Middle East Respiratory Syndrome Coronavirus Superspreading Event Involving 81 Persons, Korea 2015 (original) (raw)
Abstract
Since the first imported case of Middle East respiratory syndrome coronavirus (MERS-CoV) infection was reported on May 20, 2015 in Korea, there have been 186 laboratory-confirmed cases of MERS-CoV infection with 36 fatalities. Ninety-seven percent (181/186) of the cases had exposure to the health care facilities. We are reporting a superspreading event that transmitted MERS-CoV to 81 persons at a hospital emergency room (ER) during the Korean outbreak in 2015. The index case was a 35-yr-old man who had vigorous coughing while staying at the ER for 58 hr. As in severe acute respiratory syndrome outbreaks, superspreading events can cause a large outbreak of MERS in healthcare facilities with severe consequences. All healthcare facilities should establish and implement infection prevention and control measure as well as triage policies and procedures for early detection and isolation of suspected MERS-CoV cases.
Keywords: MERS, Coronavirus, Superspreading Event, Emergency Room, Prevention
Middle East respiratory syndrome coronavirus (MERS-CoV) infection is a severe respiratory disease that has recently emerged in the Middle East (1). As of September 30, 2015, 26 countries reported 1,589 laboratory-confirmed cases of infection with MERS-CoV, including 567 deaths, to the World Health Organization (2). Most cases of MERS-CoV infection have occurred in the Middle East, although travel-associated MERS cases have been reported by 16 countries outside the Middle East (3).
Since the first case of MERS-CoV infection in Korea was reported on May 2015 in a traveler returning from the Middle East (4), there have been 186 laboratory-confirmed cases with 36 fatalities. It is the largest outbreak outside the Middle East and 97% (181/186) of all cases were associated with healthcare facilities. The largest 4 hospital clusters (91, 36, 14, 11 cases per each hospital) account for 82% of all cases (5). Here, we report a superspreading event of MERS-CoV involving 81 persons at an emergency room (ER).
A 35-yr-old Korean man was admitted to Hospital A, on May 13, 2015, with a seven day history of fever and productive cough. He did not have any pre-existing diseases. Chest radiography and computed tomography on admission showed patchy consolidation in his left lung. Empirical antibiotics were introduced and his symptoms improved gradually but fever was rebounded on scheduled discharge date. On May 20, 2015, he was discharged with a body temperature of 38℃. During his stay at this hospital, he was unknowingly exposed to MERS-CoV during May 15 to 17, when the first imported case of MER-CoV infection in Korea was admitted to the same ward.
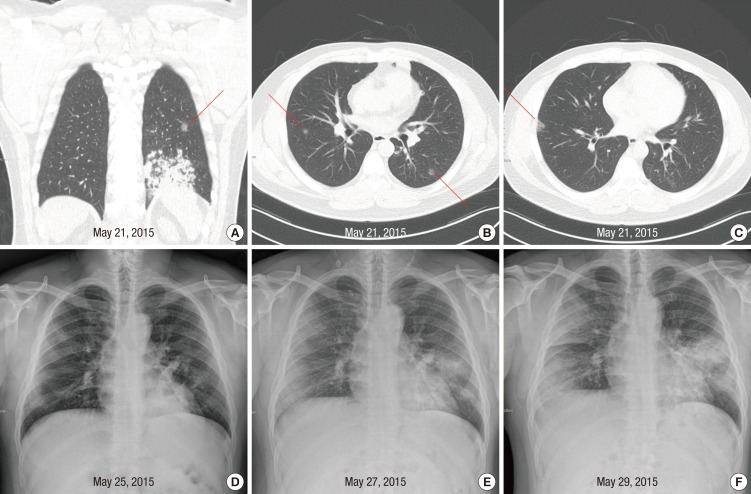
On May 21, he was readmitted to Hospital A with fever of 38.3℃. Computed tomography performed on readmission revealed slight improvement of consolidation in left lung but newly appearing small ground glass nodules in multiple lung fields (Fig. 1A, B, and C). His symptoms did not improve despite changing the antibiotics. As his fever continued and diarrhea developed, he sought another hospital (Hospital B) for treatment on May 25. Chest radiography showed faint infiltrates in both lung fields (Fig. 1D). On May 27, chest infiltrates became more prominent and intermittent tachypnea developed. The attending physician of Hospital B recommended that he visited a tertiary referral hospital.
Fig. 1. Abnormalities on chest imaging of the patient. Shown are computed tomography scans of the chest of the patient, obtained on May 21, 2015 (A, B, and C). Pre-existing pneumonic consolidation in the left lung (A) and newly appearing ground glass nodules were observed (A, B, and C, red lines). (D) is chest radiograph of the patient on May 25, 2015. Faint infiltrates were shown in both lung fields. (E) and (F) are chest radiographs of the patient on May 27 and May 29, 2015 respectively, when the patient stayed at the emergency room. Multiple patchy, opacities became more prominent on both lungs on May 27, 2015 and 2 days later, the opacities became more confluent.
On May 27, he was admitted to the ER of Hospital C, located in Seoul. On arrival, because he had pneumonia, a facemask was placed on the patient. Chest radiography showed multiple patchy opacities in both lung fields (Fig. 1E). Twelve hours later, as his dyspnea worsened and oxygen saturation decreased to 90%, oxygen supplementation at a rate of two liters per minute via nasal cannula was started. During his 58 hr stay in the ER, the location of his bed was changed several times. He walked around the ER and other places nearby while coughing frequently and oftentimes removing his facemask. He went to the toilet in the ER several times to expectorate in an effort to clear his throat. In addition, he had watery diarrhea up to seven times per 24 hr.
On May 29, KCDC notified the attending doctor that the patient had been exposed to MERS-CoV at Hospital A. Chest radiography showed rapid progression of the infiltrates (Fig. 1F). He was immediately transferred to an isolation room in the medical intensive care unit, where he was intubated for mechanical ventilation. On May 30, real-time reverse-transcriptase-polymerase-chain-reaction (RT-PCR) assay on a sputum specimen obtained on May 29 was returned as positive for MERS-CoV. As a result, he was transferred on the same day to an isolation unit of a fourth hospital, a MERS treatment hospital designated by the government.
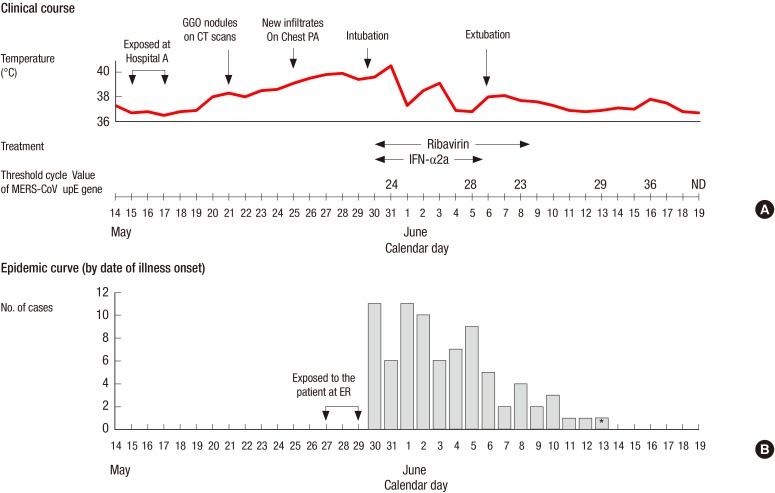
Fig. 2A shows the clinical course of the patient. After the diagnosis of MERS-CoV infection was made, pegylated interferon alpha-2a was given by subcutaneous injection at a dose of 180 µg per week for 2 weeks and oral ribavirin (1,200 mg loading dose, followed by 400 mg every 8 hr for 4 days then 200 mg every 6 hr for 6 days) was given. He received methylprednisolone 60 mg intravenously daily over 11 days for treatment of acute respiratory distress syndrome. The laboratory tests showed mild changes including leukopenia, hypoalbuminemia, proteinuria, and liver enzyme elevation (Table 1). His condition improved continuously and mechanical ventilation was discontinued on June 6. On June 21, he was removed from the isolation room after the two consecutive sputum specimens collected at 48 hr interval tested negative for MERS-CoV on RT-PCR assay. He was discharged the following day.
Fig. 2. Clinical course of the patient and the epidemic curve for the cases of Middle East respiratory syndrome coronavirus infections directly exposed to the patient. The patient had productive cough due to pneumonia in his left lung prior to the onset of MERS-CoV infection. New infiltrates on chest radiograph and dyspnea developed on May 25, 2015, and 5 days later respiratory failure developed (A). Of the cluster of 91 cases related to Hospital C, 81 had exposure to the patient at the emergency room. Among 81 cases, the date of symptoms onset was not available in four cases. The incubation period ranged from 2 to 16 days, with a median of 6 days (B). *The case had another exposure to a family member with MERS-CoV infection between 8 to 10 days prior to onset of symptom. GGO, ground glass opacity; CT, computed tomography; MERS-CoV, Middle East respiratory syndrome coronavirus; INF-α2a, interferon-alpha2a.
Table 1. Laboratory data for the patient.
| Day | White blood cells (cell/µL) | Lymphocytes (cell/µL) | Hemoglobin (g/dL) | Platelets (cell/µL) | Creatinine (mg/dL) | Albumin (g/dL) | AST (U/L) | ALT (U/L) | PT (INR) | Urine albumin in dipstick test |
|---|---|---|---|---|---|---|---|---|---|---|
| May 21 | 8,280 | 3,146 | 18.5 | 339,000 | 1.03 | 4.7 | 42 | 74 | ND | ND |
| May 25 | 5,200 | 634 | 16.6 | 164,000 | 1.0 | 4.1 | 50 | 57 | 1.26 | - |
| May 27 | 4,000 | 688 | 15.9 | 122,000 | 1.0 | 3.6 | 55 | 46 | ND | ND |
| May 30 | 5,260 | 652 | 15.9 | 138,000 | 0.66 | 3.3 | 134 | 70 | 1.01 | 2+ |
| June 2 | 6,780 | 623 | 13.7 | 230,000 | 0.89 | 2.6 | 141 | 61 | 1.09 | 2+ |
| June 6 | 8,300 | 1,307 | 13.9 | 392,000 | 0.69 | 3.1 | 64 | 131 | 1.15 | 1+ |
| June 10 | 11,220 | 2,221 | 15.5 | 453,000 | 0.67 | 3.7 | 91 | 212 | 1.08 | +/- |
| June 14 | 8,890 | 3,138 | 15.2 | 334,000 | 0.81 | 4.0 | 67 | 167 | 1.02 | +/- |
| June 18 | 6,280 | 1,720 | 13.1 | 184,000 | 0.73 | 3.6 | 29 | 66 | 1.06 | +/- |
| June 22 | 7,640 | 2,009 | 13.2 | 259,000 | 0.82 | 3.8 | 36 | 56 | 1.13 | - |
The index patient triggered a huge outbreak of MERS-CoV at Hospital C, resulting in a total of 91 cases of MERS-CoV infection. Of the 90 cases, 39% (35/90) were family members visiting the ER, and 13% (12/90) were doctors and nurses. Of the cluster of 90 cases at Hospital C, 81 cases had been exposed to the index patient at ER. These 81 cases were tertiary infection in the chain of transmission. The days of symptom onset for the 81 cases are shown in Fig. 2B. The incubation period ranged from 2 to 16 days, with a median of 6 days.
Previous study reported that person-to-person transmission of MERS-CoV can occur in health care settings (6,7,8). In a hospital outbreak of MERS-CoV in eastern Saudi Arabia, 21 cases were infected by person-to-person transmission in 3 different health care facilities (7). In the 2014 MERS-CoV outbreak in Jeddah, Saudi Arabia, 88% of the 112 symptomatic patients with MERS-CoV infection had exposure to a health care facility (8). Neither of these papers reported any superspreading events, in contrast to the many severe acute respiratory syndrome (SARS) superspreading events in hospitals in 2003 (9). Thus, this is the first report of superspreading event of MERS-CoV in health care facilities.
A superspreading event is likely to be related to the triad of the agent, host, and environment. The preliminary analysis of virus sequencing data did not found any mutations linked to transmissibility or pathogenesis (10,11). However, recent complete genome analysis of the virus showed genetic recombination events between group 3 and group 5 of clade B that may have implications for the transmissibility (12). Although the patient did not undergo any aerosol-generating procedures, he had vigorous and frequent coughing that could be related to high viral shedding or increased efficiency of transmission.
As the ER did not have private rooms with toilet, the patient walked through the ER to go to the public toilet several times a day. The social tradition of many visitors or family members staying with patients created an environment conducive to superspreading events because of allowing close contact. Thus, the behavior of the patient, the environment of ER, and social tradition contributed a large number of close contacts, leading to this superspreading event.
We found the shortest incubation period was 2 days, and half of the 81 cases developed symptoms within 7 days after the exposure. Therefore, it is of paramount importance that case identification, laboratory confirmation, and contact tracing should be done within a few days. As contact tracing would be the most time-consuming task, public disclosure of the information on the possible exposure (time and place of the index case) could facilitate a more rapid and comprehensive contact tracing. Our report highlights that all persons staying in the same ER or perhaps using the same toilet, should be considered and evaluated as having been potentially exposed to MERS-CoV. It is not always possible to early identify patients with MERS. Therefore, all health care facilities should have infection prevention and control practices in place.
Sustained human-to-human transmission did not occur in the Korean outbreak of MERS-CoV, although at least 23 cases of fourth-generation transmission were confirmed (5). As of September 30, 2015, exported cases of MERS-CoV infection have been reported by 18 countries outside the Arabian Peninsula (3), but MERS-CoV has never been spread to more than a few other people in health care facilities in these countries (13,14,15). The basic reproduction number R0 for MERS-CoV has been estimated to be less than 1, suggesting that the virus has not yet reached epidemic potential (16). However, a recent study suggested that cluster size over 150 cases should not be unexpected and there is substantial potential for superspreading events (17).
Superspreading events have contributed other infectious disease outbreaks (18), most notably with the other recent new coronavirus that caused SARS in 2003. One of the defining features of SARS-CoV transmission inside and outside hospitals was superspreading events (19,20). Until now the lack of reported superspreading events due to MERS-CoV was a reassuring difference between MERS and SARS. This report clearly demonstrates that, as in SARS outbreak, superspreading events can cause a large outbreak of MERS in health care facilities. To prevent future international hospital outbreaks of MERS-CoV, all health care facilities should establish and implement infection prevention and control measures as well as triage policies and procedures for early detection and isolation of suspected MERS-CoV cases.
Footnotes
Funding: This study was supported by research grant (2015-1980) from the Clinical Research Institute, Seoul National University Hospital.
DISCLOSURE: The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conception and design: Oh MD, Choe PG, Park WB, Kim NJ. Acquisition of data: Choe PG, Oh HS, Lee SM, Park JK, Lee SK, Song JS. Analysis and interpretation of data: Oh MD, Choe PG, Park WB, Kim NJ. Manuscript preparation: Oh MD, Choe PG, Oh HS. Manuscript approval: all authors.
References
- 1.Lee JK. MERS Countermeasures as One of Global Health Security Agenda. J Korean Med Sci. 2015;30:997–998. doi: 10.3346/jkms.2015.30.8.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Disease outbreak news: Middle East respiratory syndrome coronavirus (MERS-CoV) - Saudi Arabia, Sep 30, 2015. WHO; 2015. [accessed on 1 Oct 2015]. Available at http://www.who.int/csr/don/30-september-2015-mers-saudi-arabia/en/ [Google Scholar]
- 3.World Health Organization. Middle East Respiratory Syndrome Coronavirus (MERS-CoV): Summary of current situation, literature update and risk assessmsnet, 7 July 2015. WHO; 2015. [accessed on 15 Jun 2015]. Available at http://www.who.int/csr/disease/coronavirus_infections/risk-assessment-7july2015/en/ [Google Scholar]
- 4.Lee J. Better Understanding on MERS Corona Virus Outbreak in Korea. J Korean Med Sci. 2015;30:835–836. doi: 10.3346/jkms.2015.30.7.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korea Ministry of Health and Welfare, Korean Centers for Disease Control and Prevention. Summary of MERS statistics in the Republic of Korea. 2015. [accessed on 1 Oct 2015]. Available at http://www.mers.go.kr/mers/html/jsp/Menu_C/list_C4.jsp.
- 6.Al-Abdallat MM, Payne DC, Alqasrawi S, Rha B, Tohme RA, Abedi GR, Al Nsour M, Iblan I, Jarour N, Farag NH, et al. Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014;59:1225–1233. doi: 10.1093/cid/ciu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DA, Alabdullatif ZN, Assad M, Almulhim A, Makhdoom H, et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oboho IK, Tomczyk SM, Al-Asmari AM, Banjar AA, Al-Mugti H, Aloraini MS, Alkhaldi KZ, Almohammadi EL, Alraddadi BM, Gerber SI, et al. 2014 MERS-CoV outbreak in Jeddah--a link to health care facilities. N Engl J Med. 2015;372:846–854. doi: 10.1056/NEJMoa1408636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. how a global epidemic was stopped. Geneva, Switzerland: WHO Press; 2006. [Google Scholar]
- 10.Kim YJ, Cho YJ, Kim DW, Yang JS, Kim H, Park S, Han YW, Yun MR, Lee HS, Kim AR, et al. Complete Genome Sequence of Middle East Respiratory Syndrome Coronavirus KOR/KNIH/002_05_2015, Isolated in South Korea. Genome Announc. 2015;3 doi: 10.1128/genomeA.00787-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu R, Wang Y, Wang W, Nie K, Zhao Y, Su J, Deng Y, Zhou W, Li Y, Wang H, et al. Complete Genome Sequence of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) from the First Imported MERS-CoV Case in China. Genome Announc. 2015;3 doi: 10.1128/genomeA.00818-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Liu D, Shi W, Lu R, Wang W, Zhao Y, Deng Y, Zhou W, Ren H, Wu J, et al. Origin and Possible Genetic Recombination of the Middle East Respiratory Syndrome Coronavirus from the First Imported Case in China: Phylogenetics and Coalescence Analysis. MBio. 2015;6:e01280-15. doi: 10.1128/mBio.01280-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breakwell L, Pringle K, Chea N, Allen D, Allen S, Richards S, Pantones P, Sandoval M, Liu L, Vernon M, et al. Lack of Transmission among Close Contacts of Patient with Case of Middle East Respiratory Syndrome Imported into the United States, 2014. Emerg Infect Dis. 2015;21:1128–1134. doi: 10.3201/eid2107.150054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reuss A, Litterst A, Drosten C, Seilmaier M, Böhmer M, Graf P, Gold H, Wendtner CM, Zanuzdana A, Schaade L, et al. Contact investigation for imported case of Middle East respiratory syndrome, Germany. Emerg Infect Dis. 2014;20:620–625. doi: 10.3201/eid2004.131375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guery B, Poissy J, el Mansouf L, Séjourné C, Ettahar N, Lemaire X, Vuotto F, Goffard A, Behillil S, Enouf V, et al. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: a report of nosocomial transmission. Lancet. 2013;381:2265–2272. doi: 10.1016/S0140-6736(13)60982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breban R, Riou J, Fontanet A. Interhuman transmissibility of Middle East respiratory syndrome coronavirus: estimation of pandemic risk. Lancet. 2013;382:694–699. doi: 10.1016/S0140-6736(13)61492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kucharski AJ, Althaus CL. The role of superspreading in Middle East respiratory syndrome coronavirus (MERS-CoV) transmission. Euro Surveill. 2015;20:pii: 21167. doi: 10.2807/1560-7917.es2015.20.25.21167. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438:355–359. doi: 10.1038/nature04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poutanen SM, Low DE, Henry B, Finkelstein S, Rose D, Green K, Tellier R, Draker R, Adachi D, Ayers M, et al. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 20.Shen Z, Ning F, Zhou W, He X, Lin C, Chin DP, Zhu Z, Schuchat A. Superspreading SARS events, Beijing, 2003. Emerg Infect Dis. 2004;10:256–260. doi: 10.3201/eid1002.030732. [DOI] [PMC free article] [PubMed] [Google Scholar]