Three Types of Cortical L5 Neurons that Differ in Brain-Wide Connectivity and Function (original) (raw)
. Author manuscript; available in PMC: 2016 Dec 16.
SUMMARY
Cortical layer 5 (L5) pyramidal neurons integrate inputs from many sources and distribute outputs to cortical and subcortical structures. Previous studies demonstrate two L5 pyramid types: cortico-cortical (CC) and cortico-subcortical (CS). We characterize connectivity and function of these cell types in mouse primary visual cortex and reveal a new subtype. Unlike previously described L5 CC and CS neurons, this new subtype does not project to striatum [cortico-cortical, non-striatal (CC-NS)] and has distinct morphology, physiology and visual responses. Monosynaptic rabies tracing reveals that CC neurons preferentially receive input from higher visual areas, while CS neurons receive more input from structures implicated in top-down modulation of brain states. CS neurons are also more direction-selective and prefer faster stimuli than CC neurons. These differences suggest distinct roles as specialized output channels, with CS neurons integrating information and generating responses more relevant to movement control and CC neurons being more important in visual perception.
INTRODUCTION
The cerebral cortex is populated by numerous types of excitatory and inhibitory neurons. Excitatory pyramidal neurons (PNs) are the source of nearly all cortical outputs and thus play an essential role in mediating interactions between brain areas. In contrast, cortical inhibitory neurons make primarily local connections and modulate cortical outputs. Many studies have capitalized on cell type specific mouse lines to explore the diversity of inhibitory neuron types and their unique roles in cortical computations (Adesnik et al., 2012; Fu et al., 2014; Lee et al., 2013; Lee et al., 2012; Nienborg et al., 2013; Taniguchi et al., 2011; Wilson et al., 2012). In contrast, mouse lines for exploring the diverse contributions of different types of cortical PNs have only recently become available (Gerfen et al., 2013; Gong et al., 2007; Li et al., 2015; Olsen et al., 2012). These lines have been used to investigate the functional properties and connections of layer 6 PN types (Kim et al., 2014; Olsen et al., 2012; Vélez-Fort et al., 2014), but most previous studies of layer 5 (L5) PN types have relied on more conventional cell targeting approaches (but see Li et al., 2015). Here we identify and use mouse lines expressing Cre recombinase selectively in subtypes of L5 PNs to facilitate experiments using modern molecular, genetic, and viral tools to link distinct cell types to brain-wide connectivity and function in the visual cortex.
Previous studies of L5 PNs have revealed key details about the long-distance projections, morphology, intrinsic physiological properties, and local inputs of two major cell classes: Cortico-cortical (CC) and cortico-subcortical (CS). Importantly, CC PNs (often referred to as L5A or intratelencephalic) project to other cortical areas whereas CS neurons (L5B or pyramidal tract) project to subcortical structures including superior colliculus, thalamus, and brainstem (Bourassa and Deschenes, 1995; Groh et al., 2010; Hallman et al., 1988; Hattox and Nelson, 2007; Hubener and Bolz, 1988; Hubener et al., 1990; Kasper et al., 1994; Tsiola et al., 2003; Zarrinpar and Callaway, 2014). Both CC and CS L5 neurons project to the striatum (Cowan and Wilson, 1994; Levesque et al., 1996). L5 CC PNs have a relatively simple apical dendritic tuft, thin apical dendrite and fire action potentials in regular trains (regular spiking, RS) following somatic current injections (Groh et al., 2010; Larsen et al., 2007). In contrast, L5 CS PNs have a complex apical dendritic tuft, thick apical dendrite, and are burst spiking (BS) (Groh et al., 2010). These differences suggest that CC and CS neurons likely function as distinct information channels for mediating different perceptual and behavioral demands.
In this study, we take advantage of bacterial artificial chromosome (BAC) Cre-expressing transgenic mouse lines, in vitro whole cell recording and dye-filling, viral tracers, and two-photon calcium imaging of visual responses to define and characterize distinct types of layer 5 output neurons in mouse V1. In addition to CC and CS L5 pyramidal neurons, we identify and characterize a third type of L5 pyramidal neuron, which makes some cortico-cortical connections but does not project to striatum (CC-NS). We show that each cell class has unique in vitro electrophysiological and morphological properties. Furthermore, using monosynaptic rabies virus-based tracing methods, we show that CC neurons receive more of their synaptic inputs from higher order visual cortical areas specialized for visual image perception. CS neurons, on the other hand, receive more inputs from structures such as retrosplenial/cingulate cortex and basal forebrain, which are implicated in top-down modulation of brain states. These differences suggest possible functional differences in vivo which we evaluated using two-photon calcium imaging to assess visual responses to drifting sine wave gratings. We find that CS neurons are more direction-selective and prefer higher temporal frequency than CC neurons. Furthermore, CC-NS neurons prefer higher spatial frequencies. Our results show that each L5 projection neuron type receives differential brain-wide inputs and extracts different visual information to mediate its specialized functions.
RESULTS
Three classes of layer 5 pyramidal neurons in mouse V1 defined by distinct BAC Cre transgenic mice display different axonal projection patterns
To label distinct layer 5 pyramidal neuron subpopulations in the mouse cortex, we have identified and characterized three BAC Cre transgenic mouse lines obtained from the GENSAT project (Gerfen et al., 2013) (http://www.gensat.org/cre.jsp): Tlx3-Cre PL56, Glt25d2-Cre NF107, and Efr3a-Cre NO108. Cre recombinase expression in adult cortices of these transgenic mice is restricted to subsets of deep layer cortical neurons (Gerfen et al., 2013), consistent with results in the Allen Brain Institute transgenic atlas (http://connectivity.brain-map.org/transgenic).
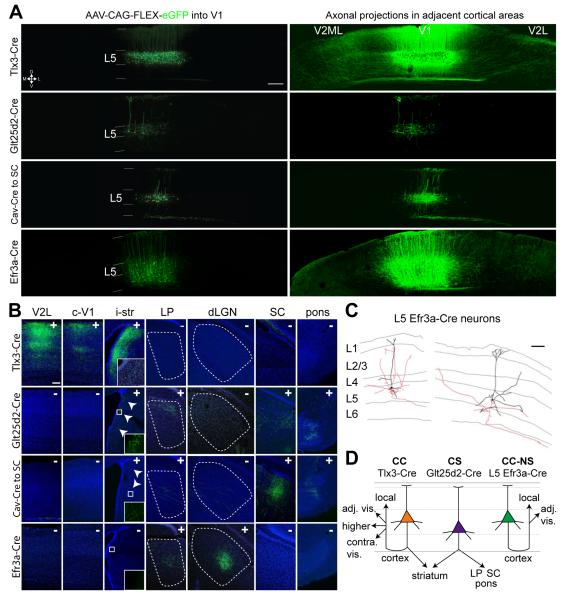
To investigate the location of Cre expressing neurons in the adult visual cortex and long-distance axonal projections of cortical neurons in each transgenic line, we injected Cre-dependent adeno-associated virus (AAV) that expresses eGFP or tdTomato (AAV-FLEX-eGFP (or tdTomato)) into the primary visual cortex (V1) of Tlx3-Cre, Glt25d2-Cre, or Efr3a-Cre mice at post-natal day 60 (P60) and harvested brains at post-injection day 21. Cell bodies expressing eGFP (or tdTomato) are located exclusively in subsets of L5 PNs in Tlx3-Cre and Glt25d2-Cre lines; in the Efr3a-Cre line cell bodies are located in both layer 5 and 6 (Figure 1A), confirming that these three mouse lines express Cre in subsets of deep layer cortical neurons. eGFP+ Tlx3-Cre+ neurons in V1 project their axons densely and predominantly to adjacent visual cortical areas such as V2L, V2ML and V2MM, as well as further cortical regions including other sensory cortices, frontal cortices, and contralateral visual cortex (Figures 1A,B and S1A-C). This suggests that Tlx3-Cre selectively labels L5 CC PNs. eGFP+ axons from Tlx3-Cre+ neurons were not found in any subcortical structures with the exception of the striatum, a known target of axon collaterals from CC neurons (Figure 1B) (Levesque et al., 1996). In contrast, eGFP+ axons from Glt25d2-Cre+ neurons in V1 do not project to other cortical areas (Figures 1A,B). Instead, their axons enter white matter and travel to target subcortical structures including the superior colliculus, lateral posterior (LP) and lateral dorsal (LD) nuclei of thalamus, pons, and ipsilateral striatum (Figures 1B and S1D-O). These axonal projections suggest Glt25d2-Cre selectively labels L5 CS PNs. We also labeled L5 CS PNs in V1 by injecting retrogradely infecting Cav2-Cre virus into the superior colliculus and AAV-FLEX-eGFP into V1. Layer 5 neurons labeled by Cre expression in the Glt25d2-Cre mouse line and those labeled following Cav2-Cre injection to superior colliculus exhibit similar cell body locations and axon target profiles (Figures 1A,B). We conclude that although Glt25d2-Cre+ neurons are sparse, they are a representative sample of CS neurons.
Figure 1. Three Distinct Classes of Layer 5 Pyramidal Neurons Defined by BAC Cre Transgenic Lines Exhibit Distinct Long Distance Axonal Projection Patterns.
(A) Coronal sections showing eGFP labeled neurons after AAV-FLEX-eGFP (or tdTomato) injection into the primary visual cortex of three BAC transgenic Cre mice, Tlx3-Cre PL56, Glt25d2-Cre NF107, Efr3a-Cre NO108 or C57BL/6 mouse injected with Cav2-Cre virus into the superior colliculus (SC). Left and right panels for each condition correspond to photographs of the same fields of view but with the panels to the left imaged at lower brightness to illustrate dendritic morphology and cell body locations and right panels at higher brightness to better reveal axonal projections. (B) Axonal projections of eGFP+ labeled V1 Cre+ neurons to V2L, contralateral V1, ipsilateral striatum (i-str), thalamic nuclei LP and dLGN, superior colliculus (SC), and pons. Inset in i-str panel of Tlx3-Cre: contralateral striatum. Insets in i-str panels of Glt25d2-Cre and Cav2-Cre to SC: magnified images. '+' or '−' indicates presence or absence of GFP+ axons in each panel. (C) Partial reconstructions of L5 Efr3a-Cre+ neurons using Neurolucida. Dendrites: black; axons: red. (D) Schematic representation illustrating three different Cre+ subpopulations of layer 5 neurons projecting their axons to different structures. Abbreviations: CC, cortico-cortical; CC-NS, cortico-cortical non-striatal; CS, cortico-subcortical; dLGN, dorsal lateral geniculate nucleus; i-str, ipsilateral striatum; L5, layer 5; LP, lateral posterior thalamic nucleus; SC, superior colliculus; V1, primary visual cortex; V2L, secondary visual cortex, lateral area. Scale bars = 200 μm (A), 100 μm (B, C).
Notably, Efr3a-Cre+ layer 5 (L5) V1 neurons lack projections to known axonal targets of layer 5 CC and CS neurons such as: superior colliculus, thalamus, brainstem, and striatum (Figure 1B). Efr3a-Cre+ neurons do project to other adjacent cortical areas, a target they share in common with layer 5 CC neurons. Dense eGFP+ labeled long distance axons are also found in known layer 6 neuron targets, including the dorsal lateral geniculate nucleus (dLGN), LD, and LP of thalamus (Figures 1B and S1J-O). In LP, a shared target region of layer 5 and 6 neurons, axon terminals of Efr3a-Cre+ neurons are thin and small type I morphology, distinct from the thick and large type II morphology of Glt25d2-Cre+ neurons (Figures S1P,Q) (Li et al., 2003). The presence of labeled neurons in both layer 5 and 6 of Efr3a-Cre mice makes it less straightforward to study the projections of L5 Efr3a-Cre+ neurons in isolation. However, several lines of evidence detailed below indicate that L5 Efr3a-Cre+ neurons do not project to the thalamus and that they include both local pyramids (not projecting out of V1) and projection neurons. Since the only targets of Efr3a-Cre+ neurons that are known to receive input from L5 rather than L6 are adjacent cortical areas (see above), L5 Efr3a-Cre+ neurons must include CC projection neurons and might therefore represent a sub-group of CC neurons. Despite the fact that both L5 Efr3a-Cre+ neurons and Tlx3-Cre+ CC cells share a common extrinsic target (adjacent cortical areas), they are clearly distinct and non-overlapping groups. In addition to differences in projections to striatum, as described in further detail below, the morphology and intrinsic physiology of L5 Efr3a-Cre+ neurons further distinguish them from Tlx3-Cre+ CC cells.
To determine whether LP projecting Efr3a-Cre+ V1 neurons are in L5, cholera toxin subunit B conjugated to Alexa Fluor 594 (a retrograde tracer) was injected into LP of Efr3a-Cre mice (Figure S1R), while AAV-FLEX-eGFP was injected in V1. Although many Alexa Flour 594 labeled neurons were found in L5, none were eGFP+ L5 Efr3a-Cre+ neurons, indicating an absence of projections to LP (Figures S1S,T). Further, in line with a previous study demonstrating that L5 neurons in mouse primary visual cortex do not project to dLGN (Bortone et al., 2014), L5 Efr3a-Cre+ neurons do not project to thalamus. To trace the axons of single L5 Efr3a-Cre+ neurons, we partially reconstructed sparsely labeled neurons (see Experimental Procedures, Figure S2B). The main descending axons of all cells were reconstructed far enough to unambiguously determine whether they extended into the white matter or clearly ended before reaching the white matter. Results from axonal reconstructions demonstrate that 8 of 15 L5 Efr3a-Cre+ neurons are local pyramids (Figure 1C, left panel), whereas 7 of 15 neurons project out of V1 (Figure 1C, right panel) and presumably continue to nearby cortical areas, as these are the only regions other than the thalamus in which axons are seen following bulk labeling (Figure 1A). Note that the long distance projections of Efr3a-Cre+ neurons also differ from both Tlx3-Cre+ and Glt25d2-Cre+ neurons in that no eGFP labeling is seen in the striatum (Figure 1B). These results are consistent with evidence for three distinct classes of L5 PNs (Figure 1D).
Morphological and electrophysiological characterization indicates labeling of three distinct layer 5 pyramidal neuron populations
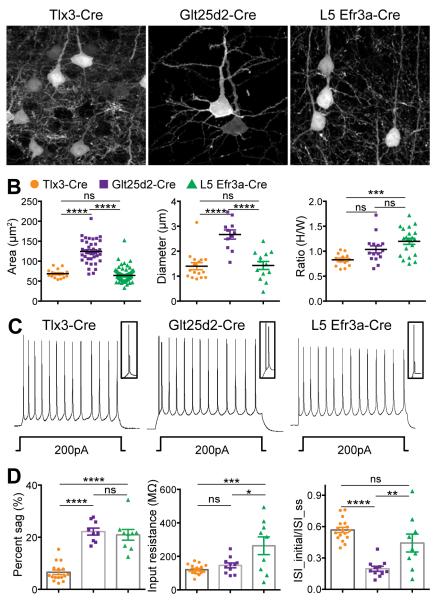
We further investigated whether L5 PNs defined by Tlx3-Cre, Glt25d2-Cre, and Efr3a-Cre are distinct morphologically and/or physiologically. First, we characterized their soma and proximal dendrite morphology using confocal microscopy after AAV-FLEX-eGFP injection into V1 (Figure 2A). In terms of both soma size and apical dendrite diameter, Tlx3-Cre+ neurons (n=16) are significantly smaller than Glt25d2-Cre+ neurons (n=39) (soma size (μm2): 68.60 ± 2.72 versus 124.0 ± 4.67, respectively, apical dendrite diameter (μm): 1.40 ± 0.14 versus 2.67 ± 0.18, respectively; mean ± SEM, one-way ANOVA with Tukey’s post-hoc test, p<0.0001, Figures 2A,B). The soma size and apical dendrite diameter of L5 Efr3a-Cre+ neurons (n=97, soma size: 64.29 ± 1.58 μm2, apical dendrite diameter: 1.43 ± 0.16 μm) are also significantly smaller than Glt25d2-Cre+ neurons (one-way ANOVA with Tukey’s post-hoc test, p<0.0001). These differences between Glt25d2-Cre+ neurons and both Tlx3-Cre+ and Efr3a-Cre+ neurons were expected based on previous comparisons of CC and CS cells (Groh et al., 2010; Kasper et al., 1994; Larkman et al., 1988). In contrast, there was no difference between soma sizes and apical dendrite diameters between Tlx3-Cre+ and Efr3a-Cre+ neurons (Figures 2A,B).
Figure 2. Morphological and Electrophysiological Properties of Three Types of Layer 5 Pyramidal Neurons.
(A) Confocal images displaying soma and proximal dendrites of eGFP+ Tlx3-Cre+, Glt25d2-Cre+ and L5 Efr3a-Cre+ neurons. (B) Dendritic and cell body morphologies of Tlx3-Cre+, Glt25d2-Cre+ and L5 Efr3a-Cre+ neurons differ in cell soma size (μm2), apical dendrite diameter (μm) at the base, and height over width (H/W) ratio. (C) Examples of action potential trains evoked by 200 pA current injections into Tlx3-Cre+, Glt25d2-Cre+, and L5 Efr3a-Cre+ neurons. (D) Intrinsic electrical property differences in (left) percent sag (%), (middle) input resistance (MΩ) and (right) interspike interval at initial over interspike interval at steady state phase of Tlx3-Cre+, Glt25d2-Cre+ and L5 Efr3a-Cre+ neurons. Values are reported as means ± SEM for each class of neurons. Statistics were calculated from one-way ANOVAs followed by Tukey’s post-hoc tests to compare means of pairs of each L5 class. Significant differences between pairs are indicated by the P value. *p < 0.05, **p < 0.01, ***p < 0.001, ****p<0.0001. Scale bar = 10 μm (A).
Despite these similarities between Tlx3-Cre+ and Efr3a-Cre+ neurons, other morphological features clearly distinguished the two populations. Upon careful visual inspection, L5 Efr3a-Cre+ cells have a different soma shape from Tlx3-Cre+ or Glt25d2-Cre+ cells: Efr3a-Cre+ cell somata (n=21) have an oval appearance whereas Tlx3-Cre+ and Glt25d2-Cre+ cell somata (n=13 and n=15, respectively) appear more pyramidal (Figure 2A). We quantified this by measuring the height and width of each cell and determining the height over width ratio (H/W) as a parameter of cell body shape. L5 Efr3a-Cre+ neurons have significantly higher H/W ratios than Tlx3-Cre+ neurons (1.20 ± 0.06 and 0.83 ± 0.04, respectively, one-way ANOVA with Tukey’s post-hoc test, p<0.001, Figure 2B), indicating that Tlx3-Cre+ and L5 Efr3a-Cre+ neurons are morphologically different. Furthermore, the greatest H/W ratio for the Tlx3-Cre+ neurons was 1.03, while 15 of 21 (71.43%) of the L5 Efr3a-Cre+ neurons had larger ratios indicating that there is less than 30% overlap between these distributions and that the two populations are largely distinct based solely on this single morphological feature.
Next, we measured intrinsic membrane properties of the three L5 PN populations to test whether they exhibit distinct electrophysiological characteristics. To perform whole-cell patch-clamp analysis, we prepared acute brain slices of P28-P50 visual cortex from Tlx3-Cre, Glt25d2-Cre, and Efr3a-Cre mice after they were either crossed with Ai14 (Cre reporter line expressing tdTomato upon Cre-mediated recombination; (Madisen et al., 2012) or injected with AAV-FLEX-eGFP (or tdTomato). Near threshold depolarizing current pulses injected to the cell bodies under current clamp conditions revealed that all Tlx3-Cre+ neurons (17 of 17) and most L5 Efr3a-Cre+ neurons (7 of 9) are regular spiking, while all Glt25d2-Cre+ neurons (11 of 11) are intrinsically bursting (see Experimental Procedures), as expected from previous descriptions of CC thin-tufted and CS thick-tufted neurons, respectively (Figure 2C) (Groh et al., 2010; Guan et al., 2015; Kasper et al., 1994; Larkman et al., 1988)
Despite the fact that both Tlx3-Cre+ and L5 Efr3a-Cre+ neurons are regular spiking, further analyses revealed that the intrinsic electrical properties of L5 Efr3a-Cre+ neurons are distinct from both Tlx3-Cre+ and Glt25d2-Cre+ neurons (Figure 2D). Analysis of interspike intervals (ISIs) at the onset of current injection divided by the interval at steady state yielded similar values for Tlx3-Cre+ and L5 Efr3a-Cre+ neurons (0.57 ± 0.02 and 0.44 ± 0.09, respectively; mean ± SEM) both of which are distinct from Glt25d2-Cre+ neurons (0.20 ± 0.02, one-way ANOVA with Tukey’s post-hoc test, p<0.0001 and p<0.01 respectively). Input resistance (MΩ) for Tlx3-Cre+ and Glt25d2-Cre+ neurons are similar (120.91 ± 6.68 and 146.37 ± 14.53, respectively). However, L5 Efr3a-Cre+ neurons exhibit significantly higher input resistances than the other two cell types (263.37 ± 52.72, one-way ANOVA with Tukey’s post-hoc test, p<0.05 to Glt25d2-Cre+ and p<0.001 to Tlx3-Cre+ neurons). Although differences in the mean values were observed between cell types for both ISIs and input resistance, there was considerable overlap in the distributions between L5 Efr3a-Cre+ neurons and both of the other groups (Figure 2D).
However, a third measure of intrinsic electrical properties clearly distinguished L5 Efr3a-Cre+ neurons from Tlx3-Cre+ neurons. Notably, L5 Efr3a-Cre+ neurons exhibited significantly higher percent sag (%) than Tlx3-Cre+ neurons (20.93 ± 2.05 and 6.57 ± 0.88, respectively. One-way ANOVA with Tukey’s post-hoc test, p<0.0001), while percent sags for L5 Efr3a-Cre+ and Glt25d2-Cre+ neurons are similar (20.93 ± 2.05 and 22.19 ± 1.34, respectively). Sag amplitudes were also significantly larger for L5 Efr3a-Cre+ and Glt25d2-Cre+ neurons than for Tlx3-Cre+ neurons (Figure S3D). Further details of percent sag measurements can be found in Figure S3. Note that there is little overlap in the distributions of percent sag values between L5 Efr3a-Cre+ neurons and Tlx3-Cre+ neurons (Figure 2D), and no overlap in sag amplitudes (Figure S3D), indicating that they are distinct populations. Furthermore, the lack of bursting in both Efr3a-Cre+ and Tlx3-Cre+ neurons distinguishes them from Glt25d2-Cre+ neurons. These physiological features, along with the morphological features described above, indicate that these three mouse lines label three distinct and non-overlapping L5 neuronal populations that must correspond to distinct cell types. Additional electrophysiological characterization, including adaptation indexes and capacitance were also evaluated for all three Cre+ cell groups and can be found in Table S1.
Altogether, extensive comparisons among three genotypically defined layer 5 PN populations demonstrate distinct morphological and physiological properties. While the classical measures of intrinsic physiology (RS versus BS) and morphology (thin-tufted versus thick-tufted) are as expected from the distant projections (CC versus CS) of each cell group, L5 Efr3a-Cre+ neurons are clearly distinct from both Tlx3-Cre+ L5 CC PNs and Glt25d2-Cre+ L5 CS PNs. The soma shapes of L5 Efr3a-Cre+ neurons in V1 are more oval, they have 3-fold greater electrical sag than Tlx3-Cre+ CC PNs (with no overlap in their distributions), and they lack projections to the striatum. Furthermore, both Tlx3-Cre+ CC PNs and L5 Efr3a-Cre+ neurons are distinct from Glt25d2-Cre+ L5 CS PNs based on their classical intrinsic firing and dendritic morphological features.
Brain-wide inputs to genetically defined layer 5 pyramidal neurons using monosynaptic rabies virus
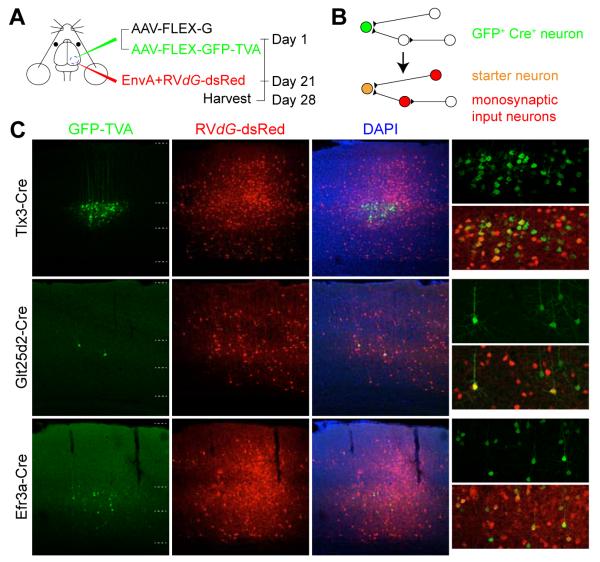
To profile brain-wide distributions of neurons directly presynaptic to Tlx3-Cre+, Glt25d2-Cre+ and Efr3a-Cre+ neurons in V1, we used Cre-dependent tracing with G-deleted rabies virus (RV_dG_) (Figures 3A,B) (Wall et al., 2010; Wickersham et al., 2007). To restrict initial RV_dG_ infection to Cre expressing starter neurons in a given region and label monosynaptic input neurons specifically, we used Cre-dependent AAV vectors to express TVA, rabies glycoprotein (G), and GFP selectively in Cre+ neurons. TVA is a receptor for the avian sarcoma leucosis virus envelope protein, EnvA, and allows selective infection of Cre+ “starter” cells with EnvA-pseudotyped RV_dG_ (EnvA+RV_dG_). Expression of G in Cre+ neurons allows for trans_-complementation in neurons infected with RV_dG such that rabies particles can be produced in starter cells and spread to their direct presynaptic inputs. GFP is used to mark cells expressing TVA, facilitating later quantitative analyses. In these experiments, we expressed these three genes in two separate AAV vectors in conjunction with a novel chimeric rabies glycoprotein that mediates more efficient _trans_-complementation and trans-synaptic spread than previous versions (see Experimental Procedures). Furthermore, one of the AAV vectors (AAV-FLEX-G) expresses G alone in order to maximize G expression, further improving _trans_complementation, and trans-synaptic spread of RV. The second AAV vector (AAV-FLEX-GFP-TVA) expresses both TVA and GFP.
Figure 3. Monosynaptic Rabies Virus Tracing of Inputs to Layer 5 Pyramidal Neurons.
(A) Schematic illustrating virus injection schemes for monosynaptic rabies virus tracing. (B) Schematic illustrating labeling of various neuronal populations following rabies virus-mediated monosynaptic input labeling. (C) Coronal sections of visual cortices of Tlx3-Cre, Glt25d2-Cre, Efr3a-Cre mice showing GFP-TVA+, dsRed+ starter neuron locations. Scale bar = 100 μm (C).
Tlx3-Cre+, Glt25d2-Cre+ and Efr3a-Cre+ mice (>42 days old at the onset of experiments) were first injected at day 0 with a mixture of AAV-FLEX-G and AAV-FLEX-GFP-TVA into V1. At day 21, EnvA+RV_dG_ expressing dsRed (EnvA+RV_dG-_dsRed) was injected at the same location. This resulted in expression of GFP, TVA, G, and dsRed in “starter” cells that were directly infected with EnvA+RV_dG_-dsRed in V1 and expression of only dsRed in distant neurons providing direct monosynaptic input to the starter cells (Figure 3). At day 28-29, animals were sacrificed, their brains sectioned and stained, and labeling patterns across the whole brain were reconstructed to create maps of the locations of dsRed+ input neurons for analysis.
To assess long distance presynaptic neurons to L5 Tlx3-Cre+, Glt25d2-Cre+ and L5/6 Efr3a-Cre+ neurons of V1, we analyzed every other coronal section of the whole brains from Bregma 2.50 mm to −5.00 mm. Three factors should be considered before analyzing and interpreting long distance presynaptic cells correctly in our paradigm. First, it should be noted that since it was not possible to selectively infect L5 Cre+ neurons in Efr3a-Cre mice (unlike Tlx3-Cre and Glt25d2-Cre mice), both L5 and L6 neurons serve as starter neurons (Figure 3C). Second, low levels of leaky TVA expression are sufficient to mediate direct infection with EnvA+RV_dG_-dsRed and dsRed expression in non-Cre+ neurons close to the injection site, however, leak expression of G is not sufficient to mediate _trans_-complementation and trans-synaptic labeling in distant neurons (Figure S4) (Miyamichi et al., 2013; Wall et al., 2010). Our virus injection sites were in the center of V1, from −3.3 to −3.5 mm along anterior-posterior axis, and from 2.4 to 2.7 mm along medial-lateral axis from bregma (Figure 4A). The adjacent visual cortical areas closest to these injections are more than 1mm away and beyond the zone where direct infection of neurons with leaky TVA expression could confound results (Figures 4A and S4A). Therefore, this caveat should not affect our long-distance monosynaptic input mapping. Third, each brain in each transgenic mouse group has different starter cell numbers and differs in total numbers of input neurons (Figure S4B). In order to make direct statistical comparisons between experiments and between transgenic lines, here we report the percentage of input in any given region over total number of input neurons across the entire brain (excluding local input neurons in V1).
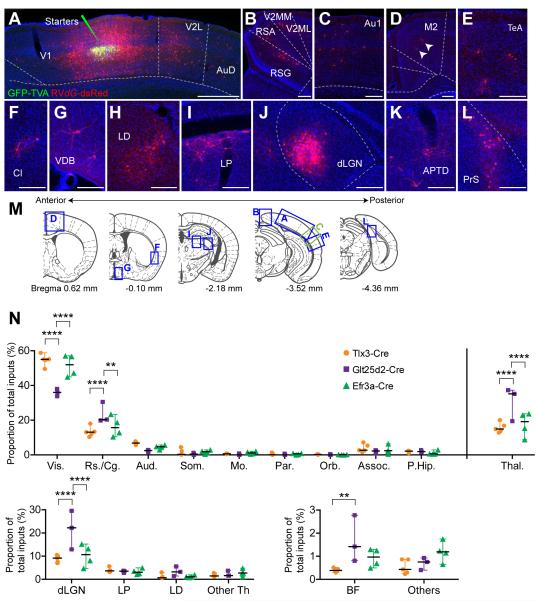
Figure 4. Brain-Wide Monosynaptic Input to Layer 5 Pyramidal Neurons Revealed by Monosynaptic Rabies Virus Tracing.
(A-L) Coronal sections of a rabies virus labeling in Tlx3-Cre+ mice showing GFP-TVA+, dsRed+ starter neurons and local inputs in V1 (A) and long-distance inputs from V2L and AuD (A), V2MM, V2ML, RSA, and RSG (B), Au1 (C), M2 (D), TeA (E), Cl (F), VDB (G), LD (H), LP (I), dLGN (J), APTD (K) and PrS (L). (M) Coronal section diagrams along the anterior-posterior axis illustrating locations of anatomical regions shown in A-L. Note that the diagrams were chosen for illustration purposes and do not necessarily correspond exactly to figures. (N) Summary of long-range monosynaptic inputs onto Tlx3-Cre+, Glt25d2-Cre+, Efr3a-Cre+ neurons in the primary visual cortex. Statistics were calculated from two-way ANOVAs with Tukey’s post-hoc tests. Significant differences between pairs are indicated by the P value. **p < 0.01 and ****p<0.0001. Abbreviations are: APTD, anterior pretectal nucleus, dorsal part; Au1, primary auditory cortex; AuD, secondary auditory cortex, dorsal area; BF, basal forebrain; Cl, claustrum; dLGN, dorsal lateral geniculate nucleus; LD, laterodorsal thalamic nucleus; LP, lateral posterior thalamic nucleus; M2, secondary motor cortex; PrS, presubiculum; RSA, retrosplenial agranular cortex; RSG, retrosplenial granular cortex; V1, primary visual cortex; VDB, nucleus of the vertical limb of the diagonal band; V2L, secondary visual cortex, lateral; V2ML, secondary visual cortex, mediolateral area; V2MM, secondary visual cortex, mediomedial area; TeA, temporal association cortex. Scale bars = 500 μm (A), 200 μm (B-L).
We counted and registered dsRed+ input neurons to the smallest possible subregion referenced in the Paxinos mouse atlas (Paxinos et al., 2001). We also used nuclear DAPI (4′,6-diamidino-2-phenylindole) counterstaining and autofluorescence background to identify various anatomical boundaries to ensure input neurons were assigned to the correct structures (see Experimental Procedures). To directly compare the proportions of inputs from each area across mouse lines, we assigned input neurons to 16 regions. These include 10 cortical subdivisions: visual (including V2L, V2ML and V2MM), retrosplenial/cingulate, auditory, somatosensory, motor, parietal, orbital, ventral-associated cortical areas, para-hippocampal area; 4 thalamic subdivisions: dLGN, LP, LD, other thalamic areas; and the basal forebrain and other areas including striatum, amygdala and hypothalamus (See Table S2). Neurons in visual cortical areas were assigned to V2L, V2ML and V2MM rather than their smaller subdivisions (e.g. P, POR, LM, AL, RL, AM, PM, M) because only the larger regions can be reliably identified using postmortem anatomical criteria. Smaller subdivisions encompassed by V2L are likely to include RL, AL, LI, LM, P and POR. Similarly, V2ML and V2MM likely include AM, PM and M (Garrett et al., 2014).
Cortical inputs to CC and CS PNs in V1
Figure 4N summarizes long range input profiles onto Tlx3-Cre+, Glt25d2-Cre+ and Efr3a-Cre+ V1 neurons. All three Cre+ populations in V1 received more input from extrastriate visual areas than any other structure (Figures 4A,N). Other relatively strong long-range cortical inputs were found in retrosplenial, cingulate, auditory and somatosensory cortices (both primary and secondary regions; Figures 4A-C,N). There were smaller numbers of input neurons found in higher cortices such as parietal, orbital, motor and ventral associational cortex (including temporal association cortex, entorhinal, ectorhinal, perirhinal cortex) and para-hippocampal areas (Figures 4D-E, N). These results indicate the presence of direct feedback from many higher association areas to V1 in the mouse, without the necessity for transfer through intermediate higher sensory cortical areas.
Although these overall input trends were similar across Tlx3-Cre+, Glt25d2-Cre+ and Efr3a-Cre+ mice, there were significant differences between mouse lines. Extrastriate visual areas provide a significantly higher proportion of the inputs to Tlx3-Cre+ and Efr3a-Cre+ neurons (54.65 ± 1.48% and 51.50 ± 3.16% respectively) than to Glt25d2-Cre+ neurons (35.94 ± 1.21%, two-way ANOVA with Tukey’s post-hoc test, p<0.0001) (Figure 4N). In contrast, Glt25d2-Cre+ V1 neurons receive a higher proportion of their inputs from retrosplenial and cingulate cortices than do Tlx3-Cre+ and Efr3a-Cre+ V1 neurons (23.59 ± 3.49%, 13.38 ± 1.29% and 16.33 ± 2.90% respectively, two-way ANOVA with Tukey’s post-hoc test, p<0.0001 (Glt25d2-Cre+ vs. Tlx3-Cre+) and p<0.01 (Glt25d2-Cre+ vs. Efr3a-Cre+) (Figure 4N).
Thalamic inputs to CC and CS PNs in V1
We next examined long-range inputs from thalamic areas, the most prominent subcortical input source to all three Cre+ L5 neuronal populations in V1, ranging from 12% to 30% of total inputs (Figures 4H-J, N and S5D-G). Among thalamic areas, dLGN functions as the primary relay from the retina to V1 (Grubb and Thompson, 2003). While the axons of LGN neurons primarily target cortical layer 4, there are also substantial projections to layers 1 and 5, and L5 PNs have dendritic branches in all of these locations. Thus, it is not surprising that previous physiological and anatomical studies have demonstrated direct dLGN input to L5 PNs (Constantinople and Bruno, 2013; Ferster and Lindstrom, 1983). To our knowledge, it is not known whether different types of L5 V1 neurons receive different proportions of dLGN inputs. Our trans-synaptic rabies tracing showed that Glt25d2-Cre+ neurons receive a much higher proportion of dLGN input (21.57 ± 4.81%) compared to Tlx3-Cre+ and Efr3a-Cre+ neurons (8.85 ± 0.67% and 10.32 ± 2.39% respectively, two-way ANOVA with Tukey’s post-hoc test, p<0.0001). Rabies tracing data for Tlx3-Cre+ and Glt25d2-Cre+ neurons also showed that both CC and CS L5 neurons receive substantial proportions of their direct input from secondary visual thalamic nuclei, LD and LP. In addition, all three Cre+ L5 PN groups in V1 reliably receive small numbers of labeled inputs from various epithalamic nuclei as well as secondary thalamic nuclei for other sensory modalities, such as Po (Figures S5D-G).
Other subcortical inputs to CC and CS PNs in V1
In addition to cortical and thalamic inputs, L5 PNs in V1 received direct long-range inputs from basal forebrain (Figures 4G,N). The basal forebrain is composed of diverse neuronal types including cholinergic neurons that project to cortex to modulate brain states such as arousal and wakefulness. Interestingly, Glt25d2-Cre+ neurons receive a significantly higher proportion of their inputs from the basal forebrain compared to Tlx3-Cre+ neurons (1.67 ± 0.58% and 0.40 ± 0.04% respectively, two-way ANOVA with Tukey’s post-hoc test, p<0.01).
By examining direct presynaptic neurons throughout the whole brain, we have also observed monosynaptic inputs to V1 from various unexpected anatomical structures, which have not been described in previous studies. These structures include caudate putamen and lateral globus pallidus in the striatum, the medial amygdaloid nucleus, and posterior or lateral hypothalamic areas (Figures S5A-C).
Most cortical inputs to V1 L5 CC and CS PNs originate from other L5 PNs
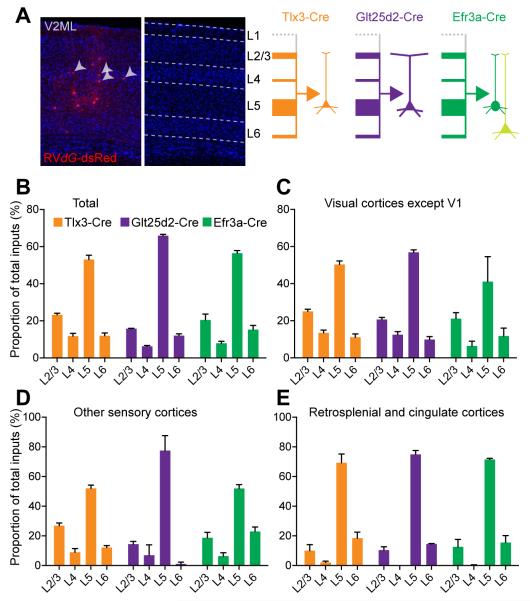
We next investigated the laminar distributions of long-range cortical input neurons to L5 V1 neurons. While many previous studies have investigated the laminar locations of neurons making feedforward or feedback cortical connections, and other studies have documented the laminar termination patterns of cortical projections, we are not aware of any studies investigating the laminar sources of inputs to neurons located in a particular cortical layer or of a particular PN type. We therefore analyzed the laminar locations of rabies-labeled cortical neurons in Tlx3-Cre, Glt25d2-Cre and Efr3a-Cre mice. Figure 5A shows the laminar pattern of input neurons in visual cortical area V2ML following rabies virus injections into V1 of a Tlx3-Cre+ mouse. We found dsRed+ rabies-labeled neurons in all layers except L1. Across the three Cre+ lines, for cortical neurons pooled from all cortical areas, the majority of long-range input neurons are located in L5 (53.05 ± 1.47% for Tlx3-Cre+, 65.93 ± 0.74 % for Glt25d2-Cre+ and 56.46 ± 1.46% for Efr3a-Cre+ neurons; mean ± SEM, Figure 5B). Laminar bias of input neurons to L5 is even more striking in retrosplenial and cingulate cortices that modulate top-down processes; 69.31 ± 5.91% of input neurons from medial cortices such as retrosplenial and cingulate cortices to Tlx3-Cre+ V1 neurons are located in L5, whereas few L2/3 neurons in retrosplenial and cingulate cortices make monosynaptic connections to L5 neurons in V1 (10.06 ± 4.09%, Figure 5E). It is also notable that 8.64 ± 1.63% of long-range input neurons are from L4 (Figures 5B-D). Studies of L4 excitatory neurons in primary sensory cortices have emphasized their roles as recipients of thalamocortical inputs, and in projecting locally to layer 2/3 without providing long-distance outputs (Douglas and Martin, 2004). This result reveals that at least some L4 neurons participate in long-range cortical-cortical connections. All three Cre+ neuronal populations show considerable similarities in terms of laminar distribution of long-range input neurons. This suggests that cell-type specific connectivity between long-range connections might be based on soma locations along layers rather than their axonal projection specificity. Together, our findings provide anatomical evidence for layer-specific long distance connection specificity at a cellular level.
Figure 5. Laminar Distributions Long-Range Cortical Inputs onto Layer 5 Pyramidal Neurons.
(A) An example coronal section showing laminar distribution of dsRed+ rabies traced input neurons in V2ML onto Tlx3-Cre+ V1 neurons. Arrowheads indicate dsRed+ presynaptic neurons in L4. (A, right panel) Schematic illustrating relative proportions of input neurons from each layer as thickness of arrows for Tlx3-Cre+, Glt25d2-Cre+ and Efr3a-Cre+ mice. (B-E) Laminar distributions of long-range cortical inputs onto Tlx3-Cre+, Glt25d2-Cre+, Efr3a-Cre+ V1 neurons as proportions of total cortical inputs (%) for all cortical areas (B), visual cortices except V1 (C), other sensory cortices (D) and retrosplenial and cingulate cortices (E). Abbreviations are V1, primary visual cortex; V2ML, secondary visual cortex, mediolateral area. Scale bar = 100 μm (A).
Visual response properties of cortical versus subcortical projecting layer 5 neurons
Because L5 CC, CC-NS and CS PNs send their outputs to different structures it is likely that they process different types of visual information. We therefore took advantage of the Tlx3-Cre, Glt25d2-Cre, and Efr3a-Cre mouse lines to investigate in vivo functional properties of L5 CC. CC-NS and CS PNs in V1. Visual response properties were characterized based on two-photon imaging of calcium dynamics in stationary, awake mice (Figure 6A). We expressed the calcium indicator GCaMP6 and tdTomato in subsets of CC, CC-NS or CS L5 PNs by injecting a 2:1 mixture of AAV-FLEX-GCaMP6 and AAV-FLEX-tdTomato in V1 of each Cre transgenic mouse (Figures 6B-C). Figure 6B displays a z-stack of two-photon microscope images from GCaMP6 and TdTomato expressing L5 CS PNs in V1 of a Glt25d2-Cre mouse; cell bodies can be clearly distinguished as well as their apical dendrites extending through the cortical depth up to the pia.
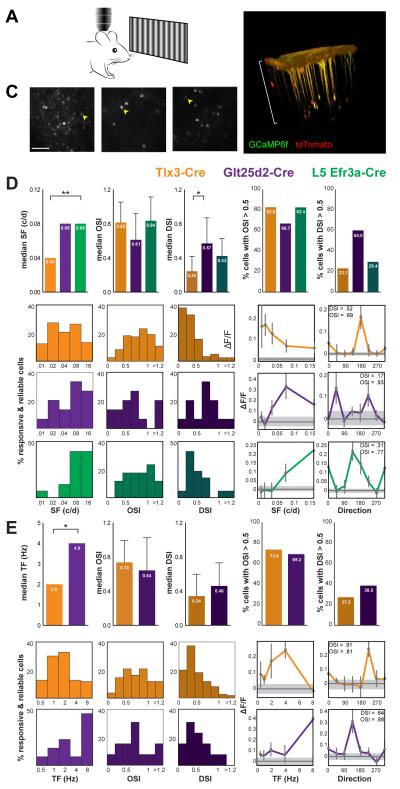
Figure 6. Visual Responses of Layer 5 Pyramidal Neurons Assayed with Two-Photon Calcium Imaging.
(A) Schematic illustration of two-photon in vivo calcium imaging set up for awake and head-fixed stationary mouse. (B) Two-photon microscope z-stack projection of Glt25d2-Cre+ mouse V1 after AAV-FLEX-GCaMP6 and AAV-FLEX-tdTomato injection. (C) Representative images of single cells expressing GCaMP6 in V1 of Tlx3-Cre+, Glt25d2-Cre+, Efr3a-Cre+ mice. Arrowheads indicate cells plotted in (D) and (E). Scale bar = 100 μm. (D) Spatial Frequency (SF) Experiments. Top: Medians for preferred SF, OSI and DSI (with interquartile ranges for OSI and DSI, at the preferred SF) for Tlx3-Cre+, Glt25d2-Cre+, L5 Efr3a-Cre+ neurons, as well as the percentage of cells with OSI or DSI > 0.5. Bottom left: Distributions of preferred SF, OSI and DSI (at the preferred SF) for Tlx3-Cre+, Glt25d2-Cre+, and L5 Efr3a-Cre+ neurons. Bottom right: SF and orientation tuning curve examples for each cell type. Values plotted as means ± SEM. Gray lines indicate average responses during blank stimulus; shading is ± SEM. (E) Temporal Frequency (TF) Experiments. Top: Medians with interquartile ranges for preferred TF, OSI and DSI (at the preferred TF) for Tlx3-Cre+ and Glt25d2-Cre+ neurons, as well as the percentage of cells with OSI or DSI > 0.5. Bottom left: Distributions of preferred TF, OSI and DSI (at the preferred TF) for Tlx3-Cre+ and Glt25d2-Cre+ neurons. Bottom right: Temporal frequency (TF) and orientation tuning curve examples for each cell type. Values plotted as means ± SEM. Gray lines indicate average responses during blank stimulus; shading is ± SEM. For median plots, statistical significances are labeled as p values after Wilcoxon rank-sum test (TF experiments) or Kruskal-Wallis test with Dunn's multiple comparisons test as post-hoc (SF experiments). *p < 0.05 and **p < 0.01. Abbreviations: c/d, cycle per degree; Hz, Hertz.
To assess tuning properties, two different stimulation paradigms were used. To quantify spatial frequency (SF) tuning, drifting sine wave gratings were varied over five different SFs (0.01 to 0.16 c/d) and eight different directions while temporal frequency (TF) was kept constant at 1 Hz. To quantify TF tuning, gratings were presented at five different TFs (0.05 to 8 Hz) and eight directions while SF was kept constant at 0.04 cycles per degree (c/d). Using these paradigms, we generated tuning curves for SF, TF, and orientation/direction (at best SF or TF) for Tlx3-Cre+, Glt25d2-Cre+ and L5 Efr3a-Cre+ neurons (Figures 6D-E, right panels).
For neurons that were visually responsive and reliable (see Experimental Procedures), various indices were calculated: orientation selectivity (OSI), direction selectivity (DSI), preferred SF, and preferred TF (Figures 6D-E). We present data for both SF and TF paradigms for Tlx3-Cre+and Glt25d2-Cre+ neurons but only for the SF paradigm for L5 Efr3a-Cre+ neurons. This is because L5 Efr3a-Cre+ neurons tend to prefer very high SF and were therefore rarely responsive to the lower SF gratings used in the TF paradigm (see details below).
To compare visual response properties between the L5 cell types in V1, we characterized the visual responses (OSI, DSI, TF, and SF) of more than 110 Tlx3-Cre+, 13 Glt25d2-Cre+ and 17 L5 Efr3a-Cre+ neurons (Figures 6D-E, Table S3). Comparisons of the distributions of preferred SF between the three cell types (Figure 6D) showed that both L5 Efr3a-Cre+ and Glt25d2-Cre+ cells tended to prefer higher SF than Tlx3-Cre+ cells (median 0.04 c/d for Tlx3-Cre+ and 0.08 c/d for both Glt25d2-Cre+ and L5 Efr3a-Cre+) but only the distribution for L5 Efr3a-Cre+ cells differed significantly from Tlx3-Cre+ cells (Kruskal-Wallis test, p = 0.0015, with Dunn's multiple comparisons L5 Efr3a-Cre+ versus Tlx3-Cre+, p=0.0019). The distributions for Tlx3-Cre+ and Glt25d2-Cre+ cells appear similar to previous reports for mouse V1 L2/3 neurons (Marshel et al., 2011; Niell and Stryker, 2008). However, L5 Efr3a-Cre+ neurons appear unique in that the great majority of cells (14/17, 82%) prefer SF of 0.08 or higher. While Glt25d2-Cre+ and Tlx3-Cre+ neurons did not differ significantly in their SF tuning, these populations did differ significantly in TF tuning (Figure 6E), with Glt25d2-Cre+ neurons preferring TFs that were nearly twice as fast as for Tlx3-Cre+ neurons (median 4.0 Hz and 2.0 Hz respectively, Wilcoxon rank-sum test, p = 0.0242). All three cell types were highly orientation tuned. Across SF and TF experiments, Glt25d2-Cre+ were the least tuned, yet still had median OSI values >0.61, and over two-thirds of cells had an OSI > 0.5. (See Figure 6D-E for values for all cell types.) Using the varied SF paradigm with TF held constant at 1 Hz, L5 CS PNs were remarkably direction selective and non-parametric statistical tests showed that Glt25d2-Cre+ neurons have higher DSI (median 0.57) than Tlx3-Cre+ (median 0.24, Kruskal-Wallis test p=0.0024, with Dunn's multiple comparisons, Tlx3-Cre+ versus Glt25d2-Cre+, p = 0.0135). 60% of Glt25d2-Cre+ cells were very sharply tuned for direction (DSI > 0.5) while less than 30% of Tlx3-Cre+ or L5 Efr3a-Cre+ cells had DSI > 0.5 (figure 6D, top, right panel). Interestingly, when TF was varied and SF was held constant at 0.04 c/d, the DSI values for Glt25d2-Cre+ neurons were lower than in the SF paradigm (median 0.46) while the DSI values were similar for Tlx3-Cre+ neurons regardless of the stimulation paradigm (median 0.34) and differences between the distributions were not statistically significant. In summary, L5 Efr3a-Cre+ neurons prefer higher SFs than Tlx3-Cre+, Glt25d2-Cre+ neurons prefer higher TFs than Tlx3-Cre+, and Glt25d2-Cre+ are more direction selective than Tlx3-Cre+ cells**.**
DISCUSSION
While previous in vitro studies have provided extensive information about the intrinsic physiology and local connectivity of specific cortical cell types, information about brain-wide connectivity and in vivo function has been more elusive. Here we took advantage of innovative molecular, viral and genetic tools to study subtypes of layer 5 (L5) pyramidal neurons in the mouse primary visual cortex. First, we identified a Cre-driver mouse line (Efr3a-Cre) that distinguishes a distinct subtype of L5 cortico-cortical, non-striatal (CCNS)/local pyramidal neurons and used in vitro physiological and anatomical approaches to characterize the intrinsic physiology and morphology of these cells, in comparison to L5 CC (Tlx3-Cre) and cortical-subcortical (CS; Glt25d2-Cre) pyramidal neurons. We then employed targeted monosynaptic rabies tracing of brain-wide inputs and GCaMP-based two-photon calcium imaging to characterize the in vivo visual function and connectivity of genetically-defined L5 CC, CC-NS and CS pyramidal neurons. Our observations reveal novel insights into the diversity of L5 pyramidal neurons, demonstrating differences in connectivity and physiology that may underlie the unique contributions of each cell type to cortico-cortical versus subcortical computations (Figure 7).
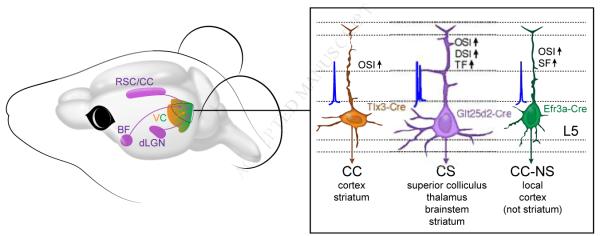
Figure 7. Connectivity and Function of Three Types of L5 Pyramidal Neurons.
Three genetically-identified populations of layer 5 pyramidal neurons in V1 of Tlx3-Cre+, Glt25d2-Cre+, and Efr3a-Cre+ mice were characterized based on monosynaptic rabies tracing of brain-wide inputs (left) and their morphologies, axonal projections, intrinsic electrophysiology and in vivo responses to drifting gratings (right). (Left) Tlx3-Cre+ and Efr3a-Cre+ V1 neurons receive preferential long-range inputs from the extrastriate visual areas (orange/green), whereas Glt25d2-Cre+ V1 neurons receive preferential inputs from the retrosplenial cortex, the basal forebrain, and dLGN (purple). (Right) CC Tlx3-Cre+, CS Glt25d2-Cre+, CC-NS Efr3a-Cre+ V1 L5 pyramidal neurons exhibit distinct axonal projections, cell morphology, electrical properties and visual responses. Abbreviations: BF, basal forebrain; CC, cortico-cortical; CC-NS, cortico-cortical non-striatal; CS, cortico-subcortical; dLGN, dorsal lateral geniculate nucleus; RSC/CC, retrosplenial and cingulate cortex; VC, visual cortex.
Heterogeneity of L5 PNs and their functions
Historically, cortical layer 5 pyramidal neurons have been classified into two types, originally defined by their distinct projections to subcortical versus cortical targets (CS and CC, respectively) (O'Leary and Koester, 1993). Since that time there have been innumerable studies of the intrinsic physiology, connectivity and function of these neuronal populations, as well as evolving nomenclature and definitions. Typically the definition used depends on the measurement methods being employed; because these diagnostic features have been well documented and are highly correlated, measurement of a single property generally allows other features to be unambiguously inferred. For example, CS neurons are invariably intrinsically bursting while CC neurons are regular spiking (Groh et al., 2010; Kasper et al., 1994), and CS neurons have a larger cell body, thicker apical dendrite and more extensive apical dendritic tuft than CC cells, leading to names such as thick- or thin-tufted, and tall- or slender-tufted (Groh et al., 2010; Kasper et al., 1994; Larkman et al., 1988). In the S1 barrel cortex, CC and CS neurons are largely confined to layers 5A and 5B, respectively (Groh et al., 2010), leading to a 5A versus 5B nomenclature. In motor cortex, CS neurons project to the pyramidal tract and have been termed PT while the CC cells are called intratelencephalic (IT) (Gerfen et al., 2013). These distinctions, however, cannot be universally applied across cortical areas or species as seen here in mouse V1 (Zarrinpar and Callaway, 2014).
Our observations comparing Efr3a-Cre+ neurons to Tlx3-Cre+ and Glt25d2-Cre+ cells reveal that L5 CC neurons in mouse V1 can be further subdivided into at least two distinct groups. Using conventional features to distinguish CC from CS cells, Efr3a-Cre+ neurons share the typical diagnostic features of CC cells, including regular spiking physiology, a relatively small cell body, and a thin-tufted morphology. However, with more ovoid cell bodies, higher input resistance, greater percent sag, a lack of projections to the striatum, and a preference for higher spatial frequency visual stimuli, Efr3a-Cre+ CC-NS cells differ in several ways from Tlx3-Cre+ CC cells. Across our measurements, percent sag, sag amplitude, and absence of a striatal projection most clearly distinguish Efr3a-Cre+ from Tlx3-Cre+ cells and can therefore be considered as diagnostic of the cell type: the percent sag and sag amplitude measures are both more than 3-fold greater on average for the Efr3a-Cre+ cells and there is very little overlap in the percent sag distributions and no overlap in the sag amplitudes. Another diagnostic feature of Efr3a-Cre+ cells in V1 is a lack of projections to the striatum; while axons projecting to the ipsilateral striatum are clearly seen from both Tlx3-Cre+ and Glt25d2-Cre+ neurons, they are not present in Efr3a-Cre+ mice. Previous studies have shown a strong correlation between percent sag and hyperpolarization-activated cyclic nucleotide gated cation channel (HCN1) and Trip8b expression in L5 projection neurons (Day et al., 2005; Sheets et al., 2011). In future studies, it would be interesting to investigate whether L5 Efr3a-Cre+ cells may also differ from Tlx3-Cre+ neurons in the expression levels of HCN1.
Previous single cell tracing and genomic profiling studies have shown that both CC and CS L5 neurons can be divided into subgroups projecting only to subsets of their multiple targets or having distinct somato-dendritic morphology, or expressing specific combinations of genes (Bourassa and Deschenes, 1995; Molyneaux et al., 2007; Sorensen et al., 2013). A population of CC neurons in the primary somatosensory cortex can consist of cells projecting to the contralateral cortex, to the contralateral cortex and striatum, or to both the contralateral and ipsilateral frontal cortex (Molyneaux et al., 2007; Sorensen et al., 2013). Individual CS neurons in the primary visual cortex can also project to different subsets of known targets of the population, including superior colliculus, ventral LGN, LD/LP, pretectum and pons (Bourassa and Deschenes, 1995). Despite the diversity of projections, these differences have not been correlated with other anatomical or physiological features as we have demonstrated for Tlx3-Cre+ versus Efr3a-Cre+ L5 PNs. It is also possible that L5 Efr3a-Cre+ neurons could comprise more than one type. For example, we have observed that some project to the white matter (and presumably to adjacent cortical areas) while others are strictly local (Figures 1C and S2B). Whether these are truly different types depends on the definition of cell type and whether future studies might correlate these anatomical features with other properties. Such identification of further subdivisions based on distinct genetic, physiological, and anatomical properties will be important to further understanding how separate groups of neurons can function as distinct channels of cortical output to other structures.
It is also important to note that, unlike Efr3a-Cre+ L5 neurons in V1, Efr3a-Cre+ neurons in other cortical areas, including extrastriate visual areas but not S1 and Au1, appear to include typical CS neurons; they have large pyramidal cell bodies and thick apical dendrites and project axons to subcortical structures (Figure S2B). There are several possible explanations for this observation: the cell group distinguished by Cre expression in V1 of Efr3a-Cre mice might be unique to primary sensory cortical areas; Efr3a-Cre expression is present in CS neurons in other cortical areas but not in primary sensory cortex; the expression of Cre does not fully recapitulate the expression pattern of Efr3a and differs between cortical areas. While the possibility that primary sensory cortices possess a cell type not present in other cortical areas is at odds with the notion of a universal cortical laminar and cellular architecture, this would certainly not be the first such observation. In the future it will be important to search for cells like the V1 Efr3a-Cre+ cells in other cortical areas and, if they are present, to identify methods to distinguish them. For example, gene expression profiling comparing Efr3a-Cre+ neurons to Tlx3-Cre+ neurons in mouse V1 might reveal differences in gene expression that more universally distinguishes the cell type across all cortical regions.
In vivo physiology and monosynaptic long distance inputs to layer 5 neurons
Our data show that L5 neurons exhibit diverse in vivo visual receptive fields. Glt25d2-Cre+ V1 neurons display higher DSI and prefer higher temporal frequencies compared to Tlx3-Cre+ V1 neurons. This indicates that Glt25d2-Cre+ neurons and Tlx3-Cre+ neurons integrate and convey different visual information to downstream target regions. Furthermore, Efr3a-Cre+ neurons prefer higher SFs than both Tlx3-Cre+ neurons, suggesting that these cells are involved in pathways requiring higher visual acuity. Lastly, we found that each of these cell types were highly orientation tuned, more so than those reported in previous studies using single-unit electrical recordings in L5 of anesthetized or awake mice (Niell and Stryker, 2008; Niell and Stryker, 2010). It should be noted that only about 10-20% of L5 neurons responded reliably to the drifting grating stimuli we used (Table S3). This is lower than the roughly 50% of visually responsive neurons in L2/3, but comparable to the low percentages in some extrastriate visual areas (Andermann et al., 2011; Marshel et al., 2011). Future studies should investigate experimental conditions and/or sensory stimuli that might generate responses in neurons that were not responsive under our experimental conditions.
As L5 neurons are long distance output neurons, it is particularly interesting to correlate their stimulus selectivity to the projection targets. L5 CC PNs may convey visual information necessary for object recognition to higher visual areas in a feedforward manner. In contrast, L5 CS PNs may convey visual motion-related information to the superior colliculus. Previous studies of functional properties and connections in primate, cat, and rodent V1 also suggest that CC PNs process and convey image-forming visual information to higher visual cortices, whereas CS PNs are involved in sensory gating associated with movement (Finlay et al., 1976; Palmer and Rosenquist, 1974; Van Essen, 2005).
We find that different types of L5 projection neurons receive different proportions of input from the many cell populations that project to mouse V1. This contrasts with previous studies which found no difference in the laminar sources of local inputs to L5 CC and CS PNs (Schubert et al., 2001; Zarrinpar and Callaway, 2014). Nevertheless, the differences in visual receptive fields described here suggest that these two populations integrate inputs from populations of neurons that impart different functional properties. Such differences might include local inputs in addition to the differences that we have observed in long-distance inputs.
It is noteworthy that L5 CC Tlx3-Cre+ PNs that provide direct feedforward input to higher visual areas receive a higher proportion of feedback inputs from those same areas when compared to L5 CS Glt25d2-Cre+ PNs. This is consistent with the potential importance of feedback in regulating levels of feedforward input. Feedback might also play a more important role in high-resolution image formation than in the generation of signals that are conveyed subcortically. In contrast, Glt25d2-Cre+ neurons receive a higher proportion of their inputs from retrosplenial and cingulate cortical areas, and cingulate cortex has recently been implicated in direct top-down attentional modulation of mouse V1 (Zhang et al., 2014). Together these observations suggest an important role for top-down modulation of V1 neurons projecting to the superior colliculus and other subcortical regions. Such an influence on neurons that project to superior colliculus is consistent with its role in regulation of spatial attention (Krauzlis et al., 2013).
Using recently available Cre driver lines, we have defined and characterized subtypes of L5 PNs in mouse V1 based on their morphology, axonal projections, and intrinsic electrophysiology, and we have correlated these features with differences in visual responses and brain-wide monosynaptic input networks. Altogether these observations provide insight into potential mechanisms by which differential inputs and integrative mechanisms create functionally distinct outputs that are specialized for the roles of each cell type. This work can serve as a foundation for future studies that are likely to further subdivide L5 PN types and probe their contributions to perception and behavior by manipulating their activity and that of their various inputs.
EXPERIMENTAL PROCEDURES
All experimental procedures using live animals followed procedures approved by the Salk Institute Animal Care and Use Committee. Tlx3-Cre PL56, Glt25d2-Cre (or Colgalt2-Cre) NF107 and Efr3a-Cre NO108 mice are GENSAT BAC transgenic lines and have been previously described (Gerfen et al., 2013; Gong et al., 2007). Mouse strains were maintained on mixed genetic backgrounds (129/C57BL6). Mice were used for analysis of: 1) brain-wide axonal projections and morphology, by injecting Cre-dependent AAVs expressing fluorescent proteins into V1 and subsequent post-mortem histological analyses; 2) brain-wide inputs, by injecting Cre-dependent AAVs and EnvA-pseudotyped, glycoprotein-deleted rabies virus into V1 and subsequent post-mortem histological analyses; 3) intrinsic physiology, by injecting Cre-dependent AAVs expressing fluorescent proteins into V1 and then recording from the fluorescent neurons using whole cell patching in brain slices; 4) visual responses, by injecting Cre-dependent AAVs expressing the fluorescent calcium indicator GCaMP6 into V1 and then two-photon imaging of visual responses in awake mice. Detailed descriptions of experimental procedures can be found in Supplemental Experimental Procedures.
Supplementary Material
1
2
ACKNOWLEDGEMENTS
We thank all Callaway lab members for discussion, B.J. Neichin, T. Ito, D. Chatterjee, S. Gilmour for technical assistance, I. Nauhaus for Matlab programming help, C. Gerfen and E. Schmidt for BAC transgenic mice search help, and L. Luo for sharing Cav2-Cre virus. We also thank the Salk viral vector and biophotonics core staff members. This work was supported by the National Institutes of Health grants EY022577 and MH063912, and the Gatsby Charitable Foundation (E.M.C). A.L.J. is supported by the NSF and Martinet Foundation. E.M.K. is supported by the Howard Hughes Medical Institute Gilliam Fellowship and the University of California, San Diego Medical Scientist Training Program T32 GM007198-40. E.J.K. is a Biogen-IDEC Fellow of the Life Science Research Foundation and a recipient of 2012 NARSAD Young Investigator Award from Brain & Behavior Research Foundation.
Footnotes
AUTHOR CONTRIBUTIONS
E.J.K. and E.M.C. designed the study. E.J.K. performed anatomical tracing studies. E.M.K. performed slice electrophysiology experiments. A.L.J. performed and analyzed two-photon calcium imaging experiments. E.J.K. and M.W.J. analyzed rabies virus tracing data. E.J.K. and E.M.C. wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adesnik H, Bruns W, Taniguchi H, Huang ZJ, Scanziani M. A neural circuit for spatial summation in visual cortex. Nature. 2012;490:226–231. doi: 10.1038/nature11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andermann ML, Kerlin AM, Roumis DK, Glickfeld LL, Reid RC. Functional specialization of mouse higher visual cortical areas. Neuron. 2011;72:1025–1039. doi: 10.1016/j.neuron.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelo K, Margrie TW. Population diversity and function of hyperpolarization-activated current in olfactory bulb mitral cells. Sci Rep. 2011;1:50. doi: 10.1038/srep00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortone DS, Olsen SR, Scanziani M. Translaminar Inhibitory Cells Recruited by Layer 6 Corticothalamic Neurons Suppress Visual Cortex. Neuron. 2014;82:474–485. doi: 10.1016/j.neuron.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourassa J, Deschenes M. Corticothalamic Projections From the Primary Visual Cortex in Rats: A Single Fiber Study Using Biocytin As An Anterograde Tracer. Neuroscience. 1995;66:253–263. doi: 10.1016/0306-4522(95)00009-8. [DOI] [PubMed] [Google Scholar]
- Constantinople CM, Bruno RM. Deep cortical layers are activated directly by thalamus. Science. 2013;340:1591–1594. doi: 10.1126/science.1236425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RL, Wilson CJ. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. J Neurophysiol. 1994;71:17–32. doi: 10.1152/jn.1994.71.1.17. [DOI] [PubMed] [Google Scholar]
- Day M, Carr DB, Ulrich S, Ilijic E, Tkatch T, Surmeier DJ. Dendritic excitability of mouse frontal cortex pyramidal neurons is shaped by the interaction among HCN, Kir2, and Kleak channels. J Neurosci. 2005;25:8776–8787. doi: 10.1523/JNEUROSCI.2650-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RJ, Martin KAC. NEURONAL CIRCUITS OF THE NEOCORTEX. Annual Review of Neuroscience. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- Ferster D, Lindstrom S. An intracellular analysis of geniculo-cortical connectivity in area 17 of the cat. J Physiol. 1983;342:181–215. doi: 10.1113/jphysiol.1983.sp014846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay BL, Schiller PH, Volman SF. Quantitative studies of single-cell properties in monkey striate cortex. IV. Corticotectal cells. J Neurophysiol. 1976;39:1352–1361. doi: 10.1152/jn.1976.39.6.1352. [DOI] [PubMed] [Google Scholar]
- Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, Huang ZJ, Stryker MP. A cortical circuit for gain control by behavioral state. Cell. 2014;156:1139–1152. doi: 10.1016/j.cell.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett ME, Nauhaus I, Marshel JH, Callaway EM. Topography and Areal Organization of Mouse Visual Cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:12587–12600. doi: 10.1523/JNEUROSCI.1124-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Paletzki R, Heintz N. GENSAT BAC Cre-Recombinase Driver Lines to Study the Functional Organization of Cerebral Cortical and Basal Ganglia Circuits. Neuron. 2013;80:1368–1383. doi: 10.1016/j.neuron.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh A, Meyer HS, Schmidt EF, Heintz N, Sakmann B, Krieger P. Cell-Type Specific Properties of Pyramidal Neurons in Neocortex Underlying a Layout that Is Modifiable Depending on the Cortical Area. Cerebral Cortex. 2010;20:826–836. doi: 10.1093/cercor/bhp152. [DOI] [PubMed] [Google Scholar]
- Grubb MS, Thompson ID. Quantitative characterization of visual response properties in the mouse dorsal lateral geniculate nucleus. J Neurophysiol. 2003;90:3594–3607. doi: 10.1152/jn.00699.2003. [DOI] [PubMed] [Google Scholar]
- Guan D, Armstrong WE, Foehring RC. Electrophysiological properties of genetically identified subtypes of layer 5 neocortical pyramidal neurons: Ca(2)(+) dependence and differential modulation by norepinephrine. J Neurophysiol. 2015;113:2014–2032. doi: 10.1152/jn.00524.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallman LE, Schofield BR, Lin CS. Dendritic morphology and axon collaterals of corticotectal, corticopontine, and callosal neurons in layer V of primary visual cortex of the hooded rat. J Comp Neurol. 1988;272:149–160. doi: 10.1002/cne.902720111. [DOI] [PubMed] [Google Scholar]
- Hattox AM, Nelson SB. Layer V Neurons in Mouse Cortex Projecting to Different Targets Have Distinct Physiological Properties. Journal of Neurophysiology. 2007;98:3330–3340. doi: 10.1152/jn.00397.2007. [DOI] [PubMed] [Google Scholar]
- Hubener M, Bolz J. Morphology of identified projection neurons in layer 5 of rat visual cortex. Neuroscience letters. 1988;94:76–81. doi: 10.1016/0304-3940(88)90273-x. [DOI] [PubMed] [Google Scholar]
- Hubener M, Schwarz C, Bolz J. Morphological types of projection neurons in layer 5 of cat visual cortex. J Comp Neurol. 1990;301:655–674. doi: 10.1002/cne.903010412. [DOI] [PubMed] [Google Scholar]
- Kasper EM, Larkman AU, Lubke J, Blakemore C. Pyramidal neurons in layer 5 of the rat visual cortex. I. Correlation among cell morphology, intrinsic electrophysiological properties, and axon targets. J Comp Neurol. 1994;339:459–474. doi: 10.1002/cne.903390402. [DOI] [PubMed] [Google Scholar]
- Kim J, Matney CJ, Blankenship A, Hestrin S, Brown SP. Layer 6 corticothalamic neurons activate a cortical output layer, layer 5a. J Neurosci. 2014;34:9656–9664. doi: 10.1523/JNEUROSCI.1325-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzlis RJ, Lovejoy LP, Zenon A. Superior colliculus and visual spatial attention. Annu Rev Neurosci. 2013;36:165–182. doi: 10.1146/annurev-neuro-062012-170249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman AU, Mason A, Blakemore C. The in vitro slice preparation for combined morphological and electrophysiological studies of rat visual cortex. Neurosci Res. 1988;6:1–19. doi: 10.1016/0168-0102(88)90002-8. [DOI] [PubMed] [Google Scholar]
- Larsen DD, Wickersham IR, Callaway EM. Retrograde tracing with recombinant rabies virus reveals correlations between projection targets and dendritic architecture in layer 5 of mouse barrel cortex. Front Neural Circuits. 2007;1:5. doi: 10.3389/neuro.04.005.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nature neuroscience. 2013;16:1662–1670. doi: 10.1038/nn.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kwan AC, Zhang S, Phoumthipphavong V, Flannery JG, Masmanidis SC, Taniguchi H, Huang ZJ, Zhang F, Boyden ES, et al. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature. 2012;488:379–383. doi: 10.1038/nature11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque M, Charara A, Gagnon S, Parent A, Deschenes M. Corticostriatal projections from layer V cells in rat are collaterals of long-range corticofugal axons. Brain research. 1996;709:311–315. doi: 10.1016/0006-8993(95)01333-4. [DOI] [PubMed] [Google Scholar]
- Li J, Wang S, Bickford ME. Comparison of the ultrastructure of cortical and retinal terminals in the rat dorsal lateral geniculate and lateral posterior nuclei. The Journal of comparative neurology. 2003;460:394–409. doi: 10.1002/cne.10646. [DOI] [PubMed] [Google Scholar]
- Li N, Chen TW, Guo ZV, Gerfen CR, Svoboda K. A motor cortex circuit for motor planning and movement. Nature. 2015;519:51–56. doi: 10.1038/nature14178. [DOI] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ, 3rd, Gu X, Zanella S, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshel JH, Garrett ME, Nauhaus I, Callaway EM. Functional Specialization of Seven Mouse Visual Cortical Areas. Neuron. 2011;72:1040–1054. doi: 10.1016/j.neuron.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason A, Larkman A. Correlations between morphology and electrophysiology of pyramidal neurons in slices of rat visual cortex. II. Electrophysiology. J Neurosci. 1990;10:1415–1428. doi: 10.1523/JNEUROSCI.10-05-01415.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamichi K, Shlomai-Fuchs Y, Shu M, Weissbourd BC, Luo L, Mizrahi A. Dissecting local circuits: parvalbumin interneurons underlie broad feedback control of olfactory bulb output. Neuron. 2013;80:1232–1245. doi: 10.1016/j.neuron.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JRL, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nature Reviews Neuroscience. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Highly Selective Receptive Fields in Mouse Visual Cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:7520–7536. doi: 10.1523/JNEUROSCI.0623-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65:472–479. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienborg H, Hasenstaub A, Nauhaus I, Taniguchi H, Huang ZJ, Callaway EM. Contrast Dependence and Differential Contributions from Somatostatin- and Parvalbumin-Expressing Neurons to Spatial Integration in Mouse V1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:11145–11154. doi: 10.1523/JNEUROSCI.5320-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary DD, Koester SE. Development of projection neuron types, axon pathways, and patterned connections of the mammalian cortex. Neuron. 1993;10:991–1006. doi: 10.1016/0896-6273(93)90049-w. [DOI] [PubMed] [Google Scholar]
- Olsen SR, Bortone DS, Adesnik H, Scanziani M. Gain control by layer six in cortical circuits of vision. Nature. 2012;483:47–52. doi: 10.1038/nature10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakada F, Callaway EM. Design and generation of recombinant rabies virus vectors. Nature protocols. 2013;8:1583–1601. doi: 10.1038/nprot.2013.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LA, Rosenquist AC. Visual receptive fields of single striate corical units projecting to the superior colliculus in the cat. Brain research. 1974;67:27–42. doi: 10.1016/0006-8993(74)90295-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2nd edn Academic Press; San Diego: 2001. [Google Scholar]
- Schubert D, Staiger JF, Cho N, Kotter R, Zilles K, Luhmann HJ. Layer-specific intracolumnar and transcolumnar functional connectivity of layer V pyramidal cells in rat barrel cortex. J Neurosci. 2001;21:3580–3592. doi: 10.1523/JNEUROSCI.21-10-03580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets PL, Suter BA, Kiritani T, Chan CS, Surmeier DJ, Shepherd GM. Corticospinal-specific HCN expression in mouse motor cortex: I(h)-dependent synaptic integration as a candidate microcircuit mechanism involved in motor control. J Neurophysiol. 2011;106:2216–2231. doi: 10.1152/jn.00232.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen SA, Bernard A, Menon V, Royall JJ, Glattfelder KJ, Desta T, Hirokawa K, Mortrud M, Miller JA, Zeng H, et al. Correlated Gene Expression and Target Specificity Demonstrate Excitatory Projection Neuron Diversity. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht243. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Schwindt PC, Flatman JA, Crill WE. Properties of subthreshold response and action potential recorded in layer V neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1984;52:244–263. doi: 10.1152/jn.1984.52.2.244. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsani D, Fu Y, Lu J, Lin Y, et al. A Resource of Cre Driver Lines for Genetic Targeting of GABAergic Neurons in Cerebral Cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiola A, Hamzei-Sichani F, Peterlin Z, Yuste R. Quantitative morphologic classification of layer 5 neurons from mouse primary visual cortex. The Journal of comparative neurology. 2003;461:415–428. doi: 10.1002/cne.10628. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. Corticocortical and thalamocortical information flow in the primate visual system. Progress in brain research. 2005;149:173–185. doi: 10.1016/S0079-6123(05)49013-5. [DOI] [PubMed] [Google Scholar]
- Vélez-Fort M, Rousseau CV, Niedworok CJ, Wickersham IR, Rancz EA, Brown APY, Strom M, Margrie TW. The stimulus selectivity and connectivity of layer six principal cells reveals cortical microcircuits underlying visual processing. Neuron. 2014;83:1431–1443. doi: 10.1016/j.neuron.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall NR, Wickersham IR, Cetin A, De La Parra M, Callaway EM. Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21848–21853. doi: 10.1073/pnas.1011756107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham IR, Lyon DC, Barnard RJO, Mori T, Finke S, Conzelmann K-K, Young JAT, Callaway EM. Monosynaptic Restriction of Transsynaptic Tracing from Single, Genetically Targeted Neurons. Neuron. 2007;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NR, Runyan CA, Wang FL, Sur M. Division and subtraction by distinct cortical inhibitory networks in vivo. Nature. 2012;488:343–348. doi: 10.1038/nature11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrinpar A, Callaway EM. Functional Local Input to Layer 5 Pyramidal Neurons in the Rat Visual Cortex. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Xu M, Kamigaki T, Hoang Do JP, Chang WC, Jenvay S, Miyamichi K, Luo L, Dan Y. Selective attention. Long-range and local circuits for top-down modulation of visual cortex processing. Science. 2014;345:660–665. doi: 10.1126/science.1254126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2