Spirodiketopiperazine-Based CCR5 Inhibitor Which Preserves CC-Chemokine/CCR5 Interactions and Exerts Potent Activity against R5 Human Immunodeficiency Virus Type 1 In Vitro (original) (raw)
Abstract
We identified a novel spirodiketopiperazine (SDP) derivative, AK602/ONO4128/GW873140, which specifically blocked the binding of macrophage inflammatory protein 1α (MIP-1α) to CCR5 with a high affinity (Kd of ≈3 nM), potently blocked human immunodeficiency virus type 1 (HIV-1) gp120/CCR5 binding and exerted potent activity against a wide spectrum of laboratory and primary R5 HIV-1 isolates, including multidrug-resistant HIV-1 (HIV-1MDR) (50% inhibitory concentration values of 0.1 to 0.6 nM) in vitro. AK602 competitively blocked the binding to CCR5 expressed on Chinese hamster ovary cells of two monoclonal antibodies, 45523, directed against multidomain epitopes of CCR5, and 45531, specific against the C-terminal half of the second extracellular loop (ECL2B) of CCR5. AK602, despite its much greater anti-HIV-1 activity than other previously published CCR5 inhibitors, including TAK-779 and SCH-C, preserved RANTES (regulated on activation normal T-cell expressed and secreted) and MIP-1β binding to CCR5+ cells and their functions, including CC-chemokine-induced chemotaxis and CCR5 internalization, while TAK-779 and SCH-C fully blocked the CC-chemokine/CCR5 interactions. Pharmacokinetic studies revealed favorable oral bioavailability in rodents. These data warrant further development of AK602 as a potential therapeutic for HIV-1 infection.
Highly active antiretroviral therapy has had a major impact on the AIDS epidemic in industrially advanced nations (5, 20); however, eradication of human immunodeficiency virus type 1 (HIV 1) appears to be currently impossible, in part due to the viral reservoirs remaining in blood and infected tissues (6, 27). The limitation of antiviral therapy of AIDS is exacerbated by complicated regimens, the development of drug-resistant HIV-1 variants (11), and a number of inherent adverse effects. Successful antiviral drugs, in theory, exert their virus-specific effects by interacting with viral receptors, virally encoded enzymes, viral structural components, viral genes, or their transcripts without disturbing cellular metabolism or function (20). However, at present, no antiretroviral drugs or agents are likely to be completely specific for HIV-1 or to be devoid of toxicity or side effects in the therapy of AIDS, which has been a critical issue because patients with AIDS and its related diseases will have to receive antiretroviral therapy for a long period of time, perhaps for the rest of their lives (6, 27). Thus, the identification of new antiretroviral drugs which have unique mechanisms of action and produce no or minimal side effects remains an important therapeutic objective (20). In this respect, it has been thought that certain chemokine receptor inhibitors might produce no or minimal toxicity.
In the present study, we designed, synthesized, and identified a novel small nonpeptidic CCR5 inhibitor, AK602/ONO4128/GW873140, and related compounds which showed high binding affinity to CCR5, potently inhibited CCR5 gp120 interactions, and had potent HIV-1-specific antiviral activity against laboratory and clinical strains of HIV-1, including highly drug-resistant HIV-1 variants. We describe here the pharmacological characteristics of AK602/ONO4128/GW873140 and itsunique feature that, despite the compound's much greater anti-HIV-1 activity compared to previously published CCR5 inhibitors, AK602/ONO4128/GW873140 preserves RANTES and MIP-1β binding to CCR5+ cells and their functions.
MATERIALS AND METHODS
Reagents.
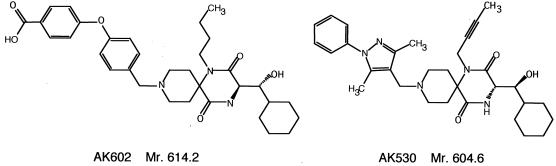
Two newly designed and synthesized spirodiketopiperazine (SDP) derivatives, AK530 [(3_S_)-1-but-2-yn-1-yl-3-[(1_S_)-cyclohexylhydroxymethyl]-9 (3,5-dimethyl-1-phenyl-1H-pyrazol-4-ylmethyl)-1,4,9-triazaspiro[5.5]undecane 2,5-dione dihydrochloride] and AK602 [4-[4-[(3_R_)-1-butyl-3-[(1_R_) cyclohexylhy-droxymethyl]-2,5-dioxo-1,4,9-triazaspiro[5.5]undec-9-yl methyl]phenoxy]benzoic acid hydrochloride], are discussed in the present report. The methods for their synthesis and physicochemical profiles will be described elsewhere. The structures of these two compounds are shown in Fig. 1. A previously reported prototypic SDP derivative, E913 (17), was used as a reference compound. E921 and AK671, which have the same structures as CCR5 inhibitors TAK-779 and SCH-351125 (SCH-C), respectively, were synthesized as previously described by others (1, 28).
FIG. 1.
Structures of AK602 and AK530.
Zidovudine was purchased from Sigma (St. Louis, Mo.). Nelfinavir and saquinavir were provided by Japan Energy (Tokyo, Japan) and Roche Products (Welwyn Garden City, United Kingdom), respectively.
125I-labeled chemokines macrophage inflammatory protein-1α (MIP-1α), macrophage inflammatory protein-1β (MIP-1β), and RANTES were purchased from Amersham Pharmacia Biotech (Little Chalfont, United Kingdom) and PerkinElmer Life Sciences, Inc. (Boston, Mass.), and three corresponding unlabeled chemokines (MIP-1α, MIP-1β, and RANTES) were purchased from PeproTech Inc. (Rocky Hill, N.J.). Recombinant HIV-1YU2 gp120 (rgp120) and human soluble CD4 (sCD4) were purchased from Immuno Diagnostics, Inc. (Woburn, Mass.).
Cells, viruses, and anti-HIV-1 assay.
Chinese hamster ovary (CHO) cells expressing CCR5 (17) were maintained in Ham's F-12 medium (Gibco-BRL, Rockville, Md.) supplemented with 10% fetal calf serum (JRH Biosciences, Lenaxa, Kans.), 50 U of penicillin per ml, and 50 μg of streptomycin per ml in the presence of 5 μg of blasticidin S hydrochloride per ml. Peripheral blood mononuclear cells were isolated from buffy coats of HIV-1-seronegative individuals with Ficoll-Hypaque density gradient centrifugation and cultured at a concentration of 106 cells/ml in RPMI 1640-based culture medium supplemented with 10% fetal calf serum and antibiotics with 10 μg of phytohemagglutinin per ml for 3 days prior to use (phytohemagglutinin-peripheral blood mononuclear cells). Cell line CCR5+ MOLT4 (18) was a kind gift from Yosuke Maeda, Kumamoto University, Japan.
A panel of HIV-1 strains was employed for drug susceptibility assays: HIV 1Ba-L (8), HIV-1JR-FL (13) HIV-1NL4-3 (34), a wild-type HIV-1MOKW isolated from a drug-naive AIDS patient (17), and two multidrug-resistant (HIV-1MDR) primary HIV-1 strains (HIV-1JSL and HIV-1MM) (36). All primary HIV-1 strains were passaged once or twice in phytohemagglutinin-peripheral blood mononuclear cell cultures, and the culture supernatants were stored at −80°C until use. Antiviral assays with phytohemagglutinin-peripheral blood mononuclear cells were conducted as previously reported (12, 17, 26).
HIV-1 gp120 binding inhibition assays.
CCR5+ CHO cells were incubated with rgp120 (5 μg/ml) and sCD4 at 5 μg/ml, biotinylated with EZ-link sulfo-NHS-SS-biotin (Pierce, Rockford, Ill.) in the presence of the indicated concentrations of a CCR5 inhibitor for 1 h at 37°C. Cells were washed, and the binding of the rgp120-sCD4 complex to CCR5+ CHO cells was determined with phycoerythrin-conjugated streptavidin (BD PharMingen, San Diego, Calif.). Nonspecific binding was determined based on the mean florescence intensity of phycoerythrin-conjugated streptavidin with sCD4 but without rgp120. Drug concentrations that brought about 50% inhibition (IC50) of mean fluorescence intensity were then determined.
Generation of 3H-labeled CCR5 inhibitors.
Five CCR5 inhibitors, AK530, AK602, E913, E921/TAK-779, and AK671/SCH-C, were tritiated by reductive amination with sodium triacetoxyborotritide (10), methylation with [3H]methyliodide, and heterogeneous catalytic exchange with tritium gas (4). Detailed description of the radiosynthesis of the inhibitors will be presented by J.M. elsewhere. In brief, [3H]E913, [3H]AK530, and [3H]AK602 were prepared by reductive amination of the corresponding aldehyde with piperidine-containing components of each inhibitor with an excess of sodium triacetoxyborotritide, and the tritium label was positioned selectively into the methylene group connecting the two components, generating inhibitors with specific activities of 10.2 Ci/mmol, 17.5 Ci/mmol, and 8.3 Ci/mmol, respectively. [3H]E921/TAK-779 was prepared by methylating the _N_-methyl precursor of E921/TAK-779 with [3H]methyliodide, generating [3H]E921/TAK-779, with a specific activity of 6.1 Ci/mmol. For the preparation of [3H]AK671/SCH-C, methyl-2,4-dimethylpyridine-3-carboxylate was tritiated by an exchange with tritium gas, catalyzed by palladium on carbon in ethanol and triethylamine. Its conversion to N-oxide and alkaline hydrolysis of the resulting ester provided [3H]2,4-dimethyl-pyridine-3-carboxylic acid. Its condensation with _N-tert_-butoxycarbonyl precursor provided [3H]AK671/SCH-C, with a specific activity of 5 Ci/mmol.
Saturation binding assay.
CCR5+ CHO cells (1.5 × 105 cells/well) were plated onto 48-well, flat-bottomed culture plates, incubated for 24 h, rinsed with Ham's F-12 medium containing 20 mM HEPES and 0.5% bovine serum albumin (Sigma), exposed to various concentrations of each 3H-labeled CCR5 inhibitor, washed thoroughly with cold phosphate-buffered saline, and lysed with 0.5 ml of 1 N NaOH, and the radioactivity in the lysates was measured. The nonspecific binding of a radiolabeled compound was determined based on the radioactivity detected in the CCR5+ CHO cell-plated wells containing the same amount of the 3H-labled CCR5 inhibitor and a 200-fold greater amount of the corresponding non radiolabeled compound. The Kd (dissociation) values of CCR5 inhibitors and the maximal binding values (_B_max = number of CCR5/cell) were calculated based on their specific radioactivity with Graphpad Prism software (Intuitive Software for Science, San Diego, Calif.). All assays were performed in duplicate, and the values shown in this report are the arithmetic means (±1 standard deviation) of 3 to 10 independently conducted assays.
Chemokine binding inhibition and chemotaxis inhibition assays.
CCR5+ CHO cells (1.5 × 105) were plated onto 48-well microculture plates, incubated for 24 h, rinsed, exposed to 3 nM [125I]MIP-1α, [125I]MIP-1β, or [125I]RANTES in the presence of various concentrations of a CCR5 inhibitor at room temperature for 1 h, thoroughly washed with phosphate-buffered saline, and lysed with 0.5 ml of 1 N NaOH, and their radioactivity was counted. The nonspecific binding of the labeled chemokine to the cells was determined based on the radioactivity detected in the wells plated with the same number of CCR5-negative CHO (CHO-K1) cells exposed to each radiolabeled chemokine (3 nM).
Chemotaxis inhibition assays were conducted with CCR5+ MOLT4 cells and the ChemTx System (Neuro Probe, Inc., Gaithersburg, Md.). In brief, CCR5+ MOLT4 cells were exposed to various concentrations of each CCR5 inhibitor for 30 min, thoroughly rinsed, plated onto the upper chamber of the ChemTx System, exposed to 0.5 nM RANTES contained in the lower chamber, and incubated for 4 h at 37°C, and the number of the cells which migrated from the upper chamber to the lower chamber was determined. Percent chemotaxis was determined with the formula 100 × [(number of CCR5 inhibitor-exposed cells which migrated to the lower chamber in the presence of RANTES) − (number of CCR5 inhibitor-unexposed cells which migrated to the lower chamber in the absence of RANTES)]/[(number of CCR5 inhibitor-unexposed cells which migrated to the lower chamber in the presence of RANTES) − (number of CCR5 inhibitor-unexposed cells which migrated to the lower chamber in the absence of RANTES)].
FACS analysis.
Fluorescence-activated cell sorting (FACS) analysis was performed as previously described (17) with minor modifications. Briefly, CCR5+ CHO cells (3 × 105) were stained with a phycoerythrin- or fluorescein isothiocyanate-conjugated anti-CCR5 monoclonal antibody 2D7 (BD PharMingen, San Diego, Calif.) or 45523 or 45531 (R&D Systems, Minneapolis, Minn.), with or without a test CCR5 inhibitor, washed, and examined with an Epics XL (Beckman Coulter, Fullerton, Calif.).
RESULTS
Potent activity of AK602 against R5 wild-type and multidrug-resistant R5 HIV-1.
We have previously reported that a prototypic SDP derivative, E913, was active against R5 HIV-1 in vitro, with IC50 values of 30 to 60 nM as tested in target phytohemagglutinin-treated peripheral blood mononuclear cells (17). Following optimization for increased potency against R5 HIV-1 and favorable pharmacokinetic features, we identified AK602 as the most potent agent among newly designed and synthesized SDP derivatives. AK602 exerted potent activity against three wild-type R5 HIV-1 strains (HIV-1Ba-L, HIV 1JR-FL and HIV-1MOKW) with IC50 values of 0.1 to 0.4 nM (Table 1). It was of note that AK602 was substantially more potent than two previously published CCR5 inhibitors, E921/TAK-779 and AK671/SCH-C (1, 28).
TABLE 1.
Anti-HIV-1 activity of SDP derivatives
| Compound | Mean IC50 (IC90) ± SD in p24 assay (nM) | CC50a (μM) | |||||
|---|---|---|---|---|---|---|---|
| HIV-1Ba-L (R5) | HIV-1JR-FL (R5) | HIV-1MOKWb (R5) | HIV-1MMb (R5MDR) | HIV-1JSLb (R5MDR) | HIV-1NL4-3 (X4) | ||
| AK602 | 0.4 ± 0.3 (12 ± 10) | 0.1 ± 0.1 (4 ± 2) | 0.2 ± 0.1 (5 ± 3) | 0.6 ± 0.2 (11 ± 2) | 0.4 ± 0.3 (7 ± 2) | >1,000 | 50 |
| AK530 | 32 ± 27 (324 ± 120) | 13 ± 4 (144 ± 60) | NDc | ND | ND | >1,000 | 60 |
| E913 | 82 ± 58 (709 ± 256) | 81 ± 46 (>1,000) | 51 ± 14 (941 ± 201) | 61 ± 28 (>1,000) | 64 ± 30 (713 ± 405) | >1,000 | 50 |
| E921/TAK-779 | 28 ± 32 (256 ± 169) | 5 ± 1 (237 ± 25) | 11 ± 7 (194 ± 168) | 14 ± 8 (352 ± 180) | 7 ± 4 (316 ± 151) | >1,000 | 50 |
| AK671/SCH-C | 4 ± 2 (79 ± 52) | 2 ± 0.5 (56 ± 57) | 2 ± 1 (54 ± 20) | 3 ± 0.5 (138 ± 25) | 2 ± 0.3 (84 ± 18) | >1,000 | >100 |
| Zidovudine | 7 ± 4 (48 ± 21) | 10 ± 9 (157 ± 72) | 6 ± 5 (47 ± 20) | 250 ± 98 (>1,000) | 70 ± 64 (>1,000) | 11 ± 5 (181 ± 90) | >100 |
| Nelfinavir | 12 ± 8 (105 ± 48) | ND | 14 ± 8 (82 ± 56) | >1,000 | >1,000 | 20 ± 7 (75 ± 52) | ND |
| Saquinavir | 11 ± 5 (60 ± 21) | ND | 5 ± 2 (49 ± 40) | 300 ± 65 (>1,000) | 350 ± 105 (>1,000) | 10 ± 4 (48 ± 2) | ND |
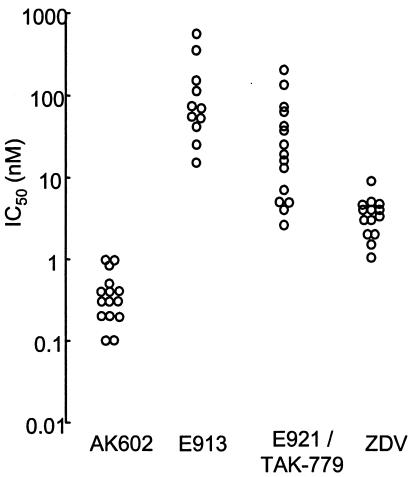
During the extended study of the antiviral activity of the prototypic E913, we noted that its activity against R5 HIV-1Ba-L in vitro varied substantially; the range of IC50 values spanned from 14 to 650 nM (Fig. 2). When we tested the activity of E921/TAK-779 in phytohemagglutinin-treated peripheral blood mononuclear cells from multiple seronegative donors, its variability was also substantial: its IC50 values varied from 2 to 200 nM. However, when we tested AK602, the variability of AK602's anti-HIV-1 activity was limited and similar to that seen for zidovudine. The difference in the range of the CCR5 inhibitor's IC50 values seems to correlate with the potency of the inhibitor examined. Indeed, we have seen a greater variability in the antiviral activity of the prototypic E913 (Fig. 2). Moreover, AK602 suppressed the infectivity and replication of two HIV-1MDR variants, HIV-1MM and HIV-1JSL (36), at extremely low concentrations (IC50 values of 0.4 to 0.6 nM), while these two R5 HIV-1 variants were less susceptible to zidovudine, nelfinavir, and saquinavir (IC50 values were greater by factors of 10 to 36, >83, and 27 to 32, respectively, compared to those against HIV-1Ba-L). As expected, none of these CCR5 inhibitors suppressed the infectivity and replication of X4 HIV-1NL4-3 in vitro. Although certain CC-chemokines reportedly enhance the replication of X4 HIV-1 (19, 22), no such enhancement of X4 HIV-1 replication was seen with the CCR5 inhibitors examined in this study at concentrations of up to 1 μM (data not shown).
FIG. 2.
Variability of anti-HIV-1 activity of AK602 in phytohemagglutinin-peripheral blood mononuclear cells. The range of IC50 values of E913 and E921/TAK-779 against HIV-1Ba-L varied substantially when examined in multiple phytohemagglutinin-peripheral blood mononuclear cells as target cells, 14 to 650 nM (n = 11) and 2 to 200 nM (n = 15), respectively, while that of AK602 was relatively narrow, 0.1 to 1 nM (n = 15), similar to that of zidovudine (ZDV), 1 to 9 nM (n = 14).
CCR5 binding properties of SDP derivatives.
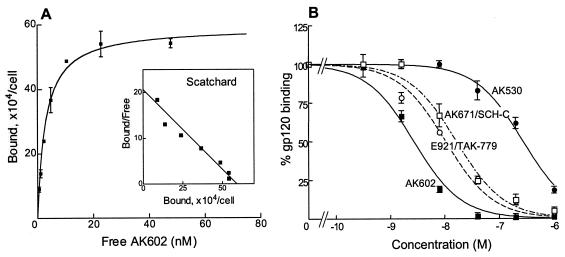
We determined the CCR5 binding profiles of SDP derivatives and compared them with those of previously published CCR5 inhibitors in saturation binding assays employing 3H-labeled compounds. Figure 3A depicts the CCR5 binding profile of AK602, showing that it binds with high affinity to CCR5. The Kd values thus determined for AK602, E913, E921/TAK-779, and AK671/SCH-C were 2.9 ± 1.0 (Fig. 3A), 111.7 ± 3.5, 32.2 ± 9.6, and 16.0 ± 1.5 nM (data not shown), respectively.
FIG. 3.
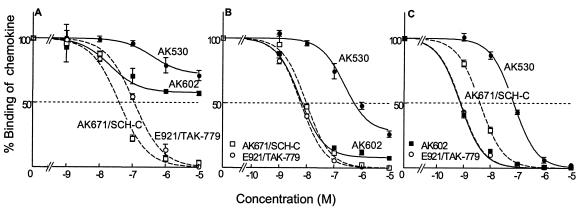
CCR5 binding profiles and rgp120 binding blocking of various CCR5 inhibitors. (A) Binding affinity of AK602 to CCR5. CCR5+ CHO cells were incubated with the 3H-labeled CCR5 inhibitors AK530, AK602, E913, E921/TAK-779, and AK671/SCH-C for 1 h. Following thorough washing, cells were lysed, the radioactivity in the lysates was determined, and _B_max and Kd values were calculated. The Kd values thus obtained were 0.4 ± 0.4, 2.9 ± 1.0, 111.7 ± 3.5, 32.2 ± 9.6, and 16.0 ± 1.5 nM, respectively. All assays were independently performed 3 to 10 times, and the values represent the arithmetic means ± 1 standard deviation. (B) AK602 potently blocks the binding of rgp120/sCD4 to CCR5. CCR5+ CHO cells were incubated with rgp120 (5 μg/ml) and sCD4 (5 μg/ml) in the presence or absence of the indicated concentrations of CCR5 inhibitors, and the binding of rgp120/sCD4 complex to CCR5+ CHO cells was determined. The 50% binding inhibition (EC50) value was determined based on the mean fluorescence intensity values obtained with or without CCR5 inhibitors. EC50 values for AK602, AK530, E921/TAK-779, and AK671/SCH-C were 2.7, 280, 12.0, and 16.5 nM, respectively.
We also asked whether the SDP derivatives blocked the binding to CCR5 of rgp120 following exposure to sCD4. As shown in Fig. 3B, AK602 potently blocked rgp120/sCD4 binding to CCR5 with an IC50 value of 2.7 nM, followed by E921/TAK-779 and AK-671/SCH-C, with IC50 values of 12.0 and 16.5 nM, respectively. When we asked whether AK602 blocked the intracellular Ca2+ mobilization induced by MIP-1α, MDC, SDF-1α, and MCP-1, whose primary receptors are CCR5, CCR4, CXCR4, and CCR2, respectively, with the method we published previously (17), AK602 completely blocked MIP-1α-induced Ca2+ mobilization at 0.1 μM and beyond; however, it failed to block Ca2+ mobilization induced with MDC, SDF-1α, and MCP-1 (data not shown).
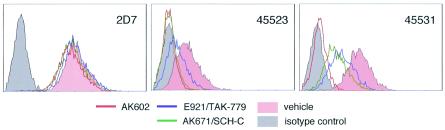
We also attempted to illustrate where AK602 binds on the CCR5 molecule by employing several monoclonal antibodies known to bind to different domains of CCR5. FACS analyses revealed that there was no AK602 inhibition of the binding of monoclonal antibody 2D7, known to bind to the N-terminal half (or domain A) of the second extracellular loop of CCR5 (14) (Fig. 4). In contrast, AK602 competitively blocked the binding of two different monoclonal antibodies, 45523, reportedly directed against multidomain epitopes of CCR5, and 45531, which is known to be specific against the C-terminal half (or domain B) of the second extracellular loop (ECL2B) of CCR5 (14), as examined with CCR5+ CHO cells (Fig. 4). These data suggest that the potent activity of AK602 against R5 HIV-1 stems from its binding to ECL2B and/or its vicinity with high affinity, resulting in inhibition of gp120/CD4 binding to CCR5. It was of note, however, that another SDP derivative, AK530, whose antiviral activity was moderate (the IC50 value against HIV-1Ba-L was 32 nM; Table 1), whose rgp120/sCD4 binding inhibition was the lowest among the inhibitors examined (IC50, 280 nM; Fig. 3B), and had only a moderate effect on the binding of monoclonal antibody 45531 to CCR5+ cells (data not shown), had the highest binding affinity to CCR5 (Kd value, 0.4 nM; data not shown) among the SDP derivatives, suggesting that the binding pocket (or subsite) of certain SDP derivatives (such as AK530) does not quite overlap that of AK602.
FIG. 4.
AK602 binds to the second extracellular loop of CCR5. AK602 at 100 nM almost completely inhibited the binding of two monoclonal antibodies, 45523, directed against multidomain epitopes of CCR5, and 45531, recognizing ECL2B of CCR5. In contrast, E921/TAK-779 and AK671/SCH-C moderately blocked the binding of 45523 and 45531. Note that there was no AK602 inhibition of the binding of a monoclonal antibody 2D7, which is known to bind to domain A of ECL2 of CCR5.
SDP derivatives bind to CCR5 but permit RANTES and MIP-1β to bind to CCR5.
We asked whether SDP derivatives blocked the binding of CC-chemokines to CCR5 expressed on the surface of CHO cells with [125I]RANTES, [125I]MIP-1α, and [125I]MIP-1β and CCR5 inhibitors AK602, AK530, E921/TAK-779, and AK671/SCH-C. As shown in Fig. 5A, the concentrations of E921/TAK-779 and AK671/SCH-C which blocked RANTES binding to CCR5 by 50% (IC50) were 110 and 40 nM, respectively, and RANTES binding was completely blocked in the presence of ≥10 μM E921/TAK-779 or AK671/SCH-C. In contrast, AK602 only partially blocked RANTES binding to CCR5 by 40% even at 10 μM (Fig. 5A). The binding of MIP-1β to CCR5 was also completely blocked by E921/TAK-779 and AK671/SCH-C; however, AK602 failed to completely block MIP-1β binding (Fig. 5B). The MIP-1β binding value in the presence of 10 μM AK602 was 10%, and no further blockade occurred at higher concentrations up to 40 μM (data not shown). AK530 also failed to completely block the binding of RANTES and MIP-1β to CCR5.
FIG. 5.
Inhibition of CC-chemokine binding to CCR5 by various CCR5 inhibitors. CCR5+ CHO cells were incubated with 3 nM [125I]RANTES (A), [125I]MIP 1 β (B), or [125I]MIP-1 α (Pnel C) in the presence and absence of various concentrations of CCR5 inhibitors. Note that while AK671/SCH-C and E921/TAK-779 completely inhibited the binding of [125I]RANTES, [125I]MIP-1α, and [125I]MIP-1β to CCR5, SDP derivatives partially blocked RANTES (A) and MIP-1β (B) binding, although they completely blocked MIP-1α binding (C).
These data suggest that the binding pockets (or subsites) of CCR5 for SDP derivatives only partially overlap the CC-chemokine binding sites of CCR5 or that the conformational changes ensuing the binding of SDP derivatives to CCR5 have only moderate effects on the binding of RANTES and MIP-1β. In the initial search for CCR5 inhibitors, lead compounds were sought as those inhibiting MIP-1α binding to CCR5 and MIP-1α-driven cytosolic Ca2+ flux, and thus, as expected, AK602 blocked MIP-1α binding to CCR5 although AK530 was substantially less potent in blocking MIP-1α binding (Fig. 5C). E921/TAK-779 and AK671/SCH-C were also found to completely block MIP-1α binding to CCR5 (Fig. 5C).
AK602 and RANTES bind simultaneously to CCR5.
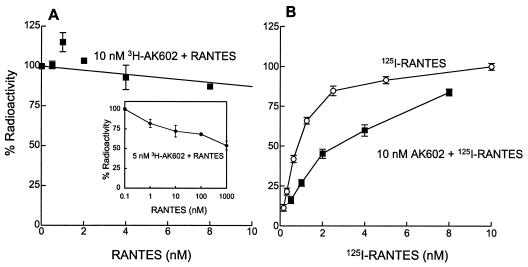
As described above, AK602 and AK530 only partially inhibited RANTES binding to CCR5+ CHO cells; however, it was not clear whether those SDP derivatives and RANTES bound simultaneously to CCR5. Therefore, competitive binding assays employing 3H-labeled and unlabeled AK602 and 125I-labeled and unlabeled RANTES were conducted. As shown in Fig. 6A, the binding of [3H]AK602 (10 nM) to CCR5 was only partially inhibited by ≥4 nM RANTES. Also, the binding of [125I]RANTES at 8 nM was only inhibited by up to 20% in the presence of 10 nM AK602 (Fig. 6B).
FIG. 6.
AK602 and RANTES bind simultaneously to CCR5. (A) CCR5+ CHO cells were exposed to 10 nM [3H]AK602 and various concentrations of unlabeled RANTES. After 1 h of incubation, the cells were washed, and the [3H]AK602 bound to the cells was measured. Note that 100% radioactivity on the ordinate denotes the radioactivity of cell-bound [3H]AK602 without RANTES and that ≈90% of CCR5 molecules are bound to AK602 at 10 nM (Fig. 3A). (B) CCR5+ CHO cells were exposed to 10 nM unlabeled AK602 and various concentrations of [125I]RANTES. After 1 h of incubation, the cells were washed, and the [125I]RANTES bound to the cells was measured. The binding profile of [125I]RANTES alone is illustrated by open circles. Note that 100% radioactivity is equated to the radioactivity of cell-bound [125I]RANTES at 10 nM. The Kd values of RANTES in the presence and absence of 10 nM AK602 were 4.5 and 0.6 nM, respectively.
The interpretation that AK602 and RANTES bind simultaneously to CCR5 was corroborated by another experiment in which a lower concentration of [3H]AK602 and much higher concentrations of RANTES were used (Fig. 6A, inset). The radioactivity counted for [3H]AK602 (5 nM) bound to CCR5+ CHO cells was only moderately blocked in the presence of 100 and 1,000 nM RANTES, by 32 and 46%, respectively (Fig. 6A, inset). These data suggest that the SDP derivatives, in particular AK602, and RANTES bind simultaneously to CCR5, although conformational changes potentially caused by either of the two might have occurred. Indeed, 15 to 25% inhibition was seen at nearly equimolar concentrations of AK602 and RANTES, which may reflect the involvement of the conformational changes caused by either of the two agents or an overlap in their binding sites (or domains).
AK602 permits RANTES-induced chemotaxis and CCR5 internalization at anti-HIV-1 activity-exerting concentrations.
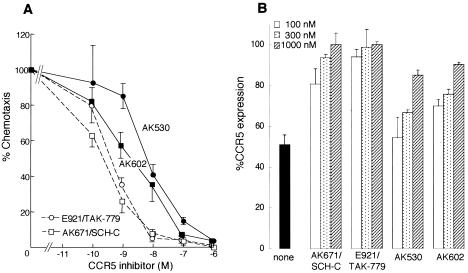
We next asked whether AK602 allowed RANTES-induced chemotaxis and CCR5 internalization with CCR5+ MOLT4 cells and CCR5+ CHO cells at its anti-HIV-1 activity-exerting concentrations. As shown in Fig. 7A, AK671/SCH-C most potently blocked chemotaxis, followed by E921/TAK-779. The chemotaxis values at the IC50s against R5 HIV-1Ba-L of AK671/SCH-C and E921/TAK-779 (4 and 24 nM, respectively: Table 1) were low, 18 and 8%, respectively, suggesting that these two inhibitors considerably blocked chemotaxis at their anti-HIV-1 IC50 concentrations as determined in peripheral blood mononuclear cells. In contrast, the chemotaxis seen at the IC50 level of AK602, 0.4 nM (see Table 1), was considerable, with 70% retained (Fig. 7A), while that seen AK530 was much less (30%).
FIG. 7.
AK602 allows RANTES-induced chemotaxis and CCR5 internalization. (A) CCR5+ MOLT4 cells were exposed to various concentrations of AK530, AK602, E921/TAK-779, or AK671/SCH-C, thoroughly washed, plated onto the upper chamber of the ChemTx System, exposed to 0.5 nM RANTES contained in the lower chamber, and incubated for 4 h; the number of the cells which migrated to the lower chamber was determined, and chemotaxis was calculated. (B) CCR5+ CHO cells were exposed to 10 nM RANTES in the presence or absence of various concentrations of each CCR5 inhibitor and washed with acidic solution for removal of the cell-bound RANTES (21). The amount of cell surface CCR5 was subsequently determined with monoclonal antibody 3A9 (BD PharMingen), which recognizes the N terminus of CCR5 and competes with none of the CCR5 inhibitors tested. In panel A, the level of chemotaxis suppression by TAK-779 and SCH C was greater than that by AK530 and AK602 at four concentrations examined, although complete suppression was seen only at the highest concentration of the AK compounds, 1 μM. However, in panel B, the level of CCR5 internalization suppression by TAK-779 and SCH-C was greater than that of the AK compounds at all three concentrations examined.
In order to corroborate the modest chemotaxis inhibition seen with AK602, the inhibition of RANTES-induced CCR5 internalization was also examined. In the absence of CCR5 inhibitors, ≈50% of CCR5 molecules were internalized from the surface of CCR5+ CHO cells incubated for 1 h at 37°C in the presence of 10 nM RANTES; however, AK671/SCH-C and E921/TAK-779 at 100 nM considerably blocked internalization, and only 19 and 6%, respectively, of CCR5 molecules were internalized. In the presence of higher concentrations of AK671/SCH-C and E921/TAK-779, 300 and 1,000 nM, virtually no CCR5 internalization occurred (Fig. 7B). In contrast, AK530 and AK602 at 100 nM allowed RANTES-induced CCR5 internalization of 46 and 30%, respectively, and even at 300 and 1,000 nM, 10 to 34% CCR5 internalization occurred (Fig. 7B).
DISCUSSION
A novel SDP derivative, AK602/ONO4128/GW873140, exhibited high affinity to CCR5, blocked rgp120/sCD4 complex binding to CCR5, and exerted potent activity against a wide spectrum of laboratory and primary R5 HIV-1 isolates, including HIV-1MDR. We recently examined AK602 against several non-clade B R5 HIV strains and found that in general AK602 is comparably active against such non-clade B strains (data not shown). It is of note that several small-molecule CCR5 inhibitors have been reported in the literature, including SCH-D (D. Schurmann et al., Abstr. 11th Conf. Retroviruses Opportunistic Infections, 2004, abstr. 140LB), UK427,857 (A. L. Pozniak et al. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., 2003, abstr. H-443), CMPD167 (32), and TAK-220 (Y. Iizawa et al., Abstr. 10th Conf. Retroviruses Opportunistic Infections, 2003, abstr. 11).
In the present study, we also demonstrated that AK602 potently blocked rgp120/sCD4 complex binding to CCR5. With respect to gp120/CD4 binding to CCR5, Olson et al. previously reported no correlation between fusion with and entry into the target cell of HIV-1 and inhibition of rgp120/sCD4 complex binding to CCR5, based on data with various anti-CCR5 monoclonal antibodies (24). However, with all small-molecule SDP derivatives examined in the present study, inhibition of HIV-1 infectivity and replication generally correlated with inhibition of the rgp120/sCD4 complex binding to CCR5, strongly suggesting that the anti-HIV-1 activity of SDP derivatives stems from their inhibition of gp120 binding to CCR5, as reported for other CCR5 inhibitors such as TAK-779 (3), although the binding pocket (or subsite) of CCR5 for certain SDP derivatives (such as AK530) apparently does not quite overlap the rgp120/sCD4 complex binding site of CCR5 (Fig. 3B). It is also possible that the conformational changes ensuing upon AK602's binding to CCR5 could differ from that ensuing upon AK530's binding to CCR5, thereby producing differences in gp120/sCD4 binding and anti-HIV activity.
It is generally noted that although the determination of any binding sites with antibodies provides “indirect” evidence, in many cases it gives good insights (14). Indeed, SCH-C has been reported to induce conformational changes in CCR5 and bind to its transmembrane (TM) domain, thereby blocking HIV-gp120 binding to CCR5. In our data, SCH-C completely blocked the binding of the “multidomain”-reactive monoclonal antibody 45523, which reportedly causes conformational changes in CCR5, while it only moderately blocked the binding of the ECL2B-specific monoclonal antibody 45531 (Fig. 4). In contrast, AK602 completely blocked the binding of both 45523 and 45531. Considering that monoclonal antibody 45531's CCR5 binding is closely linked to amino acids 184 to 189 of ECL2B, as shown by Lee and colleagues (14), it was thought that the binding site of AK602 includes ECL2B or is vicinal to it. Indeed, our recent analysis with the alanine-scanning algorithm showed that AK602 totally failed to bind to a CCR5 mutant when a K191A substitution was introduced (Maeda et al., unpublished data), corroborating and extending the idea that AK602's binding site involves the ECL2B domain.
It is noted that the IC50 of AK602 against HIV-1 as determined in peripheral blood mononuclear cells (0.4, 0.1, and 0.2 nM against HIV-1Ba-L, HIV-1JR-FL, and HIV-1MOKW, respectively: Table 1) are substantially lower than the Kd of AK602 (2.9 nM) and the IC50 of AK602 for its inhibition of rgp120/sCD4 complex binding to CCR5 (2.7 nM). The anti-HIV-1 IC50s of AK602 are also lower than the IC50s of AK602 for its inhibition of MIP-1α-induced Ca2+ influx (39.8 nM; unpublished data) and that for its inhibition of CCR5 internalization (≈300 nM; unpublished data).
One possible explanation for these inconsistencies is the different cell lines employed for each assay. However, it is of note that when we determined the IC50 values against several R5 HIV strains and Kd values of AK602 in MAGI/CCR5 cells (18), AK602's IC50s (≈0.2 nM) were reproducibly lower than AK602's Kd (3.8 nM) (data not shown). Thus, one can postulate that for the inhibition of HIV-1 infection by CCR5 inhibitors, not all CCR5 molecules might have to be occupied. In this regard, our studies with 3H-labeled AK602 and CD4+ target cells expressing CCR5, MAGI/CCR5 (18) and U373-MAGI (34), have shown that less than 30% of HIV-1 infection occurred when approximately 50% of CCR5 molecules were bound by AK602, and at its anti-HIV-1 IC50 concentration, AK602 was found to bind to 5 to 20% of CCR5 molecules on the target cells (Maeda et al., unpublished data). These data suggest that when one of the multimerized CCR5 molecules is bound or occupied by AK602, inhibition of the cell is likely to be blocked, although further stoichiometric analyses need to be conducted.
It has been thought that individuals carrying a gene encoding a mutant form of CCR5 called Delta32 are resistant to HIV-1 infection and apparently do not have significant health problems (2, 15, 23, 25). One can assume that individuals with homozygous CCR5-Delta32 might inherently have certain defenses which could compensate for the deficiency of CCR5. In this regard, there has been a report that individuals carrying homozygous CCR5-Delta32 have longer survival of renal transplants than those with other genotypes, suggesting that such individuals might have compromised graft rejection immunity (7). Moreover, Woitas et al. have reported that individuals with homozygous CCR5-Delta32 have significantly higher levels of hepatitis C virus in blood than their counterparts who have wild-type CCR5, suggesting that the CCR5-Delta32 mutation may be an adverse host factor in hepatitis C virus infection (35), although others have recently argued against a role of CCR5 in susceptibility to hepatitis C virus infection or response to antiviral therapy (9). Thus, sustained, long-term suppression of the effect of CC-chemokines/CCR5 interactions, in particular in those who carry wild-type CCR5 and might not have a possible compensatory mechanism for the absence of CCR5, might produce adverse effects, and caution should be used in the development of chemokine receptor antagonists as potential therapeutics for HIV-1 infection.
In this respect, SDP derivatives such as AK602 can preserve CC-chemokine/CCR5 interactions at their anti-HIV activity-exerting concentrations; they allow RANTES and MIP-1β binding to CCR5+ cells and their functions at anti-HIV-1 concentrations. In contrast, two previously published CCR5 inhibitors, TAK-779 and SCH-C, fully blocked CC-chemokine/CCR5 interactions (Fig. 5 and 7). It is of note that AK602's complete inhibition of the binding of MIP-1α was not surprising because in the initial search of lead compounds, we sought compounds that blocked the binding of 125I-labeled MIP-1α to CCR5+ CHO cells and MIP-1α-elicited cellular Ca2+ mobilization, as described previously (17).
In support of the above observation, the results of competitive biding assays with [3H]AK602 and [125I]RANTES and their corresponding unlabeled agents clearly indicated that AK602 and RANTES bind simultaneously to CCR5 (Fig. 6). Moreover, AK602 allowed CCR5+ MOLT4 cells to undergo RANTES-elicited chemotaxis (Fig. 7A) and CCR5+ CHO cells to internalize CCR5 in response to RANTES (Fig. 7B) at concentrations much greater than AK602's anti-HIV-1 activity-exerting concentration in peripheral blood mononuclear cells. However, it is worth noting that although AK602 blocked the binding of [125I]RANTES to CCR5+ CHO cells only by ≈40% at micromolar concentrations (Fig. 5A), it virtually completely blocked the RANTES-induced chemotaxis at micromolar concentrations, as examined in CCR5+ MOLT4 cells (Fig. 7A). This apparent inconsistency could be explained by the different cell lines employed for each assay and the fact that the number of CCR5 molecules in CCR5+ CHO cells (≈5 × 105/cell) is substantially different from that of CCR5+ MOLT4 cells (≈1 × 105/cell), and thus, AK602 could more efficiently block the chemotaxis of MOLT4 cells. It is also possible that AK602 may more effectively block CCR5 multimerization, which is reportedly important for the functionality of the G protein-coupled receptor (29), rather than the RANTES binding block to CCR5 per se. However, it is not clear yet whether AK602's unique profile that AK602 partially allows RANTES and MIP-1β to bind to CCR5 despite AK602's tight binding to CCR5 brings about a clinical advantage. This can be examined only in the setting of clinical trials and careful clinical investigation in long-term treatment with such an agent.
Several HIV-1 variants which acquired resistance to CC-chemokines, including MIP-1α and CCR5 inhibitors, have been reported. Trkola et al. described that when HIV-1 was passaged in the presence of increasing concentrations of a CCR5-specific, structurally SCH-C-related CCR5 inhibitor, AD101, an escape mutant which contained 22 amino acid substitutions in the gp120 subunits emerged as early as after 19 passages (31). This escape mutant showed a >20,000-fold resistance to AD101 and was similarly resistant to SCH-C compared with wild-type HIV-1, suggesting that HIV-1 can acquire the capability of using CCR5 bound to certain classes of CCR5 inhibitors for its entry into the target cell (31). Maeda et al. reported that HIV-1JR-FL, following in vitro selection against MIP-1α over 3 months, acquired amino acid substitutions in the V2 and V3 regions of HIV-1 gp120 and became four- to sixfold more resistant to MIP-1α, MIP-1β, and RANTES (18). In this regard, as of this writing, we have passaged HIV-1Ba-L in CD4+ CCR5+ PM1 cells (16) in the presence of moderately increasing concentrations of AK602 in one selection experiment and aggressively increasing concentrations of AK602 in another selection experiment over 22 months (45 passages); however, the virus has acquired no detectable resistance to AK602 and no significant amino acid substitutions (Nakata et al., unpublished data).
It is worth noting that the anti-HIV-1 activity of AK602 is virtually unaffected by the presence of human serum proteins. For instance, the IC50 of AK602 against HIV-1Ba-L in the presence of 10% fetal calf serum in culture medium was 0.4 ± 0.3 nM, while those of AK602 with 10 μM α1-acid glycoprotein and 45% human serum added to the culture medium were 0.8 ± 0.3 and 0.7 ± 0.7 nM, respectively. AK602 failed to induce Ca2+ flux, chemotaxis, or CCR5 internalization in CCR5+ cells (Maeda et al., unpublished data). As far as the sensitivities of our methods used in the present work, AK602 is to be categorized as a nonagonist or antagonist. The phase 1 clinical trial of AK602 in HIV-1-seronegative individuals has recently been concluded, and no significant adverse effects have been documented. Considering that AK602 potently inhibited the replication of HIV-1 in vitro and in a nonobese diabetic-SCID mouse model (Nakata et al., unpublished data) and that AK602 has a favorable oral bioavailability in rodents, averaging 20 to 30% (unpublished data), the present data strongly suggest that AK602 is a promising CCR5 inhibitor as a potential therapeutic for HIV-1 infection.
Acknowledgments
We thank Steve LaFon, Larry Boone, Jim Demarest, Eddy Arnold, Shigeyoshi Harada, Kazuhisa Yoshimura, and Yosuke Maeda for helpful discussion and critical reading of the manuscript.
This work was supported in part by a grant from the Research for the Future Program (JSPS-RFTF 97L00705) of the Japan Society for the Promotion of Science, a Grant-in-Aid for Scientific Research (Priority Areas) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Monbu-Kagakusho), and a Grant for the Promotion of AIDS Research from the Ministry of Health, Welfare, and Labor of Japan (Kosei-Rohdosho).
REFERENCES
- 1.Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96**:**5698-5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dean, M., M. Carrington, C. Winkler, G. A. Huttley, M. W. Smith, R. Allikmets, J. J. Goedert, S. P. Buchbinder, E. Vittinghoff, E. Gomperts, S. Donfield, D. Vlahov, R. Kaslow, A. Saah, C. Rinaldo, R. Detels, and S. J. O'Brien. 1996. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science 273**:**1856-1862. [DOI] [PubMed] [Google Scholar]
- 3.Dragic, T., A. Trkola, D. A. Thompson, E. G. Cormier, F. A. Kajumo, E. Maxwell, S. W. Lin, W. Ying, S. O. Smith, T. P. Sakmar, and J. P. Moore. 2000. A binding pocket for a small molecule inhibitor of HIV-1 entry within the transmembrane helices of CCR5. Proc. Natl. Acad. Sci. USA 97**:**5639-5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans, E. A. 1974. Catalytic exchange in solution, p. 271-317. In Tritium and its compounds. Wiley and Sons, New York, N.Y.
- 5.Fauci, A. S. 2003. HIV and AIDS: 20 years of science. Nat. Med. 9**:**839-843. [DOI] [PubMed] [Google Scholar]
- 6.Finzi, D., J. Blankson, J. D. Siliciano, J. B. Margolick, K. Chadwick, T. Pierson, K. Smith, J. Lisziewicz, F. Lori, C. Flexner, T. C. Quinn, R. E. Chaisson, E. Rosenberg, B. Walker, S. Gange, J. Gallant, and R. F. Siliciano. 1999. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 5**:**512-517. [DOI] [PubMed] [Google Scholar]
- 7.Fischereder, M., B. Luckow, B. Hocher, R. P. Wuthrich, U. Rothenpieler, H. Schneeberger, U. Panzer, R. A. Stahl, I. A. Hauser, K. Budde, H. Neumayer, B. K. Kramer, W. Land, and D. Schlondorff. 2001. CC chemokine receptor 5 and renal-transplant survival. Lancet 357**:**1758-1761. [DOI] [PubMed] [Google Scholar]
- 8.Gartner, S., P. Markovits, D. M. Markovitz, M. H. Kaplan, R. C. Gallo, and M. Popovic. 1986. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 233**:**215-219. [DOI] [PubMed] [Google Scholar]
- 9.Glas, J., H. P. Torok, C. Simperl, A. Konig, K. Martin, F. Schmidt, M. Schaefer, U. Schiemann, and C. Folwaczny. 2003. The Delta 32 mutation of the chemokine-receptor 5 gene neither is correlated with chronic hepatitis C nor does it predict response to therapy with interferon alpha and ribavirin. Clin. Immunol. 108**:**46-50. [DOI] [PubMed] [Google Scholar]
- 10.Gribble, G. W. 1975. Reactions of sodium borohydride in acidic media. Selective reduction of aldehydes with sodium triacetoborohydride. JCS Chem. Comm. 1975**:**535-541. [Google Scholar]
- 11.Kavlick, M. F., and H. Mitsuya. 2001. The emergence of drug-resistant human immunodeficiency virus type 1 variants and its impact on antiretroviral therapy of human immunodeficiency virus type 1 infection, p. 279-312. In E. de Clerq (ed.), The art of antiretroviral therapy. American Society for Microbiology, Washington, D.C.
- 12.Koh, Y., H. Nakata, K. Maeda, H. Ogata, G. Bilcer, T. Devasamudram, J. F. Kincaid, P. Boross, Y. F. Wang, Y. Tie, P. Volarath, L. Gaddis, R. W. Harrison, I. T. Weber, A. K. Ghosh, and H. Mitsuya. 2003. Novel bis-tetrahydrofuranylurethane-containing nonpeptidic protease inhibitor (PI) UIC-94017 (TMC114) with potent activity against multi-PI-resistant human immunodeficiency virus in vitro. Antimicrob. Agents Chemother. 47**:**3123-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koyanagi, Y., W. A. O'Brien, J. Q. Zhao, D. W. Golde, J. C. Gasson, and I. S. Chen. 1988. Cytokines alter production of HIV-1 from primary mononuclear phagocytes. Science 241**:**1673-1675. [DOI] [PubMed] [Google Scholar]
- 14.Lee, B., M. Sharron, C. Blanpain, B. J. Doranz, J. Vakili, P. Setoh, E. Berg, G. Liu, H. R. Guy, S. R. Durell, M. Parmentier, C. N. Chang, K. Price, M. Tsang, and R. W. Doms. 1999. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J. Biol. Chem. 274**:**9617-9626. [DOI] [PubMed] [Google Scholar]
- 15.Liu, R., W. A. Paxton, S. Choe, D. Ceradini, S. R. Martin, R. Horuk, M. E. MacDonald, H. Stuhlmann, R. A. Koup, and N. R. Landau. 1996. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86**:**367-377. [DOI] [PubMed] [Google Scholar]
- 16.Lusso, P., F. Cocchi, C. Balotta, P. D. Markham, A. Louie, P. Farci, R. Pal, R. C. Gallo, and M. S. Reitz, Jr. 1995. Growth of macrophage-tropic and primary human immunodeficiency virus type 1 (HIV-1) isolates in a unique CD4+ T-cell clone (PM1): failure to downregulate CD4 and to interfere with cell-line-tropic HIV-1. J. Virol. 69**:**3712-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeda, K., K. Yoshimura, S. Shibayama, H. Habashita, H. Tada, K. Sagawa, T. Miyakawa, M. Aoki, D. Fukushima, and H. Mitsuya. 2001. Novel low molecular weight spirodiketopiperazine derivatives potently inhibit R5 HIV-1 infection through their antagonistic effects on CCR5. J. Biol. Chem. 276**:**35194-35200. [DOI] [PubMed] [Google Scholar]
- 18.Maeda, Y., M. Foda, S. Matsushita, and S. Harada. 2000. Involvement of both the V2 and V3 regions of the CCR5-tropic human immunodeficiency virus type 1 envelope in reduced sensitivity to macrophage inflammatory protein 1alpha. J. Virol. 74**:**1787-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marozsan, A. J., V. S. Torre, M. Johnson, S. C. Ball, J. V. Cross, D. J. Templeton, M. E. Quinones-Mateu, R. E. Offord, and E. J. Arts. 2001. Mechanisms involved in stimulation of human immunodeficiency virus type 1 replication by aminooxypentane RANTES. J. Virol. 75**:**8624-8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitsuya, H., and J. Erickson. 1999. Discovery and development of antiretroviral therapeutics for HIV infection, p. 751-780. In T. C. Merigan, J. G. Bartlet, and D. Bolognesi (ed.), Textbook of AIDS medicine. Williams & Wilkins, Baltimore, Md.
- 21.Miyakawa, T., K. Obaru, K. Maeda, S. Harada, and H. Mitsuya. 2002. Identification of amino acid residues critical for LD78beta, a variant of human macrophage inflammatory protein-1alpha, binding to CCR5 and inhibition of R5 human immunodeficiency virus type 1 replication. J. Biol. Chem. 277**:**4649-4655. [DOI] [PubMed] [Google Scholar]
- 22.Moriuchi, H., M. Moriuchi, and A. S. Fauci. 1998. Factors secreted by human T lymphotropic virus type I (HTLV-I)-infected cells can enhance or inhibit replication of HIV-1 in HTLV-I-uninfected cells: implications for in vivo coinfection with HTLV-I and HIV-1. J. Exp. Med. 187**:**1689-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Brien, S. J., and J. P. Moore. 2000. The effect of genetic variation in chemokines and their receptors on HIV transmission and progression to AIDS. Immunol. Rev. 177**:**99-111. [DOI] [PubMed] [Google Scholar]
- 24.Olson, W. C., G. E. Rabut, K. A. Nagashima, D. N. Tran, D. J. Anselma, S. P. Monard, J. P. Segal, D. A. Thompson, F. Kajumo, Y. Guo, J. P. Moore, P. J. Maddon, and T. Dragic. 1999. Differential inhibition of human immunodeficiency virus type 1 fusion, gp120 binding, and CC-chemokine activity by monoclonal antibodies to CCR5. J. Virol. 73**:**4145-4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samson, M., F. Libert, B. J. Doranz, J. Rucker, C. Liesnard, C. M. Farber, S. Saragosti, C. Lapoumeroulie, J. Cognaux, C. Forceille, G. Muyldermans, C. Verhofstede, G. Burtonboy, M. Georges, T. Imai, S. Rana, Y. Yi, R. J. Smyth, R. G. Collman, R. W. Doms, G. Vassart, and M. Parmentier. 1996. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382**:**722-725. [DOI] [PubMed] [Google Scholar]
- 26.Shirasaka, T., M. F. Kavlick, T. Ueno, W. Y. Gao, E. Kojima, M. L. Alcaide, S. Chokekijchai, B. M. Roy, E. Arnold, R. Yarchoan, and H. Mitsuya. 1995. Emergence of human immunodeficiency virus type 1 variants with resistance to multiple dideoxynucleosides in patients receiving therapy with dideoxynucleosides. Proc. Natl. Acad. Sci. USA 92**:**2398-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siliciano, J. D., J. Kajdas, D. Finzi, T. C. Quinn, K. Chadwick, J. B. Margolick, C. Kovacs, S. J. Gange, and R. F. Siliciano. 2003. Long term follow-up studies confirm the stability of the latent reservoir for HIV 1 in resting CD4+ T cells. Nat. Med. 9**:**727-728. [DOI] [PubMed] [Google Scholar]
- 28.Strizki, J. M., S. Xu, N. E. Wagner, L. Wojcik, J. Liu, Y. Hou, M. Endres, A. Palani, S. Shapiro, J. W. Clader, W. J. Greenlee, J. R. Tagat, S. McCombie, K. Cox, A. B. Fawzi, C. C. Chou, C. Pugliese Sivo, L. Davies, M. E. Moreno, D. D. Ho, A. Trkola, C. A. Stoddart, J. P. Moore, G. R. Reyes, and B. M. Baroudy. 2001. SCH-C (SCH 351125), an orally bioavailable, small molecule antagonist of the chemokine receptor CCR5, is a potent inhibitor of HIV-1 infection in vitro and in vivo. Proc. Natl. Acad. Sci. USA 98**:**12718-12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thelen, M. 2001. Dancing to the tune of chemokines. Nat. Immunol. 2**:**129-134. [DOI] [PubMed] [Google Scholar]
- 30.Tsamis, F., S. Gavrilov, F. Kajumo, C. Seibert, S. Kuhmann, T. Ketas, A. Trkola, A. Palani, J. W. Clader, J. R. Tagat, S. McCombie, B. Baroudy, J. P. Moore, T. P. Sakmar, and T. Dragic. 2003. Analysis of the mechanism by which the small-molecule CCR5 antagonists SCH 351125 and SCH-350581 inhibit human immunodeficiency virus type 1 entry. J. Virol. 77**:**5201-5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trkola, A., S. E. Kuhmann, J. M. Strizki, E. Maxwell, T. Ketas, T. Morgan, P. Pugach, S. Xu, L. Wojcik, J. Tagat, A. Palani, S. Shapiro, J. W. Clader, S. McCombie, G. R. Reyes, B. M. Baroudy, and J. P. Moore. 2002. HIV-1 escape from a small molecule, CCR5-specific entry inhibitor does not involve CXCR4 use. Proc. Natl. Acad. Sci. USA 99**:**395-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veazey, R. S., P. J. Klasse, T. J. Ketas, J. D. Reeves, M. Piatak, Jr., K. Kunstman, S. E. Kuhmann, P. A. Marx, J. D. Lifson, J. Dufour, M. Mefford, I. Pandrea, S. M. Wolinsky, R. W. Doms, J. A. DeMartino, S. J. Siciliano, K. Lyons, M. S. Springer, and J. P. Moore. 2003. Use of a small molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection or prevent simian-human immunodeficiency virus infection. J. Exp. Med. 198**:**1551-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vodicka, M. A., W. C. Goh, L. I. Wu, M. E. Rogel, S. R. Bartz, V. L. Schweickart, C. J. Raport, and M. Emerman. 1997. Indicator cell lines for detection of primary strains of human and simian immunodeficiency viruses. Virology 233**:**193-198. [DOI] [PubMed] [Google Scholar]
- 34.Westervelt, P., H. E. Gendelman, and L. Ratner. 1991. Identification of a determinant within the human immunodeficiency virus 1 surface envelope glycoprotein critical for productive infection of primary monocytes. Proc. Natl. Acad. Sci. USA 88**:**3097-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woitas, R. P., G. Ahlenstiel, A. Iwan, J. K. Rockstroh, H. H. Brackmann, B. Kupfer, B. Matz, R. Offergeld, T. Sauerbruch, and U. Spengler. 2002. Frequency of the HIV-protective CC chemokine receptor 5-Delta32/Delta32 genotype is increased in hepatitis C. Gastroenterology 122**:**1721-1728. [DOI] [PubMed] [Google Scholar]
- 36.Yoshimura, K., R. Kato, K. Yusa, M. F. Kavlick, V. Maroun, A. Nguyen, T. Mimoto, T. Ueno, M. Shintani, J. Falloon, H. Masur, H. Hayashi, J. Erickson, and H. Mitsuya. 1999. JE-2147: a dipeptide protease inhibitor (PI) that potently inhibits multi-PI-resistant HIV-1. Proc. Natl. Acad. Sci. USA 96**:**8675-8680. [DOI] [PMC free article] [PubMed] [Google Scholar]