A Framework for personalized surgical approach to ovarian cancer (original) (raw)
. Author manuscript; available in PMC: 2016 Apr 1.
Published in final edited form as: Nat Rev Clin Oncol. 2015 Feb 24;12(4):239–245. doi: 10.1038/nrclinonc.2015.26
Abstract
The standard therapeutic approach for advanced ovarian cancer is upfront cytoreductive surgery followed by a combination of platinum and taxane-based chemotherapy. The degree of residual disease following upfront cytoreductive surgery correlates with objective response to adjuvant chemotherapy, rate of pathological complete response at second-look assessment operations, progression-free survival and overall survival. Contemporary data and meta-analyses have documented a continuous relationship between volume of residual disease and patient outcomes with those patients undergoing complete gross resection having the best outcomes, thereby focusing attention of surgical effort to remove as much disease as possible with the metric of “optimal” cytoreduction being R0 disease. Since patients with R0 resection appear to have the best overall outcomes, efforts to spare unnecessary primary debulking surgery by pre- or intra-operative assessment have abounded without external validity to incorporate into general practice. Serum CA125, physical examination and CT imaging have lacked accuracy in determining if disease can be optimally debulked. Therefore, an algorithm that identifies patients likely to achieve complete gross resection at primary surgery would be expected to improve patient survival. Herein, we review contemporary definitions of “optimal” residual disease, and discuss opportunities to personalize surgical therapy and improve the quality of surgical care delivered to patients with advanced ovarian cancer.
Introduction
Surgical cytoreduction of advanced ovarian cancer has long been considered an important tenet of effective management. Although the sequence of chemotherapy and surgical intervention is of some debate, there is broad consensus that integration of the two modalities represents the best initial strategy for women with metastatic disease. Retrospective, case-cohort and meta-analysis reports have demonstrated a strong prognostic link between degree of post-operative residual disease and objective clinical and pathological complete response rates, progression-free and overall survival [1]. The relationship appears to be strongest or most discriminative between patients with no visible residual, so called R0 resection, and those with any measure of residual disease [2, 3]. Reports have demonstrated incremental survival benefits among patients with residual disease volumes under 1 cm; however, most have shown little benefit is gained from a debulking effort if the residual disease cannot be reduced to less than 1 cm. In response to these observations, the metric for “optimal” surgical cytoreduction was defined as no tumor implants greater than 1 cm [2–5]. However, in light of more contemporary data, we feel that a strong case can be made for raising the bar for optimal cytoreduction to R0 given its substantially stronger prognostic value, lower prevalence and unambiguous assignment following surgery. Moreover, a personalized surgical approach is desirable to allow rational decision making with regard to timing of surgery.
Herein, we provide a brief overview of the historical progression of primary cytoreduction and definitions of “optimal” residual disease, highlight current gaps in knowledge, and present logical approaches for personalized surgical approaches for women with advanced ovarian cancer.
Historical evolution of the definition of optimal cytoreduction
Primary Cytoreduction
Surgical outcome among patients with advanced ovarian cancer is classically defined by the amount of postoperative residual tumor. A complete gross resection is regarded if no macroscopic residual tumor remains. If any visible tumor remains after surgery, it is classified according to the largest residual diameter. Historically, operations resulting in residual tumor up to 1 cm in diameter have been classified as “optimal” whereas those resulting in any larger residual tumor being defined as “suboptimal.” One of the first studies among patients with advanced ovarian cancer to demonstrate a survival advantage with cytoreduction was a single-institution observational study, which demonstrated survival to be inversely correlated with residual tumor size [2]. Multiple subsequent retrospective series have since validated the findings of this seminal paper firmly establishing primary cytoreduction followed by platinum-based chemotherapy as the standard management of advanced ovarian cancer for those patients with epithlelial ovarian tumors [3–6] (Table 1). The definition of “optimal” cytoreduction, however, continues to evolve and remains a critical focus of ongoing clinical investigation.
Table 1.
Rates of R0 Resection
| Author [Ref] | TotalNo. ofPatients | % R0 | % < 1 cm | % > 1 cm |
|---|---|---|---|---|
| Bristow et al [9] | 6885 | N/A | 17.2 | 82.7 |
| Winter et al [11] | 360 | 8.1 | 21.7 | 70.2 |
| du Bois, et al [12] | 3126 | 33.5 | 31.2 | 35.3 |
| Chi et al [16] | 285 | 24 | 47 | 29 |
| Vergote et al [14] | ||||
| Primary Debulking | 310 | 19.4 | 22.2 | 53 |
| Neoadjuvant Chemotherapy | 322 | 51.2 | 29.5 | 17.7 |
| Kehoe et al [15] | ||||
| Primary Debulking | 276 | 15 | N/A | N/A |
| Neoadjuvant Chemotherapy | 274 | 35 | N/A | N/A |
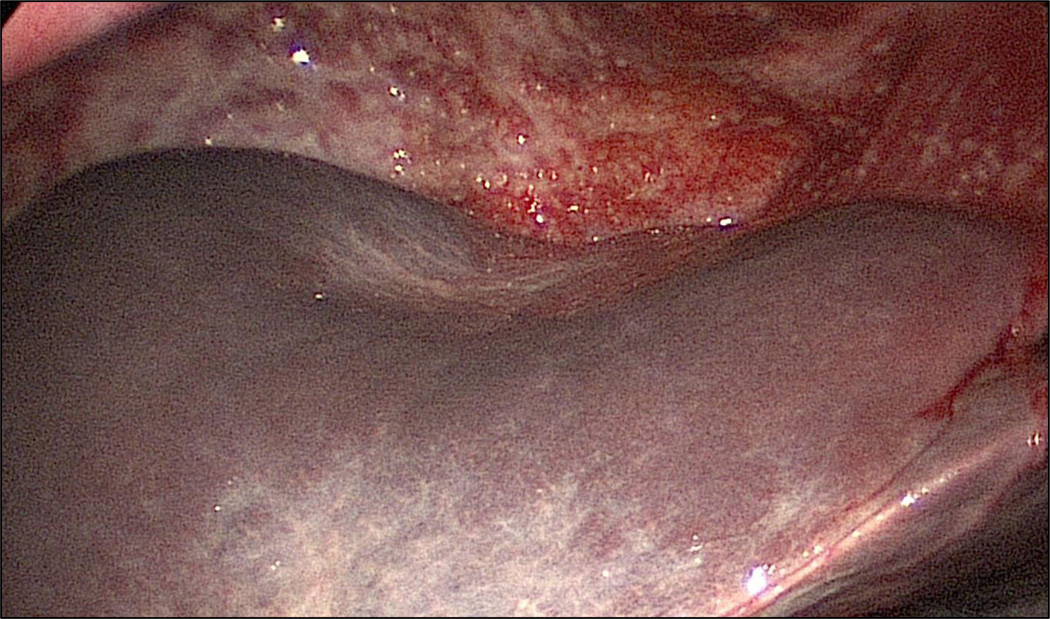
Ancillary studies of Gynecologic Oncology Group (GOG) data (GOG 52 and 97) have examined the correlation between maximal diameter of residual disease after primary cytoreduction and patient survival with the longest survival noted among those with no residual disease [7, 8]. After controlling for other prognostic variables, the maximal diameter of residual disease was found to be an independent predictor of overall survival with those with no residual disease having a 5 year survival rate of 60^ compared to those with 0.1–1 cm or 1–2 cm residual disease who had 5-year survival rates of 35% and <20%, respectively. Meta-analytical data have confirmed a significant association between maximal cytoreduction and overall survival with each 10% increase in the degree of cytoreduction resulting in a concomitant 5.5% increase in median cohort survival time [9]. However, definitions of surgical outcome that are not binary lend themselves to subjectivity. What one surgeon classifies as optimal (≤1 cm) may be classified differently by another surgeon making a binary classification of complete resection of all visible disease the most objective classifier of surgical success. In addition, plaque-like disease in the pelvis or the diaphragmatic surfaces is difficult to quantify since it may represent a thinly coated coalition of several sub-centimeter nodules (Figure 1).
Figure 1.
Plaque-like disease involving the right diaphragm
Critics of these original reports on the impact of residual disease and patient survival cite the impact of inherent tumor biology. Contemporary studies have sought to analyze the impact of complete gross resection to only microscopic residual on patient survival. Those with R0 resection had substantially improved median survival compared to those with any residual disease [4, [5]. Even among patients with the most extensive disease, performance of radical surgery and status of residual disease were the only independent prognostic factors associated with 5-year disease-specific survival. Individual surgeon effort may have an impact on outcome since significant improvements in median overall survival were found among patients treated by surgeons who frequently utilized radical procedures compared to those who infrequently utilized radical procedures. Furthermore, institutions that perform complex surgeries in greater than 25% of patients are associated with a 30% reduction in recurrence risk after primary treatment [10]. Those that have successfully incorporated upper abdominal surgery in effort to improve rates of complete or optimal cytoreduction have done so without unacceptable rates of serious morbidity and/or mortality. Chi and colleagues incorporated extensive upper abdominal procedures as part of a paradigm shift in their surgical practice without significantly increasing perioperative morbidity and mortality [11].
To avoid biases related to single institutional and individual surgeon or group experience, data from several GOG clinical trials (GOG 111, 114, 132, 152, 158, 162, and 172) were analyzed and revealed that R0 resection was achieved in 8.1% and was associated with the longest median survival compared with any visible residual disease [12]. Similarly, data from 3 European prospective randomized trials (AGO-OVAR 3, 5 and 7) demonstrated that R0 resection was associated with significantly longer median overall survival after stratifying for FIGO stage [13]. Given the survival benefit incurred with R0 resection, those patients with advanced ovarian cancer unlikely to achieve R0 resection should be considered for neoadjuvant chemotherapy (NACT) followed by interval cytoreductive surgery.
Neoadjuvant Chemotherapy and Interval Cytoreductive Surgery
Administration of chemotherapy followed by interval cytoreductive surgery offers an alternate approach to the primary management of advanced epithelial ovarian cancer and one that has been the topic of much investigation. Retrospective meta-analytical data suggested that NACT was associated with a worse outcome compared with primary debulking [14]. Recently, however, the results of two randomized controlled, prospective trials conducted by the European Organization for Research and Treatment of Cancer (EORTC) and the Medical Research Counsel (MRC) Clinical Trials Unit have investigated the potential utility of neoadjuvant chemotherapy as it compares to primary cytoreduction among patients with stage III or IV ovarian cancer [15, 16]. In both studies, patients were randomly assigned to either primary cytoreductive surgery or neoadjuvant chemotherapy. Although there was no significant difference in overall survival between the two cohorts of patients, there was a higher rate of R0 resection among those patients who underwent NACT. R0 resection at primary or interval surgery was the soundest independent prognostic variable in predicting overall survival. Subgroup analyses demonstrated improved survival among patients who underwent R0 resection with no difference in overall survival when comparing surgical outcome in upfront or interval setting. Critics of these studies have raised concerns about the absolute number of patients who underwent “optimal” cytoreduction at the participating institutions. A retrospective study has demonstrated superior survival in the primary cytoreduction cohort with an “optimal” cytoreduction rate of 71% (compared to 41.6% in the EORTC study) [17]. Although administration of NACT may yield increased rates of R0 resection, the equivalency of such surgical outcome in the primary and interval settings remains to be demonstrated.
Current Diagnostic Methods for Determining Success of Primary Cytoreduction
Despite the collective data demonstrating the survival advantage incurred with “optimal” or R0 resection at the time ovarian cancer cytoreduction, meta-analytical data from patient cohorts across the United States have demonstrated an optimal cytoreduction (variably defined) rate of 42% [9, 18]. Given that the majority of patients who undergo tumor reductive surgery will be left with visible or suboptimal disease and will not incur the same magnitude of survival advantage as those who achieve complete resection, while being exposed to morbidity resulting from an extensive surgical procedure. Reasons for incomplete cytoreduction are multifactorial and are surgeon-related and disease-specific. Surveys of practicing gynecologic oncologists reflect practice pattern variations and cite concerns regarding the safety and benefit of aggressive cytoreduction among patients with medical comorbidities and differences in tumor biology as reasons for offering patients NACT followed by interval cytoreduction [19, 20]. Further, broad adaptation of NACT lags behind, particularly in the United States where critics reference failure to incorporate upper abdominal surgery and maximal cytoreductive effort as the primary reason for lack of survival advantage with primary tumor reductive surgery [17]. These data highlight the need for a much more personalized approach with accurate predictors of R0 resection. To date, predictive models that incorporate clinical parameters, serum markers and radiographic features have demonstrated good sensitivity and specificity when evaluated in small cohorts of patients, but have failed to demonstrate generalizable applicability. Developing an algorithm that identifies patients likely to achieve complete gross resection at primary surgery would be expected to improve patient survival.
Serum Markers
Cancer Antigen 125
Preoperative CA-125 levels have been one of the most studied preoperative modalities for predicting surgical outcome among patients with advanced ovarian cancer. Given that CA-125 levels are found to be elevated in greater than 90% of patients [21] and can be a surrogate marker for extent of tumor burden, numerous studies have attempted to identify a threshold level above which optimal surgical resection is not feasible [22–38]. Of these studies, many have used a CA-125 cut point of 500 U/mL as a critical value in the analysis of the ability to predict optimal cytoreduction with varying degrees of success and reproducibility [22–24, 27–29, 31–33, 35, 36, 38]. However, with evolving surgical practices, i.e. the implementation of more radical surgical procedures to achieve optimal cytoreduction, CA-125 has been shown to no longer correlate with surgical outcome [23]. Collectively, studies evaluating the predictive ability of CA-125 have been confounded by varying degrees of surgical effort, making it hard to generalize CA-125 cut-points for predicting surgical outcomes in broader populations of women with advanced ovarian carcinoma.
Computed Tomography
Computed tomography (CT) scanning is the most commonly utilized imaging modality for the preoperative assessment of patients with advanced ovarian cancer. CT has been evaluated as a means of staging as well as a tool for monitoring for recurrent or persistent disease. Several studies have exploited CT characteristics of disease and their predictive ability to determine surgical success [39–44]. Of these, only three utilized more contemporary definitions of optimal cytoreduction (residual disease ≤1cm). Each study utilized a set of radiographic features to determine a score predictive of suboptimal tumor reductive surgery with 100% sensitivity in smaller cohorts of patients from single institutions. [41–43] However, attempts at cross-validation with other studies were largely unsuccessful. Collectively, several limitations of studies evaluating CT predictors of surgical outcome exist. These include variability in CT features (including technique and radiologist skill) evaluated and an individual surgeon’s philosophy and skill set. Moreover, CT imaging is more likely to demonstrate disease clearly visualized as unresectable, rather than resectable, making it less useful for predicting R0 resection. CT findings have also been combined with CA-125 and clinical criteria to identify those patients unlikely to achieve optimal cytoreduction [45]. On multivariate analysis, 3 clinical and six radiographic criteria were associated with suboptimal cytoreduction: age ≥60 years, CA-125 >500 U/mL, ASA 3–4, suprarenal lymphadenopathy > 1cm, diffuse small bowel thickening, or tumor >1 cm in the small bowel mesentery, root of the superior mesenteric artery, perisplenic area or lesser sac were associated with suboptimal cytoreduction. Results of this pre-treatment predictive value score remain to be validated.
Minimally Invasive Surgery
Given the limitations described for both serum biomarkers and CT imaging, the primary issue of how best to evaluate the resectability of advanced ovarian cancer remains an unmet need. Laparoscopy has recently been investigated as a means of averting cytoreduction and the potential morbidity associated with laparotomy that results in suboptimal residual disease. To test the utility of this approach, patients suspected of having advanced disease have been evaluated with laparoscopy followed by standard laparotomy [46]. The overall accuracy rate of laparoscopy in assessing optimal cytoreduction (defined as ≤1cm) was 90%. There were no cases where the judgment of unresectable disease as determined by laparoscopy was changed by laparotomy. Subsequently, a quantitative predictive model based on the pattern of disease (Table 2) was tested and validated [47]. Components of the predictive model include evaluation of the degree of peritoneal carcinomatosis, diaphragm disease, mesenteric involvement, the need for potential bowel resection, liver surface involvement, obvious neoplastic involvement of the gastric wall, and omental disease up to the level of the greater curvature of the stomach with those parameters that are present receiving a score of 2 and those parameters that are absent receiving a score of 0. The additive sum of scores results in a predictive index value (PIV) with a PIV ≥8 resulting in a predictive probability of optimal cytoreduction of 0 suggesting that the patient should be triaged to NACT. In a follow-up study, patients were prospectively assessed with laparoscopy and the accuracy of predicting optimal cytoreduction by the laparoscopic procedure ranged between 77.3 and 100% [48]. When comparing survival among those patients triaged to primary cytoreductive surgery after laparoscopic evaluation, those who achieve complete gross resection have the longest survival [49]. These results have led to the SCORPION trial, a prospective randomized controlled trial of primary debulking surgery versus NACT among those patients with PIV ≥8 (ClinicalTrials.gov Identifier: NCT01461850).
Table 2.
Laparoscopic predictive index value to determine disease distribution
| Score = 2 | Score = 0 | |
|---|---|---|
| Peritoneal carcinomatosis | Unresectable massive peritoneal involvement + miliary pattern of distribution | Carcinomatosis involving a limited area surgically removable by peritonectomy |
| Diaphragmatic disease | Wide spread infiltrating carcinomatosis or confluent nodules to most part of the diaphragmatic surface | Isolated diaphragmatic disease |
| Mesenteric disease | Large infiltrating nodules or involvement of the root of the mesentery supposed by limited movements of various intestinal segments | Small nodules potentially treatable with argon beam coagulation |
| Omental disease | Tumor diffusion up to the large curvature of the stomach | Isolated omental disease |
| Bowel infiltration | Bowel resection assumed to be required or miliary carcinomatosis on the mesenteric junction | |
| Stomach infiltration | Obvious neoplastic involvement of the gastric wall | |
| Liver metastasis | Any surface lesions |
Gaps In Knowledge
With the advent of various “omic” technologies, several investigators have sought to characterize molecular predictors of residual disease at the time of cytoreduction with varying degrees of success. Molecular predictors of surgical outcome offer the advantage of incorporating the tumor biology into medical decision-making. Collectively, such models were better at predicting incomplete cytoreduction with macroscopic residual tumor (sensitivity of 82% and a positive predictive value of 78%) [48, [50]. We have used publicly available genomic datasets (TCGA and Tothill) to discover candidate gene markers associated with a high likelihood of residual disease, finding that high tumoral FABP4 and ADH1B expression is associated with significantly higher risk of residual disease [51]. Collectively, molecular predictor studies demonstrate interesting advances in attempts to personalize surgical therapy; however, limitations exist in that external validity is yet to be established. Furthermore, compared to clinical predictors that are readily available from parameters that can be obtained prior to surgical intervention, molecular predictors thus far are based on tumoral expression. Ultimately, both molecular and clinical predictors may become equally useful in different scenarios but further investigation is warranted.
Improving the quality of the surgical management of ovarian cancer patients
Opportunities to Standardize Medical Practice
Despite the fact that the volume of tumor residuum following cytoreductive surgery remains the main prognostic factor impacting clinical outcome among patients with advanced ovarian cancer, marked disparities still exist in the definition of “optimal” resection and rates of R0 cytoreduction among centers across the U.S. The reasons for this inconsistency are multifactorial and include differences in surgeon skill and training, suboptimal infrastructure for delivery of highly specialized care and inability to accurately predict those likely to achieve surgical success defined as complete cytoreduction. However, data emphasizing the survival advantage imparted on those patients who undergo R0 cytoreduction presents a unique metric that is clearly measurable and can be exploited to improve the quality of surgical care.
The Anderson Algorithm: Personalized Surgical Therapy
To improve the proportion of patients with advanced ovarian cancer undergoing complete cytoreduction, we have introduced a quality improvement program with a goal of improving rates of R0 resection. This initiative is part of the Women’s Cancer Moon Shot Program, a comprehensive multidisciplinary research effort focused on improving survival among patients with high-grade serous ovarian cancer. Based on the breadth of data describing impact of residual disease on patient outcome, we anticipate that increasing rates of R0 resection will result in a reciprocal improvement in both progression free and overall survival.
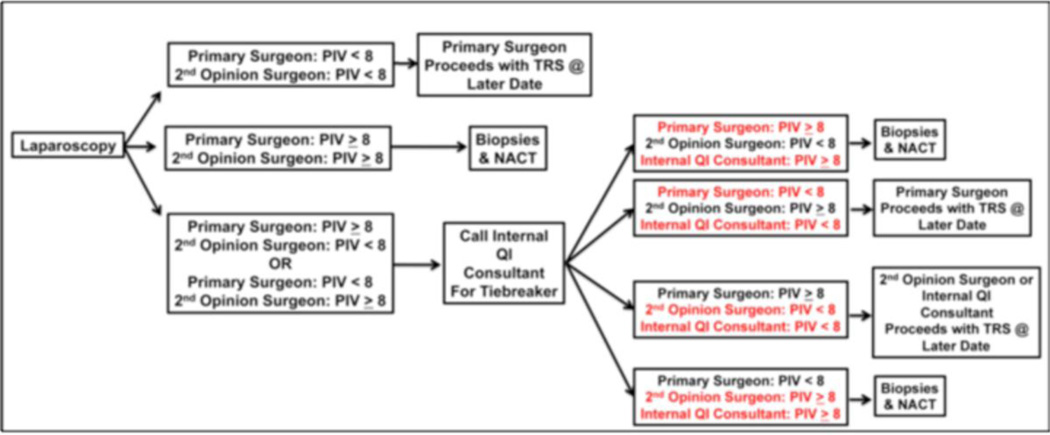
Key features of our quality improvement program are (Figure 2):
- Prospective screening and tracking of all patients with a suspected diagnosis of advanced ovarian cancer
- Surgeon education on the importance of R0 resection and consensus to offer NACT to those patients unlikely to achieve complete gross resection
- Multidisciplinary assessment of disease distribution via collaboration with hepatobiliary, thoracic, colorectal and urologic surgical oncology when indicated
- Incorporation of diagnostic laparoscopy for peritoneal disease assessment among all surgically fit patients with suspected advanced ovarian cancer to determine resectability of disease
- Inclusion of a 2 surgeon opinion at the time of diagnostic laparoscopy for peritoneal disease assessment to ensure consistent opinion of disease resectability across different surgeons in the same clinical practice
- Weekly quality improvement conferences including adherence to pre-set guidelines and reporting of morbidity and patient outcome
- Consensus among all gynecologic oncologists to follow the project’s standard operating procedures for eligible patients
Figure 2.
The Anderson Algorithm for laparoscopic disease assessment of patients with advanced ovarian cancer. PIV = predictive index value. TRS = tumor reductive surgery. NACT = neoadjuvant chemotherapy. QI = quality improvement
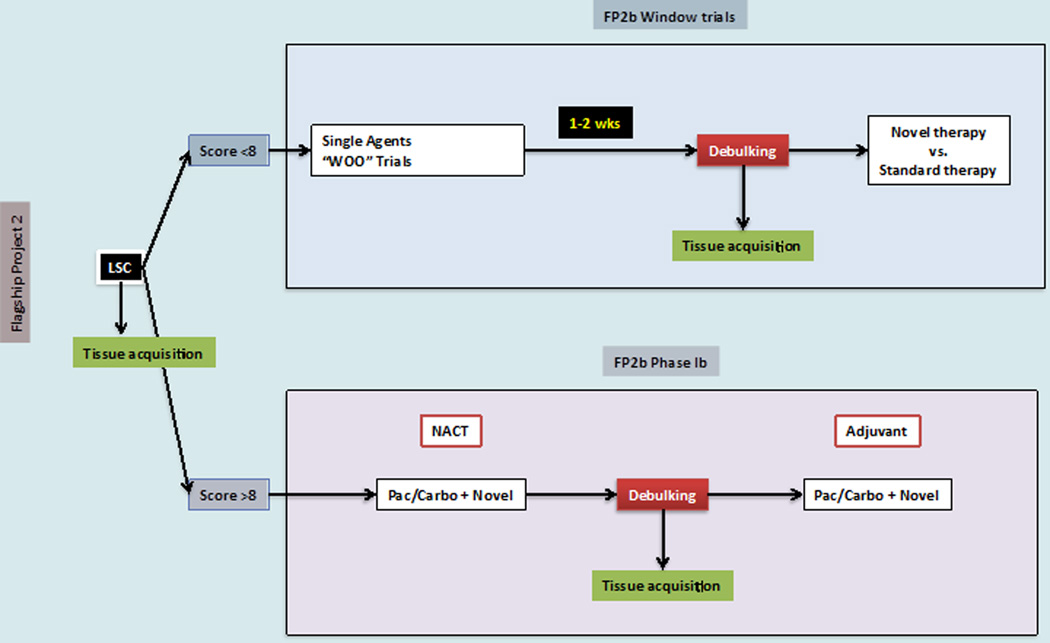
At our center, all patients presenting with presumed advanced-stage (Stage III or IV) ovarian cancer are considered for a two-surgeon laparoscopic tumor evaluation (The Anderson Algorithm). A validated composite scoring system is utilized to determine ability to resect to no gross residual disease [48]. Patients with scores < 8 proceed with primary cytoreductive surgery. Patients with scores ≥ 8 receive NACT with subsequent interval tumor reductive surgery. Fresh frozen tumor samples are obtained from the following pre-specified sites: (1) ovary (or suspected primary); (2) omentum; (3) & (4) from two additional metastatic sites at the time of primary assessment (diagnostic laparoscopy and/or primary cytoreductive surgery). Following 3 cycles of NACT, interval cytoreduction is undertaken in all patients who exhibit partial response to induction chemotherapy. For those patients with gross disease, additional fresh tissue from matching sites is obtained for comparative analysis to primary pre-chemotherapy samples for characterization of adaptive changes related to chemotherapy exposure. Several opportunities provided by this approach: (1) proceeding with upfront debulking in only those patients most likely to achieve R0 and the reciprocal anticipated survival benefit, (2) collection of untreated tumor, (3) utilization of time between laparoscopic evaluation and tumor reductive surgery to evaluate clinical and molecular impact of novel therapeutic agents among those triaged to primary cytoreductive surgery (window of opportunity trials) and (4) application of tumor collected at the time of interval cytoreductive surgery to evaluate clinical and molecular impact of novel combinations of therapeutic agents (Figure 3). The window of opportunity studies offer a unique opportunity to study end tissue effects of novel therapeutic in a manner that has never been possible among patients with advanced ovarian cancer. When evaluating the feasibility of this algorithm, we noted significant improvements in rates of complete resection at the time of primary cytoreductive surgery (R0 rates: 44% [pre-implementation] vs. 84% [post-implementation], p<0.01) with 50% of patients being offered NACT compared to primary cytoreductive surgery. In the feasibility phase, no tie-breaker opinions were required. In addition, there was a trend towards increased complete resection in patients undergoing NACT (R0 rates: 65% [pre-implementation] vs. 100% [post-implementation] p=0.15) To date, we have seen no port site metastases and no difference in surgical morbidity. We anticipate the improvement in R0 resection will translate into improved patient progression-free and overall survival (unpublished data presented at the Society of Gynecologic Oncology 2014 Annual Meeting).
Figure 3.
Novel clinical trial design for patients treated on the Anderson Algorithm. LSC = laparoscopic evaluation and score. WOO = window of opportunity. NACT = neoadjuvant chemotherapy. Pac = paclitaxel. Carbo = carboplatin.
Summary of recommendations
Survival of patients with advanced ovarian cancer is inversely proportional to the volume of residual disease. Those with no gross residual disease have the best outcome compared to those patients left with any visible residual disease. The objective of primary cytoreduction should be complete removal of all macroscopic tumors. Diagnostic laparoscopy allows rapid assessment of extent of peritoneal disease distribution and resectability of disease. Only those patients most amenable to achieve R0 resection should be offered primary cytoreductive surgery, thereby allowing a more rational and personalized surgical approach. Centers should strive to standardize the surgical approach to patients with advanced ovarian cancer with systematic quality improvement initiatives in an effort to improve surgical outcomes and patient survival. Future studies regarding the direct impact of surgical cytoreduction among patients with advanced ovarian cancer will help to determine the ideal timing of surgery as well as potential benefits of surgery beyond complete resection and reciprocal improvement in survival.
Acknowledgements
The authors thank all faculty members of the Department of Gynecologic Oncology & Reproductive Medicine for highly productive discussions and also the Women’s Cancer Moonshot Program for support. Work supported in part by the NIH (P50CA083639, U54CA151668, UH2TR000943, CA016672), CPRIT (RP110595, RP120214), Ovarian Cancer Research Fund Program Project Development Grant, Department of Defense grants (OC120547 and OC093416), the Judy Reis Ovarian Cancer Fund, the Ann Rife Cox Chair in gynecology, the Betty Ann Asche Murray Distinguished Professorship, and the Blanton-Davis Ovarian Cancer Research Program.
References
- 1.Elattar A, et al. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst Rev. 2011;(8):CD007565. doi: 10.1002/14651858.CD007565.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffiths CT, Fuller AF. Intensive surgical and chemotherapeutic management of advanced ovarian cancer. Surg Clin North Am. 1978;58(1):131–142. doi: 10.1016/s0039-6109(16)41440-4. [DOI] [PubMed] [Google Scholar]
- 3.Eisenkop SM, et al. Relative influences of tumor volume before surgery and the cytoreductive outcome on survival for patients with advanced ovarian cancer: a prospective study. Gynecol Oncol. 2003;90(2):390–396. doi: 10.1016/s0090-8258(03)00278-6. [DOI] [PubMed] [Google Scholar]
- 4.Chi DS, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103(2):559–564. doi: 10.1016/j.ygyno.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 5.Aletti GD, et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol. 2006;107(1):77–85. doi: 10.1097/01.AOG.0000192407.04428.bb. [DOI] [PubMed] [Google Scholar]
- 6.Dubois N, Willems T, Myant N. Ovarian metastasis of breast cancer: a case report. Role of cytoreductive surgery] J Gynecol Obstet Biol Reprod (Paris) 2009;38(3):242–245. doi: 10.1016/j.jgyn.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Hoskins PJ, McMurtrie E, Swenerton KD. A phase II trial of intravenous etoposide (VP-16-213) in epithelial ovarian cancer resistant to cisplatin or carboplatin: clinical and serological evidence of activity. Int J Gynecol Cancer. 1992;2(1):35–40. doi: 10.1046/j.1525-1438.1992.02010035.x. [DOI] [PubMed] [Google Scholar]
- 8.Hoskins WJ, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170(4):974–979. doi: 10.1016/s0002-9378(94)70090-7. discussion 979-80. [DOI] [PubMed] [Google Scholar]
- 9.Bristow RE, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 10.Kang S, et al. Frequent use of complex surgeries adn survival outcomes in ovarian cancer: A propensity score analysis from the Korean Gynecologic Oncology Group. J Clin Oncol. 2014;32(5S) [Google Scholar]
- 11.Chi DS, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009;114(1):26–31. doi: 10.1016/j.ygyno.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Winter WE, 3rd, et al. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2008;26(1):83–89. doi: 10.1200/JCO.2007.13.1953. [DOI] [PubMed] [Google Scholar]
- 13.du Bois A, et al. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO) Cancer. 2009;115(6):1234–1244. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 14.Tangjitgamol S, et al. Interval debulking surgery for advanced epithelial ovarian cancer. Cochrane Database Syst Rev. 2009;(2):CD006014. doi: 10.1002/14651858.CD006014.pub3. [DOI] [PubMed] [Google Scholar]
- 15.Vergote I, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 16.Kehoe S, et al. Chemotherapy or upfront surgery for newly diagnosed advanced ovarian cancer: Results from the MRC CHORUS trial. J Clin Oncol. 2013;31(suppl) abstr 5500. [Google Scholar]
- 17.Chi DS, et al. An analysis of patients with bulky advanced stage ovarian, tubal, and peritoneal carcinoma treated with primary debulking surgery (PDS) during an identical time period as the randomized EORTC-NCIC trial of PDS vs neoadjuvant chemotherapy (NACT) Gynecol Oncol. 2012;124(1):10–14. doi: 10.1016/j.ygyno.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Eisenkop SM, Friedman RL, Wang HJ. Complete cytoreductive surgery is feasible and maximizes survival in patients with advanced epithelial ovarian cancer: a prospective study. Gynecol Oncol. 1998;69(2):103–108. doi: 10.1006/gyno.1998.4955. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, et al. Discordance between beliefs and recommendations of gynecologic oncologists in ovarian cancer management. Int J Gynecol Cancer. 2004;14(6):1055–1062. doi: 10.1111/j.1048-891X.2004.14602.x. [DOI] [PubMed] [Google Scholar]
- 20.Dewdney SB, et al. The role of neoadjuvant chemotherapy in the management of patients with advanced stage ovarian cancer: survey results from members of the Society of Gynecologic Oncologists. Gynecol Oncol. 2010;119(1):18–21. doi: 10.1016/j.ygyno.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Badgwell D, Bast RC., Jr Early detection of ovarian cancer. Dis Markers. 2007;23(5–6):397–410. doi: 10.1155/2007/309382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chi DS, et al. The ability of preoperative serum CA-125 to predict optimal primary tumor cytoreduction in stage III epithelial ovarian carcinoma. Gynecol Oncol. 2000;77(2):227–231. doi: 10.1006/gyno.2000.5749. [DOI] [PubMed] [Google Scholar]
- 23.Chi DS, et al. A contemporary analysis of the ability of preoperative serum CA-125 to predict primary cytoreductive outcome in patients with advanced ovarian, tubal and peritoneal carcinoma. Gynecol Oncol. 2009;112(1):6–10. doi: 10.1016/j.ygyno.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Vorgias G, et al. Can the preoperative Ca-125 level predict optimal cytoreduction in patients with advanced ovarian carcinoma? A single institution cohort study. Gynecol Oncol. 2009;112(1):11–15. doi: 10.1016/j.ygyno.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 25.Memarzadeh S, et al. CA125 levels are a weak predictor of optimal cytoreductive surgery in patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2003;13(2):120–124. doi: 10.1046/j.1525-1438.2003.13019.x. [DOI] [PubMed] [Google Scholar]
- 26.Arits AH, et al. Preoperative serum CA125 levels do not predict suboptimal cytoreductive surgery in epithelial ovarian cancer. Int J Gynecol Cancer. 2008;18(4):621–628. doi: 10.1111/j.1525-1438.2007.01064.x. [DOI] [PubMed] [Google Scholar]
- 27.Gilani MM, et al. A study to evaluate the utility of presurgical CA125 to predict optimal tumor cytoreduction of epithelial ovarian cancer. Gynecol Oncol. 2007;105(3):780–783. doi: 10.1016/j.ygyno.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 28.Barlow TS, et al. The utility of presurgical CA125 to predict optimal tumor cytoreduction of epithelial ovarian cancer. Int J Gynecol Cancer. 2006;16(2):496–500. doi: 10.1111/j.1525-1438.2006.00573.x. [DOI] [PubMed] [Google Scholar]
- 29.Everett EN, et al. Predictors of suboptimal surgical cytoreduction in women treated with initial cytoreductive surgery for advanced stage epithelial ovarian cancer. Am J Obstet Gynecol. 2005;193(2):568–574. doi: 10.1016/j.ajog.2005.03.058. discussion 574-6. [DOI] [PubMed] [Google Scholar]
- 30.Brockbank EC, et al. Preoperative predictors of suboptimal primary surgical cytoreduction in women with clinical evidence of advanced primary epithelial ovarian cancer. Int J Gynecol Cancer. 2004;14(1):42–50. doi: 10.1111/j.1048-891x.2004.14065.x. [DOI] [PubMed] [Google Scholar]
- 31.Rossi AC, et al. A retrospective study of preoperative CA 125 levels in 82 patients with ovarian cancer. Arch Gynecol Obstet. 2004;269(4):263–265. doi: 10.1007/s00404-002-0404-6. [DOI] [PubMed] [Google Scholar]
- 32.Obeidat B, Latimer J, Crawford R. Can optimal primary cytoreduction be predicted in advanced stage epithelial ovarian cancer? Role of preoperative serum CA-125 level. Gynecol Obstet Invest. 2004;57(3):153–156. doi: 10.1159/000076236. [DOI] [PubMed] [Google Scholar]
- 33.Eltabbakh GH, et al. Factors associated with cytoreducibility among women with ovarian carcinoma. Gynecol Oncol. 2004;95(2):377–383. doi: 10.1016/j.ygyno.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 34.Alcazar JL, et al. CA-125 levels in predicting optimal cytoreductive surgery in patients with advanced epithelial ovarian carcinoma. Int J Gynaecol Obstet. 2004;84(2):173–174. doi: 10.1016/j.ijgo.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Cooper BC, et al. Preoperative CA 125 levels: an independent prognostic factor for epithelial ovarian cancer. Obstet Gynecol. 2002;100(1):59–64. doi: 10.1016/s0029-7844(02)02057-4. [DOI] [PubMed] [Google Scholar]
- 36.Saygili U, et al. Can serum CA-125 levels predict the optimal primary cytoreduction in patients with advanced ovarian carcinoma? Gynecol Oncol. 2002;86(1):57–61. doi: 10.1006/gyno.2002.6719. [DOI] [PubMed] [Google Scholar]
- 37.Gemer O, Segal S, Kopmar A. Preoperative CA-125 level as a predictor of non optimal cytoreduction of advanced epithelial ovarian cancer. Acta Obstet Gynecol Scand. 2001;80(6):583–585. [PubMed] [Google Scholar]
- 38.Gemer O, et al. A multicenter study of CA 125 level as a predictor of non-optimal primary cytoreduction of advanced epithelial ovarian cancer. Eur J Surg Oncol. 2005;31(9):1006–1010. doi: 10.1016/j.ejso.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Nelson BE, Rosenfield AT, Schwartz PE. Preoperative abdominopelvic computed tomographic prediction of optimal cytoreduction in epithelial ovarian carcinoma. J Clin Oncol. 1993;11(1):166–172. doi: 10.1200/JCO.1993.11.1.166. [DOI] [PubMed] [Google Scholar]
- 40.Meyer JI, et al. Ovarian carcinoma: value of CT in predicting success of debulking surgery. AJR Am J Roentgenol. 1995;165(4):875–878. doi: 10.2214/ajr.165.4.7676985. [DOI] [PubMed] [Google Scholar]
- 41.Dowdy SC, et al. The utility of computed tomography scans in predicting suboptimal cytoreductive surgery in women with advanced ovarian carcinoma. Cancer. 2004;101(2):346–352. doi: 10.1002/cncr.20376. [DOI] [PubMed] [Google Scholar]
- 42.Bristow RE, et al. A model for predicting surgical outcome in patients with advanced ovarian carcinoma using computed tomography. Cancer. 2000;89(7):1532–1540. doi: 10.1002/1097-0142(20001001)89:7<1532::aid-cncr17>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 43.Axtell AE, et al. Multi-institutional reciprocal validation study of computed tomography predictors of suboptimal primary cytoreduction in patients with advanced ovarian cancer. J Clin Oncol. 2007;25(4):384–389. doi: 10.1200/JCO.2006.07.7800. [DOI] [PubMed] [Google Scholar]
- 44.Byrom J, et al. Can pre-operative computed tomography predict resectability of ovarian carcinoma at primary laparotomy? BJOG. 2002;109(4):369–375. doi: 10.1111/j.1471-0528.2002.01216.x. [DOI] [PubMed] [Google Scholar]
- 45.Suidan RS, et al. A multicenter prospective trial evaluating the ability of preoperative computed tomography scan and serum CA-125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian, fallopian tube, and peritoneal cancer. Gynecol Oncol. 2014;134(3):455–461. doi: 10.1016/j.ygyno.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fagotti A, et al. Role of laparoscopy to assess the chance of optimal cytoreductive surgery in advanced ovarian cancer: a pilot study. Gynecol Oncol. 2005;96(3):729–735. doi: 10.1016/j.ygyno.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 47.Fagotti A, et al. A laparoscopy-based score to predict surgical outcome in patients with advanced ovarian carcinoma: a pilot study. Ann Surg Oncol. 2006;13(8):1156–1161. doi: 10.1245/ASO.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 48.Fagotti A, et al. Prospective validation of a laparoscopic predictive model for optimal cytoreduction in advanced ovarian carcinoma. Am J Obstet Gynecol. 2008;199(6):642 e1–642 e6. doi: 10.1016/j.ajog.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 49.Fagotti A, et al. Introduction of staging laparoscopy in the management of advanced epithelial ovarian, tubal and peritoneal cancer: impact on prognosis in a single institution experience. Gynecol Oncol. 2013;131(2):341–346. doi: 10.1016/j.ygyno.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 50.Abdallah R, Chon HS, Gonzalez Bosquet J. Gene expression and prediction of complete cytoreduction in ovarian cancer. Obstet Gynecol. 2014;123(Suppl 1):89S. [Google Scholar]
- 51.Tucker SL, et al. Molecular Biomarkers of Residual Disease after Surgical Debulking of High-Grade Serous Ovarian Cancer. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-14-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]