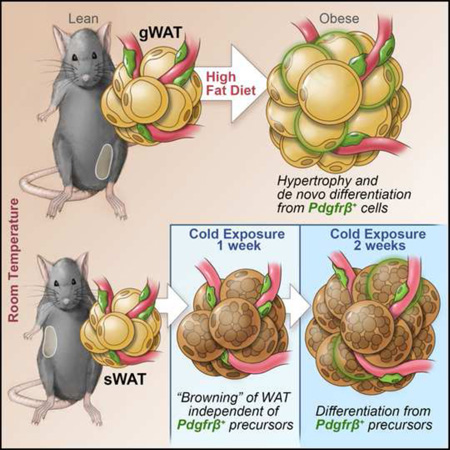
Pdgfrβ+ Mural Preadipocytes Contribute to Adipocyte Hyperplasia Induced by High-Fat Diet Feeding and Prolonged Cold Exposure in Adult Mice (original) (raw)
. Author manuscript; available in PMC: 2017 Feb 9.
Published in final edited form as: Cell Metab. 2015 Nov 25;23(2):350–359. doi: 10.1016/j.cmet.2015.10.018
Summary
The expansion of white adipose tissue (WAT) in obesity involves de novo differentiation of new adipocytes; however, the cellular origin of these cells remains unclear. Here, we utilize _Zfp423_GFP reporter mice to characterize adipose mural (Pdgfrβ+) cells with varying levels of the preadipocyte commitment factor Zfp423. We find that adipose tissue contains distinct mural populations, with levels of Zfp423 distinguishing adipogenic from inflammatory-like mural cells. Using our “MuralChaser” lineage tracking system, we uncover adipose perivascular cells as developmental precursors of adipocytes formed in obesity, with adipogenesis and precursor abundance regulated in a depot-dependent manner. Interestingly, Pdgfrβ+ cells do not significantly contribute to the initial cold-induced recruitment of beige adipocytes in WAT; it is only after prolonged cold exposure that these cells differentiate into beige adipocytes. These results provide genetic evidence for a mural cell origin of white adipocytes in obesity, and suggest that beige adipogenesis may originate from multiple sources.
Graphical abstract
Introduction
White adipose tissue (WAT) has a remarkable capacity to expand or remodel in order to meet the energy demands of the organism. In the face of caloric excess, WAT expands through the enlargement of existing white energy-storing adipocytes (adipocyte hypertrophy) as well as by the recruitment of new fat cells (adipocyte hyperplasia, or “adipogenesis”). The balance between adipocyte hyperplasia and adipocyte hypertrophy in obesity is critical for metabolic health. Preferential WAT expansion through adipocyte hyperplasia is a feature of the “metabolically healthy obese” and is associated with lower adipose inflammation and preserved insulin sensitivity (Sun et al., 2011). To date, the factors that determine the mechanisms of WAT expansion remain incompletely understood.
WAT depots can also undergo extensive remodeling in animals exposed to cold temperatures. In response to the cold, WAT can adopt a thermogenic phenotype, elicited by the emergence of “brown-like” energy-burning adipocytes, termed “beige” cells. Beige fat cells are thermogenic adipocytes that express uncoupling protein 1 (UCP1), but appear to be molecularly and developmentally distinct from brown adipocytes present in brown adipose depots formed during development (Seale et al., 2008; Wu et al., 2012). The cellular origin of active beige adipocytes in adult WAT has been intensely debated. Current hypotheses (reviewed in Kajimura et al., 2015) include trans-differentiation of white adipocytes into beige adipocytes (Himms-Hagen et al., 2000; Lee et al., 2015), activation of dormant (uni-locular, UCP1−) beige adipocytes (Rosenwald et al., 2013), and de novo differentiation from resident precursors (Long et al., 2014; Wang et al., 2013; Wang et al., 2014; Wu et al., 2012).
In recent years, tremendous progress has been made in identifying markers that select for pools of adipose precursor cells (APC) residing within adult WAT (Berry et al., 2014). However, the precise cell types from which new fat cells originate in response to various physiological conditions remains unclear. A significant barrier to advancing our understanding of adipose precursors is the relative lack of genetic tools suitable to precisely localize, fate map, and manipulate these cells in vivo under different settings. Our previous work has identified the large C2H2 zinc-finger transcription factor, Zfp423, as a factor associated with preadipocyte commitment (Gupta et al., 2010). We subsequently derived BAC transgenic mice expressing GFP under the control of the Zfp423 locus (_Zfp423_GFP mice) to use as a tool to identify potential APC populations (Gupta et al., 2012). In histological sections of adipose tissue obtained from _Zfp423_GFP mice, Zfp423/GFP expression was found in mature adipocytes, a subset of endothelial cells, and a distinct subset of perivascular cells that express the mural cell marker Pdgfrβ (Gupta et al., 2012). The perivascular localization of Zfp423 is consistent with the long-standing hypothesis that preadipocytes resemble pericyte-like cells (reviewed in (Cawthorn et al., 2012)). However, in recent years, non-perivascular origins of adipogenesis have also been suggested (Chau et al., 2014; Hudak et al., 2014; Lee et al., 2012; Ryden et al., 2015; Tran et al., 2012). Further complicating matters is that the origin of adipocytes may vary with depot, age, and may even be heterogeneous within individual depots (Chau et al., 2014; Jiang et al., 2014; Sanchez-Gurmaches and Guertin, 2014; Seale et al., 2008). As a result, whether resident mural cells contribute to adipogenesis associated with the remodeling of WAT in adult animals remains unclear.
In this study, we utilize a number of genetic tools to explore the molecular and functional properties of Zfp423+ mural cells. We find that Zfp423-expressing mural cells represent a subset of physiologically regulated and lineage-primed adipose precursors. Moreover, using our newly derived “MuralChaser” lineage tracking system, we reveal the contribution of adipose mural (Pdgfrβ+) cells to adipose remodeling in obese or cold exposed adult mice.
Results and Discussion
Adipose Zfp423+ perivascular cells express accepted cell surface markers of adipose progenitors cells
Our previously published _Zfp423_GFP BAC transgenic mouse was originally derived as a FVB strain and eventually maintained on a mixed FVB;C57BL/6 genetic background. Here, we derived a pure C57BL/6 _Zfp423_GFP transgenic line (_Zfp423_GFPB6) by microinjection of the previously modified BAC into fertilized C57BL/6 embryos. This new strain allows us to characterize Zfp423-expressing perivascular cells in context with other models utilized in our study. Consistent with the previously reported strain, the distribution of GFP mRNA across tissues in adult _Zfp423_GFPB6 mice closely followed the pattern of endogenous Zfp423 expression (Fig. 1A). Furthermore, GFP expression again localized to mature adipocytes and subsets of perivascular cells within adult adipose tissue (Fig. 1B–D). Notably, almost all of the GFP+ perivascular cells appeared in close contact with the underlying endothelium, giving them the classic pericyte-like morphology and localization (Fig. 1D).
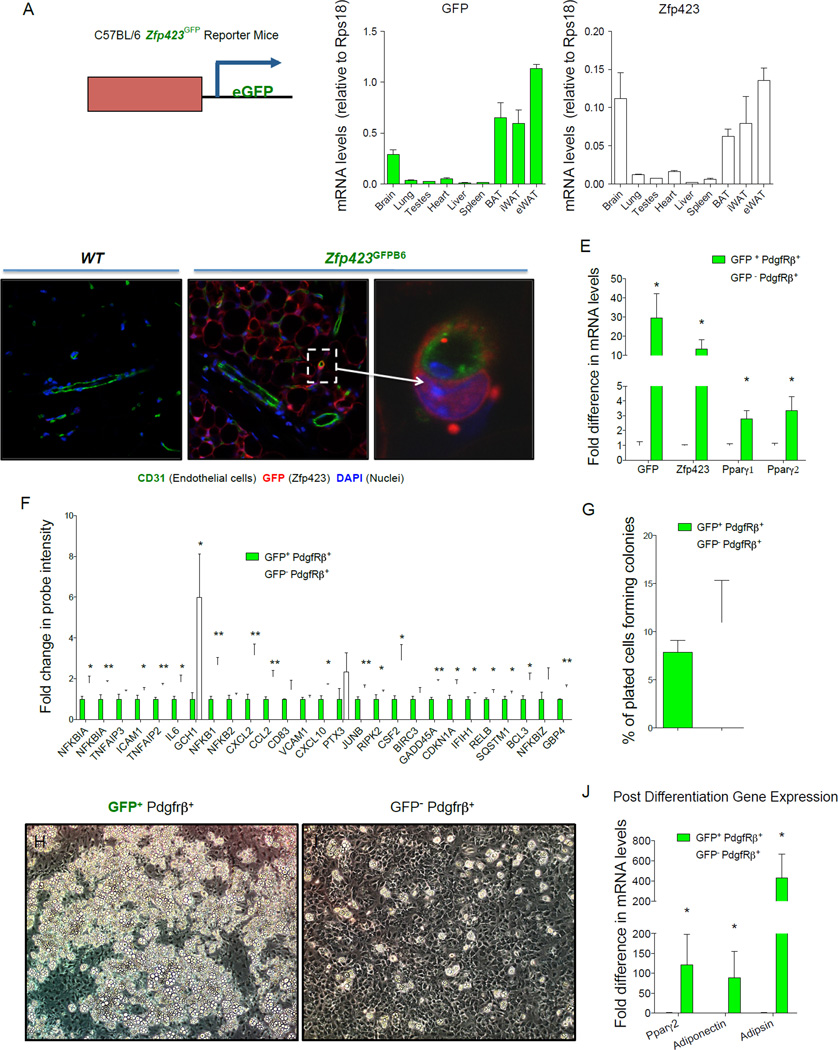
Figure 1. _Zfp423_-expressing adipose mural cells are lineage-primed adipose precursors.
(A) mRNA levels of GFP and endogenous Zfp423 in different adult tissues isolated from newly derived C57BL/6 _Zfp423_GFPB6 reporter mice in which enhanced GFP (eGFP) is expressed under the control of a _Zfp423_-containing BAC. (B–D) Confocal images of cryosectioned inguinal white adipose tissue (WAT) stained with antibodies recognizing GFP (red) and the endothelial protein CD31 (green), with nuclei counterstained with DAPI. (B) WAT from wild-type (WT) mice. (C) WAT from _Zfp423_GFPB6 mice. (D) Digital enhancement of the small blood vessel shown in (C) (white arrow). Note expression of GFP in mature adipocytes and subset of periendothelial cells. Similar results were observed in gonadal WAT (E). Quantitative PCR measurements of mRNA levels of GFP, Zfp423, and Pparγ isoforms in freshly isolated GFP+ or GFP− mural (Pdgfrβ+; Lin−) cells from the gonadal WAT of male _Zfp423_GFPB6 mice. * denotes p <0.05 from student’s t-test. Bars represent mean ± SEM. _n_= 3 samples, with each sample representing cells obtained from pooled tissue from 6–7 male mice. (F) Relative probe intensity values of key inflammatory genes with an overlapping presence in multiple gene sets enriched in GFP− mural cells. Bars represent mean ± SEM. * and ** denotes p <0.05 and p<0.005, respectively (G) Quantification of colony-forming unit potential of freshly isolated GFP+ or GFP− mural cells. _n_=8 (H–I) Phase contrast images of spontaneous differentiation in cultures of GFP+ (H) and GFP− (I) mural cells maintained at confluence in growth media containing 2% FBS and 1% ITS (insulin, transferrin, and selenium). Images captured 10 days post-confluence. (J) mRNA levels of adipocyte selective genes in differentiated cultures from (H–I) _n_=3 Bars represent mean ± SEM. * and ** denotes p <0.05.
Most adipogenic cells within WAT are of non-endothelial and non-hematopoeitic origin (CD31− and CD45−, respectively, defined as Lin−) and express stem/surface cell markers such as Sca1, CD34, CD29, and Pdgfrα (Berry et al., 2014). As a result, we focused our analysis here on the Zfp423+;Lin− population of stromal cells within WAT as candidate adipose precursors. Flow cytometry analysis of GFP+; Lin− cells freshly isolated from adipose SVF of the _Zfp423_GFPB6 transgenic animals indicates that a vast majority of these cells express the accepted markers of APCs (Sca1+, CD34+; Pdgfrα+) (Fig. S1A) (Berry and Rodeheffer, 2013). Consistent with their peri-endothelial localization in vivo, GFP+; Lin− SVF cells also express Pdgfrβ, a mural cell marker (Armulik et al., 2011). Most of the cells in the population are CD24−, suggesting that most GFP+; Lin− cells are among the committed precursor population recently described (Berry and Rodeheffer, 2013).
Levels of Zfp423 distinguish adipogenic from potential inflammatory mural cells in adipose tissue
In order to evaluate the molecular and functional properties of Zfp423-expressing mural cells, we isolated GFP+;Pdgfrβ+;Lin− stromal cells directly from the gonadal WAT SVF of Zfp423GFPB6 mice and compared them to GFP−;Pdgfrβ+;Lin− cells from the same preparations (Fig. S1B,C). GFP+;Pdgfrβ+ stromal cells had higher mRNA levels of Zfp423, confirming the ability of the reporter mice to identify and enrich for Zfp423-expressing cells (Fig. 1E). Notably, mRNA levels of both Ppar_γ_1 and Ppar_γ_2 isoforms were enriched in GFP+ mural cells, consistent with our previous studies demonstrating that Zfp423 regulates _Ppar_γ gene expression and both factors are co-enriched in adipogenic cells.
In order to define GFP+ and GFP− mural cell populations in an unbiased manner, we compared global microarray gene expression profiles obtained for these mural cell populations. Included in the lists of differentially expressed transcripts (Table S1, S2) are individual genes previously implicated in adipogenesis (e.g. Wisp2) (Hammarstedt et al., 2013). Moreover, gene set enrichment analysis (GSEA) revealed coordinate changes in sets of genes related to a number of pathways. Many of these gene signatures were related to adipocyte differentiation directly and involved in pathways previously suggested to correlate with preadipocyte commitment, such as extracellular matrix reorganization (Table S3).
The most striking revelation from GSEA is the number of gene sets statistically enriched in the GFP− mural cell population related to inflammation and stress response (Table S4). A “leading-edge analysis” within the GSEA algorithm identified a core set of genes with an overlapping presence in the enriched gene sets. Included in these gene sets are key regulators of inflammatory signaling, many of which were upregulated in GFP− mural cells (Fig. 1F). This is an unpredicted finding; however, an inflammatory-like phenotype of pericytes has been observed before, associated with brain and skin inflammation (Jansson et al., 2014; Stark et al., 2013). One emerging hypothesis is that pericytes can serve as gatekeepers for immune cell infiltration into tissues; their perivascular orientation places them in closest proximity to circulating factors and immune cells (Stark et al., 2013). Whether mural cell inflammation plays an immunomodulatory role in WAT still needs to be investigated.
We also examined the proliferative and adipogenic potential of gonadal adipose mural cells in vitro. Gonadal adipose Pdgfrβ+; Lin− cells proliferate and grow to confluence when cultured in growth medium containing 2% FBS and 1% ITS (insulin, transferin, selenium) supplement, sustaining their initial morphology in the process. Following separation by FACS, cultured gonadal GFP+ and GFP− mural cells exhibit similar colony-forming unit potential (Fig. 1G), reminiscent of mesenchymal stem-cell behavior. However, upon reaching confluence, only the GFP+ cells underwent spontaneous adipocyte differentiation at a high efficiency (>80%) (Fig. 1H–J). Remarkably, small clusters of spontaneous differentiation were even observed when GFP+ cells were cultured in as little as 0.1% FBS (Fig. S1 D,E). The further addition of FBS to the culture medium brought out latent adipogenic potential of GFP− mural cells. Under these conditions, both populations differentiate spontaneously upon reaching confluence, with ~60–70% efficiency at 7 days post-confluence (Fig. S1F–G). This is likely not due to a growth advantage of contaminating GFP+ cells as GFP− mural cells tend to replicate at a slightly higher rate (Fig. S1H). However, careful inspection of the cultured GFP− mural cells revealed that these cells activate GFP expression within days of plating in medium containing 10% FBS, suggesting that the suppression of Zfp423 is not stable in these perivascular cells when cultured in vitro under these conditions (Fig. S1I–J). These data suggest a degree of plasticity to adipose mural cells, at least in culture. Taken all together, the molecular and functional data above suggest that GFP+ mural cells represent a subpopulation of the adipose precursor pool that is primed for adipocyte differentiation.
The frequency of Zfp423-expressing mural cells is highest in WAT depots with a propensity for adipocyte hyperplasia
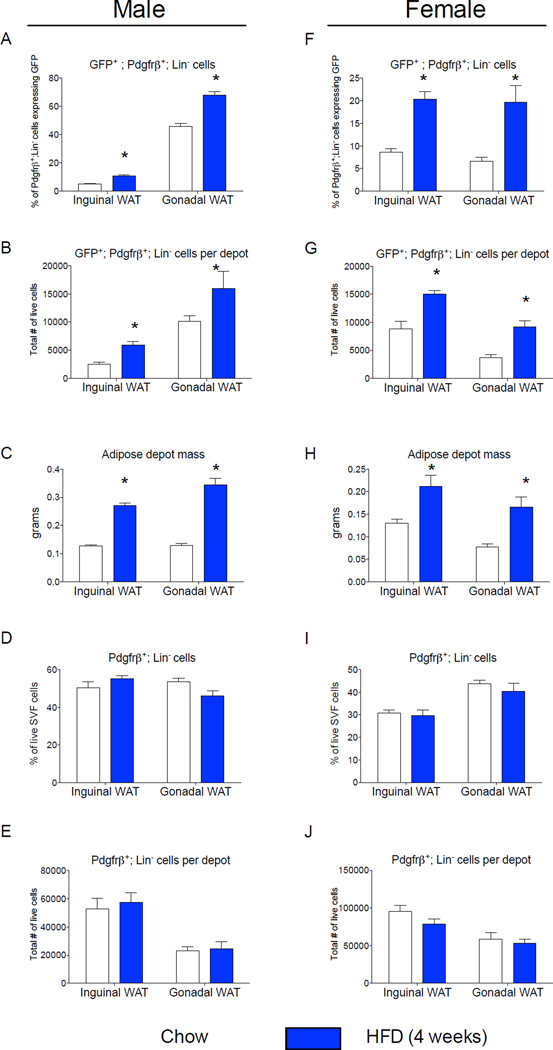
Diet-induced obesity in mice triggers WAT expansion in a depot-specific manner. Gonadal WAT depot of adult mice expands by both adipocyte hypertrophy and adipocyte hyperplasia following high-fat diet feeding. De novo adipocyte differentiation in this depot occurs after four weeks of high-fat diet feeding (Wang et al., 2013). On the other hand, inguinal WAT in the same animals expands predominantly through cellular hypertrophy (Wang et al., 2013). Adipose precursor proliferation is also regulated by high-fat diet feeding in a depot-dependent manner (Jeffery et al., 2015; Joe et al., 2009; Macotela et al., 2012). We assessed the frequency of mural cells expressing GFP (GFP+; Pdgfrβ+) in anatomically distinct fat pads of eight week-old mice that were maintained on a standard chow diet since weaning. Interestingly, the frequency and absolute number of GFP+;Pdgfrβ+ mural cells is much higher in the gonadal WAT than in the inguinal WAT of lean adult male mice (Fig. 2A,B); this correlates with the propensity of these depots to undergo adipocyte hyperplasia when switched to high-fat diet feeding. We also assessed the frequency of these cells in fat pads of age-matched mice that were instead fed a high-fat diet for four weeks after weaning, a time-point immediately prior to onset of adipocyte hyperplasia induced in obesity. High-fat diet feeding increased both inguinal and gonadal WAT mass to the same degree (Fig. 2C). Moreover, the overall number of Pdgfrβ+ cells was similar in both depots, and unaffected by four weeks of high-fat diet feeding (Fig. 2D,E). However, high-fat diet feeding led to a statistically significant increase in both the absolute number and the frequency of GFP+;Pdgfrβ+ mural cells in both depots, but importantly, to a larger degree in the gWAT where adipogenesis actually occurs (Fig. 2A,B). The frequency of gonadal adipose Pdgfrβ+ cells expressing GFP is also elevated after eight weeks of high-fat diet feeding in comparison to age-matched animals fed standard chow for the same duration (Fig. S2). However, the overall frequency of the gonadal precursors in both the chow and high-fat fed animals appears lower than what is observed in corresponding mice that were analyzed four weeks earlier (Fig. 2A). This suggests that the abundance of this preadipocyte population may be age dependent.
Figure 2. The frequency of _Zfp423_-expressing adipose mural cells is regulated in a sex- and depot-dependent manner.
(A) Percentage of Pdgfrβ+;Lin− cells expressing GFP (GFP+; Pdgfrβ+; Lin−) in WAT depots of _Zfp423_GFPB6 male mice fed chow or high fat diet (HFD) for 4 weeks following weaning. (B) Total number of GFP+; Pdgfrβ+; Lin− cells per individual WAT depot after 4 weeks of chow or HFD. (C) Adipose depot mass (grams) after 4 weeks of chow or HFD. (D) Percentage of the live cell population of the total SVF that are Pdgfrβ+;Lin− cells. (E) Total number of live Pdgfrβ+;Lin− cells per individual WAT depot after 4 weeks of chow or HFD. (F–J) Corresponding data in female animals fed chow or HFD for the same duration. * denotes p <0.05 from student’s t-test. Bars represent mean ± SEM. _n_= 5–6 animals for each group.
Interestingly, the frequency of GFP+;Pdgfrβ+ mural cells is sex-dependent. In female mice, the abundance of GFP+ mural cells is similar between iWAT and gWAT (Fig. 2F,G). Upon high-fat diet feeding, the absolute levels and frequency of these cells increases in both depots (Fig. 2,F,G). As seen in the male animals, high-fat diet feeding for 4 weeks increased both inguinal and gonadal WAT mass to the same degree (Fig. 2H). Moreover, the overall number of Pdgfrβ+ cells was also unaffected by four weeks of high-fat diet feeding (Fig. 2I,J). Thus, the frequency of GFP+ mural cells appears to be regulated in both a depot- and sex-dependent manner.
Lineage tracing reveals Pdgfrβ+ cells as a cellular origin of adipogenesis associated with high-fat diet feeding
The striking correlation between the frequency of GFP+ mural cells and the hyperplastic potential of adipose depots suggests mural cells are a cellular origin of de novo differentiated adipocytes in obesity. However, direct lineage-tracing evidence supporting a mural cell origin of adipocytes formed in association with high-fat diet feeding has been lacking. This type of analysis requires the use of an inducible system for indelible pulse labeling specifically of mural cells, and not mature adipocytes. Zfp423 is actively expressed in both mural cells and mature adipocytes; therefore, lineage tracing guided by the Zfp423 promoter is uninformative in this context (Gupta et al., 2012).
In order to specifically label and track the mural cell lineage in adults, we have derived a novel doxycycline-inducible mural cell lineage tracking system. To generate this system, we first engineered a transgenic mouse strain that expresses reverse tetracycline transactivator (rtTA) under the control of a large fragment of the _Pdgfr_β promoter (Fig. S3A). Importantly, we have confirmed that rtTA mRNA is only expressed in Pdgfrβ-expressing stromal cells (Fig. S3B). We then derived the “MuralChaser” system by breeding _Pdgfr_βrtTA transgenic mice to animals carrying the TRE-Cre transgene (Cre recombinase under the control of the Tet-Response Element) and the Rosa26R _loxP_-mtdTomato-_loxP_-mGFP (mT/mG) fluorescent reporter allele (Fig. 3A). In this tetracycline-inducible transgenic model, administration of doxycycline-containing food (DOX) results in the rtTA-mediated transactivation of Cre expression and permanent mGFP reporter expression, specifically in peri-endothelial and adventitial cells of WAT blood vessels (Fig. 3B–D). Critically, mature adipocytes and Lin+ cells are not labeled during this pulse-labeling period (Fig. 3E–F; S3C–F).
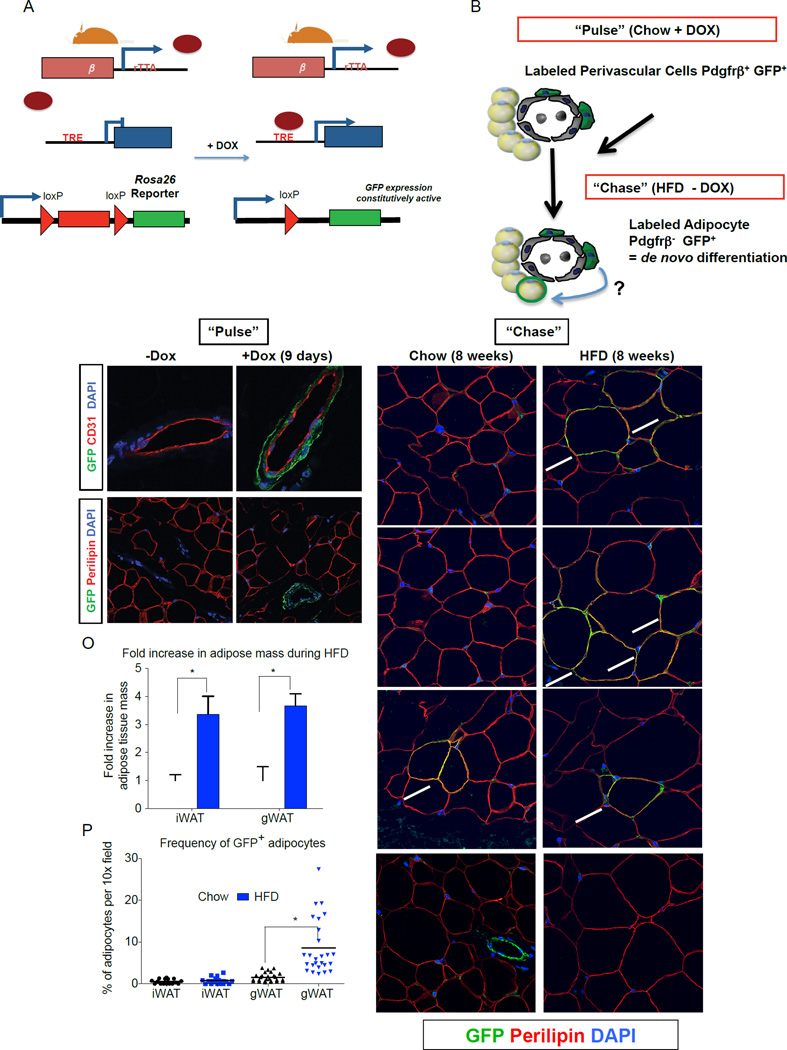
Figure 3. Lineage tracing reveals Pdgfrβ+ cells as a cellular origin of adipogenesis associated with high-fat diet feeding.
(A) Genetic alleles comprising of the “MuralChaser” lineage tracing system. In the presence of doxycycline (DOX) rtTA activates Cre expression. Cre excises the loxP-flanked membrane tdTomato cassette and allows constitutive activation of membrane GFP (mGFP) reporter expression. (B) “Pulse-Chase” lineage tracing approach. DOX-containing food (600 mg/kg) is administered to male MuralChaser mice for 9 days to label Pdgfrβ+ cells (“Pulse”). Animals are then immediately harvested (Pulse) or are switched to a high-fat diet (HFD) or chow diet for 8 weeks in the absence of DOX (Chase). mGFP+ adipocytes represent de novo differentiated fat cells formed during the 8 week period. (C–D) mGFP labeling is confined to peri-endothelial (CD31+) cells and occurs in a DOX-dependent manner. (E–F) mGFP labeling is not found in mature adipocytes (perilipin+). (G–I) Following 8 weeks of chow feeding, only few mGFP+ gonadl adipocytes (gWAT) are observed. (J–L) Following 8 weeks of HFD feeding, numerous mGFP+ perilipin+ adipocytes are observed. These cells represent de novo differentiated adipocytes formed during the 8 week period. (M–N) After 8 weeks of chow or HFD, very few mGFP+ adipocytes are observed in the inguinal WAT (iWAT), supporting the notion of very little postnatal adipocyte turnover or hyperplasia in this depot during HFD feeding. Note that antibody staining was utilized with formalin-fixed paraffin embedded sections; native mGFP and mTdTomato fluorescence are not observed here and are lost during tissue processing. (O) Fold increase in adipose tissue mass (normalized to body weight) during 8-week HFD period. Bars represent mean + stdev. * in both panels denotes p < 0.05 from student’s t-test. (P) Quantification of mGFP+ adipocytes observed in randomly chosen 10× magnification fields from gWAT and iWAT sections. * denotes p <0.05 from student’s t-test. Twenty-six random 10x magnification fields were photographed from duplicate sections of stained WAT depots from four animals of each group. All images show were photographed at 63× magnification.
We also assessed the efficiency of Cre-mediated recombination in Pdgfrβ+ mural cells of adipose depots of Dox-treated MuralChaser animals. Flow cytometry analysis indicated that ~ 50% of resident Pdgfrβ+ (antibody-labeled) cells in gWAT were marked by mGFP expression (Fig. S3G). In the inguinal WAT, labeling efficiency appears higher as >70% of antibody-labeled resident Pdgfrβ+ cells were mGFP+ (Fig. S3H). A high efficiency of Cre-mediated recombination in cells of this depot can also be observed when Dox is added in vitro to isolated SVF cells. Dox treatment of cultured iWAT SVF from _Pdgfr_βrtTA; TRE-Cre; _Zfp423_loxP/loxP mice resulted in a >80% reduction in Zfp423 mRNA levels as well as in a clear reduction in the adipogenic capacity of the cultured SVF (Fig. S3I–M). The later result is consistent with the notion that Zfp423 is both a molecular marker and functional regulator of Pdgfrβ+ preadipocytes.
To monitor de novo adipogenesis during high-fat diet feeding, we first administered DOX-containing food to eight week-old male _Pdgfr_βrtTA; TRE-Cre; _Rosa26R_mT/mG mice for nine days. Then, animals were placed on either a standard chow diet or HFD, without any DOX added to the food (Fig. 3B). Eight weeks after the removal of DOX, some mGFP+ adipocytes were observed in the gonadal WAT of chow-fed animals (Fig. 3G–I). The newly derived adipocytes reflect the normal adipocyte turnover or continued adipocyte development occurring at this age. After eight weeks of high-fat diet, many clusters of mGFP+ adipocytes were found in the gWAT (Fig. 3J–L). These labeled cells represent adipocytes formed during the high-fat diet feeding-period. In agreement with our previous studies of the AdipoChaser mouse, adipocyte hyperplasia was not observed in the inguinal WAT depot of male mice (Fig. 3M–N), despite the depot expanding by the same magnitude as the gWAT (Fig. 3O), and fairly efficient labeling of resident Pdgfrβ+ cells in this depot during the pulse-labeling period (Fig. S3H) (Wang et al., 2013). Overall, nearly 10% of adipocytes present in the gWAT after eight weeks of HFD feeding were newly derived from the mural cell lineage (Fig. 3P). As described above, ~50% of resident mural Pdgfrβ+ cells in the gonadal WAT were genetically labeled during the pulse-labeling conditions used here (Fig. S3G). Therefore, the actual contribution of Pdgfrβ+ cells to adipocyte hyperplasia may be greater than observed here. Jeffery et al. recently reported that ~20% of all gonadal adipocytes in eight-week HFD-fed animals represent de novo differentiated cells (Jeffery et al., 2015). Direct comparison to our experiment here is difficult; however, our data at least suggests that the contribution of mural cells to adipocyte hyperplasia in obesity is appreciable. The mechanisms controlling the depot-selective activation of Zfp423+ preadipocytes are not entirely clear, as is the function of these cells in lean animals if/when they are not undergoing adipogenesis. Future studies of the inguinal and gonadal GFP+;Pdgfrβ+ preadipocytes may help shed insight into this regulation. All together, these data indicate that Pdgfrβ+ mural cells represent a cellular source of new adipocytes formed in association with high-fat diet feeding.
Labeled Pdgfrβ+ mural cells contribute to beige adipocyte formation in adult WAT after prolonged cold exposure
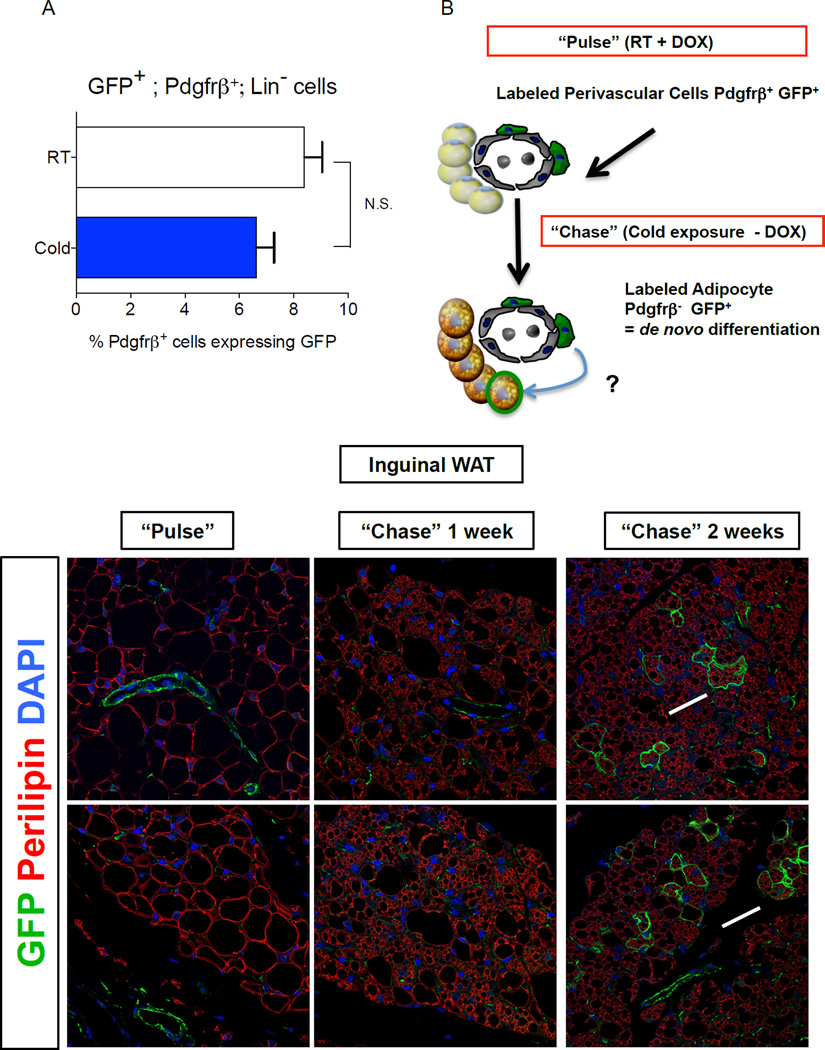
Previous fate mapping studies using the AdipoChaser model revealed that beige adipocytes can accumulate in WAT rapidly upon cold exposure, in part through de novo differentiation from precursors (Wang et al., 2013). Thus, we asked if the frequency of GFP+;Pdgfrβ+ cells is regulated by cold exposure. We found that the abundance of GFP+ mural cells in iWAT or gWAT from male _Zfp423_GFPB6 mice exposed to cold challenge for two weeks was indistinguishable from the amount found in WAT depots of _Zfp423_GFPB6 mice housed at room temperature (Fig. 4A). Thus, the abundance of the GFP+ precursor population does not appear to be significantly regulated by cold exposure in vivo, at least under these conditions.
Figure 4. Pdgfrβ+ mural cells contribute to beige adipocyte formation in adult WAT after prolonged cold exposure.
(A) Percentage of Pdgfrβ+;Lin− cells expressing GFP (GFP+; Pdgfrβ+; Lin−) in WAT depots of _Zfp423_GFPB6 male mice housed at room temperature or cold exposed (6°C) for 2 weeks. Bars represent mean ± SEM. _n_= 5–6 animals for each group. (B) “Pulse-Chase” lineage tracing approach. DOX-containing food (600 mg/kg) is administered to MuralChaser mice for 9 days at room temperature to label Pdgfrβ+ cells (“Pulse”). Afterwards, animals were immediately harvested (Pulse) or are housed in the cold (6 °C) for either 1 or 2 weeks in the absence of DOX (Chase). mGFP+ multilocular adipocytes represent de novo differentiated fat cells formed during the cold exposure period. (C–D) Confocal immunofluorescence image of inguinal WAT from MuralChaser mice dox-treated for 9 days. Parrafin–embedded sections are immunostained with antibodies recognizing mGFP and perilipin. mGFP staining is confined to the vasculature at this stage. (E–F) Confocal immunofluorescence image of inguinal WAT from MuralChaser mice after 1 week of cold exposure (after removal of DOX). mGFP staining is still largely confined to the vasculature at this stage. (G–H) Confocal immunofluorescence image of inguinal WAT from MuralChaser mice after 2 weeks of cold exposure (after removal of DOX). Several small clusters of mGFP+ multilocular adipocytes can now be observed, indicating a contribution of Pdgfrβ+ mural cells to beige adipogenesis during prolonged cold exposure. All images photographed at 63× magnification.
We also asked if any Pdgfrβ+ mural cells contribute to beige adipogenesis in the WAT. We labeled male MuralChaser mice for nine days by feeding animals a DOX-containing chow diet (Fig. 4B–D). After this initial pulse-labeling period, we placed animals at 6°C for either one or two weeks (Fig. 4B). Large regions of beige multilocular adipocytes were observed in iWAT following one week of cold exposure. However, we rarely observed mGFP+ multilocular adipocytes in the MuralChaser mice (Fig. 4E,F). Importantly, in parallel we performed the same “pulse-chase” experiments using the AdipoChaser mouse model (Fig. S4A–B). This models serves as a positive control and our experiments confirm the occurrence of de novo differentiation during this duration at 6 °C (Fig. S4C–G). The lack of significant contribution by Pdgfrβ+ mural cells during this time implies that other mechanisms are involved in the initial formation of the beige lineage in this depot. These multilocular adipocytes may represent either existing beige adipocytes present during the pulse labeling at room temperature, re-activation (UCP1 induction) of dormant beige adipocytes, bona fide trans-differentiation of existing white adipocytes into beige adipocytes, or de novo differentiation from cells not actively expressing _Pdgfr_β. Two weeks of cold exposure resulted in nearly complete browning of the iWAT depot (Fig. 4G,H). Under this condition, we now observed several small clusters of mGFP+ multilocular beige adipocytes (Fig. 4G,H). The presence of Pdgfrβ+ mural cell-derived beige fat cells following prolonged cold exposure indicates that Pdgfrβ+ cells can be recruited to become beige adipocytes, but is likely distinct from the precursors differentiating upon acute cold exposure.
Overall, these data provide additional evidence that de novo differentiation of beige cells occurs in the cold, likely in conjunction with other aforementioned mechanisms. However, the timing in which these Pdgfrβ+ cells contribute during cold exposure suggests that multiple “waves” of beige adipogenesis may occur depending on the degree of cold stress, and that this may originate from multiple precursor populations. We cannot rule out the possibility that cells differentiating early upon cold exposure were once mural cells; however, at the time of the pulse labeling most of these cells must be devoid, or at least low, in expression of Pdgfrβ. Interestingly, lineage tracing analyses and other studies point to a developmental relationship between smooth muscle-like cells and beige adipocytes in the WAT depots (Long et al., 2014; McDonald et al., 2015). Thus, one can speculate that the adipose vasculature may serve as a home for multiple types of adipocyte precursors.
In summary, we have identified Zfp423-expressing mural cells as a subset of physiologically regulated adipose precursors that are highly committed to the adipose lineage, and reveal a previously unrecognized population of inflammatory-like mural cells. Using our “MuralChaser” lineage tracking system, we uncover adipose perivascular cells as a developmental origin of adipocytes formed in obesity and upon prolonged cold exposure. Future studies using these genetic tools will help shed insight into the regulation of mural preadipocytes and adipogenesis during development and in adult mice.
Experimental Procedures
Animals and Diets
_Zfp423_GFPB6 and _Pdgfr_βrtTA transgenic mice were derived by the UT Southwestern (UTSW) Transgenic Core Facility. Details of the transgene design can be found in Supplemental Experimental Procedures. TRE-Cre and _Rosa26R_mT/mG mice were obtained from Jackson Laboratories. All animal experiments were performed according to procedures approved by the UTSW Animal Care and Use Committee. Mice were maintained on a standard rodent chow diet or specialized diets with 12 hour light and dark cycles. Detailed use of the diets for lineage tracing can be found in Supplemental Experiment Procedures.
Histological analysis of adipose tissues
White adipose tissues were harvested from perfused (4% paraformaldehyde) adult mice. Paraffin sections were prepared by the Molecular Pathology Core Facility at UTSW. Cryosections were prepared as previously described (Gupta et al., 2012). A detailed protocol for indirect immunofluorescence assays can be found in Supplementary Experimental Procedures.
Gene expression profiling
Relative expression of mRNAs was determined by quantitative PCR using SYBR Green chemistry and values were normalized to Rps18 levels using the ΔΔ-Ct method. Student's t-test was used to evaluate statistical significance. All primer sequences are available upon request. Microarray analysis was conducted utilizing the Illumina MouseWG-6 V2 BeadChip arrays (See Supplemental Experimental Procedures for details). All microarray data has been deposited to Gene Expression Omnibus (accession number GSE70789).
Isolation of adipose SVF cells for flow cytometry
Minced fat tissues were digested for two hours while shaking at 37°C in buffer containing 100mM HEPES pH 7.4, 120 mM NaCl, 50 mM KCl, 5 mM glucose, 1 m CaCl2, 1.5% BSA, and 1mg/mL collagenase D (Roche 11088882001). Digested tissues were then filtered through a 100 µm cell strainer and then centrifuged for 5 minutes at 600 × g to pellet the SV cells. Red blood cells were lysed using commercial lysis buffer (Sigma R7757). SV cells were then used for flow cytometry experiments as described in Supplemental Experimental Procedures.
Mural cell culture and adipocyte differentiation assays
Freshly isolated mural cell fractions were obtained by FACS from the SVF of 5 week-old male mice isolated as described above (see also Supplementary Experimental Procedures). For adipocyte differentiation assays, cells were plated directly onto collagen-coated dishes and grown to confluence at 10% CO2 in growth media (60% pH7–7.4 low glucose DMEM, 40% pH 7.25 MCDB201 (Sigma M6770), 2% FBS (Fisher Scientific 03-600-511 Lot FB-002) supplemented with 1% ITS premix (Insulin-Transferrin-Selenium) (BD Bioscience 354352), 0.1 mM L-ascorbic acid-2-2phosphate (Sigma A8960-5G), 10ng/mL FGF basic (R&D systems 3139-FB-025/CF), Pen/Strep, and gentamicin). Once confluent, cells were allowed to maintain in growth media for up to 10 days to observe the spontaneous adipocyte differentiation.
Supplementary Material
1
2
3
4
5
6
Acknowledgements
The authors are grateful to members of the UTSW Touchstone Diabetes Center and C. Wrann (Boston, MA) for useful discussions, and P. Scherer and W. Holland for critical reading of the manuscript. The authors thank the UTSW Animal Resource Center, Flow Cytometry core, Transgenic Core Facility, Pathology Core, Live Cell Imaging Core, and Microarray Core for excellent guidance and assistance with experiments performed here. This study was supported by NIDDK R03 DK099428, R01 DK104789, the Searle Scholars Program, and the American Heart Association 15BGIA22460021 to R.K.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicting interests statement. The authors declare that they have no competing financial interests.
Author Contributions
L.V., K.M., C.H., M.S., S.S., and C.K. conducted the experiments, Q.W., M.Y., and T.M generated critical reagents, and R.G. designed the experiments and wrote the manuscript.
References
- Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Berry R, Jeffery E, Rodeheffer MS. Weighing in on adipocyte precursors. Cell Metab. 2014;19:8–20. doi: 10.1016/j.cmet.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 2013;15:302–308. doi: 10.1038/ncb2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227–246. doi: 10.1194/jlr.R021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau YY, Bandiera R, Serrels A, Martinez-Estrada OM, Qing W, Lee M, Slight J, Thornburn A, Berry R, McHaffie S, et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol. 2014;16:367–375. doi: 10.1038/ncb2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, Roby YA, Kulaga H, Reed RR, Spiegelman BM. Transcriptional control of preadipocyte determination by Zfp423. Nature. 2010;464:619–623. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Mepani RJ, Kleiner S, Lo JC, Khandekar MJ, Cohen P, Frontini A, Bhowmick DC, Ye L, Cinti S, et al. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 2012;15:230–239. doi: 10.1016/j.cmet.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarstedt A, Hedjazifar S, Jenndahl L, Gogg S, Grunberg J, Gustafson B, Klimcakova E, Stich V, Langin D, Laakso M, et al. WISP2 regulates preadipocyte commitment and PPARgamma activation by BMP4. Proc Natl Acad Sci U S A. 2013;110:2563–2568. doi: 10.1073/pnas.1211255110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol. 2000;279:C670–C681. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- Hudak CS, Gulyaeva O, Wang Y, Park SM, Lee L, Kang C, Sul HS. Pref-1 marks very early mesenchymal precursors required for adipose tissue development and expansion. Cell Rep. 2014;8:678–687. doi: 10.1016/j.celrep.2014.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson D, Rustenhoven J, Feng S, Hurley D, Oldfield RL, Bergin PS, Mee EW, Faull RL, Dragunow M. A role for human brain pericytes in neuroinflammation. J Neuroinflammation. 2014;11:104. doi: 10.1186/1742-2094-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery E, Church CD, Holtrup B, Colman L, Rodeheffer MS. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol. 2015;17:376–385. doi: 10.1038/ncb3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Berry DC, Tang W, Graff JM. Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell Rep. 2014;9:1007–1022. doi: 10.1016/j.celrep.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe AW, Yi L, Even Y, Vogl AW, Rossi FM. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells. 2009;27:2563–2570. doi: 10.1002/stem.190. [DOI] [PubMed] [Google Scholar]
- Kajimura S, Spiegelman BM, Seale P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015;22:546–559. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Konkar AA, Granneman JG. Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J. 2015;29:286–299. doi: 10.1096/fj.14-263038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012;15:480–491. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, et al. A smooth muscle-like origin for beige adipocytes. Cell Metab. 2014;19:810–820. doi: 10.1016/j.cmet.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macotela Y, Emanuelli B, Mori MA, Gesta S, Schulz TJ, Tseng YH, Kahn CR. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes. 2012;61:1691–1699. doi: 10.2337/db11-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald ME, Li C, Bian H, Smith BD, Layne MD, Farmer SR. Myocardin-related transcription factor A regulates conversion of progenitors to beige adipocytes. Cell. 2015;160:105–118. doi: 10.1016/j.cell.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald M, Perdikari A, Rulicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013;15:659–667. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- Ryden M, Uzunel M, Hard JL, Borgstrom E, Mold JE, Arner E, Mejhert N, Andersson DP, Widlund Y, Hassan M, et al. Transplanted Bone Marrow-Derived Cells Contribute to Human Adipogenesis. Cell Metab. 2015 doi: 10.1016/j.cmet.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J, Guertin DA. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun. 2014;5:4099. doi: 10.1038/ncomms5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Bruhl ML, Gartner F, Khandoga AG, Legate KR, Pless R, et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and 'instruct' them with pattern-recognition and motility programs. Nat Immunol. 2013;14:41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran KV, Gealekman O, Frontini A, Zingaretti MC, Morroni M, Giordano A, Smorlesi A, Perugini J, De Matteis R, Sbarbati A, et al. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 2012;15:222–229. doi: 10.1016/j.cmet.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Kissig M, Rajakumari S, Huang L, Lim HW, Won KJ, Seale P. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc Natl Acad Sci U S A. 2014;111:14466–14471. doi: 10.1073/pnas.1412685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2
3
4
5
6