Plasma ApoC-III Levels, Triglycerides, and Coronary Artery Calcification in Type 2 Diabetics (original) (raw)
. Author manuscript; available in PMC: 2016 Aug 1.
Published in final edited form as: Arterioscler Thromb Vasc Biol. 2015 Jun 11;35(8):1880–1888. doi: 10.1161/ATVBAHA.115.305415
Abstract
Objective
Triglyceride-rich lipoproteins (TRL) have emerged as causal risk factors for developing coronary heart disease (CHD) independent of low-density lipoprotein cholesterol (LDL-C) levels. Apolipoprotein C-III (ApoC-III) modulates TRL metabolism through inhibition of lipoprotein lipase and hepatic uptake of TRL. Mutations causing loss-of-function of ApoC-III lower TG and reduce CHD risk, suggestive of a causal role for ApoC-III. Little data exist regarding the relationship of ApoC-III, TG, and atherosclerosis in type 2 diabetes mellitus (T2DM) patients. Here, we examined the relationships between plasma ApoC-III, TG and coronary artery calcification (CAC) in T2DM patients.
Approach & Results
Plasma ApoC-III levels were measured in a cross-sectional study of 1422 subjects with T2DM but without clinically manifest CHD. ApoC-III levels were positively associated with total cholesterol (Spearman r=0.36), TG (r=0.59), LDL-C (r=0.16), fasting glucose (r=0.16) and glycosylated hemoglobin (r=0.12) (P < 0.0001 for all). In age, gender, and race-adjusted analysis, ApoC-III levels were positively associated with CAC (Tobit regression ratio (TRR) 1.78, 95% CI 1.27–2.50 per SD-increase in ApoC-III, P <0.001). As expected for an intermediate mediator, these findings were attenuated when adjusted for both TG (TRR 1.43, 95% CI 0.94–2.18, P=0.086) and separately for VLDL-C (TRR 1.14, 95% ci 0.75–1.71, P=0.53).
Conclusions
In persons with T2DM, increased plasma ApoC-III is associated with higher TG, less favorable cardiometabolic phenotypes, and higher CAC, a measure of subclinical atherosclerosis. Therapeutic inhibition of ApoC-III may thus be a novel strategy for reducing plasma TRLs and cardiovascular risk in T2DM.
Keywords: triglyceride-rich lipoproteins, type 2 diabetes, coronary artery calcification, atherosclerosis, apolipoprotein
Introduction
Currently, the paradigm for the prevention and treatment of coronary heart disease (CHD) is lowering circulating low-density lipoprotein cholesterol (LDL-C) through statins and other therapies 1. While statins are highly efficacious in lowering LDL-C and vascular risk, significant residual risk remains for many. Also, many at-risk patients are intolerant to the adverse effects of these drugs. Plasma triglycerides (TG) have emerged as independent predictor of cardiovascular risk, as evidenced by multiple prospective epidemiological studies 2, 3. Recent human genetics studies have given credence to the concept that TG-rich lipoproteins (TRL) may be causally related to cardiovascular risk 4, 5. Genetic variation in several genes in the lipoprotein lipase pathway of plasma TG hydrolysis is associated with TG levels and CHD risk 6–10. Among the genes implicated in these studies is APOC3, which encodes apolipoprotein C-III (ApoC-III) 4, 6, 7.
ApoC-III is a small protein (8.8 kilodaltons) that is secreted from the liver and small intestine 11. ApoC-III circulates in the blood on very-low density lipoproteins (VLDLs), chylomicrons, and high-density lipoproteins (HDL). Animal models and biochemical studies have shown that apoC-III inhibits the turnover of plasma TG through potentially multiple synergistic mechanisms, including inhibition of lipoprotein lipase activity, delay of hepatic clearance of TG-rich lipoproteins (TRLs), and promotion of VLDL secretion 12–18. In humans, genetic loss-of-function of APOC3 is associated with lower plasma TG and a reduced risk of CHD and coronary calcification 6, 7, 19. Investigations of rare coding variants in APOC3 have shown that CHD-protective variants reduce circulating ApoC-III levels. These studies suggest that inhibition of ApoC-III may reduce vascular risk.
The molecular regulation of ApoC-III expression and circulating levels in metabolic disease states is complex. Several nutrient- and metabolite-activated hepatic transcription factors, including HNF4α, PPARα, Rev-Erbα, RORα, and FXR, may either positively or negatively regulate Apoc3 transcription in rodent hepatocytes 17, 20–27. Studies in a mouse model of insulin resistance demonstrated that Apoc3 gene expression increases in response to glucose via HNFα- and ChrEBP-mediated transcription 27. Apoc3 expression decreases with insulin or fibrate stimulation in vitro 20, 22, 26, 28. However, plasma ApoC-III levels are not correlated with plasma insulin in humans 17, 27. It has been suggested that glucose-mediated induction and insulin-mediated suppression of hepatic APOC3 expression may normally balance each other to regulate the total amount of ApoC-III secreted from the liver 17, 27. Likewise, in the insulin resistant state, the sensitivity of APOC3 expression to insulin may be lost and in the concomitant setting of hyperglycemia there may be unopposed activation of APOC3 expression and increased ApoC-III secretion on TRLs. This mechanism of perturbed TRL metabolism may modulate insulin resistance and cardiovascular risk in multifaceted ways.
The majority of studies of ApoC-III, TG, and CHD risk so far have been conducted in nondiabetic subjects. However, CHD is prevalent in patients with type 2 diabetes mellitus (T2DM) and is indeed the leading cause of death in this population 29. Insulin resistance and T2DM are characterized by alterations in TRL metabolism 30. In addition, the expression of APOC3 is regulated by both insulin and glucose 17, 20, 22, 27, 28. Thus, the relationship of ApoC-III to TRL metabolism and CHD in T2DM is of substantial importance. Here, we studied a sample of 1422 subjects with T2DM but without clinical CHD for the relationship of plasma ApoC-III levels with TG, related metabolic biomarkers, and coronary artery calcification (CAC), a measure of subclinical atherosclerosis.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Characteristic of participants
The characteristics of the study population are described in Table 1. Study participants (N=1422) were predominantly males of Caucasian descent. Subjects had a median age of 59 years at the time of enrollment. Mean plasma ApoC-III levels were 12.5 ± 10 mg/dL, with a median of 11.3 mg/dL (Figure 1). Subjects of African ancestry had lower ApoC-III levels than those of European ancestry (10.9 ± 12.4 vs. 13.5 ± 10, P < 1×10−3, Table 1 and Supplementary Table I). ApoC-III levels were significantly lower in women than men (11.8 ± 11 vs. 13 ± 10 mg/dL, P < 0.05, Table 1).
Table 1.
Characteristics of Study Participants
| All (N=1422) | Women (N=597) | Men (N=825) | |
|---|---|---|---|
| Age (years) | 59 (53–66)* | 57 (52–64) | 61 (54–67) |
| Caucasian (%) | 61 | 49.7 | 66.4 |
| African American (%) | 34.1 | 44.7 | 28 |
| Metabolic syndrome (%) | 78.3 | 83.2 | 75.8 |
| Hypertension (%) | 64.7 | 65.7 | 64.1 |
| Current tobacco use (%) | 48.2 | 39.9 | 52.9 |
| BMI (kg/m2) | 32.1 (28–36) | 33.8 (29.3–38.4) | 31.3 (28.1–34.8) |
| HbA1c | 6.7 (6.1–7.7) | 6.7 (6.1–7.7) | 6.7 (6.1–7.6) |
| Total Cholesterol(mg/dl) | 171 (149–197) | 179 (158–206) | 166 (145–192) |
| Triglycerides (mg/dl) | 115 (82–171) | 107 (79–149) | 121 (85–185) |
| HDL-C (mg/dl) | 45 (38–55) | 52 (44–63) | 42 (36–50) |
| LDL-C (mg/dl) | 95 (77–117) | 101 (82–121) | 92 (75–114) |
| ApoC-III (mg/dl) | 11.3 (8–15.6) | 10.7 (7.5–14.7) | 11.8 (8.5–16.2) |
| ApoA-I (mg/dl) | 130 (116–145) | 141 (127–160) | 123 (112–137) |
| ApoA-II (mg/dl) | 34 (30–38) | 35 (31–39) | 33 (30–37) |
| ApoB (mg/dl) | 81 (69–95) | 81 (69–96) | 80 (69–93) |
| Lp(a) (mg/dl) | 20 (8–51) | 29 (11–68) | 16 (7–46) |
| ApoE (mg/dl) | 3.9 (3.2–4.6) | 4.1 (3.5–4.9) | 3.7 (3.1–4.4) |
| Leptin (ng/ml) | 16 (8.5– 28.9) | 30.7 (20.3–42.5) | 10.5 (6.6–17.2) |
| Insulin (iuml) | 14.9 (10.5– 21.9) | 15.6 (10.9–23.6) | 14.7 (10.1–20.7) |
| hs CRP (mg/l) | 1.98 (0.8– 4.87) | 3.1 (1.3–6.6) | 1.4 (0.7–3.9) |
| CAC Score | 34 (0–289) | 7 (0–57) | 98 (3–427) |
| Medications (%) | All (N=1422) | Women (N=597) | Men (N=825) |
| Aspirin | 43.2 | 37.2 | 46.7 |
| Statins | 55.5 | 48.9 | 59.3 |
| Fibrates | 6.5 | 2.6 | 8.7 |
| Niacin Derivatives | 5.3 | 1.9 | 7.2 |
| Ezetimibe | 5.1 | 5.3 | 5 |
| Metformin | 62.5 | 62.7 | 62.4 |
| Sulfonylureas | 35.1 | 26.7 | 39.9 |
| Thiazolidinediones (TZDs) | 22.7 | 20.3 | 24.1 |
| Insulin | 21.7 | 24.1 | 20.3 |
| Exenatide | 4.1 | 7.2 | 2.3 |
| Sitagliptan | 4.2 | 4.7 | 3.9 |
Figure 1. Distribution of Plasma ApoC-III Levels in Study Participants.
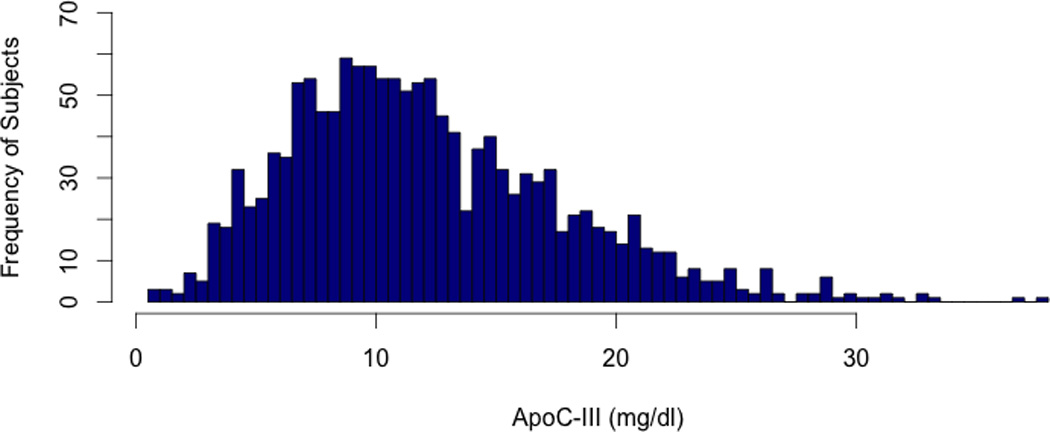
Plasma ApoC-III levels were measured by an immunoturbmidimetric assay as mentioned in the Materials & Methods section. ApoC-III levels were measured in a total of 1422 subjects.
Association of ApoC-III levels with lipid-related traits
We found a significant positive association of ApoC-III levels with TG (Spearman correlation coefficient r = 0.59, P < 1×10−4, Table 2). This association remained significant even after adjusting for age, gender, race, BMI, alcohol use, GFR, exercise, use of lipid-lowering and hypoglycemic medications in a linear regression analysis model (β=0.57, P < 1×10−4, Table 3). In a multivariate linear regression model stratified by gender, we found that TG levels were significantly associated with ApoC-III levels in both genders (Women: β=0.53, P < 1×10−4, Men: β=0.60, P < 1×10−4, Table 3).
Table 2.
Correlation of ApoC-III levels with plasma lipids and cardiometabolic phenotypes
| Measure | All (N=1422) | Women (N=597) | Men (N=825) | |||
|---|---|---|---|---|---|---|
| Spearmancoefficient (r) | P-value | Spearmancoefficient (r) | P-value | Spearmancoefficient (r) | P-value | |
| Total Cholesterol | 0.36 | P<1×10−5 | 0.38 | P<1×10−5 | 0.4 | P<1×10−5 |
| TG* | 0.59 | P<1×10−4 | 0.55 | P<1×10−5 | 0.59 | P<1×10−5 |
| LDL-C | 0.16 | P<1×10−5 | 0.2 | P<1×10−5 | 0.16 | P<1×10−5 |
| HDL-C | −0.06 | P<0.05 | 0.05 | P=0.15 | −0.08 | P<0.05 |
| ApoA-I | −0.16 | P<1×10−5 | −0.07 | P=0.07 | −0.16 | P<1×10−5 |
| ApoA-II | 0.004 | P=0.86 | 0.04 | P=0.25 | −0.009 | P=0.79 |
| ApoB | 0.34 | P<1×10−5 | 0.33 | P<1×10−5 | 0.37 | P<1×10−5 |
| ApoE | 0.25 | P<1×10−5 | 0.24 | P<1×10−5 | 0.3 | P<1×10−5 |
| BMI | −0.01 | P=0.7 | −0.03 | P=0.33 | 0.05 | P=0.14 |
| HbA1c | 0.12 | P<1×10−5 | 0.09 | P=0.02 | 0.15 | P<1×10−5 |
| Glucose | 0.16 | P<1×10−5 | 0.08 | P=0.02 | 0.2 | P<1×10−5 |
| Waist Circumference | 0.05 | 0.05 | 0.01 | P=0.70 | 0.07 | P=0.03 |
Table 3.
Association of ApoC-III with Plasma TG
| Covariates | All (N=1422)β* (P-value) | Women (N=597)β* (P-value) | Men (N=825)β* (P-value) |
|---|---|---|---|
| Unadjusted | 0.59 (1×10−4) | 0.53 (1×10−4) | 0.63 (1×10−4) |
| Age, Gender, Race | 0.58 (1×10−4) | 0.50 (1×10−4) | 0.63 (1×10−4) |
| BMI, Alcohol use, GFR, Smoking | 0.58 (1×10−4) | 0.52 (1×10−4) | 0.62 (1×10−4) |
| Medications ** | 0.57 (1×10−4) | 0.53 (1×10−4) | 0.60 (1×10−4) |
We found a positive correlation of ApoC-III levels with plasma levels of total cholesterol (Spearman correlation coefficient r = 0.36, P < 1×10−5), LDL-C (Spearman correlation coefficient r = 0.16, P < 1×10−5), ApoB (Spearman correlation coefficient r = 0.34, P < 1×10−5), and ApoE (Spearman correlation coefficient r = 0.25, P < 1×10−5, Table 2). These findings were significant after adjusting for age, gender, race, BMI, smoking, alcohol use, and use of lipid-lowering and hypoglycemic medications in linear regression (Supplementary Table II). Plasma ApoC-III levels were inversely correlated with HDL-C (Spearman correlation coefficient r = −0.06, P <0.05) and ApoA-I levels (Spearman correlation coefficient r = −0.16, P <1×10−5, Table 2). Following adjustment for age, gender, and race, the association between ApoC-III levels with HDL-C attenuated but the association with ApoA-I remained significant (Supplementary Table II).
Relationship of ApoC-III levels with glycemic and metabolic traits
Plasma ApoC-III levels correlated positively with glycosylated hemoglobin (Spearman correlation coefficient r = 0.12, P <1×10−5) and fasting glucose levels (Spearman correlation coefficient r = 0.16, P <1×10−5), two metrics of glucose homeostasis (Table 2). These associations remained significant after adjusting for age, gender and race in linear regression (Supplementary Table III). There was no association of ApoC-III levels with plasma fasting insulin. We stratified the analysis of ApoC-III levels with fasting glucose and HbA1c by plasma TG levels (Supplementary Table III) and found that the association was significant in subjects with elevated TG (>150 mg/dl) but not in those with normal TG (<150 mg/dl). To determine if this association might be dependent on the effect of ApoC-III on plasma TG, we examined the association of ApoC-III with both HbA1c and fasting glucose before and after adjusting for plasma TG (Table 4). There was no significant association of both HbA1c and fasting glucose with ApoC-III levels after adjusting for TG in both sexes (Women: β = −0.006, P=0.86; Men: β = 0.05, P=0.37, Table 4).
Table 4.
Association of ApoC-III with Glucose Phenotypes
| Covariates | All (N=1422)β* (P-value) | Women (N=597)β* (P-value) | Men (N=825)β* (P-value) | |
|---|---|---|---|---|
| HbA1c | Unadjusted | 0.13 (1×10−4) | 0.01 (0.81) | 0.24 (1×10−4) |
| Age, Gender, Race | 0.14 (1×10−4) | 0.01 (0.64) | 0.24 (1×10−4) | |
| BMI, Smoking, Alcohol use, GFR | 0.14 (1×10−4) | 0.026 (0.52) | 0.24 (1×10−4) | |
| Medications** | 0.14 (1×10−4) | 0.044 (0.24) | 0.23 (1×10−4) | |
| Triglycerides | 0.021 (0.51) | 0.012 (0.74) | 0.085 (0.19) | |
| Fasting Glucose | Unadjusted | 0.11 (1×10−4) | 0.01 (0.80) | 0.21 (1×10−4) |
| Age, Gender, Race | 0.12 (1×10−4) | 0.01 (0.65) | 0.21 (1×10−4) | |
| BMI, Smoking, Alcohol use, GFR | 0.12 (1×10−4) | 0.027 (0.49) | 0.2 (1×10−4) | |
| Medications** | 0.11 (1×10−4) | 0.03 (0.35) | 0.2 (1×10−4) | |
| Triglycerides | −0.006 (0.84) | −0.006 (0.86) | 0.05 (0.37) |
In gender-stratified analysis of the relationship of ApoC-III with waist circumference, we found no association in women (Spearman correlation coefficient r = 0.05, P=0.05) but a modest association with increased waist circumference in men (Spearman correlation coefficient r = 0.07, P=0.03, Table 2). However, ApoC-III levels were not significantly associated with body-mass index (BMI) in the combined sample (Spearman correlation coefficient r = −0.01, P=0.70, Table 2).
Association of apoC-III levels with coronary artery calcification (CAC)
In age, gender and race adjusted tobit conditional regression of ln (CAC +1) (Table 5), higher plasma ApoC-III was also associated with increasing CAC scores (Tobit regression ratio (TRR) 1.78, 95% CI 1.27- 2.50, P <0.001); this association remained significant after adjusting for smoking, GFR, BMI, alcohol, CRP, systolic blood pressure, history of hypertension and use of all lipid-lowering and hypoglycemic medications (TRR 1.91, 95% CI 1.32–2.74, P<0.01, Table 5). The association with CAC was attenuated, losing statistical significance, after including TG in the multivariate model (TRR 1.43, 95% CI 0.94–2.18, P = 0.086). Similarly, when VLDL-C was included in the multivariate model including demographics and all medications, the association between ApoC-III and CAC was even more attenuated (TRR 1.14, 95% CI 0.75–1.71, P-0.53). Consistent with analysis of ApoC-III as a continuous variable, Tobit regression of plasma ApoC-III quartiles showed that subjects within the highest ApoC-III quartile had a significantly greater likelihood of increased CAC when compared to subjects in the lowest ApoC-III quartile (Figure 2). These results were comparable in both the combined and sex-stratified analyses for both sexes (Table 5). Consistent with these results, the presence of any amount of CAC (CAC > 0) was significantly associated with higher ApoC-III levels relative to no CAC present when CAC presence or absence was treated as a dichotomous variable (OR = 1.33, 95% CI 1.05–1.69, P<0.05, Supplementary Table IV).
Table 5.
Association of ApoC-III with CAC Score in Multivariate Tobit Regression Model
| Covariates | All (N=1422) | Women (N=597) | Men (N=825) | |||
|---|---|---|---|---|---|---|
| TRR* (95% CI) | P-value | TRR* (95% CI) | P-value | TRR* (95% CI) | P-value | |
| Age, Race, Gender | 1.78 (1.27–2.50) | P<0.001 | 2.07 (1.03–4.17) | P <0.05 | 1.64 (1.11–2.45) | P<0.05 |
| BMI, Smoking, Alcohol Use, GFR, Medications**, CRP, Systolic Blood Pressure, Hypertension | 1.91 (1.32–2.74) | P<0.01 | 2.18 (1.08–4.41) | P<0.05 | 1.63 (1.08–2.43) | P<0.05 |
| VLDL-C | 1.14 (0.75–1.71) | P=0.53 | 1.27 (0.61–2.69) | P= 0.51 | 1.29 (0.80–2.1) | P=0.28 |
| Triglycerides*** | 1.43 (0.94–2.18) | P=0.086 | 1.4 (0.65–3.06) | P= 0.38 | 1.47 (0.91–2.38) | P=0.11 |
Figure 2. Relationship of ApoC-III to CAC Score Stratified by ApoC-III Quartile.
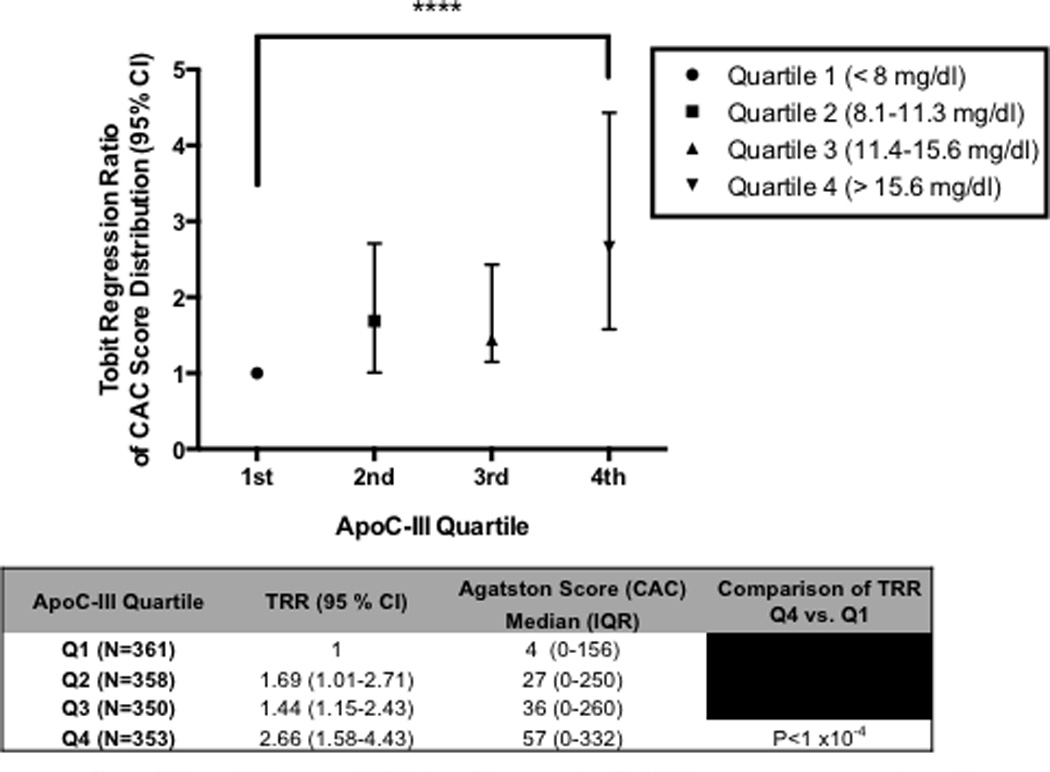
Plasma ApoC-III distribution in studied participants was separated into quartiles and CAC score distribution was measured in each quartile (Agatston CAC scores for each ApoC-III quartile given in table below the graph). Tobit regression ratios (TRR) were calculated after adjustment for age, race, gender, BMI, smoking, alcohol use, systolic blood pressure, history of hypertension, GFR, CRP, and medication use. TRR for ApoC-III quartile 4 vs. quartile 1 were compared. **** P<0.0001.
Discussion
In this study, we evaluated the relationship of plasma ApoC-III levels to plasma lipids, additional cardiometabolic phenotypes, and CAC in T2DM patients. Because insulin resistance, perturbed TRL metabolism and increased CHD risk are hallmarks of T2DM, ApoC-III may play a particularly important role in risk of heart disease in this population. As anticipated, we found strong positive relationships between plasma ApoC-III and TG, total cholesterol, ApoB and ApoE levels and a negative relationship between HDL-C and ApoA-I levels. We also found a significant positive, TG-dependent relationship between ApoC-III and fasting glucose and HbA1c. Finally, we demonstrate a robust association of ApoC-III levels with higher CAC scores and further show that this relationship is attenuated after adjustment for TG or VLDL-C, suggesting that TRLs play an intermediary role in the relationship of ApoC-III with atherosclerosis. This study represents the largest investigation to date evaluating the relationship between plasma ApoC-III levels, markers of cardiometabolic risk, and atherosclerosis in T2DM.
Here, we further advance the proposed relationship between ApoC-III, TG, and atherosclerotic burden in T2DM patients by evaluating diabetic subjects without preexisting CHD. CAC is an established pre-clinical marker and predictor of clinical CHD and future cardiac events in both the general population and in patients with T2DM 31–33. It has been previously shown that CAC scores above 100 robustly predict risk of coronary events in patients > 7-fold relative to no CAC, as per the Agatston scoring criteria for CAC, and this relationship is consistent across different ethnicities 32. We found that when we separated subjects in our study by ApoC-III quartile, the highest ApoC-III quartile had higher CAC scores than the lowest ApoC-III quartile, whose scores were largely 0. Thus, we posit that those subjects in the highest ApoC-III quartile would possess a substantially greater risk of CAD relative to those with the lowest ApoC-III levels in our study. Previously, it was suggested that the predominant predictors of CAC progression are age and baseline CAC score beyond traditional risk factors for atherosclerosis 34. Our study here did not assess the contribution of ApoC-III to the progression of CAC, yet our finding of a robust association of ApoC-III levels with CAC score after correction for multiple important demographic and pharmacological contributors warrants study of the relationship of ApoC-III to the progression of CAC over time and subsequent influence on clinically manifest vascular disease development, particularly in T2DM.
In our analysis of ApoC-III and CAC, adjustment for plasma TG levels attenuated the relationship, implying that plasma ApoC-III relates to CAC in a TG-dependent manner. This finding extends those of previous smaller studies evaluating ApoC-III levels and CHD in T2DM. Onat and colleagues showed in 857 subjects with metabolic syndrome that plasma ApoC-III levels positively correlated with multiple inflammatory biomarkers and in men were associated with increased incidence of CHD 35. Similarly, in a cohort of 188 T2DM patients, Gervaise and colleagues reported a positive relationship between both total plasma and ApoB-associated ApoC-III levels with TG and with macroangiopathy and incident CHD 36.
We also found that, like TG, VLDL-C fully attenuated the association between ApoC-III and CAC in our study. We recently showed that VLDL-C was a predictor of CAC beyond plasma TG in this study population 37. Indeed, our new findings suggest that the attenuation of the relationship between ApoC-III and CAC may have been more robust after adjusting for VLDL-C than for TG alone. This suggests that ApoC-III may mediate atherosclerotic risk most specifically by contributing to the elevation of remnant cholesterol particles, such as VLDL and chylomicron remnants. This is consistent with its known physiological role in delaying the clearance of TRL remnant particles by the liver through interplay with ApoE on these particles 13, 14, 17. Our results suggest that inhibition of ApoC-III-mediated elevation of TG and VLDL-C could reduce risk of vascular diseases in the setting of T2DM.
When taken together, our findings support decades of evidence from animal models, biochemical studies, and more recent human genetics studies showing ApoC-III as a key regulator of plasma TG and CHD. Physiological studies in proatherogenic, _APOC3_-overexpressing mice (APOC3 transgenic; Ldlr KO mice) showed that ApoC-III could promote atherogenesis in vivo38. One prior genotyping study of an APOC3 variant in the Lancaster Amish and 2 recent sequencing studies in large populations identified loss-of-function variants in APOC3 that were associated with lower plasma TG and reduced CAD risk 6, 7, 19. These 3 studies have established a direct casual genetic role for ApoC-III in mediating cardiovascular risk in humans.
Few studies have evaluated how type 2 diabetes modifies the relationship of ApoC-III to CHD risk. Crosby and colleagues attempted to evaluate this in the Framingham Heart Study and the Verona Heart Study as part of their larger exome sequencing effort, which identified APOC3 loss-of-function variants associated with disease protection 6. They tested the association of ApoC-III levels with incident CHD (Framingham Heart Study) or with cardiovascular mortality (Verona Heart Study). They tested these associations both with a model adjusting for only age and sex or a model that corrected for these in addition to diabetes, hypertension, LDL-C, HDL-C, lipid-lowering treatment and fasting glucose. For the Framingham Heart Study, association-testing using the second model with additional covariates including diabetes attenuated the positive relationship between ApoC-III and incident CHD seen when the first model was used. However, this finding was not replicated in the Verona Heart Study association analysis and inclusion of the additional covariates including diabetes status only modestly attenuated the association of ApoC-III with cardiovascular mortality. Most importantly, this analysis did not specifically test the contribution of diabetes to the association between ApoC-III and CHD as multiple other contributors to this relationship were included in the second model. Indeed, type 2 diabetes is a multifaceted state of metabolic dysregulation, which may include obesity and hypertriglyceridemia, two potential confounders to assessing whether diabetes alone impacts the relationship of ApoC-III to plasma lipids themselves or to vascular disease risk. Larger studies carefully comparing obese vs. nonobese diabetics and hypertriglycerdemic vs. normotriglycerdmic diabetics to each other as well as to nondiabetics will be crucial to definitively establish how each metabolic perturbation alters the relationship of ApoC-III to plasma lipids and CHD risk.
Prior to our study here, several smaller studies have reported that ApoC-III levels are increased with the metabolic syndrome or insulin resistance in humans. In a study of 310 T2DM patients vs. control subjects, Hiukka and colleagues found higher plasma apoC-III concentrations, higher ratios of ApoC-II-to-ApoC-III, and higher ApoC-III-to-ApoE of VLDL in T2DM than in nondiabetic subjects 39. In a study of Cherokee Native American youths, a population predisposed to insulin resistance and increased CHD risk, Blackett and colleagues found positive relationships of apoB-bound apoC-III to plasma TG and BMI 40. In insulin resistant states, higher plasma ApoC-III is robustly associated with hypertriglyceridemia. Van der Ham and colleagues reported higher plasma ApoC-III correlated with higher postprandial TG in a study of 98 men challenged to an oral fat load 41. Similarly, Lee and colleagues reported in a smaller study of 30 diabetic vs. 30 nondiabetic subjects a correlation of total plasma ApoC-III with plasma TG 42. Despite plasma ApoC-III association with glycemic traits and hypertriglyceridemia in insulin resistance, our data in a much larger T2DM sample than previously reported suggest limited association of ApoC-III with plasma adipokines or with anatomic measures of obesity (BMI and waist circumference) perhaps arguing against a primary ApoC-III modulation of adipose tissue per se.
Supporting the recent genetic studies suggesting ApoC-III loss-of-function is atheroprotective, the direct relationship of ApoC-III to CAC via its modulation of TG and VLDL-C shown by our study implicate ApoC-III as a potential therapeutic target for reduction of vascular disease risk, especially in T2DM. Currently, statins are effective therapeutics for preventing CHD in T2DM subjects, and multiple studies have reported that statins also reduce plasma ApoC-III levels, likely through promoting the clearance of TRLs as well as LDL from circulation 43. However, there remains an immense burden of CHD in patients who may already be maximally treated with statins, with approximately 1 in 7 patients treated with statins during a 5 year period exhibiting residual cardiovascular disease 44, 45. Non HDL-C is the most significant predictor of this on-statin residual vascular risk beyond LDL-C in large meta-analyses of randomized statin trials for reduction of cardiovascular events 46. There is thus a substantial need for additional synergistic therapies to reduce other causal mediators of CHD such as non HDL-C. At least one therapy targeting ApoC-III, antisense oligonucleotides silencing hepatic APOC3 expression, is in clinical development 47. Recently this therapy was shown to successfully reduce ApoC-III levels and plasma TG in 3 patients with familial chylomicronemia syndrome, a genetic cause of profoundly elevated TG due to lipoprotein lipase deficiency 48. This work provides one example of the utility of ApoC-III inhibition for reducing TG in specific clinical settings. Our results suggest there will be great interest in and need to directly evaluate the efficacy of ApoC-III-focused drugs in preventing CHD in high-risk populations particularly those insulin resistant and T2DM.
Our study has both strengths and limitations. We report here the largest evaluation of ApoC-III levels, plasma TG and other lipid and metabolic parameters, and CHD risk in T2DM subjects. Our analysis included significant proportions of non-Caucasian ethnicity and women, and we found mostly consistent results for the associations reported across these demographics, suggesting the generalizability of our findings. Limitations of our study include its cross-sectional design and lack of additional information on CHD-related outcomes in these subjects, though CAC has been demonstrated previously to be a reliable predictor of future CHD-related events in T2DM 31–33. The utility of CAC in monitoring the progression of CHD after incidence and after lipid-lowering therapy is currently being debated, and thus we could not accurately predict how ApoC-III may modulate the progression or regression of disease as measured by this surrogate metric 49. A large proportion of subjects in our study were on aspirin though they were not necessarily indicated as candidates for primary prevention of cardiovascular events. As aspirin can modulate risk of atherosclerotic disease, we did test the association of ApoC-III with CAC by Tobit regression modeling after adjusting for aspirin use and observed no attenuation in the association when it was included (data not shown). Because of limited measures and the fact that many of the subjects were on insulin therapy, we did not directly measure homeostatic models of insulin resistance (HOMA-IR) in our patient population, a measure that may have supported our findings of the relationship of ApoC-III to plasma glucose. However, we note consistency in the findings between measures of fasting glucose and HbA1c within our study cohort. Although we did not measure ApoC-III levels in specific lipoprotein subclasses, we show here that total plasma apoC-III, an easily measurable biomarker in the clinical setting, correlates with plasma TG and apoB in ways that previous studies of lipoprotein-associated apoC-III levels have suggested while total plasma apoC-III also associated positively with CAC, a predictor of CHD.
As evidence builds from human genetics and early clinical studies regarding the value of lowering apoC-III to reduce cardiovascular risk, it is critical to identify specific high-risk patient populations who would benefit most from apoC-III-targeted therapies. Here, we demonstrate convincingly that in T2DM associates with elevated cardiovascular risk factors and greater subclinical atherosclerosis in a manner related to their ApoC-III levels. Thus, therapeutic targeting of apoC-III in T2DM may provide a powerful tool for reducing the high residual risk of atherosclerotic CHD in this population.
Supplementary Material
Materials and Methods
Supplementary Tables
Significance.
Strong evidence from epidemiology, human genetics, and animal physiological studies support a role for the protein ApoC-III in promoting risk of coronary heart disease through increasing plasma triglycerides. The relationship of ApoC-III to risk of heart disease in type 2 diabetics, a population already at elevated risk of heart attacks, has been studied limitedly. In the largest cross-sectional study of blood ApoC-III levels in type 2 diabetics to date, we measure the relationship of ApoC-III to plasma lipids, glucose and metabolic traits, and coronary artery calcification (CAC), a noninvasive surrogate measure of atherosclerosis. We find a positive relationship between ApoC-III levels to triglycerides and CAC in T2DM patients, supporting a role for ApoC-III inhibition in these patients as a means to reduce vascular risk.
Acknowledgements
Sources of Funding
This work and related research was supported by K24 HL107643 to MPR and R37 HL055323 to DJR. MPR is also supported by R01 HL113147, R01 HL111694, R01 DK090505 and U01 HL108636. SAK is supported in by the University of Pennsylvania MSTP program and by the F30 HL124967 fellowship from the NIH.
Abbreviations
CHD
Coronary heart disease
LDL-C
Low-density lipoprotein cholesterol
TG
Triglycerides
TRLs
TG-rich lipoproteins
VLDL-C
Very-low density lipoprotein cholesterol
HDL-C
High-density lipoprotein cholesterol
CAC
Coronary artery calcium
T2DM
Type 2 diabetes mellitus
Footnotes
References
- 1.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 acc/aha guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 2.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. Jama. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 3.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 western prospective studies. Circulation. 2007;115:450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 4.Do R, Willer CJ, Schmidt EM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45:1345–1352. doi: 10.1038/ng.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenson RS, Davidson MH, Hirsh BJ, Kathiresan S, Gaudet D. Genetics and causality of triglyceride-rich lipoproteins in atherosclerotic cardiovascular disease. Journal of the American College of Cardiology. 2014;64:2525–2540. doi: 10.1016/j.jacc.2014.09.042. [DOI] [PubMed] [Google Scholar]
- 6.Tg, Hdl Working Group of the Exome Sequencing Project NHL. Blood I, Crosby J, Peloso GM, et al. Loss-of-function mutations in apoc3, triglycerides, and coronary disease. The New England journal of medicine. 2014;371:22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Loss-of-function mutations in apoc3 and risk of ischemic vascular disease. The New England journal of medicine. 2014;371:32–41. doi: 10.1056/NEJMoa1308027. [DOI] [PubMed] [Google Scholar]
- 8.Do R, Stitziel NO, Won H, et al. Exome sequencing identifies rare ldlr and apoa5 alleles conferring risk for myocardial infarction. Nature. 2014 doi: 10.1038/nature13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rip J, Nierman MC, Ross CJ, Jukema JW, Hayden MR, Kastelein JJ, Stroes ES, Kuivenhoven JA. Lipoprotein lipase s447x: A naturally occurring gain-of-function mutation. Arterioscler Thromb Vasc Biol. 2006;26:1236–1245. doi: 10.1161/01.ATV.0000219283.10832.43. [DOI] [PubMed] [Google Scholar]
- 10.Kastelein JJ, Ordovas JM, Wittekoek ME, Pimstone SN, Wilson WF, Gagne SE, Larson MG, Schaefer EJ, Boer JM, Gerdes C, Hayden MR. Two common mutations (d9n, n291s) in lipoprotein lipase: A cumulative analysis of their influence on plasma lipids and lipoproteins in men and women. Clinical genetics. 1999;56:297–305. doi: 10.1034/j.1399-0004.1999.560407.x. [DOI] [PubMed] [Google Scholar]
- 11.Ooi EM, Barrett PH, Chan DC, Watts GF. Apolipoprotein c-iii: Understanding an emerging cardiovascular risk factor. Clin Sci (Lond) 2008;114:611–624. doi: 10.1042/CS20070308. [DOI] [PubMed] [Google Scholar]
- 12.Eisenberg S, Patsch JR, Sparrow JT, Gotto AM, Olivecrona T. Very low density lipoprotein. Removal of apolipoproteins c-ii and c-iii-1 during lipolysis in vitro. J Biol Chem. 1979;254:12603–12608. [PubMed] [Google Scholar]
- 13.Aalto-Setala K, Fisher EA, Chen X, Chajek-Shaul T, Hayek T, Zechner R, Walsh A, Ramakrishnan R, Ginsberg HN, Breslow JL. Mechanism of hypertriglyceridemia in human apolipoprotein (apo) ciii transgenic mice. Diminished very low density lipoprotein fractional catabolic rate associated with increased apo ciii and reduced apo e on the particles. J Clin Invest. 1992;90:1889–1900. doi: 10.1172/JCI116066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aalto-Setala K, Weinstock PH, Bisgaier CL, Wu L, Smith JD, Breslow JL. Further characterization of the metabolic properties of triglyceride-rich lipoproteins from human and mouse apoc-iii transgenic mice. J Lipid Res. 1996;37:1802–1811. [PubMed] [Google Scholar]
- 15.Ebara T, Ramakrishnan R, Steiner G, Shachter NS. Chylomicronemia due to apolipoprotein ciii overexpression in apolipoprotein e-null mice. Apolipoprotein ciii-induced hypertriglyceridemia is not mediated by effects on apolipoprotein e. J Clin Invest. 1997;99:2672–2681. doi: 10.1172/JCI119456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H, Talmud PJ, Lins L, Brasseur R, Olivecrona G, Peelman F, Vandekerckhove J, Rosseneu M, Labeur C. Characterization of recombinant wild type and site-directed mutations of apolipoprotein c-iii: Lipid binding, displacement of apoe, and inhibition of lipoprotein lipase. Biochemistry. 2000;39:9201–9212. doi: 10.1021/bi0009441. [DOI] [PubMed] [Google Scholar]
- 17.Ginsberg HN, Brown WV. Apolipoprotein ciii: 42 years old and even more interesting. Arterioscler Thromb Vasc Biol. 2011;31:471–473. doi: 10.1161/ATVBAHA.110.221846. [DOI] [PubMed] [Google Scholar]
- 18.Larsson M, Vorrsjo E, Talmud P, Lookene A, Olivecrona G. Apolipoproteins c-i and c-iii inhibit lipoprotein lipase activity by displacement of the enzyme from lipid droplets. J Biol Chem. 2013;288:33997–34008. doi: 10.1074/jbc.M113.495366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB, Post W, McLenithan JC, Bielak LF, Peyser PA, Mitchell BD, Miller M, O'Connell JR, Shuldiner AR. A null mutation in human apoc3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322:1702–1705. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen M, Breslow JL, Li W, Leff T. Transcriptional regulation of the apoc-iii gene by insulin in diabetic mice: Correlation with changes in plasma triglyceride levels. J Lipid Res. 1994;35:1918–1924. [PubMed] [Google Scholar]
- 21.Reaven GM, Mondon CE, Chen YD, Breslow JL. Hypertriglyceridemic mice transgenic for the human apolipoprotein c-iii gene are neither insulin resistant nor hyperinsulinemic. J Lipid Res. 1994;35:820–824. [PubMed] [Google Scholar]
- 22.Altomonte J, Cong L, Harbaran S, Richter A, Xu J, Meseck M, Dong HH. Foxo1 mediates insulin action on apoc-iii and triglyceride metabolism. J Clin Invest. 2004;114:1493–1503. doi: 10.1172/JCI19992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claudel T, Inoue Y, Barbier O, Duran-Sandoval D, Kosykh V, Fruchart J, Fruchart JC, Gonzalez FJ, Staels B. Farnesoid × receptor agonists suppress hepatic apolipoprotein ciii expression. Gastroenterology. 2003;125:544–555. doi: 10.1016/s0016-5085(03)00896-5. [DOI] [PubMed] [Google Scholar]
- 24.Barbier O, Torra IP, Sirvent A, Claudel T, Blanquart C, Duran-Sandoval D, Kuipers F, Kosykh V, Fruchart JC, Staels B. Fxr induces the ugt2b4 enzyme in hepatocytes: A potential mechanism of negative feedback control of fxr activity. Gastroenterology. 2003;124:1926–1940. doi: 10.1016/s0016-5085(03)00388-3. [DOI] [PubMed] [Google Scholar]
- 25.Coste H, Rodriguez JC. Orphan nuclear hormone receptor rev-erbalpha regulates the human apolipoprotein ciii promoter. J Biol Chem. 2002;277:27120–27129. doi: 10.1074/jbc.M203421200. [DOI] [PubMed] [Google Scholar]
- 26.Lalloyer F, Wouters K, Baron M, Caron S, Vallez E, Vanhoutte J, Bauge E, Shiri-Sverdlov R, Hofker M, Staels B, Tailleux A. Peroxisome proliferator-activated receptor-alpha gene level differently affects lipid metabolism and inflammation in apolipoprotein e2 knock-in mice. Arterioscler Thromb Vasc Biol. 2011;31:1573–1579. doi: 10.1161/ATVBAHA.110.220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caron S, Verrijken A, Mertens I, et al. Transcriptional activation of apolipoprotein ciii expression by glucose may contribute to diabetic dyslipidemia. Arterioscler Thromb Vasc Biol. 2011;31:513–519. doi: 10.1161/ATVBAHA.110.220723. [DOI] [PubMed] [Google Scholar]
- 28.Li WW, Dammerman MM, Smith JD, Metzger S, Breslow JL, Leff T. Common genetic variation in the promoter of the human apo ciii gene abolishes regulation by insulin and may contribute to hypertriglyceridemia. J Clin Invest. 1995;96:2601–2605. doi: 10.1172/JCI118324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen MK, Bertoia ML, Cahill LE, Agarwal I, Rimm EB, Mukamal KJ. Novel metabolic biomarkers of cardiovascular disease. Nature reviews. Endocrinology. 2014 doi: 10.1038/nrendo.2014.155. [DOI] [PubMed] [Google Scholar]
- 30.Haas ME, Attie AD, Biddinger SB. The regulation of apob metabolism by insulin. Trends in endocrinology and metabolism: TEM. 2013;24:391–397. doi: 10.1016/j.tem.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. Journal of the American College of Cardiology. 2004;43:1663–1669. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 32.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. The New England journal of medicine. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 33.Kondos GT, Hoff JA, Sevrukov A, Daviglus ML, Garside DB, Devries SS, Chomka EV, Liu K. Electron-beam tomography coronary artery calcium and cardiac events: A 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107:2571–2576. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 34.Erbel R, Lehmann N, Churzidse S, et al. Progression of coronary artery calcification seems to be inevitable, but predictable - results of the heinz nixdorf recall (hnr) study. European heart journal. 2014;35:2960–2971. doi: 10.1093/eurheartj/ehu288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onat A, Hergenc G, Sansoy V, Fobker M, Ceyhan K, Toprak S, Assmann G. Apolipoprotein c-iii, a strong discriminant of coronary risk in men and a determinant of the metabolic syndrome in both genders. Atherosclerosis. 2003;168:81–89. doi: 10.1016/s0021-9150(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 36.Gervaise N, Garrigue MA, Lasfargues G, Lecomte P. Triglycerides, apo c3 and lp b:C3 and cardiovascular risk in type ii diabetes. Diabetologia. 2000;43:703–708. doi: 10.1007/s001250051366. [DOI] [PubMed] [Google Scholar]
- 37.Prenner SB, Mulvey CK, Ferguson JF, Rickels MR, Bhatt AB, Reilly MP. Very low density lipoprotein cholesterol associates with coronary artery calcification in type 2 diabetes beyond circulating levels of triglycerides. Atherosclerosis. 2014;236:244–250. doi: 10.1016/j.atherosclerosis.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masucci-Magoulas L, Goldberg IJ, Bisgaier CL, Serajuddin H, Francone OL, Breslow JL, Tall AR. A mouse model with features of familial combined hyperlipidemia. Science. 1997;275:391–394. doi: 10.1126/science.275.5298.391. [DOI] [PubMed] [Google Scholar]
- 39.Hiukka A, Fruchart-Najib J, Leinonen E, Hilden H, Fruchart JC, Taskinen MR. Alterations of lipids and apolipoprotein ciii in very low density lipoprotein subspecies in type 2 diabetes. Diabetologia. 2005;48:1207–1215. doi: 10.1007/s00125-005-1753-z. [DOI] [PubMed] [Google Scholar]
- 40.Blackett PR, Blevins KS, Quintana E, Stoddart M, Wang W, Alaupovic P, Lee ET. Apoc-iii bound to apob-containing lipoproteins increase with insulin resistance in cherokee indian youth. Metabolism: clinical and experimental. 2005;54:180–187. doi: 10.1016/j.metabol.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 41.van der Ham RL, Alizadeh Dehnavi R, Berbee JF, Putter H, de Roos A, Romijn JA, Rensen PC, Tamsma JT. Plasma apolipoprotein ci and ciii levels are associated with increased plasma triglyceride levels and decreased fat mass in men with the metabolic syndrome. Diabetes care. 2009;32:184–186. doi: 10.2337/dc08-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee SJ, Moye LA, Campos H, Williams GH, Sacks FM. Hypertriglyceridemia but not diabetes status is associated with vldl containing apolipoprotein ciii in patients with coronary heart disease. Atherosclerosis. 2003;167:293–302. doi: 10.1016/s0021-9150(03)00007-8. [DOI] [PubMed] [Google Scholar]
- 43.Dallinga-Thie GM, Berk P, II, Bootsma AH, Jansen H Diabetes Atorvastatin Lipid intervention Study G. Atorvastatin decreases apolipoprotein c-iii in apolipoprotein b-containing lipoprotein and hdl in type 2 diabetes: A potential mechanism to lower plasma triglycerides. Diabetes care. 2004;27:1358–1364. doi: 10.2337/diacare.27.6.1358. [DOI] [PubMed] [Google Scholar]
- 44.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R Cholesterol Treatment Trialists C. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 45.Cholesterol Treatment Trialists C. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of ldl cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boekholdt SM, Arsenault BJ, Mora S, et al. Association of ldl cholesterol, non-hdl cholesterol, and apolipoprotein b levels with risk of cardiovascular events among patients treated with statins: A meta-analysis. Jama. 2012;307:1302–1309. doi: 10.1001/jama.2012.366. [DOI] [PubMed] [Google Scholar]
- 47.Graham MJ, Lee RG, Bell TA, 3rd, Fu W, Mullick AE, Alexander VJ, Singleton W, Viney N, Geary R, Su J, Baker BF, Burkey J, Crooke ST, Crooke RM. Antisense oligonucleotide inhibition of apolipoprotein c-iii reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circulation research. 2013;112:1479–1490. doi: 10.1161/CIRCRESAHA.111.300367. [DOI] [PubMed] [Google Scholar]
- 48.Gaudet D, Brisson D, Tremblay K, Alexander VJ, Singleton W, Hughes SG, Geary RS, Baker BF, Graham MJ, Crooke RM, Witztum JL. Targeting apoc3 in the familial chylomicronemia syndrome. The New England journal of medicine. 2014;371:2200–2206. doi: 10.1056/NEJMoa1400284. [DOI] [PubMed] [Google Scholar]
- 49.Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR, Tuzcu EM, Nissen SE. Impact of statins on serial coronary calcification during atheroma progression and regression. Journal of the American College of Cardiology. 2015;65:1273–1282. doi: 10.1016/j.jacc.2015.01.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and Methods
Supplementary Tables