Differential Susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika Virus (original) (raw)
Abstract
Background
Since the major outbreak in 2007 in the Yap Island, Zika virus (ZIKV) causing dengue-like syndromes has affected multiple islands of the South Pacific region. In May 2015, the virus was detected in Brazil and then spread through South and Central America. In December 2015, ZIKV was detected in French Guiana and Martinique. The aim of the study was to evaluate the vector competence of the mosquito spp. Aedes aegypti and Aedes albopictus from the Caribbean (Martinique, Guadeloupe), North America (southern United States), South America (Brazil, French Guiana) for the currently circulating Asian genotype of ZIKV isolated from a patient in April 2014 in New Caledonia.
Methodology/Principal Findings
Mosquitoes were orally exposed to an Asian genotype of ZIKV (NC-2014-5132). Upon exposure, engorged mosquitoes were maintained at 28°±1°C, a 16h:8h light:dark cycle and 80% humidity. 25–30 mosquitoes were processed at 4, 7 and 14 days post-infection (dpi). Mosquito bodies (thorax and abdomen), heads and saliva were analyzed to measure infection, dissemination and transmission, respectively. High infection but lower disseminated infection and transmission rates were observed for both Ae. aegypti and Ae. albopictus. Ae. aegypti populations from Guadeloupe and French Guiana exhibited a higher dissemination of ZIKV than the other Ae. aegypti populations examined. Transmission of ZIKV was observed in both mosquito species at 14 dpi but at a low level.
Conclusions/Significance
This study suggests that although susceptible to infection, Ae. aegypti and Ae. albopictus were unexpectedly low competent vectors for ZIKV. This may suggest that other factors such as the large naïve population for ZIKV and the high densities of human-biting mosquitoes contribute to the rapid spread of ZIKV during the current outbreak.
Author Summary
Zika virus (ZIKV) is an emerging mosquito-borne arbovirus causing dengue-like symptoms. This virus was commonly detected in Africa and Asia. Since its emergence in Yap Island in Micronesia in 2007, ZIKV reemerged in the South Pacific region in 2013 and ultimately reached the American continent in 2015. The human biting mosquito Aedes aegypti and the less anthropophilic Aedes albopictus have been incriminated as vectors of ZIKV. Our study showed that American populations of Ae. aegypti and Ae. albopictus were able to become infected and disseminate ZIKV within the mosquito general cavity at early days (4, 7) post-infection (dpi). Nevertheless, transmission was unexpectedly low and only detected at 14 dpi. Our findings will help in designing more adapted vector control strategies and limiting the impact of a new emerging threat on human health in the Americas as did the chikungunya in 2014.
Introduction
Zika virus (ZIKV; family Flaviviridae, genus Flavivirus) was first isolated from a sentinel rhesus monkey in the Zika forest of Uganda in 1947 [[1]]. Since then, it has emerged outside of its natural range of distribution, Africa and Asia: Yap Island (Micronesia) in 2007 [2], French Polynesia in 2013 [3], New Caledonia in 2014 [4], Easter Island in 2014 [5], the Cook Islands in 2014 [6] and more recently, northeastern Brazil in May 2015 [7, 8], the starting point of a pandemic in the Americas with 26 American countries reporting active ZIKV transmission (http://www.cdc.gov/zika/geo/active-countries.html). Although reports indicate that most infections produce mild signs and symptoms of rash, fever, arthritis or arthralgia, and conjunctivitis, recent infections have been associated with more severe disease outcomes with neurological or auto-immune complications such as Guillain-Barre syndrome [9] and microcephaly (http://www.cdc.gov/zika/pdfs/possible-association-between-zika-virus-and-microcephaly.pdf). This virus has a high potential for geographic expansion into countries where Aedes spp. mosquitoes are present notably Aedes aegypti mosquitoes.
The primary vectors of ZIKV in Africa are Aedes mosquitoes with reported viral isolations from Ae. africanus and Ae. apicoargenteus [10], Ae. luteocephalus [11], Ae. furcifer and Ae. taylori [12], and Ae. vittatus [13]. The human-biting mosquito Ae. aegypti is usually considered as a laboratory-competent vector of ZIKV [14] and viral isolations were reported from the species in the wild [13, 15, 16]. However, transmission of ZIKV by African Ae. aegypti has been unexpectedly low to null [17], underlining the importance of genetic delineation of mosquito populations on vector competence [18, 19]. In addition, Aedes albopictus has also been shown to be an efficient laboratory vector of ZIKV [20], with viral isolations from field-collected mosquitoes [21].
This positive-sense, single-stranded RNA virus of 10,794-nt is composed of three major lineages: East African, West African, or Asian [22]. The Asian genotype is responsible for the current expansion of ZIKV in the Americas [22–24]. As the outcome of transmission depends on the specific pairing of vector and pathogen genotypes [25], we investigated the vector competence of populations of Ae. aegypti and Ae. albopictus from the Caribbean (Martinique, Guadeloupe), North America (southern United States), South America (Brazil, French Guiana) for an Asian genotype of ZIKV.
Materials and Methods
Ethics statement
The Institut Pasteur animal facility has received accreditation from the French Ministry of Agriculture to perform experiments on live animals in compliance with the French and European regulations on care and protection of laboratory animals. This study was approved by the Institutional Animal Care and Use Committee (IACUC) at the Institut Pasteur. No specific permits were required for the described field studies in locations which are not protected in any way and did not involve endangered or protected species.
Mosquito populations
Seven populations of mosquitoes (5 populations of Ae. aegypti and 2 of Ae. albopictus; (Table 1) from the Caribbean (Martinique, Guadeloupe) and continental America (southern United States, French Guiana, Brazil) were collected as larvae or using ovitraps. Eggs were hatched in dechlorinated tap water and larvae were reared under controlled conditions of 150–200 larvae per 1 liter and fed with one yeast tablet renewed every 3–4 days. Adults were kept in cages at 28°±1°C with a 16h:8h light:dark cycle, 80% relative humidity, and supplied with a 10% sucrose solution. The F1-F2 generation of mosquitoes was used for infection assays except for Ae. aegypti from Orlando (> F10) and Ae. albopictus from Vero Beach (F7).
Table 1. Mosquito populations collected in the Caribbean and continental Americas.
| Mosquito population | Collection site | Region | Country | Generation used | Mosquito species used |
|---|---|---|---|---|---|
| CAY | Cayenne, French Guiana | South America | French Guiana | F1 | AE |
| GUA | Baie-Mahault, Guadeloupe | Caribbean | Guadeloupe | F2 | AE |
| JUR | Jurujuba, Rio de Janeiro | South America | Brazil | F1 | AL |
| MAR | Pointe Chaudière, Martinique | Caribbean | Martinique | F1 | AE |
| ORL | Orlando, Florida | North America | United States | >F10 | AE |
| TUB | Tubiacanga, Rio de Janeiro | South America | Brazil | F1 | AE |
| VRB | Vero Beach, Florida | North America | United States | F7 | AL |
Viral strain
The ZIKV strain (NC-2014-5132) was isolated from a patient in April 2014 in New Caledonia. Viral stocks were prepared after five passages of the isolate onto Vero cells maintained at 37°C; cell infection was tracked by observation of cytopathic effect (CPE). Supernatants were collected and the viral titer was estimated by serial 10-fold dilutions on Vero cells expressed in TCID50/mL. The virus stock was divided into 1 mL aliquots and stored at—80°C until use. Partial sequences of the NC-2014-5132 strain showed that it is phylogenetically related to the Asian genotype as are ZIKV strains circulating in the South Pacific region [26] and Brazil [7]. Indeed, based on the NS5 gene sequence, the NC-2014-5132 strain exhibited 99.4% identity with ZIKV from Brazil (Dupont-Rouzeyrol, personal communication).
Mosquito experimental infections
Seven day-old females were fed an infectious blood-meal containing 1.4 mL of washed rabbit erythrocytes and 700 μL of viral suspension supplemented with a phagostimulant (ATP) at a final concentration of 5 mM. For each population, 4–6 boxes of 60 mosquitoes each were exposed to the ZIKV NC-2014-5132 strain. The titer of infectious blood-meals was 107 TCID50/mL. After the infectious blood-meal, engorged females were transferred to small containers and fed with 10% sucrose in a chamber maintained at 28°±1°C, a 16h:8h light:dark cycle and 80% humidity.
Infection, dissemination and transmission analysis
For each population, batches of 25–30 mosquitoes were analyzed at 4 and 7 days post-infection (dpi). Additionally, Ae. albopictus from Vero-Beach (VRB) and Ae. aegypti from Tubiacanga (TUB) were examined at 14 dpi. Each mosquito was processed as follows: abdomen and thorax were examined to estimate infection, head for dissemination and collected saliva for transmission. Abdomen and thorax, and head were individually ground in 300 μL of DMEM medium supplemented with 2% fetal bovine serum (FBS). Homogenates were centrifuged at 10,000 g for 5 min before titration. Saliva was collected from individual mosquitoes as described in [27]. Briefly, wings and legs of each mosquito were removed and the proboscis was inserted into a 20 μL tip containing 5 μL of FBS. After 45 min, FBS containing saliva was expelled in 45 μL of DMEM medium for titration.
Infection rate (IR) refers to the proportion of mosquitoes with infected body (abdomen and thorax) among tested mosquitoes. Disseminated infection rate (DIR) corresponds to the proportion of mosquitoes with infected head among the previously detected infected mosquitoes (i.e; abdomen/thorax positive). Transmission rate (TR) represents the proportion of mosquitoes with infectious saliva among mosquitoes with disseminated infection. Transmission efficiency (TE) represents the proportion of mosquitoes with infectious saliva among the total number of mosquitoes tested.
Viral titration
Body and head homogenates were serially diluted and inoculated onto monolayers of Vero cells in 96-well plates. Cells were incubated for 7 days at 37°C then stained with a solution of crystal violet (0.2% in 10% formaldehyde and 20% ethanol). Presence of viral particles was assessed by detection of CPE. Saliva was titrated on monolayer of Vero cells in 6 well plates incubated 7 days under an agarose overlay. Titers of saliva were expressed as pfu (plaque-forming unit)/saliva.
Statistical analysis
All statistical tests were conducted using the STATA software (StataCorp LP, Texas, USA). Rates were compared using Fisher’s exact test and sample distributions with the Kruskal-Wallis test. P-values>0·05 were considered non-significant.
Results
Similar dissemination of ZIKV in Ae. aegypti and Ae. albopictus at early dpi
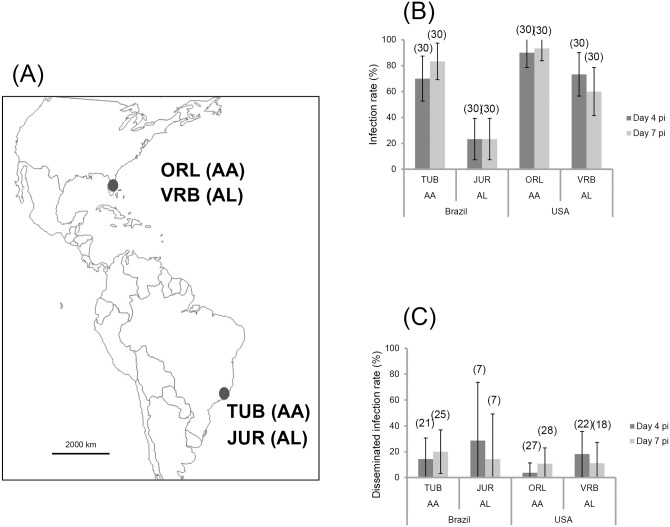
To define whether Ae. aegypti or Ae. albopictus were more likely to sustain a ZIKV outbreak, we analyzed the susceptibility to infection, as well as the ability of the virus to establish disseminated infection at 4 and 7 dpi in the two mosquito species collected from sites where they coexist, Rio de Janeiro (Brazil) and Florida (United States) (Fig 1A). When examining infection rates (IR) (Fig 1B), Ae. aegypti (TUB and ORL) were more likely to become infected than Ae. albopictus (JUR and VRB) (p < 0.001). Whereas the two _Ae_. _aegypti_ populations examined behaved similarly (TUB _versus_ ORL, p > 0.05), Ae. albopictus VRB were more infected than Ae. albopictus JUR (p = 10−3); infection rates at 4 and 7 dpi were lowest for Ae. albopictus JUR (N = 7 positive among 30 tested). When analyzing dissemination of infected mosquitoes (Fig 1C), disseminated infection rates (DIR) were low at 4 and 7 dpi regardless of the mosquito species and the collection site (p > 0.05). Transmission determined by detecting the presence of virus in mosquito saliva was not observed at early dpi (4 and 7) for any mosquito populations.
Fig 1. Mosquito populations (A), viral infection (B), dissemination (C) at days 4 and 7 after challenge of Aedes aegypti and Aedes albopictus from Continental America (Brazil and United States) with ZIKV provided at a titer of 107 TCID50/mL.
30 mosquitoes were sampled each day. The error bars represent the confidence intervals (95%). The number of individuals analyzed is given in parentheses.
Aedes aegypti and Aedes albopictus exhibit similar transmission potential for ZIKV
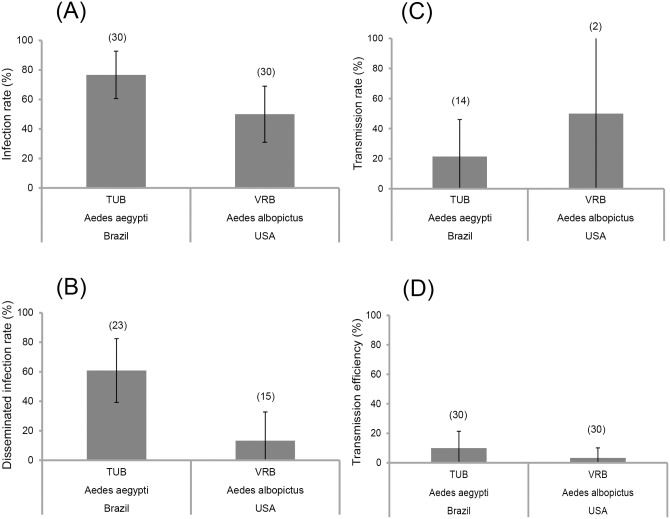
At late dpi (14), IRs, DIRs, TRs, and TEs were examined for Ae. aegypti TUB and Ae. albopictus VRB. Ae. aegypti TUB displayed higher IR and DIR than Ae. albopictus (IR: Ae. aegypti TUB: 76.7% ± 7.8 versus Ae. albopictus VRB: 50% ± 9.3, Fig 2A; DIR: Ae. aegypti TUB: 60.7% ± 10.4 versus Ae. albopictus VRB: 13.3% ± 9.1, Fig 2B). When examining the saliva of Ae. aegypti TUB and Ae. albopictus VRB at 14 dpi, TRs and viral load in saliva were higher for Ae. albopictus VRB (TR: 50% ± 50, Fig 2C; viral load: 134 ± 0 (mean ± SE), data not shown) compared to Ae. aegypti TUB (TR: 21.4% ± 11.4, Fig 2C; viral load: 18.7 ± 10.3, data not shown), even if it was not significant (P = 0.383). We should note that the number of mosquitoes examined for transmission was low despite the 30 mosquitoes initially examined. Thus we calculated the transmission efficiency showing that TEs drastically decreased to 3.3% ± 3.3 for Ae. albopictus VRB and 10% ± 5.5% for Ae. aegypti TUB (Fig 2D) suggesting that these two species were less competent to ZIKV than expected.
Fig 2. Viral infection (A), dissemination (B) and transmission (C, D) of Aedes aegypti TUB (Brazil) and Aedes albopictus VRB (United States), 14 days after oral exposure to with ZIKV.
Error bars represent the confidence intervals (95%). The number of individuals analyzed is given in parentheses.
Ae. aegypti in the French overseas territories of America disseminate ZIKV more efficiently
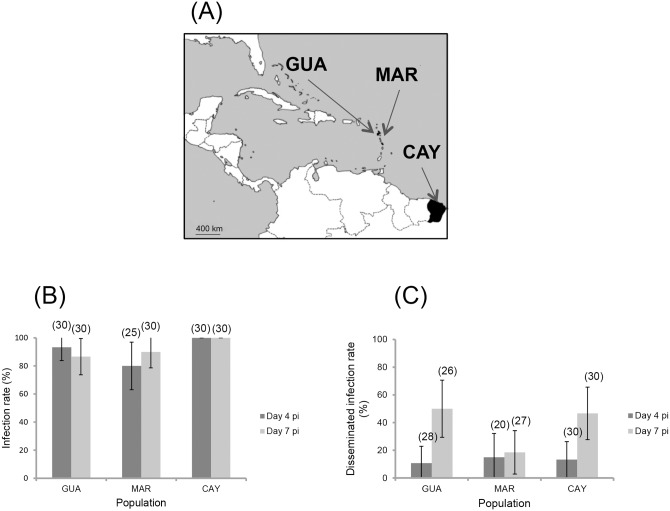
Ae. aegypti is present in French Guiana, Guadeloupe and Martinique, but where no Ae. albopictus has yet been reported (Fig 3A). We therefore determined the ability of Ae. aegypti from these territories to become infected and disseminate virus after oral exposure to ZIKV. Infection rates were high and similar regardless of dpi, and the mosquito population (Fig 3B; p > 0.05 (0.025 at 4 dpi and 0.133 at 7 dpi)). However, DIRs were reduced as compared to infection rates (Fig 3C). Viral dissemination rate, however, increased significantly with dpi except for Ae. aegypti MAR. No viral transmission was observed at 4 and 7 dpi.
Fig 3. Mosquito populations (A), viral infection (B) and dissemination (C) at days 4 and 7 after oral exposure of Aedes aegypti from the French overseas territories of America (French Guiana, Guadeloupe and Martinique) to ZIKV provided at a titer of 107 TCID50/mL.
25–30 mosquitoes were sampled each day. The error bars represent the confidence intervals (95%). The number of individuals analyzed is given in parentheses.
When comparing all five Ae. aegypti populations at 7 dpi, DIRs were significantly different (P = 0.002) and two homogeneous groups could be distinguished: Ae. aegypti from Guadeloupe and French Guiana (P = 0.803) with higher DIRs compared to Ae. aegypti from Martinique, USA and Brazil (P = 0.609).
Discussion
Zika virus has recently started to spread outside its natural range of distribution. After the South Pacific islands (Yap island, French Polynesia, New Caledonia, Cook islands, Easter island; [28]]), ZIKV was detected in the South American continent: Brazil in May 2015 [7], and since, 26 American countries (http://www.cdc.gov/zika/geo/active-countries.html). The first autochthonous cases were recently recorded in Martinique and French Guiana where the mosquito Ae. aegypti was assumed to be the unique vector. Our study showed that the Asian genotype of ZIKV infected and was disseminated by the vectors Ae. aegypti and Ae. albopictus collected in the Caribbean and continental America. Furthermore, we showed that Ae. aegypti from Rio de Janeiro in Brazil and Ae. albopictus from Vero Beach in the United States were able to transmit ZIKV at 14 dpi. Although susceptible to infection, these populations were unexpectedly low competent vectors for ZIKV.
After the emergence of chikungunya virus (CHIKV) from East Africa [29] followed by its worldwide expansion and establishment in the Americas of the Asian lineage since October 2013 [30], ZIKV became a second example of emergence of a vector-borne disease threatening a new continent. Both viruses originated in Africa where they circulate in an enzootic cycle involving non-human primates and a wide variety of zoophilic mosquitoes [17, 31]. Human outbreaks due to CHIKV involve anthropophilic vectors such as Ae. aegypti and to a lesser extent, Ae. albopictus. This latter species has been shown to be capable of transmitting at least 26 arboviruses in the laboratory and its implication as a main vector became a reality with the recent CHIKV pandemic [32]. Ae. albopictus transmits preferentially a CHIKV variant presenting an amino-acid change in an envelope glycoprotein [33, 34]. This viral variant was selected after passing through the midgut barrier, the first step in mosquito infection [35]. We showed that Ae. albopictus VRB restrained ZIKV dissemination highlighting the importance of barriers such as the midgut in Ae. albopictus mosquitoes. The examination of ZIKV infection in Ae. aegypti TUB underlined the significant role of salivary glands in transmission. Therefore, the specific role of salivary glands on ZIKV transmission by Ae. aegypti and the passage of the virus in this mosquito compartment should be explored more in detail as it has been done with CHIKV in Ae. albopictus [36]. However, the proportion of mosquitoes capable of transmitting ZIKV on the total number of tested mosquitoes, was unexpectedly low suggesting that these two species were poorly competent to ZIKV.
We also demonstrated that Ae. albopictus from Florida was at least two times more susceptible to ZIKV infection than Ae. albopictus collected in Rio de Janeiro, underlining differences depending on the mosquito population described under genotype-by-genotype (G x G) interactions where the outcome of infection depends on the specific pairing of vector and pathogen genotypes [37]. Additionally, the two Ae. albopictus populations examined from the Americas exhibited lower susceptibilities to ZIKV than Ae. albopictus from tropical Asia (i.e. Singapore) [20].
Zika disease can be confused with dengue fever and chikungunya fever, all transmitted by Ae. aegypti and Ae. albopictus. Viremia in patients was lower when infected with ZIKV, i.e. 103−106 RNA copies/mL [23] compared to viremia for dengue virus (DENV) (106−107 RNA copies/mL; [38] and CHIKV (107−109 RNA copies/mL; [39]). We thus expected a longer extrinsic incubation period (EIP) associated with the lower viremia. EIP corresponds to the time necessary for the virus to reach the mosquito saliva after an infectious blood-meal [40]. An increase of the blood-meal viral titer has been demonstrated to decrease the length of the EIP. For other flaviviruses than ZIKV, yellow fever virus and DENV, viral particles started to be detected in salivary glands of Ae. aegypti at 10 dpi [41] and at 7–9 dpi [42, 43], respectively. With ZIKV, we showed an EIP longer than 7 days with a blood-meal at 107 TCID50/mL to Ae. aegypti and Ae. albopictus from the Americas. [41]. Of note, artificial feeding systems usually need higher viral titers to reproduce infection rates observed when mosquitoes fed on viremic hosts [44]. Capable of inducing even higher viremia, CHIKV has been mainly associated with very shorter EIP, e.g. 2 days [27]. Therefore ZIKV does not present the same features as CHIKV in mosquito populations from the Americas with a longer EIP; this longer EIP would allow a broader window for implementation of vector control measures. Surveillance and control measures against ZIKV transmission in the Americas and more specifically, in Brazil, the starting point of the Zika outbreak, mainly use measures implemented for dengue control focused on Ae. aegypti (http://portalsaude.saude.gov.br/images/pdf/2015/dezembro/09/Microcefalia---Protocolo-de-vigil--ncia-e-resposta---vers--o-1----09dez2015-8h.pdf). However, if ZIKV is able to infect and be transmitted by other mosquito species (e.g. Culex spp.), their role in transmission would need to be defined to help design of more adapted vector control strategies aiming to impair the spread of the Zika outbreak in the continent.
The recent introduction of ZIKV in the Americas and its rapid spread across the continent and the Caribbean is likely attributable to the globalization of trades and travels and also the ability of local Ae. aegypti and Ae. albopictus to disseminate and then to transmit the Asian genotype of ZIKV. Contrary to the scenario with CHIKV, longer EIPs of ZIKV in the populations examined would allow implementation of more adapted vector control measures leading to improved limitation of this new emerging threat to human health in the Americas. Nevertheless, both Ae. aegypti and Ae. albopictus in the Americas do not appear to be highly efficient vectors of ZIKV, which may be balanced by the large number of susceptible humans and their close contacts with Aedes mosquitoes.
Acknowledgments
The authors thank Karima Zouache, Pei-Shi Yen, Fadila Amraoui for technical help. We thank Pei-Shi Yen for having provided mosquitoes from Orlando and L Philip Lounibos (and his laboratory) for mosquitoes collected in Vero Beach, Pascal Gaborit and Jean Issaly for helping in mosquito collections in Cayenne, and Céline Charles in Martinique. We are grateful to Joel Gustave and Isabelle Dusfour for their support, and Olivia O’Connor for virus isolation. We warmly thank Richard Paul for correcting the manuscript. We also thank the two PI of the Labex IBEID program, Pascale Cossart and Philippe Sansonetti for supporting our studies on vector-borne-diseases.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was funded by the Institut Pasteur, the French Government's Investissement d'Avenir program, Laboratoire d'Excellence "Integrative Biology of Emerging Infectious Diseases" (grant n°ANR-10-LABX-62-IBEID). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dick GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg. 1952;46(5):521–34. [DOI] [PubMed] [Google Scholar]
- 2.Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–43. 10.1056/NEJMoa0805715 [DOI] [PubMed] [Google Scholar]
- 3.Cao-Lormeau VM, Roche C, Teissier A, Robin E, Berry AL, Mallet HP, et al. Zika virus, French polynesia, South pacific, 2013. Emerg Infect Dis. 2014;20(6):1085–6. 10.3201/eid2006.140138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dupont-Rouzeyrol M, O'Connor O, Calvez E, Daures M, John M, Grangeon JP, et al. Co-infection with Zika and dengue viruses in 2 patients, New Caledonia, 2014. Emerg Infect Dis. 2015;21(2):381–2. 10.3201/eid2102.141553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tognarelli J, Ulloa S, Villagra E, Lagos J, Aguayo C, Fasce R, et al. A report on the outbreak of Zika virus on Easter Island, South Pacific, 2014. Arch Virol. 2015. [DOI] [PubMed] [Google Scholar]
- 6.Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infect. 2014;20(10):O595–6. 10.1111/1469-0691.12707 [DOI] [PubMed] [Google Scholar]
- 7.Zanluca C, de Melo VC, Mosimann AL, Dos Santos GI, Dos Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015;110(4):569–72. 10.1590/0074-02760150192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campos GS, Bandeira AC, Sardi SI. Zika Virus Outbreak, Bahia, Brazil. Emerg Infect Dis. 2015;21(10):1885–6. 10.3201/eid2110.150847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastere S, Valour F, et al. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill. 2014;19(9). [DOI] [PubMed] [Google Scholar]
- 10.McCrae AW, Kirya BG. Yellow fever and Zika virus epizootics and enzootics in Uganda. Trans R Soc Trop Med Hyg. 1982;76(4):552–62. [DOI] [PubMed] [Google Scholar]
- 11.Fagbami AH. Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State. J Hyg (Lond). 1979;83(2):213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monlun E, Zeller H, Le Guenno B, Traore-Lamizana M, Hervy JP, Adam F, et al. [Surveillance of the circulation of arbovirus of medical interest in the region of eastern Senegal]. Bull Soc Pathol Exot. 1993;86(1):21–8. [PubMed] [Google Scholar]
- 13.Diallo D, Sall AA, Diagne CT, Faye O, Faye O, Ba Y, et al. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS One. 2014;9(10):e109442 10.1371/journal.pone.0109442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li MI, Wong PS, Ng LC, Tan CH. Oral susceptibility of Singapore Aedes (Stegomyia) aegypti (Linnaeus) to Zika virus. PLoS Negl Trop Dis. 2012;6(8):e1792 10.1371/journal.pntd.0001792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am J Trop Med Hyg. 1969;18(3):411–5. [DOI] [PubMed] [Google Scholar]
- 16.Akoua-Koffi C, Diarrassouba S, Benie VB, Ngbichi JM, Bozoua T, Bosson A, et al. [Investigation surrounding a fatal case of yellow fever in Cote d'Ivoire in 1999]. Bull Soc Pathol Exot. 2001;94(3):227–30. [PubMed] [Google Scholar]
- 17.Diagne CT, Diallo D, Faye O, Ba Y, Faye O, Gaye A, et al. Potential of selected Senegalese Aedes spp. mosquitoes (Diptera: Culicidae) to transmit Zika virus. BMC Infect Dis. 2015;15:492 10.1186/s12879-015-1231-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tesh RB, Gubler DJ, Rosen L. Variation among goegraphic strains of Aedes albopictus in susceptibility to infection with chikungunya virus. Am J Trop Med Hyg. 1976;25(2):326–35. [DOI] [PubMed] [Google Scholar]
- 19.Failloux AB, Vazeille M, Rodhain F. Geographic genetic variation in populations of the dengue virus vector Aedes aegypti. J Mol Evol. 2002;55(6):653–63. [DOI] [PubMed] [Google Scholar]
- 20.Wong PS, Li MZ, Chong CS, Ng LC, Tan CH. Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Negl Trop Dis. 2013;7(8):e2348 10.1371/journal.pntd.0002348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grard G, Caron M, Mombo IM, Nkoghe D, Mboui Ondo S, Jiolle D, et al. Zika virus in Gabon (Central Africa)—2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8(2):e2681 10.1371/journal.pntd.0002681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faye O, Freire CC, Iamarino A, Faye O, de Oliveira JV, Diallo M, et al. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis. 2014;8(1):e2636 10.1371/journal.pntd.0002636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14(8):1232–9. 10.3201/eid1408.080287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, et al. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis. 2012;6(2):e1477 10.1371/journal.pntd.0001477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fansiri T, Fontaine A, Diancourt L, Caro V, Thaisomboonsuk B, Richardson JH, et al. Genetic mapping of specific interactions between Aedes aegypti mosquitoes and dengue viruses. PLoS Genet. 2013;9(8):e1003621 10.1371/journal.pgen.1003621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baronti C, Piorkowski G, Charrel RN, Boubis L, Leparc-Goffart I, de Lamballerie X. Complete coding sequence of zika virus from a French polynesia outbreak in 2013. Genome Announc. 2014;2(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubrulle M, Mousson L, Moutailler S, Vazeille M, Failloux AB. Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PLoS One. 2009;4(6):e5895 10.1371/journal.pone.0005895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musso D, Cao-Lormeau VM, Gubler DJ. Zika virus: following the path of dengue and chikungunya? Lancet. 2015;386(9990):243–4. 10.1016/S0140-6736(15)61273-9 [DOI] [PubMed] [Google Scholar]
- 29.Kariuki Njenga M, Nderitu L, Ledermann JP, Ndirangu A, Logue CH, Kelly CH, et al. Tracking epidemic Chikungunya virus into the Indian Ocean from East Africa. J Gen Virol. 2008;89(Pt 11):2754–60. 10.1099/vir.0.2008/005413-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weaver SC. Arrival of chikungunya virus in the new world: prospects for spread and impact on public health. PLoS Negl Trop Dis. 2014;8(6):e2921 10.1371/journal.pntd.0002921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coffey LL, Failloux AB, Weaver SC. Chikungunya virus-vector interactions. Viruses. 2014;6(11):4628–63. 10.3390/v6114628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paupy C, Delatte H, Bagny L, Corbel V, Fontenille D. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect. 2009;11(14–15):1177–85. d 10.1016/j.micinf.2009.05.005 [DOI] [PubMed] [Google Scholar]
- 33.Vazeille M, Moutailler S, Coudrier D, Rousseaux C, Khun H, Huerre M, et al. Two Chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS One. 2007;2(11):e1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3(12):e201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arias-Goeta C, Mousson L, Rougeon F, Failloux AB. Dissemination and transmission of the E1-226V variant of chikungunya virus in Aedes albopictus are controlled at the midgut barrier level. PLoS One. 2013;8(2):e57548 10.1371/journal.pone.0057548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vega-Rua A, Schmitt C, Bonne I, Krijnse Locker J, Failloux AB. Chikungunya Virus Replication in Salivary Glands of the Mosquito Aedes albopictus. Viruses. 2015;7(11):5902–7. 10.3390/v7112917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambrechts L, Chevillon C, Albright RG, Thaisomboonsuk B, Richardson JH, Jarman RG, et al. Genetic specificity and potential for local adaptation between dengue viruses and mosquito vectors. BMC Evol Biol. 2009;9:160 10.1186/1471-2148-9-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyet MN, Duong TH, Trung VT, Nguyen TH, Tran CN, Long VT, et al. Host and viral features of human dengue cases shape the population of infected and infectious Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A. 2013;110(22):9072–7. 10.1073/pnas.1303395110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caron M, Paupy C, Grard G, Becquart P, Mombo I, Nso BB, et al. Recent introduction and rapid dissemination of Chikungunya virus and Dengue virus serotype 2 associated with human and mosquito coinfections in Gabon, central Africa. Clin Infect Dis. 2012;55(6):e45–53. 10.1093/cid/cis530 [DOI] [PubMed] [Google Scholar]
- 40.Kramer LD, Ebel GD. Dynamics of flavivirus infection in mosquitoes. Adv Virus Res. 2003;60:187–232. [DOI] [PubMed] [Google Scholar]
- 41.McElroy KL, Girard YA, McGee CE, Tsetsarkin KA, Vanlandingham DL, Higgs S. Characterization of the antigen distribution and tissue tropisms of three phenotypically distinct yellow fever virus variants in orally infected Aedes aegypti mosquitoes. Vector Borne Zoonotic Dis. 2008;8(5):675–87. 10.1089/vbz.2007.0269 [DOI] [PubMed] [Google Scholar]
- 42.Rohani A, Wong YC, Zamre I, Lee HL, Zurainee MN. The effect of extrinsic incubation temperature on development of dengue serotype 2 and 4 viruses in Aedes aegypti (L.). Southeast Asian J Trop Med Public Health. 2009;40(5):942–50. [PubMed] [Google Scholar]
- 43.Xiao FZ, Zhang Y, Deng YQ, He S, Xie HG, Zhou XN, et al. The effect of temperature on the extrinsic incubation period and infection rate of dengue virus serotype 2 infection in Aedes albopictus. Arch Virol. 2014;159(11):3053–7. 10.1007/s00705-014-2051-1 [DOI] [PubMed] [Google Scholar]
- 44.Turell MJ. Reduced Rift Valley fever virus infection rates in mosquitoes associated with pledget feedings. Am J Trop Med Hyg. 1988;39(6):597–602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.