JAK2V617F-mediated phosphorylation of PRMT5 down-regulates its methyltransferase activity and promotes myeloproliferation (original) (raw)
. Author manuscript; available in PMC: 2015 Dec 22.
Published in final edited form as: Cancer Cell. 2011 Feb 15;19(2):283–294. doi: 10.1016/j.ccr.2010.12.020
SUMMARY
The JAK2V617F constitutively activated tyrosine kinase is found in most patients with myeloproliferative neoplasms. While examining the interaction between JAK2 and PRMT5, an arginine methyltransferase originally identified as JAK2 binding protein 1, we found that JAK2V617F (and JAK2K539L) bound PRMT5 more strongly than did wild-type JAK2. These oncogenic kinases also acquired the ability to phosphorylate PRMT5, greatly impairing its ability to methylate its histone substrates, and representing a specific gain-of-function that allows them to regulate chromatin modifications. We readily detected PRMT5 phosphorylation in JAK2V617F-positive patient samples and when we knocked down PRMT5 in human CD34+ cells using shRNA, we observed increased colony formation and erythroid differentiation. These results indicate that phosphorylation of PRMT5 contributes to the mutant JAK2-induced myeloproliferative phenotype.
Keywords: JAK2 exon12 mutations, tyrosine phosphorylation, MEP50, cross-talk, histone arginine methylation, myeloproliferative neoplasm
INTRODUCTION
The myeloproliferative neoplasms (MPNs) are stem cell disorders whose proliferation is thought to be driven by activating tyrosine kinase gene mutations, such as the BCR-ABL fusion gene in chronic myelogenous leukemia (CML) (De Keersmaecker and Cools, 2006). The JAK2 kinase V617F mutation is found in most patients with non-CML MPN (Baxter et al., 2005; James et al., 2005; Kralovics et al., 2005; Levine et al., 2005). It is a constitutively active kinase that can phosphorylate STAT5 in the absence of upstream signals, confer cytokine-independent growth to Ba/F3 cells, and induce a myeloproliferative disease in mouse models (Akada et al., 2010; James et al., 2005; Marty et al., 2010; Mullally et al., 2010; Xing et al., 2008). In addition to V617F, mutations within the exon 12 region of JAK2, such as K539L, have been observed in MPN patients, although they are much rarer (Pikman and Levine, 2007; Scott et al., 2007). Like the V617F mutation, these mutations disrupt the negative regulation of JAK2 and lead to constitutive kinase activity.
The JAK2 protein associates with the cytoplasmic domains of a number of cytokine receptors and is crucial for mediating signals triggered by several hematopoietic growth factors, including erythropoietin (Epo), thrombopoietin (Tpo) and granulocyte colony-stimulating factor (G-CSF). Murine JAK2 knockout embryos die from severe anemia on day 11 to 13 in utero, demonstrating the importance of JAK2 in hematopoietic cytokine signaling (Neubauer et al., 1998; Parganas et al., 1998). It has been reported by several groups that the transforming effects of JAK2V617F requires an intact FERM domain, which binds to homodimeric type I cytokine receptors (Lu et al., 2005; Wernig et al., 2008a). This suggests that interactions between JAK2 and cytokine receptors remain capable of regulating the biological function of JAK2V617F.
Upon activation, the receptor-bound JAK2 phosphorylates specific tyrosine residues of its downstream targets, activating cell survival/proliferation-promoting signaling pathways (Ihle and Gilliland, 2007). Several kinase cascades are activated by JAK2V617F, including the STAT5/BCL-XL, PI3K/AKT and ERK/MAPK pathways (James et al., 2005; Wang et al., 2009), however they may not completely account for the MPN phenotype.
The type II arginine methyltransferase PRMT5 was first identified as JAK2 binding protein (JBP1) in a yeast two-hybrid assay (Pollack et al., 1999). It mediates the symmetrical dimethylation of arginine residues within histones H2A, H3 and H4 (Ancelin et al., 2006; Branscombe et al., 2001; Pal et al., 2004), and methylates other cellular proteins as well, such as p53, SPT5 and MBD2 (Jansson et al., 2008; Kwak et al., 2003; Tan and Nakielny, 2006). Together with the WD40-repeat containing MEP50 protein and with pICln, PRMT5 forms a large 20S protein arginine methyltransferase complex, termed the methylosome. This complex functions in RNA processing by methylating Sm proteins and affecting snRNP biogenesis (Chari et al., 2008; Friesen et al., 2001; Friesen et al., 2002; Meister and Fischer, 2002). PRMT5 has been also found in the hSWI/SNF and NURD chromatin remodeling complexes (Le Guezennec et al., 2006; Pal et al., 2004), where it can exert transcriptional control on target gene expression.
Although first identified as JAK2 binding protein, there is no functional data linking PRMT5 with JAK2. To gain insights into JAK2V617F-induced MPN, we investigated the in vivo interaction between PRMT5 and the oncogenic mutant JAK2 kinases (JAK2V617F and JAK2V617F), and determined how this interaction contributes to the myeloproliferative phenotype that they induce.
RESULTS
PRMT5 interacts with JAK2V617F and JAK2K539L more strongly than wild-type JAK2
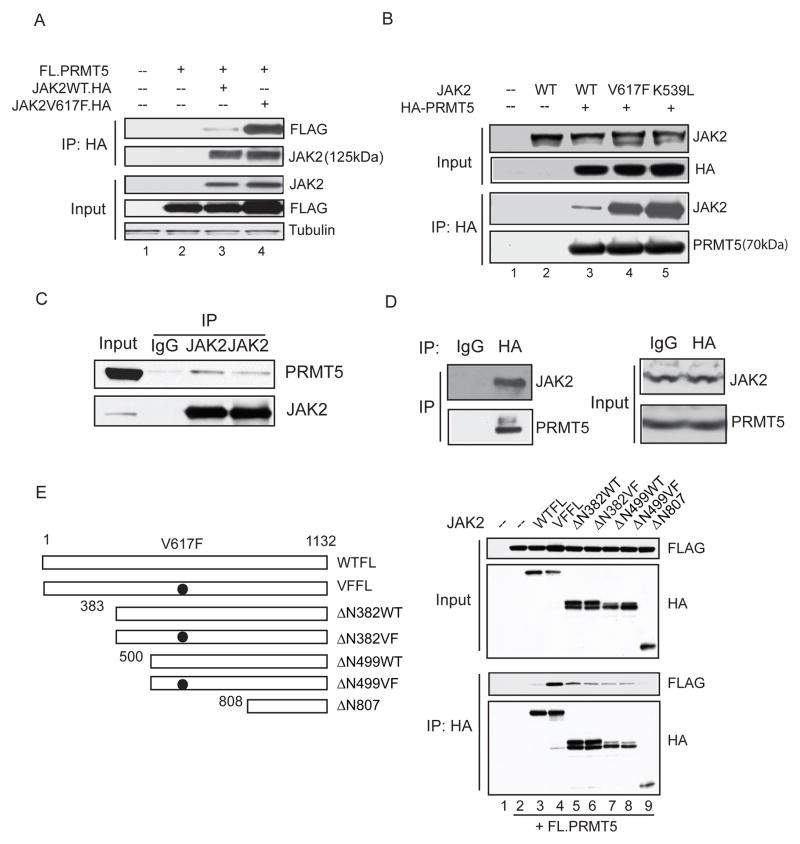
First, we examined whether PRMT5 interacts with JAK2 and if the V617F (and K539L) activating mutations in JAK2 affect this interaction. We co-expressed FLAG-PRMT5 with HA-tagged wild-type JAK2 and JAK2V617F**,** or HA-PRMT5 with non-tagged versions of the wild-type JAK2, JAK2V617F and JAK2K539L proteins in 293T cells, and found that while the wild-type JAK2 interacts with PRMT5, both the JAK2V617F and JAK2K539L mutants bound PRMT5 more strongly than wild-type JAK2 (Figure 1A and B), demonstrating that both constitutively activated forms of JAK2 have increased affinity for PRMT5. Next, to determine whether the endogenous JAK2V617F and PRMT5 proteins interact in leukemia cells, we performed co-immunoprecipitation (Co-IP) assays using two different anti-JAK2 antibodies and the JAK2V617F -positive HEL cell line: The interaction of JAK2V617F with PRMT5 was readily detected using either antibody (Figure 1C). Since none of the commercially available anti-PRMT5 antibodies efficiently immunoprecipitate PRMT5, we also utilized a HEL cell line that we engineered to stably express HA-tagged PRMT5. Using an anti-HA antibody, we could detect a robust interaction between PRMT5 and the mutant JAK2 (Figure 1D). We confirmed that the interaction between PRMT5 and JAK2V617F is stronger than the interaction between PRMT5 and wild-type JAK2 in hematopoietic cells, using Ba/F3 cell lines that stably express the wild-type or V617F mutant JAK2 proteins. Even though these cell lines express endogenous JAK2 protein (compare lanes 2 and 3 to lane 1 in Figure S1A), using an anti-JAK2 antibody for the IP, we see that the mutant JAK2V617F pulls down significantly more endogenous PRMT5 protein than does wild-type JAK2. We also determined the subcellular localization of the JAK2-PRMT5 interaction by performing Co-IP experiments using cytoplasmic and nuclear fractions of HEL cells that stably express HA-tagged PRMT5 (Figure S1C). The interaction between JAK2 and PRMT5 can be detected in the both cytoplasmic and nuclear fractions of these hematopoietic cells. This reflects their normal localization in the cell, as the endogenous JAK2 and PRMT5 proteins are found in both the cytoplasmic and nuclear fractions of HEL cells (Figure S1B).
Figure 1. The oncogenic JAK2 mutants interact more strongly with PRMT5 than wild-type JAK2 and gain the ability to phosphorylate PRMT5.
(A) The V617F mutation enhances the interaction between JAK2 and PRMT5. 293T cells were transiently transfected with vectors expressing FLAG-PRMT5 alone (lane 2) or with HA-tagged wild-type (lane 3) or V617F (lane 4) JAK2 proteins. Immunoprecipitation was performed using an anti-HA antibody and immunoblotting with anti-FLAG or anti-JAK2 antibodies.
(B) Both the V617F and K539L mutations enhance the PRMT5/JAK2 association. HA-tagged PRMT5 was co-expressed in 293T cells with wild-type (lane 3), V617F (lane 4) or K539L (lane 5) JAK2 proteins. Proteins were precipitated by anti-HA immunoprecipitation and the blots were probed with antibodies specific for HA, JAK2 or PRMT5.
(C) Endogenous interaction between PRMT5 and JAK2V617F is detected in HEL cells. Proteins were precipitated from HEL cell extracts using two different anti- JAK2 antibodies; normal rabbit IgG was used as a control for the immunoprecipitation.
(D) Interaction between HA-PRMT5 and endogenous JAK2V617F is detected in HEL cells. A Co-IP was performed using a HEL cell line stably expressing HA-PRMT5. Proteins were precipitated by either a normal mouse IgG or anti-HA antibody. Immunoblotting was performed using antibodies specific for JAK2 and PRMT5.
(E) The N-terminal region of JAK2 interacts with PRMT5. Left: diagram of full-length JAK2 and various amino-terminal deleted JAK2 proteins with or without the V617F mutation. Numbers indicate the relevant amino acids. Right: HA-tagged JAK2 full-length or various amino-terminal deletion mutants were co-expressed in 293T cells with FLAG-PRMT5. JAK2 and any associated PRMT5 were pulled down using anti-HA immunoprecipitation. Membranes were immunoblotted with an anti-HA antibody to detect JAK2 and an anti-FLAG antibody to detect PRMT5. WTFL= wild-type full-length JAK2 protein; VFFL= JAK2V617F full-length protein. See also Figure S1.
To map the region(s) in JAK2 that interacts with PRMT5, we constructed a series of N-terminal deletion mutants of HA-tagged JAK2 with or without the V617F substitution, and expressed these proteins with FLAG-tagged PRMT5 in 293T cells (Figure 1E). Co-IP experiments showed that deletion of the first 382 amino acids (which contains the receptor-binding FERM domain) from JAK2V617F greatly reduced its interaction with PRMT5. Given that the C-terminus of JAK2 (808-1132) does not bind PRMT5, this indicates that the N-terminal portion of JAK2 is responsible for binding PRMT5. The JAK2Δ382WT deletion mutant protein also binds weakly to PRMT5, suggesting that loss of the FERM domain may expose other epitopes in wild-type JAK2 that can bind PRMT5.
Oncogenic JAK2 kinases phosphorylate PRMT5 in vivo
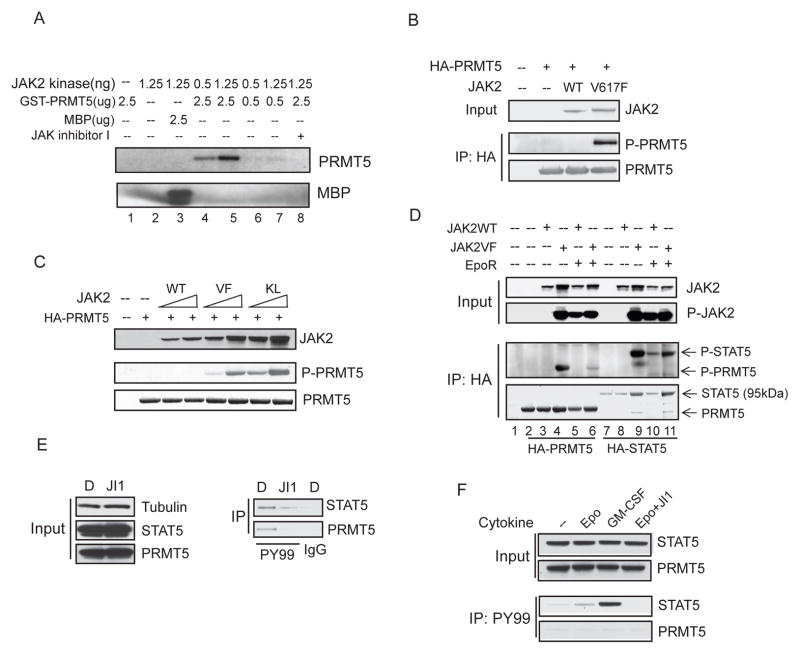
To determine if JAK2 kinase can directly phosphorylate PRMT5, we performed an in vitro kinase assay using bacterially purified GST-PRMT5 as the substrate (Figure 2A). JAK2 dependent phosphorylation of GST-PRMT5 was readily detected, as a JAK2 inhibitor (JAK Inhibitor I) (1uM) completely abrogated the phosphorylation (lane 8). We next determined if JAK2 phosphorylates PRMT5 in vivo, by co-expressing HA-PRMT5 with wild-type JAK2 or the JAK2 mutants in 293T cells, and using anti-HA immunoprecipitation followed by anti-phosphotyrosine immunoblotting. We found that PRMT5 was phosphorylated by the mutant JAK2V617F and JAK2K539L kinases, but not the wild-type JAK2 kinase (Figure 2B and 2C). The phosphorylation of PRMT5 by JAK2V617F was further confirmed using an anti-phosphotyrosine antibody to pull down proteins from 293T cells co-expressing JAK2V617F and FLAG-tagged PRMT5 (Figure S2). To determine whether wild-type JAK2 can phosphorylate PRMT5 when it is activated by signaling through the Erythropoietin receptor (EpoR), we transfected 293T cells with JAK2 wild-type or V617F mutant with or without the EpoR, and added 20 U/ml of Epo to the cells for 20 min (Figure 2D). Wild-type JAK2 was activated by the presence of Epo and its receptor (as shown by JAK2 auto-phosphorylation and phosphorylation of HA-STAT5, lane 10). However, unlike JAK2V617F, the activated wild-type JAK2 kinase did not detectably phosphorylate PRMT5, indicating that PRMT5 phosphorylation is indeed an acquired function of the mutant JAK2 kinase. Interestingly, phosphorylation of PRMT5 by JAK2V617F was reduced in cells over-expressing the EpoR, suggesting that the level of EpoR expression can affect the ability of JAK2V617F to phosphorylate PRMT5.
Figure 2. The constitutively active JAK2 mutants phosphorylate PRMT5 in vivo.
(A) JAK2 phosphorylates PRMT5 in vitro. In vitro kinase assays were performed using bacterial purified GST-PRMT5 and the active JAK2 kinase. The amount of protein in the reaction is indicated. MBP (myelin basic protein) was used as a positive control (lane 3).
(B) PRMT5 is phosphorylated by JAK2V617F in vivo. HA-PRMT5 was co-transfected into 293T cells with an empty vector or with vectors expressing wild-type or V617F mutant JAK2 protein. PRMT5 was precipitated by anti-HA immunoprecipitation and phosphorylation of PRMT5 was detected using a phosphotyrosine specific antibody (PY350).
(C) Both JAK2V617F and JAK2K539L phosphorylate PRMT5 in vivo. 293T cells were transiently transfected with HA-PRMT5 alone or co-transfected with an increasing concentration of JAK2 wild-type, JAK2V617F- or JAK2K539L expressing vector. HA-PRMT5 was purified by anti-HA immunoprecipitation, and phosphorylation of PRMT5 was detected by immunoblotting with the PY350 antibody.
(D) Activated wild-type JAK2 is unable to phosphorylate PRMT5 in vivo. 293T cells were transfected with vectors expressing the proteins indicated in the figure. Proteins were purified by anti-HA immunoprecipitation, and phosphorylation of PRMT5 and STAT5 was detected using an anti-phosphotyrosine antibody. Input was from 5% of the total lysate. JAK2 phosphorylation was detected using an antibody specific for phospho-JAK2.
(E) Endogenous PRMT5 is phosphorylated in HEL cells. HEL cells were treated with either DMSO or 2uM JAK Inhibitor I for 16 hours. Phosphorylated proteins were purified using the PY99 anti-phosphotyrosine antibody. Normal mouse IgG was used as a control for the immunoprecipitation. The precipitated STAT5 and PRMT5 proteins were detected using anti-STAT5 and anti-PRMT5 antibodies, respectively. JI1: JAK Inhibitor I.
(F) Phosphorylated PRMT5 is not detected in TF-1 cells. TF-1 cells cultured in growth medium supplemented with 2ng/ml of recombinant human IL-3 were deprived of cytokine for 16 hours. The cells (5× 106) were then treated with GM-CSF (25 ng/ml), Epo (20 U/ml) or Epo plus 2uM of JAK Inhibitor I (JI1) for 20 min. Phosphorylated STAT5 and PRMT5 proteins were immunoprecipitated using the PY99 antibody. See also Figure S2.
We next determined whether phosphorylation of endogenous PRMT5 by JAK2V617F occurs in JAK2V617F-positive HEL leukemia cells. HEL cells were treated with either DMSO or JAK Inhibitor I for 16 hours, and the phosphorylated proteins were immunoprecipitated using an anti-phosphotyrosine antibody (Figure 2E). While PRMT5 (and STAT5) are phosphorylated in DMSO treated HEL cells, and the phosphorylation of both proteins is greatly reduced by the JAK2 inhibitor, PRMT5 is not phosphorylated in the TF-1 hematopoietic cells (which express wild-type JAK2) even when the JAK2 kinase is activated by Epo (or GM-CSF), which clearly trigger STAT5 phosphorylation (Figure 2F). These results identify PRMT5 as a bona fide in vivo substrate of JAK2V617F, but not activated wild-type JAK2.
Phosphorylation of PRMT5 by JAK2V617F greatly impairs its methyltransferase activity
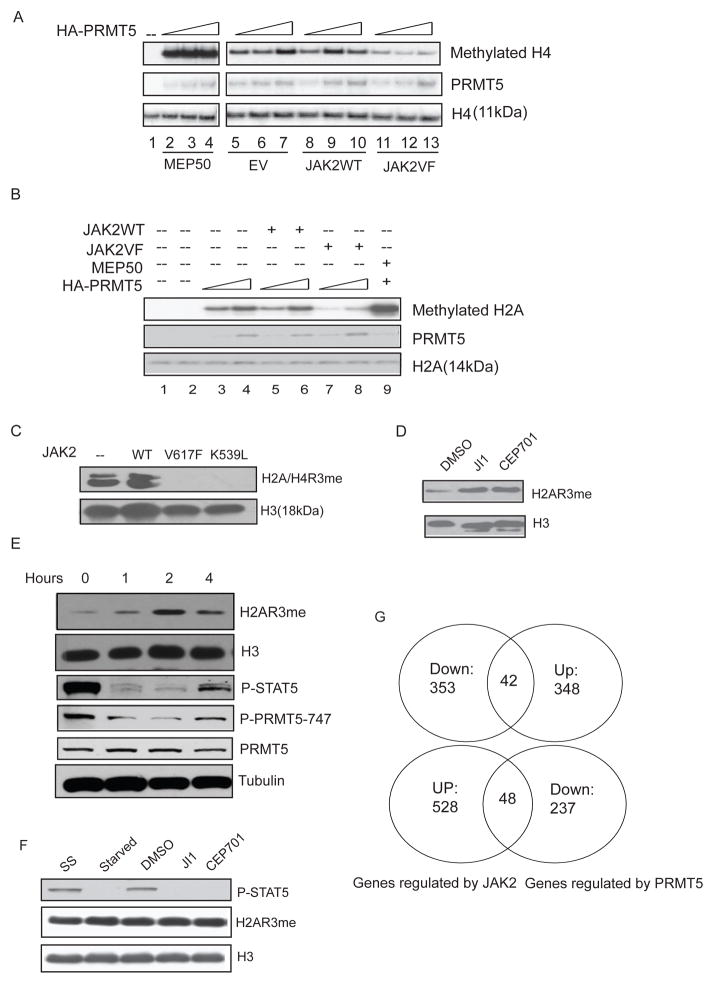
PRMT5 has been shown to methylate histones H2A, H3 and H4 in vitro and in vivo. To determine whether phosphorylation of PRMT5 affects its enzymatic activity, we purified HA-tagged PRMT5 protein from 293T cells engineered to express either wild-type or mutant JAK2 and HA-PRMT5. After we confirmed the phosphorylation of PRMT5 by JAK2V617F, we incubated the purified PRMT5 with [3H] _S_-adenosylmethionine and recombinant histone H4 (Figure 3A) or histone H2A (Figure 3B) in an in vitro methylation assay. While co-expression of wild-type JAK2 had little effect on PRMT5 methyltransferase activity (Figure 3A, lanes 8–10), JAK2V617F significantly impaired the ability of PRMT5 to methylate histone H4 (lanes 11–13). As expected, co-expression of MEP50 with PRMT5 greatly enhanced its enzymatic activity (lanes 2–4). Similar results were seen for JAK2V617F (and JAK2K539L) on histone H2A methylation (Figure 3B and data not shown).
Figure 3. Phosphorylation of PRMT5 by JAK2V617F impairs its histone methyltransferase activity.
(A) Co-expression of JAK2V617F, but not the wild-type JAK2, impairs the ability of PRMT5 to methylate histone H4 in vitro. An in vitro methylation assay was performed using HA-PRMT5 purified from 293T cells transfected with HA-PRMT5 alone (lane 5 to 7) or co-transfected with MEP50 (lanes 2 to 4), wild type JAK (lanes 8 to 10) or JAK2V617F (lanes 11 to 13). Increasing amount of proteins (10, 15 or 20ul) were added to each methylation reaction with 2.5ug recombinant H4 and 1 uCi of 3H-SAM. Even loading of the proteins (PRMT5 and histone H4) was visualized using Coomassie blue staining. EV= empty vector.
(B) In vitro methylation assays were performed with recombinant H2A and similarly purified HA-PRMT5. Increasing amount of purified protein (10 or 20 ul) was added to the reaction.
(C) Overexpression of the mutant JAK2 proteins, but not the wild-type JAK2 protein, down-regulates H2A/H4 R3 methylation. The core histones were purified from 293T cells transfected with either an empty vector, or with vectors expressing the JAK2 proteins indicated in the figure. Methylation of H2A/H4 R3 was detected using a rabbit polyclonal antibody specific for H2A/H4 R3 symmetric dimethylation (H2A/H4R3me2s). An anti-histone H3 antibody was used to show the equal loading.
(D) Inhibition of JAK2 kinase activity increases H2A R3 methylation in HEL cells. HEL cells were treated with DMSO or with the JAK2 inhibitors JAK Inhibitor I (1μM) or CEP701 (0.2μM) for 2 hours. Histone methylation was detected in cell lysates using an antibody specific for symmetric dimethylation of H2A/H4 R3. JI1: JAK Inhibitor I.
(E) Inhibition of JAK2 rapidly up-regulates H2A R3 methylation in HEL cells. HEL cells were treated with TG101348 (3uM) for 0, 1, 2 and 4 hours. JAK2 kinase inhibition was monitored by measuring STAT5 phosphorylation using an anti-phospho-STAT5 antibody. Phosphorylation of PRMT5 was detected with the phospho-specific PRMT5 antibody (P-PRMT5-747). Histones were purified by acidic extraction and H2A R3 methylation was detected using an anti-H2A/H4R3me2s antibody. The levels of histone H3 and tubulin are shown as loading controls.
(F) JAK2 inhibitors do not change the level of H2A R3 methylation in TF-1 cells. TF-1 cells growing in medium supplemented with recombinant human IL-3 were treated with DMSO or JAK2 inhibitors (as indicated in the figure) for 2 hours. Histone methylation was detected using an anti-H2A/H4R3me2s antibody and STAT5 phosphorylation detected using a phospho-STAT5 antibody. SS: steady state. Starved: cells were deprived of IL-3 for 2 hours.
(G) Gene expression profiles were generated using Affymetrix HG133 GeneChips and CEP701 (0.5uM)-treated or shPRMT5-treated HEL cells, vs. controls (DMSO or a lentivirus expressing a scrambled shRNA, respectively). Genes reciprocally up- or down-regulated ≥ 1.5 fold in both duplicate samples from drug-treated samples and the shPRMT5-treated samples are compared. Top: number of genes that are down-regulated by CEP701 treatment and up-regulated by knock down of PRMT5. Bottom: number of genes that are up-regulated by CEP701 treatment and down-regulated by PRMT5 knockdown. See also Table S1.
We next examined whether JAK2V617F and JAK2K539L expression affects global H2A/H4 R3 symmetric dimethylation level in vivo, using 293T cells transiently expressing JAK2 wild type, JAK2V617F or JAK2K539L. Wild-type JAK2 had no effect on the global level of H2A/H4 R3 methylation; however, both oncogenic JAK2 kinases nearly abolished H2A/H4 R3 methylation (Figure 3C). We next assessed histone arginine methylation levels in HEL cells, in the presence or absence of JAK Inhibitor I, CEP701, which is a JAK2 (and FLT3) inhibitor (Hexner et al., 2008) and TG101348 (Lasho et al., 2008; Wernig et al., 2008b), the most specific JAK2 inhibitor tested. Treatment with all three JAK2 inhibitors markedly increased H2A R3 symmetric dimethylation in the cell (Figure 3D and 3E) (H4 R3 symmetric dimethylation is not found in HEL cells in the presence or absence of the JAK2 inhibitor). To determine how rapidly JAK2 inhibition affects the level of H2A/H4 R3 methylation, we performed a time-course experiment using HEL cells treated with TG101348 (Figure 3E). An increase in H2A/H4 R3 methylation was seen within 1 hour, which peaked at the 2 hour time point. At 4 hours, the effect on H2A/H4 R3 methylation began to reverse, likely due to instability of the inhibitor, as both STAT5 and PRMT5 phosphorylation began to increase at 4 hour time point. In contrast, these JAK2 inhibitors had minimal effect on H2A/H4 R3 methylation levels in TF-1 cells even though they blocked STAT5 phosphorylation (Figure 3F). Thus, the oncogenic JAK2 proteins gain the ability to regulate global H2A/H4 R3 symmetric dimethylation levels, presumably via phosphorylation of PRMT5.
While JAK2 regulates gene transcription through the canonical JAK2-STAT5 pathway, as well as other pathways, our data suggests that the oncogenic JAK2 kinases (like V617F and K539L) can also regulate gene expression via repression of PRMT5 activity and possibly changes in the methylation of the histone H2A and H4 tails. We performed gene expression profile analysis on DMSO-treated vs. CEP701-treated HEL cells and on PRMT5-directed shRNA expressing HEL cells vs. control shRNA expressing cells, using Affymetrix HG133 GeneChips. We found 881genes whose mRNA levels changed more than 1.5 fold in both duplicate samples of the CEP701 treated cells, and 585 genes whose expression reproducibly changed ≥ 1.5 fold in cells where PRMT5 was knocked down. Since inhibition of JAK2 activity de-represses PRMT5 activity, we hypothesize that genes that are reciprocally regulated between the inhibitor treated samples and the shPRMT5-treated samples will be regulated by JAK2 induced PRMT5 phosphorylation. Indeed, we found 90 such genes (42 up-regulated by the inhibitor and down-regulated by the shRNA, and 48 down-regulated by the inhibitor and up-regulated by the shRNA), including genes involved in ribosomal biogenesis and autophagy (Figure 3G and Table S1).
The major phosphorylation sites in PRMT5 map to its N-terminal region
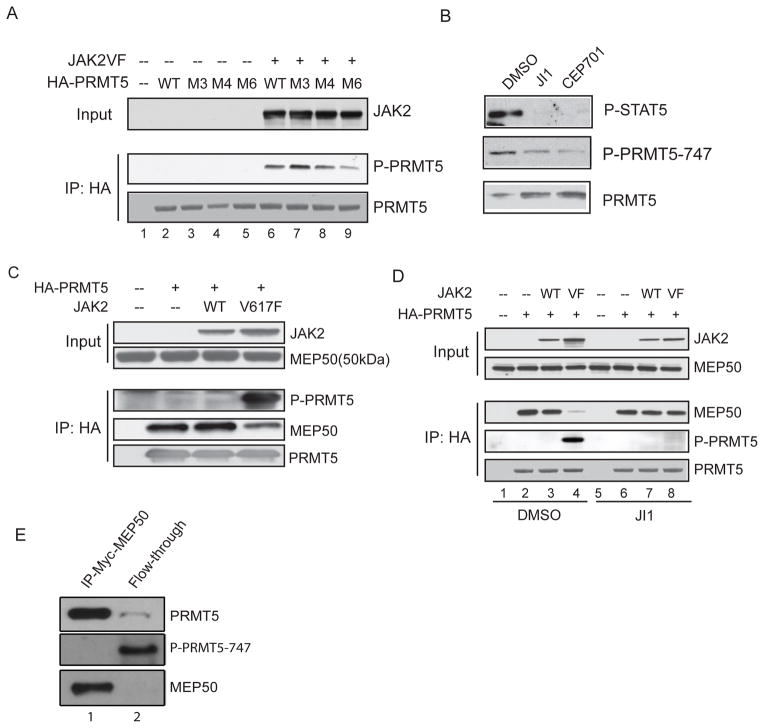
To map the site(s) in PRMT5 phosphorylated by JAK2V617F, we performed in vitro kinase assays and found that PRMT5 is phosphorylated at multiple sites located between amino acids 268 and 320 (data not shown). There are six tyrosine residues in this region (at position 280, 283, 286, 297, 304 and 307), and we mutated the first three (M3), first four (M4) and all six tyrosine residues (M6) to phenylalanine, and examined the extent of PRMT5 phosphorylation in transfected 293T cells (Figure 4A). Compared to wild-type PRMT5, the M6 form of PRMT5 had markedly reduced phosphorylation when co-expressed with JAK2V617F. The residual phosphorylation could be due to dimerization with endogenous wild-type PRMT5, or to the presence of other phosphorylation sites within the protein. In contrast, the M3 form of PRMT5 had a similar degree of phosphorylation as wild-type PRMT5, suggesting that the last three tyrosines are the major sites of JAK2 phosphorylation in PRMT5.
Figure 4. Phosphorylation of PRMT5 by JAK2V617F disrupts its association with MEP50.
(A) Tyrosine residues within PRMT5 that are potential sites of phosphorylation (279,282,285, 296,303,306) were converted to phenylalanine residues by site-directed mutagenesis. Wild type or mutant HA-PRMT5 proteins were co-expressed with JAK2V617F protein in 293T cells, and immunoprecipitated with an anti-HA antibody. Phosphorylation of PRMT5 was detected using a rabbit polyclonal anti-phosphotyrosine antibody (PY350).
(B) HEL cells were treated with DMSO, JAK Inhibitor I (1uM) or CEP701 (0.2uM) overnight and the phosphorylation of PRMT5 was detected by immunoblotting using a phospho-PRMT5 antibody (P-PRMT5-747) specific for the Y297, Y304 and Y307 phosphotyrosine residues in the PRMT5 protein. The level of PRMT5 and phospho-STAT5 are also shown.
(C) Co-expression of JAK2V617F disrupts the PRMT5/MEP50 association. The HA-PRMT5/MEP50 complex was purified using an anti-HA antibody from 293T cells expressing the proteins indicated in the figure. Phosphorylation of PRMT5 was confirmed by immunoblotting with an anti-phosphotyrosine antibody. Co-precipitated MEP50 was detected with an anti-MEP50 antibody.
(D) The kinase activity of JAK2V617F is required to disrupt the PRMT5/MEP50 complex. DMSO or 4 uM JAK Inhibitor I (JI1) was added to the 293T cells after transfection. Cells were collected 48 hours post-transfection and the HA-PRMT5/MEP50 complex was immunoprecipitated using an anti-HA antibody.
(E) Phosphorylated PRMT5 isolated from HEL cells does not bind MEP50. HEL cells stably expressing myc-tagged MEP50 were subjected to immunoprecipitation using an anti-myc antibody. After overnight incubation, the flow-through was saved, and the PRMT5/MEP50 complex was eluted from the beads. Proteins in the eluate and the flow-through were precipitated by TCA precipitation and resolved by SDS-PAGE gels. The membranes were blotted using anti-PRMT5 and anti-MEP50 antibodies, and the P-PRMT5-747 antibody, to quantify the amount of each protein in the various lanes. See also Figure S3.
To demonstrate that PRMT5 is phosphorylated at these tyrosine residues, we generated a phospho-specific anti-PRMT5 antibody (P-PRMT5-747) using a mixture of peptides containing all combinations of phosphorylated Y297, Y304 and Y307 tyrosine residues. A dot blot assay confirmed that this antibody recognizes the phosphorylated and not the un-phosphorylated peptides (data not shown). To confirm that endogenous PRMT5 is phosphorylated by JAK2V617F in HEL cells, we treated the cells with the JAK inhibitor I, CEP701 (Figure 4B) and TG101348 (Figure 3F) and performed several immunoblots. These JAK2 inhibitors significantly reduced the phosphorylation of PRMT5 and STAT5, demonstrating that endogenous PRMT5 is phosphorylated at these three tyrosine residues by JAK2V617F. These three tyrosine residues are highly conserved in PRMT5, from Xenopus to human (Figure S3A).
Phosphorylation of PRMT5 by JAK2V617F disrupts its association with MEP50
Since MEP50 markedly enhances the enzymatic activity of PRMT5 (Fig 3A and B), we examined whether JAK2V617F impairs PRMT5 activity by disrupting the PRMT5/MEP50 complex. Using 293T cells that transiently express HA-PRMT5, and either the wild-type JAK2 or the V617F mutant protein, we could readily co-immunoprecipitate endogenous MEP50 protein from cells expressing wild-type JAK2 with an anti-HA antibody (Figure 4C). However, co-expression of JAK2V617F significantly reduced the interaction between PRMT5 and MEP50. The kinase activity of JAK2V617F is required to disrupt the PRMT5/MEP50 association, as treating the cells with JAK Inhibitor I blocked the phosphorylation of PRMT5 and restored the interaction between PRMT5 and MEP50 (Figure 4D). Expression of JAK2V617F also disrupted the PRMT5/MEP50 interaction in HeLa cells and U2OS cells (Figure S3B and S3C).
To determine whether phosphorylation of PRMT5 affects the PRMT5/MEP50 complex in JAK2V617F-positive HEL cells, we established a stable HEL cell line that expresses myc-tagged MEP50 and purified the PRMT5/MEP50 complex using an anti-myc antibody (Figure 4E). While the un-phosphorylated form of PRMT5 bound to MEP50, the phosphorylated form of PRMT5 was exclusively in the flow- through following the immunoprecipitation. Thus, phosphorylation of PRMT5 by JAK2V617F blocks its association with MEP50 in hematopoietic cells.
PRMT5 negatively regulates hematopoietic stem/progenitor cell expansion and erythroid differentiation
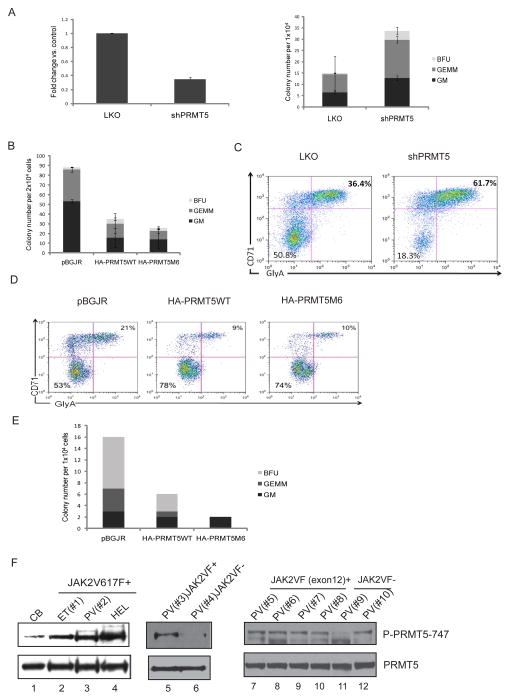
We examined whether decreased PRMT5 activity promotes myeloproliferation and/or erythroid differentiation, by knocking down PRMT5 expression in human CD34+ CB cells using shRNA. We achieved only 60% to 70% knockdown of PRMT5 mRNA, but still observed a two fold increase in CFUs (Figure 5A). We then overexpressed HA-tagged wild-type PRMT5, or the M6 mutant form of PRMT5 (PRMT5M6), in CD34+ CB cells and performed CFU assays. Consistent with the knockdown experiments, PRMT5 overexpression significantly decreased colony formation (Figure 5B), indicating that PRMT5 negatively regulates progenitor cell proliferation and expansion.
Figure 5. PRMT5 activity regulates progenitor cell expansion/differentiation in human CD34+ cells.
(A) Knockdown of PRMT5 promotes CFU formation. Isolated human CB CD34+ cells were transduced with lentiviruses expressing a scrambled shRNA or shRNA specific to PRMT5. GFP positive cells were sorted 2 days after infection. Left: PRMT5 mRNA expression was detected by real-time PCR. Right: Approximately 1×104 GFP-positive CD34+ cells were plated in methylcellulose culture supplemented with cytokines to support the formation of CFU-GM, CFU-GEMM and BFU-E. Colonies were scored 2 weeks after the plating. The results shown here are the averages of two independent experiments with error bars indicating +/- SD.
(B) Overexpression of PRMT5 inhibits colony formation. Isolated human CB CD34+ cells were transduced with lentiviruses expressing either GFP alone, or GFP together with HA-tagged wild-type PRMT5 or an HA-tagged M6 mutant form of PRMT5. GFP-positive cells were sorted and 2×104 cells were used for CFU assays. The average results of two independent experiments are shown here. The error bars indicate +/- SD.
(C) Knockdown of PRMT5 promotes erythroid differentiation. GFP+ CD34+ cells were cultured in serum-free medium supplemented with cytokines supporting erythroid differentiation for 14 days. CD71-positive/Ter-115-positive cells were determined by FACS analysis.
(D) PRMT5 overexpression inhibits erythroid differentiation. HA-PRMT5 or HA-PRMT5M6 was overexpressed in CB CD34+ cells, which were cultured in erythroid promoting medium for 7 days; erythroid differentiation was determined by FACS analysis, staining for CD71 (Y-axis) and Glycophorin A (X-axis). pBGJR refers to the control lentiviral vector.
(E) PRMT5 overexpression inhibits colony formation in JAK2V617F-positive CD34+ cells isolated from PV patients. CD34+ cells were isolated from phlebotomy units of PV patients and transduced by control lentivirus or lentiviruses expressing HA-PRMT5 or HA-PRMT5M6. 1×104 GFP+ cells were plated in methylcellulose with cytokines supporting GM, GEMM and BFU colony formation. Colonies were scored 14 days after plating.
(F) Phosphorylation of PRMT5 was detected in CD34+ cells isolated from MPN patients. CD34+ cells were isolated from human umbilical cord blood (lane 1) or from ten phlebotomy units taken from MPN patients (lanes 2 to 3 and 5 to 12). HEL cells were used as a positive control (lane 4). Phosphorylation of PRMT5 was detected using the P-PRMT5-747 antibody. The total amount of PRMT5 was also assessed by immunoblotting. ET= essential thrombocythemia; PV= polycythemia vera; HEL= HEL leukemia cells. See also Figure S4 and Table S2.
We also examined whether PRMT5 activity regulates erythroid differentiation, using in vitro liquid culture assays. We knocked down PRMT5 or overexpressed wild-type PRMT5 or the PRMT5M6 mutant in CD34+ CB cells and cultured the GFP+ cells in medium supporting erythroid differentiation for one or two weeks. While PRMT5 knockdown cells showed a significant increase in CD71/Ter-119-positive cells, overexpression of either the wild-type or the M6 mutant form of PRMT5 blocked erythroid differentiation (Figure 5C and D). The ability of PRMT5 knockdown cells to differentiate down the myeloid lineage was also assessed; a mild reduction in CD11b-positive cells was seen (Figure S4A).
To further determine whether down-regulation of PRMT5 activity is important for JAK2V617F-induced myeloproliferation, we overexpressed the wild-type or mutant PRMT5 (PRMT5M6) proteins in JAK2V617F-positive CD34+ cells isolated from therapeutic phlebotomy specimens of PV patients and performed CFU assays, using 1×104 GFP+ cells (Figure 5E). The control JAK2V617F positive CD34+ cells formed predominantly BFU-E colonies. However, the cells overexpressing PRMT5M6 showed no BFU-E or CFU-GEMM colonies, suggesting that the phosphorylation defective form of PRMT5 can abrogate the erythroid differentiation potential of these cells.
To determine if PRMT5 is phosphorylated in JAK2V617F positive patient samples, we isolated CD34+ cells from therapeutic phlebotomy specimens taken from PV patients (Figure 5F), using normal CB CD34+ cells as a control. Of the ten patient samples tested, all of the JAK2V617F- positive (or exon 12 mutation-positive) samples had higher PRMT5 phosphorylation than the CD34+ CB cells or the JAK2V617F-negative CD34+ cells. We found less phospho-PRMT5 in two of the three JAK2V617F-negative patient samples analyzed, compared to the JAK2V617F+ MPN patient samples (Figure 5F, lane 6 and 11). The clinical information on these patients (including their JAK2 mutant allele status) is summarized in Table S2. The weak positivity in CB CD34+ cells and the high level of phospho-PRMT5 in the one JAK2 wild-type patient sample suggest that other tyrosine kinases can also phosphorylate PRMT5 in hematopoietic cells. Computer-based programs, such as GPS 2.1 (Xue et al., 2008), indicate that the Y297, Y304 and Y307 tyrosine residues in PRMT5 are potentially phosphorylatable by ABL1, FGFR, Src and JAK1. In addition to examining CD34+ cells, we also examined granulocytes isolated from normal CB, or from the phlebotomy specimens of MPN patients and detected PRMT5 phosphorylation (Figure S4B).
DISCUSSION
We have determined that oncogenic mutations within the JAK2 tyrosine kinase (V617F and K539L) enhance its interaction with PRMT5, leading to PRMT5 phosphorylation in vivo. While both the wild-type and mutant forms of JAK2 proteins interact with PRMT5, phosphorylation of PRMT5 is a “gain-of-function” of the mutant JAK2 kinases, which reduces PRMT5 methyltransferase activity and decreases global histone H2A/H4 R3 methylation.
The PRMT5-interacting region in the JAK2 protein maps to its N-terminus, making it unlikely that the mutated residues in the JH2 domain play a critical role in this interaction. Furthermore, it appears that the active conformation of the mutant JAK2 proteins and not necessarily their kinase activity, allows them to bind PRMT5 more strongly, as the increased binding persists in the presence of JAK2 kinase inhibitors (data not shown).
We find that PRMT5 that is phosphorylated by JAK2V617F no longer binds MEP50, even though we can detect an interaction of MEP50 with unphosphorylated PRMT5 in HEL cells. It is possible that much of the PRMT5:MEP50 complex that contains unphosphorylated PRMT5 is cytoplasmic, rather than nuclear, as we and others have shown that both proteins are found in the nucleus and the cytoplasm and PRMT5 is known to complex with MEP50 in both locations (Liang et al., 2007). Given the enhanced proliferation seen following knockdown of PRMT5 in normal CD34+ cells, it would seem “easier” for the JAK2V617F+ mutant cells to simply degrade, or not express PRMT5. However, PRMT5 preferentially promotes p53-dependent cell cycle arrest at the expense of p53-dependent apoptosis (Jansson et al., 2008), and indeed, we find that knocking down PRMT5 in HEL cells triggers cell death (data not shown). Thus, JAK2V617F expressing hematopoietic cells may require that some PRMT5 protein be present, to complex with MEP50, and perhaps to promote dimerization of the JAK2V617F protein, similar to the role that the Epo receptor has been shown to play (Lu et al., 2005; Wernig et al., 2008a). Given our confirmation of the recent demonstrations that JAK2 can be found in the nucleus (Dawson et al., 2009; Rinaldi et al., 2010), PRMT5 phosphorylation may be differentially regulated in the nucleus vs. the cytoplasm of the cell, and in hematopoietic cells vs. other cell types.
The kinase-dependent regulation of global H2A/H4 R3 symmetric methylation reveals a link between an oncogenic tyrosine kinase and this particular chromatin modification. We have also shown the dynamic nature of this regulation. Symmetric dimethylation of histone H2A/H4 R3 has been shown to negatively, as well as positively, regulate gene expression (Fabbrizio et al., 2002; Richard et al., 2005). To gain insight into how JAK2V617F could regulate gene expression through phosphorylation of PRMT5, we performed gene expression profiling comparing the JAK2-regulated genes (defined using a JAK2 inhibitor) with the PRMT5-regulated genes (defined using shRNA directed against PRMT5) and found 90 genes that were reciprocally regulated. The recently reported interplay between histone arginine methylation and DNA methylation (Zhao et al., 2009), suggests that the oncogenic JAK2 kinases may not only regulate histone arginine methylation but also DNA methylation, thereby “maintaining” a myeloproliferative epigenetic signature that could be established by activation of the JAK/STAT pathway.
Recent work has shown an important role for arginine methylation in regulating hematopoiesis and leukemogenesis. PRMT1 (a type I arginine methyltransferase) has been shown to be an essential component of MLL fusion protein-driven leukemogenesis (Cheung et al., 2007), and to regulate AML1(Runx1) function (Zhao et al., 2008). PRMT5 has been shown to repress gamma-globin gene expression by recruiting DNMT3A and inducing additional repressive epigenetic marks, such as H4S1ph and H4K20me3 (Rank et al., 2010; Zhao et al., 2009); this suggests a role for PRMT5 in erythropoiesis (which we have demonstrated) and hematopoiesis in general. In contrast, we found that down-regulation of PRMT5 activity (a type II arginine methyltransferase) promotes progenitor cell expansion and accelerates erythroid differentiation. Forced expression of JAK2V617F induces a PV-like disease in mouse models (Akada et al., 2010; Marty et al., 2010; Mullally et al., 2010), and our studies suggest that in addition to activating the STAT5 pathway, this mutant JAK2 kinase can induce myeloproliferation and erythrocytosis by abrogating PRMT5 activity. While knockdown of PRMT5 in many cell lines, such as HEL and K562, led to apoptosis and/or growth arrest, its down-regulation in normal CB CD34+ cells provides a proliferative signal. We too find that PRMT5 regulates globin gene expression in HEL, CB CD34+ cells as well as in K562 cells (data not shown). However, the distinct effects of PRMT5 knockdown on gene expression in HEL vs. CD34+ cells (data not shown) demonstrates the importance of cell context on PRMT5 function.
While PRMT5 is most highly phosphorylated in JAK2V617F-positive MPN patient CD34+ cells (and in granulocytes), it is also phosphorylated to some degree in JAK2V617F-negative MPN and normal CB CD34+ cells. Overexpressing PRMT5 (wild-type and in particularly the M6 mutant form of PRMT5) in JAK2V617F-positive CD34+ patient cells resulted in a block in cell expansion and erythroid differentiation, confirming that phosphorylation and abrogation of PRMT5 activity is an important function of this oncogenic kinase. Nonetheless, further experiments are needed to assess the relative contribution of PRMT5 phosphorylation to the various clinical syndromes associated with the JAK2V617F mutation.
In conclusion, we have identified a “gain of function” for the constitutively activated forms of JAK2 kinase (JAK2V617F and JAK2K539L), namely phosphorylation of PRMT5, which allows them to control transcription by regulating histone H2A and H4 arginine methylation. Given the diverse functions of PRMT5 in the cell, further studies of the proteins methylated by PRMT5 and the pathways affected in JAK2V617F expressing cells, will shed additional light on the molecular pathogenesis of the myeloproliferative neoplasms.
EXPERIMENTAL PROCEDURES
Cell culture, transfection and plasmids
The human leukemia cell lines TF-1, HEL and Ba/F3 were grown in RPMI Media 1640 with 10% FBS (Invitrogen), supplemented with recombinant human IL-3 (2ng/ml) for TF-1 and Ba/F3 cells. To inhibit JAK2 activity in HEL and TF-1 cells, cells were treated with three distinct JAK2 inhibitors: JAK Inhibitor I (Calbiochem), CEP701 (LC Laboratories) and TG101348 at the indicated concentrations (DMSO was used as the diluent control). In the time-course experiment, HEL cells were treated with 3uM TG101348 for 0, 1, 2 and 4 hours. To activate JAK2 in TF-1 cells, the cells were deprived of cytokine for 12 to 16 hours, and then GM-CSF (25 ng/ml) or Epo (20 U/ml) was added to the growth medium for 20 minutes. Transient transfection of 293T cells was performed using the Polyfect Transfection Reagent (Qiagen) per the manufacturer’s instructions. To express the mutant or wild-type JAK2 proteins in 293T cells, the wild-type JAK2, JAK2V617F and JAK2K539L cDNAs were subcloned into the CMV promoter-driven pCDNA3 vector (Invitrogen) using EcoRV and Not1 sites. FLAG-tagged or HA-tagged PRMT5 and HA-tagged STAT5 cDNAs were also subcloned into the pCDNA3 vector through appropriate restriction sites. For in vitro kinase assays, the full-length PRMT5 open reading frame was subcloned into the bacterial expression vector pSBET, which contains a glutathione _S_-transferase (GST) tag and a 6 x Histidine tag at the N- and C-terminus of the inserted sequence, respectively.
In vitro kinase assay
In vitro kinase assays were performed using commercially available JAK2 kinase (Upstate), which contains only the kinase domain (from amino acid 800 to C-terminal end), and bacterial-purified GST-PRMT5. For each reaction, 0.5 or 2.5ug of GST-PRMT5, 0.5 or 1.25ng of JAK2 and 5uCi of [γ-32p] ATP were added to the kinase buffer (8mM MOPS pH 7.0, 0.2mM EDTA, 10mM MgAc and 0.1mM cold ATP). Reactions were incubated at 30°C for 20 min, and proteins resolved by gel electrophoresis.
In vitro methylation assay
The methylation assay was done as described (Zhao et al., 2008). HA-PRMT5 was purified from transfected 293T cells by anti- HA immunoprecipitation. The immobilized proteins were then incubated with 25ul of HMT buffer (20mM Tris pH 8.8, 4mM EDTA, 1mM PMSF, 0.5mM DTT) supplemented with 2.5ug of recombinant histone H4 or H2A (New England Biolabs) and 1uCi 3H-SAM (Amersham) at 30°C for 4 hours. The reaction was stopped, by adding SDS loading buffer, and the proteins were resolved on SDS-PAGE gels.
Microarray analysis
RNA was isolated from HEL cells expressing control shRNA or PRMT5-directed shRNA using the RNAeasy plus kit (Qiagen), transcribed into cDNA using random hexamer priming and Superscriptase (Invitrogen), and then hybridized to the Affymetrix HG-U133 GeneChips. The data were analyzed using Partek Genomic Suites, version 6.5, using a false discovery rate (FDR) of 1% to filter our data, and identify differentially expressed genes. Duplicate, independent samples were prepared for each condition.
Isolation of granulocytes and CD34+ cells from patient samples and/or cord blood
Granulocytes and mononuclear cells were isolated from human umbilical cord blood by Ficoll-Hypaque Plus (Sigma Chemical) density centrifugation. CD34+ cells were then purified from the mononuclear cells by positive selection using a Midi MACS separator, LS+ column and CD34 Micro Bead Kit (Miltenyi Biotec). For CFU and cell differentiation assays, CD34+ cells were cultured in IMDM (Cellgro) containing 20% BIT 9500 (Stem Cell Technology) for 24 hours before infection. The media was also supplemented with SCF (100ng/ml), FLT-3 (10ng/ml), IL-6 (20ng/ml) and TPO (100ng/ml) (all purchased from Peprotech).
For the analysis of patient samples, CD34+ cells and granulocytes were isolated from phlebotomy units taken from MPN patients treated at MSKCC, with appropriate IRB-approved informed consent. All samples were obtained as discarded samples during the routine clinical care of the patients and they were de-identified prior to analysis. To obtain enough cells for western blotting, the CD34+ cells were cultured in medium supplemented with cytokines (including SCF, IL3, IL6 and Epo) for one week before being subjected to analysis. Phosphorylation of PRMT5 was detected using the P-PRMT5-747 antibody. The same membrane was stripped and re-probed to quantify the total amount of PRMT5.
Generation of lentiviruses and infection of primary hematopoietic CD34+ cells
ShRNA targeting PRMT5 or a scrambled shRNA were cloned into the pLKO.1-GFP lentiviral vector. HA-PRMT5 WT or M6 cDNAs were cloned into the lentiviral vector pBGJR (modified by replacing the CMV promoter with the EF1α promoter). Viruses were produced by transfecting pLKO.1-GFP or pBGJR vector with helper plasmids into 293T cells, according to standard protocols. After 24 hours of growth, CD34+ cells were infected with high-titer concentrated lentiviral suspensions, in the presence of 8 μg/ml polybrene (Aldrich). GFP-positive cells were sorted 2 days after infection.
CFU assay
1 or 2×104 transduced GFP-positive CD34+ cells isolated from CB or PV patients were plated (in duplicate) in methylcellulose, supplemented with erythropoietin (5IU/ml), SCF (50ng/ml), IL-3 (20ng/ml), IL-6 (20ng/ml), G-CSF (20ng/ml) and GM-CSF (20 ng/ml). BFU-E, CFU-GM and CFU-GEMM colonies were scored 14 days after seeding.
In vitro differentiation assay
GFP positive CD34+ CB cells were cultured in 20% BIT in IMDM medium with different cytokines that support erythroid or myeloid cell differentiation for 7 or 14 days. The erythroid culture was supplemented with Epo (6IU/ml) and SCF (100ng/ml) and the myeloid culture with SCF (100ng/ml), FLT-3 (10ng/ml), IL-3 (20ng/ml), IL-6 (20ng/ml), GM-CSF (20ng/ml) and G-CSF (20ng/ml). Cells were collected and stained with CD71-APC (BD Pharmingen), Glycophorin A-PE (Invitrogen) to monitor erythroid differentiation and with CD11b-APC (BD Pharmingen) to monitor myeloid differentiation.
Supplementary Material
01
SIGNIFICANCE.
The JAK2V617F mutation has been found in most cases of MPNs, and in this study, we show that oncogenic JAK2 mutant kinases acquire the ability to phosphorylate and down-regulate PRMT5 activity. This contributes to the myeloproliferation and erythroid differentiation promoting effects of JAK2V617F and represents a gain-of –function that leads to cross-talk between oncogenic kinases and histone arginine methylation, altering the gene expression profile and the behavior of hematopoietic stem/progenitor cells. These findings provide insights into the pathogenesis and possible treatment strategies for the MPNs.
Acknowledgments
We thank Minkui Luo and the members of the Nimer lab for reading this manuscript and providing thoughtful suggestions and comments. We thank Erica Chuang for assistance in preparing this manuscript. This work was supported by a Leukemia Lymphoma Society SCOR Grant (SDN), a Starr Foundation Award (SDN) and a Clinical Scholar Grant (awarded to FL).
Footnotes
ACCESSION NUMBER
All expression profiling data have been deposited into NCBI GEO under accession number GSE25725.
The authors have no conflicts-of-interest or financial disclosures to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akada H, Yan D, Zou H, Fiering S, Hutchison RE, Mohi MG. Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood. 2010;115:3589–3597. doi: 10.1182/blood-2009-04-215848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancelin K, Lange UC, Hajkova P, Schneider R, Bannister AJ, Kouzarides T, Surani MA. Blimp1 associates with Prmt5 and directs histone arginine methylation in mouse germ cells. Nat Cell Biol. 2006;8:623–630. doi: 10.1038/ncb1413. [DOI] [PubMed] [Google Scholar]
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- Branscombe TL, Frankel A, Lee JH, Cook JR, Yang Z, Pestka S, Clarke S. PRMT5 (Janus kinase-binding protein 1) catalyzes the formation of symmetric dimethylarginine residues in proteins. J Biol Chem. 2001;276:32971–32976. doi: 10.1074/jbc.M105412200. [DOI] [PubMed] [Google Scholar]
- Chari A, Golas MM, Klingenhager M, Neuenkirchen N, Sander B, Englbrecht C, Sickmann A, Stark H, Fischer U. An assembly chaperone collaborates with the SMN complex to generate spliceosomal SnRNPs. Cell. 2008;135:497–509. doi: 10.1016/j.cell.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Cheung N, Chan LC, Thompson A, Cleary ML, So CW. Protein arginine-methyltransferase-dependent oncogenesis. Nat Cell Biol. 2007;9:1208–1215. doi: 10.1038/ncb1642. [DOI] [PubMed] [Google Scholar]
- Dawson MA, Bannister AJ, Gottgens B, Foster SD, Bartke T, Green AR, Kouzarides T. JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature. 2009;461:819–822. doi: 10.1038/nature08448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Keersmaecker K, Cools J. Chronic myeloproliferative disorders: a tyrosine kinase tale. Leukemia. 2006;20:200–205. doi: 10.1038/sj.leu.2404064. [DOI] [PubMed] [Google Scholar]
- Fabbrizio E, El Messaoudi S, Polanowska J, Paul C, Cook JR, Lee JH, Negre V, Rousset M, Pestka S, Le Cam A, Sardet C. Negative regulation of transcription by the type II arginine methyltransferase PRMT5. EMBO Rep. 2002;3:641–645. doi: 10.1093/embo-reports/kvf136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen WJ, Paushkin S, Wyce A, Massenet S, Pesiridis GS, Van Duyne G, Rappsilber J, Mann M, Dreyfuss G. The methylosome, a 20S complex containing JBP1 and pICln, produces dimethylarginine-modified Sm proteins. Mol Cell Biol. 2001;21:8289–8300. doi: 10.1128/MCB.21.24.8289-8300.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen WJ, Wyce A, Paushkin S, Abel L, Rappsilber J, Mann M, Dreyfuss G. A novel WD repeat protein component of the methylosome binds Sm proteins. J Biol Chem. 2002;277:8243–8247. doi: 10.1074/jbc.M109984200. [DOI] [PubMed] [Google Scholar]
- Hexner EO, Serdikoff C, Jan M, Swider CR, Robinson C, Yang S, Angeles T, Emerson SG, Carroll M, Ruggeri B, Dobrzanski P. Lestaurtinib (CEP701) is a JAK2 inhibitor that suppresses JAK2/STAT5 signaling and the proliferation of primary erythroid cells from patients with myeloproliferative disorders. Blood. 2008;111:5663–5671. doi: 10.1182/blood-2007-04-083402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle JN, Gilliland DG. Jak2: normal function and role in hematopoietic disorders. Curr Opin Genet Dev. 2007;17:8–14. doi: 10.1016/j.gde.2006.12.009. [DOI] [PubMed] [Google Scholar]
- James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, Garcon L, Raslova H, Berger R, Bennaceur-Griscelli A, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- Jansson M, Durant ST, Cho EC, Sheahan S, Edelmann M, Kessler B, La Thangue NB. Arginine methylation regulates the p53 response. Nat Cell Biol. 2008;10:1431–1439. doi: 10.1038/ncb1802. [DOI] [PubMed] [Google Scholar]
- Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- Kwak YT, Guo J, Prajapati S, Park KJ, Surabhi RM, Miller B, Gehrig P, Gaynor RB. Methylation of SPT5 regulates its interaction with RNA polymerase II and transcriptional elongation properties. Mol Cell. 2003;11:1055–1066. doi: 10.1016/s1097-2765(03)00101-1. [DOI] [PubMed] [Google Scholar]
- Lasho TL, Tefferi A, Hood JD, Verstovsek S, Gilliland DG, Pardanani A. TG101348, a JAK2-selective antagonist, inhibits primary hematopoietic cells derived from myeloproliferative disorder patients with JAK2V617F, MPLW515K or JAK2 exon 12 mutations as well as mutation negative patients. Leukemia. 2008;22:1790–1792. doi: 10.1038/leu.2008.56. [DOI] [PubMed] [Google Scholar]
- Le Guezennec X, Vermeulen M, Brinkman AB, Hoeijmakers WA, Cohen A, Lasonder E, Stunnenberg HG. MBD2/NuRD and MBD3/NuRD, two distinct complexes with different biochemical and functional properties. Mol Cell Biol. 2006;26:843–851. doi: 10.1128/MCB.26.3.843-851.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Liang JJ, Wang Z, Chiriboga L, Greco MA, Shapiro E, Huang H, Yang XJ, Huang J, Peng Y, Melamed J, et al. The expression and function of androgen receptor coactivator p44 and protein arginine methyltransferase 5 in the developing testis and testicular tumors. J Urol. 2007;177:1918–1922. doi: 10.1016/j.juro.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Lu X, Levine R, Tong W, Wernig G, Pikman Y, Zarnegar S, Gilliland DG, Lodish H. Expression of a homodimeric type I cytokine receptor is required for JAK2V617F-mediated transformation. Proc Natl Acad Sci U S A. 2005;102:18962–18967. doi: 10.1073/pnas.0509714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty C, Lacout C, Martin A, Hasan S, Jacquot S, Birling MC, Vainchenker W, Villeval JL. Myeloproliferative neoplasm induced by constitutive expression of JAK2V617F in knock-in mice. Blood. 2010 doi: 10.1182/blood-2009-12-257063. [DOI] [PubMed] [Google Scholar]
- Meister G, Fischer U. Assisted RNP assembly: SMN and PRMT5 complexes cooperate in the formation of spliceosomal UsnRNPs. EMBO J. 2002;21:5853–5863. doi: 10.1093/emboj/cdf585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullally A, Lane SW, Ball B, Megerdichian C, Okabe R, Al-Shahrour F, Paktinat M, Haydu JE, Housman E, Lord AM, et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell. 2010;17:584–596. doi: 10.1016/j.ccr.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer H, Cumano A, Muller M, Wu H, Huffstadt U, Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- Pal S, Vishwanath SN, Erdjument-Bromage H, Tempst P, Sif S. Human SWI/SNF-associated PRMT5 methylates histone H3 arginine 8 and negatively regulates expression of ST7 and NM23 tumor suppressor genes. Mol Cell Biol. 2004;24:9630–9645. doi: 10.1128/MCB.24.21.9630-9645.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S, Vanin EF, Bodner S, Colamonici OR, van Deursen JM, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- Pikman Y, Levine RL. Advances in the molecular characterization of Philadelphia-negative chronic myeloproliferative disorders. Curr Opin Oncol. 2007;19:628–634. doi: 10.1097/CCO.0b013e3282f0e20c. [DOI] [PubMed] [Google Scholar]
- Pollack BP, Kotenko SV, He W, Izotova LS, Barnoski BL, Pestka S. The human homologue of the yeast proteins Skb1 and Hsl7p interacts with Jak kinases and contains protein methyltransferase activity. J Biol Chem. 1999;274:31531–31542. doi: 10.1074/jbc.274.44.31531. [DOI] [PubMed] [Google Scholar]
- Rank G, Cerruti L, Simpson RJ, Moritz RL, Jane SM, Zhao Q. Identification of a PRMT5-dependent repressor complex linked to silencing of human fetal globin gene expression. Blood. 2010;116:1585–1592. doi: 10.1182/blood-2009-10-251116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard S, Morel M, Cleroux P. Arginine methylation regulates IL-2 gene expression: a role for protein arginine methyltransferase 5 (PRMT5) Biochem J. 2005;388:379–386. doi: 10.1042/BJ20040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi CR, Rinaldi P, Alagia A, Gemei M, Esposito N, Formiggini F, Martinelli V, Senyuk V, Nucifora G, Pane F. Preferential nuclear accumulation of JAK2V617F in CD34+ but not in granulocytic, megakaryocytic or erythroid cells of patients with Philadelphia-negative myeloproliferative neoplasia. Blood. 2010 doi: 10.1182/blood-2010-08-302265. [DOI] [PubMed] [Google Scholar]
- Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR, Futreal PA, Erber WN, McMullin MF, Harrison CN, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356:459–468. doi: 10.1056/NEJMoa065202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CP, Nakielny S. Control of the DNA methylation system component MBD2 by protein arginine methylation. Mol Cell Biol. 2006;26:7224–7235. doi: 10.1128/MCB.00473-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Fiskus W, Chong DG, Buckley KM, Natarajan K, Rao R, Joshi A, Balusu R, Koul S, Chen J, et al. Cotreatment with panobinostat and JAK2 inhibitor TG101209 attenuates JAK2V617F levels and signaling and exerts synergistic cytotoxic effects against human myeloproliferative neoplastic cells. Blood. 2009;114:5024–5033. doi: 10.1182/blood-2009-05-222133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig G, Gonneville JR, Crowley BJ, Rodrigues MS, Reddy MM, Hudon HE, Walz C, Reiter A, Podar K, Royer Y, et al. The Jak2V617F oncogene associated with myeloproliferative diseases requires a functional FERM domain for transformation and for expression of the Myc and Pim proto-oncogenes. Blood. 2008a;111:3751–3759. doi: 10.1182/blood-2007-07-102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig G, Kharas MG, Okabe R, Moore SA, Leeman DS, Cullen DE, Gozo M, McDowell EP, Levine RL, Doukas J, et al. Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F-induced polycythemia vera. Cancer Cell. 2008b;13:311–320. doi: 10.1016/j.ccr.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Xing S, Wanting TH, Zhao W, Ma J, Wang S, Xu X, Li Q, Fu X, Xu M, Zhao ZJ. Transgenic expression of JAK2V617F causes myeloproliferative disorders in mice. Blood. 2008;111:5109–5117. doi: 10.1182/blood-2007-05-091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Ren J, Gao X, Jin C, Wen L, Yao X. GPS 2.0, a tool to predict kinase-specific phosphorylation sites in hierarchy. Mol Cell Proteomics. 2008;7:1598–1608. doi: 10.1074/mcp.M700574-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Rank G, Tan YT, Li H, Moritz RL, Simpson RJ, Cerruti L, Curtis DJ, Patel DJ, Allis CD, et al. PRMT5-mediated methylation of histone H4R3 recruits DNMT3A, coupling histone and DNA methylation in gene silencing. Nat Struct Mol Biol. 2009;16:304–311. doi: 10.1038/nsmb.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Jankovic V, Gural A, Huang G, Pardanani A, Menendez S, Zhang J, Dunne R, Xiao A, Erdjument-Bromage H, et al. Methylation of RUNX1 by PRMT1 abrogates SIN3A binding and potentiates its transcriptional activity. Genes Dev. 2008;22:640–653. doi: 10.1101/gad.1632608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01