Analysis of 25I-NBOMe, 25B-NBOMe, 25C-NBOMe and Other Dimethoxyphenyl-N-[(2-Methoxyphenyl) Methyl]Ethanamine Derivatives on Blotter Paper (original) (raw)
Abstract
In recent years, _N_-methoxybenzyl-methoxyphenylethylamine (NBOMe) derivatives, a class of designer hallucinogenic drugs, have become popular drugs of abuse. These drugs have been the cause of severe intoxications and even deaths. They act as 5-HT2A receptors agonists and have been reported to produce serotonin-like syndrome with bizarre behavior, severe agitation and seizures persisting for as long as 3 days. The most commonly reported derivatives are 25I-NBOMe, 25B-NBOMe and 25C-NBOMe, respectively 2-(4-_iodo_-2,5-dimethoxyphenyl)-_N_-[(2-methoxyphenyl) methyl]ethanamine, _N_-(2-methoxybenzyl)-2,5-dimethoxy-4-bromophenethylamine and _N_-(2-methoxybenzyl)-2,5-dimethoxy-4-chlorophenethylamine. Like many low dose hallucinogenic drugs these compounds are often sold on blotter paper. Three different types of commercially available blotter papers reported to contain NBOMe derivatives were obtained. These blotter papers were screened using Direct Analysis in Real Time AccuTOFTM mass spectrometry followed by confirmation and quantification by high-performance liquid chromatography triple quadrapole mass spectrometry. The major drug present on each of the three blotter products was different, 25I-NBOMe, 25C-NBOMe or 25B-NBOMe. The blotter papers were also found to have minute amounts of two or three NBOMe derivative impurities of 25H-NBOMe, 25I-NBOMe, 25C-NBOMe, 25B-NBOMe and/or 25D-NBOMe.
Introduction
Recently, a new class of “2C” serotonin 5-HT2A receptor agonists designer drugs, dimethoxyphenyl-_N_-[(2-methoxyphenyl) methyl]ethanamine (NBOMe) derivatives have become easily obtainable over the Internet which has resulted in their abuse in the USA, Europe and Asia (1). Stimulation of 5-HT2A receptors is responsible for the hallucinogenic effects of recreational drugs such as lysergic acid diethylamide (LSD) and 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (2). The terminology “2C” is an acronym coined by Alexander Shulgin for the two carbon atoms between the benzene ring and the amino group on the perceptional distorting and/or hallucinogenic phenylethylamine derivatives he synthesized (3, 4). Several of these derivatives, including 2-(4-iodo-2,5-dimethoxyphenyl)-_N_-[(2-methoxyphenyl) methyl]ethanamine (25I-NBOMe) and _N_-(2-methoxybenzyl)-2,5-dimethoxy-4-bromophenethylamine (25B-NBOMe) were first synthesized by Ralf Heim at the Free University of Berlin as part of a series of pharmacological tools to study the 5-HT2A receptor (5–7). Blotter papers containing 25I-NBOMe appeared on the designer drug market beginning in 2011 (8) and since then numerous NBOMe derivatives have been identified in blotter papers seized from the illicit drug market including: 2-(2,5-dimethoxy-4-methylphenyl)-_N_-(2-methoxybenzyl)ethanamine (25D-NBOMe), 2-(4-ethyl-2,5-dimethoxyphenyl)-_N_-(2-methoxybenzyl)ethanamine (25E-NBOMe), 2-(2,5-dimethoxy-3,4 dimethylphenyl)-_N_-(2-methoxybenzyl)ethanamine (25G-NBOMe), _N_-(2-methoxybenzyl)-2,5-dimethoxy-4-chlorophenethylamine (25C-NBOMe) and 2-(4-iodo-2,5-dimethoxyphenyl)-_N_-[(2,3-methylenedioxyphenyl) methyl]ethanamine (25I-NBMD) (9–11).
Several published abstracts and clinical case reports have described signs and symptoms of 25I-NBOMe (1, 8, 12–16), 25B-NBOMe (17–20) and 25C-NBOMe (18–21) intoxication. These reports reveal NBOMe intoxicated patients are typically young males, 14–29 years old with clinical presentations of a serotonin-like syndrome with bizarre behavior and severe agitation and seizures persisting for as long as 3 days. 25I-NBOMe intoxication has been ruled the cause of death in two cases in which the drug was detected in blood and urine (22). Quantified distribution of 25I-NBOMe in body fluids and tissues from a case of traumatic death has also been reported (23). Deemed a hazard to public health and safety, the Drug Enforcement Administration placed 25I-NBOMe, 25B-NBOMe and 25C-NBOMe into Schedule 1 of the Controlled Substances Act on 10 October 2013 (24).
Currently, the most widely abused of the many NBOMe derivatives appears to be 25I-NBOMe (25–28) which is sold as a powder or on blotter paper under the names 25I-NBOMe, “N-Bomb” and “Smiles”. Anecdotal reports indicate the powder in doses of 50–250 µg may be administered sublingually, by insufflation or applied to the buccal cavity. 25I-NBOMe blotter papers usually contain higher doses of 500–800 µg, apparently due to low bioavailability of the drug. Psychoactive drugs of abuse with very high potency having effective doses in the microgram (µg) range are often dissolved in a volatile solution and dropped or soaked on to blotter paper. The paper is often perforated into tiny squares or “tabs” which can be torn or cut apart and placed under the tongue or swallowed. Historically, LSD has been distributed on blotter paper with colorful and/or unique artwork which may serve as a trademark in the illicit drug trade. NBOMe blotter paper is similarly marked with identifying artwork and is often distributed and/or sold on the street as “blotter acid”, in reference to LSD.
We present the analysis of three different types of commercial available blotter papers reported to contain NBOMe derivatives. The blotter papers were ordered over the Internet and received before 25I-NBOMe, 25C-NBOMe or 25B-NBOMe were categorized as Schedule I according to the Federal Controlled Substances Act in November 2013. The blotters were advertised as containing 500 µg of either 25I-NBOMe, 25C-NBOMe or 25B-NBOMe. The approximate price per “hit” of blotter paper was $5 USD.
These blotter papers arrived via the mail with a customs declaration form attached to the front of the package claiming to contain a “music CD”. Upon opening the package, a Christmas music CD case was found that contained three different small bags containing blotter paper (Figure 1). The first type of blotter paper pictured part of a psychedelic sun and was labeled “25C-NBOMe”; the second type of blotter paper pictured part of a pyramid with an eye and was labeled “25I-NBOMe”; and the third type of blotter paper pictured Felix the Cat and was labeled “25B-NBOMe”. These blotter papers were screened using Direct Analysis in Real Time AccuTOFTM mass spectrometry (DART-MS) followed by confirmation and quantification by high-performance liquid chromatography triple quadrapole mass spectrometry (HPLC–MS-MS). The DART-MS method for the rapid detection of NBOMe derivatives was performed on both the blotter paper and the blotter papers dissolved in methanol. Ten other NBOMe derivatives including: (E)-2-(4-iodo-2,5-dimethoxyphenyl)-_N_-(2-methoxybenzylidene)ethanamine (25I-NBOMe imine), 25I-NBOMD, _N_-(2-fluorobenzyl)-2-(4-iodo-2,5-dimethoxyphenyl)ethanamine (25I-NBF), 25G-NBOMe, 25D-NBOMe, 2-(2,5-dimethoxyphenyl)-_N_-(2-methoxybenzylidene) ethanamine (25H-NBOMe), (E)-2-(2,5-dimethoxyphenyl)-_N_-(2-methoxybenzylidene)ethanamine (25H-NBOMe imine), 25B-NBOMe, 25C-NBOMe and 2-(2,5-dimethoxy-4-(methylthio)phenyl)-_N_-(2-methoxybenzyl)ethanamine (2CT-NBOMe) were also evaluated in methanol. Confirmation and quantification were performed on the methanol dissolved blotter papers using HPLC–MS-MS.
Figure 1.
NBOMe blotter papers as delivered.
Methods
Reagents
The primary reference materials for 25I-NBOMe, 25I-NBOMe imine, 25I-NBMD, 25I-NBF, 25G-NBOMe, 25D-NBOMe, 25H-NBOMe, 25H-NBOMe imine, 25B-NBOMe, 25C-NBOMe and 2CT-NBOMe were purchased from Cayman Chemical Company (Ann Arbor, MI, USA) as hydrochloride salts. Polyethylene glycol (PEG) was purchased from ULTRA Inc. (North Kingstown, RI, USA). Melting point tubes were obtained from Corning Incorporated (Corning, NY, USA). Certified ACS ammonium acetate, formic acid, HPLC grade methanol and deionized (DI) water were purchased from Fisher Scientific (Hanover Park, IL, USA). Medical grade nitrogen and helium were purchased from National Welders Supply Company (Richmond, VA, USA). Blotter papers containing NBOMe derivative were obtained through the Internet at future-labs.eu.
Sample and reagent preparation
NBOMe derivative standards were individually prepared at 10, 25, 50 and 100 µg/mL. A 10 µg/mL NBOMe derivative mix standard containing: 25I-NBOMe, 25I-NBOMe imine, 25I-NBMD, 25I-NBF, 25G-NBOMe, 25D-NBOMe, 25H-NBOMe, 25H-NBOMe imine, 25B-NBOMe and 25C-NBOMe was also prepared. A set of the three different blotter paper products were each added to 10 mL of methanol which was then gently mixed to extract the NBOMe derivatives. All stock standards were stored at −20°C until testing.
DART-MS analysis
The screening was performed using a DART-MS operated in positive-ion mode and controlled by Mass Center software version 1.3.4m (JEOL Inc. Tokyo, Japan). The Direct Analysis in Real Time ion source had the helium gas flow rate at 2.0 L/min, gas heater temperature of 300°C, discharge electrode needle at 4,000 V, Electrode 1 was set at 150 V and Electrode 2 at 250 V. The resolving power of the mass spectrometer was 6,000 full width at half maximum. Measurements were taken with the ion guide peak voltage of 800 V, reflectron voltage of 900 V, Orifice 1 was operated at 300°C in 20, 60 or 90 V using switching mode that created a single file for all three voltages, Orifice 2 was set at 5 V and the ring lens was set at 3 V. The measured mass range was from 40 to 1,100 Da.
Sampling for the DART-MS was performed as previously described by Steiner et al. (29). In brief, a mass spectrum of PEG with average molecular weight 600 was obtained with each data acquisition set as a reference standard to enable exact mass measurements. The PEG and methanol standards containing the NBOMe derivatives were measured by dipping the closed end of a cleaned glass melting point tube into the standard. The blotter papers were analyzed by DART-MS using two different sampling methods. The first analysis did not involve any sample preparation. The blotter paper was held with a pair of forceps and placed directly into the DART-MS gas stream. The second analysis was performed by adding each blotter paper to 10 mL of methanol and gently mixing for 1 h. The methanol solutions were then sampled by dipping the closed end of a clean glass melting point tube into the methanol containing the blotter paper. The standards and samples were then moved back in forth, or wanded, in to the DART gas stream. Each of the samples or standards was wanded two times. The signal with the greatest abundance was used for the data analysis. Data were created using an averaged, background subtracted, centroided spectrum that was calibrated to the PEG + H mass reference table. The measured [M+H]+ spectra were compared with theoretical masses of each drug or fragment produced by in-source collision-induced dissociation.
The evaluation of the NBOMe derivatives in methanol was conducted over five separate days. The NBOMe derivative standards were analyzed for limit of detection (LOD), precision and selectivity. The LOD of the DART-MS was evaluated using the 10, 25, 50 and 100 µg/mL of each NBOMe derivative standard. They were analyzed ten times using different aliquots. The intra- and interday precision for the mass accuracy was calculated from the analysis of five aliquots of each NBOMe derivative standard on three separate days for a total of 15 replicates. The selectivity of the DART-MS was determined for the NBOMe derivatives using the 10 µg/mL of NBOMe derivative mix standard. The acceptance criteria for each NBOMe derivative to be considered above LOD were met if the measured mass was within the instrument manufacturer's specification of ± 5.0 mmu of the theoretical mass. Selectivity was determined if the individual NBOMe derivatives could be distinguished in with the mixture.
HPLC–MS-MS analysis
The confirmation and quantification of the NBOMe derivatives on the blotter papers was performed using a modified previously published HPLC-MS-MS method (30). In brief, the instrument was an Applied Biosystems 3200 Q trap with a turbo V source for TurbolonSpray attached to a Shimadzu SCL HPLC system controlled by Analyst 1.4.2 software. Chromatographic separation was performed on a Restek Allure Biphenyl 5 µm 100 × 3.2 mm column (Bellefonte, PA, USA). The mobile phase consisted of A: DI Water with 10 mM ammonium acetate and 0.1% formic acid and B: methanol. The following gradient was used: 0.00–1.0 min starting at 50% B with a linear gradient to 80% B, then using linear gradient ending at 10.0 min to 70% B and finally returning at 10.1 min to 50% B. The source temperature was set at 650°C and had a curtain gas flow rate of 30 mL/min. The ionspray voltage was 5,000 V, with the ion source gases 1 and 2 at flow rates of 30 mL/min. The acquisition mode used was multiple reaction monitoring. The retention times (min), declustering potential (V), transition ions (m/z) and corresponding collection energies (eV) for all the compounds can be found in Table I. The total run time for the analytical method was 13 min.
Table I.
The HPLC–MS-MS Acquisition Parameters
| Designer drug | RT (min) | DP (V) | Trans ions (m/z) | CE (eV) |
|---|---|---|---|---|
| 25H-NBOMe | 7.45 | 45 | 302 > 121 | 26 |
| 302 > 91 | 55 | |||
| 25C-NBOMe | 7.86 | 40 | 336 > 121 | 25 |
| 336 > 91 | 58 | |||
| 25I-NBF | 7.86 | 60 | 416 > 291 | 26 |
| 416 > 109 | 65 | |||
| 25D-NBOMe | 8.36 | 45 | 316 > 121 | 26 |
| 316 > 91 | 60 | |||
| 25B-NBOMe | 8.77 | 45 | 380 > 121 | 27 |
| 380 > 91 | 65 | |||
| 2CT-NBOMe | 8.86 | 45 | 348 > 121 | 28 |
| 348 > 91 | 60 | |||
| 25I-NBMD | 9.64 | 60 | 442 > 135 | 36 |
| 442 > 77 | 90 | |||
| 25G-NBOMe | 10.08 | 42 | 330 > 121 | 27 |
| 330 > 91 | 60 | |||
| 25I-NBOMe-d3 | 10.68 | 50 | 431 > 124 | 30 |
| 431 > 92 | 75 | |||
| 25I-NBOMe | 10.77 | 55 | 428 > 121 | 30 |
| 428 > 91 | 70 |
The evaluation of the HPLC–MS-MS method was performed over 3 days. A seven-point calibration curve containing 1, 2, 5, 10, 20, 50 and 100 ng/mL of the NBOMe derivatives in methanol with 20 ng/mL added 25I-NBOMe-d3 was analyzed each day. A linear regression of the ratio of the peak area counts of NBOMe derivatives and the 25I-NBOMe-d3 internal standard (ISTD) versus concentration was used to construct the calibrations curves. Each sample batch also contained drug free control (negative control) with ISTD added, a double negative control containing neither NBOMe derivatives nor ISTD, and replicates (n = 3) of the following QC specimens prepared in methanol containing the NBOMe derivatives: limit of quantification quality control (LOQC), target concentration of 1.0 ng/mL; low control, target concentration of 3.0 ng/mL; medium control, target concentration of 30 ng/mL; high control, target concentration of 75 ng/mL. All QC samples were stored at −20°C until testing. Stock standards were also evaluated for stability by allowing aliquots of the stock standards to sit at room temperature for 6 h. These aliquots and freshly prepared stock standards were analyzed by the presented HPLC–MS-MS method. The absolute areas of aliquots of NBOMe derivatives kept at room temperature for 6 h were compared with the freshly prepared stock standards. The HPLC–MS-MS analysis of the blotter papers containing the NBOMe derivatives was performed by adding each blotter paper to 10 mL of methanol and gently mixing for 1 h. Samples were then further diluted to the linear range of the assay and analyzed by the descried HPLC–MS-MS method.
Results
DART-MS analysis
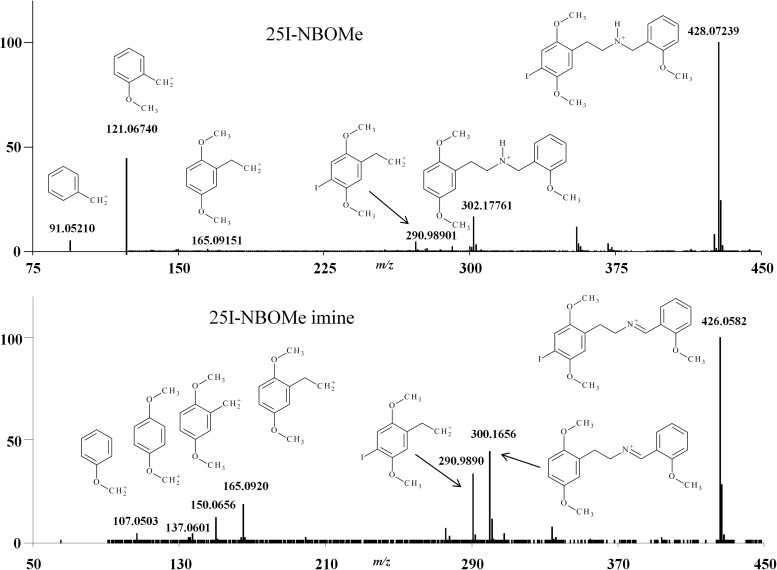
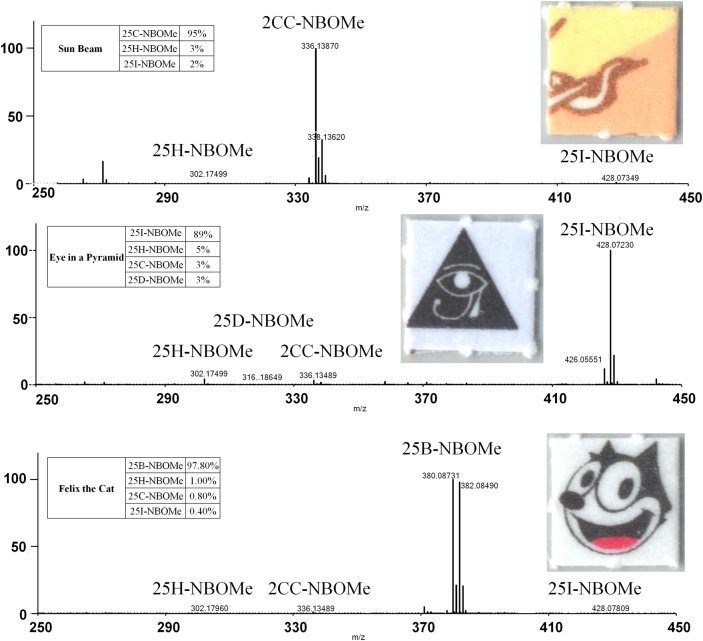
[M+H]+ ions were produced at 20 V setting for Orifice 1. Less abundant [M+H]+ ions were produced at 60 and 90 V. These voltages produced greater abundance of product ions. All of the analyzed NBOMe derivatives, with the exception of the NBOMe imines, produced the same or similar product ions. The 25H-NBOMe imine and 25I-NBOMe imine produced product ions similar to each other through the same fragmentation pattern (Figure 2). Each of the NBOMe derivatives analyzed produced [M + H]+ ions with mass accuracy of ± 5 mmu or better at the administratively set LOD of 10 µg/mL. The measured mass for all the NBOMe derivatives at 20, 60 and 90 V setting for Orifice 1 were within ± 5.0 mmu of the theoretical mass for both the inter- and intra-day precision. The selectivity of the assay was determined by the 10 µg/mL NBOMe derivative mix standard. All of the NBOMe derivatives present were distinguishable even though several of the NBOMe derivatives produced product ions with the same structure and theoretical mass. Eight of the 10 analyzed NBOMe derivatives produced product ions that were unique to that NBOMe derivative (Table II). The blotter papers and the blotter papers dissolved in methanol were analyzed by the described DART-MS method. The most abundant [M+H]+ ion peak produced with a 20 V setting was within the acceptable mass accuracy of the NBOMe derivatives. At the 60 and 90 V settings, the expected product ions were detected. As advertised, the blotter paper picturing part of a psychedelic sun was found to contain 25C-NBOMe; the second type of blotter paper picturing the pyramid with an eye was found to contain 25I-NBOMe; and the third type of blotter picturing Felix the Cat was found to contain 25B-NBOMe. These blotter papers were also determined to contain other less abundant NBOMe derivatives (Figure 3).
Figure 2.
Example of the fragmentation pattern for NBOMe derivatives using 25I-NBOMe and 25I-NBOMe imine.
Table II.
DART-MS Produced Ions of the NBOMe Derivatives
| Contributing NBOMe derivative(s) | Fragment formula | Calculated mass |
|---|---|---|
| 25I-NBMD | C18H20NO4I | 442.0515 |
| 25I-NBOMe | C18H22NO3I | 428.0723 |
| 25I-NBOMe imine | C18H20NO3I | 426.0566 |
| 25I-NBF | C17H19NO2FI | 416.0523 |
| 25B-NBOMe | C18H22BrNO3 | 380.0861 |
| 25C-NBOMe | C18H22ClNO3 | 336.1366 |
| 25G-NBOMe | C20H27NO3 | 330.2069 |
| 25D-NBOMe | C19H25NO3 | 316.1913 |
| 25H-NBOMe | C18H23NO3 | 302.1756 |
| 25B-NBOMe, 25I-NBOMe | C18H24NO3 | 302.1756 |
| 25H-NBOMe imine | C18H21NO3 | 300.16 |
| 25I-NBOMe imine | C18H22NO3 | 300.16 |
| 25I-NBF, 25I-NBMD, 25I-NBOMe, 25I-NBOMe imine | C10H12O2I | 290.9882 |
| 25B-NBOMe | C17H21NO2F | 290.1556 |
| 25C-NBOMe | C10H12BrO2 | 243.002 |
| 25G-NBOMe | C10H12ClO2 | 199.0526 |
| 25D-NBOMe | C12H17O2 | 193.1229 |
| 25H-NBOMe imine, 25I-NBF, 25H-NBOMe, 25I-NBOMe, 25I-NBOMe imine | C11H15O2 | 179.1072 |
| 25H-NBOMe imine | C10H13O2 | 165.0916 |
| 25I-NBMD | C9H11O2 | 150.0681 |
| 25H-NBOMe imine, 25I-NBOMe imine | C8H9O2 | 137.0603 |
| 25I-NBMD | C8H7O2 | 135.0446 |
| 25C-NBOMe, 25B-NBOMe, 25G-NBOMe, 25D-NBOMe,25H-NBOMe, 25I-NBOMe | C8H9O | 121.0653 |
| 25I-NBF | C7H6F | 109.0454 |
| 25H-NBOMe imine, 25I-NBOMe imine | C7H7O | 107.0497 |
| 25C-NBOMe, 25B-NBOMe, 25H-NBOMe, 25D-NBOMe, 25G-NBOMe, 25I-NBOMe | C7H7 | 91.0548 |
Figure 3.
Blotter paper DART-MS spectra at of 20 V setting for Orifice 1. This figure is available in black and white in print and in color at JAT online.
HPLC–MS-MS analysis
The linear regression correlation coefficients (_r_2) for all the NBOMe calibration curves yielded the least fit mean _r_2 of 0.9997 or better. The lower limit of quantification (LOQ) and lower LOD was administratively set at 1 ng/mL. LOQC samples were used to verify that the LOQ was within ± 10% of the target value and had a response at least 10 times greater than the signal-to-noise ratio of the methanol negative control. The precision and accuracy/bias for all nine NBOMe derivatives was determined to have %CVs that did not exceed a 7% and were within 94–104% of their expected values for all QC samples. For the assessment of carryover, none of the NBOMe derivatives were detected in the negative controls that were immediately injected after the 100 ng/mL calibrator. The absolute percent difference of the stock standards samples used to assess the stability of the NBOMe derivatives and the ISTD, 25I-NBOMe-d3 were within −6 and 5% of the expected values. Analysis of the methanol containing the blotter papers confirmed the DART-MS results. The HPLC–MS-MS analysis allowed for the quantitation of the detected NBOMe derivatives on each of the blotter papers (Table III).
Table III.
Identification and Quantification of the NBOMe Derivatives by HPLC–MS-MS
| Blotter paper | NBOMe detected | Blotter paper (µg) |
|---|---|---|
| Psychedelic sun | 25C-NBOMe | 510 |
| 25H-NBOMe | 15 | |
| 25I-NBOMe | 13 | |
| Pyramid with eye | 25I-NBOMe | 540 |
| 25H-NBOMe | 29 | |
| 25C-NBOMe | 18 | |
| 25D-NBOMe | 20 | |
| Felix the cat | 25B-NBOMe | 1,500 |
| 25H-NBOMe | 15 | |
| 25C-NBOMe | 12 | |
| 25I-NBOMe | 7 |
Discussion
Several NBOMe derivatives including 25D-NBOMe, 25E-NBOMe, 25G-NBOMe, 25C-NBOMe, 25I-NBOMe and 25C-NBOMe mixed with 25D-NBOMe have been reported as detected on blotter papers using liquid chromatographic mass spectrometry, Fourier transform spectroscopy, nuclear magnetic resonance spectroscopy and/or gas chromatography mass spectrometry methods (9, 10, 23). In contrast to these previously reported NBOMe derivatives on blotter paper, using the DART-MS method required no solvents, extractions, or sample preparation. The blotter paper was held with a pair of forceps and placed directly into the DART-MS gas stream and yielded spectra information in a few seconds. Unlike previously reported blotter paper analyses, the presented DART-MS method allows for rapid identification of the major NBOMe derivative. The DART-MS analysis of the three different blotter papers resulted in the detection of a major drug present, either 25I-NBOMe, 25C-NBOMe or 25B-NBOMe and minute amounts of two or three NBOMe derivatives as impurities, 25H-NBOMe, 25I-NBOMe, 25C-NBOMe, 25B-NBOMe and/or 25D-NBOMe. Previously published analyses of blotter papers containing NBOMe derivatives have not reported detecting trace amounts of an NBOMe derivative with a much larger concentration of a single NBOMe derivative. These impurities detected on the blotter papers are likely a result of the synthesis and/or contamination post synthesis, possibly during the production of the blotter papers. The identification of these types of impurities help explain urines values reported in the literature where a large concentration of 25I-NBOMe is reported with lower concentrations of 25C-NBOMe and/or 25H-NBOMe (8, 30) even though neither 25C-NBOMe nor 25H-NBOMe appear to be metabolites of 25I-NBOMe.
The HPLC–MS-MS analysis of the blotter papers confirmed the DART-MS results. Previously published analyses of blotter papers containing NBOMe derivatives have not reported concentrations or accuracy of the vendor-advertised concentrations. The advertised concentration of each of the blotter papers was 500 µg of 25I-NBOMe, 25C-NBOMe or 25B-NBOMe. The blotter papers were correctly labeled as to their major NBOMe derivative. The blotter paper that pictured part of a psychedelic sun was labeled “25C-NBOMe”. It was determined to be correctly labeled with the drug, containing 102% of the labeled dose and 3.3% NBOMe derivative impurities. The blotter paper that pictured a pyramid with an eye was labeled “25I-NBOMe”. It was determined to be correctly labeled with the drug, containing 108% of the labeled dose and 8.0% NBOMe derivative impurities. The blotter paper pictured Felix the Cat and was labeled “25B-NBOMe”. It was determined to be correctly labeled with the drug, containing 300% of the labeled dose and 2.2% NBOMe derivative impurities. The discrepancy in the calculated concentration versus the advertised concentrations of Felix the Cat blotter paper indicates that the advertised concentration may not be accurate and, in some cases, the concentration of the NBOMe derivative may be several times greater than expected.
Conclusion
Three different types of commercially available blotter papers were analyzed using a DART-MS and confirmed and quantitated by HPLC–MS-MS. Both methods used were able to detect and differentiate between multiple NBOMe derivatives on the same blotter paper. Each blotter paper yielded a different major NBOMe derivative, 25I-NBOMe, 25C-NBOMe or 25B-NBOMe. The blotter papers were also found to contain minute amounts of two or three NBOMe derivative impurities including 25H-NBOMe, 25I-NBOMe, 25C-NBOMe, 25B-NBOMe and/or 25D-NBOMe. The identification of these impurities may help explain reports of urine analyses which identified a large concentration of 25I-NBOMe and a much lower concentration of 25C-NBOMe and/or 25H-NBOMe. Neither 25C-NBOMe nor 25H-NBOMe appears to be metabolites of 25I-NBOMe. The discrepancy in the calculated concentration versus the advertised concentrations of Felix the Cat blotter paper indicates that the advertised concentration may not be accurate and, in some cases, the concentration of the NBOMe derivative may be several times greater than expected.
Funding
This work was supported in part by the National Institutes of Health (P30DA033934).
Conflict of interest
None declared.
Acknowledgements
The authors thank Dr Robert Cody for his helpful suggestions to improve this manuscript.
References
- 1.Dean B.V., Stellpflug S.J., Burnett S.M., Engebretsen K.M. (2013) 2C or not 2C: Phenethylamine designer drug review. Journal of Medical Toxicology, 9, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.González-Maeso J., Weisstaub N.V., Zhou M., Chan P., Ivic L., Ang R. et al. (2007) Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron, 53, 439–452. [DOI] [PubMed] [Google Scholar]
- 3.Shulgin A.T., Carter M.F. (1975) Centrally active phenethylamines. Psychopharmacol Commun, 1, 93–98. [PubMed] [Google Scholar]
- 4.Shulgin A., Shulgin A. (1991) PIHKAL: A Chemical Love Story. Transform Press, Berkeley, CA. [Google Scholar]
- 5.Heim R., Pertz H.H., Elz S. (2000) Partial 5-HT2A-receptor agonists of the phenylethanamine series: effect of a trifluoromethyl substituent. Archiv der Pharmazie – Pharmaceutical and Medicinal Chemistry, 333, 45. [Google Scholar]
- 6.Pertz H.H., Heim R., Elz S. (2000) N-Benzylated phenylethanamines are highly potent partial agonists at 5-HT2A receptors. Archiv der Pharmazie – Pharmaceutical and Medicinal Chemistry, 333, 30. [Google Scholar]
- 7.Heim R. (2013) Synthese und pharmakologie potenter 5-HT2A-rezeptoragonisten mit N-2-methoxybenzyl-partialstruktur. Entwicklung eines neuen struktur-wirkungskonzepts. Free University of Berlin, Berlin. [Google Scholar]
- 8.Stellpflug S.J., Kealey S.E., Hegarty C.B., Janis G.C. (2014) 2-(4-Iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25I-NBOMe): clinical case with unique confirmatory testing. Journal of Medical Toxicology, 10, 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuba D., Sekuła K. (2013) Analytical characterization of three hallucinogenic N-(2-methoxy)benzyl derivatives of the 2C-series of phenethylamine drugs. Drug Testing and Analysis, 8, 634–645. [DOI] [PubMed] [Google Scholar]
- 10.Zuba D., Sekula K., Buczek A. (2013) 25C-NBOMe – new potent hallucinogenic substance identified on the drug market. Forensic Science International, 227, 7–14. [DOI] [PubMed] [Google Scholar]
- 11.Sekuła K., Zuba D. (2013) Structural elucidation and identification of a new derivative of phenethylamine using quadrupole time-of-flight mass spectrometry. Rapid Communications in Mass Spectrometry, 18, 2081–2090. [DOI] [PubMed] [Google Scholar]
- 12.Kelly A., Eisenga B., Riley B., Judge B. (2012) Case series of 25I-NBOMe exposures with laboratory confirmation. Clinical Toxicology (Philadelphia), 50, 702. [Google Scholar]
- 13.Rose S.R., Cumpston K.L., Stromberg P.E., Wills B.K. (2012) Severe poisoning following self-reported use of 25-I, a novel substituted amphetamine. Clinical Toxicology (Philadelphia), 50, 707–708. [Google Scholar]
- 14.Rose R.S., Poklis J.L., Poklis A. (2013) A case of 25I-NBOMe (25-I) intoxication: a new potent 5HT2a agonist designer drug. Clinical Toxicology (Philadelphia), 51, 174–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill S.L., Doris T., Gurung S., Katebe S., Lomas A., Dunn M. et al. (2013) Severe clinical toxicity associated with analytically confirmed recreational use of 25I-NBOMe: case series. Clinical Toxicology (Philadelphia), 51, 487–492. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki J., Poklis J.L., Poklis A. (2014) “My friend said it was good LSD”: a suicide attempt following analytically confirmed 25I-NBOMe ingestion. Journal of Psychoactive Drugs, 46, 379–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poklis J.L., Nanco C.R., Troendle M.M., Wolf C.E., Poklis A. (2014) Determination of 4-bromo-2,5-dimethoxy-N-[(2-methoxyphenyl)methyl]-benzeneethanamine (25B-NBOMe) in serum and urine by high performance liquid chromatography with tandem mass spectrometry in a case of severe intoxication. Drug Testing and Analysis, 6, 764–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang M.H., Ching C.K., Tsui M.S., Chu F.K., Mak T.W. (2014) Two cases of severe intoxication associated with analytically confirmed use of the novel psychoactive substances 25B-NBOMe and 25C-NBOMe. Clinical Toxicology (Philadelphia), 52, 561–565. [DOI] [PubMed] [Google Scholar]
- 19.Laskowski L.K., Elbakoush F., Calvo J., Exantus-Bernard G., Fong J., Poklis J.L. et al. (2015) Evolution of the NBOMes: 25C- and 25B-sold as 25I-NBOMe. Journal of Medical Toxicology, 11, 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson R.D., Botch-Jones S.R., Flowers T., Lewis C.A. (2014) An evaluation of 25B-, 25C-, 25D-, 25H-, 25I- and 25T2-NBOMe via LC-MS-MS: method validation and analyte stability. Journal of Analytical Toxicology, 38, 479–484. [DOI] [PubMed] [Google Scholar]
- 21.Grautoff S., Kähler J. (2014) Near fatal intoxication with the novel psychoactive substance 25C-NBOMe. Medizinische Klinik – Intensivmedizin und Notfallmedizin, 109, 271–275. [DOI] [PubMed] [Google Scholar]
- 22.Walterscheid J.P., Phillips G.T., Lopez A.E., Gonsoulin M.L., Chen H.H., Sanchez L.A. (2014) Pathological findings in 2 cases of fatal 25I-NBOMe toxicity. The American Journal of Forensic Medicine and Pathology, 35, 20–25. [DOI] [PubMed] [Google Scholar]
- 23.Poklis J.L., Devers K.G., Arbefeville E.F., Julia M., Pearson J.M., Houston E. et al. (2014) Postmortem detection of 25I-NBOMe [2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine] in fluids and tissues determined by high performance liquid chromatography with tandem mass spectrometry from a traumatic death. Forensic Science International, 234, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drug Enforcement Administration. Department of Justice (2014) Schedules of controlled substances: temporary placement of three synthetic phenethylamines into Schedule, I. Final order. Drug Enforcement Administration. Department of Justice. Federal Register, 78, 716–719. [PubMed] [Google Scholar]
- 25.Forrester M.B. (2014) NBOMe designer drug exposures reported to Texas poison centers. Journal of Addictive Diseases, 33, 196–201. [DOI] [PubMed] [Google Scholar]
- 26.Nikolaou P., Papoutsis I., Stefanidou M., Spiliopoulou C., Athanaselis S. (2015) 2C-I-NBOMe, an “N-bomb” that kills with “Smiles”. Toxicological and legislative aspects. Drug and Chemical Toxicology, 38, 113–119. [DOI] [PubMed] [Google Scholar]
- 27.Lawn W., Barratt M., Williams M., Horne A., Winstock A. (2014) The NBOMe hallucinogenic drug series: patterns of use, characteristics of users and self-reported effects in a large international sample. Journal of Psychopharmacology, 28, 780–788. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki J., Dekker M.A., Valenti E.S., Arbelo Cruz F.A., Correa A.M., Poklis J.L. et al. (2015) Toxicities associated with NBOMe ingestion, a novel class of potent hallucinogens: a review of the literature. Psychosomatics, 56, 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steiner R.R., Larson R.L. (2009) Validation of the direct analysis in real time source for use in forensic drug screening. Journal of Forensic Science, 54, 617–622. [DOI] [PubMed] [Google Scholar]
- 30.Poklis J.L., Clay D.J., Poklis A. (2014) High-performance liquid chromatography with tandem mass spectrometry for the determination of nine hallucinogenic 25-NBOMe designer drugs in urine specimens. Journal of Analytical Toxicology, 35, 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]