How Imaging Glutamate, GABA, and Dopamine Can Inform the Clinical Treatment of Alcohol Dependence and Withdrawal (original) (raw)
. Author manuscript; available in PMC: 2016 Dec 1.
Published in final edited form as: Alcohol Clin Exp Res. 2015 Oct 28;39(12):2268–2282. doi: 10.1111/acer.12893
Abstract
Neuroimaging studies have dramatically advanced our understanding of the neurochemical basis of alcohol dependence, a major public health issue. In this paper we review the research generated from neurochemical-specific imaging modalities including magnetic resonance spectrometry (MRS), positron emission tomography (PET), and single photon emission computed tomography (SPECT) in studies of alcohol dependence and withdrawal. We focus on studies interrogating γ-aminobutryic acid (GABA), glutamate, and dopamine, as these are prominent neurotransmitter systems implicated in alcohol dependence. Highlighted findings include diminished dopaminergic functioning and modulation of the GABA system by tobacco smoking during alcohol withdrawal. Then, we consider how these findings impact the clinical treatment of alcohol dependence and discuss directions for future experiments to address existing gaps in the literature, e.g., sex differences and smoking comorbidity. These and other considerations provide opportunities to build upon the current neurochemistry imaging literature of alcohol dependence and withdrawal, which may usher in improved therapeutic and relapse prevention strategies.
1. Introduction
Compulsive alcohol seeking and use despite negative consequences characterizes alcohol dependence, which also features the hallmarks of craving, tolerance, withdrawal, and high rates of relapse. However, the neurochemical basis of these alcohol dependence characteristics is not fully understood. This information is important for the development of efficacious alcohol interventions since current alcohol pharmacotherapies have only small to moderate effects on drinking (Kranzler and Van Kirk 2001, Pettinati and Rabinowitz 2006). Brain imaging allows us to longitudinally measure neurochemical changes in alcohol dependence and withdrawal, observe neurochemical factors contributing to withdrawal symptoms, correlate neurochemistry with behavioral measures of alcohol dependence and propensity for relapse, distinguish pharmacological targets for treatment, and evaluate therapeutic interventions. Thus, neuroimaging is a powerful tool that can be used to bridge preclinical findings with clinical correlates of alcohol dependence and treatment strategies (Everitt and Robbins 2005, Volkow and Baler 2013).
During alcohol dependence and withdrawal several major neurotransmitter systems including the γ-aminobutryic acid (GABA), glutamate, and dopamine systems are recruited. These neurotransmitters have been the focus of many neuroimaging studies. GABA and glutamate are the primary inhibitory and excitatory neurotransmitters in the brain, respectively. The GABA and glutamate systems together regulate critical dopaminergic projections from the ventral tegmental area to striatal and limbic regions (Spanagel 2009) and balance inhibitory (GABA) and excitatory (glutamate) tone throughout the brain. The dopamine system, particularly the stimulus salience and reward processing mesolimbic circuitry (Berridge 2007), is involved in the development and maintenance of alcohol dependence and drug dependence in general (Volkow et al. 2011). A summary of hypothesized longitudinal changes to GABA, glutamate, and dopamine receptor system function during alcohol dependence and withdrawal is featured in Table 1, and more detailed reviews are available elsewhere (Harris et al. 2008, Koob and Volkow 2010, Krystal et al. 2003, Krystal et al. 2006, Kumar et al. 2009, Lovinger et al. 1989).
TABLE 1.
Hypothesized Changes to GABA, Glutamate, and Dopamine Systems During Alcohol Dependence and Withdrawal
| Alcohol Dependence | Early Withdrawal | Extended Withdrawal | ||
|---|---|---|---|---|
| GABA | Extracellular Signaling | Increased neurosteroidlevels | Elevated GABAlevels | Normalizedneurosteroid levels |
| Normalized (reduced?)GABA levels | ||||
| Receptor Expression | Altered GABAAreceptor subunitcomposition | Compensatoryrecruitment | Decreasedextrasynaptic GABAAreceptors | |
| Altered GABAAfunctionality | Increased synapticGABAA receptors | Normalized (reduced?)GABAA receptors | ||
| Downstream Effects | Less effective inhibitorysignaling | Diminished inhibitorytone | ||
| Increased alcoholtolerance | Withdrawalsymptoms | |||
| Glutamate | Extracellular Signaling | Elevated extracellularglutamate | “Hyperglutamatergic”State | Normalized (reduced?)glutamate levels |
| Enhancedglutamate release | ||||
| Receptor Expression | Increased NMDARexpression | Elevated NMDARs | ||
| Reduced NMDARsensitivity to alcohol | Enhanced NMDARfunctionality | |||
| Downstream Effects | More effectiveexcitatory signaling | Heightened excitatory tone | ||
| Increased tolerance | Withdrawal symptoms | |||
| Dopamine | Extracellular Signaling | Blunted DopamineRelease | Blunted dopaminerelease | |
| Receptor Expression | Reduced dopamineD2/D3 receptor availability | Reduced dopamineD2/D3 receptoravailability | ||
| Reduced DATavailability | Normalized DATavailability | |||
| Downstream Effects | Dopamine D2/D3availability correlateswith severity | Possible vulnerability toearly relapse |
Chronic alcohol exposure modifies GABAA receptor composition to a state of reduced function (Follesa et al. 2006, Liang et al. 2007), while _N_-methyl-D-aspartate (NMDA) receptor expression is upregulated (Gass and Olive 2008), and glutamatergic tone is heightened (Tsai and Coyle 1998). When chronic alcohol is removed, as in withdrawal, a “hyperglutamatergic” state develops (Holmes et al. 2013), which in combination with reduced GABA function produces excessive excitatory signaling, contributing to the potentially life-threatening alcohol withdrawal syndrome. Additionally, downregulation of striatal dopamine D2/D3 receptor number and blunted dopaminergic signaling contribute to dysfunctional reward and salience circuitry during alcohol dependence and withdrawal (Volkow et al. 2012). The GABA, glutamate, and dopamine systems therefore provide important targets for understanding the neurochemical basis of alcohol dependence. Neuroimaging technologies provide critical tools to study neurotransmitter-specific function in clinically diagnosed alcohol dependent patients with minimal invasiveness. Here, we critically review neuroimaging studies examining GABA, glutamate, and dopamine in alcohol dependence and withdrawal with the goal of providing recommendations for future research. Additionally, since tobacco smoking frequently co-occurs with alcohol dependence, neuroimaging studies examining the influence of tobacco smoking on alcohol dependence and withdrawal are examined.
2. Neurochemical imaging modalities
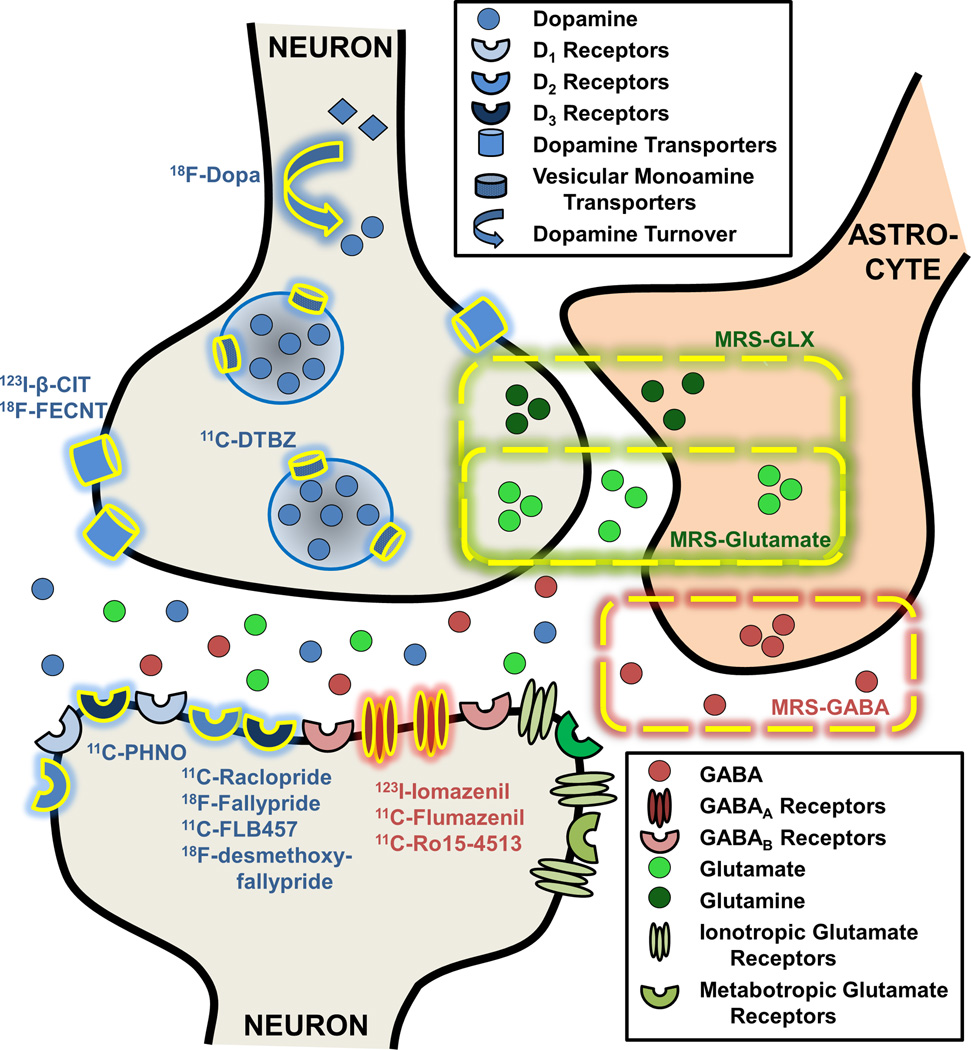
Neuroimaging uniquely enables observation of neurochemically-specific phenomena in alcohol dependent individuals. These neuroimaging experiments help to elucidate possible biochemical processes underlying cognitive and behavioral abnormalities specific to alcohol dependence. In addition, neuroimaging provides a crucial bridge accelerating translation of fully invasive but pharmacologically precise animal experiments to use in clinical research by evaluating pharamacologically specific effects of alcohol dependence treatments. The imaging modalities used to measure brain chemistry include magnetic resonance spectroscopy (MRS), positron emission tomography (PET), and single photon emission computed tomography (SPECT), with their targets illustrated in Figure 1.
FIGURE 1. Neuroimaging Targets Specific to the GABA, Glutamate, and Dopamine Systems in Alcohol Dependence.
Dashed yellow lines indicate neurotransmitter concentrations measured with magnetic resonance spectroscopy (MRS). Specific targets highlighted in yellow denote targets imaged with positron emission tomography (PET) or single photon computed emission tomography (SPECT).
MRS data, acquired with magnetic resonance imaging (MRI) scanners, yield spectra of different metabolite species based on their molecular structure. The area under the spectral curve is directly related to a variety of factors, including the metabolite’s concentration, allowing for measures of relative or absolute metabolite concentrations in vivo, including the neurotransmitters glutamate and GABA (Mason and Krystal 2006). The resonances of glutamate undergo what is called strong coupling, resulting in a complicated pattern of multiple peaks, which overlap with glutamine and GABA, in addition to contamination by underlying macromolecular resonances. Consequently, many MRS measurements of glutamatergic function do not distinguish these peaks and report summed glutamate + glutamine + GABA levels, often called Glx. The detection of GABA is technically challenging because of the nearly complete overlap of its resonances with other far more abundant neurochemicals, so techniques called “spectral editing” are commonly used to measures GABA. Because of the low sensitivity of MRS compared to radiotracer imaging, MRS measurements of glutamate and GABA are typically acquired from large tissue volumes of 8–20 cc. The meaning of an abnormality or change in the amount of GABA or glutamate in the brain remains under investigation. Although the quantity of glutamate at any given instant packaged for neurotransmission is a minority of the total glutamate pool, 13C MRS studies show that the entire pool of glutamate, glutamine, and probably GABA pass through the active neurotransmitter pools (Rothman et al. 2011). As such, there is potential for the total concentration of glutamate to be related to neurotransmission, although at this time the relationship remains unknown.
In PET and SPECT experiments subjects are injected with a compound of biological interest tagged with a radioactive label (radiotracer). The energy emitted when the radioactive label decays is detected by the PET or SPECT camera. The raw data is reconstructed and analyzed to provide measurements of kinetic properties of the radiotracer, most commonly ligand-receptor binding kinetic properties, but other processes such as glucose metabolism or presynaptic dopamine turnover can also be measured. PET and SPECT acquire measurements of physiologically-specific radiotracer uptake throughout the brain at resolutions of 3–5 mm3 and ~1 cm3, respectively.
Here, we focus on PET and SPECT studies of the glutamate, GABA, and dopaminergic systems. PET/SPECT studies examining other neurotransmitter systems including 5-hydroxytryptamine (serotonin), opioid, cannabinoid, and acetylcholine are summarized elsewhere (Ravan et al. 2014), while glutamate-specific PET imaging has not yet been performed in alcohol dependent participants (see discussion). The PET and SPECT target specific to the GABA system that has been successfully imaged in alcohol dependence is the GABAA receptor. The radiotracers 11C-flumazenil and 123I-iomazenil bind with roughly equivalent affinity for GABAA receptors containing the α1, α2, α3, and/or α5 subtypes, while 11C-Ro15-4513 is more specific to GABAA receptors containing the α5 subtype (Lingford-Hughes et al. 2002). Neuroimaging of the dopamine system primarily utilizes radioligands binding to dopamine D2/D3 receptors. Owing to their different pharmacokinetic properties, data from the radiotracers 11C-raclopride and 18F-desmethoxyfallypride are spatially restricted to the D2/D3-rich basal ganglia, while data from 11C-FLB-457 and 18F-fallypride are most appropriate for extra-striatal regions. In addition, the radiotracer 11C-PHNO is a more D3-preferring radioligand, of particular interest to the D3-rich regions in the midbrain (Erritzoe et al. 2014). Radiotracers binding to the dopamine transporter (DAT) include 123I-β-CIT, which binds to DAT in the striatum and serotonin transporters in the diencephalon and midbrain (Seibyl et al. 1996), and 18F-FECNT, which binds more preferentially to DAT than serotonin transporters (Goodman et al. 2000). Finally, vesicular monoamine transporters, type 2 (VMAT2) can be imaged with 11C-DTBZ. Since over 95% of striatal VMAT2 vesicles are dopaminergic, striatal 11C-DTBZ uptake is generally attributed to presynaptic dopaminergic vesicular packaging (Frey et al. 2001). The outcome measure of these radioligand-receptor binding experiments, binding potential, (_BP_ND) is referred to as “receptor availability” since _BP_ND reflects the number of receptors available to the radioligand as binding sites. Thus receptor availability as indexed by BPND is sensitive to a variety of factors including receptor density, affinity, and changes in receptor occupancy by exogenous drugs or synaptic neurotransmitter concentrations. Finally, 18F-DOPA measures the rate of DOPA’s conversion to dopamine, or dopamine turnover (Barrio et al. 1990)
In addition to the scientific considerations to neuroimaging presented here, logistical factors must also be considered. The analysis of MRS, PET, and SPECT data all require considerable modality-specific expertise in the critical steps of scanning protocol design, and advanced computational skills are required for proper quantitative evaluation of all data sets. Further, considerable cost and infrastructure are required to support MRS, PET, and SPECT imaging laboratories. MRS measurements of glutamate resolved from glutamine require higher field magnets or special methodologies such as TE-averaging (Hurd et al. 1998). Both scanning procedures require extensive scanning time. MRS can require 10–15 min for positioning and calibration, plus anywhere from a few minutes for glutamate 1H MRS measures to two hours for 13C kinetic scans. PET and SPECT scans can require up to two hours in the camera, and even longer in exceptional scanning protocols. Molecular imaging studies can also require arterial blood sampling for accurate quantification, which can be mildly invasive to the subject and requires additional laboratory equipment. Finally, the administration of radioactivity to the subject for signal detection in PET and SPECT scans limits the amount of annual scans participants can receive as mandated by the FDA. However, the subject dose from a typical PET scan is less than the annual natural background dose received from living in certain areas of the world with high background radiation. Despite these obstacles to neurotransmitter-specific neuroimaging, their immense power to interrogate pharmacologically precise phenomena in vivo has driven many laboratories to conduct neuroimaging studies, resulting in great benefits to many fields including alcohol research.
3. Imaging the Neurochemistry of Alcohol Dependence
3. A. Cross-Sectional Studies
Most imaging studies investigating alcohol dependence and withdrawal are cross-sectional comparisons of alcohol dependent participants at various stages of alcohol abstinence compared to healthy controls. Typical alcohol withdrawal symptoms which may include autonomic arousal, seizures, anxiety, tremors, and delirium present within 24 hours of the last drink, peak in severity around 72 hours after the last drink and generally subside over the following weeks (Kosten and O’Connor 2003). A full summary of the work reviewed here is shown in Table 2.
Table 2.
| Reference | EtOHAbstinence | NonsmokingPatients | FemalePatients | BenzosUsed? | Modality | Target | Primary Finding | Notes |
|---|---|---|---|---|---|---|---|---|
| <24 Hours Withdrawal | ||||||||
| Glutamate | ||||||||
| Ende et al., 2013 | BAC = 0 | ??? | 8/23 | N/A | MRS | Glu - Frontal CortexWhite Matter | ↓ Glu for AD vs. HC | |
| Yeo et al., 2013 | BAC = 0 | 98/213 | 60/213 | N/A | MRS | Glx - Anterior CingulateCortex | Trend ↑ Glx for AD vs.HC | |
| DA | ||||||||
| Albrecht et al., 2013 | BrAC = 0 | 0/27 | 7/27 | No | PET | D2-D3 Receptors -Striatum | ↓ D2-D3 avail. for AD vs.HC | ↓ D2-D3 avail. for HCfor smokers vs.nonsmokers |
| 1–14 Days Withdrawal | ||||||||
| Glutamate | ||||||||
| Bauer et al., 2013 | > 10 Days | 27/29 | ??? | 20/29; > 1 Day | MRS | Glu; Gln; Glx - NucleusAccumbens; AnteriorCingulate | ↑ Glu in NAcc for AD vs.HC; No differences in ACC; Controlled forsmoking | ↑ Glu associated with ↑craving |
| DA | ||||||||
| Cosgrove et al., 2009 | 1–5 Days | 8/14 | 5/14 | No | SPECT | DAT - striatum | ↑ DAT avail. for ADnonsmokers vs. HC;No DAT avail. difference forAD smokers vs. HC | |
| >14 Days Withdrawal | ||||||||
| GABA | ||||||||
| Gilman et al., 1996 | > 1 Month | ??? | 0/17 | 5/17; > 2Weeks | PET | GABAA Receptors;Glucose Metabolism | ↓ GABAA avail. & cMRGlc inmFC for AD vs. HC | |
| Abi-Dargham et al., 1998 | 1–6 Months | ??? | 0/11 | > 4Weeks | SPECT | GABAA Receptors | ↓ GABAA avail. in pFC, aCG,CB for Type II AD vs. HC | |
| Lingford-Hughes et al., 1998 | > 3 Months | ??? | 0/12 | ??? | SPECT | GABAA Receptors | ↓ GABAA avail. in FC, PC,TC for AD vs. HC | ↓ GABAA avail.associated with moresevere dependence |
| Lingford-Hughes et al., 2000 | > 3 Months | ??? | 9/21 | > 3Months | SPECT | GABAA Receptors | ↓ GABAA avail. in PC, OC,CB for AD vs. HC | Trend ↓ GABAA avail.in PC, OC, CB forfemale AD vs. maleAD |
| Lingford-Hughes et al., 2012 | > 2 Months | 5/8 | 0/8 | > 6Weeks | PET | GABAA Receptors | ↓ GABAA avail. in nAcc, HPfor AD vs. HC | ↑ HP GABAA avail.correlated with betterdelayed memory recallfor AD but not HC |
| Volkow et al., 1997 | 2–3 Weeks | 0/10 | 0/10 | 2–3Weeks | PET | Glucose Metabolism | ↓ cMRGlc after lorazepamfor AD vs. HCTrend more radioliganddisplacement at GABAAreceptor from midazolam forAD vs. HC | |
| Lingford-Hughes et al., 2005 | > 1.5Months | ??? | 0/10 | > 6Weeks | PET | GABAA Receptors | Trend more radioliganddisplacement at GABAAreceptor from midazolam forAD vs. HC | |
| Glutamate | ||||||||
| Thoma et al., 2011 | > 2 Days | 3/13 | 3/13 | ??? | MRS | Glu; Gln - Medial FrontalCortex | ↓ Glu; ↑ Gln for AD vs. HC | |
| Bagga et al., 2014 | > 2 Weeks | 0/35 | 0/35 | ??? | MRS | Glu/Cr - Occipital Cortex | ↓ Glu/Cr for AD vs. HC | |
| DA | ||||||||
| Hietala et al., 1994 | 1–68 Weeks | ??? | 0/9 | 3/9; > 20 Hours | PET | D2-D3 Receptors -Striatum | ↓ D2-D3 avail. for AD vs. HC | Nonsignificant trendattributing differenceto Bmax |
| Volkow et al., 1996 | 1–14 Weeks | ??? | 1/10 | No | PET | D2-D3 Receptors -StriatumDAT - Striatum | ↓ D2-D3 avail. for AD vs. HCNo difference in DAT avail. | |
| Heinz et al., 2005 | 2–4 Weeks | ??? | 0/12 | ??? | PET | D2-D3 Receptors -Striatum | ↓ D2-D3 avail. for AD vs. HC | ↓ D2-D3 avail.correlated with↑ alcohol craving |
| Erritzoe et al., 2014 | > 4 Weeks | 5/16 | 0/16 | Yes | PET | D3 Receptors -Subcortical | ↑ D3 avail. in HT; trend in TH,PL for AD vs. HC | D3 avail. correlatedwith lifetime alcoholconsumed in CA, TH |
| Tiihonen et al., 1995 | 1–200Weeks | ??? | 2/29 | 8/29; > 2Months | SPECT | DAT - Striatum | ↓ DAT avail. for non-violentAD vs. HC | Trend ↑ DAT avail. inviolent AD vs. HC |
| Heinz et al., 2000 | 3–5 Weeks | ??? | 3/14 | ??? | SPECT | DAT - Striatum | No difference in DAT avail. | VNTR polymorphisminfluenced DAT avail. |
| Kumakura et al., 2013 | 2–9 Weeks | 0/12 | 0/12 | ??? | PET | Presynaptic DopamineTurnover – Striatum | ↓ DA turnover in l CA for ADvs. HC | |
| Gilman et al., 1998 | 3–9 Months | 1/7 | 0/7 | Yes; > 6Weeks | PET | VMAT2 - Striatum | ↓ VMAT2 avail. in striatumfor AD vs. HC | Trend in CA; Significantin PU |
| Martinez et al., 2005 | 2 Weeks | 6/15 | 2/15 | > 1Week | PET | DA Release(Methamphetamine) -Striatum | ↓ D2-D3 baseline avail. forAD vs. HC | ↓ baseline D2-D3 avail.associated with ↑Benzo Dose |
| ↓ DA release in ventralstriatum for AD vs. HC | ||||||||
| Volkow et al., 2007 | 4–17 Weeks | 4/20 | 0/20 | ??? | PET | DA Release(Methylphenidate) –Striatum | ↓ D2-D3 baseline avail. invST for AD vs. HC | |
| ↓ DA release in vST, PU forAD vs. HC | ||||||||
| Narendran et al., 2014 | > 2 Weeks | 9/21 | 5/21 | No | PET | DA Release(Methamphetamine) -Extrastriatal | ↓ DA Release in cortex forAD vs. HC | |
| Longitudinal Studies | ||||||||
| GABA | ||||||||
| Staley et al., 2005 | 2.5–8.5 Days | 8/23 | 0/23 | No | SPECT | GABAA Receptors | ↑ GABAA avail. in cortex forAD nonsmokers vs.HC | No difference inGABAA avail. for ADsmokers vs. HC |
| 22–37 Days | > 1Month | Normalized GABAA avail. incortex to HC levels for ADnonsmokers | ||||||
| Mason et al., 2006 | 2–9 Days | 7/12 | 0/12 | No | MRS | GABA; Glx - OccipitalCortex | ↑ GABA in cortex for ADnonsmokers vs. HC | No difference inGABA for ADsmokers vs. HC |
| 21–41 Days | No | Normalized GABA in cortexto HC levels for ADnonsmokers | ↑ Glu+Gln forsmokers vs.nonsmokers -independent of AD | |||||
| Cosgrove et al., 2014 | 1.4–3.6 days | 10/27 | 5/27 | 2/27 | SPECT | GABAA Receptors | ↑ GABAA avail. in restrictedcortical regions for ADnonsmokers vs. HC | ↑ GABAA avail. inrestricted corticalregions for ADsmokers vs. HC |
| 7.6–12 days | ↑ GABAA avail. in robustcortical regions for AD | ↑ GABAA avail. inrestricted corticalsmokers vs. HC; Nolongitudinal change | ||||||
| 26.1–32.7 | Normalized GABAA avail. incortex to TP1 levels for ADnonsmokers | ↑ GABAA avail. inrestricted corticalregions for ADsmokers vs. HC; Nolongitudinal change | ||||||
| Glutamate | ||||||||
| Hermann et al., 2012 | BAC = 0 | 8/47 | 9/47 | No | MRS | Glu - Prefrontal Cortex | ↑ Glu for AD vs. HC | Glu correlated withseverity ofdependence |
| 14 Days | Yes | Normalized Glu to HC levels | ||||||
| Mon et al., 2012 | 5–13 Days | 7/20 | 3/20 | 2/20 | MRS | Glu - Anterior Cingulate;Right Prefrontal Cortex;Parietal/Occipital Cortex | ↓ Glu in ACC for AD vs. HC | MANOVA controlledfor smoking |
| 27–41 Days | ???/47 | ???/47 | Yes | Normalized Glu in ACC toHC levels | ||||
| Umhau et al., 2010 | 4 Days | 28/33 | 14/33 | 20/33 | MRS | Glu/Cr - AnteriorCingulate | ||
| 4 Weeks | ↓ Glu/Cr from 4 days inacomprasate but notplacebo group | |||||||
| DA | ||||||||
| Volkow et al., 2002 | 6–27 Days | 1/14 | 1/14 | ??? | PET | D2-D3 receptors -Striatum | ↓ D2-D3 avail. for Type II ADvs. HC | |
| 1–4 Months | ↓ D2-D3 avail. maintained forType II AD vs. HC | |||||||
| Rominger et al., 2011 | BAC = 0 | 3/17 | 0/17 | 1/17 | PET | D2-D3 receptors -Extrastriatal | ↓ D2-D3 avail. in TH, IC, HP,TC for AD vs. HC | |
| 7 – 14 Days | ???/14 | 0/14 | Yes; > 3 Days | ↓ D2-D3 avail. maintained forAD vs. HC | ||||
| 1 Year | ???/4 | 0/4 | ↑ D2-D3 avail. (30%) relativeto baseline abstinence scan | |||||
| Laine et al., 1999 | < 4 Days | ??? | 5/27 | 22/27 | SPECT | DAT - Striatum | ↓ DAT avail. in ST for AD vs.HC | |
| 4 Weeks | Normalized DAT to HClevels | ↑ change in DATavail. correlated with↓ time since last drink |
3.A.i. Pre-Withdrawal (Less than 24 Hours Abstinence)
Neuroimaging studies conducted in alcohol dependent participants within 24 hours of the last drink reflect an individual’s state immediately prior to significant withdrawal symptoms. A large MRS study (n=213) reported a trend for higher Glx (glutamate + glutamine) levels in the anterior cingulate of alcohol dependent participants relative to controls. A longer history of drinking was associated with higher Glx levels, as were more cigarettes smoked per day; however, an interaction between smoking status and alcohol dependence was not examined (Yeo et al. 2013). This trend level result may reflect subtle differences in glutamate homeostasis just prior to significant alcohol withdrawal symptoms, as preclinical results indicate heightened glutamate levels during early withdrawal (Fliegel et al. 2013). In another study a trend towards lower glutamate levels in the white matter of the frontal cortex in heavy drinkers was reported compared to light drinkers, where heavier drinking and greater self-reported loss of control accompanied lower glutamate levels (Ende et al. 2013); however, the importance of glutamate levels in areas of predominantly white matter requires further study.
Using 11C-raclopride PET, lower striatal dopamine D2/D3 receptor availability in non-treatment seeking alcohol dependent individuals was observed compared to social drinkers. Additionally, smokers in both groups had lower striatal dopamine D2/D3 receptor availability compared to nonsmokers regardless of alcohol use (Albrecht et al. 2013). Lower striatal dopamine D2/D3 receptor availability was reported in otherwise healthy tobacco smokers at and within 24 hours of abstinence (Brown et al. 2012, Fehr et al. 2008), highlighting the need to tease apart smoking and alcohol dependence in order to understand dopaminergic abnormalities likely associated with a prolonged dysfunctional reward system (Volkow et al. 2012).
3.A.ii. Early Phase Withdrawal (1–14 Days Withdrawal)
The period of 1–14 days after the last drink is a critical yet logistically difficult timeframe to image alcohol dependent participants because alcohol withdrawal symptoms are most severe during this time. Higher glutamate levels were reported in alcohol dependent participants (1–10 days abstinence) compared to controls in the nucleus accumbens (but not cingulate) (Bauer et al. 2013). Alcohol dependent participants with higher glutamate levels in nucleus accumbens and cingulate also reported higher craving for alcohol (Bauer et al. 2013). Meanwhile, elevated striatal dopamine transporter availability in nonsmoking alcohol dependent participants was observed compared to both healthy controls and smoking alcohol dependent participants at 1–5 days abstinence (Cosgrove et al. 2009), further highlighting an influence of comorbid tobacco smoking on dopamine function during alcohol withdrawal.
3.A.iii. Late Phase Withdrawal (>14 Days)
While heightened glutamate concentrations were found during early withdrawal, lower glutamate levels in alcohol dependent participants relative to healthy controls were reported during prolonged withdrawal (2–50 days since last drink) in medial frontal cortex (Thoma et al. 2011) and occipital cortex (Bagga et al. 2014). This has led to speculation that a glutamate-glutamine cycling imbalance may occur during alcohol withdrawal with possible associations to neurotoxicity. However, no subsequent studies have confirmed this hypothesis, and additional in vivo research is required to determine the importance of glutamate in clinical alcohol dependence.
Studies of the GABAA receptor system during prolonged withdrawal (at least 4 weeks) consistently provided evidence of lower GABAA receptor availability in alcohol dependent participants relative to controls, primarily in regions of cortex and cerebellum (Abi-Dargham et al. 1998, Gilman et al. 1996, Lingford-Hughes et al. 2012, Lingford-Hughes et al. 2000, Lingford-Hughes et al. 1998). Lower GABAA receptor availability in alcohol dependent participants relative to controls was also reported in the nucleus accumbens and hippocampus, and reduced hippocampal GABAA receptor availability was associated with declining performance on a delayed memory task (Lingford-Hughes et al. 2012) suggesting prolonged GABA-ergic dysfunction may impede cognitive function. Diminished glucose metabolism rates in areas of the frontal cortex corresponding to lower GABAA receptor availability were also observed (Gilman et al. 1996), and a blunted metabolic response of alcohol dependent participants to the benzodiazepine lorazepam was reported (N. D. Volkow et al. 1997). Interestingly, one study found evidence of a trend towards regional differences between female (cerebellum, occipital cortex, parietal cortex) and male (frontal cortex) alcohol dependent participants compared to sex-matched control groups (Lingford-Hughes et al. 2000); however, the study was underpowered to detect significant sex differences. While these studies consistently reported lower GABAA availability in alcohol dependent participants during extended abstinence compared to controls, a caveat is that it remains unknown whether this lower availability is pre-existing or reflects a neurochemical consequence of prolonged alcohol use. Due to the practical difficulties in performing prospective studies and limitations in using radioactivity to image adolescents, this is a critical area where preclinical studies must augment the clinical literature. Preclinical evidence suggests reduced dopamine function may be a pre-existing trait rather than a neurochemical modification in response to chronic alcohol exposure (Everitt et al. 2008), however, given some disparities between preclinical and neuroimaging studies on dopaminergic function following chronic drug use (Narendran and Martinez 2008), further studies are necessary.
Neuroimaging studies of the dopamine system have revealed dopaminergic dysfunction in alcohol dependent participants. Lower dopamine D2/D3 receptor availability was observed in striatal regions of alcohol dependent participants compared to controls (Heinz et al. 2005, Hietala et al. 1994, Martinez et al. 2005, Volkow et al. 1996, Volkow et al. 2007), with one study attributing reduced receptor availability to lower receptor numbers (Hietala et al. 1994). Additionally, reduced striatal dopamine D2/D3 availability was associated with greater alcohol craving, (Heinz et al. 2005) more daily alcohol consumed, and the need for higher benzodiazepine doses to treat withdrawal symptoms (Martinez et al. 2005). One study indicated lower DAT availability in the striatum of non-violent (but not habitually violent) alcohol dependent participants compared to controls (Tiihonen et al. 1995), which implicates dysfunctional dopamine tone. On the other hand, no difference in striatal DAT availability between alcohol dependent participants and controls was reported in two other studies (Heinz et al. 2000, Volkow et al. 1996). Diminished presynaptic dopamine synthesis capacity was observed in the caudate of alcohol dependent participants (Kumakura et al. 2013), as were reduced type 2 vesicular monoamine transporters, indicating reduced presynaptic dopamine vesicle integrity (Gilman et al. 1998). Finally, a paradigm used to probe dopamine neurotransmission involves the administration of stimulants such as amphetamine and methylphenidate, which reverse and block dopamine transporters, respectively, leading to robust increases in synaptic dopamine levels. Using this paradigm in alcohol-dependent participants, dopaminergic neurotransmission was blunted in the striatum (Martinez et al. 2005, Volkow et al. 2007) and cortex (Narendran et al. 2014) compared to healthy controls. A comprehensive view of these neuroimaging studies provides strong evidence of reduced dopaminergic function, from postsynaptic receptor availability to presynaptic functioning and dopaminergic release, in alcohol withdrawal, which likely underlies reward dysfunction (Volkow et al. 2012) and may contribute to the prolonged propensity for relapse.
Advances in PET have led to the development of radioligands with higher affinity for dopamine D3 vs. D2 receptors, which allows measurement in extrastriatal regions. Higher dopamine D3 receptor availability in the hypothalamus for alcohol dependent participants in relation to controls was reported, and higher availability was related to greater lifetime alcohol consumption (Erritzoe et al. 2014). The role of dopamine D3 receptors, and regional fractions of dopamine D3/D2 receptors are of interest for future neuroimaging studies, particularly because the dopamine D3 receptor is a potential medication target (Le Foll et al. 2014).
3.B. Longitudinal Studies
Cross-sectional studies of alcohol withdrawal are more practically feasible, but provide only a brief snapshot in time of the full picture of the recovery from alcohol dependence. Longitudinal neuroimaging studies, although logistically challenging, have yielded diverse and robust findings. These studies are critical to situate cross-sectional imaging findings in the context of a progression of neurochemical adaptations during alcohol dependence and withdrawal.
3.B.i. Longitudinal Studies of Alcohol Withdrawal
Longitudinal MRS studies measuring glutamate provide evidence of glutamatergic alterations during alcohol abstinence (Tsai and Coyle 1998). Higher anterior cingulate glutamate levels in alcohol dependent participants on the day after their last drink were reported and associated with greater severity of alcohol dependence and number of heavy drinking days in the month prior to abstinence. After two weeks abstinence, the heightened glutamate levels returned to control levels (Hermann et al. 2012). This study observed similar results with data obtained in chronic alcohol-exposed rats, where extracellular glutamate levels during intoxication were similar to pre-alcohol measures, but increased by 12 hours of abstinence and remained elevated at 60 hours of abstinence. In contrast, lower anterior cingulate glutamate levels were reported in alcohol dependent participants at 5–13 days withdrawal compared to controls, which increased to control levels after 5 weeks of abstinence (Mon et al. 2012). Similarly, trends of increased glutamate/creatine ratios were indicated in the anterior cingulate of alcohol dependent participants scanned at 4 days and 4 weeks abstinence (Umhau et al. 2010). Taken together these studies suggest increased glutamate levels during early withdrawal, which then fluctuate prior to normalization.
Given the role of glutamate in neuroplasticity and alcohol withdrawal (Krystal et al. 2003), glutamatergic drugs have been identified as promising therapeutics for suppressing withdrawal symptoms and alcohol craving (Holmes et al. 2013). Acamprosate, a N-methyl-D-aspartate (NMDA-R) modulator and GABAB partial agonist, is one of only three approved medications for alcohol dependence, but has had mixed efficacy in clinical trials (Kufahl et al. 2014). Understanding the neurochemical effects of drugs such as acamprosate and the relationship to relapse can help disentangle mixed clinical efficacy results and possibly present new treatment targets. In a brain imaging study in alcohol dependent participants, acamprosate decreased glutamate/creatine ratios in anterior cingulate from 4 days to 4 weeks abstinence, while placebo treatment led to trends of increased glutamate/creatine ratios (Umhau et al. 2010). This finding suggests that acamprosate may modulate glutamate levels during alcohol withdrawal and illustrates the powerful application of neuroimaging to evaluate the pharmacological effects of drugs developed for alcohol dependent participants.
Persistent deficits in the dopamine system have also been documented. A longitudinal study reported lower striatal DAT availability in alcohol dependent participants after less than 4 days of withdrawal that increased to control levels after 4 weeks of abstinence (Laine et al. 1999). Importantly, the participants imaged most recently since their last drink exhibited the greatest changes in DAT availability by 4 weeks abstinence. Since it is unknown how many of these participants were smokers, it is unclear how these findings relate to the findings of increased DAT availability mediated by smoking during acute alcohol withdrawal (Cosgrove et al. 2009). Yet, it is clear that the response of DAT availability to alcohol withdrawal occurs rapidly during abstinence, highlighting the importance of scan timing during alcohol withdrawal.
Impairments in the dopamine system appear to be long-lasting and a hallmark feature of drug and alcohol dependence (Brown et al. 2012, Martinez et al. 2012, N. D. Volkow et al. 1997). Lower striatal dopamine D2/D3 receptor availability was observed at 6–27 days of withdrawal in alcohol dependent participants vs. controls, and this persisted and remained lower 1–4 months later (Volkow et al. 2002). Lower dopamine D2/D3 receptor availability was also found in extrastriatal regions (thalamus, insular cortex, hippocampus, and temporal cortex) at both one day and two weeks of alcohol withdrawal compared to controls (Rominger et al. 2012). In four individuals who maintained abstinence for one year, dopamine D2/D3 receptor availability recovered from levels observed during acute withdrawal, increasing by 30%, consistent with healthy control levels. This intriguing preliminary finding raises the possibility that dopamine D2/D3 availability may recover during prolonged alcohol abstinence, but the small sample size demands more exploration of this research question. The possibility of long-term deficits in dopaminergic function has critical implications for long-term sobriety and suggests extended treatment focused on this system.
3.B.ii. Smoking Influences on Longitudinal Studies of Alcohol Withdrawal
Neuroimaging studies of the GABA system have yielded critical findings defining the time course of GABA neuroadaptations during alcohol withdrawal, including the role of tobacco smoking comorbidity in these neuroadaptations. The role of smoking in treating alcohol dependence is critical, as concomitant alcohol and nicotine abstinence can intensify withdrawal severity (Perez et al. 2015). An MRS study of GABA concentration in the occipital cortex of alcohol dependent participants found increased GABA levels in nonsmoking participants at 1 week withdrawal relative to smoking participants that decreased to control levels after 4 weeks, while no differences from control levels were observed in smoking participants (Mason et al. 2006). These findings may suggest that smoking blunted the higher GABA levels seen in 1-week abstinent alcohol dependent participants. Similarly, molecular imaging studies performed by our group observed significantly higher GABAA receptor availability for nonsmoking alcohol dependent participants at one week withdrawal that returned to control levels after 4 weeks of abstinence, whereas smoking participants had comparable GABAA receptor availability to controls at both timepoints (Staley et al. 2005). These MRS and GABAA receptor data reflect different components of the GABA system that are both indirectly measured in different ways. Yet, both show convincing changes in the GABA system during alcohol withdrawal in nonsmoking participants that appear altered by smoking, reflecting a critical need to account for smoking and smoking abstinence in treating alcohol dependence.
We recently extended these findings with further SPECT experiments in a larger sample size. This study reported higher GABAA receptor availability in nonsmoking alcohol dependent participants compared to nonsmoking controls at approximately 3 days withdrawal that further heightened at 10 days withdrawal. By 4 weeks of withdrawal, GABAA availability began to recover in nonsmokers, returning to levels observed at 3 days of withdrawal. In contrast, smoking alcohol dependent participants exhibited higher GABAA receptor availability compared to smoking controls, but less than nonsmoking participants. This moderately elevated GABAA receptor availability persisted throughout withdrawal with no evidence of recovery. Further, alcohol dependent smokers had significantly higher levels of alcohol craving compared to the nonsmoker participants, which positively correlated with GABAA availability (Cosgrove et al. 2014). A parallel study in nonhuman primates revealed a similar time course of GABAA receptor availability during alcohol withdrawal to that observed in nonsmoking participants, and found that continued nicotine exposure did not alter the time course of GABAA availability during alcohol withdrawal. Together these studies suggest that a component of tobacco smoke other than nicotine interacts with the GABA system to block critical neuroadaptations during alcohol withdrawal (Cosgrove et al. 2014). Importantly, because there is evidence that concurrent alcohol and nicotine cessation leads to worse withdrawal symptoms than alcohol cessation alone (Perez et al. 2015) and because smoking, but not nicotine, likely blocks normalization of GABAA receptors, nicotine replacement therapy in alcohol dependent individuals who wish to quit smoking is indicated. Finally, GABAA receptor availability may be a trait marker of nicotine and alcohol dependence (Stokes et al. 2013), thus smoking status is a critical consideration for both treating alcohol withdrawal and mitigating relapse risk.
4. Commentary and Future Directions
Imaging studies have greatly advanced the field of alcohol dependence by contributing neurochemical information in living individuals during alcohol dependence and withdrawal that can be situated within the framework of known behavioral correlates, such as the temporal changes in withdrawal, craving and cognitive dysfunction. Critical findings include an apparent downregulation of dopamine D2/D3 receptor availability, peak glutamate levels during acute withdrawal followed by fluctuating concentrations, and potentially detrimental effects of smoking on neuroadaptations in the GABA system. Next, we aim to identify lessons from the currently published imaging literature to guide future neuroimaging and preclinical studies.
4.A. Preclinical studies are needed
An obvious limitation in neuroimaging studies is the inability to determine whether particular neurochemical states are a cause or consequence of alcohol dependence. Most studies discussed herein imaged participants after 2 weeks withdrawal (Table 2), although a handful of studies conducted during the first two weeks of alcohol withdrawal were reported. The logistical impracticality of prospectively recruiting and imaging human participants prior to substance dependence, and the limitations of imaging individuals during severe alcohol withdrawal can be circumvented with the use of preclinical studies. Well-validated models of alcohol drinking have been developed in nonhuman primates (Grant et al. 2008, Vivian et al. 2001) and rodents (Bell et al. 2014), which when combined with neuroimaging technology can provide unique opportunities to study alcohol dependence. Such preclinical experiments can provide baseline measurements in an alcohol-naive state, followed by measures of alcohol drinking in free-choice paradigms (Hillmer et al. 2014a, Hillmer et al. 2014b), to answer questions such as whether low receptor availability is a pre-existing trait of elevated alcohol consumption, a result of chronic alcohol exposure, or a combination of the two. These findings could profoundly guide our understanding of how and why alcohol dependence develops and significantly impact treatment strategies. Animal models also allow for prospective studies of adolescent subjects to predict future alcohol dependence. Further, many confounding factors present in clinical patients, such as drug comorbidity, can be isolated and systematically studied (Cosgrove et al. 2014). Improved precision in imaging outcome measures, such as receptor density or apparent radioligand affinity, is also possible with preclinical subjects (Morris et al. 2004). Although significant limitations exist in scaling the complexity of preclinical models of alcohol dependence to mimic human dependence, these experiments can isolate pharmacological factors to complement interpretation and understanding of human imaging studies.
4.B. Smoking needs to be carefully controlled
Tobacco smoking is an essential consideration when investigating alcohol dependence, since smoking and alcohol rates are dramatically increased in instances of comorbidity (Dawson 2000, York and Hirsch 1995). Smoking modulates the GABA system during alcohol withdrawal (Cosgrove et al. 2009) possibly by muting withdrawal symptoms while raising relapse risk (Cosgrove et al. 2014). Preclinical studies demonstrate that chronic nicotine exposure upregulates NMDA and AMPA receptor expression (Wang et al. 2007) and blocks glutamatergic transmission (Li et al. 2014), and neuroimaging studies point to an influence of smoking on glutamate levels (Mason et al. 2006, O’Neill et al. 2014, Yeo et al. 2013). Reduced striatal dopamine D2/D3 availability in smokers relative to nonsmokers has also been reported (Albrecht et al. 2013, Fehr et al. 2008), however, possible interactions between smoking and alcohol in the dopamine system are underexamined. MRS studies have indicated significant relationships between smoking and alcohol dependence in non-neurotransmitter specific metabolite levels such as N-acetylaspartate and choline (Durazzo and Meyerhoff 2007). Additionally, alcohol interacts with α4β2*- nicotinic acetylcholine receptors (nAChRs), the primary site of nicotine’s direct action on the brain (Larsson and Engel 2004), and preclinical receptor imaging studies observed reduced receptor availability following chronic alcohol exposure (Cosgrove et al. 2010, Hillmer et al. 2014a). Indeed, the α4β2*-nAChR partial agonist varenicline shows promise as a therapeutic for alcohol dependence (Feduccia et al. 2014, Schacht et al. 2014), thus further imaging of this receptor system is of interest for future studies. This wide variety of neurochemical effects from smoking underscores the need to control both smoking status and smoking withdrawal status in neuroimaging. Moreover, by including status as a covariate in analysis improved individualized treatment options may be discerned to improve cessation rates for both alcohol and tobacco smoking.
4.C. Other Comorbidities
Alcohol dependence is comorbid with many disorders in addition to tobacco smoking, such as other substance use disorders and mood and anxiety disorders, including persistent depression, major depressive disorder, and posttraumatic stress disorder (Debell et al. 2014, Grant et al. 2015). In the literature, these comorbidities have been considered even less than tobacco smoking, and should be more closely examined in future studies. For example, clinical measures of psychiatric history, current mood and anxiety symptoms and trauma exposure can be obtained and considered in conjunction with neuroimaging data. Moreover, clinical diagnosis of mood and anxiety disorders must be accounted for in neuroimaging studies of alcohol dependence.
4.D. Sex Differences
There is substantial literature demonstrating sex differences in the trajectory and consequences of alcohol dependence (Greenfield et al. 2007, Mann et al. 2005), accompanied by preclinical evidence for sex differences in the response of both NMDA-R and GABAA receptor pharmacology in chronic alcohol exposure and withdrawal (Alele and Devaud 2005, Sharrett-Field et al. 2013). In addition, neurosteroid levels, implicated in modulating the GABA system during chronic alcohol exposure, fluctuated significantly more in response to chronic alcohol exposure in male than female rodents (Janis et al. 1998), which may explain more frequent and severe withdrawal symptoms observed in males (Devaud et al. 2006). There is further evidence of higher GABAA receptor availability in healthy women than men (Esterlis et al. 2013), suggesting sex differences may play a role in alcohol’s effects on the GABA system. Yet, only one neuroimaging study presented here directly investigated sex differences in alcohol dependence (Lingford-Hughes et al. 2000), showing a trend of regional differences in reduced GABAA receptor availability during extended alcohol withdrawal between men and women. Sex differences in alcohol dependence thus merit closer investigation from neuroimaging researchers.
4.E. Treatment response
The evaluation of pharmacological treatments for alcohol dependence is a powerful application of neuroimaging. For example, an MRS study observed acamprosate-induced suppression of glutamate levels (Umhau et al. 2010), while PET studies with the opioid receptor targeting drug naltrexone discerned opioid receptor subtype differences in naltrexone occupancy that may influence patient response (Weerts et al. 2008). Such experiments are vital to determine optimal treatment dose or individual differences in neurochemical response mediating differences in treatment response. Further, imaging can determine neurochemical endophenotypes such as genetically altered neurochemical function that may mediate individual dependence susceptibility or treatment response, which could improve individualized therapeutic strategies.
Conversely, pharmacological treatment also presents a critical covariate not well controlled in previous studies. Specifically, benzodiazepines, which act at GABAA receptors (Fujita et al. 1999), are frequently administered prophylactically to treat alcohol withdrawal symptoms. However, close monitoring of inpatient research participants with clear guidelines can reduce the need for benzodiazepine treatment in moderately dependent participants (Reoux and Miller 2000). Patient’s history of benzodiazepine treatment, particularly recent doses, may influence neuroimaging outcomes. Thus, benzodiazepine treatment should be more closely reported and statistically considered in future studies to properly interpret neuroimaging outcomes of alcohol dependence.
4.F. Additional imaging targets
Additional neuroimaging targets are needed to fully understand the neurochemistry of alcohol dependence. Promising PET radioligands to image glutamatergic targets such as metabotropic glutamate receptors 1 (Li and Huang 2014) and 5 (DeLorenzo et al. 2011, Sullivan et al. 2013), have recently emerged. Subunit specific radioligands for GABAA (Lingford-Hughes et al. 2012) and dopamine D3 (Erritzoe et al. 2014) receptors have been used, and development of PET radioligands with improved properties to image dopamine D1 receptors and GABAB receptors would augment current studies of alcohol dependence. Refinement of experimental techniques to use tiagabine (Frankle et al. 2012) and ketamine (DeLorenzo et al. 2015) to interrogate the function of GABA and glutamate systems, respectively, in a manner analogous to amphetamine challenge with the dopamine system (Laruelle 2000) could detect fluctuations of exclusively extracellular neurotransmitter concentrations, improving upon MRS measurements that include both extracellular and intracellular concentrations. Finally, multimodal neuroimaging incorporating both MRS and PET/SPECT to provide a comprehensive view of both metabolite concentrations and receptor availability represents a powerful application that could be exploited to enhance understanding of dynamic changes in the GABA and glutamate systems in alcohol dependence, withdrawal, and treatment.
5. Conclusion
Neurochemical brain imaging has informed our understanding of the neurochemical contributions of GABA, glutamate, and dopamine to alcohol dependence. Particularly important findings include evidence of a long-lasting dysfunctional dopaminergic system, elevated glutamate tone in acute withdrawal, and an interaction of smoking with the GABA system during alcohol withdrawal. Future experiments should minimize possible confounds from smoking comorbidity, sex, and therapeutic benzodiazepines. Moreover, focusing on neurochemical states predisposing individuals to alcohol dependence and evaluating pharmacological therapeutics represent underutilized applications of neuroimaging. These uses of neuroimaging will greatly advance our understanding of alcohol dependence, withdrawal, and treatment.
Acknowledgments
Funding: This research was supported in part by National Institute of Health grants T32 DA022975 (Mason, Hillmer), K05 AA014715 (O’Malley) and R01 AA17464; K02 DA031750 (Cosgrove). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
References
- Abi-Dargham A, Krystal JH, Anjilvel S, Scanley BE, Zoghbi S, Baldwin RM, Rajeevan N, Ellis S, Petrakis IL, Seibyl JP, Charney DS, Laruelle M, Innis RB. Alterations of benzodiazepine receptors in type II alcoholic subjects measured with SPECT and [123I]iomazenil. Am J Psychiatry. 1998;155(11):1550–1555. doi: 10.1176/ajp.155.11.1550. [DOI] [PubMed] [Google Scholar]
- Albrecht DS, Kareken DA, Yoder KK. Effects of smoking on D(2)/D(3) striatal receptor availability in alcoholics and social drinkers. Brain Imaging Behav. 2013;7(3):326–334. doi: 10.1007/s11682-013-9233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alele PE, Devaud LL. Differential adaptations in GABAergic and glutamatergic systems during ethanol withdrawal in male and female rats. Alcohol Clin Exp Res. 2005;29(6):1027–1034. doi: 10.1097/01.alc.0000167743.96121.40. [DOI] [PubMed] [Google Scholar]
- Bagga D, Khushu S, Modi S, Kaur P, Bhattacharya D, Garg ML, Singh N. Impaired visual information processing in alcohol-dependent subjects: a proton magnetic resonance spectroscopy study of the primary visual cortex. J Stud Alcohol Drugs. 2014;75(5):817–826. doi: 10.15288/jsad.2014.75.817. [DOI] [PubMed] [Google Scholar]
- Barrio JR, Huang SC, Melega WP, Yu DC, Hoffman JM, Schneider JS, Satyamurthy N, Mazziotta JC, Phelps ME. 6-[18F]fluoro-L-dopa probes dopamine turnover rates in central dopaminergic structures. J Neurosci Res. 1990;27(4):487–493. doi: 10.1002/jnr.490270408. [DOI] [PubMed] [Google Scholar]
- Bauer J, Pedersen A, Scherbaum N, Bening J, Patschke J, Kugel H, Heindel W, Arolt V, Ohrmann P. Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology. 2013;38(8):1401–1408. doi: 10.1038/npp.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Engleman EA, Toalston JE, McBride WJ. Scheduled access alcohol drinking by alcohol-preferring (P) and high-alcohol-drinking (HAD) rats: modeling adolescent and adult binge-like drinking. Alcohol. 2014;48(3):225–234. doi: 10.1016/j.alcohol.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Brown AK, Mandelkern MA, Farahi J, Robertson C, Ghahremani DG, Sumerel B, Moallem N, London ED. Sex differences in striatal dopamine D2/D3 receptor availability in smokers and non-smokers. Int J Neuropsychopharmacol. 2012;15(7):989–994. doi: 10.1017/S1461145711001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Esterlis I, Mason GF, Bois F, O’Malley SS, Krystal JH. Neuroimaging insights into the role of cortical GABA systems and the influence of nicotine on the recovery from alcohol dependence. Neuropharmacology. 2011;60(7–8):1318–1325. doi: 10.1016/j.neuropharm.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Kloczynski T, Bois F, Pittman B, Tamagnan G, Seibyl JP, Krystal JH, Staley JK. Decreased Beta(2)*-nicotinic acetylcholine receptor availability after chronic ethanol exposure in nonhuman primates. Synapse. 2010;64(9):729–732. doi: 10.1002/syn.20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Krantzler E, Frohlich EB, Stiklus S, Pittman B, Tamagnan GD, Baldwin RM, Bois F, Seibyl JP, Krystal JH, O’Malley SS, Staley JK. Dopamine and serotonin transporter availability during acute alcohol withdrawal: effects of comorbid tobacco smoking. Neuropsychopharmacology. 2009;34(10):2218–2226. doi: 10.1038/npp.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, McKay R, Esterlis I, Kloczynski T, Perkins E, Bois F, Pittman B, Lancaster J, Glahn DC, O’Malley S, Carson RE, Krystal JH. Tobacco smoking interferes with GABAA receptor neuroadaptations during prolonged alcohol withdrawal. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1413947111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA. Drinking as a risk factor for sustained smoking. Drug Alcohol Depend. 2000;59(3):235–249. doi: 10.1016/s0376-8716(99)00130-1. [DOI] [PubMed] [Google Scholar]
- Debell F, Fear NT, Head M, Batt-Rawden S, Greenberg N, Wessely S, Goodwin L. A systematic review of the comorbidity between PTSD and alcohol misuse. Soc Psychiatry Psychiatr Epidemiol. 2014;49(9):1401–1425. doi: 10.1007/s00127-014-0855-7. [DOI] [PubMed] [Google Scholar]
- DeLorenzo C, DellaGioia N, Bloch M, Sanacora G, Nabulsi N, Abdallah C, Yang J, Wen R, Mann JJ, Krystal JH, Parsey RV, Carson RE, Esterlis I. In vivo ketamine-induced changes in [(1)(1)C]ABP688 binding to metabotropic glutamate receptor subtype 5. Biol Psychiatry. 2015;77(3):266–275. doi: 10.1016/j.biopsych.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzo C, Kumar JS, Mann JJ, Parsey RV. In vivo variation in metabotropic glutamate receptor subtype 5 binding using positron emission tomography and [11C]ABP688. J Cereb Blood Flow Metab. 2011;31(11):2169–2180. doi: 10.1038/jcbfm.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud LL, Risinger FO, Selvage D. Impact of the hormonal milieu on the neurobiology of alcohol dependence and withdrawal. J Gen Psychol. 2006;133(4):337–356. doi: 10.3200/GENP.133.4.337-356. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ. Neurobiological and neurocognitive effects of chronic cigarette smoking and alcoholism. Front Biosci. 2007;12:4079–4100. doi: 10.2741/2373. [DOI] [PubMed] [Google Scholar]
- Ende G, Hermann D, Demirakca T, Hoerst M, Tunc-Skarka N, Weber-Fahr W, Wichert S, Rabinstein J, Frischknecht U, Mann K, Vollstadt-Klein S. Loss of control of alcohol use and severity of alcohol dependence in non-treatment-seeking heavy drinkers are related to lower glutamate in frontal white matter. Alcohol Clin Exp Res. 2013;37(10):1643–1649. doi: 10.1111/acer.12149. [DOI] [PubMed] [Google Scholar]
- Erritzoe D, Tziortzi A, Bargiela D, Colasanti A, Searle GE, Gunn RN, Beaver JD, Waldman A, Nutt DJ, Bani M, Merlo-Pich E, Rabiner EA, Lingford-Hughes A. In vivo imaging of cerebral dopamine D3 receptors in alcoholism. Neuropsychopharmacology. 2014;39(7):1703–1712. doi: 10.1038/npp.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterlis I, McKee SA, Kirk K, Lee D, Bois F, Stiklus SM, Seibyl JP, Krishnan-Sarin S, O’Malley SS, Staley JK, Cosgrove KP. Sex-specific differences in GABA(A) -benzodiazepine receptor availability: relationship with sensitivity to pain and tobacco smoking craving. Addict Biol. 2013;18(2):370–378. doi: 10.1111/j.1369-1600.2011.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363(1507):3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Feduccia AA, Simms JA, Mill D, Yi HY, Bartlett SE. Varenicline decreases ethanol intake and increases dopamine release via neuronal nicotinic acetylcholine receptors in the nucleus accumbens. Br J Pharmacol. 2014;171(14):3420–3431. doi: 10.1111/bph.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr C, Yakushev I, Hohmann N, Buchholz HG, Landvogt C, Deckers H, Eberhardt A, Klager M, Smolka MN, Scheurich A, Dielentheis T, Schmidt LG, Rosch F, Bartenstein P, Grunder G, Schreckenberger M. Association of low striatal dopamine d2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am J Psychiatry. 2008;165(4):507–514. doi: 10.1176/appi.ajp.2007.07020352. [DOI] [PubMed] [Google Scholar]
- Fliegel S, Brand I, Spanagel R, Noori HR. Ethanol-induced alterations of amino acids measured by in vivo microdialysis in rats: a meta-analysis. In Silico Pharmacol. 2013;1:7. doi: 10.1186/2193-9616-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follesa P, Biggio F, Talani G, Murru L, Serra M, Sanna E, Biggio G. Neurosteroids, GABAA receptors, and ethanol dependence. Psychopharmacology (Berl) 2006;186(3):267–280. doi: 10.1007/s00213-005-0126-0. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Cho RY, Mason NS, Chen CM, Himes M, Walker C, Lewis DA, Mathis CA, Narendran R. [11C]flumazenil binding is increased in a dose-dependent manner with tiagabine-induced elevations in GABA levels. PLoS One. 2012;7(2):e32443. doi: 10.1371/journal.pone.0032443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey KA, Koeppe RA, Kilbourn MR. Imaging the vesicular monoamine transporter. Adv Neurol. 2001;86:237–247. [PubMed] [Google Scholar]
- Fujita M, Woods SW, Verhoeff NP, Abi-Dargham A, Baldwin RM, Zoghbi SS, Soares JC, Jatlow PA, Krystal JH, Rajeevan N, Charney DS, Seibyl JP, Innis RB. Changes of benzodiazepine receptors during chronic benzodiazepine administration in humans. Eur J Pharmacol. 1999;368(2–3):161–172. doi: 10.1016/s0014-2999(99)00013-8. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75(1):218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S, Koeppe RA, Adams K, Johnson-Greene D, Junck L, Kluin KJ, Brunberg J, Martorello S, Lohman M. Positron emission tomographic studies of cerebral benzodiazepine-receptor binding in chronic alcoholics. Ann Neurol. 1996;40(2):163–171. doi: 10.1002/ana.410400207. [DOI] [PubMed] [Google Scholar]
- Gilman S, Koeppe RA, Adams KM, Junck L, Kluin KJ, Johnson-Greene D, Martorello S, Heumann M, Bandekar R. Decreased striatal monoaminergic terminals in severe chronic alcoholism demonstrated with (+)[11C]dihydrotetrabenazine and positron emission tomography. Ann Neurol. 1998;44(3):326–333. doi: 10.1002/ana.410440307. [DOI] [PubMed] [Google Scholar]
- Goodman MM, Kilts CD, Keil R, Shi B, Martarello L, Xing D, Votaw J, Ely TD, Lambert P, Owens MJ, Camp VM, Malveaux E, Hoffman JM. 18F–labeled FECNT: a selective radioligand for PET imaging of brain dopamine transporters. Nucl Med Biol. 2000;27(1):1–12. doi: 10.1016/s0969-8051(99)00080-3. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of dsm-5 alcohol use disorder: Results from the national epidemiologic survey on alcohol and related conditions iii. JAMA psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res. 2008;32(10):1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Brooks AJ, Gordon SM, Green CA, Kropp F, McHugh RK, Lincoln M, Hien D, Miele GM. Substance abuse treatment entry, retention, and outcome in women: A review of the literature. Drug Alcohol Depend. 2007;86(1):1–21. doi: 10.1016/j.drugalcdep.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA, Trudell JR, Mihic SJ. Ethanol’s molecular targets. Sci Signal. 2008;1(28):re7. doi: 10.1126/scisignal.128re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, Lee KS, Linnoila M, Weinberger DR. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22(2):133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Buchholz HG, Grunder G, Kumakura Y, Cumming P, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am J Psychiatry. 2005;162(8):1515–1520. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- Hermann D, Weber-Fahr W, Sartorius A, Hoerst M, Frischknecht U, Tunc-Skarka N, Perreau-Lenz S, Hansson AC, Krumm B, Kiefer F, Spanagel R, Mann K, Ende G, Sommer WH. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol Psychiatry. 2012;71(11):1015–1021. doi: 10.1016/j.biopsych.2011.07.034. [DOI] [PubMed] [Google Scholar]
- Hietala J, West C, Syvalahti E, Nagren K, Lehikoinen P, Sonninen P, Ruotsalainen U. Striatal D2 dopamine receptor binding characteristics in vivo in patients with alcohol dependence. Psychopharmacology (Berl) 1994;116(3):285–290. doi: 10.1007/BF02245330. [DOI] [PubMed] [Google Scholar]
- Hillmer AT, Tudorascu DL, Wooten DW, Lao PJ, Barnhart TE, Ahlers EO, Resch LM, Larson JA, Converse AK, Moore CF, Schneider ML, Christian BT. Changes in the alpha4beta2* nicotinic acetylcholine system during chronic controlled alcohol exposure in nonhuman primates. Drug Alcohol Depend. 2014a;138:216–219. doi: 10.1016/j.drugalcdep.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmer AT, Wooten DW, Tudorascu DL, Barnhart TE, Ahlers EO, Resch LM, Larson JA, Converse AK, Moore CF, Schneider ML, Christian BT. The effects of chronic alcohol self-administration on serotonin-1A receptor binding in nonhuman primates. Drug Alcohol Depend. 2014b;144:119–126. doi: 10.1016/j.drugalcdep.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Spanagel R, Krystal JH. Glutamatergic targets for new alcohol medications. Psychopharmacology (Berl) 2013;229(3):539–554. doi: 10.1007/s00213-013-3226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd RE, Gurr D, Sailasuta N. Proton spectroscopy without water suppression: the oversampled J-resolved experiment. Magn Reson Med. 1998;40(3):343–347. doi: 10.1002/mrm.1910400302. [DOI] [PubMed] [Google Scholar]
- Janis GC, Devaud LL, Mitsuyama H, Morrow AL. Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one in male and female rats. Alcohol Clin Exp Res. 1998;22(9):2055–2061. [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, O’Connor PG. Management of Drug and Alcohol Withdrawal. New England Journal of Medicine. 2003;348(18):1786–1795. doi: 10.1056/NEJMra020617. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: a meta-analysis. Alcohol Clin Exp Res. 2001;25(9):1335–1341. [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D’Souza DC. N-methyl-d-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacology & Therapeutics. 2003;99(1):79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Staley J, Mason G, Petrakis IL, Kaufman J, Harris RA, Gelernter J, Lappalainen J. Gamma-aminobutyric acid type A receptors and alcoholism: intoxication, dependence, vulnerability, and treatment. Arch Gen Psychiatry. 2006;63(9):957–968. doi: 10.1001/archpsyc.63.9.957. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Watterson LR, Olive MF. The development of acamprosate as a treatment against alcohol relapse. Expert Opin Drug Discov. 2014;9(11):1355–1369. doi: 10.1517/17460441.2014.960840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumakura Y, Gjedde A, Caprioli D, Kienast T, Beck A, Plotkin M, Schlagenhauf F, Vernaleken I, Grunder G, Bartenstein P, Heinz A, Cumming P. Increased turnover of dopamine in caudate nucleus of detoxified alcoholic patients. PLoS One. 2013;8(9):e73903. doi: 10.1371/journal.pone.0073903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009;205(4):529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine TP, Ahonen A, Torniainen P, Heikkila J, Pyhtinen J, Rasanen P, Niemela O, Hillbom M. Dopamine transporters increase in human brain after alcohol withdrawal. Mol Psychiatry. 1999;4(2):189–191. 104–105. doi: 10.1038/sj.mp.4000514. [DOI] [PubMed] [Google Scholar]
- Larsson A, Engel JA. Neurochemical and behavioral studies on ethanol and nicotine interactions. Neurosci Biobehav Rev. 2004;27(8):713–720. doi: 10.1016/j.neubiorev.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20(3):423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Collo G, Rabiner EA, Boileau I, Merlo Pich E, Sokoloff P. Dopamine D3 receptor ligands for drug addiction treatment: update on recent findings. Prog Brain Res. 2014;211:255–275. doi: 10.1016/B978-0-444-63425-2.00011-8. [DOI] [PubMed] [Google Scholar]
- Li S, Huang Y. In vivo imaging of the metabotropic glutamate receptor 1 (mGluR1) with positron emission tomography: recent advance and perspective. Curr Med Chem. 2014;21(1):113–123. doi: 10.2174/09298673113209990217. [DOI] [PubMed] [Google Scholar]
- Li X, Semenova S, D’Souza MS, Stoker AK, Markou A. Involvement of glutamatergic and GABAergic systems in nicotine dependence: Implications for novel pharmacotherapies for smoking cessation. Neuropharmacology. 2014;76(Pt B):554–565. doi: 10.1016/j.neuropharm.2013.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Suryanarayanan A, Abriam A, Snyder B, Olsen RW, Spigelman I. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci. 2007;27(45):12367–12377. doi: 10.1523/JNEUROSCI.2786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingford-Hughes A, Hume SP, Feeney A, Hirani E, Osman S, Cunningham VJ, Pike VW, Brooks DJ, Nutt DJ. Imaging the GABA-Benzodiazepine Receptor Subtype Containing the [agr]5-Subunit In Vivo With [lsqb]11C[rsqb]Ro15 4513 Positron Emission Tomography. J Cereb Blood Flow Metab. 2002;22(7):878–889. doi: 10.1097/00004647-200207000-00013. [DOI] [PubMed] [Google Scholar]
- Lingford-Hughes A, Reid AG, Myers J, Feeney A, Hammers A, Taylor LG, Rosso L, Turkheimer F, Brooks DJ, Grasby P, Nutt DJ. A [11C]Ro15 4513 PET study suggests that alcohol dependence in man is associated with reduced alpha5 benzodiazepine receptors in limbic regions. J Psychopharmacol. 2012;26(2):273–281. doi: 10.1177/0269881110379509. [DOI] [PubMed] [Google Scholar]
- Lingford-Hughes AR, Acton PD, Gacinovic S, Boddington SJ, Costa DC, Pilowsky LS, Ell PJ, Marshall EJ, Kerwin RW. Levels of gamma-aminobutyric acid-benzodiazepine receptors in abstinent, alcohol-dependent women: preliminary findings from an 123I–iomazenil single photon emission tomography study. Alcohol Clin Exp Res. 2000;24(9):1449–1455. [PubMed] [Google Scholar]
- Lingford-Hughes AR, Acton PD, Gacinovic S, Suckling J, Busatto GF, Boddington SJ, Bullmore E, Woodruff PW, Costa DC, Pilowsky LS, Ell PJ, Marshall EJ, Kerwin RW. Reduced levels of GABA-benzodiazepine receptor in alcohol dependency in the absence of grey matter atrophy. The British Journal of Psychiatry. 1998;173(2):116–122. doi: 10.1192/bjp.173.2.116. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243(4899):1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A. Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcoholism: Clinical and Experimental Research. 2005;29(5):896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J, Laruelle M, Abi-Dargham A. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58(10):779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Martinez D, Saccone PA, Liu F, Slifstein M, Orlowska D, Grassetti A, Cook S, Broft A, Van Heertum R, Comer SD. Deficits in dopamine D 2 receptors and presynaptic dopamine in heroin dependence: commonalities and differences with other types of addiction. Biol Psychiatry. 2012;71(3):192–198. doi: 10.1016/j.biopsych.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason GF, Krystal JH. MR spectroscopy: its potential role for drug development for the treatment of psychiatric diseases. NMR Biomed. 2006;19(6):690–701. doi: 10.1002/nbm.1080. [DOI] [PubMed] [Google Scholar]
- Mason GF, Petrakis IL, de Graaf RA, Gueorguieva R, Guidone E, Coric V, Epperson CN, Rothman DL, Krystal JH. Cortical gamma-aminobutyric acid levels and the recovery from ethanol dependence: preliminary evidence of modification by cigarette smoking. Biol Psychiatry. 2006;59(1):85–93. doi: 10.1016/j.biopsych.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Meyerhoff DJ. Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug Alcohol Depend. 2012;125(1–2):27–36. doi: 10.1016/j.drugalcdep.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris ED, Christian BT, Yoder KK, Muzic RF., Jr Estimation of local receptor density, B’max, and other parameters via multiple-injection positron emission tomography experiments. Methods Enzymol. 2004;385:184–213. doi: 10.1016/S0076-6879(04)85011-0. [DOI] [PubMed] [Google Scholar]
- Narendran R, Martinez D. Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse. 2008;62(11):851–869. doi: 10.1002/syn.20566. [DOI] [PubMed] [Google Scholar]
- Narendran R, Mason NS, Paris J, Himes ML, Douaihy AB, Frankle WG. Decreased prefrontal cortical dopamine transmission in alcoholism. Am J Psychiatry. 2014;171(8):881–888. doi: 10.1176/appi.ajp.2014.13121581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J, Tobias MC, Hudkins M, Oh EY, Hellemann GS, Nurmi EL, London ED. Thalamic glutamate decreases with cigarette smoking. Psychopharmacology (Berl) 2014;231(13):2717–2724. doi: 10.1007/s00213-014-3441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez E, Quijano-Carde N, De Biasi M. Nicotinic Mechanisms Modulate Ethanol Withdrawal and Modify Time Course and Symptoms Severity of Simultaneous Withdrawal from Alcohol and Nicotine. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinati HM, Rabinowitz AR. Choosing the right medication for the treatment of alcoholism. Curr Psychiatry Rep. 2006;8(5):383–388. doi: 10.1007/s11920-006-0040-0. [DOI] [PubMed] [Google Scholar]
- Ravan S, Martinez D, Slifstein M, Anissa AD. Molecular imaging in alcohol dependence. Handb Clin Neurol. 2014;125:293–311. doi: 10.1016/B978-0-444-62619-6.00018-5. [DOI] [PubMed] [Google Scholar]
- Reoux JP, Miller K. Routine hospital alcohol detoxification practice compared to symptom triggered management with an Objective Withdrawal Scale (CIWA-Ar) Am J Addict. 2000;9(2):135–144. doi: 10.1080/10550490050173208. [DOI] [PubMed] [Google Scholar]
- Rominger A, Cumming P, Xiong G, Koller G, Boning G, Wulff M, Zwergal A, Forster S, Reilhac A, Munk O, Soyka M, Wangler B, Bartenstein P, la Fougere C, Pogarell O. [18F]Fallypride PET measurement of striatal and extrastriatal dopamine D 2/3 receptor availability in recently abstinent alcoholics. Addict Biol. 2012;17(2):490–503. doi: 10.1111/j.1369-1600.2011.00355.x. [DOI] [PubMed] [Google Scholar]
- Rothman DL, De Feyter HM, de Graaf RA, Mason GF, Behar KL. 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR Biomed. 2011;24(8):943–957. doi: 10.1002/nbm.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H. Varenicline effects on drinking, craving and neural reward processing among non-treatment-seeking alcohol-dependent individuals. Psychopharmacology (Berl) 2014;231(18):3799–3807. doi: 10.1007/s00213-014-3518-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibyl JP, Laruelle M, van Dyck CH, Wallace E, Baldwin RM, Zoghbi S, Zea-Ponce Y, Neumeyer JL, Charney DS, Hoffer PB, Innis RB. Reproducibility of iodine-123-beta-CIT SPECT brain measurement of dopamine transporters. J Nucl Med. 1996;37(2):222–228. [PubMed] [Google Scholar]
- Sharrett-Field L, Butler TR, Reynolds AR, Berry JN, Prendergast MA. Sex differences in neuroadaptation to alcohol and withdrawal neurotoxicity. Pflugers Arch. 2013;465(5):643–654. doi: 10.1007/s00424-013-1266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89(2):649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Staley JK, Gottschalk C, Petrakis IL, Gueorguieva R, O’Malley S, Baldwin R, Jatlow P, Verhoeff NP, Perry E, Weinzimmer D, Frohlich E, Ruff E, van Dyck CH, Seibyl JP, Innis RB, Krystal JH. Cortical gamma-aminobutyric acid type A-benzodiazepine receptors in recovery from alcohol dependence: relationship to features of alcohol dependence and cigarette smoking. Arch Gen Psychiatry. 2005;62(8):877–888. doi: 10.1001/archpsyc.62.8.877. [DOI] [PubMed] [Google Scholar]
- Stokes PR, Benecke A, Myers J, Erritzoe D, Watson BJ, Kalk N, Barros DR, Hammers A, Nutt DJ, Lingford-Hughes AR. History of cigarette smoking is associated with higher limbic GABAA receptor availability. Neuroimage. 2013;69:70–77. doi: 10.1016/j.neuroimage.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Lim K, Labaree D, Lin SF, McCarthy TJ, Seibyl JP, Tamagnan G, Huang Y, Carson RE, Ding YS, Morris ED. Kinetic analysis of the metabotropic glutamate subtype 5 tracer [(18)F]FPEB in bolus and bolus-plus-constant-infusion studies in humans. J Cereb Blood Flow Metab. 2013;33(4):532–541. doi: 10.1038/jcbfm.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma R, Mullins P, Ruhl D, Monnig M, Yeo RA, Caprihan A, Bogenschutz M, Lysne P, Tonigan S, Kalyanam R, Gasparovic C. Perturbation of the glutamate-glutamine system in alcohol dependence and remission. Neuropsychopharmacology. 2011;36(7):1359–1365. doi: 10.1038/npp.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Kuikka J, Bergstrom K, Hakola P, Karhu J, Ryynanen OP, Fohr J. Altered striatal dopamine re-uptake site densities in habitually violent and non-violent alcoholics. Nat Med. 1995;1(7):654–657. doi: 10.1038/nm0795-654. [DOI] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu Rev Med. 1998;49:173–184. doi: 10.1146/annurev.med.49.1.173. [DOI] [PubMed] [Google Scholar]
- Umhau JC, Momenan R, Schwandt ML, Singley E, Lifshitz M, Doty L, Adams LJ, Vengeliene V, Spanagel R, Zhang Y, Shen J, George DT, Hommer D, Heilig M. Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: a randomized controlled experimental medicine study. Arch Gen Psychiatry. 2010;67(10):1069–1077. doi: 10.1001/archgenpsychiatry.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol Clin Exp Res. 2001;25(8):1087–1097. [PubMed] [Google Scholar]
- Volkow ND, Baler RD. BRain imaging biomarkers to predict relapse in alcohol addiction. JAMA Psychiatry. 2013;70(7):661–663. doi: 10.1001/jamapsychiatry.2013.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler J, Logan J, Gatley S, Hitzemann R, Chen A, Dewey S, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. 1997 doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20(9):1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108(37):15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Fowler JS, Jayne B, Telang F, Logan J, Ding YS, Gatley SJ, Hitzemann R, Wong C, Pappas N. Effects of alcohol detoxification on dopamine D2 receptors in alcoholics: a preliminary study. Psychiatry Res. 2002;116(3):163–172. doi: 10.1016/s0925-4927(02)00087-2. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Overall JE, Hitzemann R, Fowler JS, Pappas N, Frecska E, Piscani K. Regional brain metabolic response to lorazepam in alcoholics during early and late alcohol detoxification. Alcohol Clin Exp Res. 1997;21(7):1278–1284. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27(46):12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Chen H, Steketee JD, Sharp BM. Upregulation of ionotropic glutamate receptor subunits within specific mesocorticolimbic regions during chronic nicotine self-administration. Neuropsychopharmacology. 2007;32(1):103–109. doi: 10.1038/sj.npp.1301033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerts EM, Kim YK, Wand GS, Dannals RF, Lee JS, Frost JJ, McCaul ME. Differences in delta- and mu-opioid receptor blockade measured by positron emission tomography in naltrexone-treated recently abstinent alcohol-dependent subjects. Neuropsychopharmacology. 2008;33(3):653–665. doi: 10.1038/sj.npp.1301440. [DOI] [PubMed] [Google Scholar]
- Yeo RA, Thoma RJ, Gasparovic C, Monnig M, Harlaar N, Calhoun VD, Kalyanam R, Mayer AR, Durazzo TC, Hutchison KE. Neurometabolite concentration and clinical features of chronic alcohol use: a proton magnetic resonance spectroscopy study. Psychiatry Res. 2013;211(2):141–147. doi: 10.1016/j.pscychresns.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York JL, Hirsch JA. Drinking patterns and health status in smoking and nonsmoking alcoholics. Alcohol Clin Exp Res. 1995;19(3):666–673. doi: 10.1111/j.1530-0277.1995.tb01565.x. [DOI] [PubMed] [Google Scholar]