The multidrug resistance (mdr1) gene product functions as an ATP channel (original) (raw)
Abstract
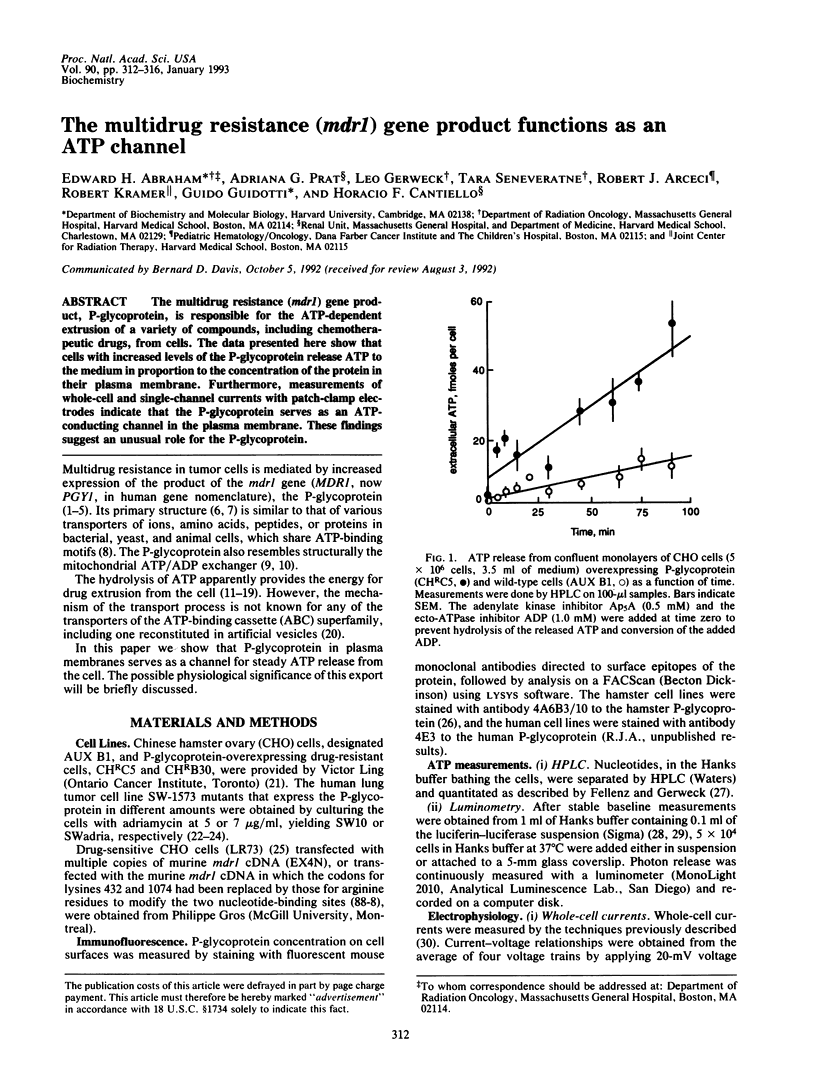
The multidrug resistance (mdr1) gene product, P-glycoprotein, is responsible for the ATP-dependent extrusion of a variety of compounds, including chemotherapeutic drugs, from cells. The data presented here show that cells with increased levels of the P-glycoprotein release ATP to the medium in proportion to the concentration of the protein in their plasma membrane. Furthermore, measurements of whole-cell and single-channel currents with patch-clamp electrodes indicate that the P-glycoprotein serves as an ATP-conducting channel in the plasma membrane. These findings suggest an unusual role for the P-glycoprotein.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzaria M., Schurr E., Gros P. Discrete mutations introduced in the predicted nucleotide-binding sites of the mdr1 gene abolish its ability to confer multidrug resistance. Mol Cell Biol. 1989 Dec;9(12):5289–5297. doi: 10.1128/mcb.9.12.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas F., Borst P. The tissue dependent expression of hamster P-glycoprotein genes. FEBS Lett. 1988 Mar 14;229(2):329–332. doi: 10.1016/0014-5793(88)81150-5. [DOI] [PubMed] [Google Scholar]
- Betcher S. L., Forrest J. N., Jr, Knickelbein R. G., Dobbins J. W. Sodium-adenosine cotransport in brush-border membranes from rabbit ileum. Am J Physiol. 1990 Sep;259(3 Pt 1):G504–G510. doi: 10.1152/ajpgi.1990.259.3.G504. [DOI] [PubMed] [Google Scholar]
- Bishop L., Agbayani R., Jr, Ambudkar S. V., Maloney P. C., Ames G. F. Reconstitution of a bacterial periplasmic permease in proteoliposomes and demonstration of ATP hydrolysis concomitant with transport. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6953–6957. doi: 10.1073/pnas.86.18.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury N. A., Jilling T., Berta G., Sorscher E. J., Bridges R. J., Kirk K. L. Regulation of plasma membrane recycling by CFTR. Science. 1992 Apr 24;256(5056):530–532. doi: 10.1126/science.1373908. [DOI] [PubMed] [Google Scholar]
- Broxterman H. J., Pinedo H. M., Kuiper C. M., Kaptein L. C., Schuurhuis G. J., Lankelma J. Induction by verapamil of a rapid increase in ATP consumption in multidrug-resistant tumor cells. FASEB J. 1988 Apr;2(7):2278–2282. doi: 10.1096/fasebj.2.7.3350243. [DOI] [PubMed] [Google Scholar]
- Broxterman H. J., Pinedo H. M., Kuiper C. M., van der Hoeven J. J., de Lange P., Quak J. J., Scheper R. J., Keizer H. G., Schuurhuis G. J., Lankelma J. Immunohistochemical detection of P-glycoprotein in human tumor cells with a low degree of drug resistance. Int J Cancer. 1989 Feb 15;43(2):340–343. doi: 10.1002/ijc.2910430229. [DOI] [PubMed] [Google Scholar]
- Brunton L. L., Mayer S. E. Extrusion of cyclic AMP from pigeon erythrocytes. J Biol Chem. 1979 Oct 10;254(19):9714–9720. [PubMed] [Google Scholar]
- Cantiello H. F., Patenaude C. R., Codina J., Birnbaumer L., Ausiello D. A. G alpha i-3 regulates epithelial Na+ channels by activation of phospholipase A2 and lipoxygenase pathways. J Biol Chem. 1990 Dec 15;265(35):21624–21628. [PubMed] [Google Scholar]
- Chan H. S., Haddad G., Thorner P. S., DeBoer G., Lin Y. P., Ondrusek N., Yeger H., Ling V. P-glycoprotein expression as a predictor of the outcome of therapy for neuroblastoma. N Engl J Med. 1991 Dec 5;325(23):1608–1614. doi: 10.1056/NEJM199112053252304. [DOI] [PubMed] [Google Scholar]
- Che M., Nishida T., Gatmaitan Z., Arias I. M. A nucleoside transporter is functionally linked to ectonucleotidases in rat liver canalicular membrane. J Biol Chem. 1992 May 15;267(14):9684–9688. [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Clairmont C. A., De Maio A., Hirschberg C. B. Translocation of ATP into the lumen of rough endoplasmic reticulum-derived vesicles and its binding to luminal proteins including BiP (GRP 78) and GRP 94. J Biol Chem. 1992 Feb 25;267(6):3983–3990. [PubMed] [Google Scholar]
- Cornwell M. M., Tsuruo T., Gottesman M. M., Pastan I. ATP-binding properties of P glycoprotein from multidrug-resistant KB cells. FASEB J. 1987 Jul;1(1):51–54. doi: 10.1096/fasebj.1.1.2886389. [DOI] [PubMed] [Google Scholar]
- Croop J. M., Raymond M., Haber D., Devault A., Arceci R. J., Gros P., Housman D. E. The three mouse multidrug resistance (mdr) genes are expressed in a tissue-specific manner in normal mouse tissues. Mol Cell Biol. 1989 Mar;9(3):1346–1350. doi: 10.1128/mcb.9.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierks T., Salentin A., Heberger C., Krämer R. The mitochondrial aspartate/glutamate and ADP/ATP carrier switch from obligate counterexchange to unidirectional transport after modification by SH-reagents. Biochim Biophys Acta. 1990 Oct 19;1028(3):268–280. doi: 10.1016/0005-2736(90)90176-o. [DOI] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Fellenz M. P., Gerweck L. E. Influence of extracellular pH on intracellular pH and cell energy status: relationship to hyperthermic sensitivity. Radiat Res. 1988 Nov;116(2):305–312. [PubMed] [Google Scholar]
- Gerisch G. Cyclic AMP and other signals controlling cell development and differentiation in Dictyostelium. Annu Rev Biochem. 1987;56:853–879. doi: 10.1146/annurev.bi.56.070187.004225. [DOI] [PubMed] [Google Scholar]
- Gill D. R., Hyde S. C., Higgins C. F., Valverde M. A., Mintenig G. M., Sepúlveda F. V. Separation of drug transport and chloride channel functions of the human multidrug resistance P-glycoprotein. Cell. 1992 Oct 2;71(1):23–32. doi: 10.1016/0092-8674(92)90263-c. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. The multidrug transporter, a double-edged sword. J Biol Chem. 1988 Sep 5;263(25):12163–12166. [PubMed] [Google Scholar]
- Gros P., Croop J., Housman D. Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell. 1986 Nov 7;47(3):371–380. doi: 10.1016/0092-8674(86)90594-5. [DOI] [PubMed] [Google Scholar]
- Gros P., Talbot F., Tang-Wai D., Bibi E., Kaback H. R. Lipophilic cations: a group of model substrates for the multidrug-resistance transporter. Biochemistry. 1992 Feb 25;31(7):1992–1998. doi: 10.1021/bi00122a014. [DOI] [PubMed] [Google Scholar]
- Hamada H., Tsuruo T. Purification of the 170- to 180-kilodalton membrane glycoprotein associated with multidrug resistance. 170- to 180-kilodalton membrane glycoprotein is an ATPase. J Biol Chem. 1988 Jan 25;263(3):1454–1458. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Gottesman M. M. Is the multidrug transporter a flippase? Trends Biochem Sci. 1992 Jan;17(1):18–21. doi: 10.1016/0968-0004(92)90419-a. [DOI] [PubMed] [Google Scholar]
- Horio M., Gottesman M. M., Pastan I. ATP-dependent transport of vinblastine in vesicles from human multidrug-resistant cells. Proc Natl Acad Sci U S A. 1988 May;85(10):3580–3584. doi: 10.1073/pnas.85.10.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde S. C., Emsley P., Hartshorn M. J., Mimmack M. M., Gileadi U., Pearce S. R., Gallagher M. P., Gill D. R., Hubbard R. E., Higgins C. F. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990 Jul 26;346(6282):362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- Jabs C. M., Ferrell W. J., Robb H. J. Microdetermination of plasma ATP and creatine phosphate concentrations with a luminescence biometer. Clin Chem. 1977 Dec;23(12):2254–2257. [PubMed] [Google Scholar]
- Juranka P. F., Zastawny R. L., Ling V. P-glycoprotein: multidrug-resistance and a superfamily of membrane-associated transport proteins. FASEB J. 1989 Dec;3(14):2583–2592. doi: 10.1096/fasebj.3.14.2574119. [DOI] [PubMed] [Google Scholar]
- Kamimoto Y., Gatmaitan Z., Hsu J., Arias I. M. The function of Gp170, the multidrug resistance gene product, in rat liver canalicular membrane vesicles. J Biol Chem. 1989 Jul 15;264(20):11693–11698. [PubMed] [Google Scholar]
- Keizer H. G., Schuurhuis G. J., Broxterman H. J., Lankelma J., Schoonen W. G., van Rijn J., Pinedo H. M., Joenje H. Correlation of multidrug resistance with decreased drug accumulation, altered subcellular drug distribution, and increased P-glycoprotein expression in cultured SW-1573 human lung tumor cells. Cancer Res. 1989 Jun 1;49(11):2988–2993. [PubMed] [Google Scholar]
- Krupinski J., Coussen F., Bakalyar H. A., Tang W. J., Feinstein P. G., Orth K., Slaughter C., Reed R. R., Gilman A. G. Adenylyl cyclase amino acid sequence: possible channel- or transporter-like structure. Science. 1989 Jun 30;244(4912):1558–1564. doi: 10.1126/science.2472670. [DOI] [PubMed] [Google Scholar]
- Lin S. H., Guidotti G. Cloning and expression of a cDNA coding for a rat liver plasma membrane ecto-ATPase. The primary structure of the ecto-ATPase is similar to that of the human biliary glycoprotein I. J Biol Chem. 1989 Aug 25;264(24):14408–14414. [PubMed] [Google Scholar]
- Lin S. H. Localization of the ecto-ATPase (ecto-nucleotidase) in the rat hepatocyte plasma membrane. Implications for the functions of the ecto-ATPase. J Biol Chem. 1989 Aug 25;264(24):14403–14407. [PubMed] [Google Scholar]
- Ling V., Thompson L. H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol. 1974 Feb;83(1):103–116. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- Naito M., Hamada H., Tsuruo T. ATP/Mg2+-dependent binding of vincristine to the plasma membrane of multidrug-resistant K562 cells. J Biol Chem. 1988 Aug 25;263(24):11887–11891. [PubMed] [Google Scholar]
- Powell S. J., Medd S. M., Runswick M. J., Walker J. E. Two bovine genes for mitochondrial ADP/ATP translocase expressed differences in various tissues. Biochemistry. 1989 Jan 24;28(2):866–873. doi: 10.1021/bi00428a069. [DOI] [PubMed] [Google Scholar]
- Raymond M., Gros P., Whiteway M., Thomas D. Y. Functional complementation of yeast ste6 by a mammalian multidrug resistance mdr gene. Science. 1992 Apr 10;256(5054):232–234. doi: 10.1126/science.1348873. [DOI] [PubMed] [Google Scholar]
- Rojas E., Ceña V., Stutzin A., Forsberg E., Pollard H. B. Characteristics of receptor-operated and membrane potential-dependent ATP secretion from adrenal medullary chromaffin cells. Ann N Y Acad Sci. 1990;603:311–323. doi: 10.1111/j.1749-6632.1990.tb37682.x. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Price E. M., Boucher R. C., Germann U. A., Scarborough G. A. Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated membrane ATPase. J Biol Chem. 1992 Mar 5;267(7):4854–4858. [PubMed] [Google Scholar]
- Schurr E., Raymond M., Bell J. C., Gros P. Characterization of the multidrug resistance protein expressed in cell clones stably transfected with the mouse mdr1 cDNA. Cancer Res. 1989 May 15;49(10):2729–2733. [PubMed] [Google Scholar]
- Shimabuku A. M., Nishimoto T., Ueda K., Komano T. P-glycoprotein. ATP hydrolysis by the N-terminal nucleotide-binding domain. J Biol Chem. 1992 Mar 5;267(7):4308–4311. [PubMed] [Google Scholar]
- Smith M. E., Dickinson J. R., Wheals A. E. Intracellular and extracellular levels of cyclic AMP during the cell cycle of Saccharomyces cerevisiae. Yeast. 1990 Jan-Feb;6(1):53–60. doi: 10.1002/yea.320060106. [DOI] [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde M. A., Díaz M., Sepúlveda F. V., Gill D. R., Hyde S. C., Higgins C. F. Volume-regulated chloride channels associated with the human multidrug-resistance P-glycoprotein. Nature. 1992 Feb 27;355(6363):830–833. doi: 10.1038/355830a0. [DOI] [PubMed] [Google Scholar]
- Williams T. C., Jarvis S. M. Multiple sodium-dependent nucleoside transport systems in bovine renal brush-border membrane vesicles. Biochem J. 1991 Feb 15;274(Pt 1):27–33. doi: 10.1042/bj2740027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H., Apps D. K., Fischer-Colbrie R. The molecular function of adrenal chromaffin granules: established facts and unresolved topics. Neuroscience. 1986 Jun;18(2):261–290. doi: 10.1016/0306-4522(86)90154-5. [DOI] [PubMed] [Google Scholar]
- Wisher M. H., Evans W. H. Functional polarity of the rat hepatocyte surface membrane. Isolation and characterization of plasma-membrane subfractions from the blood-sinusoidal, bile-Canalicular and contiguous surfaces of the hepatocyte. Biochem J. 1975 Feb;146(2):375–388. doi: 10.1042/bj1460375. [DOI] [PMC free article] [PubMed] [Google Scholar]