Obesity and Cancer: An Angiogenic and Inflammatory Link (original) (raw)
. Author manuscript; available in PMC: 2017 Apr 1.
Published in final edited form as: Microcirculation. 2016 Apr;23(3):191–206. doi: 10.1111/micc.12270
Abstract
With the current epidemic of obesity, a large number of patients diagnosed with cancer are overweight or obese. Importantly, this excess body weight is associated with tumor progression and poor prognosis. The mechanisms for this worse outcome, however, remain poorly understood. We review here the epidemiological evidence for the association between obesity and cancer, and discuss potential mechanisms focusing on angiogenesis and inflammation. In particular, we will discuss how the dysfunctional angiogenesis and inflammation occurring in adipose tissue in obesity may promote tumor progression, resistance to chemotherapy, and targeted therapies such as anti-angiogenic and immune therapies. Better understanding of how obesity fuels tumor progression and therapy resistance is essential to improve the current standard of care and the clinical outcome of cancer patients. To this end, we will discuss how an anti-diabetic drug such as metformin can overcome these adverse effects of obesity on the progression and treatment resistance of tumors.
Keywords: obesity, immune environment, desmoplasia, metformin, hypoxia, cytokines, IL-6, IL-1_β_, VEGFR-1
INTRODUCTION—THE OBESITY EPIDEMIC
Excess body weight has become a major public health problem worldwide, and its incidence is increasing at an alarming rate [176]. According to the World Health Organization, the prevalence of obesity has nearly doubled in the last three decades [176]. Excess body weight comprises overweight (25 ≥ BMI <30 kg/m2) and obesity [BMI ≥30 kg/m2]. The latest global survey shows that more than 1.4 billion adults are overweight, and more than 500 million of them are clinically obese [176]. In the United States alone, nearly 70% of the adult population are either overweight or obese [122]. The high rates of obesity and overweight are a consequence of the “western lifestyle.” The latter is mainly attributed to a positive energy balance, a combination of dietary excess energy intake along with a lack of exercise, manifesting itself as obesity. Obese adults have substantially decreased life expectancies, and with obesity-associated diseases accounting for 400,000 deaths per year in the US, we are observing the largest growth in mortality over the past decade and an explosion of medical costs [113,130]. Obesity is considered as a direct cause of the decrease in life expectancy of Americans for the first time in generations [125]. Among the reasons for obesity to associate with increased mortality, is its intimate relationship with cancer incidence and prevalence. In this review, we summarize the epidemiological and experimental evidence demonstrating an association of obesity with cancer and highlight some of the molecular mechanisms that play a pivotal role in the tumor microenvironment to initiate and propagate tumor growth, with emphasis on angiogenesis and inflammation.
THE LINK BETWEEN OBESITY AND CANCER
Based on epidemiological, clinical, and experimental data, the International Agency for Research on Cancer (IARC) in 2002 was able to evaluate the link between obesity and cancer risk [17]. IARC concluded that preventing weight gain and promoting physical activity could partially avert esophagus, colon, endometrial, and postmenopausal BC. Remarkably, a recent study found that women who gained 55 pounds or more after age 18 had almost 50% greater risk of BC after menopause compared to those who maintained their weight, making obesity the most significant risk and prognostic factor for post-menopausal BC [44]. Since the IARC report, various studies have shown that more cancers are linked to obesity than previously described. Presently, obesity and overweight are well-established major risk factors for cancer, accounting for a ~40% increase in the incidence of certain cancers [176]. In particular, an association between obesity and increased risk of gallbladder cancer, hepatocellular carcinoma, pancreatic cancer, gastric cancer, non-Hodgkin's lymphoma, cervical cancer, and prostate cancer has been described [14]. Furthermore, a number of large-scale studies have demonstrated that obesity leads to an increase in not only incidence but also cancer-related mortality [8,72,135,136]. In BC, obesity appears to be a negative prognostic factor for several cancer-related events. Obesity negatively affects disease free interval, overall survival, and outcome of second primary cancer, and is an independent predictive parameter of cancer stage [103,106]. Even if obese patients present with more advanced tumors at diagnosis, after multivariate analysis, obesity remains as an independent prognostic factor [44,103,106]. In fact, the American Cancer Society estimated that in the United States, up to 14% of all deaths from cancer in men and 20% of those in women could be caused by overweight and obesity [15]. The list of cancers associated with increased BMI and their relative mortality risk estimates as well as some potential mechanisms are shown in Tables 1 and 2.
Table 1.
Summary of cancer mortality associated with increased BMI in men.
| Cancer type | BMI | RR | Potential causal mechanism |
|---|---|---|---|
| Liver | ≥35 | 4.52 | Microvascular invasion, fatty liver [13,149] |
| Pancreas | ≥35 | 2.61* | Insulin resistance [175] |
| Stomach | ≥35 | 1.94 | Augmented immune response to Helicobacter infection [45] |
| Esophagus | ≥30 | 1.91* | IGF-1 axis [41] |
| Colon and rectum | ≥35 | 1.84 | Elevated TNF-at Wnt pathway [101] |
| Gallbladder | ≥30 | 1.76 | Cholelithiasis [173] |
| Multiple myeloma | ≥35 | 1.71 | Inflammatory pathway- IL6 [34] |
| Kidney | ≥35 | 1.70 | Renal hypertension, hypoxia, and lipid peroxidation [111] |
| All other cancers | ≥30 | 1.68* | |
| All cancers | ≥40 | 1.52 | |
| Non-Hodgkin's lymphoma | ≥35 | 1.49 | Inflammatory pathway- IL10 [32] |
| Prostate | ≥35 | 1.34 | Unclear |
Table 2.
Summary of cancer mortality associated with increased BMI in women.
| Cancer type | BMI | RR | Causal mechanism |
|---|---|---|---|
| Uterus | ≥40 | 6.25 | Insulin resistance-induced hormonal changes [114] |
| Kidney | ≥40 | 4.75 | Renal hypertension, hypoxia, and lipid peroxidation |
| Cervix | ≥35 | 3.20 | |
| Pancreas | ≥40 | 2.76 | Insulin resistance |
| Esophagus | ≥30 | 2.64* | IGF-1 axis |
| All other cancers | ≥40 | 2.51* | |
| Gallbladder | ≥30 | 2.13 | Cholelithiasis |
| Breast | ≥40 | 2.12 | Insulin resistance-induced hormonal changes [143] |
| Non-Hodgkin's lymphoma | ≥35 | 1.95 | Inflammatory pathway- IL10 |
| All cancers | ≥40 | 1.88* | |
| Liver | ≥35 | 1.68 | Microvascular invasion, fatty liver |
| Ovary | ≥35 | 1.51 | Insulin resistance-induced hormonal changes |
| Colon and rectum | ≥40 | 1.46 | Elevated TNF-tumWnt pathway |
| Multiple myeloma | ≥35 | 1.44 | Inflammatory pathway- IL6 |
The correlation between several types of cancer with obesity has been recapitulated in animal models [9,26,59,72,73,121]. In a spontaneous model of BC, mammary tumor incidence and tumor weight were increased in obese mice when compared to normal weighted mice. Further, the latency of mammary tumor development also decreased [39,83]. In addition, experiments using several xenograft models have shown that a HFD, which leads to an obese phenotype, induced a higher rate of primary tumor growth and metastasis [30,59,85,142,184]. In one particular study, obesity-associated changes in the hepatic microenvironment sustained metastasizing tumor cells in the liver and resulted in increased incidence of hepatic metastases after intrasplenic/portal inoculation of colon carcinoma cells [177]. Despite these findings, the mechanistic causes for the obesity–cancer association have yet to be fully elucidated. As a consequence of the obesity pandemic, the majority of cancer patients present with excess weight at diagnosis [8,95]. Thus, understanding why obesity confers worse prognosis, would lead to novel treatments and enhance the outcome of current therapies.
OBESITY AND CANCER: MOLECULAR MECHANISMS IN THE TUMOR MICROENVIRONMENT
It is widely accepted that a systemic metabolic derangement like obesity creates a supportive environment for tumor cell proliferation. The most prominently investigated mechanisms to explain the obesity–cancer link are insulin resistance with a concomitant increase in insulin and IGF-1 [134]; the production of endogenous hormones (e.g., estrogen) by adipocytes [172]; and the secretion of adipokines such as leptin [72]. Additional mechanisms comprise of angiogenesis, obesity-induced hypoxia, immune modulation, and inflammatory cytokines [72,75]. Here, we will briefly review the above-mentioned mechanisms as well as discuss the potential involvement of obesity-induced inflammation and tumor angiogenesis in therapy failure in cancer.
Established Links Between Obesity and Cancer
Insulin, insulin-like growth factor 1
Insulin and IGF-1 axis could partly explain the link between obesity and cancer. Obese subjects typically present with some degree of insulin resistance, which leads to increased systemic levels of insulin and IGF-1 [93]. In systemic insulin resistance, serum insulin levels are elevated to counteract hyperglycemic state [156]. As a consequence, hepatocytes produce excessive levels of IGF-1 via increased growth hormone receptors’ signaling. Moreover, obese individuals tend to have significantly higher levels of free (bioavailable) IGF-1 when compared to lean subjects [140]. Importantly, several clinical studies have demonstrated that patients with increased serum levels of insulin and IGF-1 have an increased risk of cancers such as breast, prostate, and colorectal cancer [133]. Insulin binds to insulin receptors and IGF-1/IGF-2 bind to specific IGF-receptors (IGF-1R/IGF-2R). Overexpression of IGF-1R in breast and pancreatic tumors was also reported [67,180]. Insulin directly promotes DNA/RNA/protein synthesis in human BC cells [128]. Insulin-IGF-1 axis inhibits apoptosis, stimulates cell proliferation, and survival through downstream signaling namely via PI3K-AKT-mTOR and Ras/Raf/MEK pathways [4,12]. Taken together, increased serum levels of insulin and IGF-1 in obesity and/or metabolic disease patients would facilitate tumorigenesis, proliferation, and survival, and appear to be a major mechanism linking obesity to cancer.
Adipokines
Research over the past decade has revealed that adipocytes are not merely inert storage deposits but also secrete variety of biologically active proteins. These secreted proteins, collectively called adipokines, play a key role in endocrine, metabolic, and inflammatory pathways that regulate cellular homeostasis [110]. More than 50 different hormone-like substances, cytokines, and chemokines are grouped in the adipokine family. Most prominent of which are adiponectin and leptin.
Adiponectin
Adiponectin is a collagen-like protein and a product of the APM1 gene [104]. Exclusively derived from adipocytes, adiponectin has anti-inflammatory and insulin-sensitizing effects. In obese patients, plasma concentrations of adiponectin are paradoxically reduced [5]. Several case–control studies have established an inverse relationship between adiponectin plasma levels and increased risk of BC in both pre- and post-menopause women [odds ratio (OR) OR 3.62 (1.61–8.19)] [7], endometrial cancer [OR 2.75 (1.16–6.54)] [167], and pancreatic cancer [OR 2.81 (1.04–7.59)] [155]. Although direct etiological evidence is yet to be established, the role of adiponectin in cancer appears to be protective against carcinogenesis. The anti-carcinogenic effects are mediated through AMPK/AKT system, activated mainly through two receptors, AdipoR1 and AdipoR2. Activated AMPK system plays a vital role in the regulation of energy metabolism and cellular quiescence [22]. Furthermore, independent of signaling, adiponectin decreases production of reactive oxygen species and thereby inhibits proliferation of cells [49]. A study with adipose tissue depleted mice—AZIP/F1 that are diabetic and do not have measurable levels of adiponectin—has demonstrated that this particular strain of mice is more susceptible to carcinogen-induced tumorigenesis when compared to wild-type mice [74]. Therefore, decreased plasma levels of adiponectin in obesity may contribute to increased risk of cancer among obese individuals.
Leptin
Leptin is a 16 kDa protein secreted by adipocytes, which plays a key role in tightly regulating body weight by inhibiting appetite and increasing energy consumption [162]. Defects in leptin production lead to morbid obesity in humans and rodents. Systemic leptin levels are proportional to body adiposity [54], and clinical studies have suggested an association between systemic levels of leptin and colorectal [163] as well as endometrial cancer risk [35]. Binding of leptin to LepRb—the major signaling form of the leptin receptor—leads to activation of PI3K, MAPK, and STAT signaling pathways and plays a critical role in proliferation, survival, and differentiation of cancer cells [182]. Furthermore, leptin has immunoregulatory functions that include innate immune cell activation, thymic home-ostasis, Th1 cell differentiation, and cytokine production [108].
Potential New Links Between Obesity and Cancer: Obesity is a Pro-angiogenic and Inflammatory Condition
Reciprocal regulation between adipogenesis and angiogenesis
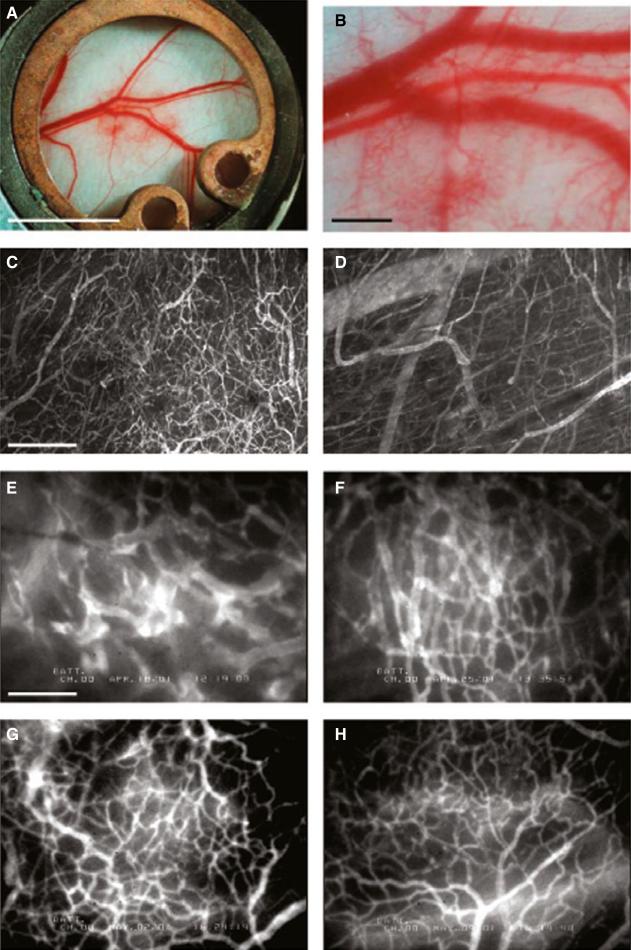
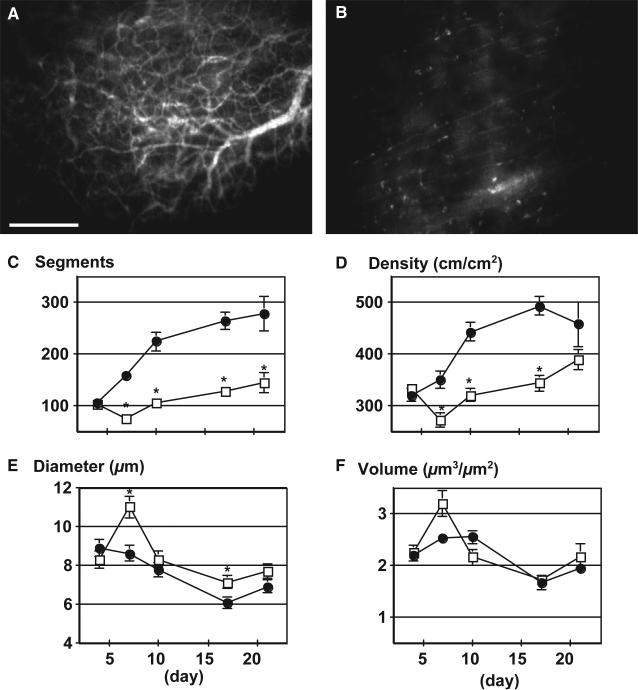
Adipose tissue produces angiogenic factors [e.g., VEGF, PLGF] and exhibits angiogenic activity [18,33,99]. In particular, we have shown that implantation of murine preadipocytes—mesenchymal precursor cells committed to adipocyte lineage—led to vigorous angiogenesis and formed subcutaneous fat pads in a mouse dorsal skinfold chamber [52] (Figure 1). While preadipocytes differentiated into adipocytes and developed adipose tissues, new blood vessels formation also occurred. These vessels were initially immature, but subsequently remodeled/differentiated into a mature vessel network consisting of all vascular units, namely arterioles, capillaries, and venules. Importantly, inhibition of adipocyte differentiation not only abrogated fat tissue formation but also reduced angiogenesis [52]. Conversely, it has been demonstrated that established adipose tissue mass can be regulated through the vasculature [63,109,183]. In fact, we found that inhibition of angiogenesis by VEGFR-2 blocking antibody not only reduced angiogenesis and tissue growth but also inhibited differentiation of preadipocytes (Figure 2) [52]. Furthermore, we found that part of this inhibition stems from the paracrine interaction between EC and preadipocytes and that VEGF–VEGFR-2 signaling in ECs, but not in preadipocytes, mediates this process.
Figure 1.
Angiogenesis and vessel remodeling during adipogenesis in the mouse dorsal skinfold chamber after 3T3-F442A cell implantation. (A, B) Macroscopic images 9 days after implantation. (C, D) Multiphoton laser-scanning microscopy images 28 days after preadipocyte implantation. Images were obtained by maximum intensity projection of 31 optical slices, each 5 _μ_m thick: the top 150-_μ_m de novo adipose tissue layer (C) and the bottom 150-_μ_m host subcutaneous layer (D). (E through H) High-power microscopic images of fluorescence contrast-enhanced blood vessels at 7 days (E), 14 days (F), 21 days (G), and 28 days (H) after implantation. Bars indicate 5 mm (A), 0.5 mm (B), 200 _μ_m (C, D), and 100 _μ_m (E through H), respectively. This figure is reproduced from Fukumura et al., Circ Res, 2003, with permission of the publisher.
Figure 2.
Effect of VEGFR2 blockade on angiogenesis and adipogenesis. (A, B) Visualization of rhodamine–dextran contrast-enhanced blood vessels 21 days after preadipocyte implantation with control rat IgG (A) and DC101, rat monoclonal anti-mouse VEGFR2 antibody (B) treatments. (C through F) Quantitative analyzes of tissue neovascularization; C, number of vessel segments; D, vascular length density; E, vessel diameter; F, vessel volume. Filled circles represent control IgG treatment (n = 6 mice); open squares, DC101 treatment (n = 6 mice). *P<0.01 as compared with IgG by two-tailed _t_-test. This figure is reproduced from Fukumura et al., Circ Res, 2003, with permission from the publisher.
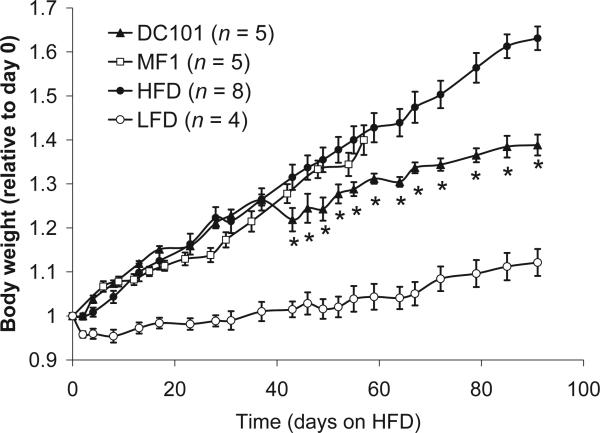
These findings revealed a reciprocal regulation between adipogenesis and angiogenesis, and suggested that blockade of angiogenesis with inhibitors of VEGF signaling can interfere with in adipose tissue formation in vivo. Indeed, we subsequently demonstrated that VEGFR-2 blockade significantly reduced body weight gain in diet-induced obese mice as compared to the control animals (Figure 3) [161]. Importantly, a recent study has shown that the consequences of modulation of angiogenic activity could be context dependent [159]. In agreement with the long-term inhibition of VEGFR2 in HFD, anti-VEGF treatment significantly reduced body weight gain in genetically engineered obesity model (ob/ob)—an effect likely mediated by the ablation of dysfunctional pro-inflammatory adipocytes [159]. However, during early stages of HFD, VEGF overexpression improved adipose tissue metabolism and resulted in reduced body weight gain, whereas anti-VEGF treatment induced adverse effects.
Figure 3.
Effects of VEGFR1 (MF1) and VEGFR2 (DC101) blockade in mice with DIO. (A) Body weight gain relative to weight at day 0 for mice given different diets and treatments. Male C57BL6 mice, 10–12 weeks old at time 0, were used for all groups. All diets and treatments began at day 0, at dosages and schedules as described in the Methods section. DC101 + HFD, DC101 treatment, white triangles (n = 5). MF1 + HFD, MF1 treatment, black squares (n = 5). HFD controls, no treatment (n = 4) or PBS treatment (n = 4), white circles. LFD: standard diet controls, crosses (n = 4). All data reported as mean ± SEM. Asterisks denote significant difference between DC101 + HFD and HFD groups (p, 0.05). This figure is reproduced from Tam et al., PLOS one, 2009, with permission from the publisher.
Dysfunctional adipose tissue angiogenesis in obesity causes inflammation
A widely accepted notion is that obesity is an inflammatory condition [73,81,157], and hypoxia is one of the most plausible causes. Adipose tissues often have inadequate vascularization, perfusion, and oxygen delivery presumably because angiogenesis cannot catch up with tissue expansion rate [62,75]. In addition, the excessive uptake of lipids by adipocytes in obesity leads to cellular hypertrophy, to the point where oxygen diffusion is no longer effective [62,75]. All of these result in a hypoxic microenvironment and eventually necrosis [72,75]. The former induces local production of angiogenic factors and inflammatory cytokines such as interleukin-1_β_, TNF-α, and MCP-1, which in turn increase infiltration of macrophages responding to the distress signals of enlarged adipocytes [33,52,53,70,72,73,99,165].
Abnormal angiogenesis in obesity and cancer
As mentioned above, angiogenesis, inflammation, and immune cell infiltration are signature features of expanding adipose tissues in obesity [33,70,73,75,117,150,174,179]. Interestingly, it was recently suggested that obesity promotes these processes also in the tumor microenvironment to facilitate tumor progression [27,123]. Another experimental study concluded that hypercholesterolemia in mice after HFD also induces angiogenesis and accelerates growth of orthotopically implanted breast tumor [131]. VEGF family such as VEGF and PlGF that have been associated with tumor progression may mediate this process. In fact, pre-clinical and clinical evidence suggests a relationship between these angiogenic factors and obesity [92,100,132,169]. Furthermore, leptin secreted by adipocytes induces activation, proliferation, and migration of EC by upregulating VEGF and VEGFR-2, and transactivates VEGFR-2 independently of VEGF [57]. Similarly, it has been shown that adipose CD34+ progenitor cells promote angiogenesis and cancer growth [107,126]. In addition, VEGFR-1 expressed in ECs and macrophages, binds to VEGF and PlGF, and has been shown to promote tumor angiogenesis, and recruitment and activation (e.g., cytokine production) of macrophages [48,68,96,115,116,148]. On the other hand, PlGF/VEGFR-1 signaling may regulate energy metabolism. Indeed, mice genetically deficient in PlGF, a ligand of VEGFR-1 and NRP-1, gained less weight during HFD-feeding than wild-type animals [100], but presented with insulin resistance and hyperinsulinemia [65]. Overall, VEGFR-1 appears to be involved in key processes both in the tumor microenvironment and adipose tissues, which suggests that, it can be a new link in the obesity–cancer connection.
Obesity-associated inflammation causes tumor progression and therapeutic failure
Similar to angiogenesis, it is well established that chronic inflammation is important for cancer initiation and progression. In fact, many of the adipocyte-produced inflammatory factors mentioned above have been shown to associate with worse prognosis in cancer patients, and to promote cancer initiation and progression in pre-clinical models [6,8,72]. In addition, obesity-associated inflammation may also affect response to chemotherapeutic agents as well as targeted therapy. For instance, in pancreatic cancer, the limited effectiveness of therapy may be attributed to the fibro-inflammatory microenvironment, which causes vessel compression that limits perfusion and directly hinders drug penetration [2,23,24,37,66,120,124]. Indeed, PDACs—the most common type of pancreatic cancer—are hypovascularized, poorly perfused, and hypoxic [71,88,124], which contributes to poor outcome of chemotherapy [25,66]. Importantly, obesity induces a pro-inflammatory state not only in adipose tissue as described above but also in visceral organs. In the normal pancreas, obesity induces pancreatic steatosis—abnormal cellular lipid retention—leading to increased expression of pro-inflammatory cytokines such as IL-1_β_ and IL-6 [72,151]. The inflammatory state leads to immune cell infiltration, ECM remodeling, and eventually fibrosis in the pancreas [73,136]. This is consistent with increased AngII production and AT1 signaling in obesity, which can be promoted by inflammatory mediators [124]. Whether these changes extend to the microenvironment of PDAC is currently unknown. In addition, PDACs in obese mice and patients also have increased adipocyte content [69,184]. Of clinical relevance, the interaction of cancer cells with adipocytes—both in the form of accumulation of fat in pancreas (pancreatic steatosis) and as invasion of cancer cells into local adipose tissue at the expanding edge of the tumor—is associated with worse outcomes in PDAC patients [64,151]. Further, the pro-inflammatory effects of adipokines (e.g., leptin) may also facilitate inflammation and immunosuppression [108]. Although the precise role of intratumor adipocytes during obesity-induced tumor progression is still unclear, it seems plausible that obesity-induced adipocyte infiltration induces tumor inflammation and aggravates desmoplasia to worsen the treatment response in PDACs and other desmoplastic tumors.
Obesity-induced resistance to anti-angiogenic therapy
Hypoxia, immune cell infiltration and the upregulation of inflammatory and angiogenic pathways, e.g., IL-6 and GF-2, have been proposed as mechanisms of resistance to anti-VEGF therapy, since they can sustain angiogenesis and tumor progression despite VEGF blockade [19,20,42,43,50,79]. In metastatic kidney or colon cancer, obesity is associated with reduced survival, specifically in patients receiving bevacizumab, an anti-VEGF antibody [53,60,78,80,91]. The effect of obesity on response to anti-VEGF therapy in BC is currently unknown. In this particular case, breast adipose tissue is the second major depot of fat in the body particularly in obese individuals and tends to be invaded by BC [10,171]. Since adipose tissue is a large component of BC, it is plausible that obesity-induced inflammation in the BC microenvironment may promote resistance to anti-VEGF therapies via these mechanisms.
It has been shown that macrophages and IL-6 expression are typically found in close proximity to dead adipocytes in breast adipose tissue [166]. Furthermore, adipocytes located near tumor cells upregulate IL-6 production in patients [10,38,158]. Indeed, adipose tissue produces 35% of the circulating levels of IL-6 [84] and IL-6 levels correlate with multiple parameters of obesity [11,129]. It is noteworthy that IL-6 can influence all stages of tumor development and metastasis [87]. In addition, IL-6 can also regulate trafficking and recruitment of inflammatory cells, which are a significant source of pro-inflammatory and pro-tumorigenic cytokines [137,138]. Not surprisingly, high levels of plasma IL-6 have also been associated with poor outcomes in cancer patients (kidney and liver cancer) treated with anti-angiogenic agents [42,43,87]. Importantly, IL-6 inhibition improved response to anti-VEGF therapy in a glioma mouse model [145]. Besides IL-6, other studies have shown that FGF2 is increased in cancer patients following anti-VEGF or VEGFR tyrosine kinase inhibitors therapy [21,61,98]. Furthermore, the FGF pathway activation has been proposed as a mechanism of escape from VEGF-targeted therapies. FGF-2 is also produced abundantly by adipocytes, and not surprisingly plasma levels of FGF-2 correlate with BMI [55,98]. Hence, it is possible that obesity can interfere with the response to targeted therapy such as anti-angiogenic agents by overproduction of alternative angiogenic and inflammatory factors.
Overcoming obesity-associated tumor progression: repurposing metformin
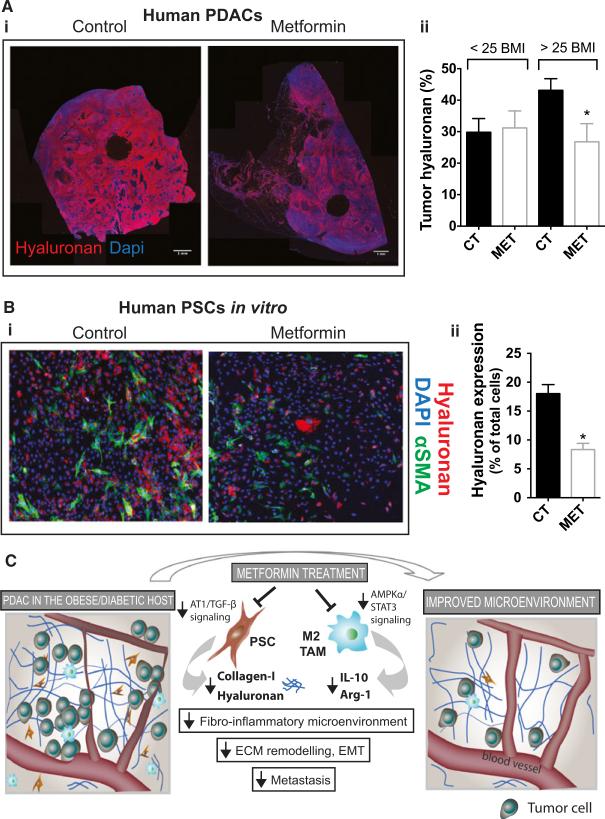
It would be ideal if we could develop a strategy to curb the effects of obesity on tumor progression using drugs that are accessible in the clinic—that are used to interfere with obesity-related disorders. One such agent, currently under intense clinical investigation, is metformin, the most widely prescribed anti-diabetic generic drug that is also frequently administered to diabetic PDAC patients [47]. Metformin can improve treatment outcomes in preclinical models of cancer, particularly in the obese setting [1,3,58,86,112,118,127,152,164,170,178,181]. In addition, it reduces the incidence of cancer in diabetic patients as well as improves survival in newly diagnosed cases [28,94,97,102,144,152]. Metformin has been shown to target cancer cells, transcription factors, microRNAs, DNA damage, cancer stem cells, and metabolism [29,40,56,77,119,160]. In addition, we recently performed a study in our laboratory establishing novel effects of metformin on pancreatic cancer. Using samples from pancreatic cancer patients, complemented with mouse models of pancreatic cancer and in vitro studies, we found that in overweight/obese condition, metformin reprograms the fibro-inflammatory tumor microenvironment and ultimately reduces metastasis in pancreatic cancer models [76]. We found that metformin at clinically relevant doses directly reduced ANG II receptor 1 expression and PDGF-β/TGF-β signaling and ECM production by PSCs—preferentially hyaluronan. It should be noted that ANG II signaling is also associated with coagulopathy [146]. Therefore, metformin treatment may produce an additional beneficial effect through prevention of thrombotic events that frequently occur in pancreatic cancer patients and contribute to the poor prognosis [153]. Furthermore, metformin treatment can restore EC function and production of NO [31,36,147,168]. NO is a potent vasodilator and has been shown to maintain/improve tumor perfusion and enhance drug delivery [51,105]. These preventative effects of metformin against vascular and thrombotic events could collectively improve tumor perfusion, even though anti-angiogenic effects of metformin on ECs have been reported [36,46,82,154,168]. Indeed, we found that metformin increases tumor perfusion while not affecting vessel density (unpublished).
Metformin also reduces inflammation—another key element of desmoplasia—through reduction of cytokine production, and recruitment and M2-polarization of TAMs. This was associated with AMPK activation and STAT3 signaling inhibition in macrophages. Finally, the alleviation of desmoplasia by metformin was associated with reduced ECM remodeling, EMT, and systemic metastasis (Figure 4). Importantly, the effects on desmoplasia observed in human samples seem restricted to an overweight/obese population, which appear to have tumors with increased content of ECM components. Indeed, a recent retrospective [141] and the first two prospective studies [89,90,139] indicated that metformin might not be beneficial to an unstratified patient population. The benefit in some but not all studies suggests that a subset of patients may respond better to metformin and that careful selection of patients may be required for metformin to be effective. We found that metformin's effect on desmoplasia in patients only occurred when their BMI was higher than 25 (overweight and obese patients), which supports the enhanced anti-cancer effects in obese compared to normal weight mice. This indicates that metformin may not be beneficial in normal weight patients, and suggests that BMI should be explored as a potential biomarker of response to this drug. With nearly 200 trials ongoing to address the effect of metformin on diabetic and non-diabetic cancer patients, understanding the yet elusive mechanisms of action of metformin may provide an opportunity to uncover potential biomarkers of response and define strategies of patient stratification for the judicious use of this highly promising yet generic drug.
Figure 4.
(A) Metformin treatment associates with reduced hyaluronan levels in human pancreatic cancers in overweight/obese patients. (i) Representative histology images showing the effect of metformin on tumor hyaluronan levels in normal weight or overweight/obese patients (n = 22 controls, 7 metformin). (ii) Immunohistochemical analysis of total tumor hyaluronan levels. Metformin decreases the hyaluronan-positive area fraction (%) in patients with BMI >25. Data are presented as the mean ± standard error. *p < 0.05 vs. control in patients with BMI >25. (B) Metformin reduces hyaluronan production by PSC. (i) PSCs were incubated in vitro with metformin (1 mM) for 48 hours. Representative immunocytochemistry images showing the effect of metformin on tumor hyaluronan and COL-I levels in human PSCs in vitro (n = 2). (ii) Quantification of hyaluronan expression in PSCs. Metformin decreases the expression of hyaluronan on PSCs. _α_SMA denotes activated PSCs. (C) Metformin inactivates PSCs and TAMs, alleviates the fibroinflammatory tumor microenvironment and reduces metastasis. Metformin treatment reduces COL-I and HA production by PSCs, leading to decreased fibrosis in PDACs. Metformin treatment also reduces cytokine production, infiltration, and M2 polarization of TAMs, leading to decreased inflammation. These lead to improved desmoplasia and reduced ECM remodeling, EMT, and metastasis. This figure is reproduced from Incio et al., PLOS One, 2015, with permission from the publisher.
CONCLUSION
Epidemiological studies clearly indicate that obesity is associated with increased risk of cancer and worsened treatment outcome [17,176]. Given the prevalence of obesity, understanding the mechanisms that underlie the poorer prognosis of obese pancreatic cancer patients is of paramount importance. A combination of hampered metabolic state and concurrent insulin resistance with factors produced by adipocytes (i.e., hormones, adipokines) in the obese setting may contribute to a tumor microenvironment that facilitates tumor proliferation, evades host immune response, and induces resistance to therapy [72,75,134,172]. In addition, obesity is a pro-angiogenic condition, as evidenced in previous work from our laboratory and others [52,63,109,183]. Despite that, the vasculature in expanding adipose tissues is insufficient and dysfunctional, leading to hypoxia and inflammation [73,81,157]. This induces production of multiple angiogenic and inflammatory factors that may abrogate the efficacy of anti-angiogenic treatment as a cancer agent. Furthermore, the local and systemic inflammation in obesity may also promote cancer progression via inflammatory cytokine signaling as well as the consequence of increased fibrosis, and may reduce the efficacy of chemotherapeutic agents. Today, for several cancer types including of breast and pancreas, majority of the patients are overweight or obese at diagnosis. We therefore should incorporate the important parameter of body weight in the design of pre-clinical studies in order to better recapitulate the clinical reality of (lack of) response to novel targeted therapies such as anti-VEGF as well as conventional therapies (e.g., chemotherapy) (Figure 5). Most importantly, by better understanding the mechanisms by which obesity promotes tumor progression and resistance to therapy, we will be able to improve the current standard of care for cancer patients. To this end, targeting fibrosis, inflammation, and metabolic pathways holds the promise to improve the clinical outcome of the major subset of cancer patients (Figure 6).
Figure 5.
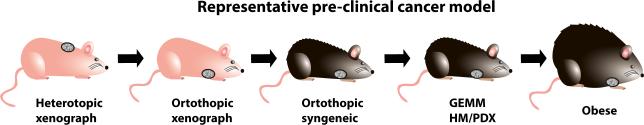
The evolution of the mouse tumor model. For preclinical cancer study, we have observed an evolution of mouse models to better represent the clinical setting: It evolves from a heterotopic to an orthotopic xenograft, from xenograft tumors in immunodeficient mice to syngeneic models, from transplantation model to genetic mouse models that better reflect the initial stages of tumor development or patient-derived xenografts to accurately represent tumor heterogeneity. Finally, we need to incorporate the obese mouse model as another dimension of improvement of the mouse model to better mimic patient population.
Figure 6.
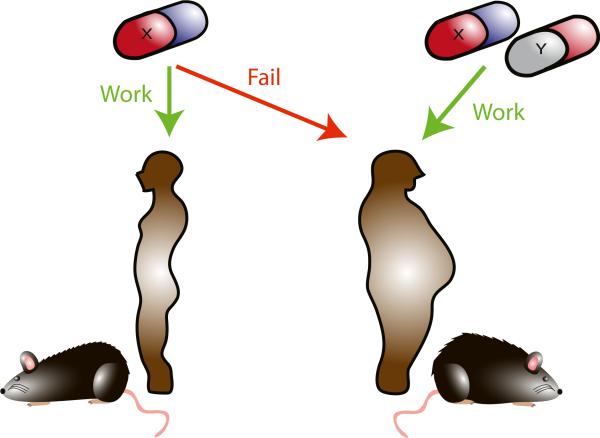
Obesity-tailored rethinking the approach to pre-clinical cancer research in light of the current obesity epidemics. Obesity patients may respond differently to a treatment or need additional therapy in order to make the treatment effective. The development of such strategy requires the use of animal models that better mimic biology and microenvironment of obese patients.
PERSPECTIVE.
Obesity is associated with inflammatory and angiogenic alterations in tumors that may affect response to conventional and targeted therapies in cancer patients.
ACKNOWLEDGMENTS
We thank Shan Min Chin for help with illustrations.
SOURCES OF FUNDING
The authors’ work was supported by a fellowship from the Foundation for Science and Technology (http://www.fct.pt/; Portugal, POPH/FSE funding program) (JI) and funds from the National Institutes of Health (http://nih.gov/; CA080124, CA085140, CA096915, CA115767, CA126642, CA197743), the US Department of Defense (W81XWH-10-1-0016), and the Lustgarten Foundation.
Abbrevations used
AdipoR1
adiponectin-receptor 1
AKT
protein kinase B
AMPK
adenosine mono phosphate kinase
ANG II
angiotensin II
APM1
adipose-most-abundant-gene-transcript 1
AT1
angiotensin II type-1 receptor
AZIP/F1
transgenic lipoatrophic diabetic mouse model
BC
breast cancer
BMI
body mass index
COL-I
collagen-I
DC101
anti VEGFR-2 antibody
DIO
diet-induced obesity
EC
endothelial cell
ECM
extracellular matrix
EMT
epithelial to mesenchymal transition
FGF-2
fibroblast growth factor 2
GEMM
genetically engineered mouse model
HA
hyaluronan
HFD
high-fat diet
HM
humanized mouse model
IGF-1
insulin growth factor 1
IgG
immunoglobulin
IL-6
interleukin 6
LepRb
leptin receptor long isoform
MCP-1
monocyte chemoattractant protein 1
MF1
anti VEGFR-1 antibody
mTOR
mammalian target of rapamycin
NO
nitric oxide
NRP-1
neuropilin 1
ob/ob
leptin-deficient mouse model
PBS
phosphate-buffered saline
PDAC
pancreatic ductal adenocarcinoma
PDX
patient-derived xenographt model
PI3K
phosphatidylinositol 3-kinase
PlGF
placental growth factor
PDGF
platelet-derived growth factor
PSC
pancreatic stellate cell
RR
relative risk
STAT3
signal transducer and activator of transcription 3
TAM
tumor associated macrophage
TGF
transforming growth factor
TNF-α
tumor necrosis factor α
VEGFR-1
vascular endothelial growth factor receptor 1
VEGFR-2
vascular endothelial growth factor receptor 2
VEGF
vascular endothelial growth factor
_α_SMA
alpha smooth muscle antigen
AUTHOR BIOGRAPHIES
 Dai Fukumura, MD, PhD, is an Associate Professor and the Deputy Director of the Edwin L. Steele Laboratories Department of Radiation Oncology, Massachusetts General Hospital and Harvard Medical School. He is an internationally recognized expert in imaging, angiogenesis, vascular and tumor biology. Dr. Fukumura and his colleagues have developed various innovative intravital optical imaging techniques and sophisticated animal models—which more faithfully represent the clinical behavior of tumors—in collaboration with world-renowned experts. Dr. Fukumura's research areas include (i) role of host–tumor interaction (microenvironment) in angiogenesis, tumor growth, metastasis and treatment response; (ii) role of nitric oxide in vessel formation, function, and normalization; (iii) probing and exploiting tumor microenvironment using nanotechnology; (iv) role of obesity in angiogenesis and tumor progression; and (v) tissue-engineered blood vessels. For more information, go to <http://steele.mgh.harvard.edu/dai_fukumura/pi_bio>.
Dai Fukumura, MD, PhD, is an Associate Professor and the Deputy Director of the Edwin L. Steele Laboratories Department of Radiation Oncology, Massachusetts General Hospital and Harvard Medical School. He is an internationally recognized expert in imaging, angiogenesis, vascular and tumor biology. Dr. Fukumura and his colleagues have developed various innovative intravital optical imaging techniques and sophisticated animal models—which more faithfully represent the clinical behavior of tumors—in collaboration with world-renowned experts. Dr. Fukumura's research areas include (i) role of host–tumor interaction (microenvironment) in angiogenesis, tumor growth, metastasis and treatment response; (ii) role of nitric oxide in vessel formation, function, and normalization; (iii) probing and exploiting tumor microenvironment using nanotechnology; (iv) role of obesity in angiogenesis and tumor progression; and (v) tissue-engineered blood vessels. For more information, go to <http://steele.mgh.harvard.edu/dai_fukumura/pi_bio>.
 Joao Incio obtained his MD degree in 2007 from Porto Medical School, Porto University, in the foggy yet beautiful city in the north of Portugal where Port wine comes from. He initiated his clinical training in internal medicine in Porto, and in 2010 he joined the Edwin L. Steele Laboratories as a PhD student under the supervision of Professors Dai Fukumura and Rakesh K. Jain. He has been studying the mechanisms of action of the anti-diabetic drug metformin in cancer. In addition, he has been deciphering the impact of inflammation and immunosuppression on cancer progression and therapy resistance using the inflammation-augmented obese mouse model.
Joao Incio obtained his MD degree in 2007 from Porto Medical School, Porto University, in the foggy yet beautiful city in the north of Portugal where Port wine comes from. He initiated his clinical training in internal medicine in Porto, and in 2010 he joined the Edwin L. Steele Laboratories as a PhD student under the supervision of Professors Dai Fukumura and Rakesh K. Jain. He has been studying the mechanisms of action of the anti-diabetic drug metformin in cancer. In addition, he has been deciphering the impact of inflammation and immunosuppression on cancer progression and therapy resistance using the inflammation-augmented obese mouse model.
 Ram C. Shankaraiah is a PhD student in the laboratory of Prof. Massimo Negrini at Department of Morphology, Surgery and Experimental Medicine and Laboratory for Technologies of Advanced Therapies (LTTA), University of Ferrara, Ferrara, Italy. He obtained his Master in Oncology in August 2014 at Vrije University, Amsterdam. In the past, he worked with Professor Dai Fukumura and Professor Rakesh Jain at the Edwin L. Steele Laboratories at the Department of Radiation Oncology, Massachusetts General Hospital, Boston, in the field of breast cancer and brain metastases. His current research focus is to investigate the role of non-coding RNAs in hepatocellular cancer.
Ram C. Shankaraiah is a PhD student in the laboratory of Prof. Massimo Negrini at Department of Morphology, Surgery and Experimental Medicine and Laboratory for Technologies of Advanced Therapies (LTTA), University of Ferrara, Ferrara, Italy. He obtained his Master in Oncology in August 2014 at Vrije University, Amsterdam. In the past, he worked with Professor Dai Fukumura and Professor Rakesh Jain at the Edwin L. Steele Laboratories at the Department of Radiation Oncology, Massachusetts General Hospital, Boston, in the field of breast cancer and brain metastases. His current research focus is to investigate the role of non-coding RNAs in hepatocellular cancer.
 Rakesh K. Jain is Andrew Werk Cook Professor of Tumor Biology and director of the Edwin L. Steele Laboratories for Tumor Biology in the radiation oncology department of Massachusetts General Hospital and Harvard Medical School. He is a member of the National Academy of Sciences, the National Academy of Engineering and the National Academy of Medicine, and a recipient of the US National Medal of Science. Dr. Jain is regarded as a pioneer in the area of tumor microenvironment and widely recognized for his seminal discoveries in tumor biology, drug delivery, in vivo imaging, bioengineering, and bench-to-bedside translation. These include uncovering the barriers to the delivery and efficacy of molecular and nano-medicines in tumors; developing new strategies to overcome these barriers; and then translating these strategies from bench to bedside.
Rakesh K. Jain is Andrew Werk Cook Professor of Tumor Biology and director of the Edwin L. Steele Laboratories for Tumor Biology in the radiation oncology department of Massachusetts General Hospital and Harvard Medical School. He is a member of the National Academy of Sciences, the National Academy of Engineering and the National Academy of Medicine, and a recipient of the US National Medal of Science. Dr. Jain is regarded as a pioneer in the area of tumor microenvironment and widely recognized for his seminal discoveries in tumor biology, drug delivery, in vivo imaging, bioengineering, and bench-to-bedside translation. These include uncovering the barriers to the delivery and efficacy of molecular and nano-medicines in tumors; developing new strategies to overcome these barriers; and then translating these strategies from bench to bedside.
Footnotes
CONFLICT OF INTEREST
MGH has filed a patent application on the work presented here on metformin.
REFERENCES
- 1.Algire C, Zakikhani M, Blouin MJ, Shuai JH, Pollak M. Metformin attenuates the stimulatory effect of a high-energy diet on in vivo LLC1 carcinoma growth. Endocr Relat Cancer. 2008;15:833–839. doi: 10.1677/ERC-08-0038. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez R, Musteanu M, Garcia-Garcia E, Lopez-Casas PP, Megias D, Guerra C, Munoz M, Quijano Y, Cubillo A, Rodriguez-Pascual J, Plaza C, de Vicente E, Prados S, Tabernero S, Barbacid M, Lopez-Rios F, Hidalgo M. Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br J Cancer. 2013;109:926–933. doi: 10.1038/bjc.2013.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anisimov VN. Metformin for prevention and treatment of colon cancer: a reappraisal of experimental and clinical data. Curr Drug Targets. 2016;17:439–446. doi: 10.2174/1389450116666150309113305. [DOI] [PubMed] [Google Scholar]
- 4.Anisimov VN, Bartke A. The key role of growth hormone-insulin-IGF-1 signaling in aging and cancer. Crit Rev Oncol Hematol. 2013;87:201–223. doi: 10.1016/j.critrevonc.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. 1999. Biochem Biophys Res Commun. 2012;425:560–564. doi: 10.1016/j.bbrc.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Bao B, Wang Z, Li Y, Kong D, Ali S, Banerjee S, Ahmad A, Sarkar FH. The complexities of obesity and diabetes with the development and progression of pancreatic cancer. Biochim Biophys Acta. 2011;1815:135–146. doi: 10.1016/j.bbcan.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86:s858–s866. doi: 10.1093/ajcn/86.3.858S. [DOI] [PubMed] [Google Scholar]
- 8.Berger NA. Obesity and cancer pathogenesis. Ann N Y Acad Sci. 2014;1311:57–76. doi: 10.1111/nyas.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blando J, Moore T, Hursting S, Jiang G, Saha A, Beltran L, Shen J, Repass J, Strom S, DiGiovanni J. Dietary energy balance modulates prostate cancer progression in Hi-Myc mice. Cancer Prev Res. 2011;4:2002–2014. doi: 10.1158/1940-6207.CAPR-11-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bochet L, Meulle A, Imbert S, Salles B, Valet P, Muller C. Cancer-associated adipocytes promotes breast tumor radioresistance. Biochem Biophys Res Commun. 2011;411:102–106. doi: 10.1016/j.bbrc.2011.06.101. [DOI] [PubMed] [Google Scholar]
- 11.Bowers LW, Cavazos DA, Maximo IX, Brenner AJ, Hursting SD, deGraffenried LA. Obesity enhances nongenomic estrogen receptor crosstalk with the PI3K/Akt and MAPK pathways to promote in vitro measures of breast cancer progression. Breast Cancer Res. 2013;15:R59. doi: 10.1186/bcr3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowers LW, Rossi EL, O'Flanagan CH, deGraffenried LA, Hursting SD. The role of the insulin/IGF system in cancer: lessons learned from clinical trials and the energy balance-cancer link. Front Endocrinol. 2015;6:77. doi: 10.3389/fendo.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldwell SH, Crespo DM, Kang HS, Al-Osaimi AM. Obesity and hepatocellular carcinoma. Gastroenterology. 2004;127:S97–S103. doi: 10.1053/j.gastro.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 14.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 15.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 16.Calle EE, Thun MJ. Obesity and cancer. Oncogene. 2004;23:6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 17.Cancer IAfRo . Weight Control and Physical Activity. Vol. 6. published : IARC press 2002 International Agency for Research on Cancer- WHO; Lyon: 2002. IARC Handbook of Cancer Prevention. [Google Scholar]
- 18.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 19.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discovery. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 21.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone? Diabetes Care. 2003;26:2442–2450. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- 23.Chauhan VP, Jain RK. Strategies for advancing cancer nanomedicine. Nat Mater. 2013;12:958–962. doi: 10.1038/nmat3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, Stylianopoulos T, Mousa A, Han X, Adstamongkonkul P, Popovi c Z, Huang P, Bawendi MG, Boucher Y, Jain RK. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumor blood vessels. Nat Commun. 2013;4:2516. doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chauhan VP, Stylianopoulos T, Boucher Y, Jain RK. Delivery of molecular and nanos cale medicine to tumors: transport barriers and strategies. Annu Rev Chem Biomol Eng. 2011;2:281–298. doi: 10.1146/annurev-chembioeng-061010-114300. [DOI] [PubMed] [Google Scholar]
- 26.Checkley LA, Rho O, Angel JM, Cho J, Blando J, Beltran L, Hursting SD, DiGiovanni J. Metformin inhibits skin tumor promotion in overweight and obese mice. Cancer Prev Res. 2014;7:54–64. doi: 10.1158/1940-6207.CAPR-13-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen CT, Du Y, Yamaguchi H, Hsu JM, Kuo HP, Hortobagyi GN, Hung MC. Targeting the IKKbeta/mTOR/VEGF signaling pathway as a potential therapeutic strategy for obesity-related breast cancer. Mol Cancer Ther. 2012;11:2212–2221. doi: 10.1158/1535-7163.MCT-12-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi Y, Kim TY, Oh DY, Lee KH, Han SW, Im SA, Kim TY, Bang YJ. The impact of diabetes mellitus and metformin treatment on survival of patients with advanced pancreatic cancer undergoing chemotherapy. Cancer Res Treat. 2016;48:171–179. doi: 10.4143/crt.2014.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi YK, Park KG. Metabolic roles of AMPK and metformin in cancer cells. Mol Cells. 2013;36:279–287. doi: 10.1007/s10059-013-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cifarelli V, Lashinger LM, Devlin KL, Dunlap SM, Huang J, Kaaks R, Pollak MN, Hursting SD. Metformin and rapamycin reduce pancreatic cancer growth in obese prediabetic mice by distinct microRNA-regulated mechanisms. Diabetes. 2015;64:1632–1642. doi: 10.2337/db14-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cittadini A, Napoli R, Monti MG, Rea D, Longobardi S, Netti PA, Walser M, Sama M, Aimaretti G, Isgaard J, Sacca L. Metformin prevents the development of chronic heart failure in the SHHF rat model. Diabetes. 2012;61:944–953. doi: 10.2337/db11-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conroy SM, Maskarinec G, Morimoto Y, Franke AA, Cooney RV, Wilkens LR, Goodman MT, Hernadez BY, Le Marchand L, Henderson BE, Kolonel LN. Non-hodgkin lymphoma and circulating markers of inflammation and adiposity in a nested case-control study: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2013;22:337–347. doi: 10.1158/1055-9965.EPI-12-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costa C, Incio J, Soares R. Angiogenesis and chronic inflammation: cause or consequence? Angiogenesis. 2007;10:149–166. doi: 10.1007/s10456-007-9074-0. [DOI] [PubMed] [Google Scholar]
- 34.Cozen W, Gebregziabher M, Conti DV, Van Den Berg DJ, Coetzee GA, Wang SS, Rothman N, Bernstein L, Hartge P, Morhbacher A, Coetzee SG, Salam MT, Wang W, Zadnick J, Ingles SA. Interleukin-6-related genotypes, body mass index, and risk of multiple myeloma and plasmacytoma. Cancer Epidemiol Biomarkers Prev. 2006;15:2285–2291. doi: 10.1158/1055-9965.EPI-06-0446. [DOI] [PubMed] [Google Scholar]
- 35.Cymbaluk A, Chudecka-Glaz A, Rzepka-Gorska I. Leptin levels in serum depending on body mass index in patients with endometrial hyperplasia and cancer. Eur J Obstet Gynecol Reprod Biol. 2008;136:74–77. doi: 10.1016/j.ejogrb.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Dallaglio K, Bruno A, Cantelmo AR, Esposito AI, Ruggiero L, Orecchioni S, Calleri A, Bertolini F, Pfeffer U, Noonan DM, Albini A. Paradoxic effects of metformin on endothelial cells and angiogenesis. Carcinogenesis. 2014;35:1055–1066. doi: 10.1093/carcin/bgu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci USA. 2011;108:2909–2914. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, Garrido I, Escourrou G, Valet P, Muller C. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 39.Dogan S, Hu X, Zhang Y, Maihle NJ, Grande JP, Cleary MP. Effects of high-fat diet and/or body weight on mammary tumor leptin and apoptosis signaling pathways in MMTV-TGF-alpha mice. Breast Cancer Res. 2007;9:R91. doi: 10.1186/bcr1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dowling RJ, Niraula S, Stambolic V, Goodwin PJ. Metformin in cancer: translational challenges. J Mol Endocrinol. 2012;48:R31–R43. doi: 10.1530/JME-12-0007. [DOI] [PubMed] [Google Scholar]
- 41.Doyle SL, Donohoe CL, Finn SP, Howard JM, Lithander FE, Reynolds JV, Pidgeon GP, Lysaght J. IGF-1 and its receptor in esophageal cancer: association with adenocarcinoma and visceral obesity. Am J Gastroenterol. 2012;107:196–204. doi: 10.1038/ajg.2011.417. [DOI] [PubMed] [Google Scholar]
- 42.Duda DG, Ancukiewicz M, Jain RK. Biomarkers of antiangiogenic therapy: how do we move from candidate biomarkers to valid biomarkers? J Clin Oncol. 2010;28:183–185. doi: 10.1200/JCO.2009.24.8021. [DOI] [PubMed] [Google Scholar]
- 43.Duda DG, Munn LL, Jain RK. Can we identify predictive biomarkers for antiangiogenic therapy of cancer using mathematical modeling? J Natl Cancer Inst. 2013;105:762–765. doi: 10.1093/jnci/djt114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296:193–201. doi: 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- 45.Ericksen RE, Rose S, Westphalen CB, Shibata W, Muthupalani S, Tailor Y, Friedman RA, Han W, Fox JG, Ferrante AW, Jr, Wang TC. Obesity accelerates Helicobacter felis-induced gastric carcinogenesis by enhancing immature myeloid cell trafficking and TH17 response. Gut. 2014;63:385–394. doi: 10.1136/gutjnl-2013-305092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esfahanian N, Shakiba Y, Nikbin B, Soraya H, Maleki-Dizaji N, Ghazi-Khansari M, Garjani A. Effect of metformin on the proliferation, migration, and MMP-2 and -9 expression of human umbilical vein endothelial cells. Mol Med Rep. 2012;5:1068–1074. doi: 10.3892/mmr.2012.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8:942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 49.Fujimoto A, Akifusa S, Kamio N, Hirofuji T, Nonaka K, Yamashita Y. Involvement of mTOR in globular adiponectin-induced generation of reactive oxygen species. Free Radic Res. 2010;44:128–134. doi: 10.3109/10715760903348328. [DOI] [PubMed] [Google Scholar]
- 50.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74:72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer. 2006;6:521–534. doi: 10.1038/nrc1910. [DOI] [PubMed] [Google Scholar]
- 52.Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, Chatterjee K, Garkavtsev I, Jain RK. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res. 2003;93:e88–e97. doi: 10.1161/01.RES.0000099243.20096.FA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gati A, Kouidhi S, Marrakchi R, El Gaaied A, Kourda N, Derouiche A, Chebil M, Caignard A, Perier A. Obesity and renal cancer: role of adipokines in the tumor-immune system conflict. Oncoimmunology. 2014;3:e27810. doi: 10.4161/onci.27810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerozissis K. Brain insulin, energy and glucose homeostasis; genes, environment and metabolic pathologies. Eur J Pharmacol. 2008;585:38–49. doi: 10.1016/j.ejphar.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 55.Giavazzi R, Sennino B, Coltrini D, Garofalo A, Dossi R, Ronca R, Tosatti MP, Presta M. Distinct role of fibroblast growth factor-2 and vascular endothelial growth factor on tumor growth and angiogenesis. Am J Pathol. 2003;162:1913–1926. doi: 10.1016/S0002-9440(10)64325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gong J, Robbins LA, Lugea A, Waldron RT, Jeon CY, Pandol SJ. Diabetes, pancreatic cancer, and metformin therapy. Front Physiol. 2014;5:426. doi: 10.3389/fphys.2014.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzalez-Perez RR, Lanier V, Newman G. Leptin's pro-angiogenic signature in breast cancer. Cancers. 2013;5:1140–1162. doi: 10.3390/cancers5031140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goodwin PJ, Stambolic V. Obesity and insulin resistance in breast cancer - chemoprevention strategies with a focus on metformin. Breast. 2011;20(Suppl. 3):S31–S35. doi: 10.1016/S0960-9776(11)70291-0. [DOI] [PubMed] [Google Scholar]
- 59.Gordon RR, Hunter KW, Sorensen P, Pomp D. Genotype X diet interactions in mice predisposed to mammary cancer. I. Body weight and fat. Mamm Genome. 2008;19:163–178. doi: 10.1007/s00335-008-9095-z. [DOI] [PubMed] [Google Scholar]
- 60.Guiu B, Petit JM, Bonnetain F, Ladoire S, Guiu S, Cercueil JP, Krause D, Hillon P, Borg C, Chauffert B, Ghiringhelli F. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut. 2010;59:341–347. doi: 10.1136/gut.2009.188946. [DOI] [PubMed] [Google Scholar]
- 61.Gyanchandani R, Ortega Alves MV, Myers JN, Kim S. A proangiogenic signature is revealed in FGF-mediated bevacizumab-resistant head and neck squamous cell carcinoma. Mol Cancer Res. 2013;11:1585–1596. doi: 10.1158/1541-7786.MCR-13-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci. 2004;82:925–934. doi: 10.2527/2004.823925x. [DOI] [PubMed] [Google Scholar]
- 64.Hefetz-Sela S, Scherer PE. Adipocytes: impact on tumor growth and potential sites for therapeutic intervention. Pharmacol Ther. 2013;138:197–210. doi: 10.1016/j.pharmthera.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hemmeryckx B, van Bree R, Van Hoef B, Vercruysse L, Lijnen HR, Verhaeghe J. Adverse adipose phenotype and hyperinsulinemia in gravid mice deficient in placental growth factor. Endocrinology. 2008;149:2176–2183. doi: 10.1210/en.2007-1272. [DOI] [PubMed] [Google Scholar]
- 66.Hidalgo M, Von Hoff DD. Translational therapeutic opportunities in ductal adenocarcinoma of the pancreas. Clin Cancer Res. 2012;18:4249–4256. doi: 10.1158/1078-0432.CCR-12-1327. [DOI] [PubMed] [Google Scholar]
- 67.Hirakawa T, Yashiro M, Murata A, Hirata K, Kimura K, Amano R, Yamada N, Nakata B, Hirakawa K. IGF-1 receptor and IGF binding protein-3 might predict prognosis of patients with resectable pancreatic cancer. BMC Cancer. 2013;13:392. doi: 10.1186/1471-2407-13-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hiratsuka S, Duda DG, Huang Y, Goel S, Sugiyama T, Nagasawa T, Fukumura D, Jain RK. C-X-C receptor type 4 promotes metastasis by activating p38 mitogen-activated protein kinase in myeloid differentiation antigen (Gr-1)-positive cells. Proc Natl Acad Sci USA. 2011;108:302–307. doi: 10.1073/pnas.1016917108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hori M, Takahashi M, Hiraoka N, Yamaji T, Mutoh M, Ishigamori R, Furuta K, Okusaka T, Shimada K, Kosuge T, Kanai Y, Nakagama H. Association of pancreatic fatty infiltration with pancreatic ductal adenocarcinoma. Clin Transl Gastroenterol. 2014;5:e53. doi: 10.1038/ctg.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 71.Hosoki T. Dynamic CT of pancreatic tumors. AJR Am J Roentgenol. 1983;140:959–965. doi: 10.2214/ajr.140.5.959. [DOI] [PubMed] [Google Scholar]
- 72.Hursting SD. Minireview: the year in obesity and cancer. Mol Endocrinol. 2012;26:1961–1966. doi: 10.1210/me.2012-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hursting SD, Dunlap SM. Obesity, metabolic dysregulation, and cancer: a growing concern and an inflammatory (and microenvironmental) issue. Ann N Y Acad Sci. 2012;1271:82–87. doi: 10.1111/j.1749-6632.2012.06737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–152. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- 75.Incio J, Soares R. Obesity, diabetes and metabolic syndrome impact on tumor angiogenesis. In: Ruben R, Gonzalez-Perez BRR, editors. Tumor Angiogenesis Regulators. CRC Press; New York, USA: 2013. [Google Scholar]
- 76.Incio J, Suboj P, Chin SM, Vardam-Kaur T, Liu H, Hato T, Babykutty S, Chen I, Deshpande V, Jain RK, Fukumura D. Metformin reduces desmoplasia in pancreatic cancer by reprogramming stellate cells and tumor-associated macrophages. PLoS ONE. 2015;10:e0141392. doi: 10.1371/journal.pone.0141392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Isoda K, Young JL, Zirlik A, MacFarlane LA, Tsuboi N, Gerdes N, Schonbeck U, Libby P. Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler Thromb Vasc Biol. 2006;26:611–617. doi: 10.1161/01.ATV.0000201938.78044.75. [DOI] [PubMed] [Google Scholar]
- 78.Jain KK. Cancer biomarkers: current issues and future directions. Curr Opin Mol Ther. 2007;9:563–571. [PubMed] [Google Scholar]
- 79.Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31:2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jain RK, Duda DG, Willett CG, Sahani DV, Zhu AX, Loeffler JS, Batchelor TT, Sorensen AG. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol. 2009;6:327–338. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kanasaki K, Koya D. Biology of obesity: lessons from animal models of obesity. J Biomed Biotechnol. 2011;2011:197636. doi: 10.1155/2011/197636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kasznicki J, Sliwinska A, Drzewoski J. Metformin in cancer prevention and therapy. Ann Transl Med. 2014;2:57. doi: 10.3978/j.issn.2305-5839.2014.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khalid S, Hwang D, Babichev Y, Kolli R, Altamentova S, Koren S, Goodwin PJ, Ennis M, Pollak M, Sonenberg N, Fantus IG. Evidence for a tumor promoting effect of high-fat diet independent of insulin resistance in HER2/Neu mammary carcinogenesis. Breast Cancer Res Treat. 2009;122:647–659. doi: 10.1007/s10549-009-0586-8. [DOI] [PubMed] [Google Scholar]
- 84.Kim JH, Bachmann RA, Chen J. Interleukin-6 and insulin resistance. Vitam Horm. 2009;80:613–633. doi: 10.1016/S0083-6729(08)00621-3. [DOI] [PubMed] [Google Scholar]
- 85.Kimura Y, Sumiyoshi M. High-fat, high-sucrose, and high-cholesterol diets accelerate tumor growth and metastasis in tumor-bearing mice. Nutr Cancer. 2007;59:207–216. doi: 10.1080/01635580701499537. [DOI] [PubMed] [Google Scholar]
- 86.Kisfalvi K, Moro A, Sinnett-Smith J, Eibl G, Rozengurt E. Metformin inhibits the growth of human pancreatic cancer xenografts. Pancreas. 2013;42:781–785. doi: 10.1097/MPA.0b013e31827aec40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Knupfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review). Breast Cancer Res Treat. 2007;102:129–135. doi: 10.1007/s10549-006-9328-3. [DOI] [PubMed] [Google Scholar]
- 88.Komar G, Kauhanen S, Liukko K, Seppanen M, Kajander S, Ovaska J, Nuutila P, Minn H. Decreased blood flow with increased metabolic activity: a novel sign of pancreatic tumor aggressiveness. Clin Cancer Res. 2009;15:5511–5517. doi: 10.1158/1078-0432.CCR-09-0414. [DOI] [PubMed] [Google Scholar]
- 89.Kordes S, Pollak MN, Zwinderman AH, Mathot RA, Weterman MJ, Beeker A, Punt CJ, Richel DJ, Wilmink JW. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16:839–847. doi: 10.1016/S1470-2045(15)00027-3. [DOI] [PubMed] [Google Scholar]
- 90.Kordes S, Pollak MN, Zwinderman AH, Mathot RA, Weterman MJ, Beeker A, Punt CJ, Richel DJ, Wilmink JW. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16:839–847. doi: 10.1016/S1470-2045(15)00027-3. [DOI] [PubMed] [Google Scholar]
- 91.Ladoire S, Bonnetain F, Gauthier M, Zanetta S, Petit JM, Guiu S, Kermarrec I, Mourey E, Michel F, Krause D, Hillon P, Cormier L, Ghiringhelli F, Guiu B. Visceral fat area as a new independent predictive factor of survival in patients with meta-static renal cell carcinoma treated with antiangiogenic agents. Oncologist. 2011;16:71–81. doi: 10.1634/theoncologist.2010-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lappas M. Markers of endothelial cell dysfunction are increased in human omental adipose tissue from women with pre-existing maternal obesity and gestational diabetes. Metab, Clin Exp. 2014;63:860–873. doi: 10.1016/j.metabol.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 93.Lashinger LM, Harrison LM, Rasmussen AJ, Logsdon CD, Fischer SM, McArthur MJ, Hursting SD. Dietary energy balance modulation of Kras- and Ink4a/Arf+/– driven pancreatic cancer: the role of insulin-like growth factor-I. Cancer Prev Res. 2013;6:1046–1055. doi: 10.1158/1940-6207.CAPR-13-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leone A, Di Gennaro E, Bruzzese F, Avallone A, Budillon A. New perspective for an old antidiabetic drug: metformin as anticancer agent. Cancer Treat Res. 2014;159:355–376. doi: 10.1007/978-3-642-38007-5_21. [DOI] [PubMed] [Google Scholar]
- 95.Li D, Morris JS, Liu J, Hassan MM, Day RS, Bondy ML, Abbruzzese JL. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301:2553–2562. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li B, Sharpe EE, Maupin AB, Teleron AA, Pyle AL, Carmeliet P, Young PP. VEGF and PlGF promote adult vasculogenesis by enhancing EPC recruitment and vessel formation at the site of tumor neovascularization. Faseb J. 2006;20:1495–1497. doi: 10.1096/fj.05-5137fje. [DOI] [PubMed] [Google Scholar]
- 97.Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–488. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lieu C, Heymach J, Overman M, Tran H, Kopetz S. Beyond VEGF: inhibition of the fibroblast growth factor pathway and antiangiogenesis. Clin Cancer Res. 2011;17:6130–6139. doi: 10.1158/1078-0432.CCR-11-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lijnen HR. Angiogenesis and obesity. Cardiovasc Res. 2008;78:286–293. doi: 10.1093/cvr/cvm007. [DOI] [PubMed] [Google Scholar]
- 100.Lijnen HR, Christiaens V, Scroyen I, Voros G, Tjwa M, Carmeliet P, Collen D. Impaired adipose tissue development in mice with inactivation of placental growth factor function. Diabetes. 2006;55:2698–2704. doi: 10.2337/db06-0526. [DOI] [PubMed] [Google Scholar]
- 101.Liu Z, Brooks RS, Ciappio ED, Kim SJ, Crott JW, Bennett G, Greenberg AS, Mason JB. Diet-induced obesity elevates colonic TNF-alpha in mice and is accompanied by an activation of Wnt signaling: a mechanism for obesity-associated colorectal cancer. J Nutr Biochem. 2012;23:1207–1213. doi: 10.1016/j.jnutbio.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lutz SZ, Staiger H, Fritsche A, Haring HU. Antihyperglycaemic therapies and cancer risk. Diab Vasc Dis Res. 2014;11:371–389. doi: 10.1177/1479164114549553. [DOI] [PubMed] [Google Scholar]
- 103.Maccio A, Madeddu C, Gramignano G, Mulas C, Floris C, Massa D, Astara G, Chessa P, Mantovani G. Correlation of body mass index and leptin with tumor size and stage of disease in hormone-dependent postmenopausal breast cancer: preliminary results and therapeutic implications. J Mol Med (Berl) 2010;88:677–686. doi: 10.1007/s00109-010-0611-8. [DOI] [PubMed] [Google Scholar]
- 104.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 105.Maeda H, Tsukigawa K, Fang J. A Retrospective 30 years after discovery of the EPR effect of solid tumors: next-generation chemotherapeutics and photodynamic-therapy-problems, solutions, prospects. Microcirculation. 2015 doi: 10.1111/micc.12228. doi:10.1111/micc.12228. [DOI] [PubMed] [Google Scholar]
- 106.Majed B, Moreau T, Senouci K, Salmon RJ, Fourquet A, Asselain B. Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res Treat. 2008;111:329–342. doi: 10.1007/s10549-007-9785-3. [DOI] [PubMed] [Google Scholar]
- 107.Martin-Padura I, Gregato G, Marighetti P, Mancuso P, Calleri A, Corsini C, Pruneri G, Manzotti M, Lohsiriwat V, Rietjens M, Petit JY, Bertolini F. The white adipose tissue used in lipotransfer procedures is a rich reservoir of CD34 + progenitors able to promote cancer progression. Cancer Res. 2012;72:325–334. doi: 10.1158/0008-5472.CAN-11-1739. [DOI] [PubMed] [Google Scholar]
- 108.Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J Immunol. 2005;174:3137–3142. doi: 10.4049/jimmunol.174.6.3137. [DOI] [PubMed] [Google Scholar]
- 109.Matsuda K, Falkenberg KJ, Woods AA, Choi YS, Morrison WA, Dilley RJ. Adi-pose-derived stem cells promote angio-genesis and tissue formation for in vivo tissue engineering. Tissue Eng Part A. 2013;19:1327–1335. doi: 10.1089/ten.tea.2012.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Matsuzawa Y, Funahashi T, Nakamura T. Molecular mechanism of metabolic syndrome X: contribution of adipocytokines adipocyte-derived bioactive substances. Ann N Y Acad Sci. 1999;892:146–154. doi: 10.1111/j.1749-6632.1999.tb07793.x. [DOI] [PubMed] [Google Scholar]
- 111.McGuire BB, Fitzpatrick JM. BMI and the risk of renal cell carcinoma. Curr Opin Urol. 2011;21:356–361. doi: 10.1097/MOU.0b013e32834962d5. [DOI] [PubMed] [Google Scholar]
- 112.Mohammed A, Janakiram NB, Brewer M, Ritchie RL, Marya A, Lightfoot S, Steele VE, Rao CV. Antidiabetic drug metformin prevents progression of pancreatic cancer by targeting in part cancer stem cells and signaling. Transl Oncol. 2013;6:649–659. doi: 10.1593/tlo.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 114.Mu N, Zhu Y, Wang Y, Zhang H, Xue F. Insulin resistance: a significant risk factor of endometrial cancer. Gynecol Oncol. 2012;125:751–757. doi: 10.1016/j.ygyno.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 115.Murakami M, Iwai S, Hiratsuka S, Yamauchi M, Nakamura K, Iwakura Y, Shibuya M. Signaling of vascular endothelial growth factor receptor-1 tyrosine kinase promotes rheumatoid arthritis through activation of monocytes/macrophages. Blood. 2006;108:1849–1856. doi: 10.1182/blood-2006-04-016030. [DOI] [PubMed] [Google Scholar]
- 116.Murakami M, Zheng Y, Hirashima M, Suda T, Morita Y, Ooehara J, Ema H, Fong GH, Shibuya M. VEGFR1 tyrosine kinase signaling promotes lymphangiogenesis as well as angiogenesis indirectly via macrophage recruitment. Arterioscler Thromb Vasc Biol. 2008;28:658–664. doi: 10.1161/ATVBAHA.107.150433. [DOI] [PubMed] [Google Scholar]
- 117.Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, Cinti S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 2008;49:1562–1568. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 118.Nair V, Pathi S, Jutooru I, Sreevalsan S, Basha R, Abdelrahim M, Samudio I, Safe S. Metformin inhibits pancreatic cancer cell and tumor growth and downregulates Sp transcription factors. Carcinogenesis. 2013;34:2870–2879. doi: 10.1093/carcin/bgt231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nair V, Sreevalsan S, Basha R, Abdelrahim M, Abudayyeh A, Rodrigues Hoffman A, Safe S. Mechanism of metformin-dependent inhibition of mammalian target of rapamycin (mTOR) and Ras activity in pancreatic cancer: role of specificity protein (Sp) transcription factors. J Biol Chem. 2014;289:27692–27701. doi: 10.1074/jbc.M114.592576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Neesse A, Frese KK, Bapiro TE, Nakagawa T, Sternlicht MD, Seeley TW, Pilarsky C, Jodrell DI, Spong SM, Tuveson DA. CTGF antagonism with mAb FG-3019 enhances chemotherapy response without increasing drug delivery in murine ductal pancreas cancer. Proc Natl Acad Sci. 2013;110:12325–12330. doi: 10.1073/pnas.1300415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nunez NP, Perkins SN, Smith NC, Berrigan D, Berendes DM, Varticovski L, Barrett JC, Hursting SD. Obesity accelerates mouse mammary tumor growth in the absence of ovarian hormones. Nutr Cancer. 2008;60:534–541. doi: 10.1080/01635580801966195. [DOI] [PubMed] [Google Scholar]
- 122.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Okwan-Duodu D, Umpierrez GE, Brawley OW, Diaz R. Obesity-driven inflammation and cancer risk: role of myeloid derived suppressor cells and alternately activated macrophages. Am J Cancer Res. 2013;3:21–33. [PMC free article] [PubMed] [Google Scholar]
- 124.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 126.Orecchioni S, Gregato G, Martin-Padura I, Reggiani F, Braidotti P, Mancuso P, Calleri A, Quarna J, Marighetti P, Aldeni C, Pruneri G, Martella S, Manconi A, Petit JY, Rietjens M, Bertolini F. Complementary populations of human adipose CD34 + progenitor cells promote growth, angiogenesis, and metastasis of breast cancer. Cancer Res. 2013;73:5880–5891. doi: 10.1158/0008-5472.CAN-13-0821. [DOI] [PubMed] [Google Scholar]
- 127.Orecchioni S, Reggiani F, Talarico G, Mancuso P, Calleri A, Gregato G, Labanca V, Noonan DM, Dallaglio K, Albini A, Bertolini F. The biguanides metformin and phenformin inhibit angiogenesis, local and metastatic growth of breast cancer by targeting both neoplastic and microenvironment cells. Int J Cancer. 2015;136:E534–E544. doi: 10.1002/ijc.29193. [DOI] [PubMed] [Google Scholar]
- 128.Osborne CK, Bolan G, Monaco ME, Lippman ME. Hormone responsive human breast cancer in long-term tissue culture: effect of insulin. Proc Natl Acad Sci USA. 1976;73:4536–4540. doi: 10.1073/pnas.73.12.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69:29–35. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 130.Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003;138:24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 131.Pelton K, Coticchia CM, Curatolo AS, Schaffner CP, Zurakowski D, Solomon KR, Moses MA. Hypercholesterolemia induces angiogenesis and accelerates growth of breast tumors in vivo. Am J Pathol. 2014;184:2099–2110. doi: 10.1016/j.ajpath.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pervanidou P, Chouliaras G, Akalestos A, Bastaki D, Apostolakou F, Papassotiriou I, Chrousos GP. Increased placental growth factor (PlGF) concentrations in children and adolescents with obesity and the metabolic syndrome. Hormones. 2014;13:369–374. doi: 10.14310/horm.2002.1491. [DOI] [PubMed] [Google Scholar]
- 133.Pollak MN. Insulin-like growth factors and neoplasia. Novartis Found Symp. 2004;262:84–98. discussion 98–107, 265–108. [PubMed] [Google Scholar]
- 134.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 135.Preziosi G, Oben JA, Fusai G. Obesity and pancreatic cancer. Surg Oncol. 2014;23:61–71. doi: 10.1016/j.suronc.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 136.Ramos-Nino ME. The role of chronic inflammation in obesity-associated cancers. ISRN Oncol. 2013;2013:697521. doi: 10.1155/2013/697521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Remick DG. Interleukin-8. Crit Care Med. 2005;33:S466–S467. doi: 10.1097/01.ccm.0000186783.34908.18. [DOI] [PubMed] [Google Scholar]
- 138.Remick DG, Bolgos G, Copeland S, Siddiqui J. Role of interleukin-6 in mortality from and physiologic response to sepsis. Infect Immun. 2005;73:2751–2757. doi: 10.1128/IAI.73.5.2751-2757.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reni M, Dugnani E, Cereda S, Belli C, Balzano G, Nicoletti R, Liberati D, Pasquale V, Scavini M, Maggiora P, Sordi V, Lampasona V, Ceraulo D, Di Terlizzi G, Doglioni C, Falconi M, Piemonti L. (Ir) relevance of metformin treatment in patients with metastatic pancreatic cancer: an open-label, randomized phase 2 trial. Clin Cancer Res. :CCR-15–1722. 2015. doi: 10.1158/1078-0432.CCR-15-1722. [DOI] [PubMed] [Google Scholar]
- 140.Ricart W, Fernandez-Real JM. No decrease in free IGF-I with increasing insulin in obesity-related insulin resistance. Obes Res. 2001;9:631–636. doi: 10.1038/oby.2001.83. [DOI] [PubMed] [Google Scholar]
- 141.Chaiteerakij R, Zhen DB, Burch PA, Chaffee KG, Bamlet WR, Oberg AL, Roberts LR, Petersen GM. Proceedings of the 106th Annual Meeting of the American Association for Cancer Research. AACR; Philadelphia: Metformin use does not increase survival of pancreatic cancer patients: a cautionary lesson [Abstract]. pp. 18–22. [Google Scholar]; Cancer Res. 2015;75(Suppl 15) Abstract nr LB-183. [Google Scholar]
- 142.Rose DP, Connolly JM, Meschter CL. Effect of dietary fat on human breast cancer growth and lung metastasis in nude mice. J Natl Cancer Inst. 1991;83:1491–1495. doi: 10.1093/jnci/83.20.1491. [DOI] [PubMed] [Google Scholar]
- 143.Rose DP, Haffner SM, Baillargeon J. Adiposity, the metabolic syndrome, and breast cancer in African-American and white American women. Endocr Rev. 2007;28:763–777. doi: 10.1210/er.2006-0019. [DOI] [PubMed] [Google Scholar]
- 144.Sadeghi N, Abbruzzese JL, Yeung SC, Hassan M, Li D. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res. 2012;18:2905–2912. doi: 10.1158/1078-0432.CCR-11-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saidi A, Hagedorn M, Allain N, Verpelli C, Sala C, Bello L, Bikfalvi A, Javerzat S. Combined targeting of interleukin-6 and vascular endothelial growth factor potently inhibits glioma growth and invasiveness. Int J Cancer. 2009;125:1054–1064. doi: 10.1002/ijc.24380. [DOI] [PubMed] [Google Scholar]
- 146.Sakamoto T, Kudoh T, Sakamoto K, Matsui K, Ogawa H. Antithrombotic effects of losartan in patients with hyper-tension complicated by atrial fibrillation: 4A (Angiotensin II Antagonist of platelet Aggregation in patients with Atrial fibrillation), a pilot study. Hypertens Res. 2014;37:513–518. doi: 10.1038/hr.2014.22. [DOI] [PubMed] [Google Scholar]
- 147.Sena CM, Matafome P, Louro T, Nunes E, Fernandes R, Seica RM. Metformin restores endothelial function in aorta of diabetic rats. Br J Pharmacol. 2011;163:424–437. doi: 10.1111/j.1476-5381.2011.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shibuya M. Involvement of Flt-1 (VEGF receptor-1) in cancer and preeclampsia. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:167–178. doi: 10.2183/pjab.87.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Siegel AB, Wang S, Jacobson JS, Hershman DL, Lim EA, Yu J, Ferrante L, Devaraj KM, Remotti H, Scrudato S, Halazun K, Emond J, Dove L, Brown RS, Jr, Neugut AI. Obesity and microvascular invasion in hepatocellular carcinoma. Cancer Invest. 2010;28:1063–1069. doi: 10.3109/07357907.2010.483500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Silverman KJ, Lund DP, Zetter BR, Lainey LL, Shahood JA, Freiman DG, Folkman J, Barger AC. Angiogenic activity of adipose tissue. Biochem Biophys Res Commun. 1988;153:347–352. doi: 10.1016/s0006-291x(88)81229-4. [DOI] [PubMed] [Google Scholar]
- 151.Smits MM, van Geenen EJ. The clinical significance of pancreatic steatosis. Nat Rev Gastroenterol Hepatol. 2011;8:169–177. doi: 10.1038/nrgastro.2011.4. [DOI] [PubMed] [Google Scholar]
- 152.Snima KS, Pillai P, Cherian AM, Nair SV, Lakshmanan VK. Anti-diabetic drug metformin: challenges and perspectives for cancer therapy. Curr Cancer Drug Targets. 2014;14:727–736. doi: 10.2174/1568009614666141020105502. [DOI] [PubMed] [Google Scholar]
- 153.Sohail MA, Saif MW. Role of anticoagulation in the management of pancreatic cancer. JOP. 2009;10:82–87. [PubMed] [Google Scholar]
- 154.Soraya H, Esfahanian N, Shakiba Y, Ghazi-Khansari M, Nikbin B, Hafezzadeh H, Maleki Dizaji N, Garjani A. Anti-angiogenic effects of metformin, an AMPK activator, on human umbilical vein endothelial cells and on granulation tissue in rat. Iran J Basic Med Sci. 2012;15:1202–1209. [PMC free article] [PubMed] [Google Scholar]
- 155.Stolzenberg-Solomon RZ, Weinstein S, Pollak M, Tao Y, Taylor PR, Virtamo J, Albanes D. Prediagnostic adiponectin concentrations and pancreatic cancer risk in male smokers. Am J Epidemiol. 2008;168:1047–1055. doi: 10.1093/aje/kwn221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Strain G, Zumoff B, Rosner W, Pi-Sunyer X. The relationship between serum levels of insulin and sex hormone-binding globulin in men: the effect of weight loss. J Clin Endocrinol Metab. 1994;79:1173–1176. doi: 10.1210/jcem.79.4.7962291. [DOI] [PubMed] [Google Scholar]
- 157.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, Zhou XK, Blaho VA, Hla T, Yang P, Kopelovich L, Hudis CA, Dannenberg AJ. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res. 2011;4:329–346. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 158.Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, Oberyszyn TM, Hall BM. Interleukin-6 induces an epithelialmesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sun K, Wernstedt Asterholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, Brekken RA, Scherer PE. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci USA. 2012;109:5874–5879. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Takahashi A, Kimura F, Yamanaka A, Takebayashi A, Kita N, Takahashi K, Murakami T. Metformin impairs growth of endometrial cancer cells via cell cycle arrest and concomitant autophagy and apoptosis. Cancer Cell Int. 2014;14:53. doi: 10.1186/1475-2867-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tam J, Duda DG, Perentes JY, Quadri RS, Fukumura D, Jain RK. Blockade of VEGFR2 and not VEGFR1 can limit diet-induced fat tissue expansion: role of local versus bone marrow-derived endothelial cells. PLoS ONE. 2009;4:e4974. doi: 10.1371/journal.pone.0004974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tam J, Fukumura D, Jain RK. A mathematical model of murine metabolic regulation by leptin: energy balance and defense of a stable body weight. Cell Metab. 2009;9:52–63. doi: 10.1016/j.cmet.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tamakoshi K, Toyoshima H, Wakai K, Kojima M, Suzuki K, Watanabe Y, Hayakawa N, Yatsuya H, Kondo T, Tokudome S, Hashimoto S, Suzuki S, Kawado M, Ozasa K, Ito Y, Tamakoshi A. Leptin is associated with an increased female colorectal cancer risk: a nested case-control study in Japan. Oncology. 2005;68:454–461. doi: 10.1159/000086988. [DOI] [PubMed] [Google Scholar]
- 164.Tan XL, Bhattacharyya KK, Dutta SK, Bamlet WR, Rabe KG, Wang E, Smyrk TC, Oberg AL, Petersen GM, Mukhopadhyay D. Metformin suppresses pancreatic tumor growth with inhibition of NFkappaB/STAT3 inflammatory signaling. Pancreas. 2015;44:636–647. doi: 10.1097/MPA.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93:1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 166.Trayhurn P, Wang B, Wood IS. Hypoxia and the endocrine and signalling role of white adipose tissue. Arch Physiol Biochem. 2008;114:267–276. doi: 10.1080/13813450802306602. [DOI] [PubMed] [Google Scholar]
- 167.Tworoger SS, Eliassen AH, Kelesidis T, Colditz GA, Willett WC, Mantzoros CS, Hankinson SE. Plasma adiponectin concentrations and risk of incident breast cancer. J Clin Endocrinol Metab. 2007;92:1510–1516. doi: 10.1210/jc.2006-1975. [DOI] [PubMed] [Google Scholar]
- 168.Venna VR, Li J, Hammond MD, Mancini NS, McCullough LD. Chronic metformin treatment improves post-stroke angio-genesis and recovery after experimental stroke. Eur J Neuorsci. 2014;39:2129–2138. doi: 10.1111/ejn.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Voros G, Maquoi E, Demeulemeester D, Clerx N, Collen D, Lijnen HR. Modulation of angiogenesis during adipose tissue development in murine models of obesity. Endocrinology. 2005;146:4545–4554. doi: 10.1210/en.2005-0532. [DOI] [PubMed] [Google Scholar]
- 170.Wairagu PM, Phan AN, Kim MK, Han J, Kim HW, Choi JW, Kim KW, Cha SK, Park KH, Jeong Y. Insulin priming effect on estradiol-induced breast cancer metabolism and growth. Cancer Biol Ther. 2015 doi: 10.1080/15384047.2015.1016660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang YY, Lehuede C, Laurent V, Dirat B, Dauvillier S, Bochet L, Le Gonidec S, Escourrou G, Valet P, Muller C. Adipose tissue and breast epithelial cells: a dangerous dynamic duo in breast cancer. Cancer Lett. 2012;324:142–151. doi: 10.1016/j.canlet.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 172.Wang X, Simpson ER, Brown KA. Aromatase overexpression in dysfunctional adipose tissue links obesity to post-menopausal breast cancer. J Steroid Biochem Mol Biol. 2015;153:35–44. doi: 10.1016/j.jsbmb.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 173.Wang F, Wang B, Qiao L. Association between obesity and gallbladder cancer. Front Biosci. 2012;17:2550–2558. doi: 10.2741/4070. [DOI] [PubMed] [Google Scholar]
- 174.Weisberg SP, McCann D, Desai M, Rosen-baum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wolpin BM, Bao Y, Qian ZR, Wu C, Kraft P, Ogino S, Stampfer MJ, Sato K, Ma J, Buring JE, Sesso HD, Lee IM, Gaziano JM, McTiernan A, Phillips LS, Cochrane BB, Pollak MN, Manson JE, Giovannucci EL, Fuchs CS. Hyperglycemia, insulin resistance, impaired pancreatic beta-cell function, and risk of pancreatic cancer. J Natl Cancer Inst. 2013;105:1027–1035. doi: 10.1093/jnci/djt123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.World Health Organization Obesity and overweight. [Online]. http://www.who.int/mediacentre/factsheets/fs311/en/. [20 May 2014]
- 177.Wu Y, Brodt P, Sun H, Mejia W, Novosyadlyy R, Nunez N, Chen X, Mendoza A, Hong SH, Khanna C, Yakar S. Insulin-like growth factor-I regulates the liver microenvironment in obese mice and promotes liver metastasis. Cancer Res. 2010;70:57–67. doi: 10.1158/0008-5472.CAN-09-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xie Y, Wang JL, Ji M, Yuan ZF, Peng Z, Zhang Y, Wen JG, Shi HR. Regulation of insulin-like growth factor signaling by metformin in endometrial cancer cells. Oncol Lett. 2014;8:1993–1999. doi: 10.3892/ol.2014.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yerushalmi R, Gelmon KA, Leung S, Gao D, Cheang M, Pollak M, Turashvili G, Gilks BC, Kennecke H. Insulin-like growth factor receptor (IGF-1R) in breast cancer subtypes. Breast Cancer Res Treat. 2012;132:131–142. doi: 10.1007/s10549-011-1529-8. [DOI] [PubMed] [Google Scholar]
- 181.Zechner D, Radecke T, Amme J, Burtin F, Albert AC, Partecke LI, Vollmar B. Impact of diabetes type II and chronic inflammation on pancreatic cancer. BMC Cancer. 2015;15:51. doi: 10.1186/s12885-015-1047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zheng Q, Dunlap SM, Zhu J, Downs-Kelly E, Rich J, Hursting SD, Berger NA, Reizes O. Leptin deficiency suppresses MMTVWnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocr Relat Cancer. 2011;18:491–503. doi: 10.1530/ERC-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zubkova ES, Beloglazova IB, Makarevich PI, Boldyreva MA, Sukhareva OY, Shestakova MV, Dergilev KV, Parfyonova YV, Menshikov MY. Regulation of adipose tissue stem cells angiogenic potential by tumor necrosis factor-alpha. J Cell Biochem. 2016;117:180–96. doi: 10.1002/jcb.25263. [DOI] [PubMed] [Google Scholar]
- 184.Zyromski NJ, Mathur A, Pitt HA, Wade TE, Wang S, Nakshatri P, Swartz-Basile DA, Nakshatri H. Obesity potentiates the growth and dissemination of pancreatic cancer. Surgery. 2009;146:258–263. doi: 10.1016/j.surg.2009.02.024. [DOI] [PubMed] [Google Scholar]