.0042656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. The Journal of experimental medicine. 2012;209:139–155. doi: 10.1084/jem.20101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabat R. IL-10 family of cytokines. Cytokine Growth Factor Rev. 2010;21:315–324. doi: 10.1016/j.cytogfr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nature reviews. Immunology. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- Shouval DS, Ouahed J, Biswas A, Goettel JA, Horwitz BH, Muise AM, Snapper SB. Interleukin 10 Receptor Signaling: Master Regulator of Intestinal Homeostasis in Mice and Humans. Advances in Immunology. 2014 doi: 10.1016/B978-0-12-800267-4.00005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SD, Di Marco F, Hooley J, Pitts-Meek S, Bauer M, Ryan AM, Sordat B, Gibbs VC, Aguet M. The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor. The Journal of experimental medicine. 1998;187:571–578. doi: 10.1084/jem.187.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- Tamoutounour S, Henri S, Lelouard H, de Bovis B, de Haar C, van der Woude CJ, Woltman AM, Reyal Y, Bonnet D, Sichien D, et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. European journal of immunology. 2012;42:3150–3166. doi: 10.1002/eji.201242847. [DOI] [PubMed] [Google Scholar]
- Zenewicz LA, Yin X, Wang G, Elinav E, Hao L, Zhao L, Flavell RA. IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. J Immunol. 2013;190:5306–5312. doi: 10.4049/jimmunol.1300016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chung Y, Bishop C, Daugherty B, Chute H, Holst P, Kurahara C, Lott F, Sun N, Welcher AA, Dong C. Regulation of T cell activation and tolerance by PDL2. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11695–11700. doi: 10.1073/pnas.0601347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, Mack M, Shpigel N, Boneca IG, Murphy KM, et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37:1076–1090. doi: 10.1016/j.immuni.2012.08.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01