The Docking Protein Gab1 Is an Essential Component of an Indirect Mechanism for Fibroblast Growth Factor Stimulation of the Phosphatidylinositol 3-Kinase/Akt Antiapoptotic Pathway (original) (raw)
Abstract
The docking protein Gab1 has been implicated as a mediator of multiple signaling pathways that are activated by a variety of receptor tyrosine kinases and cytokines. We have previously proposed that fibroblast growth factor 1 (FGF1) stimulation of tyrosine phosphorylation of Gab1 and recruitment of phosphatidylinositol (PI) 3-kinase are mediated by an indirect mechanism in which the docking protein fibroblast receptor substrate 2α (FRS2α) plays a critical role. In this report, we explore the role of Gab1 in FGF1 signaling by using mouse embryo fibroblasts (MEFs) derived from Gab1−/− or FRS2α−/− mice. We demonstrate that Gab1 is essential for FGF1 stimulation of both PI 3-kinase and the antiapoptotic protein kinase Akt, while FGF1-induced mitogen-activated protein kinase (MAPK) stimulation is not affected by Gab1 deficiency. To test the indirect mechanism for FGF1 stimulation of PI 3-kinase and Akt, we use a chimeric docking protein composed of the membrane targeting signal and the phosphotyrosine-binding domain of FRS2α fused to the C-terminal portion of Gab1, the region including the binding sites for the complement of signaling proteins that are recruited by Gab1. We demonstrate that expression of the chimeric docking protein in Gab1−/− MEFs rescues PI 3-kinase and the Akt responses, while expression of the chimeric docking protein in FRS2α−/− MEFs rescues stimulation of both Akt and MAPK. These experiments underscore the essential role of Gab1 in FGF1 stimulation of the PI 3-kinase/Akt signaling pathway and provide further support for the indirect mechanism for FGF1 stimulation of PI 3-kinase involving regulated assembly of a multiprotein complex.
Fibroblast growth factors (FGFs) play a critical role in the control of diverse cellular processes essential for cell proliferation, differentiation, migration, and survival (5, 25). FGFs mediate their responses by engaging a family of four receptor tyrosine kinases (RTKs) designated FGF receptors 1 to 4 (FGFRs 1 to 4) (5, 25, 29). Both genetic and biochemical studies have shown that the docking protein fibroblast receptor substrate 2α (FRS2α) functions as a key mediator of signaling via FGFRs (17). It has been shown that FRS2α is localized in the cell membrane via a myristyl anchor and interacts constitutively with FGFRs through a phosphotyrosine-binding domain (PTB) (7, 17, 35). Upon FGF stimulation, FRS2α is phosphorylated on multiple tyrosine residues to provide four binding sites for the adaptor protein Grb2 and two binding sites for the protein tyrosine phosphatase Shp2 (8, 17). At least two additional Grb2 molecules are recruited by FRS2α indirectly through binding to Shp2 molecules that are complexed with tyrosine-phosphorylated FRS2α (8).
The physiological significance of FRS2α in FGF1 signaling was revealed by targeted disruption of the FRS2α gene in mice (9). FRS2α deficiency results in embryonic lethality at embryonic day 7.5 (E7.5) to E8. Moreover, fibroblasts isolated from FRS2α−/− embryos are deficient in FGF1 stimulation of the mitogen-activated protein kinase (MAPK), phosphatidylinositol (PI) 3-kinase, and cell proliferation (9). We have proposed that FRS2α is also required for FGF1-induced tyrosine phosphorylation of the docking protein Gab1 (Grb2-associated binder 1) by an indirect mechanism in which Grb2 bound to Gab1 via its C-terminal SH3 domain forms a complex by means of its SH2 domain with tyrosine-phosphorylated FRS2α (or tyrosine-phosphorylated Shp2) in response to FGF1 stimulation (9, 24).
Gab1 is a member of a family of three docking proteins designated Gab1, Gab2, and Gab3 (4, 6, 11, 23, 32, 34, 38). Gab1 consists of a pleckstrin homology (PH) domain, followed by a proline-rich region and multiple tyrosine phosphorylation sites that serve as binding sites for the SH2 domains of PI 3-kinase, phospholipase Cγ, Shp2, and CrkL (10, 19). Gab1 is tyrosine phosphorylated in response to stimulation of a variety of growth factors, hormones, and cytokines, including epidermal growth factor (EGF), insulin, platelet-derived growth factor (PDGF), interleukin-3 (IL-3) and IL-6, resulting in activation of both the Ras/MAPK and PI 3-kinase/Akt signaling cascades (10, 15, 19, 37). The disruption of Gab1 genes causes severe defects in cardiac, placenta, and skin development resulting in embryonic lethality (14, 27). Since Gab1−/− fibroblasts exhibit a markedly reduced MAPK response after EGF, PDGF, or hepatocyte growth factor (HGF) stimulation, it was proposed that Gab1 is an important mediator of the MAPK cascade (14, 36).
In this report, we explore the role of Gab1 in signaling via FGFR by using mouse embryo fibroblasts (MEFs) derived from Gab1−/− (27) or FRS2α−/− embryos (9). We provide evidence that Gab1 is required for FGF1-induced PI 3-kinase and Akt activation but not for FGF1 stimulation of MAPK. We also explore the indirect mechanism of FGF1 stimulation of PI 3-kinase and Akt by expressing a chimeric docking protein consisting of the membrane targeting signal and the PTB domain of FRS2α fused to the C-terminal portion of Gab1 that includes the binding sites for a variety of signaling proteins in Gab1−/− or FRS2α−/− cells. We demonstrate that the chimeric docking protein is able to rescue the PI 3-kinase and Akt responses in FGF1-stimulated Gab1−/− cells and both the Akt and MAPK responses in FRS2α−/− cells after FGF1 stimulation. Overall, our results reveal the essential requirement of Gab1 in activation of the PI 3-kinase/Akt pathway in FGF1 signaling and provide further support for the coordinated assembly of signaling molecules that takes place during FGF1 stimulation of PI 3-kinase.
MATERIALS AND METHODS
Reagents.
Anti-FRS2α, anti-Grb2, or antiphosphotyrosine antibodies were previously described (17). Anti-phospho-Akt, anti-phospho-Erk, anti-Akt, anti-Erk, and anti-phospho-Thr/Ser MAPK substrates were purchased from Cell Signaling Technology (Beverly, Mass.). Anti-Gab1 and anti-p85 antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.), and anti-Flag (M2) antibodies were from Sigma (St. Louis, Mo.).
Cell lines and culture.
Wild-type (WT) MEFs and Gab1-deficient, (Gab1−/−) cells were established from WT embryos or Gab1−/− embryos (27), respectively, essentially by the method of Sell et al. (30). Briefly, fibroblasts were prepared from E13.5 stage embryos and cultured in Dulbecco's modified Eagle's medium supplemented with 10% calf serum and 100 μg each of penicillin and streptomycin (all from GIBCO/BRL). Cells were split 1:3 every 3 days to maintain subconfluency. Gab1−/− cells went into crisis after 7 or 8 passages and resumed growth after 20 to 25 passages. FRS2α-deficient (FRS2α−/−) MEFs were previously described (9). MEFs were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and 100 μg each of penicillin and streptomycin (all from GIBCO/BRL).
Expression constructs.
Expression vectors for WT Gab1 (Gab1-WT) and for the triple Y446F Y472F Y589F mutant of Gab1 in the binding sites for PI 3-kinase (Gab1-3YF) were previously described (24). The cDNA of Gab1-WT (pcDNA3-Gab1-WT) or Gab1-3YF (pcDNA3-Gab1-3YF) was subcloned in the mammalian vector pBabe containing a gene for puromycin resistance. A plasmid was constructed to express a chimeric protein consisting of the myristylation and PTB domain of FRS2α (residues 1 to 133) fused to the tail of Gab1 (Gab1-WT or Gab1-3YF) without its PH domain (residues 116 to 694). Specific primers that contained BamHI (5′) and SacII (3′) sites or SacII (5′) and SalI (3′) sites were used in PCRs to amplify fragments from mouse FRS2α (pRK5-FRS2α) (17) and Gab1 (pcDNA3-Gab1-WT or pcDNA-Gab1-3YF), respectively. The PCR products were digested with appropriate enzymes and ligated into pBabe at the BamHI-SalI sites. All plasmids were subjected to PCR analysis to confirm the fused fragments. Expression vectors for WT and mutant chimeric FRS2α/Gab1 proteins were designated FG and FG-3YF, respectively.
Pools of stable cell lines.
The mammalian vector pBabe, containing a puromycin resistance gene was used for expression of Gab1-WT, Gab1-3YF, FG-WT, and FG-3YF. A high-titer stock of virus was produced for each construct and used for cell infection. Gab1-deficient MEFs (Gab1−/−) were infected with the virus that expressed the vector alone [Gab1−/−(Vect)] or with virus that expressed Gab1-WT [Gab1−/−(Gab1)], Gab1-3YF [Gab1−/−(Gab1-3YF)], or FG-WT [Gab1−/−(FG)], or FG-3YF [Gab1−/−(FG-3YF)]. FRS2α-deficient MEFs (FRS2α−/−) were infected with the virus that expressed the vector alone [FRS2α−/−(Vect)] or that expressed FG-WT [FRS2α−/−(FG)]. Cells were selected in medium supplemented with puromycin, and pools of selected cell cultures were used for the experiments. Prior to FGF1 stimulation, cells were starved in serum-free medium.
Immunoprecipitation and immunoblot analysis.
Control or FGF1-stimulated cells were lysed in a solution consisting of 20 mM HEPES, 150 mM NaCl, 1 mM EDTA, 1% Triton, 5% glycerol, 10 mM pyrophosphate, 1 mM orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 5 μg of aprotinin per ml, and 5 μg of leupeptin (pH 7.5) per ml. Procedures for cell solubilization, immunoprecipitation, and immunoblotting with the different antibodies were described previously (1, 21).
Generation of GST-p85 (N+C-SH2) fusion proteins and in vitro binding assay.
Glutathione _S_-transferase (GST)-N+C-SH2 fusion protein, containing the amino-terminal and carboxy-terminal SH2 domains of p85, was constructed. Specific primers were used in PCR to amplify fragment from human p85 (pRK5-p85) (31). The amplified DNAs encoding these SH2 domains were cloned into PGEX-2T. GST fusion protein was expressed in BL21 bacteria. The bacteria were sonicated in a solution consisting of 20 mM Tris, 200 mM NaCl, 10% glycerol, 0.5% Nonidet P-40 (NP-40), 2 μg of aprotinin per ml, 2 μg of leupeptin per ml, and 1 mM dithiothreitol. The supernatant was collected and incubated with a 50% slurry of glutathione-agarose 4B (Sigma). The beads were washed and resuspended in 50 mM Tris, 2 mM NaCl, and 1 mM dithiothreitol until use. For pull-down assays, GST-N+C-SH2 (p85) immobilized on gluthathione-agarose beads was incubated with 1 mg of cell lysate for 2 h at 4°C. Bound proteins were washed, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to nitrocellulose membranes for immunoblotting.
Determination of PI 3-kinase activity.
The PI 3-kinase assay was performed as described previously (13, 33). Briefly, cells were serum starved for 18 h, stimulated with FGF1, washed with cold phosphate-buffered saline, and solubilized in lysis buffer (20 mM HEPES, 150 mM NaCl, 1 mM EDTA, 1% Triton, 5% glycerol, 10 mM pyrophosphate, 1 mM orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 5 μg of aprotinin per ml, 5 μg of leupeptin [pH 7.5] per ml). The lysates were immunoprecipitated using anti-FRS2α or anti-Gab1 antibodies and protein A-Sepharose for 2 h at 4°C. The immunoprecipitates were washed three times with buffer 1 (1× phosphate-buffered saline, 1% NP-40), twice with buffer 2 (0.5 M LiCl, 0.1 M Tris [pH 7.6]), twice with TNE (10 mM Tris [pH 7.6], 100 mM NaCl, 1 mM EDTA [pH 8]), and twice with buffer 4 (20 mM HEPES [pH 7.5], 50 mM NaCl, 5 mM EDTA [pH 8], 0.03% NP-40, 30 mM tetrasodium pyrophosphate). l-α-Phosphatidylinositol (Sigma) was added (10 μl of a sonicated solution at 10 mg/ml in 20 mM HEPES [pH 7.5]), and the reaction was initiated by the addition of 40 μl of kinase buffer (20 mM Tris [pH 7.6], 75 mM NaCl, 10 mM MgCl2, 10 μM ATP, 100 mM adenosine, 10 μCi of [γ-32 P]ATP) per sample. After a 15-min incubation at room temperature with shaking, the reaction was stopped by adding 100 μl of 1 N HCl and 200 μl of CHCl3-CH3OH (1:1). The samples were centrifuged, and the lower organic phases containing phospholipids were dried. After 90 min at room temperature, the samples were resuspended in 10 μl of PI-4-P standard (0.5 ml of CHCl3, 0.5 ml of CH3OH, 2.5 μl of HCl, 1 mg of l-α-phosphatidylinositol 4-monophosphate [Sigma]) and subjected to thin-layer chromatography (VWR) performed in CHCl3-CH3OH-NH4OH-H2O (45:35:7:3), and the thin-layer chromatography plates were exposed to X-ray films for 24 h. Radioactive bands were quantitated with a PhosphorImager (Molecular Dynamics, Sunnydale, Calif.) using Image Quant software.
RESULTS
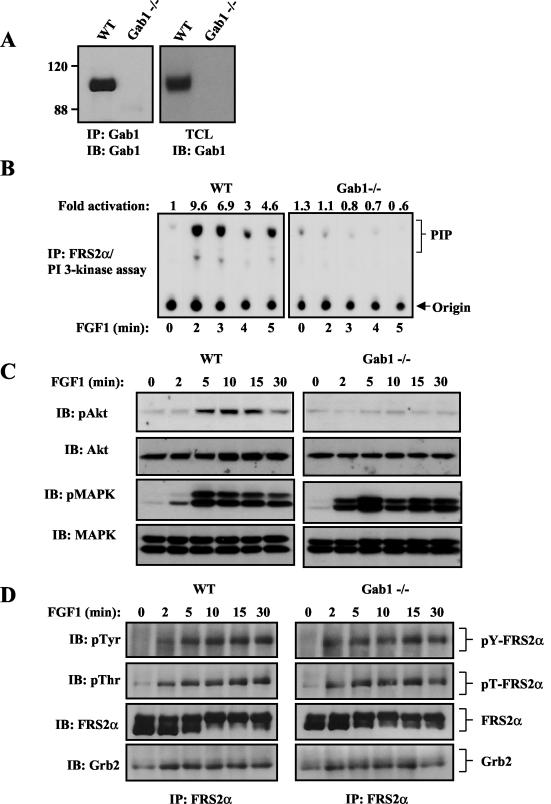
Gab1 has been implicated as a major link between a variety of growth factor and cytokine receptors and both the Ras/MAPK and PI 3-kinase/Akt signaling cascades (10, 19, 37). In order to determine the role of Gab1 in FGF1 signaling, we have analyzed the intracellular signaling pathways that are activated by FGF1 in MEFs derived from Gab1-deficient (Gab1−/−) embryos (27). An immunoblotting experiment with anti-Gab1 antibodies of lysates from WT or Gab1−/− MEFs confirmed that the mutant MEFs are indeed deficient in Gab1 (Fig. 1A).
FIG. 1.
Gab1 is required for FGF1-induced PI 3-kinase and Akt activation, but not for FGF1-induced MAPK activation. (A) Expression of Gab1 in WT and Gab1-deficient MEFs (Gab1−/−) was analyzed by immunoblotting (IB) with anti-Gab1 antibodies of Gab1 immunoprecipitations (IP) or from total cell lysates (TCL). The positions of molecular size standards (in kilodaltons) are given to the left of the blot. (B) Serum-starved WT or Gab1−/− MEFs were treated for the indicated times with FGF1 (1 ng/ml), and PI 3-kinase assay was performed on FRS2α immunoprecipitates. (C) Serum-starved WT and Gab1−/− MEFs were stimulated for the indicated times with FGF1 (1 ng/ml). Cells were lysed, and proteins were resolved by SDS-PAGE and immunoblotted with the indicated phospho-specific antibodies. The same blots were stripped and reprobed with the indicated antibodies. (D) WT or Gab1−/− MEFs were stimulated as described above for panel C, and cell lysates were immunoprecipitated with anti-FRS2α antibodies and then subjected to SDS-PAGE and immunoblotting with the indicated antibodies. pTyr, antiphosphotyrosine antibody; pThr, antiphosphothreonine antibodies.
Gab1 is required for FGF1-induced PI 3-kinase and Akt activation but not for MAPK activation.
We analyzed the capacity of FGF1 to stimulate PI 3-kinase, Akt, and MAPK responses in Gab1−/− MEFs. Compared to WT MEFs, FGF1-induced PI 3-kinase (Fig. 1B) or Akt (Fig. 1C) activation was severely compromised in Gab1−/− MEFs. Furthermore, no measurable increase in Akt stimulation was detected in Gab1-deficient MEFs for up to 2 h after FGF1 stimulation (data not shown), demonstrating that the loss of PI 3-kinase/Akt stimulation was not a consequence of a delayed response. However, FGF1 stimulation of MAPK was not affected by Gab1 deficiency; a similar MAPK response was detected in WT or Gab1−/− MEFs (Fig. 1C). Furthermore, no measurable difference in FGF1-induced MAPK stimulation was observed for up to 2 h after stimulation with 0.1 or 1 ng of FGF1 per ml (data not shown). These experiments demonstrate that Gab1 is indispensable for FGF1-induced PI 3-kinase/Akt activation and dispensable for FGF1-induced MAPK response.
Expression and tyrosine phosphorylation of FRS2α are normal in Gab1−/− MEFs.
We have previously demonstrated that FGF1-induced tyrosine phosphorylation of Gab1 and recruitment of PI 3-kinase were compromised in FRS2α−/− MEFs (9). We next examined the effect of Gab1 deficiency on FGF1-induced tyrosine phosphorylation of FRS2α. Compared to WT MEFs, FRS2α expression and FGF1-induced tyrosine phosphorylation of FRS2α were not influenced by Gab1 deficiency (Fig. 1D). Additionally, equivalent amounts of Grb2 (Fig. 1D) or Shp2 (data not shown) form a complex with tyrosine-phosphorylated FRS2α in lysates from WT or Gab1-deficient MEFs, even in response to low concentrations of FGF1 (data not shown). Furthermore, FGF1 stimulation induced a typical shift in the electrophoretic mobility of FRS2α mediated by MAPK-dependent phosphorylation of FRS2α (18) in either WT or Gab1−/− MEFs (Fig. 1D), even in response to low concentrations of FGF1 (data not shown). Tyrosine phosphorylation of FRS2α, recruitment of Grb2 and Shp2, and MAPK-dependent threonine phosphorylation of FRS2α occur in Gab1−/− cells. While FGF1-induced tyrosine phosphorylation of Gab1 is dependent on FRS2α (9), FGF1-induced tyrosine phosphorylation of FRS2α proceeds normally in Gab1−/− MEFs.
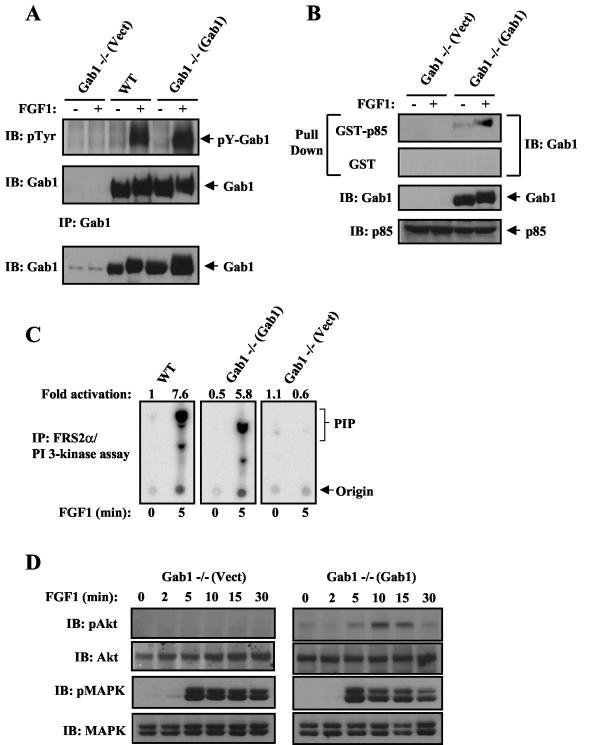
Rescue of PI 3-kinase and Akt activation upon ectopic expression of Gab1 in Gab1−/− MEFs.
We next examined the effect of ectopic expression of Gab1 in Gab1−/− MEFs on FGF1-induced PI 3-kinase and Akt as well as MAPK activation. For this purpose, Gab1-deficient MEFs were infected with WT Gab1 or with empty retrovirus as a control, and puromycin-resistant pools of cell were selected. Lysates from unstimulated or FGF1-stimulated cells were subjected to immunoprecipitation with anti-Gab1 antibodies, followed by immunoblotting with antiphosphotyrosine antibodies or by a pull-down assay with GST fusion proteins containing the two SH2 domains of p85, the regulatory subunit of PI 3-kinase [GST-p85 (N+C SH2)]. As shown in Fig. 2A and B, FGF1 induced the tyrosine phosphorylation of Gab1, as well as the formation of a complex between tyrosine-phosphorylated Gab1 and the SH2 domain of p85 in lysates from Gab1−/− cells expressing Gab1 [Gab1−/−(Gab1)]. Consequently, FGF1 stimulation of PI 3-kinase (Fig. 2C) and Akt (Fig. 2D) activation after FGF1 stimulation was fully restored in Gab1−/− cells expressing WT Gab1 compared to that in Gab1−/− cells infected with empty retrovirus [Gab1−/−(Vect)]. However, FGF1-induced MAPK stimulation was comparable in Gab1−/−(Vect) or Gab1−/−(Gab1) cells (Fig. 2D). These results confirm that Gab1 is indispensable for FGF1-induced PI 3-kinase/Akt stimulation but not required for FGF1 stimulation of MAPK.
FIG. 2.
Rescue of PI 3-kinase and Akt activation upon ectopic expression of Gab1 in Gab1-deficient MEFs. (A) The indicated cell lines were serum starved and then stimulated with FGF1 (1 ng/ml) for 10 min (+) or left unstimulated (−). Cell lysates were immunoprecipitated with anti-Gab1 and then subjected to SDS-PAGE and immunoblotting with the indicated antibodies. Expression of Gab1 in the total cell lysates is shown in the bottom blot. Abbreviations: IB, immunoblotting; pTyr, phosphotyrosine antibody; IP, immunoprecipitation. (B) Cells were treated as described above for panel A, and lysates were incubated with immobilized GST or GST-p85 containing only the N+C SH2 domains of p85. Bound proteins were eluted with SDS sample buffer and resolved by SDS-PAGE and immunoblotting with anti-Gab1 antibodies. Expression of Gab1 and p85 in the total cell lysates is shown in the bottom two blots. (C) The indicated cell lines were treated with FGF1 (1 ng/ml) and analyzed for PI 3-kinase activity as described in the legend to Fig. 1B. (D) The indicated cell lines were treated with FGF1 (1 ng/ml) and analyzed for Akt and MAPK activation as described in the legend to Fig. 1C.
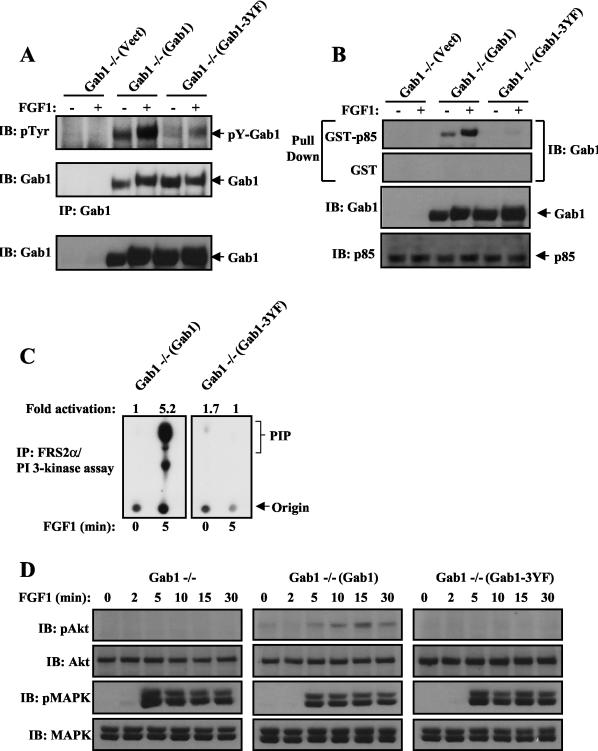
The p85-binding sites on Gab1 are required for FGF1-induced Akt activation.
Gab1 contains three potential binding sites (pYXXM) for the SH2 domain of p85 at Tyr-448, Tyr-473, and Tyr-590. To examine the possibility that Gab1-mediated recruitment and activation of PI 3-kinase are responsible for FGF1 stimulation of Akt, we have expressed a mutant Gab1 in which the three canonical binding sites for p85 were replaced by phenylalanine residues (Gab1-3YF) in Gab1−/− MEFs (Gab1−/−[Gab1-3YF]). Comparison of tyrosine phosphorylation of WT Gab1 and the Gab1-3YF mutant indicates that these tyrosine phosphorylation sites, which serve as binding sites for p85, are the major tyrosine phosphorylation sites of Gab1 after FGF1 stimulation (Fig. 3A). An in vitro pull-down assay demonstrated that WT Gab1, but not Gab1-3YF, formed a complex with the GST-p85 (N+C SH2) fusion protein (Fig. 3B). Furthermore, neither Gab1 nor Gab1-3YF forms a complex with GST, confirming the specificity of the pull-down assay (Fig. 3B). Furthermore, FGF1 stimulation of PI 3-kinase and Akt was prevented in Gab1−/−(Gab1-3YF) MEFs, while typical stimulation of PI 3-kinase and Akt was observed in Gab1−/−(Gab1) MEFs (Fig. 3C and D). However, FGF1-induced MAPK activation was similar in Gab1−/−(Gab1-3YF) and Gab1−/−(Gab1) cells (Fig. 3D). These experiments demonstrate that the binding sites for p85 on Gab1 are required for FGF1-induced Gab1-mediated stimulation of Akt.
FIG. 3.
Gab1-p85-binding mutant (Gab1-3YF) fails to rescue FGF1-mediated p85 association with Gab1 and PI 3-kinase/Akt activation. (A) The indicated cell lines were serum starved and then stimulated with FGF1 (1 ng/ml) for 10 min (+) or left unstimulated (−). Cell lysates were immunoprecipitated with anti-Gab1 and then subjected to SDS-PAGE and immunoblotting with the indicated antibodies. Expression of Gab1 in the total cell lysates is shown in the bottom blot. Abbreviations: IB, immunoblotting; pTyr, phosphotyrosine antibody; IP, immunoprecipitation. (B) Lysates from FGF1-stimulated cells were incubated with immobilized GST or GST-p85 fusion protein containing the two SH2 domains of p85. Bound proteins were eluted with SDS sample buffer and resolved by SDS-PAGE, followed by immunoblotting with anti-Gab1 antibodies. Expression of Gab1 and p85 in the total cell lysates is shown in the bottom two blots. (C) Serum-starved Gab1−/−(Gab1) or Gab1−/−(Gab1-3YF) MEFs were treated for the indicated times with FGF1 (1 ng/ml), and PI 3-kinase assay was performed on FRS2α immunoprecipitates. (D) Analysis of FGF1 stimulation of Akt and MAPK activity. The indicated cell lines were treated and processed as described in the legend to Fig. 1C.
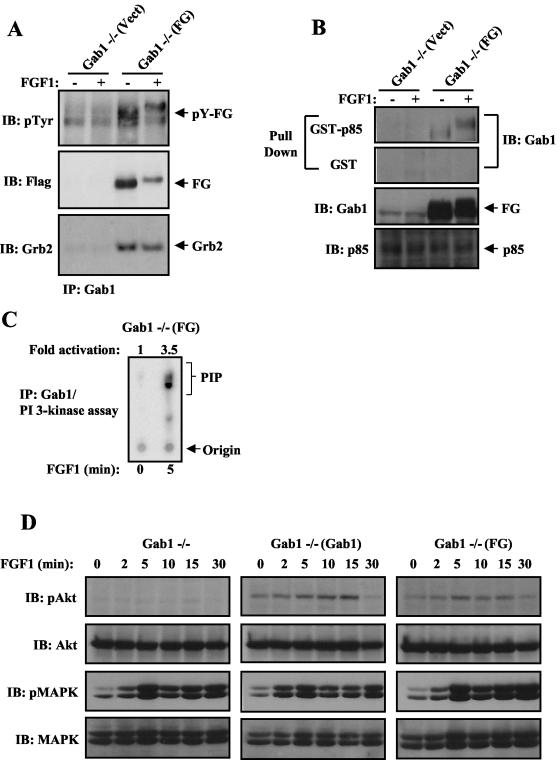
Rescue of FGF1-induced PI 3-kinase/Akt activation by expression of a chimeric FRS2α/Gab1 docking protein in Gab1−/− cells.
We have previously proposed that FGF1 induces tyrosine phosphorylation of Gab1 by an indirect mechanism in which a constitutive Grb2/Gab1 complex is recruited by tyrosine-phosphorylated FRS2α, resulting in tyrosine phosphorylation of Gab1 by the tyrosine kinase of FGFR (9, 24). To test this mechanism, we have generated a chimeric docking protein consisting of the myristoylation site and PTB domain of FRS2α in place of the PH domain of Gab1. In addition, a Flag tag was added to the C terminus of the chimeric docking protein that was designated the FG chimera.
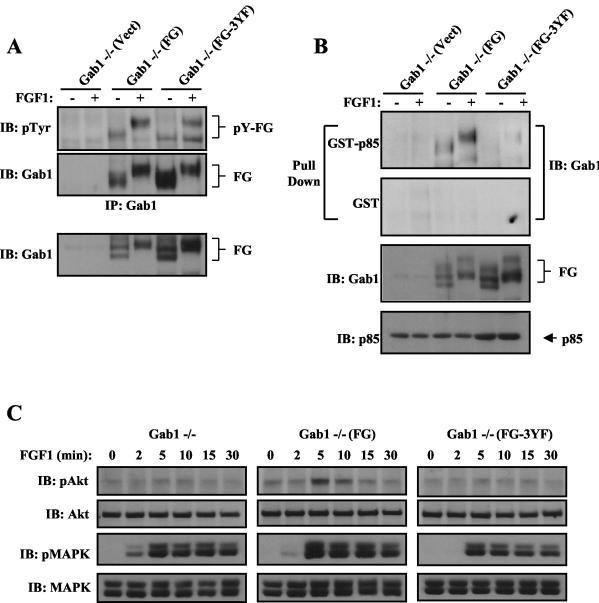
It is expected that FGF1-induced Akt stimulation will be rescued, in either Gab1−/− or FRS2α−/− MEFs expressing the chimeric FG docking protein. Gab1−/− cells were infected with the FG retrovirus, and a stable puromycin-resistant pool of Gab1−/− MEFs expressing FG was selected [Gab1−/−(FG)]. Both anti-Gab1 and anti-Flag antibodies recognized the chimeric docking protein as revealed by immunoblotting experiments with anti-Gab1 or anti-Flag, respectively (Fig. 4A). Like Gab1, the FG chimera binds constitutively to Grb2, and FGF1 induced its tyrosine phosphorylation (Fig. 4A and B). As shown in Fig. 4B, FGF1 stimulation induced the formation of a complex between tyrosine-phosphorylated FG and GST-p85 (N+C-SH2). Furthermore, ectopic expression of FG in Gab1−/− MEFs restored FGF1-induced PI 3-kinase activation (Fig. 4C) and Akt activation to a level comparable to the level of Akt stimulation achieved by ectopic expression of Gab1 in the mutant cells (Fig. 4D). However, FGF1 induced a similar MAPK activation in Gab1−/−(FG) and Gab1−/−(Gab1) cells (Fig. 4D). Additionally, a mutant form of FG lacking the p85-binding sites (FG-3YF) (Fig. 5B) is tyrosine phosphorylated in response to FGF1 stimulation (Fig. 5A) but is unable to rescue FGF1 stimulation of Akt (Fig. 5C). However, a similar MAPK response was observed upon FGF1 stimulation of Gab1−/− cells expressing either FG or FG-3YF [Gab1−/−(FG-3YF) cells] (Fig. 5C). These experiments reveal the ability of the FG chimera to substitute for Gab1 in FGF1 stimulation of PI 3-kinase and Akt and that the three canonical PI 3-kinase-binding sites are essential for the rescued responses.
FIG. 4.
FGF1-induced responses are rescued in Gab1−/− MEFs by ectopic expression of a chimeric FRS2α/Gab1 (FG) docking protein. (A) The indicated cell lines were serum starved and then stimulated with FGF1 (1 ng/ml) for 10 min (+) or left unstimulated (−). Cell lysates were immunoprecipitated with anti-Gab1 and then subjected to SDS-PAGE and immunoblotting with the indicated antibodies. Abbreviations: IB, immunoblotting; pTyr, phosphotyrosine antibody; IP, immunoprecipitation. (B) Lysates from FGF1-stimulated cells were subjected to a pull-down assay with immobilized GST fusion protein containing the two SH2 domains of p85. Cells were treated and processed as described in the legend to Fig. 2B. Expression of FG and p85 in the total cell lysates is shown in the bottom two blots. (C) Serum-starved Gab1−/− MEFs expressing the chimera FG were treated for the indicated times with FGF1 (1 ng/ml), and PI 3-kinase assay was performed on Gab1 immunoprecipitates. (D) The chimeric protein FG rescues FGF1-induced Akt activation in Gab1−/− MEFs. The indicated cell lines were treated and processed as described in the legend to Fig. 1C.
FIG. 5.
The p85-binding sites on the chimera FG are required for rescuing FGF1-mediated Akt activation in Gab1-deficient MEFs. (A) The indicated cell lines were serum starved and then stimulated with FGF1 (1 ng/ml) for 10 min (+) or left unstimulated (−). Cell lysates were immunoprecipitated with anti-Gab1 and then subjected to SDS-PAGE and immunoblotting with the indicated antibodies. Expression of FG in the cell lysates is shown in the bottom blot. Abbreviations: IB, immunoblotting; pTyr, phosphotyrosine antibody; IP, immunoprecipitation. (B) Lysates from FGF1-stimulated cells were subjected to a pull-down assay with immobilized GST fusion protein containing the SH2 domains of p85. Cells were treated and processed as described in the legend to Fig. 2B. Expression of FG and p85 in the total cell lysates is shown in the bottom two blots. (C) The p85-binding sites in the chimeric protein FG are required to rescue FGF1-induced Akt activation in Gab1-deficient MEFs. The indicated cell lines were treated and processed as described in the legend to Fig. 1C.
Rescue of FGF1-induced Akt and MAPK stimulation by expression of the FG chimera in FRS2α−/− cells.
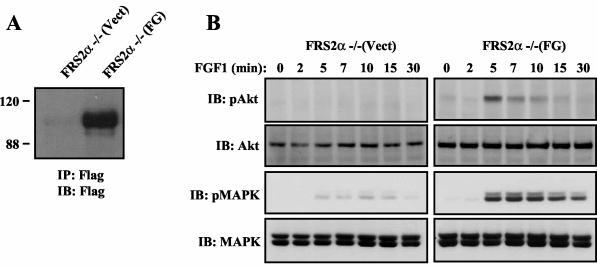
We next examined the effect of ectopic expression of the FG chimeric docking protein (Fig. 6A) on FGF1 (1 ng/ml) stimulation of Akt and MAPK responses in MEFs deficient in FRS2α. As shown in Fig. 6B, both FGF1-induced Akt and MAPK stimulation are fully restored in FRS2α−/− cells expressing the FG chimeric protein [FRS2α−/−(FG)] compared to FRS2α−/− infected with empty retrovirus [FRS2α−/−(Vect)]. Previous reports demonstrating deficiency in MAPK stimulation in Gab1−/− MEFs stimulated by EGF, PDGF, or HGF have revealed the capacity of Gab1 to function as a mediator of the MAPK response (14, 36). Furthermore, it was proposed that growth factor stimulation of the Ras/MAPK response is mediated by recruitment of Shp2 by tyrosine-phosphorylated Gab1. We have previously demonstrated that FGFRs can mediate both FRS2α-dependent and FRS2α-independent stimulation of MAPK and that the MAPK response is strongly compromised when FRS2−/− MEFs are stimulated with low concentrations of FGF1 (9). In addition, it is likely that the FG chimera rescues FGF1-induced MAPK response by recruitment of Shp2 to compensate for the loss of the FRS2α-dependent component of MAPK responses in FRS2α−/− MEFs (9).
FIG. 6.
Expression of the chimeric FG docking protein in FRS2α−/− MEFs rescues FGF1-induced Akt and MAPK activation. (A) Expression of FG in FRS2α-deficient MEFs (FRS2α−/−) was analyzed by immunoblotting (IB) with antibodies specific for the Flag tag of anti-Flag immunoprecipitates (IP). The positions of molecular size standards (in kilodaltons) are given to the left of the blot. (B) The chimeric protein FG rescues FGF1-induced Akt and MAPK activation in FRS2α−/− MEFs. The indicated cell lines were treated and processed as described in the legend to Fig. 1C.
DISCUSSION
Docking proteins function as critical mediators of signaling by RTKs. For example, the four members of the insulin receptor substrate (IRS) family of docking proteins mediate many of the intracellular responses of insulin or insulin-like growth factor 1 receptors (15), while the FRS2 family of docking proteins are key mediators of FGF signaling and signaling by the neurotrophic factors nerve growth factor, brain-derived neurotrophic factor, and glial cell-derived neurotrophic factor (7).
In this report, we use genetic and biochemical approaches to demonstrate that the docking protein Gab1 is required for FGF1-induced stimulation of PI 3-kinase and Akt. We demonstrate that FGFR activates PI 3-kinase by an indirect mechanism involving a coordinated unidirectional assembly of the FRS2α/Grb2/Gab1 complex, resulting in tyrosine phosphorylation of Gab1, followed by recruitment and activation of PI 3-kinase. While growth factor receptors, such as PDGF receptor or erbB3, possess specific binding sites for multiple SH2 domains of signaling, including canonical pYXXM-binding motifs resulting in direct recruitment of PI 3-kinase (12, 16), the insulin and insulin-like growth factor 1 receptor recruit PI 3-kinase by tyrosine phosphorylation of closely associated members of the IRS family of docking proteins (15). The link between FGFR and PI 3-kinase is indirect and more complicated, as it involves the coordinated assembly of at least two docking proteins prior to recruitment and activation of PI 3-kinase after FGF1 stimulation. Furthermore, recruitment of PI 3-kinase by Gab1 results in a positive-feedback loop mediated by binding of the PH domain of Gab1 to the product of PI 3-kinase activation, phosphatidylinositol-3,4,5-triphosphate, enabling an additional layer of control (26).
Experiments with cells derived from Gab1−/− embryos have demonstrated that Gab1 plays an important role in EGF-, PDGF-, or HGF-induced stimulation of MAPK (14, 36). Moreover, Gab1-dependent stimulation of the Ras/MAPK cascade in response to lysophosphatidic acid, EGF, or HGF stimulation is thought to be mediated by recruitment of the tyrosine phosphatase Shp2 (2, 3, 20, 22, 28, 36). However, in this report we demonstrate that Gab1 is dispensable for FGF1 stimulation of the MAPK signaling cascade. This is probably due to the central role played by FRS2α in mediating the MAPK response (9). We have previously used FRS2α-deficient fibroblasts to demonstrate that FRS2α plays a critical role in the control of multiple cellular processes, including the MAPK response (9). Like FRS2α in FGF signaling and IRSs in insulin signaling, Gab1 appears to play an important role in EGF signaling. Upon EGF stimulation, a constitutive Grb2/Gab1 complex is recruited by activated EGF receptor, resulting in tyrosine phosphorylation of Gab1, followed by recruitment of a complement of signaling proteins. Recruitment of PI 3-kinase leads to activation of the Akt-dependent antiapoptotic pathway, and recruitment of Shp2 leads to activation of the Ras/MAPK cascade.
We have tested the FGF1-induced indirect mechanism for recruitment of PI 3-kinase by expressing in Gab1−/− or FRS2α−/− cells a chimeric docking protein composed of the membrane and receptor targeting signals of FRS2α (myristyl anchor and PTB domain) fused to sequences from Gab1 that are responsible for recruitment of the complement of signaling molecules that are normally recruited by tyrosine-phosphorylated Gab1 (the C-terminal tail of Gab1). We reasoned that the FG chimeric docking protein will be able to rescue the Akt response in both Gab1−/− and FRS2α−/− cells after FGF1 stimulation, as FG will be targeted to the juxtamembrane domain of FGFR via the PTB domain of FRS2α and after FG is tyrosine phosphorylated, FG will recruit PI 3-kinase by means of the canonical p85-binding sites which were borrowed from Gab1. The experiments presented in this report demonstrate that FG is capable of bypassing the indirect activation of PI 3-kinase and Akt by FGF1-induced stimulation of a ternary FRS2α/Grb2/Gab1 complex. Full restoration of FGF1-induced stimulation of Akt is observed in Gab1−/− cells expressing the FG chimera. Moreover, the FG-3YF mutant that does not form a complex with p85 and does not recruit PI 3-kinase is unable to mediate Akt activation after FGF1 stimulation in Gab1−/− MEFs. However, expressing the chimeric FG docking protein in these cells did not influence FGF1-induced MAPK stimulation in Gab1−/− MEFs. We have also demonstrated that expression of the chimeric FG docking protein in FRS2α−/− cells results in restoration of both FGF1-dependent Akt and MAPK responses. Although the MAPK response in FRS2α−/− cells stimulated with FGF1 is severely compromised, a partial MAPK response can be detected in these mutants even upon stimulation with low concentrations of FGF1 (9). FGFR is able to induce MAPK stimulation in FRS2α−/− cells, probably by inducing tyrosine phosphorylation of the scaffold protein Shc, which forms a complex with Grb2, resulting in an alternative avenue for activation of the Ras/MAPK cascade. The experiment presented in this report shows that the FG chimera is able to contribute towards the partial MAPK response induced by FGF1 in FRS2α−/− cells probably by recruitment of the tyrosine phosphatase Shp2.
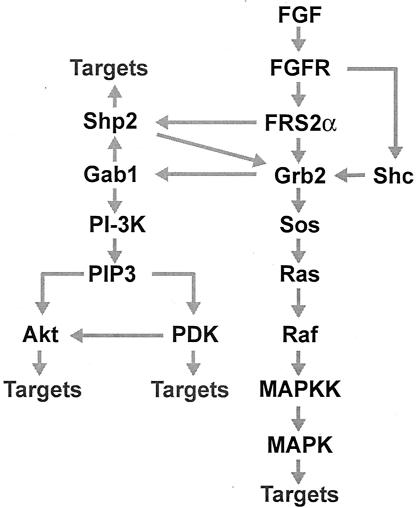
On the basis of the experiments described in this report and in earlier studies (9, 24), it is possible to propose a wiring diagram for the multiple interactions downstream of FGFR in which FRS2α and Gab1 play a critical role in mediating the MAPK kinase signaling cascade and the PI 3-kinase- and Akt-dependent antiapoptotic signaling pathway. Figure 7 depicts a connection scheme depicting the various interactions that take place downstream of FGFR that are responsible for stimulation of these intracellular pathways.
FIG. 7.
A wiring diagram describing the multiple interactions downstream of FGFR that lead to activation of the Ras/MAPK and PI 3-kinase/Akt signaling pathways. The adaptor protein Grb2 is recruited by FRS2α directly and indirectly by means of Shp2 molecules in complex with FRS2α. Recruitment of Grb2 by tyrosine-phosphorylated Shc provides an alternative FRS2α-independent mechanism for MAPK activation. FGF stimulation of coordinated assembly of a ternary Gab1/Grb2/FRS2α complex results in tyrosine phosphorylation of Gab1, followed by recruitment and activation of PI 3-kinase (PI-3K). Phosphatidylinositol-3,4,5-triphosphate (PIP3), the reaction product of PI 3-kinase, is responsible for activation of phosphoinositide-dependent kinase (PDK) and the antiapoptotic protein kinase Akt. In addition, the binding of the PH domain of Gab1 to PIP3 results in a feedback loop for recruitment of Gab1-bound signaling proteins. MAPKK, MAPK kinase.
Acknowledgments
We thank Bryant G. Darnay for useful discussions and suggestions during the course of this study.
REFERENCES
- 1.Batzer, A. G., D. Rotin, J. M. Urena, E. Y. Skolnik, and J. Schlessinger. 1994. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol. Cell. Biol. 14**:**5192-5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunnick, J. M., J. F. Dorsey, T. Munoz-Antonia, L. Mei, and J. Wu. 2000. Requirement of Shp2 binding to Grb2-associated binder-1 for mitogen-activated protein kinase activation in response to lysophosphatidic acid and epidermal growth factor. J. Biol. Chem. 275**:**13842-13848. [DOI] [PubMed] [Google Scholar]
- 3.Cunnick, J. M., L. Mei, C. A. Doupnik, and J. Wu. 2001. Phosphotyrosines 627 and 659 of Gab1 constitute a bisphosphoryl tyrosine-based activation motif (BTAM) conferring binding and activation of Shp2. J. Biol. Chem. 276**:**24380-24387. [DOI] [PubMed] [Google Scholar]
- 4.Fixman, E. D., M. Holgado-Madruga, L. Nguyen, D. M. Kamikura, T. M. Fournier, A. J. Wong, and M. Park. 1997. Efficient cellular transformation by the Met oncoprotein requires a functional Grb2 binding site and correlates with phosphorylation of the Grb2-associated proteins, Cbl and Gab1. J. Biol. Chem. 272**:**20167-20172. [DOI] [PubMed] [Google Scholar]
- 5.Goldfarb, M. 1996. Functions of fibroblast growth factors in vertebrate development. Cytokine Growth Factor Rev. 7**:**311-325. [DOI] [PubMed] [Google Scholar]
- 6.Gu, H., J. C. Pratt, S. J. Burakoff, and B. G. Neel. 1998. Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol. Cell 2**:**729-740. [DOI] [PubMed] [Google Scholar]
- 7.Guy, G. R., P. Yusoff, D. Bangarusamy, C. W. Fong, and E. S. Wong. 2002. Dockers at the crossroads. Cell. Signal. 14**:**11-20. [DOI] [PubMed] [Google Scholar]
- 8.Hadari, Y. R., H. Kouhara, I. Lax, and J. Schlessinger. 1998. Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol. Cell. Biol. 18**:**3966-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadari, Y. R., N. Gotoh, H. Kouhara, I. Lax, and J. Schlessinger. 2001. Critical role for the docking-protein FRS2 alpha in FGF receptor-mediated signal transduction pathways. Proc. Natl. Acad. Sci. USA 98**:**8578-8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hibi, M., and T. Hirano. 2000. Gab-family adapter molecules in signal transduction of cytokine and growth factor receptors, and T and B cell antigen receptors. Leuk. Lymphoma 37**:**299-307. [DOI] [PubMed] [Google Scholar]
- 11.Holgado-Madruga, M., D. R. Emlet, D. K. Moscatello, A. K. Godwin, and A. J. Wong. 1996. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature (London) 379**:**560-564. [DOI] [PubMed] [Google Scholar]
- 12.Hu, P., B. Margolis, E. Y. Skolnik, R. Lammers, A. Ullrich, and J. Schlessinger. 1992. Interaction of phosphatidylinositol 3-kinase-associated p85 with epidermal growth factor and platelet-derived growth factor receptors. Mol. Cell. Biol. 12**:**981-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu, P., and J. Schlessinger. 1994. Direct association of p110 beta phosphatidylinositol 3-kinase with p85 is mediated by an N-terminal fragment of p110 beta. Mol. Cell. Biol. 14**:**2577-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itoh, M., Y. Yoshida, K. Nishida, M. Narimatsu, M. Hibi, and T. Hirano. 2000. Role of Gab1 in heart, placenta, and skin development and growth factor- and cytokine-induced extracellular signal-regulated kinase mitogen-activated protein kinase activation. Mol. Cell. Biol. 20**:**3695-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadowaki, T., K. Tobe, R. Honda-Yamamoto, H. Tamemoto, Y. Kaburagi, K. Momomura, K. Ueki, Y. Takahashi, T. Yamauchi, Y. Akanuma, and Y. Yazaki. 1996. Signal transduction mechanism of insulin and insulin-like growth factor-1. Endocr. J. 43(Suppl.)**:**S33—S41. [DOI] [PubMed] [Google Scholar]
- 16.Kim, H. H., S. L. Sierke, and J. G. Koland. 1994. Epidermal growth factor-dependent association of phosphatidylinositol 3-kinase with the erbB3 gene product. J. Biol. Chem. 269**:**24747-24755. [PubMed] [Google Scholar]
- 17.Kouhara, H., Y. R. Hadari, T. Spivak-Kroizman, J. Schilling, D. Bar-Sagi, I. Lax, and J. Schlessinger. 1997. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 89**:**693-702. [DOI] [PubMed] [Google Scholar]
- 18.Lax, I., A. Wong, B. Lamothe, A. Lee, A. Frost, J. Hawes, and J. Schlessinger. 2002. The docking protein FRS2 alpha controls a MAP kinase-mediated negative feedback mechanism for signaling by FGF receptors. Mol. Cell 10**:**709-719. [DOI] [PubMed] [Google Scholar]
- 19.Liu, Y., and L. R. Rohrschneider. 2002. The gift of Gab. FEBS Lett. 515**:**1-7. [DOI] [PubMed] [Google Scholar]
- 20.Maroun, C. R., M. A. Naujokas, M. Holgado-Madruga, A. J. Wong, and M. Park. 2000. The tyrosine phosphatase Shp2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the Met receptor tyrosine kinase. Mol. Cell. Biol. 20**:**8513-8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammadi, M., I. Dikic, A. Sorokin, W. H. Burgess, M. Jaye, and J. Schlessinger. 1996. Identification of six novel autophosphorylation sites on fibroblast growth factor receptor 1 and elucidation of their importance in receptor activation and signal transduction. Mol. Cell. Biol. 16**:**977-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neel, B. G., H. Gu, and L. Pao. 2003. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 28**:**284-293. [DOI] [PubMed] [Google Scholar]
- 23.Nishida, K., Y. Yoshida, M. Itoh, T. Fukada, T. Ohtani, T. Shirogane, T. Atsumi, M. Takahashi-Tezuka, K. Ishihara, M. Hibi, and T. Hirano. 1999. Gab-family adapter proteins act downstream of cytokine and growth factor receptors and T- and B-cell antigen receptors. Blood 93**:**1809-1816. [PubMed] [Google Scholar]
- 24.Ong, S. H., Y. R. Hadari, N. Gotoh, G. R. Guy, J. Schlessinger, and I. Lax. 2001. Stimulation of phosphatidylinositol 3-kinase by fibroblast growth factor receptors is mediated by coordinated recruitment of multiple docking proteins. Proc. Natl. Acad. Sci. USA 98**:**6074-6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powers, C. J., S. W. McLeskey, and A. Wellstein. 2000. Fibroblast growth factors, their receptors and signaling. Endocr. Relat. Cancer 7**:**165-197. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigues, G. A., M. Falasca, Z. Zhang, S. H. Ong, and J. Schlessinger. 2000. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol. Cell. Biol. 20**:**1448-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sachs, M., H. Brohmann, D. Zechner, T. Muller, J. Hulsken, I. Walther, U. Schaeper, C. Birchmeier, and W. Birchmeier. 2000. Essential role of Gab1 for signaling by the c-Met receptor in vivo. J. Cell Biol. 150**:**1375-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaeper, U., N. H. Gehring, K. P. Fuchs, M. Sachs, B. Kempkes, and W. Birchmeier. 2000. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J. Cell Biol. 149**:**1419-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlessinger, J. 2000. Cell signaling by receptor tyrosine kinases. Cell 103**:**211-225. [DOI] [PubMed] [Google Scholar]
- 30.Sell, C., M. Rubini, R. Rubin, J.-P. Liu, A. Efstratiadis, and R. Baserga. 1993. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type 1 insulin-like growth factor receptor. Proc. Natl. Acad. Sci. USA 90**:**11217-11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skolnik, E. Y., B. Margolis, M. Mohammadi, E. Lowenstein, R. Fischer, A. Drepps, A. Ullrich, and J. Schlessinger. 1991. Cloning of PI3 kinase-associated p85 utilizing a novel method for expression/cloning of target proteins for receptor tyrosine kinases. Cell 65**:**83-90. [DOI] [PubMed] [Google Scholar]
- 32.Weider, K. M., S. Di Cesare, M. Sachs, V. Brinkmann, J. Behrens, and W. Birchmeier. 1996. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature (London) 384**:**173-176. [DOI] [PubMed] [Google Scholar]
- 33.Whitman, M., D. R. Kaplan, B. Schaffhausen, L. Cantley, and T. M. Roberts. 1985. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature (London) 315**:**239-242. [DOI] [PubMed] [Google Scholar]
- 34.Wolf, I., B. J. Jenkins, Y. Liu, M. Seiffert, J. M. Custodio, P. Young, and L. R. Rohrschneider. 2002. Gab3, a new DOS/Gab family member, facilitates macrophage differentiation. Mol. Cell. Biol. 22**:**231-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu, H., K. W. Lee, and M. Goldfarb. 1998. Novel recognition motif on fibroblast growth factor receptor mediates direct association and activation of SNT adapter proteins. J. Biol. Chem. 273**:**17987-17990. [DOI] [PubMed] [Google Scholar]
- 36.Yamasaki, S., K. Nishida, Y. Yoshida, M. Itoh, M. Hibi, and T. Hirano. 2003. Gab1 is required for EGF receptor signaling and the transformation by activated ErbB2. Oncogene 22**:**1546-1556. [DOI] [PubMed] [Google Scholar]
- 37.Yart, A., P. Mayeux, and P. Raynal. 2003. Gab1, Shp2 and other novel regulators of Ras: targets for anticancer drug discovery? Curr. Cancer Drug Targets 3**:**177-192. [DOI] [PubMed] [Google Scholar]
- 38.Zhao, C., D. H. Yu, R. Shen, and G. S. Feng. 1999. Gab2, a new pleckstrin homology domain-containing adapter protein, acts to uncouple signaling from ERK kinase to Elk-1. J. Biol. Chem. 274**:**19649-19654. [DOI] [PubMed] [Google Scholar]