Interferons and the Immunogenic Effects of Cancer Therapy (original) (raw)
. Author manuscript; available in PMC: 2016 Nov 1.
Published in final edited form as: Trends Immunol. 2015 Nov;36(11):725–737. doi: 10.1016/j.it.2015.09.007
Abstract
Much of our understanding on resistance mechanisms to conventional cancer therapies such as chemotherapy and radiation has focused on cell intrinsic properties that antagonize the detrimental effects of DNA and other cellular damage. However, it is becoming clear that the immune system and/or innate immune signaling pathways can integrate with these intrinsic mechanisms to profoundly influence treatment efficacy. In this context, recent evidence indicates that interferon (IFN) signaling has an important role in this integration by influencing immune and intrinsic/non-immune determinants of therapy response. However, IFN signaling can be both immunostimulatory and immunosuppressive, and the factors determining these outcomes in different disease settings are unclear. Here I discuss the regulation and molecular events in cancer that are associated with these dichotomous functions.
INTRODUCTION
For decades, cytotoxic chemotherapy and radiation have been part of the standard treatment for the majority of different cancer types. In patients with localized cancer, these therapies can be administered prior to surgical removal of the primary tumor (neoadjuvant), after removal of the primary tumor (adjuvant), or used in place of surgery with curative intent (definitive). In the setting of localized cancer, both therapies can be effective and significantly improve overall survival. For example, adjuvant chemotherapy or radiation therapy for breast cancer improves survival in approximately 30% of patients with residual disease [1,2]. In the definitive setting, the combination of radiation and chemotherapy can effectively eradicate the primary tumor in cancers of the head and neck and in early stage lung cancer [3,4]. However, in patients with metastatic disease, chemotherapy and radiation are rarely curative and are often used to merely delay progression and/or for palliation. Newer genomically targeted agents that inhibit proteins with driver mutations, such as BRAF inhibitors, and other biologics that target critical cancer-related proteins can often be associated with impressive responses in the metastatic setting; however, relapses with these targeted therapies almost always occur [5]. Considered in totality, conventional cancer therapies can be effective at eradicating cancer but resistance to treatment has remained a major barrier to improving cancer survival.
In most cases, nuclear DNA has long been considered the main target of chemotherapy and radiation that determines whether cancer cells live or die [6]. Either through indirect effects on DNA replication, such as disruption of nucleotide biosynthesis by 5-fluorouracil, or through direct effects that damage DNA, such as ionizing radiation or DNA cross-linking agents, the ability to interfere with the fidelity of DNA replication and with genome integrity are considered the main modes of action. As such, cell intrinsic factors that regulate cell cycle checkpoints, repair DNA damage, eliminate the damaging agent, or programmed cell death have been intensely studied as mechanisms controlling therapy response. As a result, our understanding of how cancer cells react to and repair DNA damage has led to the rational development of new agents such as PARP inhibitors [7].
A growing body of evidence argues that in addition to cell intrinsic factors, a major determinant of whether a tumor relapses or responds to cancer therapy is how the immune system can be marshaled to participate in the cytotoxic effect. Understanding this cell extrinsic mechanism has received increasing attention, particularly as immunotherapy becomes a part of the cancer treatment armamentarium and combinatorial therapies are being explored. As such, an important goal is to identify key immune and/or cancer pathways that critically regulate the ability of the immune system to facilitate an anti-tumor response. One pathway that has unexpectedly emerged is interferon (IFN) and IFN-related signaling (hereafter referred to as IFN signaling). Generally considered a pathway that stimulates the immune response, recent evidence indicates that IFN signaling can also lead to immunosuppression. The cellular and molecular events that activate IFN signaling are diverse. Immunogenic forms of cell death and multiple pattern recognition receptors (PRRs) can originate from multiple cell types in the tumor microenvironment to converge on interferon signaling. Thus, these observations raise the question as to how IFN signaling can result in either immune stimulation or suppression. In this Review, the role of the immune system in response to genotoxic cancer therapy is briefly discussed (for excellent and comprehensive reviews see [8,9]). Then, recent advances on a critical and dual role for IFN signaling in regulating immune effects of anti-cancer therapies and the ability of cancer cells to respond to genotoxic damage are discussed.
THE IMMUNE SYSTEM AND RESPONSE TO CONVENTIONAL GENOTOXIC THERAPY
Clinical Evidence: A Re-Interpretation
Given the attention to improving therapy response by understanding and manipulating cancer cell intrinsic mechanisms, it can come as a surprise when anecdotal cases reveal tumor regression that seem to implicate cancer cell extrinisic regulation. Radiation therapy is considered a local treatment and generally unable to influence tumors outside of the irradiated field. Yet, randomized clinical trials that test the role of adjuvant radiation in locally advanced breast cancer with a high risk for occult metastatic disease clearly demonstrate that radiation therapy can improve patient survival [10]. The typical thinking is that radiation to the post-surgical primary tumor bed and draining lymph nodes prevents relapse in these areas and hence the subsequent metastasis of recurrent disease from these post-surgical sites; however, alternative explanations exist.
One alternative explanation is that radiation can have a systemic effect. Consistent with this notion, there have been multiple case reports of patients with multiple metastatic tumors undergoing radiation to one tumor and experiencing marked regression of a tumor outside the irradiated field [10,11]. These types of “abscopal” effects, from Greek meaning “away from the target,” are thought to be due to the radiation triggering an immune-mediated response of the abscopal tumors. Likewise, although an immune contribution to antitumor responses may not be as apparent using systemic chemotherapy compared to assessing an abscopal tumor after local radiation, the importance of the immune system in chemotherapy response has also been clinically implicated [8]. In colorectal cancer, a high density of tumor-infiltrating lymphocytes including various T cell subsets is predictive of extended relapse-free survival after conventional chemotherapy. From retrospective studies and randomized clinical trials for breast cancer treated with adjuvant or neoadjuvant chemotherapy, high lymphocyte infiltration or their genetic markers also are predictive of response [12–14]. Thus, clinical evidence suggests that at least in some patients, the immune system may contribute to the antitumor efficacy of radiation and/or chemotherapy.
Evidence from Animal Tumor Models
In support of the abovementioned interpretation of the clinical evidence, experimental animal models demonstrate that the adaptive immune system can indeed be mobilized to help eradicate tumors after radiation. In the B16 melanoma model, flank tumors respond to 20 Gy of radiation in immunocompetent mice but not in athymic nude mice or in mice with CD8 T cells depleted [15]. This is a particularly striking result given that considerable DNA damage is expected with 20 Gy regardless of immune status. Similar results have been observed with TS/A breast cancer cells derived from a spontaneous mammary adenocarcinoma [16]. The importance of the immune system in chemotherapy response has also been observed in multiple syngeneic murine cancer models and with a wide variety of chemotherapies including 5-fluorouracil, anthracyclines, oxaliplatin, and gemcitabine [8,16].
Recent development of immune checkpoint inhibitors and testing in animal models highlights how restoring immune activation can augment response to genotoxic agents. Several studies looking at response of an irradiated tumor show improvement when checkpoint blockade is used [17,18]. Studies using multiple flank or lung tumors and employing single tumor irradiation combined with systemic immune checkpoint therapy also reveal improved CD8-dependent T cell responses in the unirradiated tumors [19–21]. Thus, immune activation, particularly of CD8 T cells, can enhance response to genotoxic therapy and genotoxic therapy can instigate immune activation.
Cell Death and Immune Activation
How can cytotoxic chemotherapy and radiation marshal the immune system to eradicate cancer cells? An important factor is how tumor cells die. An early hallmark of apoptosis was thought to be its immunologically silent property [22]. In contrast, necrosis, which was considered an accidental form of cell death, would promptly alert the immune system of cellular injury. However, we now appreciate that both apoptosis and necrosis can result in immune activation [22].
When cells undergo apoptosis, various intrinsic (e.g. DNA damage) and extrinsic signals (e.g., death receptor activation) ultimately converge onto the activation of caspases [22]. Under many circumstances, the initiator caspase, Caspase 9, forms an apoptosome with APAF1 and cytochrome c after the latter is released due to mitochondrial outer membrane permeabilization (MOMP). The propensity to undergo MOMP is promoted by pro-apoptotic BCL-2 proteins BAX and BAK and antagonized by anti-apoptotic BCL-2 and BCL-XL. Alternatively, Caspase 8 can be directly activated by death receptors such as FAS through assembly of the Death-Inducing Signaling Complex (DISC).
It is now appreciated that like apoptosis, necrosis can also be regulated [23]. Various forms of regulated necrosis exist. The best understood form, called necroptosis, depends on RIPK1, RIPK3, and MLKL. RIPK3 recruits and phosphorylates MLKL, which may either recruit ion channels or directly form a pore-forming unit to execute cell death. Radiation therapy and likely some forms of chemotherapy can induce necroptosis [24]. At least for cancer, necroptosis through the TNF family of receptors is currently the best characterized. For macrophages and other immune cells that can be part of the tumor microenvironment, various other receptors that are activated by cellular damage and infection can initiate necroptosis [23]. These receptors include toll-like receptors (TLRs), retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs), and IFN receptors. Thus, as with apoptosis, necrosis can ensue as a dedicated cellular response to DNA damage or external stimuli such as engagement of specific receptors, rather than merely result from unanticipated trauma.
Apoptosis, necroptosis, and other forms of regulated cell death clearly can impact the activation state of the immune system. It is generally thought that necroptosis is more inflammatory than apoptosis. RIPK1-deficient keratinocytes trigger inflammation through necroptosis but not apoptosis [25]. Activation of pro-apoptotic proteins BAX and BAK release mitochondrial DNA that activates the PRR and cytosolic DNA sensor cyclic GMP-AMP synthase (cGAS). cGAS activates STING, which can promote immune recognition by enhancing IFN production; however, concurrent activation of apoptotic caspases (such as Caspase 3, 7, and 9) inhibit STING, rendering cell death more immunologically silent [26,27]. Overall, the relative contribution of apoptosis versus regulated necrosis in stimulating the immune system and in the cancer setting has not been well characterized. Nonetheless, it is clear that a critical factor orchestrating the extent of immune involvement is the molecules released from dying cells.
Immunogenic Cell Death
Death of tumor cells after therapy that generates an immune dependent response and immunological memory define an immunogenic cell death (ICD) [8]. Several chemotherapeutic agents are well-studied inducers of ICD, including anthracylines and oxaliplatin. Radiation therapy can also result in an ICD [33]. One principle signal that dying cells use to alert the immune system is Damage Associate Molecular Patterns (DAMPs). DAMPs are molecules and proteins that are released or exposed as cells die. Well-characterized DAMPs include, the exposure of the endoplasmic reticulum protein calreticulin (CRT) to the cell surface, the extracellular secretion of ATP, and the extracellular release of the non-histone chromatin protein HMGB1. DAMPs such as HMGB1 can activate PRRs that include TLR4 to stimulate DCs. Increasing evidence from transplantation models, including seminal work from Zitvogel and Kroemer, have demonstrated in detail that DAMPs can dictate whether tumors are resistant to therapy or whether an ICD drives response and immunity [8]. Notably, chemotherapy response of spontaneous tumors from genetically engineered mice has been shown to occur independently of the adaptive immune system, arguing that organ site and/or interaction between cancer cells and host during the process of tumorigenesis can be important factors [9,28].
OPPOSING FUNCTIONS OF INTERFERON SIGNALING IN CANCER
Given that there are multiple DAMPs, PRRs, and regulated forms of cell death that differentially impact DAMP/PRR signals to influence immune activation, this raises the question as to what downstream signal(s) lie at a nexus that governs ICD. Recent evidence highlights the IFN pathway, which is a common outcome of DAMP signaling [29], as one critical pathway. After IFN levels are induced by TLRs, RLRs, and other receptors for DAMPs, IFNs dimerize and activate high-affinity IFN receptors, resulting in STAT1 phosphorylation by receptor-bound JAKs [30]. Phosphorylated STAT1 (p-STAT1) can either form a heterodimer with STAT2 and IRF9 (type I IFN, IFNa/b) or homodimerize (type II IFN, IFNg) to act as a transcription factor. Generally, the former transcriptional complex binds to ISRE DNA elements in the promoters of interferon-stimulated genes (ISGs), while the latter binds to GAS elements in ISG promoters.
Several observations support the concept that IFN signaling may lie at the nexus that controls ICD and include: 1) the widespread expression of ISGs across cancer and its association with radiation and chemotherapy response, 2) the requirement for PRRs and downstream IFNs for effective tumor response after chemotherapy and radiation in mouse models, and 3) recent evidence from infectious disease studies showing a critical role of IFNs and associated PRRs in dictating immune activation, including after DNA damage. However, despite these observations that support a critical role for IFN signaling in enhancing ICD and chemotherapy/radiation response, there are also seemingly paradoxical roles for IFNs whereby they orchestrate resistance to therapy. Factors that influence these dichotomous roles include whether the targets of the IFN effects are cancer cells or immune cells, the nature of the IFN signaling resulting in post-translational modifications of STAT1, and the ISGs expressed. Below, these opposing functions of IFN signaling in either promoting ICD or increasing therapy resistance to genotoxic and immunotherapies are discussed, along with possible mechanisms that govern the dichotomous outcomes.
Widespread Expression of Interferon-Stimulated Genes in Cancer
Since the early studies that employed genome-wide profiling of cancer transcriptomes, many have observed expression of immune-related cytokines and inflammatory genes [31,32]. In particular, ISGs are broadly expressed across various tumor types [33]. These ISGs often reflect the activation of T cells and an “inflamed tumor microenvironment” [34] that produce IFN and its downstream target genes that are driven by STAT1. As such, the absence of this signature can be associated with poor response to conventional and immune therapies [35–38]. However, high expression of these ISGs also has been clinically associated with poor response to therapy and immune suppression [33,39,40]. Multiple cell types within the tumor microenvironment can exhibit high expression of ISGs [41,42]; however, most studies do not distinguish between expression by the cancer cells, immune cells, and/or stromal cells.
Immunostimulatory Effects of Interferons After Genotoxic Damage
After the induction of ICD by chemotherapy, antigen-specific T cells will produce IFNg. IFNg has multiple functions that promote tumor surveillance, immune activation, and anti-tumor activity. These include, driving Th1 rather than Th2 differentiation, activating NK cells, increasing MHC and immunoproteosome expression to enhance antigen presentation, and exerting anti-proliferative, anti-angiogenic, and pro-apoptotic effects on cancer cells [30]. In addition to IFNg, type one IFNs can be prominently associated with effective ICD. For example, in the B16 melanoma model radiation therapy increases type one IFN in the tumor microenvironment, and tumors implanted into type one IFN receptor knockout mice fail to regress after radiation due to a defect in the ability of dendritic cells to cross-prime T cells [43]. Adenovirus delivery of IFNb recapitulates the T cell-dependent anti-tumor response to radiation, arguing that the initial effect goes through type one IFN. Subsequent studies using STING knockout mice and conditional knockouts of the type one IFN receptor, revealed that STING and cGAS, but not MYD88 or TRIF, are required for the ability of radiation to induce IFNb and for IFNb to signal DC priming of T cells to achieve a therapeutic response [44]. At later time points, IFNg may help to maintain response, as antibody neutralization can cause relapse after radiation [45]. A requirement for cGAS, STING, and type one IFN suggests that in response to radiation, a cytosolic DNA acts as a DAMP to drive ICD through the activation of DCs.
Similar to radiation, treatment of various tumor types such as MCA205 fibrosarcomas with the anthracyline class of ICD chemotherapy also gives rise to the rapid production of type one IFN needed for optimal chemosensitization [46]. However, in contrast to radiation where the importance of IFN signaling was defined in immune cells, gene expression profiling in this model revealed that IFN signaling and induction of ISGs occurred in the cancer cells early after treatment. Tumor cells lacking the type one IFN receptor failed to respond to chemotherapy. Using tumors derived form knockout mice, IFN signaling and chemotherapy response was shown to be dependent on TLR3 (but not MDA5), which normally functions as an endosomal sensor for viral RNA. For as yet unclear reasons, the ICD function of IFN signaling was dependent on its ability to enhance expression of CXCL10, which was able to restore anthracycline sensitivity in tumors lacking the type one IFN receptor. Moreover, exogenous CXCL10 or type one IFN, but not IFNg, increased tumor sensitivity to cisplatin, which normally poorly induces an ICD. The pre-clinical findings were corroborated by examination of breast cancer patients. Here, complete response to neoadjuvant chemotherapy and better survival after adjuvant chemotherapy was associated with high expression of MX1, an ISG induced by anthracyline treatment. In total, these observations suggests that “viral mimicry” involving an unidentified self-RNA that functions as a DAMP for TLR3, may enhance the ICD effects of chemotherapy through type one IFN signaling on tumors cells.
Unexpectedly, examining pathways that enhance anti-viral response against Vesicular stomatitis virus (VSV) has recently provided additional support that DNA damage can result in IFN and STING-driven immune stimulation [47]. It was observed that the sera from infection-free Ataxia-telangiectasia (AT) patients could activate ISGs, and fibroblasts from AT patients elicited higher levels of type one IFN after viral infection. Bone-marrow-derived macorphages from ATM knockout mice similarly showed enhanced anti-viral response to VSV and bacterial challenge. This effect was attributed to the higher levels of DNA damage occurring in an ATM mutant background and could be reproduced by radiation/chemotherapy treatment of wild type cells. The priming of the innate immune system and type one IFN response was dependent on STING (but not MYD88, TICAM, or IPS1) and the sensing of DNA damage-associated single-stranded DNA by cGAS and possibly other cytosolic DNA sensors. Whether tumors with ATM defects are more likely to elicit ICD after therapy is unclear; however, patients with mutations in the same homologous recombination pathway, such as BRCA1 and BRCA2, have tumors with increased immune infiltrates and high ISG expression [48,49].
Immunosuppressive Effects of Interferons and Adaptive Resistance
Although much evidence supports the anti-tumor and immunostimulatory effects of IFNs, early studies began revealing that IFNs can also be detrimental by promoting tumor growth. For example, resistance to NK cells and enhanced metastatic ability were observed after low-dose or autocrine IFNg exposure of melanoma and breast cancer cells [50,51]. Other undesirable effects associated with IFNg include upregulation of nonclassical MHC class I molecules that inhibit NK and T cell killing [52,53], loss of antigen expression [54], and induction of immunosuppressive regulators (discussed more below). Such pro-tumor effects of IFNg are supported by clinical evidence that IFNs are associated with poor outcome in some melanoma patients [55].
Some of the effects of IFN on promoting tumor growth and immune evasion culminate in “adaptive resistance”, which was originally described by Pardoll as a phenomenon that triggers immune suppression due to cross-talk between cancer cells and T cells [56]. Here, it is proposed that antigen-specific T cells corral the cancer cells but the cancer cells respond through immunosuppressive mechanisms. One such mechanism may be the upregulation of PD-L1 by IFNg thought to be derived from T cells [57]; however, type one IFNs can also upregulate tumor PD-L1 [58]. The resulting high expression of PD-L1 is particularly notable along the T cell-rich invasive margin of the tumor [59,60] and is distinct from constitutive PD-L1 resulting from oncogenic pathways such as EGFR [61] and PI3K [62]. The IFN-driven elevation of PD-L1 is then thought to engage PD-1 on T cells, leading to T cell exhaustion [21]. PD-L1 can also be expressed on immune cells within the tumor microenvironment, and clinical evidence suggests that this may be an important source for PD-L1 in mediating immune suppression [63].
The potential of adaptive resistance and ISG expression to disable the anti-tumor function of T cells suggests that immune-driven therapies could fair poorly when tumors express high ISGs. Indeed, selection for melanoma and breast cancer tumors that are resistance to anti-CTLA4-based combination therapy resulted in upregulation of ISGs by the tumor that included PD-L1 [21]. Data from both pre-clinical mouse models and patients from a clinical trial demonstrated that tumor PD-L1 is a major resistance mechanism, and PD-L1/PD-1 blockade by antibody or genetic knockout reverses resistance by reinvigorating exhausted T cells. The ability of PD-L1/PD-1 blockade to effectively antagonize adaptive resistance likely explains why anti-PD-L1/PD-1 therapies work well in tumors infiltrated by dysfunctional T cells [60,63,64]. Additional ISGs acting as immune suppressive factors include indolamine 2,3 dioxygenase (IDO) [65] and the TIM-3 interaction partner CEACAM1 [66]. As with PD-L1, blocking IDO and TIM-3 with small molecules and antibodies can improve anti-tumor activity [67,68], further demonstrating that ISGs can drive immune-mediated therapy resistance.
Why would IFN have both immunostimulatory and immunosuppressive effects in cancer? This duality may reflect what is normally a robust homeostatic mechanism that evolved to mitigate excessive host tissue damage during prolonged or unresolved inflammation [69]. In fact, recent studies have provided compelling evidence examining the function and timing of type one IFNs in mouse models of acute versus chronic LCMV infection [39,40]. Transcriptomic profiling of spleen from mice with chronic infection revealed high expression of ISGs including STAT1 and PD-L1. Blocking the type one IFN receptor with antagonistic antibodies improved viral titers of chronically infected mice and required CD4 T cells. Viral clearance after blocking type one IFN signaling was associated with improved lymphoid architecture and dampening of various immune suppressive factors like PD-L1 and IL-10. Whether down-modulating type one IFN signaling acts directly (e.g., through T cell activation/differentiation) or indirectly (e.g., through DCs, macrophages, or virally infected cells) to enhance adaptive immunity is still unclear. Although this immune suppressive side of IFN signaling was observed in a viral infection setting, the parallels with adaptive resistance described in cancer cells highlights a broad context for the dual role of IFNs.
Interferon-Related Signaling Drive Non-Immune-Mediated Resistance to Chemotherapy and Radiation
In addition to effects on the immune system, high levels of ISG expression can also promote cancer cell intrinsic resistance to radiation and chemotherapy. In a variety of cancer types, STAT1 drives the expression of a subset of ISGs called the Interferon-Related DNA Damage Resistance Signature (IRDS), and augmenting or crippling IRDS expression through STAT1 influences sensitivity to genotoxic agents in vitro [33,70]. Although it is not clear how high ISG expression can promote intrinsic resistance to radiation and chemotherapy, certain ISGs such as ISG15 are involved in DNA repair [71], which may contribute.
Non-immune cells such as stromal fibroblasts comprise a large proportion of cells in the tumor microenvironment. These stromal cells can also contribute to therapy resistance, often through poorly defined mechanisms [72]. Recently, ISGs and the IRDS were shown to be involved in stroma-mediated resistance as well [73]. High ISG/IRDS expression in tumors can be induced through stromal cell-derived exosomes, which are enriched in non-coding RNA and repeat/transposable elements. The transfer of stromal exosomes to cancer cells results in the activation of RIG-I and subsequent STAT1-driven ISG expression. STAT1 amplifies the transcriptional response to NOTCH3 activation, which is a juxtacrine pathway that is concurrently engaged through stromal expression of the NOTCH3 ligand JAGGED1. Evidence from both mice and breast cancer patients support the notion that the cooperation between STAT1 and NOTCH3 leads to the expansion of therapy-resistant tumor initiating cells and tumor relapse. Since athymic nude mice were used in this study, effects of stroma-regulated ISG expression on the adaptive immune system were excluded. In total, these observations reveal that besides regulating immune-mediated anti-tumor effects, IFN-related signaling can influence cell intrinsic and non-immune cell extrinsic determinants of therapy response.
Activating Interferon Signaling in a Sterile Tumor Microenvironment
In the case of pathogen infection, immune cells are the primary source of IFNg, while production of type one IFNs are less restricted by cell type. An important signal to initiate IFN signaling after infection is viral RNA and cytosolic pathogen DNA [29]. However, in a sterile tumor microenvironment, the nature of the DAMP that instigates and/or maintains IFN signaling is still poorly understood. Activation of STING occurs through DNA and the DNA sensor cGAS. In DCs responsible for spontaneous T cell responses, tumor DNA is transferred to the APC in order to stimulate STING and type one IFN production [74]. STING-dependent type one IFN production can also be activated by single-stranded DNA resulting from DNA damage [47], by mitochondrial DNA liberated from apoptotic MOMP [27], or possibly by retroelements that are not properly metabolized by the endonuclease TREX1 [47,75].
For the RNA sensing TLRs and RLRs, the nature the RNA ligand generated after chemotherapy or radiation is also unclear. After ICD-inducing anthracyline chemotherapy, TLR3 appears to bind an unknown single-stranded RNA [46]. After ultraviolet radiation, TLR3 is capable of binding U1 RNA, possibly through stem-loop structures within the RNA that form double-stranded regions [76]. Radiation and chemotherapy are known to derepress retrotransposons and transposable elements [77], which can be associated with high levels of ISGs and immune activation if improperly metabolized by TREX1 or ADAR1 [75,78]. Recently, dsRNA that include endogenous retroviruses were shown to drive an IFN response after treatment with DNA methyltransferase inhibitors [79,80]. Similar to the viral mimicry proposed to result in tumor type one IFN production after ICD chemotherapy, this IFN response was controlled by PRRs that include TLR3 and MDA5/MAVS. In the tumor microenvironment, RLRs can be activated by 5′-triphosphate RNA in exosomes transferred from stromal cells to cancer cells [73]. The RNA in the exosomes is also enriched in repetitive elements such as transposable elements.
In total, current observations indicate that the process of cell death after radiation and chemotherapy releases DAMPs that mimic pathogen associated molecular pattern (PAMPs) to activate overlapping receptors. More detailed understanding of these DAMPs and how they contribute to the output of IFN signaling and ultimately to ICD await further studies.
Phosphorylation Status of STAT1 and Duration of Interferon Signaling
What are the molecular mechanisms and signaling events that dictate whether IFN leads to immune stimulation versus immune suppression, or DNA damage sensitivity versus resistance? Recent work by Stark and colleagues highlight that besides canonical IFN signaling events that leads to p-STAT1 and expression of ISGs, a non-canonical pathway controlled by unphosphorylated STAT1 (U-STAT1) also exists [30].
Both type one and type two IFNs can drive expression of U-STAT1, which begins to increase after p-STAT1 levels decline [81]. In contrast to the previous notion that U-STAT1 is a latent cytoplasmic transcription factor, recent studies reveal that U-STAT1 functions as an active transcription factor that selectively controls a subset of ISGs compared to p-STAT1. Through expression studies, use of knockout cells, and reconstitution with STAT1 mutants that cannot be phosphorylated on Y701, it was determined that the U-STAT1-regulated set of ISGs emerge after prolonged low-dose or single high-dose exposure of cells to type one or type two IFNs [82]. This subset of ISGs offered some protection against various viruses but rendered cells resistant to chemotherapy and radiation. Accordingly, this subset of ISGs overlaps with the ISGs elevated during chronic viral infection models in mice and in patients with chronic viral infection [39,40]. Moreover, the ISGs denoted as the IRDS that are broadly expressed across common human cancers and identify patients who are resistant to radiation and chemotherapy also overlaps with the subset of ISGs selectively under the control of U-STAT1 [33,82]. This select regulation of the IRDS and subset of ISGs is associated with distinct ISREs, as determined by promoter analysis.
In total, the ability of STAT1, a key interferon-regulated transcription factor, to modulate the expression of distinct sets of ISGs through differences in post-translational modification, IFN signaling, timing, and duration, raise the possibility that the dual and opposing roles of IFNs in immune regulation and therapy resistance may be governed by such events. The importance of timing in regulating the outcome of type one IFN events was recently illustrated in simian immunodeficiency virus infection in rhesus macaques [83]. Whether viral infection was antagonized or accelerated depended on the timing and duration of type one IFN signaling. Other factors that can control properties of IFN signaling and impact outcome include various negative regulatory proteins like RNF125 that can influence the ubiquitination of PRRs [84], OASL that physically inhibits RIG-I [85,86], USP18 that displaces JAK2 from the type one IFN receptor [87], and multiple other proteins that dynamically regulate IFN signaling [88].
CONCLUDING REMARKS
Ultimately, the magnitude of tumor response after therapy will be dictated by cell intrinsic determinants and by cell extrinsic and immune-mediated effects. Interestingly, IFN signaling can influence all of these categories of response determinants (Figure 1). However, the observation that IFN signaling can drive resistance or promote response makes the complexity of the pathway a challenge to understand and to interpret as a potential biomarker. To dissect this complexity, a first step is to understand the extent to which cell intrinsic factors dominate versus anti-tumor effects directed by the immune system. If cancer cells are inherently sensitive to therapy, the ability to elicit an ICD may be of little consequence. In this regard, tumors whereby the cancer cells show low expression of the ISG subset that overlaps with the IRDS would be predicted to be sensitive to chemotherapy/radiation. This is likely the situation with certain breast cancer patients who have been tumors described as IRDS-negative, and who are sensitive to chemotherapy and radiation [33]. On the other hand, if cancer cells are intrinsically resistant to genotoxic agents, the contribution of the immune system and the ability to instigate an ICD becomes paramount for response. Under this situation, the outcome may critically depend on whether IFN signaling favors immune stimulation or immune suppression. This is the second step in dissecting the complexity of how IFN signaling influences the likelihood of an ICD and therapy response. How tumor cells die in vivo, the DAMPs that are liberated, the PRRs that are activated, the cellular target and kinetics of signaling, and the function of specific ISGs, all can be instrumental in dictating the outcome and whether an ICD ensues (Outstanding Questions Box).
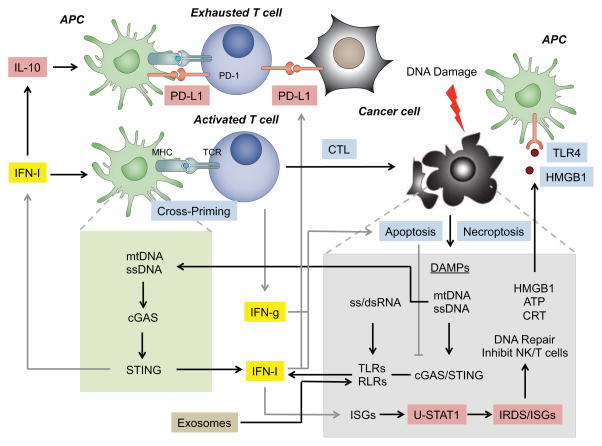
Figure 1. Cancer cell intrinsic and immune determinants of response to chemotherapy and radiation can be regulated by the opposing functions of interferon signaling.
DNA damage caused by chemotherapy or radiation initiates either apoptosis or regulated necrosis to release DAMPs. These DAMPs can be well-known immune activators such as HMGB1, ATP, or calreticulin (CRT) that get either secreted extracellularly or exposed on the plasma membrane. These DAMPs activate antigen presenting cells (APCs), with HMGB1 binding to TLR4 shown as an example. Intracellular DAMPs can be nucleic acids that include mitochondrial DNA (mtDNA), single-stranded DNA (ssDNA), ssRNA, or double-stranded RNA (dsRNA). The DNA is sensed by the cytosolic DNA sensor cGAS to activate STING, while the RNA is detected by TLRs and RLRs. Additionally, DNA from the tumor can be transferred to antigen presenting cells (APCs) where it can activate STING as well. STING and TLRs/RLRs increase type one interferon (IFN-I) production. Like HMGB1/TLR4, this enhances APC cross-priming of T cells. Tumor-reactive T cells produce high levels of interferon gamma (IFNg) and lyse cancer cells. Both IFN-I and IFNg have additional anti-tumor effects such as promoting cancer cell death. However, both IFN-I and IFNg can also promote immunosuppression and cancer cell intrinsic resistance to therapy. IFN-I can enhance IL-10 production and upregulate PD-L1 on APCs, making them immune suppressive. IFN-I and IFNg can upregulate PD-L1 on cancer cells in a phenomenon called adaptive resistance that leads to T cell exhaustion. In cancer cells, unphosphorylated STAT1 (U-STAT1) accumulates and sustains the expression of a subset of ISGs that include the Interferon-Related DNA Damage Resistance Signature (IRDS). The IRDS mediates resistance to radiation and chemotherapy, possibly by increasing DNA repair or inhibiting NK and T cell function through unclear ISGs and mechanisms. However, one mechanism involves the activation of RLRs by stromal cell-derived exosomes (tan box). This also increases IRDS and enhances the transcription of NOTCH3 target genes, which expands therapy-resistant tumor-initiating cells (not shown). Blue boxes indicate anti-tumor and immunostimulatory effects. Cyan boxes indicate pro-tumor and immuosuppressive effects.
OUTSTANDING QUESTIONS BOX.
- What is the relative contribution of type one and type two IFN signaling in immune suppression? Although both can regulate immune suppressive events, there is more evidence for type one IFNs. However, IFNγ is thought to increase ISGs during adaptive resistance of cancer cells to T cell attack, which suggests IFNγ can regulate an important resistance mechanism for immunotherapy.
- How important is the mode of regulated cell death in instigating immunogenic cell death and how does this relate to different cancer therapies? Recent studies reveal mechanisms on how the cell death machinery influences the DAMP/PRR signaling that activates IFNs. This can regulate whether cell death is immunogenic or immunologically silent.
- How do PRRs recognize the RNA/DNA DAMPs that are unleashed after genotoxic stress? The nature of the double-stranded and single-stranded RNA/DNA that activates RIG-like receptors, toll-like receptors, and cGAS/STING is largely unknown.
- How does the timing of IFN signaling and STAT1 control immune activation versus suppression? The timing of IFN stimulation can influence the phosphorylation state of STAT1 and the subset of ISGs expressed; however, the molecular details of this effect are not well characterized.
- What are the roles of different cell types that receive IFN signals in dictating immune suppression versus activation? As cancer cells, immune cells, and non-immune cells of the microenvironment respond to IFN, the individual effector pathways that are triggered likely influence the immune system in different ways.
- What are the various functions of the ISGs associated with therapy resistance and immune suppression? As is the case with viral and pathogen infection, the function of these ISGs in cancer are largely unknown
The extent to which the mode of cancer cell death in vivo influences ICD and likelihood of relapse after chemotherapy or radiation is an as yet underexplored field. We are beginning to appreciate mechanisms that regulate how apoptotic capspases can interfere with the STING pathway in order to render cell death more immunologically silent [26,27]. These new findings would suggest that cancer therapies that are less favorable to activation of caspases or combining agents with caspase inhibitors might improve anti-tumor response. Similarly, some therapies may favor activation of regulated forms of cell death like necroptosis that may be more inherently immune stimulatory. Alternatively, effective ways to enforce necroptosis may also prove effective [23].
As cancer cells die in response to therapy, the nature of the DAMPs that activate PRRs and lead to IFN production are poorly characterized. An emerging theme is that cancer therapies may provoke viral mimicry whereby endogenous DNA and RNA function as DAMPs after DNA damage or after “infection” by exosomes that enhance therapy resistance. Like viruses, nucleic acid sensing PRRs are engaged and activate IFN signaling. It is interesting that autoimmune diseases such as Aicardi-Goutières Syndrome can result from the failure to metabolize retroelements and consequently activate IFN signaling [75,89]. Cellular stress after genotoxic damage also de-represses similar endogenous transcripts [77], and several aforementioned studies show that this is associated with IFN pathway activation through PRRs [79,80]. The nature of these endogenous DNAs and RNAs, the molecular events that lead to the expression of these self nucleic acids after therapy, the molecular patterns that they possess that overlaps with pathogens, and how they are recognized are important questions.
Finally, understanding the regulation, function, and importance of the duality of IFN signaling is timely, especially with the availability of targeted agents such as immune checkpoint inhibitors and immune agonists. Several strategies that combine activation of IFN signaling using synthetic DAMPs with immunotherapies such as checkpoint blockade are being tested and showing cooperation [90,91]. Such approaches also underscore the need to understand when IFN can flip to become immunosuppressive. Parallels between IFN signaling in cancer and IFN signaling in viral and pathogen infection will likely provide key insight. In both cases, the levels of type one and two IFNs that are expressed, the activation strength, and the timing can determine the nature of the T cell response [83,92]. Moreover, in pathogen infections, whether the cell type receiving the signal is a non-immune cell versus an immune cell can reveal evidence of opposing functions [92]. Similarly, when type one IFNs are acting to enhance cross-priming of T cells and IFNg is driving T cell differentiaton, the same IFNs may be exploited by cancer cells to counter such effects. The adaptive resistance concept through IFN-driven PD-L1 induction is one such example of cancer-mediated countermeasures; however, details need to be further clarified. Molecular mechanisms that influence IFN signaling properties in different cellular contexts may depend on the expression of negative regulators [88]. Such variation in signaling may determine whether p-STAT1 or U-STAT1 dominates and the subsequent ISGs expressed. It is intriguing that the subset of ISGs regulated by U-STAT1 is associated with both chronic viral persistence and cancer therapy resistance (IRDS genes); however, the function of these ISGs is largely unknown. The ability of these ISGs to additionally orchestrate cancer cell intrinsic resistance and non-immune resistance mechanisms only adds to the potential importance of acquiring a deep understanding of the roles of IFN signaling in cancer.
TRENDS BOX.
- Genotoxic cancer therapy can instigate immune activation, thus enhancing response to genotoxic therapy. Response to chemotherapy and radiation can require IFN signaling and T cell activation. Combining conventional cancer therapies with immune checkpoint blockade can work cooperatively to promote tumor response.
- ISGs are widely expressed in human cancers and can predict therapy response. Various human cancers display an ISG signature indicative of IFN signaling; however, its expression can be associated with good response or poor response to cancer therapy.
- IFN signaling can regulate immune suppressive events. Evidence from pathogen infection and cancer models suggests that IFNs can stimulate or suppress immune responses.
- RNA and DNA can function as DAMPs, activating IFN signaling in cancer. In a sterile tumor microenvironment, cancer cells can activate RNA and DNA pattern recognition receptors to induce IFN signaling and ISG expression.
- Timing and duration of IFN signaling and phosphorylation status of STAT1 can influence immune response. After IFN-induced phosphorylated STAT1 declines, unphosphorylated STAT1 increases and can transcriptionally regulate a subset of ISGs associated with persistent viral infection and cancer therapy resistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. The Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. The Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 3.Yom SS. Radiation treatment of head and neck cancer. Surg Oncol Clin N Am. 2015;24:423–436. doi: 10.1016/j.soc.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Rusthoven CG, et al. Improved survival with stereotactic ablative radiotherapy (SABR) over lobectomy for early stage non-small cell lung cancer (NSCLC): addressing the fallout of disruptive randomized data. Ann Transl Med. 2015;3:149. doi: 10.3978/j.issn.2305-5839.2015.06.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman PB, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helleday T, et al. DNA repair pathways as targets for cancer therapy. Nature Reviews Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- 7.Farmer H, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 8.Kroemer G, et al. Immunogenic Cell Death in Cancer Therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 9.Coffelt SB, de Visser KE. Immune-mediated mechanisms influencing the efficacy of anticancer therapies. Trends Immunol. 2015;36:198–216. doi: 10.1016/j.it.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Siva S, et al. Abscopal effects of radiation therapy: A clinical review for the radiobiologist. Cancer Lett. 2015;356:82–90. doi: 10.1016/j.canlet.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Postow MA, et al. Immunologic Correlates of the Abscopal Effect in a Patient with Melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loi S, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 13.Denkert C, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 14.Ruffell B, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apetoh L, et al. Toll-like receptor 4–dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 17.Deng L, et al. Irradiation and anti–PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verbrugge I, et al. Radiotherapy Increases the Permissiveness of Established Mammary Tumors to Rejection by Immunomodulatory Antibodies. Cancer Research. 2012;72:3163–3174. doi: 10.1158/0008-5472.CAN-12-0210. [DOI] [PubMed] [Google Scholar]
- 19.Demaria S, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- 20.Dewan MZ, et al. Fractionated but Not Single-Dose Radiotherapy Induces an Immune-Mediated Abscopal Effect when Combined with Anti-CTLA-4 Antibody. Clin Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Twyman-Saint Victor CTS, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green DR, et al. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 24.Nehs MA, et al. Necroptosis is a novel mechanism of radiation-induced cell death in anaplastic thyroid and adrenocortical cancers. Surgery. 2011;150:1032–1039. doi: 10.1016/j.surg.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Dannappel M, et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014;513:90–94. doi: 10.1038/nature13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rongvaux A, et al. Apoptotic Caspases Prevent the Induction of Type I Interferons by Mitochondrial DNA. Cell. 2014;159:1563–1577. doi: 10.1016/j.cell.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White MJ, et al. Apoptotic Caspases Suppress mtDNA-Induced STING-Mediated Type I IFN Production. Cell. 2014;159:1549–1562. doi: 10.1016/j.cell.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciampricotti M, et al. Chemotherapy response of spontaneous mammary tumors is independent of the adaptive immune system. Nat Med. 2012;18:344–6. doi: 10.1038/nm.2652. author reply 346. [DOI] [PubMed] [Google Scholar]
- 29.Takeuchi O, Akira S. Pattern Recognition Receptors and Inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 30.Cheon H, et al. Interferons and Their Stimulated Genes in the Tumor Microenvironment. Seminars in Oncology. 2014;41:156–173. doi: 10.1053/j.seminoncol.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perou CM, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 32.Wolf DM, et al. Gene co-expression modules as clinically relevant hallmarks of breast cancer diversity. PLoS ONE. 2014;9:e88309. doi: 10.1371/journal.pone.0088309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weichselbaum RR, et al. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc Natl Acad Sci USA. 2008;105:18490–18495. doi: 10.1073/pnas.0809242105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harlin H, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Research. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galon J, et al. The Continuum of Cancer Immunosurveillance: Prognostic, Predictive, and Mechanistic Signatures. Immunity. 2013;39:11–26. doi: 10.1016/j.immuni.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Ulloa-Montoya F, et al. Predictive Gene Signature in MAGE-A3 Antigen-Specific Cancer Immunotherapy. Journal of Clinical Oncology. 2013;31:2388–2395. doi: 10.1200/JCO.2012.44.3762. [DOI] [PubMed] [Google Scholar]
- 37.Gajewski T, et al. Association of gene expression profile in metastatic melanoma and survival to a dendritic cell-based vaccine. ASCO Meeting Abstracts. 2009;27:9002. [Google Scholar]
- 38.Gajewski TF, et al. Molecular profiling to identify relevant immune resistance mechanisms in the tumor microenvironment. Current Opinion in Immunology. 2011;23:286–292. doi: 10.1016/j.coi.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson EB, et al. Blockade of Chronic Type I Interferon Signaling to Control Persistent LCMV Infection. Science. 2013;340:202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teijaro JR, et al. Persistent LCMV Infection Is Controlled by Blockade of Type I Interferon Signaling. Science. 2013;340:207–211. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finak G, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- 42.Farmer P, et al. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med. 2009;15:68–74. doi: 10.1038/nm.1908. [DOI] [PubMed] [Google Scholar]
- 43.Burnette BC, et al. The Efficacy of Radiotherapy Relies upon Induction of Type I Interferon-Dependent Innate and Adaptive Immunity. Cancer Research. 2011;71:2488–2496. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng L, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41:843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang H, et al. Radiation-Induced Equilibrium Is a Balance between Tumor Cell Proliferation and T Cell-Mediated Killing. The Journal of Immunology. 2013;190:5874–5881. doi: 10.4049/jimmunol.1202612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sistigu A, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20:1301–1309. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- 47.Härtlova A, et al. DNA Damage Primes the Type I Interferon System via the Cytosolic DNA Sensor STING to Promote Anti- Microbial Innate Immunity. Immunity. 2015;42:332–343. doi: 10.1016/j.immuni.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 48.McAlpine JN, et al. BRCA1 and BRCA2 mutations correlate with TP53 abnormalities and presence of immune cell infiltrates in ovarian high-grade serous carcinoma. Mod Pathol. 2012;25:740–750. doi: 10.1038/modpathol.2011.211. [DOI] [PubMed] [Google Scholar]
- 49.Jazaeri AA, et al. Gene expression profiles of BRCA1-linked, BRCA2-linked, and sporadic ovarian cancers. JNCI Journal of the National Cancer Institute. 2002;94:990–1000. doi: 10.1093/jnci/94.13.990. [DOI] [PubMed] [Google Scholar]
- 50.Taniguchi K, et al. Interferon gamma induces lung colonization by intravenously inoculated B16 melanoma cells in parallel with enhanced expression of class I major histocompatibility complex antigens. presented at the Proceedings of the …; 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lollini PL, et al. Inhibition of tumor growth and enhancement of metastasis after transfection of the γ-interferon gene. Int J Cancer. 1993;55:320–329. doi: 10.1002/ijc.2910550224. [DOI] [PubMed] [Google Scholar]
- 52.Gobin S, van den Elsen PJ. Transcriptional regulation of the MHC class ib genes HLA-E, HLA-F, and HLA-G. Human immunology. 2000 doi: 10.1016/S0198-8859(00)00198-1. [DOI] [PubMed] [Google Scholar]
- 53.Chen LJ, et al. Inhibition of HLA-G Expression Via RNAi Abolishes Resistance of Extravillous Trophoblast Cell Line TEV-1 to NK Lysis. Placenta. 2010;31:519–527. doi: 10.1016/j.placenta.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 54.Morel S, et al. Processing of some antigens by the standard proteasome but not by the immunoproteasome results in poor presentation by dendritic cells. Immunity. 2000 doi: 10.1016/S1074-7613(00)80163-6. [DOI] [PubMed] [Google Scholar]
- 55.Porter GA, et al. Significance of plasma cytokine levels in melanoma patients with histologically negative sentinel lymph nodes. Annals of surgical …. 2001;8:116–122. doi: 10.1007/s10434-001-0116-3. [DOI] [PubMed] [Google Scholar]
- 56.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee SJ, et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274) FEBS Letters. 2006;580:755–762. doi: 10.1016/j.febslet.2005.12.093. [DOI] [PubMed] [Google Scholar]
- 58.Yang X, et al. Targeting the tumor microenvironment with interferon-β bridges innate and adaptive immune responses. Cancer Cell. 2014;25:37–48. doi: 10.1016/j.ccr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taube JM, et al. Colocalization of Inflammatory Response with B7-H1 Expression in Human Melanocytic Lesions Supports an Adaptive Resistance Mechanism of Immune Escape. Sci Transl Med. 2012;4:127ra37–127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tumeh PC, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akbay EA, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Atefi M, et al. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin Cancer Res. 2014;20:3446–3457. doi: 10.1158/1078-0432.CCR-13-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herbst RS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Powles T, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 65.Spranger S, et al. Up-Regulation of PD-L1, IDO, and Tregs in the Melanoma Tumor Microenvironment Is Driven by CD8+ T Cells. Sci Transl Med. 2013;5:200ra116–200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Markel G, et al. Dynamic expression of protective CEACAM1 on melanoma cells during specific immune attack. Immunology. 2009;126:186–200. doi: 10.1111/j.1365-2567.2008.02888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holmgaard RB, et al. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. Journal of Experimental Medicine. 2013;210:1389–1402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang YH, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517:386–390. doi: 10.1038/nature13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crouse J, et al. Regulation of antiviral T cell responses by type I interferons. Nature Publishing Group. 2015;15:231–242. doi: 10.1038/nri3806. [DOI] [PubMed] [Google Scholar]
- 70.Khodarev NN, et al. Signal Transducer and Activator of Transcription 1 Regulates Both Cytotoxic and Prosurvival Functions in Tumor Cells. Cancer Research. 2007;67:9214–9220. doi: 10.1158/0008-5472.CAN-07-1019. [DOI] [PubMed] [Google Scholar]
- 71.Park JM, et al. Modification of PCNA by ISG15 Plays a Crucial Role in Termination of Error-Prone Translesion DNA Synthesis. Molecular Cell. 2014;54:626–638. doi: 10.1016/j.molcel.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 72.Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends in Cell Biology. 2015;25:198–213. doi: 10.1016/j.tcb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boelens MC, et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014;159:499–513. doi: 10.1016/j.cell.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woo SR, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41:830–842. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stetson DB, et al. Trex1 Prevents Cell-Intrinsic Initiation of Autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bernard JJ, et al. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat Med. 2012;18:1286–1290. doi: 10.1038/nm.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rudin CM, Thompson CB. Transcriptional activation of short interspersed elements by DNA-damaging agents. Genes Chromosomes Cancer. 2001;30:64–71. [PubMed] [Google Scholar]
- 78.Liddicoat BJ, et al. RNA editing by ADAR1 prevents MDA5 sensing of endogenous dsRNA as nonself. Science. 2015;349:1115–1120. doi: 10.1126/science.aac7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chiappinelli KB, et al. Inhibiting DNA Methylation Causes an Interferon Response in Cancer via dsRNA Including Endogenous Retroviruses. Cell. 2015;162:974–986. doi: 10.1016/j.cell.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roulois D, et al. DNA-Demethylating Agents Target Colorectal Cancer Cells by Inducing Viral Mimicry by Endogenous Transcripts. Cell. 2015;162:961–973. doi: 10.1016/j.cell.2015.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheon H, Stark GR. Unphosphorylated STAT1 prolongs the expression of interferon-induced immune regulatory genes. Proc Natl Acad Sci USA. 2009;106:9373–9378. doi: 10.1073/pnas.0903487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cheon H, et al. IFNβ-dependent increases in STAT1, STAT2, and IRF9 mediate resistance to viruses and DNA damage. EMBO J. 2013;32:2751–2763. doi: 10.1038/emboj.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sandler NG, et al. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature. 2014;511:601–605. doi: 10.1038/nature13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arimoto K-I, et al. Negative regulation of the RIG-I signaling by the ubiquitin ligase RNF125. Proc Natl Acad Sci USA. 2007;104:7500–7505. doi: 10.1073/pnas.0611551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu J, et al. Antiviral activity of human OASL protein is mediated by enhancing signaling of the RIG-I RNA sensor. Immunity. 2014;40:936–948. doi: 10.1016/j.immuni.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ibsen MS, et al. Structural and functional analysis reveals that human OASL binds dsRNA to enhance RIG-I signaling. Nucleic Acids Research. 2015;43:5236–5248. doi: 10.1093/nar/gkv389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malakhova OA, et al. UBP43 is a novel regulator of interferon signaling independent of its ISG15 isopeptidase activity. EMBO J. 2006;25:2358–2367. doi: 10.1038/sj.emboj.7601149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Porritt RA, Hertzog PJ. Dynamic control of type I IFN signalling by an integrated network of negative regulators. Trends Immunol. 2015;36:150–160. doi: 10.1016/j.it.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 89.Rice GI, et al. Mutations in ADAR1 cause Aicardi-Goutières syndrome associated with a type I interferon signature. Nat Genet. 2012;44:1243–1248. doi: 10.1038/ng.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bald T, et al. Immune cell-poor melanomas benefit from PD-1 blockade after targeted type I IFN activation. Cancer Discov. 2014;4:674–687. doi: 10.1158/2159-8290.CD-13-0458. [DOI] [PubMed] [Google Scholar]
- 91.Fu J, et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med. 2015;7:283ra52–283ra52. doi: 10.1126/scitranslmed.aaa4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McNab F, et al. Type I interferons in infectious disease. Vol. 15. Nature Publishing Group; 2015. pp. 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]