Crossroads of stress responses, development and flowering regulation—the multiple roles of Cyclic Nucleotide Gated Ion Channel 2 (original) (raw)
Abstract
The Arabidopsis autoimmune mutant, defense-no death 1 (dnd1) is a null mutant of CYCLIC NUCLEOTIDE-GATED ION CHANNEL2 (AtCNGC2). dnd1 exhibits constitutive pathogen resistance responses including higher levels of endogenous salicylic acid (SA), which is an important signaling molecule for pathogen defense responses. Recently we have reported that dnd1 exhibits a significantly delayed flowering phenotype, indicating the involvement of AtCNGC2 in flowering transition. However, since SA has been known to influence flowering timing as a positive regulator, the delayed flowering phenotype in dnd1 was unexpected. In this study, we have asked whether SA is involved in the _dnd1_-mediated delayed flowering phenotype. In addition, in order to gain insight into the involvement of SA and CNGCs in flowering transition, we analyzed the flowering transition of cpr22, another CNGC mutant with a similar autoimmune phenotype as dnd1 (including high SA accumulation), and null mutants of several other CNGCs. Our data suggest that dnd1 does not require SA or SA signaling for its delayed flowering phenotype, while SA was responsible for the early flowering phenotype of cpr22. None of the other CNGC mutants besides AtCNGC41 displayed an alteration in flowering transition. This indicates that AtCNGC2 and AtCNGC4 have a unique role controlling flowering timing and this function is independent from its role in pathogen defense.
Keywords: cyclic nucleotide-gated ion channel, CNGC, cpr22, dnd1, defense-no-death 1, flowering, salicylic acid
Abbreviation
CNGC
cyclic nucleotide-gated ion channel
SA
salicylic acid
Cyclic nucleotide-gated ion channels (CNGCs) are non-selective cation channels that were first identified in animals, where they play key roles in light and olfactory signaling. In mammals, there are six genes that encode CNGCs and the typical mammalian CNGC consists of 4 CNGC subunits. The predicted structures of plant CNGCs are similar to their animal counterparts; however, in plants an expansion of the CNGC family occurred. The Arabidopsis thaliana genome has 20 members in the CNGC family, which are classified into four groups (group I-IV), where group IV is further divided into subgroup IVA and IVB.2 This expansion may indicate diverse biological roles of CNGCs in plants. They have been implicated in a diverse range of biological phenomena such as defense responses, pollen tube growth, ion homeostasis and thermo-tolerance.2,3 In addition, recent electrophysiological studies showed that plant CNGCs are likely Ca2+ permeable channels that are involved in a variety of physiological phenomena.3-5
Group IVB comprises only the 2 most divergent plant CNGCs, AtCNGC2 and AtCNGC4. Both are reported to be involved in pathogen defense responses, as loss-of-function mutants of AtCNGC2 or AtCNGC4 show remarkably similar autoimmune phenotypes. The null mutant of AtCNGC2, “_defense, no death1_” (dnd1), has been extensively characterized and is known as a rare autoimmune mutant with impaired hypersensitive responses (HR).6 The HR is a characteristic defense response, which is a type of programmed cell death around the sites of pathogen entry. Despite the impairment of HR upon pathogen infection, the dnd1 mutant displays constitutive defense responses, such as elevated expression of Pathogenesis-Related (PR) genes, high levels of salicylic acid (SA) - an important signaling molecule for resistance against biotrophic pathogens, and conditional HR-like spontaneous lesions without pathogen infection. Consequently, dnd1 plants show enhanced broad spectrum resistance against several taxonomically unrelated pathogens. In addition, it exhibits characteristic morphological phenotypes, such as small stature and senescence-like chlorosis at the tips of the leaves, indicating roles of AtCNGC2 in both defense and development.7
Recently, we discovered a novel phenotype in dnd1, which is a delayed flowering transition observed under both long and short day conditions, although enhanced in the latter condition1 (Fig. 1A). Flowering transition is tightly regulated by endogenous and external cues. In addition, it is known that various stresses, such as ultraviolet-C radiation, pathogen infection and extreme temperatures can promote flowering.8 Interestingly, it has been reported that SA positively regulates flowering timing in Arabidopsis.9 SA-deficient mutants, such as nahG, sid2 and eds5/sid1, exhibit late flowering phenotypes, while SA hyper-accumulating mutants, such as acd6 or siz1 show early flowering transition, supporting this notion.9,10,11
Figure 1.
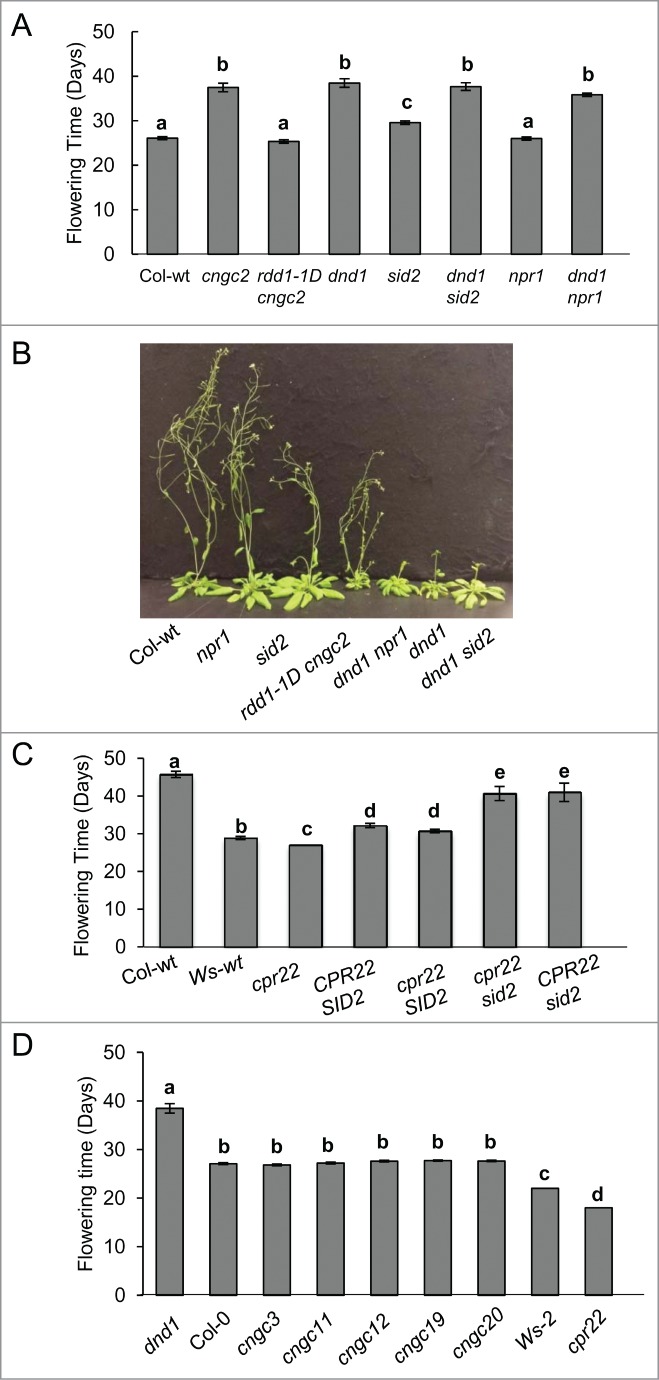
Flowering phenotypes in various CNGC-related mutants. (A) The delayed flowering phenotype in dnd1 is SA/NPR1 independent. rdd1–1D cngc2, the suppressor of dnd1, served as a control. n = 12–25. (B) Flowering phenotype of about 5 week old Col-wt and mutant plants. (C) The early flowering phenotype of cpr22 is SA-dependent. cpr22 has Ws and sid2 has Col ecotype background. n = 10–35. (D) Flowering phenotype of various CNGC T-DNA Insertion lines, n = 21–31. Flowering time was measured as described in Chin et al. (2013). Error bars = SE, Bars marked with the same letter indicate no significant difference (Student's t-test, P < 0.05). Plants were grown in Sunshine Mix #1 with a photoperiod of 16 h light and 8 h dark. All experiments have been repeated at least 3 times with similar results.
Contrary to the positive role of SA, HOPW1-INTERACTING3 (WIN3), which positively regulates broad-spectrum disease resistance through SA signaling, supresses flowering transition.10 Thus, the relationship of SA, defense activation and flowering timing is complex. This raises several questions regarding the delayed flowering phenotype in dnd1 in spite of the high levels of SA accumulation: 1) does SA play a role in the delayed flowering transition phenotype in dnd1?, 2) is the flowering transition phenotype in dnd1 a by-product of hyper-activation of defense signaling_?,_ and 3) are other CNGCs also involved in the regulation of flowering? To address the first and second questions, we monitored the timing of flowering transition in double mutants of dnd1 with SA-deficient and defense signaling mutants. SID2 (ICS1) is a major SA biosynthesis gene for defense responses; thus, dnd1 sid2 exhibits reduced levels of SA compared to the dnd1 single mutant.12 NPR1 is a major component of SA signaling and npr1 mutants show a deficiency in SA-induced defense responses.13 It has been reported that dnd1 npr1 exhibits similar susceptibility to wild type plants against pathogens; thus, the enhanced pathogen resistance of dnd1 is _NPR1_-dependent.12 As shown in Fig. 1A and B, both double mutants, dnd1 sid2 and dnd1 npr1, exhibited no significant difference in flowering transition from the dnd1 single mutant, indicating that the delayed flowering transition phenotype in dnd1 is independent from SA accumulation or _NPR1_-mediated defense activation.
To test whether other CNGC mutants that are related to SA and defense responses also show similar delayed flowering transition phenotypes, we monitored constitutive expresser of PR genes22 (cpr22). cpr22 displays autoimmune phenotypes with increased SA accumulation and constitutive PR gene expression, similar to dnd1.14 It is a gain-of-function mutant and its phenotype is due to the expression of the chimeric AtCNGC 11/12 gene.14 As show in Fig. 1C, cpr22 does not show delayed flowering transition. Rather we observed a consistent early flowering phenotype in cpr22 compared to its wild type, (Wassilewskija (Ws)). This indicates that elevated SA levels in cpr22 promote flowering transition, as expected by the positive role of SA in flowering transition.9 To further address this question, we monitored flowering transition in the double mutant of cpr22 and sid2. Since cpr22 has a Ws and sid2 has a Columbia ecotype background, we used mixed background lines from a cpr22 x sid2 cross for this analysis. As expected, cpr22 SID2 showed earlier flowering transition than CPR22 SID2 wild type by a few days (Fig. 1C). Also, CPR22 sid2 showed delayed flowering, as expected. Interestingly, the double mutant cpr22 sid2 showed almost the same flowering transition as CPR22 sid2, indicating that the earlier flowering phenotype in cpr22 is due to its SA accumulation. This agrees well with the reported positive role of SA in flowering transition, unlike what we observe in dnd1.
Although they share similar autoimmune phenotypes, cpr_22 (AtCNGC 11/12) is a gain-of-function and dnd1 (atcngc2) is a loss-of-function mutant of CNGCs. In addition, the loss-of-function mutants for AtCNGC11 and 12 (atcngc 11 and atcngc12) show a partial breakdown of pathogen resistance.14. These data indicate a striking difference in the molecular mechanisms that govern defense signaling mediated by AtCNGC11 and 12 from that of AtCNGC2.14,15 Thus, the flowering phenotype difference between cpr22 and dnd1 is not surprising. However, it is possible that some CNGC members share a common role in flowering transition and the loss-of-function of any CNGCs (loss of their channel function) might cause a similar late flowering phenotype that is not related to SA. To address this point, we have monitored various CNGC loss-of-function mutants including cngc11 and cngc12. However, as shown in Figure 1D, knockout mutants for AtCNGC3, 11, 12, 19 and 20 did not exhibit any significant delay in flowering transition, suggesting that it is not a common feature in CNGC knockout mutants. Recently, we showed that null mutants of AtCNGC4, which display very similar autoimmune phenotypes as dnd1, also have delayed flowering phenotypes,_ and that AtCNGC2 and 4 likely form a channel complex together.1 In other words, these data suggested that the 2 class IVB CNGCs have a unique role in flowering transition.
The extensive analyses of the dnd1 mutant makes _At_CNGC2 the best-characterized CNGC member and it has been suggested that AtCNGC2 transduces the Ca2+ signal after pathogen infection upon recognition of Pathogen Associated Molecular Patterns (PAMPs).16 In this work, we demonstrate that the novel delayed flowering phenotype in dnd1 is not a by-product of its autoimmune phenotype or SA accumulation. It is likely another authentic biological role of AtCNGC2 (and AtCNGC4) and is likely unique among CNGCs. Further analysis of the molecular mechanism of the delayed flowering transition in dnd1 and cngc4 will shed light on this novel biological function of CNGCs in flowering.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We greatly appreciate Dr. Andrew Bent for providing the seeds of various double mutants of dnd1.
Funding
This project was supported by a Discovery Grant from NSERC (Natural Science and Engineering Research Council of Canada), CFI (Canadian Foundation for Innovation), and ORF (Ontario Research Fund) to KY, and a graduate student fellowship from the Ontario government to KC and HU.
References
- 1.Chin K, DeFalco TA, Moeder W, Yoshioka K. The Arabidopsis cyclic nucleotide-gated ion channels AtCNGC2 and AtCNGC4 work in the same signaling pathway to regulate pathogen defense and floral transition. Plant Physiol 2013; 16: 611-24; PMID:24027242; http://dx.doi.org/ 10.1104/pp.113.225680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chin K, Moeder W, Yoshioka K. Biological roles of cyclic-nucleotide-gated ion channels in plants: what we know and don't know about this 20 member ion channel family. Botany 2009; 87: 668-77; http://dx.doi.org/ 10.1139/B08-147 [DOI] [Google Scholar]
- 3.Finka A, Cuendet AF, Maathuis FJ, Saidi Y, Goloubinoff P. Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 2012; 24: 3333-3348; PMID:22904147; http://dx.doi.org/ 10.1105/tpc.112.095844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao QF, Fei CF, Dong JY, Gu LL, Wang YF1. Arabidopsis CNGC18 Is a Ca2+-permeable channel. Molecular Plant 2014; 7: 739-43; PMID:24380879; http://dx.doi.org/ 10.1093/mp/sst174 [DOI] [PubMed] [Google Scholar]
- 5.Zhou L, Lan W, Jiang Y, Fang W, Luan S. A calcium-dependent protein kinase interacts with and activates a calcium channel to regulate pollen tube growth. Mol Plant 2014; 7: 369-76; PMID:24121288; http://dx.doi.org/ 10.1093/mp/sst125 [DOI] [PubMed] [Google Scholar]
- 6.Clough SJ, Fengler KA, Yu IC, Lippok B, Smith RK, Jr, Bent AF. The Arabidopsis dnd1 ‘defense, no death’ gene encodes a mutated cyclic nucleotide-gated ion channel. Proc Natl Acad Sci USA 2000; 97: 9323-8; PMID:10900264; http://dx.doi.org/ 10.1073/pnas.150005697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma W, Smigel A, Walker RK, Moeder W, Yoshioka K, Berkowitz GA. Leaf senescence signaling: The Ca2+-conducting Arabidopsis cyclic nucleotide gated channel2 acts through nitric oxide to repress senescence programming. Plant Physiol 2010; 154: 733-43; PMID:20699402; http://dx.doi.org/ 10.1104/pp.110.161356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raskin I. Role of salicylic acid in plants. Annu Rev Plant Physiol Plant Mol Biol 1992; 43: 439-63; http://dx.doi.org/ 10.1146/annurev.pp.43.060192.002255 [DOI] [Google Scholar]
- 9.Martínez C, Pons E, Prats G, León J. Salicylic acid regulates flowering time and links defence responses and reproductive development. Plant J 2004; 37: 209-17; PMID:14690505; http://dx.doi.org/ 10.1046/j.1365-313X.2003.01954.x [DOI] [PubMed] [Google Scholar]
- 10.Wang GF, Seabolt S, Hamdoun S, Ng G, Park J, Lu H. Multiple roles of WIN3 in regulating disease resistance, cell death, and flowering time in Arabidopsis. Plant Physiol 2011; 1563: 1508-19; PMID:21543726; http://dx.doi.org/ 10.1104/pp.111.176776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin JB, Jin YH, Lee J, Miura K, Yoo CY, Kim WY, Van Oosten M, Hyun Y, Somers DE, Lee I, et al. The SUMO E3 ligase, AtSIZ1, regulates flowering by controlling a salicylic acid-mediated floral promotion pathway and through affects on FLC chromatin structure. Plant J 2008; 53: 530-40; PMID:18069938; http://dx.doi.org/ 10.1111/j.1365-313X.2007.03359.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genger RK, Jurkowski GI, McDowell JM, Lu H, Jung HW, Greenberg JT, Bent AF. Signaling pathways that regulate the enhanced disease resistance of Arabidopsis ‘defense, no death’ mutants. Mol Plant Microbe Interact 2008; 10: 1285-96; PMID:18785824; http://dx.doi.org/ 10.1094/MPMI-21-10-1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao H, Bowling SA, Gordon AS, Dong X. Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 1994; 6: 1583-92; PMID:12244227; http://dx.doi.org/ 10.1105/tpc.6.11.1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshioka K, Moeder W, Kang HG, Kachroo P, Masmoudi K, Berkowitz G, Klessig DF. The chimeric Arabidopsis CYCLIC NUCLEOTIDE-GATED ION CHANNEL11/12 activates multiple pathogen resistance responses. Plant Cell 2006; 18: 747-63; PMID:16461580; http://dx.doi.org/ 10.1105/tpc.105.038786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moeder W, Urquhart W, Ung H, Yoshioka K. The role of cyclic nucleotide-gated ion channels in plant immunity. Mol Plant 2011; 4: 442-52; PMID:21459831; http://dx.doi.org/ 10.1093/mp/ssr018 [DOI] [PubMed] [Google Scholar]
- 16.Ali R, Ma W, Lemtiri-Chlieh F, Tsaltas D, Leng Q, von Bodman S, Berkowitz GA. Death don't have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATEDCHANNEL2 and innate immunity. Plant Cell 2007; 19: 1082-95; PMID:17384171; http://dx.doi.org/ 10.1105/tpc.106.045096 [DOI] [PMC free article] [PubMed] [Google Scholar]