Human Milk Components Modulate Toll-Like Receptor–Mediated Inflammation (original) (raw)
Abstract
Toll-like receptor (TLR) signaling is central to innate immunity. Aberrant expression of TLRs is found in neonatal inflammatory diseases. Several bioactive components of human milk modulate TLR expression and signaling pathways, including soluble toll-like receptors (sTLRs), soluble cluster of differentiation (sCD) 14, glycoproteins, small peptides, and oligosaccharides. Some milk components, such as sialyl (α2,3) lactose and lacto-_N_-fucopentaose III, are reported to increase TLR signaling; under some circumstances this might contribute toward immunologic balance. Human milk on the whole is strongly anti-inflammatory, and contains abundant components that depress TLR signaling pathways: sTLR2 and sCD14 inhibit TLR2 signaling; sCD14, lactadherin, lactoferrin, and 2′-fucosyllactose attenuate TLR4 signaling; 3′-galactosyllactose inhibits TLR3 signaling, and β-defensin 2 inhibits TLR7 signaling. Feeding human milk to neonates decreases their risk of sepsis and necrotizing enterocolitis. Thus, the TLR regulatory components found in human milk hold promise as benign oral prophylactic and therapeutic treatments for the many gastrointestinal inflammatory disorders mediated by abnormal TLR signaling.
Keywords: immune function, toll like receptors, inflammation, human milk, glycans
Introduction
Human milk is a putative innate immune system
Providing milk to their infants via the mammary gland is a major evolutionary advantage for mammals to increase survival of their offspring (1). As a food, human milk nutrients are in quantities and proportions that support rapid brain, enteric, and immunologic development of term neonates. More recent research on the thousands of distinct bioactive molecules of human milk, many of them indigestible, is increasing recognition of the importance of human milk in modulating innate immune signaling to exposure by unfamiliar microbes. These bioactive components function to protect infants against infection and inflammation, and contribute to healthy microbial colonization of the neonatal intestine, immune maturation, and organ development (2). These considerations lead to the hypothesis that human milk is an innate immune system whereby breastfeeding mothers protect their offspring (3, 4) through 3 major innate immune functions: 1) the inhibition of pathogen binding, 2) prebiotic activity, and 3) immune regulation and modulation of inflammation.
Because of their structural homology to host cell surface receptors, human milk glycans inhibit the binding of pathogens to their mucosal cell surface receptors, the first crucial step of pathogenesis. Human milk, especially colostrum, contains abundant glycans that inhibit such binding by Escherichia coli, Campylobacter jejuni (5), norovirus (GII.4 and GII.10), HIV, rotovirus, and toxins, including cholera toxin, E. coli stable toxin and labile toxin, and Shiga-like toxin (6, 7).
In addition to inhibiting pathogen binding, a negative event, human milk components can stimulate intestinal colonization, a positive event. Despite the inoculum of vaginal and fecal microbiota during delivery, the numbers and diversity of microbes in the neonatal intestine are low (8). The human milk microbiome (9, 10), maternal skin, and the mother’s saliva provide additional inocula, whereas human milk oligosaccharides (HMOSs)5 selectively promote development of mutualist intestinal microbiota communities (11). HMOSs, like other prebiotics, are dietary carbohydrates that essentially are not digested and arrive intact in the distal intestine, where they selectively support growth of mutualist bacteria such as Bifidobacterium bifidum in vitro (12). The resulting beneficent community of microbes, the intestinal microbiota, communicates with the mucosa to regulate the intestinal immune system and support homeostasis (13–15). In breastfed preterm babies and in animal models (16, 17), prebiotics improve fecal quality, reduce the risk of gastrointestinal infection, and decrease the incidence of allergic symptoms (11, 18). The synergy between pathogen inhibition and prebiotic activity provides resilience to a spectrum of insults that is further reinforced by inhibition of inflammation per se.
The immature intestinal mucosa is prone to exaggerated responses to proinflammatory stimuli, increasing the risk of inflammatory pathology in the neonatal intestine (19). During pregnancy, T helper 2 responses prevent adverse immunologic reactions between the mother and her fetus (20). Thus, the immature neonatal intestine, relative to the mature intestine, contains fewer total T cells, which are skewed toward a Th2 bias (21). Innate inflammatory genes, such as nuclear transcription factor κB (NFκB), myeloid differentiation primary response 88 (MYD88), toll-like receptor (TLR) 2, TLR4, and TNF receptor–associated factors (TRAF), are overexpressed, and negative feedback regulator genes are underexpressed (19), resulting in the neonatal’s mucosa being overly sensitive to bacterial infection and food allergy (22).
Human milk contains classic innate immune effectors, such as antibodies (20, 23), cytokines (24, 25), and cells (26, 27), reducing exposure to antigens, modulating the response to these antigens, and enhancing development and maturation of the immature immune system. Moreover, human milk glycoconjugates modulate expression of immune signaling genes, repress inflammation at the mucosal surface (28), alter leukocyte function, and modulate cytokine and TLR expression in intestine epithelial cells.
Human milk components regulate TLRs
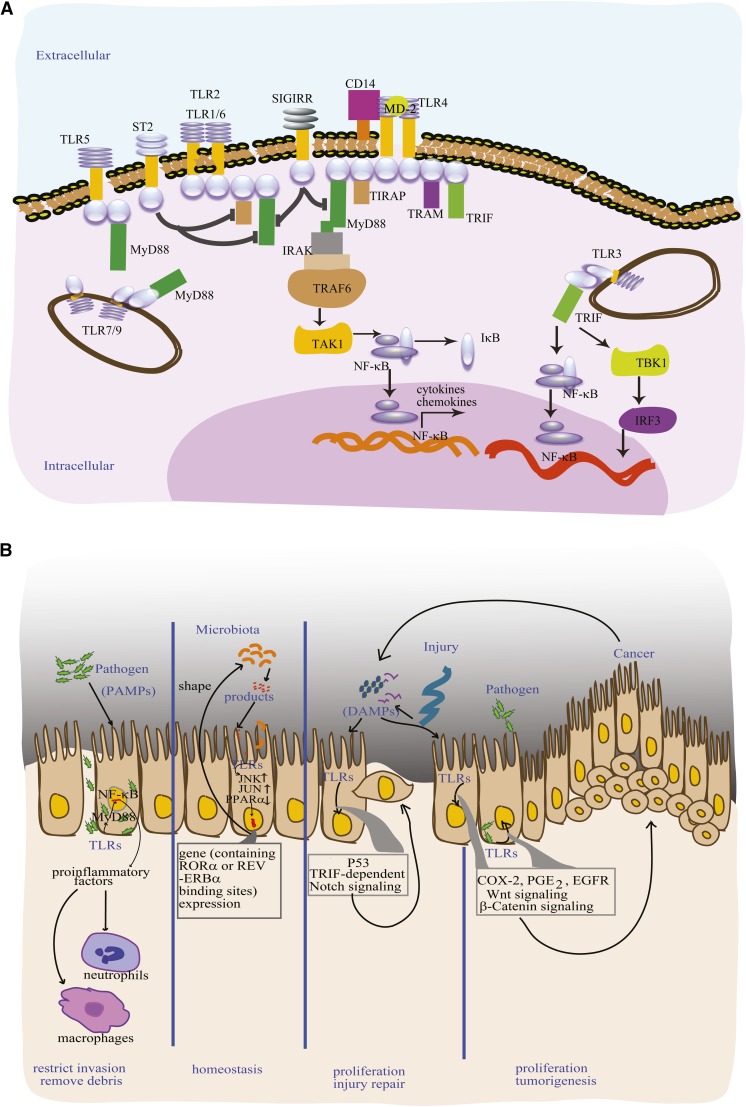
Eleven members of the TLR family are found in humans (29), and they play central roles in innate immune signaling (Figure 1A). TLRs are the major pattern recognition receptors of the innate immune system, and recognize a wide range of pathogen-associated molecular patterns (PAMPs) that are expressed by microorganisms (29, 30). After recognizing and binding a specific PAMP, each of the TLRs initiates inflammatory signaling through NF-κB. These signaling cascades result in the secretion of proinflammatory factors that recruit immune cells, neutrophils, and macrophages, eventually leading to clear pathogens in infected individuals (29) (Figure 1B). Commensal bacterial PAMPs have structures that are different from those of pathogen PAMPs; not only are they tolerated by TLRs (31–33), but TLR recognition of commensal microflora is required for intestinal homeostasis. For example, microbiota cues transduced by TLRs stimulate circadian clock expression of genes containing retinoic acid receptor–related orphan receptor α (RORA) or NR1D1 (nuclear receptor subfamily 1, group D, member 1) gene (REV-ERBα) (34–36), which, in turn, can influence microbiota colonization (Figure 1B). Also, pioneer bacterial colonization stimulates extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase signaling pathways. Amplified ERK and c-Jun N-terminal kinase results in elevation in nuclear transcription factors activating transcription factor 2 and phosphorylated c-jun (p-c-jun), respectively, which induce fucosyltransferase 2 (FUT2) gene transcription. At the intestinal mucosa surface, FUT2 gene activation leads to fucosylation of surface proteins, including TLR4; the formation of this highly fucosylated niche in the intestinal mucosa promotes further colonization by microbiota that utilize fucose (37).
FIGURE 1.
TLRs play critical roles in gastrointestinal inflammatory disorders at the mucosa. Major TLR signaling pathways (A). TLR signaling mediates major biological functions at the mucosal epithelium (B). PAMPs from pathogens bind TLRs and activate MYD88-dependent pathways, stimulate proinflammatory secretions, and recruit immune cells to the site of infection, where immune cells remove cellular debris and restrict new invasion. Through TLRs, bacteria control epithelial cell gene expression, and epithelial cell products shape the composition of the microbiota. TLRs recognize DAMPs, which are signal molecules released during cell injury and by cancer cells. DAMPs activate TLRs and the p53/TRIF–dependent notch signaling pathway, promoting the epithelial cell proliferation involved in regeneration after mucosal injury. TLR activation by PAMPs or DAMPs can activate COX-2, PGE2, EGFR, Wnt, and β-catenin signaling, promote epithelial cell proliferation, but the growth mediated by these pathways can also lead to tumorigenesis. CD, cluster of differentiation; COX-2, cyclooxygenase 2; DAMP, damage-associated molecular pattern; EGFR, epidermal growth factor receptor; IκB, inhibitor of NF-κB; IRAK, interleukin 1 receptor–associated kinase; IRF, interferon regulatory factor; MD2, lymphocyte antigen 96; MYD88, myeloid differentiation primary response 88; PAMP, pathogen-associated molecular pattern; SIGIRR, single immunoglobulin and toll–interleukin 1 receptor; ST2, iterleukin 1 receptor-like 1; TAK, TGF β activated kinase; TBK, TRAF family member-associated NF-κB activator binding kinase; TIRAP, toll-interleukin 1 receptor domain–containing adaptor protein; TLR, toll-like receptor; TRAF, tumor necrosis factor receptor–associated factor; TRAM, translocation-associated membrane protein; TRIF, TIR domain–containing adapter-inducing interferon β.
TLRs also recognize damage-associated molecular patterns (DAMPs), endogenous signals from damaged tissue. DAMP binding to TLR4 activates a p53/MYD88–independent pathway that mediates crypt proliferation, promoting wound healing (38). But if damage is excessively frequent, prolonged, or elevated, DAMP activation of distinct signaling pathways, such as TLR4 activation of the β-catenin or Wnt signaling cascade, can promote intestinal neoplasia (39, 40) (Figure 1B). Aberrant expression of TLRs is associated with risk of disease (41). TLR expression and TLR signaling pathway activation are potential therapeutic targets for treatment of inflammatory diseases (42).
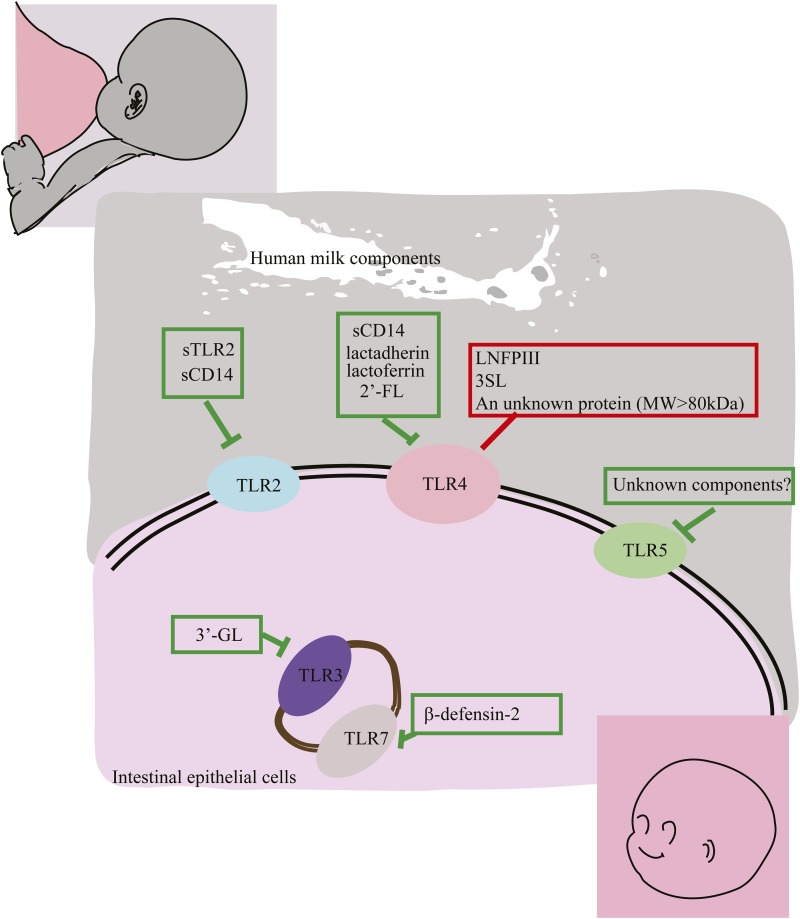
Relative to term infants or adults, preterm infants express higher concentrations of TLRs, which are associated with a higher risk of neonatal sepsis or necrotizing enterocolitis (NEC) (19, 43). The components of human milk that modulate TLR receptor expression or signaling, summarized in Table 1, show promise for suppressing the inflammation associated with neonatal sepsis and NEC. There are 2 groups of TLR modulatory human milk components. One group includes lacto-_N_-fucopentaose III (LNFP III) and sialyl (α2,3) lactose (3SL), which increase TLR signaling, possibly toward immunologic balance. Another group includes the preponderance of human milk bioactive components, such as soluble toll-like receptors (sTLRs), soluble cluster of differentiation (sCD) 14, lactadherin and lactoferrin, β-defensin 2, and several oligosaccharides, which depress TLR signaling. This quenches inflammation at the mucosal surface, contributing to the overall anti-inflammatory activity of human milk. The specific TLR signaling pathways that can be modulated by these bioactive human milk components are summarized in Figure 2.
TABLE 1.
Human milk components modulate TLR signaling1
| Molecules in human milk | Identified in milk | TLR signaling pathways modulated | References |
|---|---|---|---|
| sTLR signal pathway inhibitors | |||
| sTLR2 | Yes | Anti-inflammatory via TLR2 | (44, 45) |
| sCD14 | Yes | Anti-inflammatory via TLR2/4 | (46, 47) |
| sTLR4 | No | Anti-inflammatory via TLR4 | (48, 49) |
| sTLR5 | No | Anti-inflammatory via TLR5 | (50) |
| Glycoproteins | |||
| Lactadherin | Yes | Anti-inflammatory (TLR4) | (51–53) |
| Lactoferrin | Yes | Anti-inflammatory via TLR4 | (54, 55) |
| Unknown protein (>80 kDa) | Yes | Proinflammatory via TLR4 | (56) |
| Peptides | |||
| β-defensin 2 | Yes | Anti-inflammatory via TLR7 | (57) |
| Oligosaccharides | |||
| 3′-GL | Yes | Anti-inflammatory via TLR3 | (58) |
| 2′-GL | Yes | Anti-inflammatory via TLR4 (CD14)/STAT3/SOCS2 | (3) |
| LNFP III | Yes | Proinflammatory via TLR4/ ERK/MAPK | (59) |
| 3SL | Yes | Proinflammatory via TLR4 | (60) |
| DSLNT | Yes | Anti-inflammatory; TLR targets not known | (61) |
FIGURE 2.
Human milk components that modulate TLRs. To date, several human milk components have been identified that modulate TLRs. Human milk components that suppress TLR signaling include sTLR2 and sCD14, which dampen TLR2 signaling; sCD14, lactadherin, lactoferrin, and 2′-FL, which attenuate TLR4 signaling; 3′-GL, which suppresses TLR3 signaling; and β-defensin 2, which inhibits TLR7 signaling. Human milk depresses TLR5 signaling, but the molecule responsible remains unknown. Human milk components that simulate TLRs include LNFP III, which promotes the Th2 response; 3SL, which stimulates CD11C+ dendritic cells; and a large protein (>80 kDa) that stimulates cytokine release, all of which act through TLR4 signaling. CD, cluster of differentiation; LNFP III, lacto-_N_-fucopentaose; sCD, soluble cluster of differentiation; sTLR, soluble toll-like receptor; Th, T helper; TLR, toll-like receptor; MW, molecular weight; 2′FL, 2′-fucosyllactose; 3′GL, 3′-galactosyllactose; 3SL, sialyl (α2,3) lactose.
sTLRs and sCD14.
The responses of membrane-bound TLRs to agonist ligands can be amplified or suppressed by soluble isoforms. For example, sTLR4 from human saliva blocks the interaction between TLR4 and its coreceptors, inhibiting formation of their active complexes, thereby reducing TLR4 signaling. sTLR4 can modulate TNF-α secretion through macrophage-like cells (47). In fish, sTLR5 inhibits the acute phase reaction induced by flagellin (50). TLR2 recognizes a wide array of cell-wall microbial ligands across broad groups of species, including lipopolysaccharides from Gram-negative bacteria (62), peptidoglycan, lipoteichoic acid and lipoprotein from Gram-positive bacteria (63, 64), lipoarabinomannan from mycobacteria (65), and zymosan from yeast (66). TLR2 expression is elevated in a neonatal rat model of NEC (67, 68). Therefore, TLR2 antagonists may suppress inflammation.
Human milk sTLR2 occurs in 6 isoforms from 20–85 kDa (44). In vitro, human milk sTLR2 is capable of modulating TLR2 signaling activation by bacterial lipopeptide (69). The major function of sTLR2 is for its ectodomain to act as a decoy receptor, suppressing TLR2 activation, thereby decreasing IL-8 and TNF-α release (45). sTLR2 reduces inflammation by disrupting TLR2 triggering without compromising bacterial clearance (69).
TLR2 and CD 14 can physically interact and are sometimes coexpressed. CD14 has 2 forms: membrane CD14 and sCD14. sCD14 (molecular weight 48 kDa) was detected in human milk at a concentration of 53 ± 24 μg/mL, 10-fold higher than that in plasma (46). Milk sCD14 mediates microbial recognition in the neonatal intestine (46). However, in tissues distant from the site of inflammation, circulating sCD14 exhibits anti-inflammatory effects (46). The overall role of human milk sCD14 in infant homeostasis remains an enigma.
Glycoproteins
Human milk contains glycoproteins that can regulate TLR4 signaling. Several PAMPs stimulate TLR4, including lipopolysaccharide, the fusion protein of respiratory syncytial virus, and the envelope protein from mouse mammary tumor virus (70). TLR4 binding to lipopolysaccharide, a structural protein of the Gram-negative bacterial cell wall, initiates the MYD88-dependent signaling pathway, leading to activation of NF-κB and proinflammatory secretions. Excessive signaling from lipopolysaccharide activation can induce systemic inflammation and sepsis (71). Increased TLR4 expression was detected in a neonatal rat NEC model (67, 68) and tissues from NEC patients (72).
Lactadherin, a 46 kDa mucin-associated glycoprotein of the milk fat globule membrane, binds specifically to rotavirus, inhibits rotavirus replication, and protects against symptoms of rotavirus infection (73). The intrinsic endogenous activities of lactadherin include promotion of mucosal wound healing and attenuation of intestinal inflammation in vivo and in vitro (51, 52, 74, 75). Lactadherin induces IL-10 and TGF-β release from regulatory T cells and promotes intestinal dendritic cell development (51). Lactadherin enhances the ability of macrophages to phagocytize apoptotic cells, thereby ameliorating inflammatory process induced by NF-κB and mitogen-activated protein kinase (53). Moreover, lactadherin activates the signal transducer and activator of transcription 3–suppressor of cytokine signaling 3 pathways, directly quenching lipopolysaccharide-induced TNF-α production (51–53).
Lactoferrin (molecular weight ∼75–90 kDa) is a major glycoprotein in human milk, and it is found at its highest concentrations (5–6.7 g/L) in colostrum (76). Lactoferrin exhibits many disparate activities, including iron binding, antibacterial and antiparasitic activity, and stimulating proliferation and differentiation of intestinal epithelial cells (48, 77, 78). Lactoferrin, by virtue of its glycans, is prebiotic, promoting colonization by beneficial bacteria, thereby limiting pathogen colonization of the intestinal tract (79, 80). Lactoferrin strongly inhibits inflammatory cytokine production at local sites of inflammation of the gastrointestinal tract (81). In vivo, lactoferrin protects against intestinal infection and inflammation (82). In very-low-birth-weight (VLBW) and extremely-low-birth-weight (ELBW) preterm infants, oral administration of lactoferrin reduces the risk of late-onset sepsis (83). Although treatment with lactoferrin in vitro can activate TRAF 6–dependent NF-κB, a TLR4 proinflammatory signaling pathway, lactoferrin simultaneously inhibits the ability of lipopolysaccharide binding protein to adhere to TLR4; the net result is that lactoferrin inhibits lipopolysaccharide-stimulated TLR4 signaling and depresses endotoxemia (54, 55).
Among the many glycoproteins of human milk are also an unidentified component of molecular weight >80 kDa that, in vitro, elevates concentrations of IL-8, IL-6, and TNF-α in a TLR4-dependent manner (56). That notwithstanding, the overall activity of human milk is strongly anti-inflammatory.
β-defensin 2.
Human milk contains small bioactive peptides, including defensins; of these, β-defensin 2 affects TLR signaling. β-defensin 2 is present at ∼8.5 μg/mL in colostrum and ∼1 μg/mL in mature milk, and it displays broad antimicrobial activity against pathogenic bacteria (84). This suggests that the presence of β-defensin 2 in milk may help defend both the mammary gland and the infant intestine. The β-defensin 2 supplied by milk can be augmented by β-defensin 2 from the mucosa, and mucosal release of β-defensin 2 can be further elicited by human milk hyaluronan. β-defensin 2 enhances TLR4/CD44–dependent intestinal epithelial defense against pathogens (85). For example, low concentrations of β-defensin 2 are associated with lower TLR4/lymphocyte antigen 96 concentrations and more severe NEC (86). In addition, human milk β-defensin 2 suppresses TLR7 expression in breast and colon epithelial cells (57). TLR7, expressed in endosomes, is stimulated by single-stranded RNA, and during a virus infection TLR7 mediates signaling that leads to release of IFN, the innate immune response to viral attack (87). High TLR7 expression occurs in a rat NEC-like model (68). The ability of β-defensin 2 to inhibit TLR7 leads to the hypothesis that β-defensin 2 in milk may contribute to a decrease in the long-term risk of gastrointestinal inflammatory diseases in the breastfed infant and a reduced risk of breast cancer in mothers who had breastfed (57).
HMOSs.
HMOSs, a heterogeneous mixture of complex carbohydrate structures appended to a lactose or a polylactosamine backbone, are the third most abundant solid component of human milk (6). The HMOSs are composed of at least 200 individual oligosaccharides and exhibit the biological activities of human milk. HMOS preparations stimulate immune-modulatory activity on the neonatal intestinal mucosal surface (88–91) and modulate cytokine production (58, 92–95). Colostrum HMOSs modulate TLR3-, TLR5-, and IL-1β–dependent PAMP signaling pathways, depressing acute phase inflammatory cytokine protein expression and elevating cytokines involved in tissue repair and homeostasis (58). One oligosaccharide found in especially high concentrations in colostrum compared with mature milk, 3′-galactosyllactose, specifically quenches polyinosine-polycytidylic acid (ligand for TLR3)–induced IL-8 concentrations (58).
2′-Fucosyllactose, representing ∼30% of total HMOSs from most human milk, inhibits binding and infection of several distinct enteropathogens. Many human pathobionts bind to human mucosal surface receptors that terminate in α1,2-linked fucose as their essential first step of pathogenesis; 2′-fucosyllactose competitively inhibits this binding and protects against infection (5). Moreover, 2′-fucosyllactose depresses CD14 mRNA levels and reduces membrane-bound CD14, a coreceptor of lipopolysaccharide. This attenuates lipopolysaccharide-induced inflammation during infection by type I pili E. coli, including adherent and invasive E. coli, enterotoxigenic E. coli, and uropathogenic E. coli (3).
LNFP III is an oligosaccharide that contains the Lewis X epitope. LNFP III promotes the recruitment of suppressor macrophages and maturation of the dendritic cell 2 phenotype, releasing IL-4 and IFN-γ, which foster Th2 responses (59). The immune-modulatory activities of LNFP III that are mediated through TLR4 signaling include the activation of ERK and mitogen-activated protein kinase signaling pathways (59). LNFP III binds the dendritic cell–specific intercellular adhesion molecule 3–grabbing nonintegrin and inhibits HIV-1 transfer to CD4+ T lymphocytes (96).
In contradistinction to most other HMOSs, 3SL is reported to exhibit proinflammatory characteristics (60). 3SL stimulation of mesenteric lymph node CD11c+ dendritic cells causes the release of cytokines that expand Th1 and Th17 T cell populations. This modulation by 3SL is mediated through TLR4 signaling (60).
Other individual HMOSs that suppress inflammation of the intestinal mucosa have been identified. For example, disialyllacto-_N_-tetraose suppresses NEC-like inflammation in neonatal rats (61), but whether the mechanism involves TLRs is not known. Overall, the biological activities reported for HMOSs are predominantly the attenuation of inflammatory signaling and processes.
Other milk components.
In some investigations, human milk decreased TLR2 and TLR3 signaling but increased TLR4 and TLR5 signaling. The components responsible for these activities and their mechanisms of TLR modulation remain unknown (56). Isolation of the active components and mechanistic studies that follow from the availability of pure effector molecules may allow additional TLR modulators to be identified from human milk. Molecules of human milk whose oral consumption modulates intestinal inflammatory disorders have strong potential to yield clinically useful prophylactic and therapeutic agents.
Translating Findings on TLR Regulators into Treatments for Neonatal Inflammatory Diseases
NEC
In VLBW (<1500 g) and ELBW (<1000 g) preterm infants, NEC and sepsis are the 2 most prevalent gastrointestinal medical emergencies. The incidence of NEC was 7%, and the mortality rate of infected neonates was as high as 20–30% (97). Preterm infants fed donor human milk have a 58% lower incidence of NEC relative to those fed a cow milk–based formula (98); likewise, those receiving their mothers’ own milk exhibited a 77% lower risk of NEC relative to those fed formula (99). The likelihood of NEC decreased by a factor of 0.83 for each 10% increase in the proportion of total intake as human milk (100). In a cohort of 1272 ELBW infants, the feeding of human milk was associated with a reduction in the risk of NEC or death within the first 2 wk of life in a dose-dependent manner (100). A meta-analysis from 4 small clinical trials revealed that infants receiving human milk have one-quarter of the risk of developing clinically confirmed NEC and one-third of the risk of developing any NEC as infants fed formula (101).
Consensus on the underlying etiology of NEC has not yet been achieved. A prevalent hypothesis for NEC pathogenesis is that abnormal intestinal colonization provokes an inappropriately heightened inflammatory response in the immature intestinal epithelium (58). Increased concentrations of proinflammatory cytokines are found in intestinal samples from NEC patients (102), suggesting that they play an important role in the pathobiology of NEC. Elevated TLR activation results in increased concentrations of cytokines in infants (19). An experimental rat model of NEC overexpresses the majority of TLRs in the ileum: TLR2 and TLR4 concentrations were highly upregulated; TLRs 1, 3, 7, and 9 were moderately elevated; TLR6 was slightly elevated; and TLR5 expression was suppressed (67, 68). Other data from human, rat, and murine tissues also support the association between TLR expression and the onset of NEC (72, 103, 104). Among the most common bacterial PAMPs observed in NEC patients are lipopolysaccharides, the primary ligand of TLR4 (44). Thus, the role of TLR4 in NEC is under intense investigation (19, 47, 63, 67, 103–109). TLR4 expression in the rat intestinal mucosa increases during hypoxia and exposure to lipopolysaccharide (67, 72), whereas in mice, TLR4 expression increases, but that of TLR9 decreases (104). Mice deficient in TLR4 expression have, along with a reduced proinflammatory response, a lower propensity toward NEC (105). In view of these data, TLR4 has been proposed as a primary target for NEC treatment (110).
Human milk components known to regulate TLR4 include lactoferrin (Figure 2). Because lactoferrin also exhibits many other protective functions at the mucosal surface, it is a major candidate for NEC therapeutics, and it and its derivatives have proceeded to clinical trials (111). For example, after wide testing in vitro and in vivo, talactoferrin, a human recombinant lactoferrin, was granted investigational new drug status from the FDA (112). Clinical trials have been undertaken in VLBW Turkish neonates to test the efficacy of bovine lactoferrin for the prevention of NEC (111).
Neonatal sepsis.
Another major cause of neonatal death is sepsis, a systemic infection that causes tissue injury and inflammation. Neonatal sepsis is classified into 2 types: early- and late-onset. Early-onset sepsis, mostly caused by the maternal intrapartum transmission of invasive organisms, initiates at <3 d of life and has an incidence of ∼0.77 cases per 1000 live births in North America, with a mortality rate of 24% (112). Late-onset sepsis initiates at >3 d of life, with VLBW premature infants being at highest risk, and has a death rate of ∼36% (113). The high risk of sepsis in the neonate is commonly attributed to its hyperinflammatory immature immune system, in combination with abnormal colonization by pathogens: group B streptococcus and E. coli are more associated with early-onset sepsis (114), and coagulase-negative staphylococci, Gram-negative bacilli and fungi are more associated with late-onset sepsis (115). As the number of multidrug resistant Gram-negative microorganisms increase in neonatal intensive care units (116), treating neonatal sepsis with broad-spectrum antibiotics is becoming problematic, and novel drugs are needed. Drugs that would target TLR signaling seem especially promising as a potential new generation of therapies against neonatal sepsis.
Targeting TLR signaling follows from the postulate that exaggerated proinflammatory signal pathway activation by TLR recognition of PAMPs is central to the pathogenesis of neonatal sepsis. Polymorphisms in TLR2 and TLR5 are associated with preterm sepsis (115). Blood phagocytic cells in neonates that have already initiated neonatal sepsis express elevated concentrations of TLR2 (117). Twenty-four hours after sepsis was induced in mice by cecal ligation and puncture, TLR2 and TLR4 gene transcription and elevated TLR4 protein translation were apparent in the liver, lung and spleen, and the degree of expression was correlated with mortality (118). Mice with lethal sepsis by Gram-negative bacteria are protected by anti-TLR4 antibodies, illustrating that TLRs could be promising therapeutic targets (119). Initiation of breastfeeding within 1 h after birth reduces the incidence of sepsis in neonates at high risk (120). Bioactive milk components that are under investigation include bovine lactoferrin. The multiple activities of lactoferrin includes repression of TLR4 signaling, and lactoferrin administration reduces the risk of the first episode of culture-proven late-onset sepsis in low-birth-weight neonates from 13% to 3% (121). Thus, lactoferrin and other human milk components known to modulate TLR expression or activity show promise as orally administered benign agents for prophylaxis and therapy against inflammatory conditions of neonates.
Conclusions
TLR signaling is central to innate immunity, and neonatal inflammatory diseases involve the loss of TLR signaling homeostatic control. Human milk contains components, including sTLRs, sCD14, glycoproteins, small peptides, and oligosaccharides, that modulate the immune system and suppress inflammation. In contradistinction, 3SL, LNFP III, and a glycoprotein of >80 kDa increase TLR signaling, but these could contribute toward balancing complex signaling networks of the innate immune system. Beyond initiating inflammatory processes through NF-κB cascades, TLRs also function in mucosal homeostasis and inflammation through the activation of innate and adaptive immunity, promotion of cell proliferation, maintenance of the mucosal intestinal epithelial barrier, and coordination of mucosal homeostasis. Human milk on the whole is strongly anti-inflammatory. sTLR2 and sCD14 inhibit TLR2 signaling; sCD14, lactadherin, lactoferrin, and 2′-fucosyllactose inhibit TLR4 signaling; 3′-galactosyllactose inhibits TLR3 signaling, and β-defensin 2 inhibits TLR7 signaling, thereby quenching inflammation at the mucosal surface. Feeding human milk to neonates decreases their risk of sepsis and NEC. TLR regulatory components in human milk hold promise as benign oral prophylactic and therapeutic treatments for the many gastrointestinal inflammatory disorders mediated by abnormal TLR signaling. Their molecular characterization and synthesis will allow preclinical and clinical studies to test their efficacy for the prevention or amelioration of NEC, neonatal sepsis, inflammatory bowel diseases, and other gastrointestinal inflammatory disorders of diverse etiologies.
Acknowledgments
We thank Dr. David R Hill and Paul McCabe for their assistance in editing the manuscript. All authors read and approved the final manuscript.
Footnotes
5
Abbreviations used: DAMP, damage-associated molecular pattern; ELBW, extremely-low-birth-weight; ERK, extracellular signal-regulated kinase; FUT2, fucosyltransferase 2; HMOS, human milk oligosaccharide; LNFP III, lacto-_N_-fucopentaose III; MYD88, myeloid differentiation primary response 88; NEC, necrotizing enterocolitis; NFκB, nuclear transcription factor κB; PAMP, pathogen-associated molecular pattern; p-c-jun, phosphorylated c-jun; REV-ERBα, NR1D1 (nuclear receptor subfamily 1, group D, member 1) gene; RORA, retinoic acid receptor–related orphan receptor α sCD, soluble cluster of differentiation; sTLR, soluble toll-like receptor; TLR, toll-like receptor; TRAF, TNF receptor–associated factors; VLBW, very-low-birth-weight; 3SL, sialyl (α2,3) lactose.
References
- 1.Newburg DS. Glycobiology of human milk. Biochemistry 2013;78:771–85. [DOI] [PubMed] [Google Scholar]
- 2.Ballard O, Morrow AL. Human milk composition: Nutrients and bioactive factors. Pediatr Clin North Am 2013;60:49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He Y, Liu S, Kling DE, Leone S, Lawlor NT, Huang Y, Feinberg SB, Hill DR, Newburg DS. The human milk oligosaccharide 2′-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut 2014;gutjnl-2014. [DOI] [PubMed] [Google Scholar]
- 4.Newburg DS, Grave G. Recent advances in human milk glycobiology. Pediatr Res 2014;75:675–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruiz-Palacios GM, Cervantes LE, Ramos P, Chavez-Munguia B, Newburg DS. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J Biol Chem 2003;278:14112–20. [DOI] [PubMed] [Google Scholar]
- 6.Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr 2005;25:37–58. [DOI] [PubMed] [Google Scholar]
- 7.Liu B, Newburg DS. Human milk glycoproteins protect infants against human pathogens. Breastfeed Med 2013;8:354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newburg DS, Morelli L. Human milk and infant intestinal mucosal glycans guide succession of the neonatal intestinal microbiota. Pediatr Res 2015;77:115–20. [DOI] [PubMed] [Google Scholar]
- 9.Bode L, McGuire M, Rodriguez JM, Geddes DT, Hassiotou F, Hartmann PE, McGuire MK. It’s alive: Microbes and cells in human milk and their potential benefits to mother and infant. Adv Nutr 2014;5:571–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt KM, Foster JA, Forney LJ, Schutte UM, Beck DL, Abdo Z, Fox LK, Williams JE, McGuire MK, McGuire MA. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS One 2011;6:e21313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sela DA, Mills DA. Nursing our microbiota: molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol 2010;18:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu ZT, Chen C, Kling DE, Liu B, McCoy JM, Merighi M, Heidtman M, Newburg DS. The principal fucosylated oligosaccharides of human milk exhibit prebiotic properties on cultured infant microbiota. Glycobiology 2013;23:169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012;489:231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cebra JJ. Influences of microbiota on intestinal immune system development. Am J Clin Nutr 1999;69:1046S–51S. [DOI] [PubMed] [Google Scholar]
- 15.Nanthakumar NN, Dai D, Newburg DS, Walker WA. The role of indigenous microflora in the development of murine intestinal fucosyl- and sialyltransferases. FASEB J 2003;17:44–6. [DOI] [PubMed] [Google Scholar]
- 16.Shen Q, Tuohy KM, Gibson GR, Ward RE. In vitro measurement of the impact of human milk oligosaccharides on the faecal microbiota of weaned formula-fed infants compared to a mixture of prebiotic fructooligosaccharides and galactooligosaccharides. Lett Appl Microbiol 2011;52:337–43. [DOI] [PubMed] [Google Scholar]
- 17.Weiss GA, Chassard C, Hennet T. Selective proliferation of intestinal Barnesiella under fucosyllactose supplementation in mice. Br J Nutr 2014;111:1602–10. [DOI] [PubMed] [Google Scholar]
- 18.Rinne M, Kalliomaki M, Arvilommi H, Salminen S, Isolauri E. Effect of probiotics and breastfeeding on the bifidobacterium and lactobacillus/enterococcus microbiota and humoral immune responses. J Pediatr 2005;147:186–91. [DOI] [PubMed] [Google Scholar]
- 19.Nanthakumar N, Meng D, Goldstein AM, Zhu W, Lu L, Uauy R, Llanos A, Claud EC, Walker WA. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. PLoS One 2011;6:e17776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol 2007;7:379–90. [DOI] [PubMed] [Google Scholar]
- 21.Kuo S, El Guindy A, Panwala CM, Hagan PM, Camerini V. Differential appearance of T cell subsets in the large and small intestine of neonatal mice. Pediatr Res 2001;49:543–51. [DOI] [PubMed] [Google Scholar]
- 22.Abrahamsson TR, Sandberg Abelius M, Forsberg A, Bjorksten B, Jenmalm MCA. Th1/Th2-associated chemokine imbalance during infancy in children developing eczema, wheeze and sensitization. Clin Exp Allergy 2011;41:1729–39. [DOI] [PubMed] [Google Scholar]
- 23.Thomas JE, Austin S, Dale A, McClean P, Harding M, Coward WA, Weaver LT. Protection by human milk IgA against Helicobacter pylori infection in infancy. Lancet 1993;342:121. [DOI] [PubMed] [Google Scholar]
- 24.Garofalo R. Cytokines in human milk. J Pediatr 2010;156:S36–40. [DOI] [PubMed] [Google Scholar]
- 25.Kverka M, Burianova J, Lodinova-Zadnikova R, Kocourkova I, Cinova J, Tuckova L, Tlaskalova-Hogenova H. Cytokine profiling in human colostrum and milk by protein array. Clin Chem 2007;53:955–62. [DOI] [PubMed] [Google Scholar]
- 26.Speer CP, Schatz R, Gahr M. [Function of breast milk macrophages] Monatsschr Kinderheilkd 1985;133:913–7. [PubMed] [Google Scholar]
- 27.Ichikawa M, Sugita M, Takahashi M, Satomi M, Takeshita T, Araki T, Takahashi H. Breast milk macrophages spontaneously produce granulocyte-macrophage colony-stimulating factor and differentiate into dendritic cells in the presence of exogenous interleukin-4 alone. Immunology 2003;108:189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buescher ES. Anti-inflammatory characteristics of human milk: How, where, why. Adv Exp Med Biol 2001;501:207–22. [DOI] [PubMed] [Google Scholar]
- 29.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol 2004;4:499–511. [DOI] [PubMed] [Google Scholar]
- 30.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol 2001;1:135–45. [DOI] [PubMed] [Google Scholar]
- 31.Serino L, Virji M. Phosphorylcholine decoration of lipopolysaccharide differentiates commensal Neisseriae from pathogenic strains: Identification of licA-type genes in commensal Neisseriae. Mol Microbiol 2000;35:1550–9. [DOI] [PubMed] [Google Scholar]
- 32.Lebeer S, Vanderleyden J, De Keersmaecker SC. Host interactions of probiotic bacterial surface molecules: Comparison with commensals and pathogens. Nat Rev Microbiol 2010;8:171–84. [DOI] [PubMed] [Google Scholar]
- 33.Cario E, Podolsky DK. Intestinal epithelial TOLLerance versus inTOLLerance of commensals. Mol Immunol 2005;42:887–93. [DOI] [PubMed] [Google Scholar]
- 34.Bordon Y. Mucosal immunology: TLRs get rhythm. Nat Rev Immunol 2013;13:392. [DOI] [PubMed] [Google Scholar]
- 35.Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell 2013;153:812–27. [DOI] [PubMed] [Google Scholar]
- 36.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004;118:229–41. [DOI] [PubMed] [Google Scholar]
- 37.Nanthakumar NN, Meng D, Newburg DS. Glucocorticoids and microbiota regulate ontogeny of intestinal fucosyltransferase 2 requisite for gut homeostasis. Glycobiology 2013;23:1131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neal MD, Sodhi CP, Jia H, Dyer M, Egan CE, Yazji I, Good M, Afrazi A, Marino R, Slagle D, et al. Toll-like receptor 4 is expressed on intestinal stem cells and regulates their proliferation and apoptosis via the p53 up-regulated modulator of apoptosis. J Biol Chem 2012;287:37296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santaolalla R, Sussman DA, Ruiz JR, Davies JM, Pastorini C, Espana CL, et al. TLR4 activates the beta-catenin pathway to cause intestinal neoplasia. PLoS One 2013;8:e63298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gersemann M, Wehkamp J, Stange EF. Innate immune dysfunction in inflammatory bowel disease. J Intern Med 2012;271:421–8. [DOI] [PubMed] [Google Scholar]
- 41.Cario E. Toll-like receptors in inflammatory bowel diseases: A decade later. Inflamm Bowel Dis 2010;16:1583–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hennessy EJ, Parker AE, O’Neill LA. Targeting Toll-like receptors: Emerging therapeutics? Nat Rev Drug Discov 2010;9:293–307. [DOI] [PubMed] [Google Scholar]
- 43.Glaser K, Speer CP. Toll-like receptor signaling in neonatal sepsis and inflammation: A matter of orchestration and conditioning. Expert Rev Clin Immunol 2013;9:1239–52. [DOI] [PubMed] [Google Scholar]
- 44.LeBouder E, Rey-Nores JE, Rushmere NK, Grigorov M, Lawn SD, Affolter M, Griffin GE, Ferrara P, Schiffrin EJ, Morgan BP, et al. Soluble forms of Toll-like receptor (TLR)2 capable of modulating TLR2 signaling are present in human plasma and breast milk. J Immunol 2003;171:6680–9. [DOI] [PubMed] [Google Scholar]
- 45.Langjahr P, Díaz-Jiménez D, De la Fuente M, Rubio E, Golenbock D, Bronfman FC, et al. Metalloproteinase-dependent TLR2 ectodomain shedding is involved in soluble toll-like receptor 2 (sTLR2) Production. PLoS One 2014;9:e104624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Labéta MO, Vidal K, Nores JE, Arias M, Vita N, Morgan BP, Guillemot JC, Loyaux D, Ferrara P, Schmid D, et al. Innate recognition of bacteria in human milk is mediated by a milk-derived highly expressed pattern recognition receptor, soluble CD14. J Exp Med 2000;191:1807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kitchens RL, Thompson PA. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J Endotoxin Res 2005;11:225–9. [DOI] [PubMed] [Google Scholar]
- 48.Zunt SL, Burton LV, Goldblatt LI, Dobbins EE, Srinivasan M. Soluble forms of toll-like receptor 4 are present in human saliva and modulate tumour necrosis factor-alpha secretion by macrophage-like cells. Clin Exp Immunol 2009;156:285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adlerova L, Bartskova A, Raldyna M. Lactoferrin: A review. Vet Med (Praha) 2008;9:457–65. [Google Scholar]
- 50.Tsukada H, Fukui A, Tsujita T, Matsumoto M, Iida T, Seya T. Fish soluble toll-like receptor 5 (TLR5S) is an acute-phase protein with integral flagellin-recognition activity. Int J Mol Med 2005;15:519–25. [PubMed] [Google Scholar]
- 51.Kusunoki R, Ishihara S, Aziz M, Oka A, Tada Y, Kinoshita Y. Roles of milk fat globule-epidermal growth factor 8 in intestinal inflammation. Digestion 2012;85:103–7. [DOI] [PubMed] [Google Scholar]
- 52.Chogle A, Bu HF, Wang X, Brown JB, Chou PM, Tan XD. Milk fat globule-EGF factor 8 is a critical protein for healing of dextran sodium sulfate-induced acute colitis in mice. Mol Med 2011;17:502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aziz M, Jacob A, Matsuda A, Wang P. Review: Milk fat globule-EGF factor 8 expression, function and plausible signal transduction in resolving inflammation. Apoptosis 2011;16:1077–86. [DOI] [PubMed] [Google Scholar]
- 54.Ando K, Hasegawa K, Shindo K, Furusawa T, Fujino T, Kikugawa K, Nakano H, Takeuchi O, Akira S, Akiyama T, et al. Human lactoferrin activates NF-kappaB through the toll-like receptor 4 pathway while it interferes with the lipopolysaccharide-stimulated TLR4 signaling. FEBS J 2010;277:2051–66. [DOI] [PubMed] [Google Scholar]
- 55.Legrand D, Elass E, Carpentier M, Mazurier J. Lactoferrin: A modulator of immune and inflammatory responses. Cell Mol Life Sci 2005;62:2549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.LeBouder E, Rey-Nores JE, Raby AC, Affolter M, Vidal K, Thornton CA, Labéta MO. Modulation of neonatal microbial recognition: TLR-mediated innate immune responses are specifically and differentially modulated by human milk. J Immunol 2006;176:3742–52. [DOI] [PubMed] [Google Scholar]
- 57.Stroinigg N, Srivastava MD. Modulation of toll-like receptor 7 and LL-37 expression in colon and breast epithelial cells by human beta-defensin-2. Allergy Asthma Proc 2005;26:299–309. [PubMed] [Google Scholar]
- 58.Chen AC, Chung MY, Chang JH, Lin HC. Pathogenesis implication for necrotizing enterocolitis prevention in preterm very-low-birth-weight infants. J Pediatr Gastroenterol Nutr 2014;58:7–11. [DOI] [PubMed] [Google Scholar]
- 59.Thomas PG, Carter MR, Atochina O, Da’Dara AA, Piskorska D, McGuire E, Harn DA. Maturation of dendritic cell 2 phenotype by a helminth glycan uses a toll-like receptor 4-dependent mechanism. J Immunol 2003;171:5837–41. [DOI] [PubMed] [Google Scholar]
- 60.Kurakevich E, Hennet T, Hausmann M, Rogler G, Borsig L. Milk oligosaccharide sialyl(alpha2,3)lactose activates intestinal CD11c+ cells through TLR4. Proc Natl Acad Sci USA 2013;110:17444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jantscher-Krenn E, Zherebtsov M, Nissan C, Goth K, Guner YS, Naidu N, Choudhury B, Grishin AV, Ford HR, Bode L. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut 2012;61:1417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang RB, Mark MR, Gray A, Huang A, Xie MH, Zhang M, Goddard A, Wood WI, Gurney AL, Godowski PJ. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature 1998;395:284–8. [DOI] [PubMed] [Google Scholar]
- 63.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem 1999;274:17406–9. [DOI] [PubMed] [Google Scholar]
- 64.Hashimoto M, Tawaratsumida K, Kariya H, Aoyama K, Tamura T, Suda Y. Lipoprotein is a predominant toll-like receptor 2 ligand in Staphylococcus aureus cell wall components. Int Immunol 2006;18:355–62. [DOI] [PubMed] [Google Scholar]
- 65.Tapping RI, Tobias PS. Mycobacterial lipoarabinomannan mediates physical interactions between TLR1 and TLR2 to induce signaling. J Endotoxin Res 2003;9:264–8. [DOI] [PubMed] [Google Scholar]
- 66.Sato M, Sano H, Iwaki D, Kudo K, Konishi M, Takahashi H, Takahashi T, Imaizumi H, Asai Y, Kuroki Y. Direct binding of toll-like receptor 2 to zymosan, and zymosan-induced NF-kappa B activation and TNF-alpha secretion are down-regulated by lung collectin surfactant protein A. J Immunol 2003;171:417–25. [DOI] [PubMed] [Google Scholar]
- 67.Le Mandat Schultz A, Bonnard A, Barreau F, Aigrain Y, Pierre-Louis C, Berrebi D, Peuchmaur M. Expression of TLR-2, TLR-4, NOD2 and pNF-kappaB in a neonatal rat model of necrotizing enterocolitis. PLoS One 2007;2:e1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y, Zhu L, Fatheree NY, Liu X, Pacheco SE, Tatevian N, Rhoads JM. Changes in intestinal toll-like receptors and cytokines precede histological injury in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 2009;297:G442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raby AC, Le Bouder E, Colmont C, Davies J, Richards P, Coles B, George CH, Jones SA, Brennan P, Topley N, et al. Soluble TLR2 reduces inflammation without compromising bacterial clearance by disrupting TLR2 triggering. J Immunol 2009;183:506–17. [DOI] [PubMed] [Google Scholar]
- 70.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine 2008;42:145–51. [DOI] [PubMed] [Google Scholar]
- 71.Beutler B, Rietschel ET. Innate immune sensing and its roots: The story of endotoxin. Nat Rev Immunol 2003;3:169–76. [DOI] [PubMed] [Google Scholar]
- 72.Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol 2007;179:4808–20. [DOI] [PubMed] [Google Scholar]
- 73.Newburg DS, Peterson JA, Ruiz-Palacios GM, Matson DO, Morrow AL, Shults J, Guerrero ML, Chaturvedi P, Newburg SO, Scallan CD, et al. Role of human-milk lactadherin in protection against symptomatic rotavirus infection. Lancet 1998;351:1160–4. [DOI] [PubMed] [Google Scholar]
- 74.Garcia C, Duan RD, Brevaut-Malaty V, Gire C, Millet V, Simeoni U, Bernard M, Armand M. Bioactive compounds in human milk and intestinal health and maturity in preterm newborn: An overview. Cell Mol Biol 2013;59:108–31. [PubMed] [Google Scholar]
- 75.Zhou YJ, Gao J, Yang HM, Yuan XL, Chen TX, He ZJ. The role of the lactadherin in promoting intestinal DCs development in vivo and vitro. Clin Dev Immunol 2010;2010:357541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Queiroz VA, Assis AM, and R Júnior Hda C. Protective effect of human lactoferrin in the gastrointestinal tract. Rev Paul Pediatr 2013;31:90–5. [DOI] [PubMed] [Google Scholar]
- 77.Brock JH. Lactoferrin in human milk: Its role in iron absorption and protection against enteric infection in the newborn infant. Arch Dis Child 1980;55:417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res 2007;61:2–8. [DOI] [PubMed] [Google Scholar]
- 79.Arnold RR, Brewer M, Gauthier JJ. Bactericidal activity of human lactoferrin: Sensitivity of a variety of microorganisms. Infect Immun 1980;28:893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Petschow BW, Talbott RD, Batema RP. Ability of lactoferrin to promote the growth of Bifidobacterium spp. in vitro is independent of receptor binding capacity and iron saturation level. J Med Microbiol 1999;48:541–9. [DOI] [PubMed] [Google Scholar]
- 81.Håversen L, Ohlsson BG, Hahn-Zoric M, Hanson LA, Mattsby-Baltzer I. Lactoferrin down-regulates the LPS-induced cytokine production in monocytic cells via NF-kappa B. Cell Immunol 2002;220:83–95. [DOI] [PubMed] [Google Scholar]
- 82.Venkatesh MP, Abrams SA. Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2010;5:CD007137. [DOI] [PubMed] [Google Scholar]
- 83.Pammi M, Abrams SA. Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2015;2:CD007137. [DOI] [PubMed] [Google Scholar]
- 84.Baricelli J, Rocafull MA, Vazquez D, Bastidas B, Baez-Ramirez E, Thomas LE. β-defensin-2 in breast milk displays a broad antimicrobial activity against pathogenic bacteria. J Pediatr (Rio J) 2015;91:36–43. [DOI] [PubMed] [Google Scholar]
- 85.Hill DR, Rho HK, Kessler SP, Amin R, Homer CR, McDonald C, Cowman MK, de la Motte CA. Human milk hyaluronan enhances innate defense of the intestinal epithelium. J Biol Chem 2013;288:29090–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jenke AC, Zilbauer M, Postberg J, Wirth S. Human beta-defensin 2 expression in ELBW infants with severe necrotizing enterocolitis. Pediatr Res 2012;72:513–20. [DOI] [PubMed] [Google Scholar]
- 87.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 2004;303:1529–31. [DOI] [PubMed] [Google Scholar]
- 88.Chichlowski M, German JB, Lebrilla CB, Mills DA. The influence of milk oligosaccharides on microbiota of infants: Opportunities for formulas. Annu Rev Food Sci Technol 2011;2:331–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schumacher G, Bendas G, Stahl B, Beermann C. Human milk oligosaccharides affect P-selectin binding capacities: In vitro investigation. Nutrition 2006;22:620–7. [DOI] [PubMed] [Google Scholar]
- 90.Bode L, Kunz C, Muhly-Reinholz M, Mayer K, Seeger W, Rudloff S. Inhibition of monocyte, lymphocyte, and neutrophil adhesion to endothelial cells by human milk oligosaccharides. Thromb Haemost 2004;92:1402–10. [DOI] [PubMed] [Google Scholar]
- 91.Vos AP, M’Rabet L, Stahl B, Boehm G, Garssen J. Immune-modulatory effects and potential working mechanisms of orally applied nondigestible carbohydrates. Crit Rev Immunol 2007;27:97–140. [DOI] [PubMed] [Google Scholar]
- 92.Velupillai P, Harn DA. Oligosaccharide-specific induction of interleukin 10 production by B220+ cells from schistosome-infected mice: A mechanism for regulation of CD4+ T-cell subsets. Proc Natl Acad Sci USA 1994;91:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Terrazas LI, Walsh KL, Piskorska D, McGuire E, Harn DA Jr. The schistosome oligosaccharide lacto-N-neotetraose expands Gr1(+) cells that secrete anti-inflammatory cytokines and inhibit proliferation of naive CD4(+) cells: a potential mechanism for immune polarization in helminth infections. J Immunol 2001;167:5294–303. [DOI] [PubMed] [Google Scholar]
- 94.Eiwegger T, Stahl B, Haidl P, Schmitt J, Boehm G, Dehlink E, Urbanek R, Szépfalusi Z. Prebiotic oligosaccharides: In vitro evidence for gastrointestinal epithelial transfer and immunomodulatory properties. Pediatr Allergy Immunol 2010;21:1179–88. [DOI] [PubMed] [Google Scholar]
- 95.Lane JA, O’Callaghan J, Carrington SD, Hickey RM. Transcriptional response of HT-29 intestinal epithelial cells to human and bovine milk oligosaccharides. Br J Nutr 2013;110:2127–37. [DOI] [PubMed] [Google Scholar]
- 96.Naarding MA, Ludwig IS, Groot F, Berkhout B, Geijtenbeek TB, Pollakis G, Paxton WA. Lewis X component in human milk binds DC-SIGN and inhibits HIV-1 transfer to CD4+ T lymphocytes. J Clin Invest 2005;115:3256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fitzgibbons SC, Ching Y, Yu D, Carpenter J, Kenny M, Weldon C, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg 2009;44:1072–5. [DOI] [PubMed] [Google Scholar]
- 98.Breastfeeding and the use of human milk. Pediatrics 2012;129:e827–41. [DOI] [PubMed] [Google Scholar]
- 99.Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawöger R, Kiechl-Kohlendorfer U, Chan GM, Blanco CL, Abrams S, Cotten CM. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr 2010;156:562–7.e1. [DOI] [PubMed] [Google Scholar]
- 100.Meinzen-Derr J, Poindexter B, Wrage L, Morrow AL, Stoll B, Donovan EF. Role of human milk in extremely low birth weight infants’ risk of necrotizing enterocolitis or death. J Perinatol 2009;29:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McGuire W, Anthony MY. Donor human milk versus formula for preventing necrotising enterocolitis in preterm infants: systematic review. Arch Dis Child Fetal Neonatal Ed 2003;88:F11–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nanthakumar NN, Fusunyan RD, Sanderson I, Walker WA. Inflammation in the developing human intestine: A possible pathophysiologic contribution to necrotizing enterocolitis. Proc Natl Acad Sci USA 2000;97:6043–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sodhi CP, Neal MD, Siggers R, Sho S, Ma C, Branca MF, Prindle T Jr, Russo AM, Afrazi A, Good M, et al. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 2012;143:708–18e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gribar SC, Sodhi CP, Richardson WM, Anand RJ, Gittes GK, Branca MF, Jakub A, Shi XH, Shah S, Ozolek JA. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J Immunol 2009;182:636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Caplan MS, Simon D, Jilling T. The role of PAF, TLR, and the inflammatory response in neonatal necrotizing enterocolitis. Semin Pediatr Surg 2005;14:145–51. [DOI] [PubMed] [Google Scholar]
- 106.Hackam DJ, Afrazi A, Good M, Sodhi CP. Innate immune signaling in the pathogenesis of necrotizing enterocolitis. Clin Dev Immunol 2013;2013:475415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Soliman A, Michelsen KS, Karahashi H, Lu J, Meng FJ, Qu X, Crother TR, Rabizadeh S, Chen S, Caplan MS. Platelet-activating factor induces TLR4 expression in intestinal epithelial cells: Implication for the pathogenesis of necrotizing enterocolitis. PLoS One 2010;5:E15044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chan KL, Wong KF, Luk JM. Role of LPS/CD14/TLR4-mediated inflammation in necrotizing enterocolitis: pathogenesis and therapeutic implications. World J Gastroenterol 2009;15:4745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, Thomson RB, Soliman A, Arditi M, Caplan MS. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol 2006;177:3273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Afrazi A, Sodhi CP, Richardson W, Neal M, Good M, Siggers R, Hackam DJ. New insights into the pathogenesis and treatment of necrotizing enterocolitis: Toll-like receptors and beyond. Pediatr Res 2011;69:183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sherman MP. Lactoferrin and necrotizing enterocolitis. Clin Perinatol 2013;40:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weston EJ, Pondo T, Lewis MM, Martell-Cleary P, Morin C, Jewell B, Daily P, Apostol M, Petit S, Farley M, et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005–2008. Pediatr Infect Dis J 2011;30:937–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126:443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stoll BJ, Hansen NI, Sanchez PJ, Faix RG, Poindexter BB, Van Meurs KP, Bizzarro MJ, Goldberg RN, Frantz ID III, Hale EC, et al. Early onset neonatal sepsis: The burden of group B Streptococcal and E. coli disease continues. Pediatrics 2011;127:817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abu-Maziad A, Schaa K, Bell EF, Dagle JM, Cooper M, Marazita ML, et al. Role of polymorphic variants as genetic modulators of infection in neonatal sepsis. Pediatr Res 2010;68:323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Graham PL 3rd, Begg MD, Larson E, Della-Latta P, Allen A, Saiman L. Risk factors for late onset gram-negative sepsis in low birth weight infants hospitalized in the neonatal intensive care unit. Pediatr Infect Dis J 2006;25:113–7. [DOI] [PubMed] [Google Scholar]
- 117.Viemann D, Dubbel G, Schleifenbaum S, Harms E, Sorg C, Roth J. Expression of toll-like receptors in neonatal sepsis. Pediatr Res 2005;58:654–9. [DOI] [PubMed] [Google Scholar]
- 118.Williams DL, Ha T, Li C, Kalbfleisch JH, Schweitzer J, Vogt W, Browder IW. Modulation of tissue toll-like receptor 2 and 4 during the early phases of polymicrobial sepsis correlates with mortality. Crit Care Med 2003;31:1808–18. [DOI] [PubMed] [Google Scholar]
- 119.Roger T, Froidevaux C, Le Roy D, Reymond MK, Chanson AL, Mauri D, et al. Protection from lethal Gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc Natl Acad Sci USA 2009;106:2348–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ashraf RN, Jalil F, Zaman S, Karlberg J, Khan SR, Lindblad BS, Hanson LA. Breast feeding and protection against neonatal sepsis in a high risk population. Arch Dis Child 1991;66:488–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kaur G, Gathwala G. Efficacy of bovine lactoferrin supplementation in preventing late-onset sepsis in low birth weight neonates: A randomized placebo-controlled clinical trial. J Trop Pediatr 2015;61:370–6. [DOI] [PubMed] [Google Scholar]