Paired TCRαβ analysis of virus-specific CD8+ T cells exposes diversity in a previously defined ‘narrow’ repertoire (original) (raw)
. Author manuscript; available in PMC: 2016 Apr 1.
Published in final edited form as: Immunol Cell Biol. 2015 Mar 25;93(9):804–814. doi: 10.1038/icb.2015.44
Abstract
T cell receptor (TCR) usage plays an important role in determining the outcome of CD8+ cytotoxic T lymphocyte (CTL) responses to viruses and other pathogens. However, the characterization of TCR usage from which such conclusions are drawn, is based on exclusive analysis of either the TCRα or, more commonly, the TCRβ chain. Here, we have used a multiplexed RT-PCR protocol to analyse the CDR3 regions of both TCRα and β chains from single naïve or immune epitope-specific cells to provide a comprehensive picture of epitope-specific TCR usage and selection into the immune response. Analysis of TCR repertoires specific for three influenza-derived epitopes (DbNP366, DbPA224, and DbPB1-F262), showed preferential usage of particular TCRαβ proteins in the immune relative to the naïve repertoire, in some cases resulting in a complete shift in TRBV preference or CDR3 length, and restricted repertoire diversity. The NP366-specific TCRαβ repertoire, previously defined as clonally restricted based on TCRβ analysis, was similarly diverse as the PA224- and PB1-F262-specific repertoires. Intriguingly, preferred TCR characteristics (Variable (V) gene usage, CDR3 length and Junctional (J) gene usage) appeared able to confer specificity either independently or in concert with one another, depending on the epitope specificity. These data have implications for established correlations between the nature of the TCR repertoire and response outcomes after infection, and suggest that analysis of a subset of cells or a single TCR chain does not accurately depict the nature of the antigen-specific TCRαβ repertoire.
Introduction
Biased usage of TCRs is a fundamental characteristic of antigen-specific T cell responses, and has been observed against a wide spectrum of antigen types, including pathogen, tumor-derived, as well as innocuous environmental and self antigens (reviewed in 1, 2). Such biases in TCR usage may be classified (according to the extent of bias) as Type I, II, III, or IV1, 2 ranging from preferential use of a particular Vα or Vβ gene segment only (Type I), through to usage of identical TCRα or β clonotypes (V region, CDR3 region, and J region) (Type III)2. Biases may be present in the preimmune repertoire3, 4, due to a combination of structural constraints imposed by the need for peptide + Major Histocompatibility Complex (pMHC) recognition5, 6, and convergent recombination, a process that results in the prevalence of more easily generated TCRs7, 8. Preferential expansion of particular T cell clones from the naïve into the immune repertoire can then further skew the usage of particular TCRs or TCR characteristics3, 9–13, thereby exacerbating antigen-driven TCR bias. Whether continued antigenic stimulation, such as in chronic infection, continues to narrow the TCR repertoire, as has been suggested for the HLA-B8 restricted response to Epstein Barr Virus (EBV) EBNA-3A FLRGRAYGL peptide where the vast majority of the antigen-specific response is clonal14, or whether such extreme bias occurs upon initial antigen encounter15–17, is unclear.
Interestingly, while the nature of the TCR bias varies with antigen, antigen driven biases are highly conserved across individuals (in both animal models and humans), indicating the capacity of these biased subsets to mediate superior recognition of peptide + Major Histocompatibility Complex I complexes (pMHCI). Why is it important to understand bias in antigen-specific T cell responses? The extent to which an antigen-specific T cell response can utilize a broad range of TCRs, or relies only on a narrow subset of TCRs, has been shown to correlate with the outcome of infection. For example, in a number of viral infections, diversity in TCR usage has been positively linked to effective viral control, prevention of immune escape, and/or improved recognition of heterologous viruses18–24. This has been suggested to be due to an increased structural capacity to recognize variant epitopes25 or an increased likelihood that high affinity TCRs will be present22. In any case, it is clear that the composition of TCRs that make up an antigen-specific T cell repertoire impacts substantially on disease outcomes.
Interrogation of antigen-specific repertoires to date have predominantly relied on analysis of the TCR β chain, partly due to the early perception that this chain made a greater contribution to peptide binding, and therefore to pMHCI specificity2. This was partly based on the greater diversity inherent in the CDR3β due to the additional D region element, but also on the fact that CDR3β loops contributed more than CDR3α loops to peptide binding in several early TCR-pMHCI structures (mouse and human)26–29. However, subsequent analyses of a growing number of TCR-pMHCI crystal structures (34 available to date) demonstrate that both CDR3α and β are able to mediate significant contact with the peptide fragment and MHCI molecule, with 15 of those structures demonstrating a contribution of CDR3α that is equivalent to or greater than that of CDR3β5, 6, 30. In addition, multiple studies have shown an important functional role for the TCRα chain in conferring specificity31–35. Thus, TCR specificity can be viewed as a product of complex interactions between the TCRα and β chains. Analysis of TCRβ chain repertoires has largely been limited to bulk T cell populations, or a previously identified dominant TRBV+ subset in single cell analyses. Even deep sequencing of antigen-specific TCRs, if performed using a multiplexed approach36, 37, is prone to unequal amplification and does not typically provide information on TCRαβ pairing. Only recently have strategies begun to emerge for analysis of both TCR α and β chains from individual cells24, 38–42, and therefore accurate information on clonal frequency and TCR αβ pairing is still lacking for the vast majority of antigen-specific T cell repertoires.
Following influenza virus infection, influenza virus derived NP366-, PA224-, and PB1-F262-specific responses show clear preference for TRBV13-1+, 29+, and 19+ TCRs, respectively43–45. Previous characterization of TCRβ usage within these epitope specificities has largely been limited to these dominant TRBV+ subsets, and has also identified preferential usage of Jβ regions (NP366 - TRBJ1-1/2-2, PA224 - TRBJ1-1/2-6), and CDR3β lengths (NP366 9 amino acids (aa), PA224 6 aa, PB1-F262 8 aa), that have come to characterize these responses44, 46–48. Importantly, based on these analyses the NP366-specific response is characterized as narrow (utilizing relatively few TCRβ clonotypes) and highly public (using clonotypes observed in multiple individuals)47, 48. In contrast, the PA224- and PB1-F262-specific TCRβ repertoires are more diverse and typically unique to individuals (private)44, 46. Given the biological significance of such characteristics of virus-specific T cell repertoires, we have used a multiplexed, nested RT-PCR protocol to amplify the CDR3 regions of the full range of both TCR α and β chains expressed by individual, epitope-specific CTLs both before and after infection, to get a complete and unbiased picture of three virus-specific CTL TCRαβ repertoires, and to gain a better understanding of how key characteristics of antigen-specific TCRαβ repertoires are selected in response to virus challenge.
Results
TRAV and TRBV usage is restricted in antiviral immune CTL TCR repertoires, compared to their naïve counterparts
Biased TRBV usage has been documented for all of the NP366-, PA224-, and PB1-F262-specific populations. Analysis of TRBV usage from multiplex RT-PCR analysis of single antigen-specific cells38, confirmed both the previously described bias toward TRBV13-143, 2945, and 1944 usage in NP366-, PA224-, and PB1-F262-specific responses, respectively, and the exacerbation of these biases from naïve to immune populations3, 49 (Figure 1A-C and Supp. Figure 1A-C). In contrast, there is relatively little known about the TCRα chain usage in these populations, although a previous bulk analysis of NP366-specific TCRα chains suggests the usage is relatively diverse50.
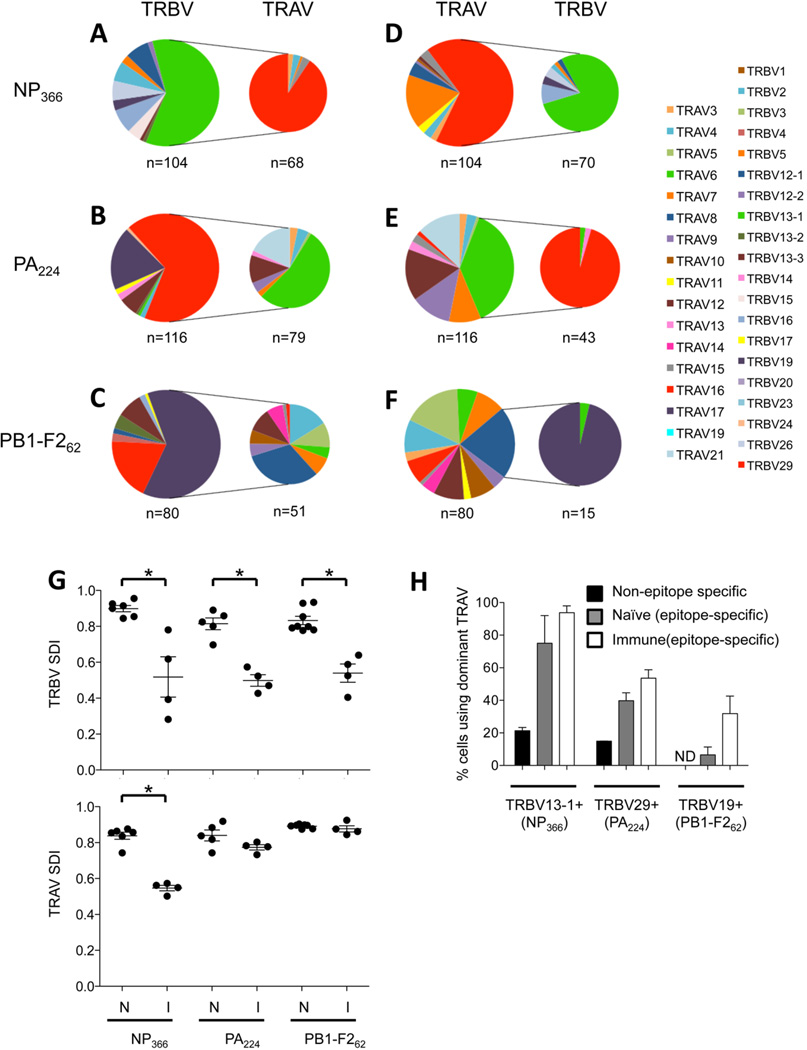
Figure 1. TRAV and TRBV bias, pairing, and diversity in immune antiviral CTL responses.
TRAV and TRBV usage was determined from CDR3 sequences generated using multiplexed nested RT-PCR from individual NP366-, PA224-, or PB1-F262-specific CD8+ T cells from d10 immune splenocytes, as described. Shown is data from all cells that yielded both CDR3α and CDR3β sequences. (A-C) TRBV usage in the total epitope-specific populations and TRAV usage within the dominant TRBV subset. (D-F) TRAV usage in the total epitope-specific populations and TRBV usage within the dominant TRAV subset. (G) SDI analysis of TRAV and TRBV usage in naïve and immune epitope-specific CD8+ T cell populations. (H) Analysis of TRAV16, TRAV6, and TRAV8 usage within the TRBV13-1+, TRBV29+, and TRBV19+ subsets, respectively, of non-epitope specific, naive epitope-specific and immune epitope-specific populations. Shown are the data obtained from 4, 4, and 4 immune mice and 6, 5, and 8 naive mice, for NP366, PA224, and PB1-F262, respectively. N=69 NP366-specific, 98 PA224-specific, and 139 PB1-F262-specific naïve sequences analysed. Non-antigen-specific TCRαβ sequences were obtained by single cell sorting on TRBV13-1+ (n=83), TRBV29+ (n=107) or TRBV19+ (n=121) populations from 3 uninfected B6 mice and performing nested RT-PCR using TRBVspecific oligos and a panel of multiplexed TRAV oligos. ND = not detected.
Multiplexed analysis of TCRα chain usage in NP366-, PA224-, and PB1-F262-specific immune populations also showed biased usage of particular TCRα chains (Figure 1D-F). NP366- and PA224-specific cells showed biased usage of TRAV16 (65±2.4%) and TRAV6 (38±5%), respectively, while PB1-F262-specific CTLs exhibited less dramatic preferences for TRAV5 (17±8%) or TRAV8 (21±16%). Each of these preferences was substantially increased in the immune response relative to the corresponding naïve populations (Supp. Figure 1D-F), as was observed for TRBV bias. With the exception of NP366-specific CD8+ T cells, the TRAV biases were not as pronounced as was typically observed for TRBV usage, nonetheless such TCRα chain enrichment implies its importance in conferring pMHC specificity.
The elevated usage of particular TRAV or TRBV chains in the immune, relative to the naïve, repertoire resulted in some cases in the specific diminution in usage of other chains (Supp. Figure 2A-F). For example, the enrichment of TRBV13-1 usage from naïve to immune NP366-specific cells occurred at the specific expense of TRBV17 usage, which was the most prevalent TRBV in the naïve set (26±13%), but was observed only once in the immune repertoire (0.8±1.5%)(p<0.01) (Supp. Figure 2A). These data suggest that some TCRs available for recognition in the naïve repertoire are avoided in the immune response.
We used Simpson’s Diversity Index (SDI) to determine how such antigen-driven biases influence the diversity of TRAV and TRBV usage. SDI assesses both ‘richness’ (number of V gene segments used) and ‘evenness’ (the distribution of those V gene segments) to provide a measure of overall diversity51, 52. All three epitope-specific populations showed significantly less diversity of TRBV usage in the immune compared to the naïve repertoires (Figure 1G). The effect on TRAV diversity was subtler, with only the NP366-specific population exhibiting significantly reduced diversity in TRAV usage from naïve to immune sets (p<0.01), despite a similar trend for PA224 (p=0.11) (Figure 1G). Thus, the selective expansion of CTL clones using particular TRBV and, to a lesser extent, TRAV chains, leads to more restricted TCR usage in response to antigen challenge, relative to what is available in the preimmune repertoire.
CDR3 length and J region analysis
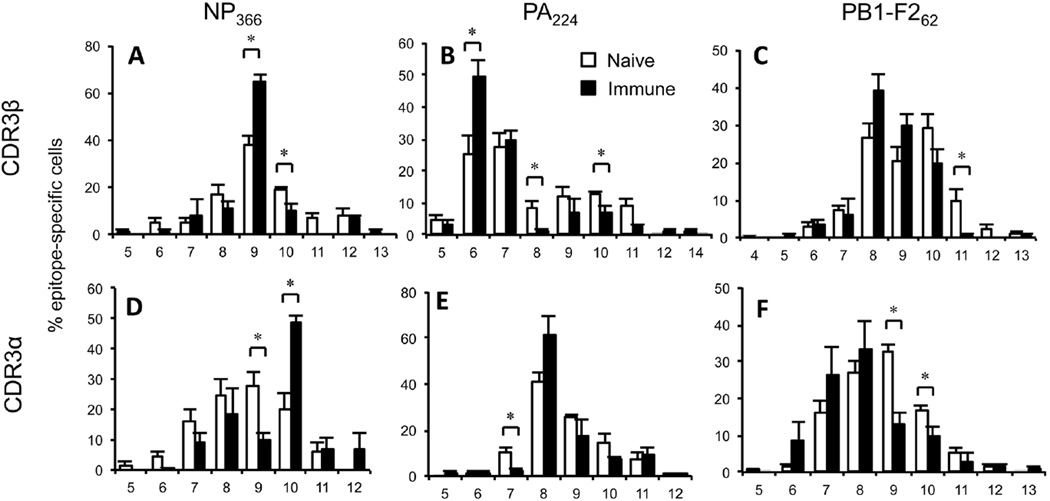
Prior analysis of TCR CDR3β loop length in influenza-specific CD8+ T cells showed preferential usage of CDR3β lengths of 9, 6, and 8 aa in TRBV13-1+ NP366-, TRBV29+ PA224-, and TRBV19+ PB1-F262-specific cells, respectively43–45. Multiplex analysis of total NP366-, PA224-, and PB1-F262-specific TCRβ repertoires also revealed these CDR3β length preferences (Figure 2A-C). TCRα chains specific for NP366, PA224, and PB1-F262 showed substantial biases toward CDR3α lengths of 10, 8, and 7–8 aa, respectively (Figure 2D-F), to a similar extent as those observed for CDR3β lengths. The prevalence of the dominant CDR3β and α lengths observed in the immune populations was significantly increased relative to naïve repertoires (Figure 2). Interestingly, the corresponding naïve repertoires often showed distinct patterns of CDR3 length usage to those observed in the immune repertoires (Figure 2B, C, D, F). These data suggest that the highly reproducible CDR3 α and β length biases observed in epitope-specific CTL responses, while partly reflected in the naïve CTLp populations, are predominantly driven by preferential clonal expansion during the immune response.
Figure 2. CDR3α and β length preferences in naïve and immune epitope-specific CD8+ T cell populations.
CDR3β (A-C) and CDR3α (D-F) lengths were determined from CDR3 sequences generated using multiplexed nested RT-PCR from individual NP366-, PA224-, or PB1-F262-specific CD8+ T cells from naïve and d10 immune splenocytes, as described. Shown is data from all CDR3α and β sequences (irrespective of whether paired sequences were detected). Number of sequences are as follows: NP366 CDR3β: naïve n=83, immune n=149; NP366 CDR3α: naïve n=81, immune n=174; PA224 CDR3β: naïve n=133, immune n=166; PA224 CDR3α: naïve n=155, immune n=161; PB1-F262 CDR3β: naïve n=269, immune n=127; PB1-F262 CDR3α: naïve n=271, immune n=136.
Analysis of J region usage in the immune repertoires revealed a strong NP366-specific preference for TRBJ2-2, a PA224-specific preference for TRBJ2-7 and a slight bias toward TRBJ2-1 usage in the PB1-F262-specific population (Supp. Figure 2G-I). The NP366-specific population exhibited a strong bias toward TRAJ42 (Supp. Figure 2J). While there appeared to be some biases noted in TRAJ usage for PA224- and PB1-F262-specific cells, they were less convincing and far more variable between mice (Supp. Figure 2K, L). Thus, unlike the NP366-specific population, which exhibited TRBJ and TRAJ biases to a similar extent, the PA224- and PB1-F262-specific populations showed no reproducible TRAJ preferences.
Pairing of dominant TRAVs and TRBVs
An important aspect of studying TCR at the single cell level is that it enables analysis of TCRαβ pairing, thereby providing the full extent of epitope-specific TCR repertoire diversity. Given the strong antigenic selection for both TCRβ and α chains in the antiviral response, we were interested in whether these chains were selected together or independently. For all 3 epitope-specific populations and in all mice analysed, usage of the dominant TCRβ family was increased within the dominant TRAV populations compared to the total population (NP366 - total 60±29 vs TRAV16+ 78±36 [p=0.06]; PA224 - total 68±5 vs TRAV6+ 95±9.1 [p=0.01]; PB1-F262 - total 63±7.7 vs TRAV8+ 96±6.4 [p=0.07], using Students paired t-test) (Figure 1, compare left pie charts to right pie charts in each of A vs D, B vs E, and C vs F).
In contrast, enrichment of the dominant TRAV within the dominant TRBV population was less readily observed (Figure 1, compare right pie charts to left pie charts in each of A vs D, B vs E, and C vs F). Certainly, usage of TRAV16+ within the dominant TRBV13-1+ NP366-specific set was greatly increased relative to total (total 67±3.4 vs TRBV13-1+ 91±14 [p=0.001]). However there was only slight enrichment of TRAV6 and TRAV8 usage in the dominant TRBV29+ PA224-specific and TRBV19+ PB1-F262-specific populations, respectively, compared to the total set (PA224 - total 38±5.1 vs TRBV29+ 54±10 [p=0.02]; PB1-F262 - total 21±16 vs TRBV19+ 32±19 [p=0.08]). These data collectively indicate that dominance of both TRAV16 and TRBV13-1 was due to their co-dependence in the NP366-specific population, however, while the dominant TCRα chains were largely dependent on the dominant TCRβ chains to confer PA224 or PB1-F262 specificity, their TCRβ chains were able to confer specificity when paired with a broad range of TCRα chains.
While these data indicate that selection of dominant TCRα and β chains is, to varying extents, co-dependent, it is possible that the dominance of the TCRα chain is simply due to its superior ability to pair with the dominant TRBV, and contributes minimally to pMHCI specificity. Analysis of TRAV usage in non-antigen-specific TRBV13-1+, TRBV29+, and TRBV19+ populations shows significantly reduced TRAV16, TRAV6, and TRAV8 usage, respectively, than in antigen-specific sets (Figure 1H), clearly demonstrating that the extent of pairing observed between the dominant TRAV and TRBV bearing TCR chains is antigen driven.
Collectively, these data suggest that there is an active selection of both the TRBV and TRAV families in antigen-specific CTL populations, and that the combination of preferred TCRα and β chains plays a role in conferring epitope specificity. However, the stringency of selection of particular TCRαβ pairs occurs to different extents, with far greater flexibility in TRAV usage generally (and in the dominant TRBV+ set) in the PA224- and in particular in the PB1-F262-specific populations, compared to NP366. Such differences may indicate a more important role for the TCRα chain in NP366, relative to PA224 or PB1-F262 recognition, or may simply indicate a greater range of TCRα chains with the capacity to impart PA224 and PB1-F262 specificity.
Relationship between characteristic features of epitope-specific TCRs
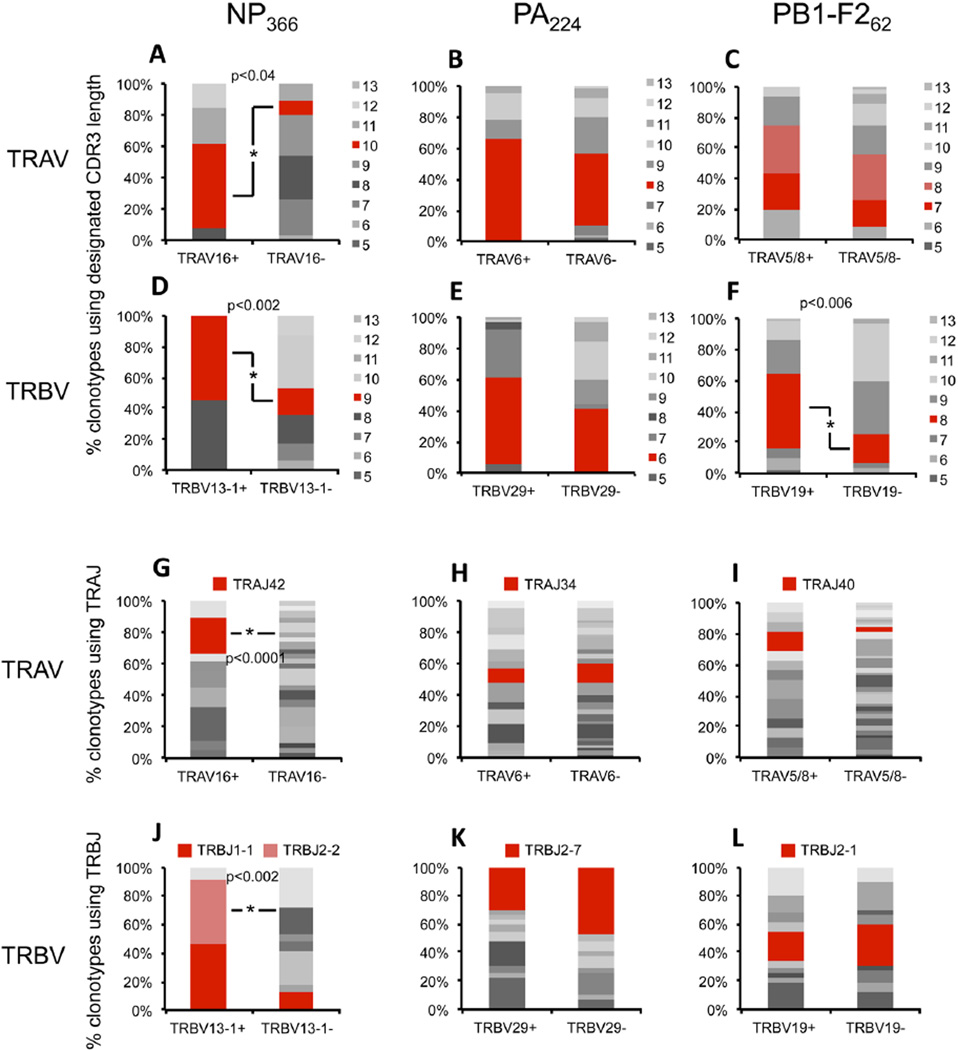
To further determine whether the canonical features that characterize each of the epitope-specific immune CTL populations (V region usage, CDR3 length, J region usage) are co-selected, we analysed CDR3 length and J region usage as a function of V region usage (Figure 3). Importantly, this analysis was done irrespective of clonal abundance in order to eliminate the effect of individual clonal expansions. For the NP366-specific population, both the preferred TRAV16+ and TRBV13-1+ TCRs showed an enrichment of TCRs with the preferred CDR3 lengths of 10 and 9 aa, respectively (Figure 3A, D). Similarly, analysis of preferred J region usage (TRAJ42 and TRBJ2-2/1-1) showed a substantial enrichment, in particular in the TRBV13-1+ (relative to TRBV13-1-) population, but also in the TRAV16+ population (Figure 3G, J). This indicates that the canonical features associated with the NP366-specific TCRβ chain (TRBV13-1 usage, 9 aa CDR3β length and TRBJ1-1/TRBJ2-2 usage) and TCRα chain (TRAV16 usage, 10 aa CDR3α length, and TRAJ42 usage) are selected together, suggesting that these features contribute to optimal pMHCI binding in a coordinated inter-dependent fashion.
Figure 3. Co-selection of TCR biases in epitope-specific populations.
TCR clonotypes from d10 immune epitope-specific populations were used to plot CDR3 length or J region usage for cells using the dominant TRAV or TRBV, and compared to CDR3 length and J region usage in cells outside of the dominant TRAV or TRBV subsets. The analysis was done irrespective of clonotype abundance. CDR3α length distribution (A-C) and TRAJ usage (G-I) in TRAV16+/-, TRAV6+/-, and TRAV5 or 8 +/- subsets of NP366-, PA224-, and PB1-F262-specific populations, respectively. CDR3β length distribution (D-F) and TRBJ usage (J-L) in TRBV13+/-, TRBV29+/, and TRBV19 +/- subsets of NP366-, PA224-, and PB1-F262-specific populations, respectively. CDR3α clonotypes: NP366 - n=48, PA224 - n=71, PB1-F262 - n=78; CDR3β clonotypes: NP366 - n=28, PA224 - n=83, PB1-F262 - n=76.
In contrast, for the PA224-specific population, the dominant TRAV6+ and TRBV29+ subsets showed no selective enrichment of preferred CDR3 lengths (Figure 3B, E) or J region elements (Figure 3H, K) relative to the rest of the epitope-specific population. Similarly, for PB1-F262-specific cells, the only co-segregation of bias observed was an increased prevalence of 8aa CDR3β lengths in the TRBV19+ (compared to TRBV19-) subset (Figure 3F), with all other preferences being observed equivalently in TRAV5/8+ compared to TRAV5/8-, or TRBV19+ compared to TRBV19-, subsets (Figure 3C, I, L). Thus, it appears that, for the PA224-and PB1-F262-specific populations, the preferred elements observed in the TCR repertoire are more likely to either contribute independently to the recognition of pMHCI, or mediate coordinated pMHCI recognition in combination with a variety of different TCR elements.
Flexibility of CDR3α and β pairing
One of the caveats of TCRβ or α chain analyses performed independently of one another is that it provides no information on the promiscuity of pairing and therefore the true extent of clonal diversity. There is a wealth of data indicating that it is the combination of TCRα and β chains (rather than either chain alone) that imparts epitope specificity (reviewed in 6, 30). Moreover, a recent study has demonstrated key structural interactions specifically between CDR3α and CDR3β regions that influence pMHC recognition53. Our analysis of the extent of flexibility in CDR3α and β pairing revealed that of the 11 CDR3β that were observed more than once either within or between mice for NP366, 6 of these were found to pair with multiple CDR3α sequences, including CDR3β sequences previously classified as ‘public’ (e.g. SGGANTGQL, SGGGNTGQL, SGGSNTGQL) (Table 1, Supp. Table 1). Indeed, even dominance of a particular CDR3β across multiple mice was not necessarily representative of a single TCRαβ clonotype (e.g. SGGSNTGQL) (Supp. Table 1). Similarly, 9/23 PA224-specific and 3/15 PB1-F262-specific CDR3β clonotypes that were observed more than once within or between mice, were found to pair with multiple CDR3α sequences (Table 1, Supp. Tables 2 & 3).
Table 1.
Degeneracy of TCRαβ pairing in epitope-specific immune populations
| NP366-specific | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TCRβ chain | Paired TCRα chains | TCRα chain | Paired TCRβ chains | ||||||||
| Vβ | CDR3β | Jβ | Vα | CDR3α | Jα | Vα | NP CDR3α | Jα | Vβ | CDR3β | Jβ |
| 13-1 | SGGSNTGQL | 2-2 | 16 | RAAGGSNAKL | 42 | 7 | MSNYNVL | 21 | 12-2 | FPDRRNSYNSPL | 1-6 |
| 16 | REALGEGGRAL | 15 | 16 | SFGAANTEV | 1-1 | ||||||
| 16 | REPSGTYQ | 13 | |||||||||
| 16 | RETRSGTYQ | 13 | |||||||||
| 16 | RGSGGSNAKL | 42 | |||||||||
| 16 | RNSGGSNAKL | 42 | |||||||||
| 16 | RVSGGSNAKL | 42 | |||||||||
| 15-1 | WELDTNAYKV | 30 | |||||||||
| 13-1 | SGGANTGQL | 2-2 | 16 | RDQGGRAL | 15 | 16 | REALGEGGRAL | 15 | 13-1 | SGGGNTGQL | 2-2 |
| 16 | RGNSGTYQ | 13 | 13-1 | SGGSNTGQL | 2-2 | ||||||
| 16 | RGSGGSNAKL | 42 | |||||||||
| 16 | RVGSGTYQ | 13 | |||||||||
| 16 | RVSGGSNAKL | 42 | |||||||||
| 2 | SQDRRNSYNSPL | 1-6 | 16 | RVSGGSNAKL | 42 | 16 | REGKGGGSNYKL | 53 | 19 | RMGVYNSPL | 1-6 |
| 7-4 | SAIASSSFSKL | 50 | 19 | SPLGGGAETL | 2-3 | ||||||
| 7-3 | SGQGGRAL | 15 | 5 | SQDLGGTYEQ | 2-7 | ||||||
| 7-4 | SPSSGSWQL | 22 | 12-1 | SPWGEYEQ | 2-7 | ||||||
| 7-4 | STMNNNAGAKL | 39 | |||||||||
| 16 | SFGAANTEV | 1-1 | 7 | MSNYNVL | 21 | 16 | RGSGGSNAKL | 42 | 16 | SFGAANTEV | 1-1 |
| 16 | REPSGTYQ | 13 | 13-1 | SGGANTGQL | 2-2 | ||||||
| 16 | RGSGGSNAKL | 42 | 13-1 | SGGSNTGQL | 2-2 | ||||||
| 16 | RVSGGSNAKL | 42 | 26 | SLWRDTL | 2-4 | ||||||
| 26 | SLWRDTL | 2-4 | 7-3 | RAQTGFASAL | 35 | 16 | RVSGGSNAKL | 42 | 16 | SFGAANTEV | 1-1 |
| 16 | RGSGGSNAKL | 42 | 13-1 | SGGANTGQL | 2-2 | ||||||
| 6 | RRNTGGLSGKL | 2 | 13-1 | SGGGNTGQL | 2-2 | ||||||
| 16 | VSGGSNAKL | 42 | 13-1 | SGGSNTGQL | 2-2 | ||||||
| 13-1 | SGGGNTGQL | 2-2 | 16 | RVSGGSNAKL | 42 | ||||||
| 16 | REALGEGGRAL | 15 |
| PA224-specific | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TCRβ chain | Paired TCRα chains | TCRα chain | Paired TCRβ chains | ||||||||
| Vβ | CDR3β | Jβ | Vα | CDR3α | Jα | Vα | NP CDR3α | Jα | Vβ | CDR3β | Jβ |
| 29 | SHDRGQL | 2-2 | 6 | GAQGGRAL | 15 | 6 | GAQGGRAL | 15 | 13-1 | SGNAEQ | 2-1 |
| 21 | RVGATGGNNKL | 56 | 29 | SHDRGQL | 2-2 | ||||||
| 19 | SIGDEQ | 2-7 | 13-1 | SNNRI | 31 | 6 | GGGSNYKL | 53 | 29 | SLGERL | 1-4 |
| 7-2 | SRGSNNRI | 31 | 29 | TAGERL | 1-4 | ||||||
| 19 | SIGGEV | 1-1 | 9D-4 | SAWGGRAL | 15 | 6 | HSGGSNAKL | 42 | 29 | SSYEQ | 2-7 |
| 7-2 | SRGSNNRI | 31 | 29 | TMGERL | 1-4 | ||||||
| 15D-1 | WEPDTNAYKV | 30 | |||||||||
| 29 | SLGERL | 1-4 | 6 | GGGSNYKL | 53 | 6 | IASSGSWQL | 22 | 29 | SGGKAP | 1-5 |
| 9D-4 | SPSNTNKV | 34 | 29 | SGGQAP | 1-5 | ||||||
| 29 | SLSGFEQ | 2-7 | 6D-4 | APSNTNKV | 34 | 21 | NGGSNYKL | 53 | 29 | SFGQAP | 1-5 |
| 6D-4 | VPSNTNKV | 34 | 29 | SPDRGAL | 2-4 | ||||||
| 29 | SPDRGAL | 2-4 | 21 | NGGSNYKL | 53 | 21 | RVGATGGNNKL | 56 | 29 | SHDRGQL | 2-2 |
| 21 | RVGATGGSSKL | 56 | 29 | SLDRGQV | 1-1 | ||||||
| 3-4 | SGGATGGNNKL | 56 | 29 | SPDRGRL | 1-1 | ||||||
| 29 | SSGETL | 2-3 | 6 | GAGSNYKL | 33 | 21 | SGGSNYKL | 53 | 29 | SLGAEQ | 2-1 |
| 7 | TDHQPGTGSNR | 28 | 19 | SWGGEQ | 2-7 | ||||||
| L | 29 | SWGGEQ | 2-7 | ||||||||
| 29 | SWGVEQ | 2-5 | |||||||||
| 29 | SSYEQ | 2-7 | 6 | GEGSNYQL | 33 | 7 | SRGSNNRI | 31 | 19 | SIGDEQ | 2-7 |
| 6 | HSGGSNAKL | 42 | 19 | SIGGEV | 1-1 | ||||||
| 19 | SIGNEQ | 2-7 | |||||||||
| 19 | SIGSEQ | 2-7 | |||||||||
| 29 | SLGGYEQ | 2-7 | |||||||||
| 19 | SMGNEQ | 2-7 | |||||||||
| 29 | SWGGEQ | 2-7 | 53 | SGGSNYKL | 19 | 12 | SRTNAYKV | 30 | 29 | SAGSDY | 1-2 |
| 53 | SKTNTNKV | 29 | 29 | SLHTEV | 1-1 | ||||||
| 29 | TGTEEQ | 2-7 | |||||||||
| 6 | VPSNTNKV | 34 | 29 | LGGDNQAP | 1-5 | ||||||
| 29 | SLSGFEQ | 2-7 | |||||||||
| 29 | SLSGYEQ | 2-7 |
| PB1-F262-specific | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TCRβ chain | Paired TCRα chains | TCRα chain | Paired TCRβ chains | ||||||||
| Vβ | CDR3β | Jβ | Vα | CDR3α | Jα | Vα | NP CDR3α | Jα | Vβ | CDR3β | Jβ |
| 19 | SIGDSYEQ | 2-7 | 8-2 | DTGNYKY | 40 | 8 | DTGNYKY | 40 | 13-2 | GELGASAETL | 2-3 |
| 6-6 | GENNYAQGL | 26 | 19 | SIGDNYEQ | 2-7 | ||||||
| 19 | SIGDSYEQ | 2-7 | |||||||||
| 19 | SIGDTTEV | 1-1 | |||||||||
| 19 | SMGTDTQ | 2-5 | |||||||||
| 19 | SPGDYAEQ | 2-1 | |||||||||
| 19 | SRGDYAEQ | 2-1 | |||||||||
| 19 | SWGDYAEQ | 2-1 | |||||||||
| 29 | SLLQLQDTQ | 2-5 | 5 | SAANKM | 47 | 12 | SDYGSSGNKL | 32 | 19 | SMGAEV | 1-1 |
| 10 | SSGGNYKP | 6 | 19 | SMGMEAP | 1-5 | ||||||
| 19 | SWGDYAEQ | 2-1 | 4-3 | DQDSNYQL | 33 | ||||||
| 8 | DTGNYKY | 40 | |||||||||
| 9 | RNSNYQL | 33 |
Analysis of the CDR3α clonotypes revealed a similar situation with 6/17, 10/20, and 2/11 clonotypes observed more than once in the NP366-, PA224-, and PB1-F262-specific repertoires, able to pair with more than one CDR3β clonotype (Table 1). Collectively, these data demonstrate a large degree of promiscuity in CDR3αβ pairing, even for NP366-specific TCRs where the TRBV13-1/TRAV16 pairing requirement is relatively stringent (Figure 1A, D). As a consequence, there appears to be substantial diversity in TCRαβ clonotypes that were previously defined as ‘restricted’ by CDR3β alone47, 48.
Diversity and sharing in influenza-specific TCRαβ repertoires
TCR repertoires are often characterised by the extent of clonotype diversity within individuals (the number and distribution of different clonotypes) and between individuals (the extent to which the repertoire is observed in multiple individuals (shared) or is unique to an individual (private))54. Previous analyses of TCRβ diversity in immune NP366-, PA224-, and PB1-F262-specific repertoires (limited to the dominant TRBV families), clearly show that the TRBV13-1+ NP366-specific repertoire is significantly more restricted in clonal diversity than either the TRBV29+ PA224- or TRBV19+ PB1-F262-specific populations, which are both characterised as diverse and private44, 46, 47. To determine whether these characteristics hold up upon analysis of the entire immune epitope-specific population and upon inclusion of both TCRα and β chains, we analysed diversity (using SDI) in the global TCRα and β chain repertoires for each of these specificities.
Looking within the dominant TRBV13-1+ NP366-specific, TRBV29+ PA224-specific, and TRBV19+ PB1-F262-specific sets, it is clear that TCRβ chain diversity is significantly lower in the NP366-specific population, compared to the relatively diverse PA224- (p<0.027) and PB1-F262- (p<0.003) specific sets (Figure 4A-C, compare white bars, right plots). Diversity in the combined TCRαβ clonotypes within the dominant TRBV+ subsets, remained relatively low for NP366-, compared to PA224- or PB1-F262-specific populations (Figure 4A-C, right plots), due to the relatively stringent TRAV16 usage in this population and the associated restriction in CDR3α diversity therein (Figure 1) (Supp. Table 1).
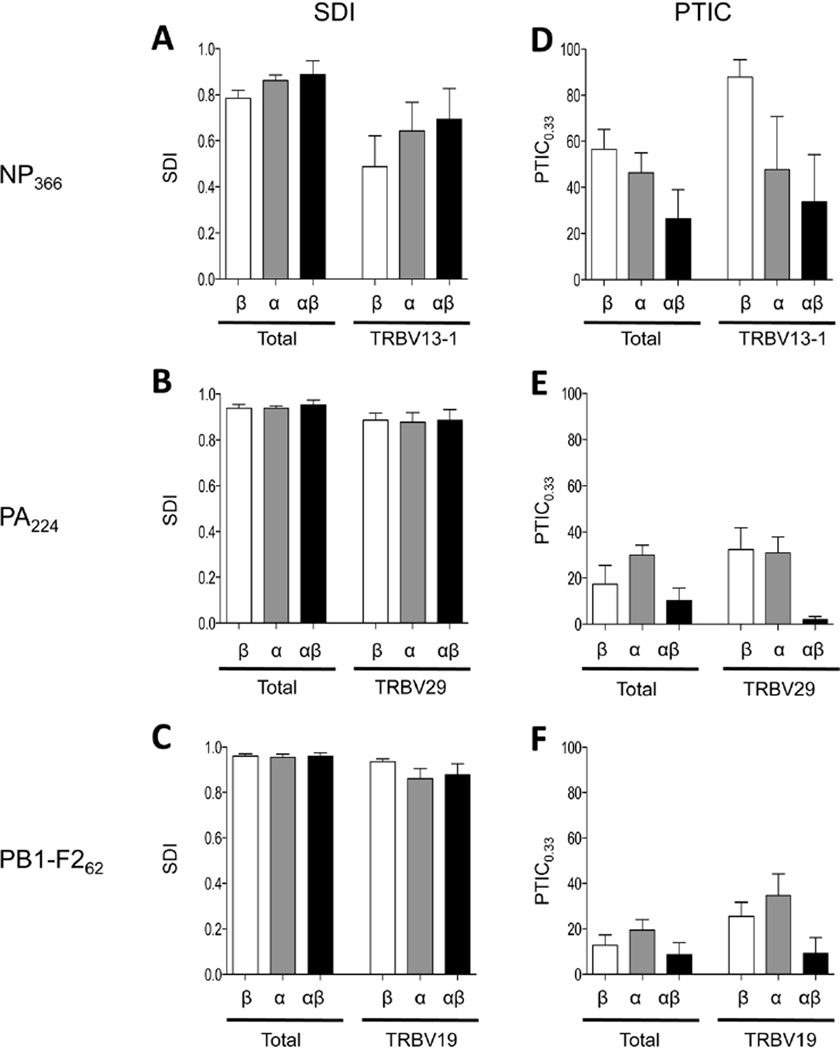
Figure 4. TCRαβ diversity and sharing in global epitope-specific CTL repertoires.
Epitope-specific CDR3α, β, and αβ clonotypes determined by single cell multiplexed RT-PCR, were analysed for diversity using SDI (A-C), and for sharing between individuals using PTIC0.33 (D-F), as described in the Methods. Number of sequences are as follows: TOTAL: NP366 β (n=149) α (n=174) αβ (n=104); PA224 β (n=166) α (n=161) αβ (n=116); PB1-F262 β (n=127) α (n=136) αβ (n=80). TRBV13-1+ NP366 β (n=93) α (n=68) αβ (n=68); TRBV29+ PA224 β (n=112) α (n=79) αβ (n=79); TRBV19+ PB1-F262 β (n=84) α (n=51) αβ (n=51). Statistical analyses were performed using Student’s t-test.
Analysis of the total epitope-specific TCRαβ repertoire (Figure 4A-C, left plots) revealed that while TCRαβ diversity was generally increased (compared to the dominant TRBV subset) for all three epitope specificities (Figure 4A-C, total v TRBV sets), this increase was most pronounced for the NP366-specific set. This was a consequence of the fact that TCRαβs outside of the TRBV13+ subset were characteristically distinct from those within it, and showed far greater CDR3α and β diversity (Supp. Table 1).
Consequently, analysis of the absolute diversity for each of the epitope-specific populations (that is, unrestricted to the dominant TRBV set and inclusive of TCRα chain), demonstrates that the NP366-, PA224-, and PB1-F262-specific populations show similar levels of overall TCR diversity (0.89, 0.95, 0.96, respectively) (p>0.2 comparing NP366- to PA224- or PB1-F262-specific sets) (Figure 4A-C, left plots, compare black bars). This contrasts with previous characterisations of the NP366-specific repertoire, based exclusively on CDR3β clonotype analysis within the TRBV13-1+ set, as being highly restricted in diversity. Indeed, comparison of SDI between NP366-specific TRBV13-1+ TCRβ and total TCRαβ reveals a 1.8-fold difference (p=0.03), while comparison of SDI between PA224-specific TRBV29+ TCRβ and total TCRαβ, or between PB1-F262-specific TRBV19+ TCRβ and total TCRαβ showed little difference (1.07-fold [p=0.12] and 1.02-fold [p=0.27], respectively).
As previously documented7, 47, 48, a significantly larger proportion of the TRBV13-1+ NP366-specific TCRβ chain sequences (88%) were observed in multiple mice (i.e. ‘shared’) compared to TRBV29+ PA224-specific (32%) (p=0.004) or TRBV19+ PB1-F262-specific (26%) (p=0.0002) sequences (Figure 4D-F, white bars, right plots). However, the extent to which TRBV13-1+ CDR3β clonotypes are shared between animals is atypical, since sharing of TCRα sequences (as defined by the Proportion of TCRs in Common (PTIC)) within dominant TRBV was more similar for the three epitope-specificities (Figure 4D-F, grey bars, right plots). Because of the influence of the TCRβ chain, however, overall the extent of TCRαβ sharing in the dominant TRBV population remained relatively (although not significantly) high for NP366-specific cells (Figure 4D-F, right plots, black bars).
Analysis of TCRαβ clone sharing in total epitope-specific responses revealed that while the NP366-specific response was still the most shared response (27%, compared to 10% and 9% for PA224 and PB1-F262, respectively), the extent to which it was shared was drastically reduced compared to what one might have predicted based on TCRβ analyses within the TRBV13-1+ population (Figure 4D, right plot, white bar) or globally (Figure 4D, left plot, white bar). The general trend of a drop in αβ clonotype sharing, relative to α or β clonotype alone (Figure 4D-F), reflects the promiscuity of TCRα/TCRβ pairing, which is most obvious for the large public NP366-specific TCRβ clonotypes (Table 1).
In summary, analysis of TCR diversity and sharing in the global TCRαβ repertoire reveals that the three epitope-specific CTL populations, formerly described as having distinct profiles of TCR diversity and sharing, are similarly diverse and are shared between individuals to a greater extent than would be predicted based on TCRβ analysis alone.
Discussion
We describe the analysis of paired TCRαβ clonotype usage in CD8+ T cell populations specific for 3 different Db restricted influenza A virus-derived epitopes. These 3 repertoires were previously distinguished on the basis of CDR3β clonotype diversity and sharing within the dominant TRBV subset, with the NP366-specific repertoire characterized as restricted and public (highly shared), while the PA224- and PB1-F262-specific repertoires are considered to exhibit diverse and private (unique to individuals) CDR3β usage. The present analysis of the total TCRαβ epitope-specific repertoire revealed that, unlike for PA224- and PB1-F262-specific cells, the restricted pairing and TCR usage in the prevalent TRBV13-1+ subset of NP366-specific cells bore little resemblance to the remainder of the NP366-specific population. As such, analysis across the total epitope-specific TCRαβ repertoires revealed that TCRαβ clonotype diversity was remarkably similar for all 3 epitope specific CTL populations.
Prior analyses of the NP366, PA224- and PB1-F262-specific predominant Vβ subpopulations (TRBV13-1, 29, and 19, respectively) reveal that the TRBV13-1+ NP366-specific repertoire is notable for its lack of clonal diversity and high level of repertoire sharing between individuals, compared to the PA224- and PB1-F262-specific repertoires. Moreover, for each of these epitope-specific Vβ subsets, preferred Jβ region usage, CDR3β lengths and conserved aa motifs have all been described44, 46–48. However, much less is known about TCRα chain usage in these populations. An analysis of CDR3α usage in bulk TRBV13-1+ NP366-specific T cells demonstrated that TCRα clonal diversity was substantially greater than TCRβ diversity in this subset of cells50. Moreover, our own recent studies utilizing single chain retrogenic mice expressing a TRBV13-1+ or TRBV29+ TCRβ specific for either NP366 or PA224, respectively, demonstrated that the TCRα chain was important for imparting specificity (only a small minority of the retrogenic TCRβ-expressing cells were epitope specific), and that a range of selected TRAVs were able to do so55. However, the current study indicates that TCRα usage is even more diverse than observed in NP366- or PA224-specific single TCRβ chain retrogenic mice and, for all specificities, TCRα usage is at least as diverse as the corresponding TCRβ chains.
Importantly, in the original description of the single cell multiplexed RT-PCR technique it was noted that 10% of all immune CD8+ cells expressed dual in-frame TCRα chains38. While both TCRα chains have the potential to pair with the expressed TCRβ chain, only one is likely to confer specificity. Thus, it remains a possibility that, from some cells within that subset, we are detecting the sequence of an irrelevant TCRα (from a viral antigen perspective). Despite this, we have previously randomly selected and expressed TCRαβ heterodimers identified using this strategy56, and all show the expected epitope specificity. Therefore, we feel confident that this strategy is providing an accurate global representation of epitope-specific TCRαβ prevalence and pairing.
Our previous analysis of pMHCI crystal structures show that both the PA224 and PB1-F262 peptides bound to Db present a relatively ‘featured’ surface for recognition by specific TCRs, by virtue of a prominent arginine residue at positions 7 and 4, respectively. In contrast, the lack of any such prominent features in the NP366 peptide was associated with the highly restricted clonotype usage observed in the TRBV13-1+ NP366-specific TCR repertoire and was thought to reflect the difficulty of navigating recognition of this relatively flat pMHCI complex57. The current study demonstrates that there is in fact a wide range of TCRs capable of DbNP366 recognition, and the overall diversity of clonotype usage in this repertoire is not significantly different to that used in the highly diverse PA224 and PB1-F262 repertoires. However, despite the breadth of TCR clonotype usage, the clones that are apparently able to confer optimal NP366 recognition (based on their preferential expansion in the immune repertoire) are a restricted and relatively uniform subset of NP366-specific clonotypes (TRBV13-1+ set). Indeed, a key finding from this study is that the key characteristics of the prominent TRBV13-1+ subset in the NP366-specific population (CDR3 length, TRAV pairing, J region usage) are distinct from the features associated with the remainder of the NP366-specific population. This is not true of the PA224- and PB1-F262-specific populations, whose preferred TCR characteristics are prominent throughout the epitope-specific population. Thus, TCR recognition of NP366 may not necessarily restrict the number of clones capable of recognition, but may limit the potential docking modes of NP366-specific TCRs, with a consequence being that the TCR populations exhibit restricted and synchronized characteristics.
A number of studies have demonstrated a link between TCR diversity and improved viral control18–24, thought to be due to the larger number of TCR structures increasing the flexibility with which variant epitopes may be recognized 25. The observation that escape mutations occur more frequently in the NP366, compared to the PA224 epitope 58, was thus originally attributed to the restricted clonal diversity of the NP366-specific population, which was thought to be less capable of structural flexibility as a consequence. Our observation that the global NP366-specific TCR repertoire is not less clonally diverse than the PA224- and PB1-F262-specific repertoires suggests that the presence or absence of clonal diversity per se may be an inadequate correlate of protection from virus. Rather, the number of ways that TCRs can recognize a specific pMHCI may be the key determinant of preventing immune evasion. As such, we would argue that despite its clonal diversity, TCR recognition of NP366 is relatively inflexible, as evidenced by the stringent co-segregation of preferred TCR elements, and thus TCR ‘structural diversity’ (rather than clonal diversity) may represent a better correlate of immune protection.
Alternatively, other studies showing that particular TCRs are associated with improved control of virus infection59–63, suggest that any benefit conferred by TCR diversity is likely to be due to the inclusion of TCRs with high affinity for pMHCI 11, 22, 64. Thus, it is possible that despite the clonal diversity of NP366-specific TCRs, the ready emergence of viral variants for this epitope may be due to a poorer quality T cell response, rather than a lack of TCR diversity, compared to PA224. This is partly supported by the observation that polyclonal NP366-specific CTLs exhibit a more rapid off rate and greater dependence on the CD8 co-receptor relative to their PA224-specific counterparts65–67.
The strong bias toward TRAV16 in the NP366-specific repertoire, contrasted somewhat with the less extensive TRAV biases in the PA224- and PB1-F262-specific repertoires and inferred a greater importance of the TCRα chain in conferring pMHCI specificity in the NP366-specific set. In fact, previous structural analysis of a single Db PA224-TCRαβ ternary complex (TRBV29/BJ27, TRAV21/AJ53) showed that the CDR3α loop mediated the majority (68%) of the PA224 peptide contacts, while the CDR3β loop provided only 17% of the peptide contacts68. Moreover, a CDR3α-encoded motif (SNY) mediated critical interactions with the PA224 peptide, while the CDR3β loop engaged the peptide only via main chain interactions. Thus it was suggested that the PA224-specific CDR3β length of 6 aa was important for recognition, while the CDR3α aa sequence (in particular, Tyr112) was critical for PA224 recognition. It remains to be seen whether similar pMHCI docking modes are utilized by all PA224-specific TCRs. While our data certainly shows a broad preference for a CDR3β length of 6 aa, the CDR3α-encoded SNY motif was only found in 20% of all PA224-specific CDR3α clonotypes, with the critical Y aa in this position found in only 32% of those clonotypes (representing only a minor enrichment from 20% in non-antigen specific TCRα chains). Thus, either the mode of TCRαβ docking is substantially altered between PA224-specific TCRs, or other CDR3α encoded aa residues (e.g. Asp, Arg, Lys) provide these seminal contacts to preserve the docking mode.
Collectively, this study is the first analysis of both naïve and immune epitope-specific TCRαβ repertoires. Comparison of these repertoires clearly demonstrates a more restricted TCR usage in the immune, relative to naïve, epitope-specific populations, indicative of selective recruitment and/or expansion of CTLs with preferred TCR characteristics. The data also suggests that, in some cases, analysis of a subset of epitope-specific TCRs does not provide an accurate representation of TCR characteristics or diversity within the total epitope-specific repertoire. Moreover, while all epitope-specific populations analysed exhibited preferences for particular TCR characteristics, the stringency of the context in which those characteristics can be utilized to impart recognition may better define the flexibility of a TCR repertoire, despite similar levels of clonal diversity.
Methods
Mice and virus infections
Female C57BL/6J (H-2b) mice were bred and housed in the animal facility of the Department of Microbiology and Immunology at the University of Melbourne (Parkville, Australia). Naïve 6–10 week-old mice were anesthetized by isofluorane inhalation and infected intranasally (i.n.) with 1 × 104 PFU of the HKx31 (H3N2) influenza A virus in 30μl of PBS. All animal experimentation was conducted following the Australian National Health and Medical Research Council Code of Practice for the Care and Use of Animals for Scientific Purposes guidelines for housing and care of laboratory animals and performed in accordance with Institutional regulations after pertinent review and approval by the University of Melbourne Animal Ethics Committee.
Tetramer and antibody staining
Spleens were collected from mice 10 d after primary influenza virus infection, processed into single cell suspension through a 70 μm sieve, and enriched for T cells by panning on plates coated with Affinipure anti-mouse IgG/IgM Abs (200ug/ml) for 1h at 37°C to remove B cells. Epitope-specific CD8+ T cells were identified by staining with PE conjugated tetrameric complexes of H-2Db MHC class I glycoprotein + nucleoprotein derived NP366–374 (ASNENMETM), acid polymerase derived PA224–233 (SSLENFRAYV), or PB1-F262–70 (LSLRNPILV), from the +1 reading frame of the influenza viral polymerase B subunit peptides (ImmunoID, University of Melbourne) for 1h at RT. Cells were then co-stained with anti-CD8α-FITC before entire samples were acquired on a FACSAria III cell sorter using FACSDiva software (BD Immunocytometry Systems, San Jose, CA, USA). Data were analysed with FlowJo software (Tree Star, Ashland, OR, USA).
Magnetic enrichment and isolation of epitope-specific CD8+ T cells
Tetramer-based magnetic enrichment was used for identification of naïve epitope-specific CD8+ T cell precursors, as described in detail previously 3, 4, 69. Briefly, single cell suspensions of pooled spleen and major LNs (auxiliary, brachial, cervical, inguinal and mesenteric) from naïve mice were stained with PE-labelled influenza-specific NP366, PA224, or PB1-F262 tetramers, washed and labelled with anti-PE conjugated magnetic microbeads, and tetramer-bound cells enriched over a magnetic LS column (Miltenyi Biotec). Enriched cells were then stained with a cocktail of conjugated antibodies to identify epitope-specific cells (CD8α+ CD3ε+ CD62Lhi CD11b- CD11c- B220- F4/80- CD4-). Entire samples were acquired on a FACSAria III cell sorter with FACSDiva software (BD Immunocytometry Systems, San Jose, CA, USA).
Single cell multiplexed RT-PCR
Individual NP366-, PA224- or PB1-F262-specific CD8+ T cells were sorted into wells of a 96-well plate using a BD FACSAria (BD Biosciences). Multiplex single-cell reverse transcription and PCR amplification of TCR CDR3α and CDR3β regions was performed using a panel of TRBV and TRAV specific oligonucleotides, as described 38. Briefly, mRNA was reverse transcribed in 2.5 μl using the Superscript III VILO cDNA Synthesis Kit (containing 1 × Vilo reaction mix, 1 × superscript RT, 0.1% Triton X-100), and incubated at 25°C for 10 min, 42°C for 120 min, and 85°C for 5 min. The entire volume was then used in a 25 μl first round PCR reaction with 1.5U Taq DNA polymerase, 1 × PCR buffer, 1.5 mM MgCL2, 0.25mM dNTPs and a mix of 23 TRAV external sense primers and a TRAC external antisense primer 38, along with 19 TRBV external sense primers and a TRBC external antisense primer (each at 5 pmol/μl) 38, using standard PCR conditions. For the second round nested PCR, a 2.5 μl aliquot of the first round PCR product was used in separate TRBV and TRAV specific PCRs, using the same reaction mix described above, however a set of 23 TRAV internal sense primers and a TRAC internal antisense primer, or a set of 19 TRBV internal sense primers and a TRBV internal antisense primer, were used 38. Second round PCR products were visualized on a gel and positive reactions were purified with ExoSAP-IT reagent. Purified products were used as template in sequencing reactions with internal TRAC or TRBC antisense primers, as described 38, 46. TCR gene segments were assigned using the International ImMunoGeneTics (IMGT) database 70.
Statistical analyses
Simpon’s Diversity Index (SDI) was used to measure the extent of diversity (i.e. a combination of the number and distribution of species) in TRBV and TRAV gene segment usage, and clonotype usage 49, 51, 52. SDI is calculated as D=Σi[(ni(ni-1))/(N(N-1))] where ni is the number of sequences in the _i_th clonotype and N is the total number of sequences in the whole population. To measure clonotype sharing, we determined the proportion of sequences that are found in a specific percentage (q) of the mice analysed, referred to as PTICq (Proportion of TCRs in Common) 49, 71. The Mann-Whitney test, or Students unpaired t-test, was used to determine significance for all individual comparisons.
Supplementary Material
01
Acknowledgments
The authors wish to thank Prof. Stephen J. Turner for critical review of the manuscript. This work was supported by National Health and Medical Research (NHMRC) Project grants AI628316 and AI1046333 (to NLG), a Sylvia and Charles Viertel Senior Medical Research Fellowship (NLG), an Australian Research Council Future Fellowship (SG), an NHMRC Biomedical Postgraduate Scholarship ID520643 (to TC), and an National Institutes of Health grant AI107625 (to PGT).
References
- 1.Miles JJ, Douek DC, Price DA. Bias in the alphabeta T-cell repertoire: implications for disease pathogenesis and vaccination. Immunol Cell Biol. 2011;89(3):375–387. doi: 10.1038/icb.2010.139. [DOI] [PubMed] [Google Scholar]
- 2.Turner SJ, Doherty PC, McCluskey J, Rossjohn J. Structural determinants of T-cell receptor bias in immunity. Nat Rev Immunol. 2006;6(12):883–894. doi: 10.1038/nri1977. [DOI] [PubMed] [Google Scholar]
- 3.La Gruta NL, Rothwell WT, Cukalac T, Swan NG, Valkenburg SA, Kedzierska K, et al. Primary CTL response magnitude in mice is determined by the extent of naive T cell recruitment and subsequent clonal expansion. J Clin Invest. 2010;120(6):1885–1894. doi: 10.1172/JCI41538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27(2):203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gras S, Kjer-Nielsen L, Burrows SR, McCluskey J, Rossjohn J. T-cell receptor bias and immunity. Curr Opin Immunol. 2008;20(1):119–125. doi: 10.1016/j.coi.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 7.Venturi V, Kedzierska K, Price DA, Doherty PC, Douek DC, Turner SJ, et al. Sharing of T cell receptors in antigen-specific responses is driven by convergent recombination. Proc Natl Acad Sci U S A. 2006;103(49):18691–18696. doi: 10.1073/pnas.0608907103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venturi V, Price DA, Douek DC, Davenport MP. The molecular basis for public T-cell responses? Nat Rev Immunol. 2008;8(3):231–238. doi: 10.1038/nri2260. [DOI] [PubMed] [Google Scholar]
- 9.Busch DH, Pamer EG. T cell affinity maturation by selective expansion during infection. The Journal of experimental medicine. 1999;189(4):701–710. doi: 10.1084/jem.189.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day EK, Carmichael AJ, ten Berge IJ, Waller EC, Sissons JG, Wills MR. Rapid CD8+ T cell repertoire focusing and selection of high-affinity clones into memory following primary infection with a persistent human virus: human cytomegalovirus. J Immunol. 2007;179(5):3203–3213. doi: 10.4049/jimmunol.179.5.3203. [DOI] [PubMed] [Google Scholar]
- 11.La Gruta NL, Thomas PG. Interrogating the relationship between naive and immune antiviral T cell repertoires. Curr Opin Virol. 2013;3(4):447–451. doi: 10.1016/j.coviro.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malherbe L, Hausl C, Teyton L, McHeyzer-Williams MG. Clonal selection of helper T cells is determined by an affinity threshold with no further skewing of TCR binding properties. Immunity. 2004;21(5):669–679. doi: 10.1016/j.immuni.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Price DA, Brenchley JM, Ruff LE, Betts MR, Hill BJ, Roederer M, et al. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. The Journal of experimental medicine. 2005;202(10):1349–1361. doi: 10.1084/jem.20051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argaet VP, Schmidt CW, Burrows SR, Silins SL, Kurilla MG, Doolan DL, et al. Dominant selection of an invariant T cell antigen receptor in response to persistent infection by Epstein-Barr virus. J Exp Med. 1994;180(6):2335–2340. doi: 10.1084/jem.180.6.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levitsky V, de Campos-Lima PO, Frisan T, Masucci MG. The clonal composition of a peptide-specific oligoclonal CTL repertoire selected in response to persistent EBV infection is stable over time. J Immunol. 1998;161(2):594–601. [PubMed] [Google Scholar]
- 16.Cohen GB, Islam SA, Noble MS, Lau C, Brander C, Altfeld MA, et al. Clonotype tracking of TCR repertoires during chronic virus infections. Virology. 2002;304(2):474–484. doi: 10.1006/viro.2002.1743. [DOI] [PubMed] [Google Scholar]
- 17.Klarenbeek PL, Remmerswaal EB, ten Berge IJ, Doorenspleet ME, van Schaik BD, Esveldt RE, et al. Deep sequencing of antiviral T-cell responses to HCMV and EBV in humans reveals a stable repertoire that is maintained for many years. PLoS Pathog. 2012;8(9):e1002889. doi: 10.1371/journal.ppat.1002889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charini WA, Kuroda MJ, Schmitz JE, Beaudry KR, Lin W, Lifton MA, et al. Clonally diverse CTL response to a dominant viral epitope recognizes potential epitope variants. J Immunol. 2001;167(9):4996–5003. doi: 10.4049/jimmunol.167.9.4996. [DOI] [PubMed] [Google Scholar]
- 19.Cornberg M, Chen AT, Wilkinson LA, Brehm MA, Kim SK, Calcagno C, et al. Narrowed TCR repertoire and viral escape as a consequence of heterologous immunity. J Clin Invest. 2006;116(5):1443–1456. doi: 10.1172/JCI27804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kosmrlj A, Read EL, Qi Y, Allen TM, Altfeld M, Deeks SG, et al. Effects of thymic selection of the T-cell repertoire on HLA class I-associated control of HIV infection. Nature. 2010;465(7296):350–354. doi: 10.1038/nature08997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo W, Su J, Zhang XB, Yang Z, Zhou MQ, Jiang ZM, et al. Limited T cell receptor repertoire diversity in tuberculosis patients correlates with clinical severity. PloS one. 2012;7(10):e48117. doi: 10.1371/journal.pone.0048117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messaoudi I, Guevara Patino JA, Dyall R, LeMaoult J, Nikolich-Zugich J. Direct link between mhc polymorphism, T cell avidity, and diversity in immune defense. Science. 2002;298(5599):1797–1800. doi: 10.1126/science.1076064. [DOI] [PubMed] [Google Scholar]
- 23.Price DA, West SM, Betts MR, Ruff LE, Brenchley JM, Ambrozak DR, et al. T cell receptor recognition motifs govern immune escape patterns in acute SIV infection. Immunity. 2004;21(6):793–803. doi: 10.1016/j.immuni.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Wang GC, Dash P, McCullers JA, Doherty PC, Thomas PG. T cell receptor alphabeta diversity inversely correlates with pathogen-specific antibody levels in human cytomegalovirus infection. Science translational medicine. 2012;4(128):128ra42. doi: 10.1126/scitranslmed.3003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ladell K, Hashimoto M, Iglesias MC, Wilmann PG, McLaren JE, Gras S, et al. A molecular basis for the control of preimmune escape variants by HIV-specific CD8+ T cells. Immunity. 2013;38(3):425–436. doi: 10.1016/j.immuni.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Ding YH, Smith KJ, Garboczi DN, Utz U, Biddison WE, Wiley DC. Two human T cell receptors bind in a similar diagonal mode to the HLA-A2/Tax peptide complex using different TCR amino acids. Immunity. 1998;8(4):403–411. doi: 10.1016/s1074-7613(00)80546-4. [DOI] [PubMed] [Google Scholar]
- 27.Garboczi DN, Ghosh P, Utz U, Fan QR, Biddison WE, Wiley DC. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996;384(6605):134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- 28.Reiser JB, Gregoire C, Darnault C, Mosser T, Guimezanes A, Schmitt-Verhulst AM, et al. A T cell receptor CDR3beta loop undergoes conformational changes of unprecedented magnitude upon binding to a peptide/MHC class I complex. Immunity. 2002;16(3):345–354. doi: 10.1016/s1074-7613(02)00288-1. [DOI] [PubMed] [Google Scholar]
- 29.Reiser JB, Darnault C, Guimezanes A, Gregoire C, Mosser T, Schmitt-Verhulst AM, et al. Crystal structure of a T cell receptor bound to an allogeneic MHC molecule. Nature immunology. 2000;1(4):291–297. doi: 10.1038/79728. [DOI] [PubMed] [Google Scholar]
- 30.Rossjohn J, Gras S, Miles JJ, Turner SJ, Godfrey DI, McCluskey J. T cell antigen receptor recognition of antigen-presenting molecules. Annu Rev Immunol. 2015;33 doi: 10.1146/annurev-immunol-032414-112334. [DOI] [PubMed] [Google Scholar]
- 31.Yokosuka T, Takase K, Suzuki M, Nakagawa Y, Taki S, Takahashi H, et al. Predominant role of T cell receptor (TCR)-alpha chain in forming preimmune TCR repertoire revealed by clonal TCR reconstitution system. J Exp Med. 2002;195(8):991–1001. doi: 10.1084/jem.20010809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner SJ, Cose SC, Carbone FR. TCR alpha-chain usage can determine antigen-selected TCR beta-chain repertoire diversity. J Immunol. 1996;157(11):4979–4985. [PubMed] [Google Scholar]
- 33.Mikszta JA, McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-driven selection of TCR In vivo: related TCR alpha-chains pair with diverse TCR beta-chains. J Immunol. 1999;163(11):5978–5988. [PubMed] [Google Scholar]
- 34.Coles RM, Jones CM, Brooks AG, Cameron PU, Heath WR, Carbone FR. Virus infection expands a biased subset of T cells that bind tetrameric class I peptide complexes. Eur J Immunol. 2003;33(6):1557–1567. doi: 10.1002/eji.200323715. [DOI] [PubMed] [Google Scholar]
- 35.Hasegawa A, Moriya C, Liu H, Charini WA, Vinet HC, Subbramanian RA, et al. Analysis of TCRalphabeta combinations used by simian immunodeficiency virus-specific CD8+ T cells in rhesus monkeys: implications for CTL immunodominance. J Immunol. 2007;178(6):3409–3417. doi: 10.4049/jimmunol.178.6.3409. [DOI] [PubMed] [Google Scholar]
- 36.Robins HS, Campregher PV, Srivastava SK, Wacher A, Turtle CJ, Kahsai O, et al. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood. 2009;114(19):4099–4107. doi: 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang C, Sanders CM, Yang Q, Schroeder HW, Jr., Wang E, Babrzadeh F, et al. High throughput sequencing reveals a complex pattern of dynamic interrelationships among human T cell subsets. Proc Natl Acad Sci U S A. 2010;107(4):1518–1523. doi: 10.1073/pnas.0913939107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dash P, McClaren JL, Oguin TH, 3rd, Rothwell W, Todd B, Morris MY, et al. Paired analysis of TCRalpha and TCRbeta chains at the single-cell level in mice. J Clin Invest. 2011;121(1):288–295. doi: 10.1172/JCI44752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eugster A, Lindner A, Heninger AK, Wilhelm C, Dietz S, Catani M, et al. Measuring T cell receptor and T cell gene expression diversity in antigen-responsive human CD4+ T cells. Journal of immunological methods. 2013;400–401:13–22. doi: 10.1016/j.jim.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Ozawa T, Tajiri K, Kishi H, Muraguchi A. Comprehensive analysis of the functional TCR repertoire at the single-cell level. Biochem Biophys Res Commun. 2008;367(4):820–825. doi: 10.1016/j.bbrc.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Sun X, Saito M, Sato Y, Chikata T, Naruto T, Ozawa T, et al. Unbiased analysis of TCRalpha/beta chains at the single-cell level in human CD8+ T-cell subsets. PloS one. 2012;7(7):e40386. doi: 10.1371/journal.pone.0040386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobayashi E, Mizukoshi E, Kishi H, Ozawa T, Hamana H, Nagai T, et al. A new cloning and expression system yields and validates TCRs from blood lymphocytes of patients with cancer within 10 days. Nat Med. 2013;19(11):1542–1546. doi: 10.1038/nm.3358. [DOI] [PubMed] [Google Scholar]
- 43.Deckhut AM, Allan W, McMickle A, Eichelberger M, Blackman MA, Doherty PC, et al. Prominent usage of V beta 8.3 T cells in the H-2Db-restricted response to an influenza A virus nucleoprotein epitope. J Immunol. 1993;151(5):2658–2666. [PubMed] [Google Scholar]
- 44.La Gruta NL, Thomas PG, Webb AI, Dunstone MA, Cukalac T, Doherty PC, et al. Epitope-specific TCRbeta repertoire diversity imparts no functional advantage on the CD8+ T cell response to cognate viral peptides. Proc Natl Acad Sci U S A. 2008;105(6):2034–2039. doi: 10.1073/pnas.0711682102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belz GT, Stevenson PG, Doherty PC. Contemporary analysis of MHC-related immunodominance hierarchies in the CD8+ T cell response to influenza A viruses. J Immunol. 2000;165(5):2404–2409. doi: 10.4049/jimmunol.165.5.2404. [DOI] [PubMed] [Google Scholar]
- 46.Turner SJ, Diaz G, Cross R, Doherty PC. Analysis of clonotype distribution and persistence for an influenza virus-specific CD8+ T cell response. Immunity. 2003;18(4):549–559. doi: 10.1016/s1074-7613(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 47.Kedzierska K, Turner SJ, Doherty PC. Conserved T cell receptor usage in primary and recall responses to an immunodominant influenza virus nucleoprotein epitope. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(14):4942–4947. doi: 10.1073/pnas.0401279101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhong W, Reinherz EL. In vivo selection of a TCR Vbeta repertoire directed against an immunodominant influenza virus CTL epitope. Int Immunol. 2004;16(11):1549–1559. doi: 10.1093/intimm/dxh156. [DOI] [PubMed] [Google Scholar]
- 49.Thomas PG, Handel A, Doherty PC, La Gruta NL. Ecological analysis of antigen-specific CTL repertoires defines the relationship between naive and immune T-cell populations. Proc Natl Acad Sci U S A. 2013;110(5):1839–1844. doi: 10.1073/pnas.1222149110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhong W, Dixit SB, Mallis RJ, Arthanari H, Lugovskoy AA, Beveridge DL, et al. CTL recognition of a protective immunodominant influenza A virus nucleoprotein epitope utilizes a highly restricted Vbeta but diverse Valpha repertoire: functional and structural implications. J Mol Biol. 2007;372(2):535–548. doi: 10.1016/j.jmb.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 51.Magurran AE. Measuring biological diversity. Blackwell Publishing; 2004. [Google Scholar]
- 52.Venturi V, Kedzierska K, Turner SJ, Doherty PC, Davenport MP. Methods for comparing the diversity of samples of the T cell receptor repertoire. Journal of immunological methods. 2007;321(1–2):182–195. doi: 10.1016/j.jim.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 53.Petersen J, Montserrat V, Mujico JR, Loh KL, Beringer DX, van Lummel M, et al. T-cell receptor recognition of HLA-DQ2-gliadin complexes associated with celiac disease. Nat Struct Mol Biol. 2014;21(5):480–488. doi: 10.1038/nsmb.2817. [DOI] [PubMed] [Google Scholar]
- 54.Cibotti R, Cabaniols JP, Pannetier C, Delarbre C, Vergnon I, Kanellopoulos JM, et al. Public and private V beta T cell receptor repertoires against hen egg white lysozyme (HEL) in nontransgenic versus HEL transgenic mice. J Exp Med. 1994;180(3):861–872. doi: 10.1084/jem.180.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clemens EB, Doherty PC, La Gruta NL, Turner SJ. Fixed Expression of Single Influenza Virus-Specific TCR Chains Demonstrates the Capacity for TCR alpha- and beta-Chain Diversity in the Face of Peptide-MHC Class I Specificity. J Immunol. 2015;194(3):898–910. doi: 10.4049/jimmunol.1401792. [DOI] [PubMed] [Google Scholar]
- 56.Cukalac T, Chadderton J, Handel A, Doherty PC, Turner SJ, Thomas PG, et al. Reproducible selection of high avidity CD8+ T-cell clones following secondary acute virus infection. Proc Natl Acad Sci U S A. 2014;111(4):1485–1490. doi: 10.1073/pnas.1323736111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turner SJ, Kedzierska K, Komodromou H, La Gruta NL, Dunstone MA, Webb AI, et al. Lack of prominent peptide-major histocompatibility complex features limits repertoire diversity in virus-specific CD8+ T cell populations. Nature immunology. 2005;6(4):382–389. doi: 10.1038/ni1175. [DOI] [PubMed] [Google Scholar]
- 58.Valkenburg SA, Quinones-Parra S, Gras S, Komadina N, McVernon J, Wang Z, et al. Acute emergence and reversion of influenza A virus quasispecies within CD8+ T cell antigenic peptides. Nat Commun. 2013;4:2663. doi: 10.1038/ncomms3663. [DOI] [PubMed] [Google Scholar]
- 59.Dong T, Stewart-Jones G, Chen N, Easterbrook P, Xu X, Papagno L, et al. HIV-specific cytotoxic T cells from long-term survivors select a unique T cell receptor. The Journal of experimental medicine. 2004;200(12):1547–1557. doi: 10.1084/jem.20032044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gillespie GM, Stewart-Jones G, Rengasamy J, Beattie T, Bwayo JJ, Plummer FA, et al. Strong TCR conservation and altered T cell cross-reactivity characterize a B*57-restricted immune response in HIV-1 infection. Journal of immunology. 2006;177(6):3893–3902. doi: 10.4049/jimmunol.177.6.3893. [DOI] [PubMed] [Google Scholar]
- 61.Yu XG, Lichterfeld M, Chetty S, Williams KL, Mui SK, Miura T, et al. Mutually exclusive T-cell receptor induction and differential susceptibility to human immunodeficiency virus type 1 mutational escape associated with a two-amino-acid difference between HLA class I subtypes. J Virol. 2007;81(4):1619–1631. doi: 10.1128/JVI.01580-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Bockel DJ, Price DA, Munier ML, Venturi V, Asher TE, Ladell K, et al. Persistent survival of prevalent clonotypes within an immunodominant HIV gag-specific CD8+ T cell response. Journal of immunology. 2011;186(1):359–371. doi: 10.4049/jimmunol.1001807. [DOI] [PubMed] [Google Scholar]
- 63.Chen H, Ndhlovu ZM, Liu D, Porter LC, Fang JW, Darko S, et al. TCR clonotypes modulate the protective effect of HLA class I molecules in HIV-1 infection. Nature immunology. 2012;13(7):691–700. doi: 10.1038/ni.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Derby M, Alexander-Miller M, Tse R, Berzofsky J. High-avidity CTL exploit two complementary mechanisms to provide better protection against viral infection than low-avidity CTL. Journal of immunology. 2001;166(3):1690–1697. doi: 10.4049/jimmunol.166.3.1690. [DOI] [PubMed] [Google Scholar]
- 65.Turner SJ, La Gruta NL, Stambas J, Diaz G, Doherty PC. Differential tumor necrosis factor receptor 2-mediated editing of virus-specific CD8+ effector T cells. Proc Natl Acad Sci U S A. 2004;101(10):3545–3550. doi: 10.1073/pnas.0307347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.La Gruta NL, Doherty PC, Turner SJ. A correlation between function and selected measures of T cell avidity in influenza virus-specific CD8+ T cell responses. Eur J Immunol. 2006;36(11):2951–2959. doi: 10.1002/eji.200636390. [DOI] [PubMed] [Google Scholar]
- 67.La Gruta NL, Turner SJ, Doherty PC. Hierarchies in cytokine expression profiles for acute and resolving influenza virus-specific CD8+ T cell responses: correlation of cytokine profile and TCR avidity. J Immunol. 2004;172(9):5553–5560. doi: 10.4049/jimmunol.172.9.5553. [DOI] [PubMed] [Google Scholar]
- 68.Day EB, Guillonneau C, Gras S, La Gruta NL, Vignali DA, Doherty PC, et al. Structural basis for enabling T-cell receptor diversity within biased virus-specific CD8+ T-cell responses. Proc Natl Acad Sci U S A. 2011;108(23):9536–9541. doi: 10.1073/pnas.1106851108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28(6):859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lefranc MP. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 2001;29(1):207–209. doi: 10.1093/nar/29.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kedzierska K, Thomas PG, Venturi V, Davenport MP, Doherty PC, Turner SJ, et al. Terminal deoxynucleotidyltransferase is required for the establishment of private virus-specific CD8+ TCR repertoires and facilitates optimal CTL responses. J Immunol. 2008;181(4):2556–2562. doi: 10.4049/jimmunol.181.4.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01