Comparable Performance of the Kansas City Cardiomyopathy Questionnaire in Heart Failure Patients with Preserved and Reduced Ejection Fraction (original) (raw)
. Author manuscript; available in PMC: 2015 Aug 22.
Abstract
Background
Despite the growing epidemic of heart failure with preserved ejection fraction (HFpEF), no valid measure of patients’ health status (symptoms, function and quality of life) exists. We evaluated the Kansas City Cardiomyopathy Questionnaire (KCCQ), a validated measure of heart failure with reduced ejection fraction (HFrEF), in HFpEF patients.
Methods and Results
Using a prospective HF registry, we dichotomized patients into HFrEF (EF ≤ 40) and HFpEF (EF ≥ 50). The associations between NYHA class, a commonly used criterion standard, and KCCQ Overall Summary and Total Symptom domains were evaluated using Spearman correlations and two-way ANOVA with differences between HFrEF and HFpEF patients tested with interaction terms. Predictive validity of the KCCQ Overall Summary scores was assessed with Kaplan-Meier curves for death and all-cause hospitalization. Covariate adjustment was made using Cox proportional hazards models. Internal reliability was assessed with Cronbach’s α.
Conclusions
Among 849 patients, 200 (24%) had HFpEF. KCCQ summary scores were strongly associated with NYHA class in both HFpEF (r = −0.62, p < .001) and HFrEF patients (r = −0.55; p=0.27 for interaction). One-year event-free rates by KCCQ category among HFpEF patients were 0–25=13.8%, 26–50=59.1%, 51–75=73.8%, and 76–100=77.8%, (log rank p < .001), with no significant interaction by EF (p=0.37). The KCCQ domains demonstrated high internal consistency among HFpEF patients (Cronbach’s α = 0.96 for overall summary and ≥ 0.69 in all sub-domains).
Conclusion
Among patients with HFpEF, the KCCQ appears to be a valid and reliable measure of health status and offers excellent prognostic ability. Future studies should extend and replicate our findings, including the establishment of its responsiveness to clinical change.
Keywords: Heart Failure, Preserved Ejection Fraction, HFpEF, KCCQ, Quality of Life, Outcomes, Diastolic Heart Failure
Introduction
Heart failure has become a major global epidemic, with increasing prevalence, clinical impact and cost, with approximately half due to diastolic dysfunction; commonly referred to as HF with preserved ejection fraction (HFpEF)1–3. Beyond its effects on mortality, HF, regardless of its type, can also have a profound impact on patients’ health status—their symptoms, function and quality of life. To date, however, there have been no established methods for reliably and validly quantifying the health status of HFpEF patients. Understanding the health status outcomes of HFpEF from patients’ perspectives is especially important to accurately assess the success of treatment, both in clinical trials and routine care.
Quantifying patients’ health status requires the development of psychometrically sound instruments to reliably and sensitively elicit from patients how the disease impacts their symptoms, function and quality of life.4, 5 Once developed, these assessments can then be used to support clinical research, clinical practice, quality assessment and public policy.6 While validated health status questionnaires exist for HF with reduced ejection fraction (HFrEF) 7–10, none have been developed or tested in patients with HFpEF. The Kansas City Cardiomyopathy Questionnaire (KCCQ) has proven to be a valid, reliable, responsive, and prognostically important measure of patients’ health status for patients with HFrEF.7, 11–14 The purpose of this study was to examine the psychometric performance of the KCCQ in patients with HFpEF.
Methods
Patient Population
Patients were drawn from the Washington University Heart Failure Registry, a prospective registry of inpatients and outpatients carrying a clinical diagnosis of HF, regardless of EF or etiology, evaluated at Washington University School of Medicine or Barnes Jewish Hospital, St. Louis, MO. Detailed patient information was prospectively collected, including heart disease onset, etiology, clinical stage and severity, medications, device therapies, co-morbidities, demographics, and health status. All patients provided informed consent, and the study was approved by the Washington University Institutional Review Board.
Study Measures
Patients were assessed for clinical factors and health status at the time of study enrollment or baseline, and subsequent clinical outcomes were collected at 6, 12, 18 and 24 months. Disease-specific health status was assessed with the KCCQ,11 a 23-item, self-administered questionnaire that quantifies multiple domains by which HF can impact patients’ lives, including their physical and social limitations, symptom frequency and severity, quality of life, recent changes in symptom status, and self-efficacy. The first 4 domains can be combined into an overall summary scale, which has previously been shown to be valid, reliable, sensitive to clinical change and associated with survival, hospitalization and costs in patients with HFrEF.8, 9, 11, 15–19 Values for all domains range from 0 to 100, with higher scores indicating lower symptom burden and better quality of life. Scores were divided into ranges of 0–25, 26–50, 51–75, and 76–100, which corresponds to severe, moderate, fair, and little to no disability, respectively7, 11, 20. New York Heart Association (NYHA) Functional Class, a physician-assigned assessment of patients’ symptoms and function, was also recorded for each patient at baseline.
Measuring clinical outcomes and follow-up
Clinical outcomes after enrollment were collected by telephone interviews at home or by in-person interviews at clinic visits. These were supplemented with medical record reviews at 6, 12, 18, and 24 months. For patients who were unreachable by telephone and did not have any documentation of contact in their medical record, vital status was determined with the social security death index. Data collected for each clinical event included date and reason for death and hospitalization(s), categorized as one of the following: HF, other cardiac, or non-cardiac reason for admission.
Definition of HFpEF
The EF used to differentiate HFpEF from HFrEF is variably defined as >40%, >45%, or >50% in the medical literature.21–25 Given the uncertainty of how to classify patients with an EF between 40–50%, our primary analysis was designed to focus on groups in whom there would be no controversy about the classification of their HF. We therefore considered HFrEF as those with an EF <40% and compared the performance of the KCCQ to those with HFpEF, defined as an EF >50%. A sensitivity analysis was performed to determine whether the definition of HFpEF used above may have biased the data analysis. We therefore repeated the analysis with HFpEF defined as EF >40%. We also examined the relationship between KCCQ and NYHA among intermediate EF patients (EF 41–49%). EF was obtained from chart review, using the most recent echocardiogram, nuclear scan, ventriculogram, or cardiac MRI performed prior to the health status assessment.
Statistical Methods
In the primary analysis, patients were grouped into HFrEF (EF ≤ 40%) and HFpEF (EF ≥ 50%). In the supplementary analysis, patients were analyzed as EF greater or less than 40%. Patients who did not complete the KCCQ were excluded from the analysis (32 (13.8%) of 232 HFpEF patients and 116 (12%) or 965 HFrEF patients). Among the 28 variables listed in Table 1, there were no significant differences between HFpEF patients who did and did not complete the health status assessments, except that those who participated in this study were more likely to be taking β-blockers (87% vs. 69%, p=0.01) and less likely to have had a prior history of cerebrovascular disease (10% vs. 29%, p=0.01). Descriptive statistics were provided for patient characteristics by EF group. Student’s t-tests for independent groups and Fisher’s exact tests were used to compare continuous and categorical data, respectively. All statistical analyses were performed with the use of SAS software, version 9.2 (SAS Institute, Inc., Cary, NC).
Table 1.
Patient characteristics
| Variable | Overall(N=849) | HFrEF (EF≤40) (N=649) | HFpEF (EF≥50)(N=200) | P-value* |
|---|---|---|---|---|
| Age | 56.6± 13.1 | 55.6 ± 13.0 | 59.8 ± 13.0 | <.001 |
| Female, No. (%) | 293 (35%) | 194 (30%) | 99 (50%) | <.001 |
| White, No. (%) | 622 (73%) | 470 (72%) | 152 (76%) | 0.36 |
| HR | 77.9± 15.2 | 79.5 ± 15.4 | 72.8 ± 13.5 | <.001 |
| BP, systolic | 114.3± 19.2 | 111.7 ± 18.0 | 122.8 ± 20.4 | <.001 |
| BP, diastolic | 71.0± 11.3 | 70.8 ± 11.1 | 71.7 ± 11.9 | 0.30 |
| eGFR | 71.2± 27.1 | 70.4 ± 27.0 | 73.9 ± 27.2 | 0.12 |
| Na | 139.0± 3.7 | 138.7 ± 3.8 | 140.0 ± 3.1 | <.001 |
| BMI | 31.9± 8.5 | 31.2 ± 7.6 | 34.0 ± 10.7 | <.001 |
| Ef | 32.2± 15.9 | 24.5 ± 8.2 | 57.3 ± 5.8 | <.001 |
| NYHA, No. (%) | <.001 | |||
| . I | 55 (6%) | 26 (4%) | 29 (15%) | |
| . II | 403 (47%) | 294 (45%) | 109 (55%) | |
| . III | 304 (36%) | 251 (39%) | 53 (27%) | |
| . IV | 87 (10%) | 78 (12%) | 9 (5%) | |
| Inpatient, No. (%) | 136 (16%) | 100 (15%) | 36 (18%) | 0.38 |
| Afib, No. (%) | 320 (38%) | 255 (40%) | 65 (33%) | 0.07 |
| DM, No. (%) | 279 (33%) | 216 (33%) | 63 (32%) | 0.67 |
| HTN, No. (%) | 510 (60%) | 384 (59%) | 126 (63%) | 0.36 |
| HLD, No. (%) | 394 (46%) | 306 (47%) | 88 (44%) | 0.47 |
| Stroke/TIA, No. (%) | 96 (11%) | 76 (12%) | 20 (10%) | 0.61 |
| Ischemic, No. (%) | 287 (34%) | 259 (40%) | 28 (14%) | <.001 |
| Smoker, No. (%) | 558 (67%) | 433 (68%) | 125 (64%) | 0.26 |
| BB, No. (%) | 712 (84%) | 539 (83%) | 173 (87%) | 0.27 |
| ACE/ARB, No. (%) | 686 (81%) | 520 (80%) | 166 (83%) | 0.41 |
| Diuretics, No. (%) | 729 (86%) | 572 (88%) | 157 (79%) | 0.001 |
| Dig, No. (%) | 285 (34%) | 260 (40%) | 25 (13%) | <.001 |
| CCB, No. (%) | 89 (10%) | 43 (7%) | 46 (23%) | <.001 |
| Nitrates, No. (%) | 245 (29%) | 194 (30%) | 51 (26%) | 0.25 |
| Antiarrythmics, No. (%) | 185 (22%) | 166 (26%) | 19 (10%) | <.001 |
| Hydralazine, No. (%) | 133 (16%) | 102 (16%) | 31 (16%) | 1.00 |
| Antiplatlets, No. (%) | 590 (69%) | 460 (71%) | 130 (65%) | 0.12 |
| Coumadin or Pradaxa, No. (%) | 298 (35%) | 261 (40%) | 37 (19%) | <.001 |
Internal reliability of the KCCQ
Cronbach’s alpha was used to determine the internal consistency of the KCCQ domains among patients with HFrEF and HFpEF, separately. Cronbach’s alpha evaluates the internal consistency, or the average correlation, of the items within a domain.26 Values range from 0–1 with larger values providing greater consistency of the items within the scale.
Criterion Validity of the KCCQ in HFpEF
The KCCQ overall summary and total symptoms domains were compared across categories of NYHA functional class. Spearman rank-order correlations were used to describe the overall trend between KCCQ and NYHA. To examine whether or not the association between KCCQ and NYHA differed between those with HFpEF and HFrEF, a two-way ANOVA with a test for the interaction by type of HF was conducted.
Predictive validity of the KCCQ in patients with HFpEF
To assess the prognostic ability of the KCCQ, patients were followed from the date of enrollment until date of death or first hospitalization, up to 2 years. Kaplan-Meier (KM) curves were created for the composite of death and all-cause hospitalization by KCCQ overall summary scores category: 0–25, 26–50, 51–75, and 76–100. KM estimates for 1-year event-free rates were provided for HFrEF and HFpEF patients, and the log rank test was used to identify differences in survival distributions. KM curves were also created for death, all-cause hospitalization, and hospitalization for HF separately.
A Cox proportional hazards model evaluated the association of KCCQ overall summary scores (as a continuous variable) at enrollment, while adjusting for age, body mass index (BMI), race, gender, inpatient/outpatient status at time of enrollment, HF etiology, history of atrial fibrillation or atrial flutter, diabetes mellitus (DM), smoking status, and EF group. The interaction between KCCQ and EF group was also included to determine if the association between KCCQ and the time until death or hospitalization varied with respect to type of HF (HFpEF vs. HFrEF). While adjusting for the same covariates, a separate Cox regression model examined the relationship between death or hospitalization and KCCQ overall summary score when categorized into ranges (0–25, 26–50, 51–75, and 76–100), including an interaction term for KCCQ category and EF group. The c-statistic for KCCQ overall summary score as a predictor of death or hospitalization was calculated for HFrEF and HFpEF separately. The C-statistic evaluates the discriminatory ability of the KCCQ to differentiate events and non-events.27
Because inpatients with HF tend to have rapidly changing symptoms, there is a potential risk for variable responses depending on the understanding of patients completing the KCCQ. To determine if the predictive ability of the KCCQ differed with respect to inpatient/outpatient status, a separate multivariable Cox model was created that included an interaction term for KCCQ and inpatient/outpatient status, along with the same adjustment variables as before.
Supplementary analysis
In order to evaluate the sensitivity of EF cut-point when defining HFpEF, EF ≤40% vs. EF >40%, thereby including those with an EF between 40–50%, was evaluated in a similar fashion as the primary analysis above. In addition, the KCCQ overall summary score and total symptoms score were examined by NYHA class for HFrEF (EF ≤ 40) vs. intermediate (EF = 41–49) vs. HFpEF (EF ≥ 50) using a 2-way ANOVA with interaction.
Results
For the primary analysis, there were 849 patients available for analysis, of which, 200 (24%) were identified as having HFpEF (EF≥50%), and 649 (76%) with HFrEF (EF≤40%). Participants were enrolled between March 2010 and September 2012, providing an average follow-up time of 16.6 ± 6.7 months. Table 1 contains detailed patient characteristics and comparisons between HFrEF and HFpEF patients. Consistent with prior literature, patients with HFpEF were older, had higher blood pressure and a greater proportion of women as compared with HFrEF patients. The median time between EF assessment and enrollment in the study was approximately 4 months with an IQR of 11 months. There were minimal amounts of missing data with only 6.7% missing more than 1 data element. The most frequently missing variable was smoking status, which was missing in 2% of the patients. Missing data variables are listed in Supplementary Table 7.
Internal Reliability of the KCCQ in HFpEF
In patients with HFpEF, the internal consistency assessed with Cronbach’s alpha of each KCCQ domain was good to excellent (α > 0.80), and acceptable in the self-efficacy domain (α = 0.69). This pattern that was mirrored among patients with HFrEF (Table 2) and there were minimal differences in the internal consistency of the KCCQ domains between the 2 populations.
Table 2.
Internal consistency of the KCCQ domains
| Cronbach’s Alpha | ||
|---|---|---|
| HFrEF(EF≤40)* | HFpEF(EF≥50)* | |
| Overall summary | 0.94 | 0.96 |
| Clinical summary | 0.92 | 0.93 |
| Physical limitation | 0.87 | 0.88 |
| Symptoms | 0.89 | 0.91 |
| Self-efficacy | 0.64 | 0.69 |
| Quality of life | 0.82 | 0.85 |
| Social limitation | 0.88 | 0.89 |
Criterion Validity of the KCCQ in HFpEF
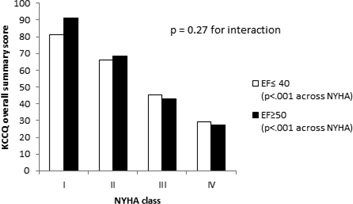
As shown in Table 3 and Figure 1, KCCQ overall summary scores were highly correlated to NYHA class for both HFrEF (r = −0.55, p < .001) and HFpEF (r = −0.62, p < .001), with large and statistically significant differences in mean KCCQ overall summary scores between NYHA classes in both groups. No significant interaction (p=0.27) was identified between HF type and NYHA class, suggesting that the association between NYHA and KCCQ overall summary score is similar in HFrEF and HFpEF patients. Similarly, the KCCQ total symptoms domain scores were highly correlated to NYHA class, (HFpEF patients: r = −0.61, p < .001; HFrEF patients: r = −0.54; p < .001), with no significant interaction between HFpEF and HFrEF; p=0.20 (Table 4).
Table 3.
Mean KCCQ overall summary and symptoms score by NYHA class
| KCCQ overall summary score | KCCQ symptoms score | |||
|---|---|---|---|---|
| NYHA | HFrEF(EF≤40) | HFpEF(EF≥50) | HFrEF(EF≤40) | HFpEF(EF≥50) |
| I | 81.3 | 91.3 | 89.9 | 94.1 |
| II | 66.1 | 68.4 | 73.2 | 72.3 |
| III | 45.3 | 43.0 | 52.8 | 44.2 |
| IV | 29.2 | 27.3 | 34.5 | 29.9 |
| p-value | <.001 | <.001 | <.001 | <.001 |
Figure 1.
KCCQ Summary Score Association with NYHA Class in HFrEF and HFpEF
Table 4.
Event-free rates for HFrEF and HFpEF
| 1-year rates | 2-year rates | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HFrEF (EF ≤ 40) | HFpEF (EF ≥ 50) | HFrEF (EF ≤ 40) | HFpEF (EF ≥ 50) | ||||||
| KCCQ | Rate % | 95% CI | Rate % | 95% CI | Rate % | 95% CI | Rate % | 95% CI | |
| Death/hosp | 0–25 | 15.5 | (8.3, 24.7) | 13.8 | (1.2, 41.6) | 9.7 | (4.0, 18.5) | NA | NA |
| 26–50 | 33.2 | (26.6, 39.8) | 59.1 | (43.1, 72.0) | 24.0 | (17.5, 31.0) | 45.1 | (28.2, 60.5) | |
| 51–75 | 48.8 | (41.4, 55.8) | 73.8 | (57.7, 84.6) | 30.8 | (23.0), 38.9) | 59.7 | (40.8, 74.4) | |
| 76–100 | 69.2 | (61.1, 76.0) | 77.8 | (67.1, 85.4) | 49.6 | (38.7, 59.6) | 61.2 | (47.8, 72.1) | |
| Death | 0–25 | 74.2 | (62.5, 82.8) | 77.7 | (43.8, 92.6) | 57.7 | (42.7, 70.0) | 77.7 | (43.8, 92.6) |
| 26–50 | 86.7 | (81.1, 90.8) | 95.6 | (83.4, 98.9) | 78.4 | (71.2, 84.1) | 92.3 | (77.4, 97.5) | |
| 51–75 | 91.3 | (86.2, 94.6) | 97.6 | (84.3, 99.7) | 83.6 | (75.7, 89.2) | 97.6 | (84.3, 99.7) | |
| 76–100 | 96.7 | (92.2, 98.6) | 1.00 | (1.00, 1.00) | 92.8 | (85.0, 96.6) | 94.7 | (78.6, 98.8) | |
| Hospitalization | 0–25 | 16.8 | (9.1, 26.6) | 20.0 | (1.7, 52.9) | 10.5 | (4.3, 19.9) | NA | NA |
| (all cause) | 26–50 | 35.7 | (28.8, 42.6) | 62.1 | (45.6, 74.9) | 26.1 | (19.0, 33.7) | 47.4 | (29.7, 63.1) |
| 51–75 | 49.9 | (42.5, 56.9) | 73.8 | (57.7, 84.6) | 33.8 | (25.8, 41.9) | 59.7 | (40.8, 74.4) | |
| 76–100 | 70.3 | (62.2, 77.0) | 77.8 | (67.1, 85.4) | 51.2 | (40.0, 61.2) | 61.2 | (47.8, 72.1) | |
| Hospitalization | 0–25 | 42.2 | (30.3, 53.6) | 53.5 | (20.3, 78.3) | 37.3 | (25.2, 49.3) | NA | NA |
| (CHF) | 26–50 | 66.0 | (58.7, 72.4) | 90.2 | (75.9, 97.7) | 59.0 | (50.8, 66.3) | 78.1 | (57.5, 89.5) |
| 51–75 | 81.6 | (75.1, 86.6) | 97.6 | (84.3, 99.7) | 75.1 | (67.5, 81.2) | 97.6 | (84.3, 99.7) | |
| 76–100 | 93.2 | (87.7, 96.3) | 93.9 | (86.0, 97.4) | 79.6 | (69.5, 86.7) | 90.7 | (81.3, 95.5) |
Predictive Validity of the KCCQ in HFpEF
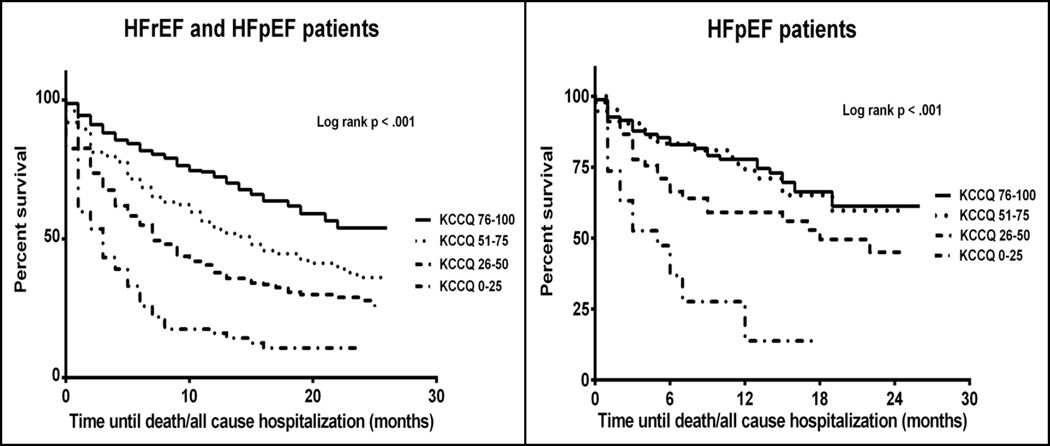
During the study period, there were 469 adverse events (deaths or hospitalizations), 391 events in 621 patients with HFrEF (63% of cohort) and 78 events in 188 patients with HFpEF (41% of cohort). As this is an on-going registry, there were 40 recently enrolled subjects for which no follow up information was available at the time of analysis (28 HFrEF and 12 HFpEF). Lower KCCQ overall summary scores at enrollment were associated with lower event-free survival rates than higher KCCQ summary scores (p<0.001) for patients with HFpEF, as well as for the entire study cohort (Figure 2). Among HFpEF patients, the KM-estimated 1-year event-free survival rates by KCCQ score quartile were: KCCQ 0–25 = 13.8%, 26–50 = 59.1%, 51–75 = 73.8%, and 76–100 = 77.8% (Table 4). After multivariable adjustment, KCCQ overall summary scores were significantly associated with time until death or hospitalization, a 10-point increase in KCCQ overall summary score resulted in a 12% decrease in the hazard of death/hospitalization (HR = 0.88, 95% CI = 0.81–0.96). A similar association was found among HFrEF patients (HR = 0.84 per10-point higher KCCQ score, 95% CI = 0.81–0.88)], with no significant interaction between HFpEF and KCCQ (p = 0.37).
Figure 2.
Kaplan-Meier Curves for Death or Hospitalization
Panel A: Entire study cohort, HFrEF and HFpEF, Panel B: In HFpEF (EF>50%)
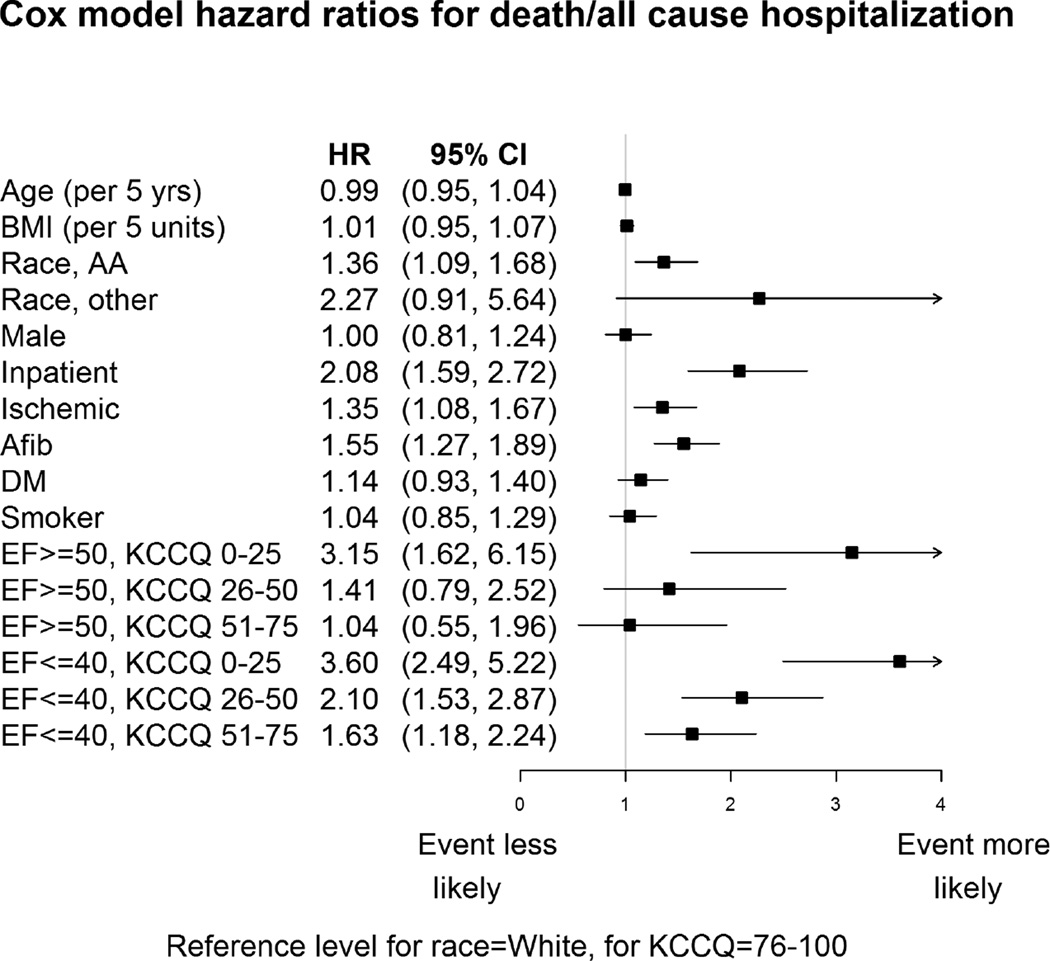
Analyzing KCCQ as a categorical variable, HFpEF patients with KCCQ summary scores of 0–25 had a 3-fold greater risk of death/hospitalization as compared with patients with scores 76–100 (adjusted HR=3.15, 95% CI = 1.62–6.15). Risk decreased as KCCQ score ranges increased, although the differences were not statistically significant (KCCQ 26–50 adjusted HR = 1.41, 95% CI = 0.79–2.52); KCCQ 51–75 adjusted HR = 1.04, 95% CI=0.55–1.96); Figure 3). The C-statistic for KCCQ as a predictor of death or hospitalization was comparable between types of HF (HFrEF: 0.69; 95% CI=0.65, 0.73; HFpEF: 0.67; 95% CI=0.59, 0.76).
Figure 3.
Cox Proportional Hazards Model for Death and Hospitalization in HFpEF and HFrEF
Reference levels: race = White, KCCQ = 76–100.
When examining the outcomes individually, there were 105 deaths (HFrEF = 96, HFpEF = 9) and 442 all-cause hospitalizations (HFrEF = 368, HFpEF = 74). There were significant differences across KCCQ score ranges for both individual endpoints (log-rank p<0.05 for both death and all-cause hospitalization for HFrEF and HFpEF patients; Table 4).
We found that the association of KCCQ and outcome did not vary with respect to inpatient/outpatient enrollment status (p-value for interaction=0. 0.21). Among outpatients that were admitted during the study period (N=369), the median time to admission after KCCQ administration was 5 months (IQR=8 months.) Among all outpatients in the cohort, the Kaplan-Meier follow up period was approximately 16 months.
Supplementary analyses
Adding the intermediate EF patients and dichotomizing the total sample N=940 into HFrEF (EF ≤ 40) vs. HFpEF (EF > 40), the number of HFpEF patients increased to N = 291 (31%). The above analyses were repeated and the results were consistent with the primary analyses (Supplementary tables 1–4 and corresponding graphs). The mean KCCQ overall summary scores and total symptoms scores for HFrEF (EF ≤ 40), intermediate (EF = 41–49), and HFpEF (EF ≥ 50) showed similar significant trends across NYHA classes regardless of EF category (p = 0.57 and p = 0.51 for interaction, respectively, Supplementary tables 5 and 6).
Discussion
Given the growing importance of understanding the impact of disease on patients’ perceptions of their health status,6, 9, 10, 16 and the increasing prevalence of HFpEF,22, 23 there is a pressing need to have a validated measure for quantifying health status in this population. In this study, we investigated several standard psychometric properties of the KCCQ, which was originally developed for patients with HFrEF, in patients with HFpEF. Using data from a prospective HF registry, we found similar criterion validity, as assessed by the associations between the KCCQ NYHA class functional, and similar internal reliability in HFpEF and HFrEF patients. We also noted similar predictive validity, with lower KCCQ scores being associated with worse event-free survival (all cause death and HF hospitalization) at one year. Moreover, the sensitivity analyses suggested that the KCCQ reliably predicted clinical outcomes in patients across all EF spectra. Viewed together, these results provide important preliminary evidence that the KCCQ has similar psychometric properties across a broad spectrum of heart failure patients, regardless of patients’ ejection fraction.
Approximately half of hospitalizations in the United States for the clinical syndrome of HF occur in patients with a preserved or normal EF, and the incidence of hospitalization in this patient group has been steadily increasing over the past 2 decades.1, 30, 31 Unfortunately, no treatment options have been proven to reduce mortality in HFpEF.32, 33 To improve the care of patients with HFpEF, a means of monitoring the impact of the disease on their health status is needed to supplement the traditional outcomes of hospitalization and mortality. Although it is tempting to employ measures validated in the HFrEF population, patients with HFpEF have distinct pathophysiology and comorbidities. In fact, controversy exists over whether the disease is part of a continuum with HFrEF, possesses a unique pathophysiology, or is a group of diverse disorders each of which lead to the clinical syndrome of HF22, 23, 34. Patients with HFpEF are more likely to be of an older age, female, and hypertensive, and they are less likely to have coronary artery disease compared with patients with HFrEF. For these reasons, extrapolation from established HFrEF metrics to the HFpEF population without prior validation may be inappropriate.
The goal of this study was to evaluate a potential measure, the KCCQ, for evaluating disease status and progression in HFpEF. Given that the HFpEF is primarily defined by the same symptom complex as HFrEF, we anticipated that the KCCQ, which measures patients’ health status from their perspectives, would be equally valid in HFpEF as it is in HFrEF. Finding virtually identical psychometric properties of the KCCQ in both populations of HF patients suggests that the KCCQ can be used to measure patients’ health status in HFpEF. The availability of a tool to measure health status in HFpEF patients is important in light of increasing evidence suggesting that quality of life may be of equally great, or even greater, importance to some patients than decreased mortality, particularly in older patients with HF35–37. As such, QOL is increasingly recognized as a key endpoint in recent HF studies, especially in older adults33, 38, 39, and was recently found to be valid in a cohort of patients with aortic stenosis from the PARTNERS trial.40 In addition, because the mortality rate in HFpEF is probably lower than that in HFrEF1, 41, 42, a valid measure of health status and quality of life will greatly decrease the time required for clinical trials to assess for meaningful treatment effects, further facilitating clinical investigation.
Although it has been shown that symptom burden and quality of life are equally impaired in HFpEF as in HFrEF,32, 43–45 little is known about the validity and prognostic ability of existing HF status questionnaires in HFpEF. Nevertheless, even in the absence of such data, ongoing large randomized clinical studies in HFpEF, such as the NIH funded, multicenter trial, TOPCAT (Treatment Of Preserved Cardiac function heart failure with an Aldosterone anTagonist), are utilizing disease-specific QOL questionnaires as metrics of disease progress.33 Our findings support the use of the KCCQ in TOPCAT and suggest that it can fill the pressing need for an established metric of disease progression in HFpEF. Once this study is completed, additional data supporting the validity, reliability, predictive validity, responsiveness and interpretability of the KCCQ in HFpEF will be available.
Our results should be interpreted in the context of the following potential limitations. First, in this registry, the KCCQ was administered only at initial enrollment. Thus, there was no serial testing for test-retest reliability, nor was there the explicit demonstration of responsiveness to clinical change. Future studies will need to address this persistent gap in psychometric evaluation of the KCCQ for patients with HFpEF. Second, this was a single-center study. However, there was a good representation of women and minorities, and there are no a priori reasons to expect that patients with HFpEF at Washington University would differ in their responses to the KCCQ compared with patients at other institutions. Finding similar performance at our center in patients with HFrEF with those reported in the literature further adds to the generalizability of our findings. Next, we did not conduct formal, qualitative solicitations of patients’ symptoms and there may be additional domains relevant to the assessment of patient-reported outcomes in HFpEF patients. Regardless, those domains that were measured in the KCCQ showed excellent validation, internal reliability and prognostic significance, suggesting that they are relevant and important to patients with HFpEF. Future studies may seek to explore whether other domains ought to be assessed to further improve the content validity of the KCCQ in patients with HFpEF. An additional concern would be the inclusion of acutely decompensated HF patients who may have had difficulty reflecting on the 2-week recall period of the KCCQ. Finding evidence of the criterion and predictive validity, despite this potential limitation, and no difference between inpatients and outpatients, suggests that the KCCQ may perform well in clinical trials or quality assessment among hospitalized patients with HFpEF. Lastly, exercise capacity was not measured in the study, nor was there a comparison with additional quality of life instruments; thus, we could not more reliably measure convergent validity in this study. However, the extensive prior analyses to establish convergent validity in HFrEF and the similarity of the association of the KCCQ with NYHA and prognosis suggest that the KCCQ is likely going to have similar convergent validity in HFpEF patients as well. This needs to be established in future registries or clinical trails, such as TOPCAT.
Conclusions
The results of this study suggest that the KCCQ is a valid and reliable instrument to measure health status and quality of life in patients with HFpEF, with virtually identical performance characteristics as previously observed in patients with HFrEF. While future studies are needed to replicate and extend our findings, including the establishment of the instrument’s responsiveness in HFpEF, our preliminary findings suggest that the KCCQ represents a potentially important research and clinical tool to measure disease status in all patients with HF.
Supplementary Material
Supplement
Acknowledgments
This research was supported by research funds from the N.I.H. (RC2-HL102222)
Footnotes
Author Disclosures:
Dr. John Spertus owns the copyright to the KCCQ.
The authors have no other relevant disclosures to report.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. The New England journal of medicine. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Hogg K, McMurray J. The treatment of heart failure with preserved ejection fraction ("diastolic heart failure") Heart failure reviews. 2006;11:141–146. doi: 10.1007/s10741-006-9488-6. [DOI] [PubMed] [Google Scholar]
- 3.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. Journal of the American College of Cardiology. 2004;43:317–327. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 4.McLeod LD, Coon CD, Martin SA, Fehnel SE, Hays RD. Interpreting patient-reported outcome results: Us fda guidance and emerging methods. Expert review of pharmacoeconomics & outcomes research. 2011;11:163–169. doi: 10.1586/erp.11.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Speight J, Barendse SM. Fda guidance on patient reported outcomes. Bmj. 2010;340:c2921. doi: 10.1136/bmj.c2921. [DOI] [PubMed] [Google Scholar]
- 6.Spertus JA. Evolving applications for patient-centered health status measures. Circulation. 2008;118:2103–2110. doi: 10.1161/CIRCULATIONAHA.107.747568. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan MD, Levy WC, Russo JE, Crane B, Spertus JA. Summary health status measures in advanced heart failure: Relationship to clinical variables and outcome. Journal of cardiac failure. 2007;13:560–568. doi: 10.1016/j.cardfail.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Luther SA, McCullough PA, Havranek EP, Rumsfeld JS, Jones PG, Heidenreich PA, Peterson ED, Rathore SS, Krumholz HM, Weintraub WS, Spertus JA, Masoudi FA for the Cardiovascular Outcomes Research C. The relationship between b-type natriuretic peptide and health status in patients with heart failure. Journal of cardiac failure. 2005;11:414–421. doi: 10.1016/j.cardfail.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Chan PS, Soto G, Jones PG, Nallamothu BK, Zhang Z, Weintraub WS, Spertus JA. Patient health status and costs in heart failure: Insights from the eplerenone post-acute myocardial infarction heart failure efficacy and survival study (ephesus) Circulation. 2009;119:398–407. doi: 10.1161/CIRCULATIONAHA.108.820472. [DOI] [PubMed] [Google Scholar]
- 10.Spertus JA, Jones PG, Kim J, Globe D. Validity, reliability, and responsiveness of the kansas city cardiomyopathy questionnaire in anemic heart failure patients. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2008;17:291–298. doi: 10.1007/s11136-007-9302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the kansas city cardiomyopathy questionnaire: A new health status measure for heart failure. Journal of the American College of Cardiology. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 12.Myers J, Zaheer N, Quaglietti S, Madhavan R, Froelicher V, Heidenreich P. Association of functional and health status measures in heart failure. Journal of cardiac failure. 2006;12:439–445. doi: 10.1016/j.cardfail.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Parissis JT, Nikolaou M, Farmakis D, Paraskevaidis IA, Bistola V, Venetsanou K, Katsaras D, Filippatos G, Kremastinos DT. Self-assessment of health status is associated with inflammatory activation and predicts long-term outcomes in chronic heart failure. European journal of heart failure. 2009;11:163–169. doi: 10.1093/eurjhf/hfn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation. 2004;110:546–551. doi: 10.1161/01.CIR.0000136991.85540.A9. [DOI] [PubMed] [Google Scholar]
- 15.Faller H, Steinbuchel T, Schowalter M, Spertus JA, Stork S, Angermann CE. [the kansas city cardiomyopathy questionnaire (kccq) -- a new disease-specific quality of life measure for patients with chronic heart failure] Psychotherapie, Psychosomatik, medizinische Psychologie. 2005;55:200–208. doi: 10.1055/s-2004-834597. [DOI] [PubMed] [Google Scholar]
- 16.Flynn KE, Lin L, Moe GW, Howlett JG, Fine LJ, Spertus JA, McConnell TR, Pina IL, Weinfurt KP. Relationships between changes in patient-reported health status and functional capacity in outpatients with heart failure. American heart journal. 2012;163:88–94. e83. doi: 10.1016/j.ahj.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flynn KE, Pina IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, Kraus WE, Miller NH, Schulman KA, Spertus JA, O'Connor CM, Weinfurt KP, Investigators H-A. Effects of exercise training on health status in patients with chronic heart failure: Hf-action randomized controlled trial. JAMA : the journal of the American Medical Association. 2009;301:1451–1459. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heidenreich PA, Spertus JA, Jones PG, Weintraub WS, Rumsfeld JS, Rathore SS, Peterson ED, Masoudi FA, Krumholz HM, Havranek EP, Conard MW, Williams RE. Cardiovascular Outcomes Research C. Health status identifies heart failure outpatients at risk for hospitalization or death. Journal of the American College of Cardiology. 2006;47:752–756. doi: 10.1016/j.jacc.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Kosiborod M, Soto GE, Jones PG, Krumholz HM, Weintraub WS, Deedwania P, Spertus JA. Identifying heart failure patients at high risk for near-term cardiovascular events with serial health status assessments. Circulation. 2007;115:1975–1981. doi: 10.1161/CIRCULATIONAHA.106.670901. [DOI] [PubMed] [Google Scholar]
- 20.Arnold SV, Spertus JA, Jones PG, Xiao L, Cohen DJ. The impact of dyspnea on health-related quality of life in patients with coronary artery disease: Results from the premier registry. American heart journal. 2009;157:1042–1049. e1041. doi: 10.1016/j.ahj.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 21.Heart Failure Society of A. Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, Teerlink JR, Walsh MN. Hfsa 2010 comprehensive heart failure practice guideline. Journal of cardiac failure. 2010;16:e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation. 2011;123:2006–2013. doi: 10.1161/CIRCULATIONAHA.110.954388. discussion 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: Pathophysiology, diagnosis, and treatment. European heart journal. 2011;32:670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zile MR. Heart failure with preserved ejection fraction: Is this diastolic heart failure? Journal of the American College of Cardiology. 2003;41:1519–1522. doi: 10.1016/s0735-1097(03)00186-4. [DOI] [PubMed] [Google Scholar]
- 25.Burkhoff D, Maurer MS, Packer M. Heart failure with a normal ejection fraction: Is it really a disorder of diastolic function? Circulation. 2003;107:656–658. doi: 10.1161/01.cir.0000053947.82595.03. [DOI] [PubMed] [Google Scholar]
- 26.Bland JM, Altman DG. Cronbach's alpha. Bmj. 1997;314:572. doi: 10.1136/bmj.314.7080.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guller U, DeLong ER. Interpreting statistics in medical literature: A vade mecum for surgeons. Journal of the American College of Surgeons. 2004;198:441–458. doi: 10.1016/j.jamcollsurg.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Senni M, Redfield MM. Heart failure with preserved systolic function. A different natural history? Journal of the American College of Cardiology. 2001;38:1277–1282. doi: 10.1016/s0735-1097(01)01567-4. [DOI] [PubMed] [Google Scholar]
- 29.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from olmsted county, minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA : the journal of the American Medical Association. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 31.Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Progress in cardiovascular diseases. 2005;47:320–332. doi: 10.1016/j.pcad.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Hoekstra T, Jaarsma T, van Veldhuisen DJ, Hillege HL, Sanderman R, Lesman-Leegte I. Quality of life and survival in patients with heart failure. European journal of heart failure. 2012 doi: 10.1093/eurjhf/hfs148. [DOI] [PubMed] [Google Scholar]
- 33.Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, McKinlay S, O'Meara E, Shaburishvili T, Pitt B, Pfeffer MA. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: A randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. American heart journal. 2011;162:966–972. e910. doi: 10.1016/j.ahj.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circulation. Heart failure. 2012;5:720–726. doi: 10.1161/CIRCHEARTFAILURE.111.966366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dracup K, Walden JA, Stevenson LW, Brecht ML. Quality of life in patients with advanced heart failure. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 1992;11:273–279. [PubMed] [Google Scholar]
- 36.Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2001;20:1016–1024. doi: 10.1016/s1053-2498(01)00298-4. [DOI] [PubMed] [Google Scholar]
- 37.Stevenson LW. Counting performance with therapies for heart failure: Aiming for quality or quantity? Circulation. 2010;122:561–566. doi: 10.1161/CIRCULATIONAHA.110.970061. [DOI] [PubMed] [Google Scholar]
- 38.Parthasarathy HK, Pieske B, Weisskopf M, Andrews CD, Brunel P, Struthers AD, MacDonald TM. A randomized, double-blind, placebo-controlled study to determine the effects of valsartan on exercise time in patients with symptomatic heart failure with preserved ejection fraction. European journal of heart failure. 2009;11:980–989. doi: 10.1093/eurjhf/hfp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daniel KR, Wells G, Stewart K, Moore B, Kitzman DW. Effect of aldosterone antagonism on exercise tolerance, doppler diastolic function, and quality of life in older women with diastolic heart failure. Congestive heart failure. 2009;15:68–74. doi: 10.1111/j.1751-7133.2009.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnold SV, Spertus JA, Lei Y, Allen KB, Chhatriwalla AK, Leon MB, Smith CR, Reynolds MR, Webb JG, Svensson LG, Cohen DJ. Use of the kansas city cardiomyopathy questionnaire for monitoring health status in patients with aortic stenosis. Circulation. Heart failure. 2013;6:61–67. doi: 10.1161/CIRCHEARTFAILURE.112.970053. [DOI] [PubMed] [Google Scholar]
- 41.Somaratne JB, Berry C, McMurray JJ, Poppe KK, Doughty RN, Whalley GA. The prognostic significance of heart failure with preserved left ventricular ejection fraction: A literature-based meta-analysis. European journal of heart failure. 2009;11:855–862. doi: 10.1093/eurjhf/hfp103. [DOI] [PubMed] [Google Scholar]
- 42.Meta-analysis Global Group in Chronic Heart F. The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: An individual patient data meta-analysis. European heart journal. 2012;33:1750–1757. doi: 10.1093/eurheartj/ehr254. [DOI] [PubMed] [Google Scholar]
- 43.Lewis EF, Lamas GA, O'Meara E, Granger CB, Dunlap ME, McKelvie RS, Probstfield JL, Young JB, Michelson EL, Halling K, Carlsson J, Olofsson B, McMurray JJ, Yusuf S, Swedberg K, Pfeffer MA, Investigators C. Characterization of health-related quality of life in heart failure patients with preserved versus low ejection fraction in charm. European journal of heart failure. 2007;9:83–91. doi: 10.1016/j.ejheart.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 44.O'Meara E, Lewis E, Granger C, Dunlap ME, McKelvie RS, Probstfield JL, Young JB, Michelson EL, Ostergren J, Carlsson J, Olofsson B, McMurray J, Yusuf S, Swedberg K, Pfeffer MA. Patient perception of the effect of treatment with candesartan in heart failure. Results of the candesartan in heart failure: Assessment of reduction in mortality and morbidity (charm) programme. European journal of heart failure. 2005;7:650–656. doi: 10.1016/j.ejheart.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 45.Hoekstra T, Lesman-Leegte I, van Veldhuisen DJ, Sanderman R, Jaarsma T. Quality of life is impaired similarly in heart failure patients with preserved and reduced ejection fraction. European journal of heart failure. 2011;13:1013–1018. doi: 10.1093/eurjhf/hfr072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement