Prospective: Database of genomic biomarkers for cancer drugs and clinical targetability in solid tumors (original) (raw)
. Author manuscript; available in PMC: 2016 Apr 8.
Abstract
Comprehensive genomic profiling is expected to revolutionize cancer therapy. In this Prospective, we present the prevalence of mutations and copy number alterations with predictive associations across solid tumors at different levels of stringency for gene-drug targetability. More than 90% of TCGA samples have potentially targetable alterations, the majority with multiple events, illustrating the challenges for treatment prioritization given the complexity of the genomic landscape. Nearly 80% of the variants in rarely mutated oncogenes are of uncertain functional significance, reflecting the gap in our understanding of the relevance of many alterations potentially linked to therapeutic actions. Access to targeted agents in early clinical trials could affect treatment decision in 75% of cancer patients. Prospective implementation of large-scale molecular profiling and standardized reports of predictive biomarkers are fundamental steps for making precision cancer medicine a reality.
Keywords: cancer, clinical genomics, predictive, targetable alterations
Introduction
The Cancer Genome Atlas (TCGA) project has provided many biological insights through genomic, transcriptomic, epigenomic and proteomic profiling from a large number of patient samples in many cancer types. However, in-depth clinical tie-ins to TCGA data have yet to be thoroughly performed. A recent study has shown that integrating large-scale molecular profiling data to clinical variables yields statistically significant improvements in prognostic estimates for selected cancer patients (1). In addition, investigators have found that nearly 90% of TCGA samples harbor alterations in genes linked to predictive, prognostic or diagnostic associations (1,2). But the value of comprehensive genomic tests for medical oncologists planning to optimally guide therapy by matching every possible gene alteration to targeted agents remains largely unknown.
In this context, massively parallel sequencing of the whole exome/genome as opposed to targeted sequencing (hotspots) frequently identifies mutations of unknown functional consequence in hundreds of genes, most of which are likely to be neutral or passenger alterations (3). Nonetheless, these interrogations also detect low-frequency alterations (rare variants) in cancer genes that have well-established clinical utility and are known drivers of tumor progression, such as EGFR and BRAF. Furthermore, these gene alterations have been repeatedly observed in unexpected tumor types, emphasizing the potential benefit of comprehensive molecular profiling irrespective of cancer histopathology (4-8). Importantly, consensus definitions for clinically “actionable” or “targetable” are not yet available. “Actionable” usually refers to variants with predictive, prognostic and/or diagnostic associations. Similarly, “targetable” might refer to specific genomic variants linked to approved drugs in selected tumor types (companion diagnostic), to more inclusive off-label use of targeted drugs (translating the knowledge from one tumor type to others), or to variants linked to drugs being investigated in clinical trials (with predictive associations based on early clinical evidence or even preclinical data only) (9). We believe that the most inclusive definition of a “targetable” genomic event is appropriate, which would include variants that support treatment recommendation or enrollment in a particular clinical study. However, knowing that there is significant heterogeneity in the “targetability” classification of genomic events, we propose a clinical targetability index, with varying levels of stringency to define a gene alteration as “targetable”. In this Prospective we present the prevalence of alterations that may have an impact on patient treatment options when moving from the most relaxed to the strictest criteria to match a gene alteration with a drug. In contrast to previous disease-specific molecular epidemiology efforts, we explore pan-cancer TCGA data and perform a detailed assessment of the direct clinical utility of large-scale molecular profiling by: (i) limiting the analysis to “targetable” events – excluding gene variants whose “actionability” is limited to prognostic and/or diagnostic associations; (ii) looking at both mutations and somatic copy number events across multiple solid tumor types; (iii) carefully assessing whether specific variants in oncogenes have been functionally validated; and (iv) taking into consideration the specific tumors types for which the gene-drug association has been described in the literature.
Gene-Drug Knowledge Database and Clinical Targetability Index
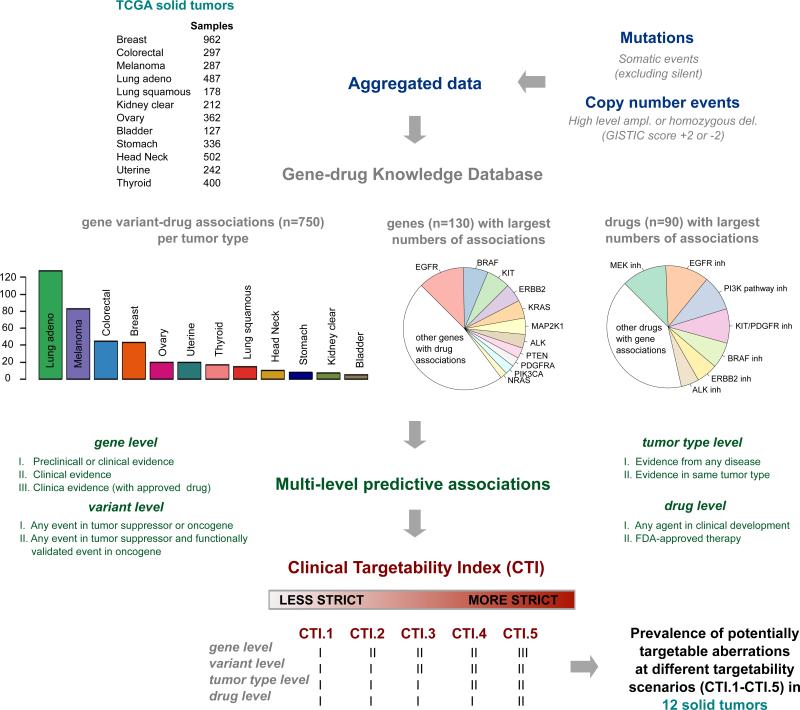
In order to perform a comprehensive clinically-oriented analysis of somatic cancer variants we developed a Gene-Drug Knowledge Database (GDKD) of predictive genomic biomarkers in oncology – a manually curated database that integrates different layers of annotations: tumor types, genes, variants, and sensitivity/resistance patterns to approved and experimental agents under clinical investigation. All associations are described using standardized terminology in a structured format and linked to PubMed identifiers. Regular updates are made publicly available through Synapse (10). More than 700 variant-specific gene-drug interactions with consensus or emerging therapeutic relevance were curated for this effort, as shown in Figure 1. We performed an extensive literature search for predictive associations on the most frequent and relevant somatic cancer variants, encompassing 130 genes, 90 drugs and 40 malignancies. Of note, the GDKB aggregates information from publicly available resources such as My Cancer Genome, Targeted Cancer Care, Personalized Cancer Therapy and other efforts (2).
Figure 1.
Workflow of analytic pipeline integrating Gene-Drug Knowledge Database (GDKD) and Clinical Targetability Index (CTI) to pan-cancer TCGA data. Statistics of GDKD: distribution of gene variant – drug associations across solid tumor types, most frequent genes and drugs with predictive associations. ampl: amplifications; del: deletions.
For a detailed estimation of targetability, we assessed genomic markers at multiple levels:
- Gene – any gene that, when somatically altered in cancer, predicts response or resistance to a specific therapy was considered a targetable gene. We defined three gene lists based on the strength of evidence for predictive associations (10):
- Genes whose associations are based on preclinical or clinical studies (n=122);
- Genes with clinically proven associations based on consensus guidelines, clinical trials or case reports (n=50);
- Genes with associations that have been clinically validated and that guided Food and Drug Administration (FDA)-approval of targeted drugs in solid tumors (n=7).
- Variant – two degrees of restrictiveness were defined:
- Relaxed: for tumor suppressors (e.g. PTEN), any non-silent mutation or copy number loss (GISTIC score −2) in the gene was considered an event. For oncogenes with targetable amplifications (e.g. MET), only GISTIC scores +2 were considered events. For oncogenes with targetable mutations, any non-synonymous variant in the gene was considered an event;
- Strict: Same as above for tumor suppressors and amplifications/deletions, but for oncogenes with targetable mutations, only functionally validated activating variants were considered events – either preclinical or clinical evidence of sensitivity/resistance to any matched targeted therapy.
- Tumor type – two levels of associations:
- Unrestricted evidence: knowledge from one tumor type is translated to all malignancies (e.g., targetability of NRAS codon 61 mutations in melanoma is applied to endometrial cancer);
- Restricted evidence: tumor-type specific knowledge on targetability of genomic events.
- Drug: only associations with agents that are currently in clinical development were considered (we excluded drugs that have not yet been translated to the clinic):
- Any targeted drug in phase 1-3 clinical trials or that received regulatory approval;
- Genomic markers linked to FDA-approved agents.
Using these criteria we defined the Clinical Targetability Index (CTI), with increasing levels of evidence for predictive associations of genomic biomarkers, as summarized in Figure 1. Briefly, in CTI.1 preclinical studies are taken into consideration when defining a biomarker, such as ERBB3 mutations (11); in CTI.2 we limited the analysis to gene alterations that have clinical associations described in the literature, such as FGFR1 amplifications (12); in CTI.3 we excluded variants in oncogenes that are of uncertain significance; in CTI.4 we focused on predictive evidence derived from studies performed in the same tumor type; and in CTI.5 we considered only associations linked to FDA approved agents. We then used gene-drug associations from the GDKD as “genomic biomarker filters” to assess the prevalence of potentially targetable events at different CTI scenarios. TCGA mutation calls were downloaded from Synapse TCGA Live data portal (13) and copy number GISTIC scores from Firehose Broad website (14) on June 12th 2014.
Prevalence of potentially targetable events in different scenarios
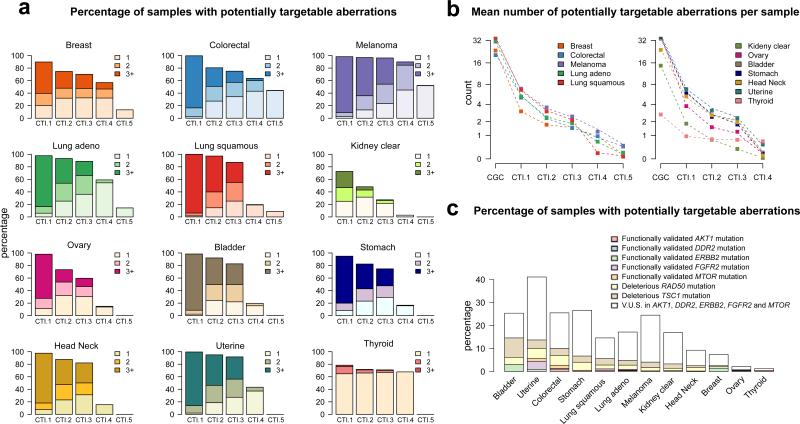
Global surveys of mutational and copy number patterns in clinically relevant genes may have a major impact on treatment selection. As shown in Figure 2a, according to the most relaxed scenario (CTI.1), on average 93% of cancer samples have targetable alterations, with most samples (69%) having three or more events per tumor, underscoring the complexity of cancer in terms of multiplicity of potentially driving events. The same is true in scenario CTI.2, when considering only clinically validated genomic alterations. In overall, 83% of the samples have targetable events, with kidney clear cell carcinomas presenting the lowest rate (50%). A different pattern is seen in thyroid cancer: 65% of the samples have only one targetable event and less than 2% have three or more alterations per sample. Notably, nearly 75% of the patients still have at least one targetable event according to CTI.3, but only 20% of the tumors have three or more events. This scenario illustrates what medical oncologists working at large research institutions with comprehensive tumor genotyping may face on a daily basis, trying to match many gene alterations that still are of unknown predictive value (emerging evidence derived from early clinical data from a variety of tumor types) with drugs in clinical trials. Surprisingly, a substantial proportion (>50%) of the patients with relatively rare malignancies – bladder, head and neck, stomach and uterine cancer – would potentially benefit from an expanded mutation/copy number analysis pipeline in order to identify alterations in genes that have emerging associations. Examples include genomic events in receptor tyrosine kinases (ERBB2, ERBB3, MET), PI3K pathway (PIK3CA, PIK3R1, PTEN, MTOR, TSC1), FGFR pathway (FGFR1, FGFR2, FGFR3), and DNA damage repair signaling (BRCA1, BRCA2, RAD50, ERCC2).
Figure 2.
a. Prevalence of targetable events according to different Clinical Targetability Indices – CTI.1 to CTI.5 – across solid tumors in TCGA. b. Mean number of alterations per sample, starting from mutations or copy number events in Cancer Gene Census (CGC) genes and then looking at potentially targetable events according to different targetability scenarios – CTI.1 to CTI.5. Tumors in the right side do not have a gene alteration linked to FDA-approved drug (i.e. no CTI.5). c. Prevalence of gene variants associated with dramatic responses to targeted therapies (case reports) across solid tumors. For oncogenes, the proportion of variants that have been functionally validated and that remain of uncertain significance is depicted. V.U.S: variants of unknown significance.
We then assessed the impact on predictive genomic biomarker frequency when moving from relaxed to stricter targetability criteria. Figure 2b depicts the mean number of alterations per sample in different scenarios, starting with any mutation or high-level amplification or deletion in genes that have been casually implicated in cancer, as catalogued by the Cancer Gene Census (CGC) and other groups (15,16). On average, solid tumors have nearly 30 cancer-related alterations and only 17% of them are considered targetable according to CTI.1. Thyroid cancer is again an outlier, with only 3 alterations in cancer genes per sample. Notably, when excluding variants of uncertain significance in oncogenes (scenario CTI.2 to CTI.3), the mean reduction in the number of targetable events is 25%, but with significant differences across solid tumors. As expected, diseases with higher prevalence of events in rarely mutated oncogenes are more likely to be affected (more than 50% reduction in targetability rate), since the majority of non-hotspot events have not been functionally validated, as discussed later. Examples include stomach, uterine and kidney clear cell carcinomas with ERBB2, FGFR2 and MTOR mutations. Of note, the largest impact on the prevalence of targetable alterations occurs when we ignore genomic events that have been matched to targeted drugs in different malignancies. Diseases in which the targetability of genomic events has been understudied (with more than a 90% drop when moving from scenario CTI.3 to CTI.4) include bladder, stomach, kidney clear cell carcinoma, squamous lung and head and neck cancers. Further preclinical-clinical validation of potential targets is needed in these tumor types. In scenario CTI.4, 39% of the patients have at least one targetable event.
By looking at scenario CTI.5, which represents the strictest criteria to match gene alterations to approved targeted agents, we confirmed that the distributions of TCGA genomic alterations are concordant with the molecular epidemiology of validated biomarkers in cancer: (i) 13% of lung carcinomas with activating EGFR mutations (sensitivity to erlotinib, afatinib); (ii) 48% of melanoma samples with BRAF V600 mutations (sensitivity to vemurafenib, dabrafenib or trametinib) and 4% with activating KIT mutations (sensitivity to imatinib); (iii) 13% of breast cancer samples with ERBB2 amplifications (sensitivity to trastuzumab, pertuzumab, ado-trastuzumab emtansine, lapatinib) – GISTIC scores +2 are likely the best approximation to the FDA-approved biomarkers (in situ hybridization or protein overexpression); and (iv) 45% of colorectal cancer samples with KRAS or NRAS mutations (resistance to cetuximab and panitumumab). These five tumor types have targeted drugs recommended by consensus guidelines and a companion biomarker diagnostic that tests positive in approximately 25% of the samples. If clinicians could add to the therapeutic armamentarium unapproved drugs linked to emerging biomarkers in the same tumor types, this number would increase to 55%. This larger population is indeed eligible for clinical trials testing the predictive value of genomic biomarkers.
Case reports and variants of unknown significance in oncogenes
It is already known that validated actionable alterations, such as BRAF V600E in melanoma, can be found in unexpected tumor types and occasionally predict for benefit with matched therapies (4,17). Case reports of dramatic responses to targeted agents are increasingly being published, not only in the setting of validated biomarkers in one tumor type being translated to other diseases, but also with rare genomic events not previously linked to benefit with a particular drug. Examples include activating mutations in AKT1 (AKT inhibitors) (18), DDR2 (dasatinib plus erlotinib) (19), ERBB2 (anti-HER2 agents) (20,21), FGFR2 (pazopanib) (6), MTOR (everolimus plus pazopanib) (22), as well as inactivating events in RAD50 (irinotecan plus CHK1/2 inhibitor) (23) or TSC1 (everolimus) (24). We decided to study the prevalence of these genomic events across solid tumors, as shown in Figure 2c. When considering only functionally validated mutations in the oncogenes AKT1, DDR2, ERBB2, FGFR2 and MTOR plus deleterious mutations in the tumor suppressors RAD50 and TSC1, on average 6% of cancer samples test positive for a potentially relevant genomic biomarker. Surprisingly, even though none of the cases of spectacular tumor response linked to this set of genes was reported in uterine cancer, translating the information derived from other tumor types could have a direct therapeutic impact in 15% of the patients. Interestingly, 80% of all mutations found in this selected list of rarely mutated oncogenes is of uncertain significance, reflecting the gap in our understanding of the clinical relevance of a large percentage of alterations in genes potentially linked to clinical actions.
Prospects in Precision Cancer Medicine
Molecular alterations in cancer cells are driving anticancer drug discovery and development for more than two decades, with substantial progress in recent years (25). Comprehensive genomic profiling has the potential to tremendously affect clinical practice in terms of guiding patient management. Caregivers will soon be faced with a large number of genomic alterations that are potentially relevant to understanding cancer progression and improving clinical decision-making. Most samples have multiple concurrent targetable events, and how to prioritize therapy and account for coexisting resistance mechanisms remains largely a heuristic task. Given that targetable events are not mutually exclusive or independent, our efforts should focus on investigating how these alterations define a molecular context that allows increased sensitivity to a particular drug or combination of targeted agents.
It is important to emphasize that not all alterations in clinically relevant genes described here will act as “drivers” and portend response to therapeutic targeting. In addition, many of the rare oncogene variants, with nearly 2% prevalence across solid tumors, are of unknown functional significance, requiring further experimental validation. In this context, we believe that clinical validation of a potential biomarker (novel mutation in oncogene matched to targeted drug) is acceptable only when mechanisms to annotate response or lack of benefit are available. Ultimately, prospective implementation of global genomic profiling in clinical trials will define its general applicability and whether matching gene alterations to targeted drugs is associated with improved patient outcomes. The Molecular Analysis for Therapy Choice (MATCH) NCI initiative, an umbrella protocol for multiple single-arm trials, plans to assign over 1,000 patients that progress after one line of standard therapy to matched agents based on next generation sequencing. Other examples include the Michigan Oncology Sequencing Project (MI-ONCOSEQ), Canadian IMPACT protocol and French MOSCATO trial, with systematic use of comprehensive genomic profiling to guide early clinical trial enrollment. We believe that in order to best inform clinical practice, reports of next generation sequencing tests should include standardized classifications of predictive genomic biomarkers, with multilevel associations to define targetability.
We have shown here the magnitude of change of targetability when applying increasingly stringent criteria for a predictive genomic biomarker. Overall, 85% of potentially targetable events are rejected with the strictest definition. Thyroid cancer is an outlier, with only 28% overall drop in the targetability rate, most likely related to its lower complexity at the genomic level as compared to the other solid tumors included in this report. One clear limitation of genomic biomarker identification and characterization in our study is the lack of fusion genes in the final list of targetable events (e.g., ALK, ROS1 and NTRK1, known sensitizers to crizotinib in lung adenocarcinomas) (26-28). However, the rarity of these events indicates that the results presented here should not be largely affected. Defining the prevalence of these events across solid tumors and providing easy access to fusion calls through TCGA data portal would be highly beneficial to the cancer research community (29).
Finally, biological and clinical interpretation of genomic events is primarily a challenge in data standards and management. We were able to aggregate in our structured GDKB the fragmented knowledge of uncoordinated and overlapping efforts from multiple institutions. Given that these efforts do not share a common set of standards, the products of each group could not be easily integrated. As proposed by Good et al (30), the only alternative moving forward is the creation of a community-based open resource to connect cancer genomic events with the necessary evidence to evaluate their biological and clinical significance. The information should be released in an interactive web-based tool, subjected to editing, validation and critique from the medical community. Most importantly, knowing that our collective understanding of predictive genomic events grows on a daily basis, the database should aim to reincorporate these advances in a timely manner. Lastly, we believe that integrating this resource to clinical trial databases, with expansion of ClinicalTrials.gov, will substantially increase therapeutic options for cancer patients.
Significance.
We developed a structured database of genomic biomarkers for cancer drugs (Gene-Drug Knowledge Database) and propose a taxonomy for clinical targetability that connects molecular events with levels of evidence to evaluate their biological and predictive significance. The clinical utility of TCGA data in 12 solid tumors is explored in detail, with a comprehensive analysis of the prevalence of genomic events that may have an impact on patient treatment options when moving from the most relaxed to the strictest criteria to match a gene alteration with a drug.
Acknowledgments
Obi Griffith for Gene-Drug Knowledge Database support, Larsson Omberg for technical help in data acquisition.
Funding: R. Dienstmann is a recipient of “La Caixa International Program for Cancer Research & Education”.
Footnotes
Author contributions
All authors involved in conception, design and methodology development. R. Dienstmann, I.S. Jang, B. Bot and J. Guinney contributed to data acquisition, analysis and interpretation. R. Dienstmann, B. Bot and J. Guinney wrote the manuscript. S. Friend and J. Guinney supervised the work. All authors made substantial contribution to discussion of content, reviewed and edited the final manuscript before submission.
Conflicts of interest: Authors declare no competing interests in relation to the work described.
References
- 1.Yuan Y, Van Allen EM, Omberg L, Wagle N, Amin-Mansour A, Sokolov A, et al. Assessing the clinical utility of cancer genomic and proteomic data across tumor types. Nat Med. 2014;32:644–52. doi: 10.1038/nbt.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Allen EM, Wagle N, Stojanov P, Perrin DL, Cibulskis K, Marlow S, et al. Whole-exome sequencing and clinical interpretation of formalin-fixed, paraffin-embedded tumor samples to guide precision cancer medicine. Nat Biotechnol. 2014;20:682–8. doi: 10.1038/nm.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dienstmann R, Rodon J, Barretina J, Tabernero J. Genomic medicine frontier in human solid tumors: prospects and challenges. J Clin Oncol. 2013;31:1874–84. doi: 10.1200/JCO.2012.45.2268. [DOI] [PubMed] [Google Scholar]
- 4.Falchook GS, Trent JC, Heinrich MC, Beadling C, Patterson J, Bastida CC, et al. BRAF mutant gastrointestinal stromal tumor: first report of regression with BRAF inhibitor dabrafenib (GSK2118436) and whole exomic sequencing for analysis of acquired resistance. Oncotarget. 2013;4:310–5. doi: 10.18632/oncotarget.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu TL, Deng X, Huang F, Tucker M, Crosby K, Rimkunas V, et al. Survey of tyrosine kinase signaling reveals ROS kinase fusions in human cholangiocarcinoma. PloS One. 2011;6:e15640. doi: 10.1371/journal.pone.0015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao RG, Jung J, Tchaicha J, Wilkerson MD, Sivachenko A, Beauchamp EM, et al. Inhibitor-sensitive FGFR2 and FGFR3 mutations in lung squamous cell carcinoma. Cancer Res. 2013;73:5195–205. doi: 10.1158/0008-5472.CAN-12-3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nardi V, Sadow PM, Juric D, Zhao D, Cosper AK, Bergethon K, et al. Detection of novel actionable genetic changes in salivary duct carcinoma helps direct patient treatment. Clin Cancer Res. 2013;19:480–90. doi: 10.1158/1078-0432.CCR-12-1842. [DOI] [PubMed] [Google Scholar]
- 8.Bahassiel M, Li YQ, Wise-Draper TM, Deng L, Wang J, Darnell CN, et al. A patient-derived somatic mutation in the epidermal growth factor receptor ligand-binding domain confers increased sensitivity to cetuximab in head and neck cancer. Eur J Cancer. 2013;49:2345–55. doi: 10.1016/j.ejca.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dienstmann R, Dong F, Borger D, Dias-Santagata D, Ellisen LW, Le LP, et al. Standardized decision support in next generation sequencing reports of somatic cancer variants. Mol Oncol. 2014;8:859–73. doi: 10.1016/j.molonc.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Synapse [Internet] Gene Drug Knowledge Database. Available from: https://www.synapse.org/Synapse:syn2370773.
- 11.Jaiswal BS, Kljavin NM, Stawiski EW, Chan E, Parikh C, Durinck S, et al. Oncogenic ERBB3 mutations in human cancers. Cancer Cell. 2013;23:603–17. doi: 10.1016/j.ccr.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Andre F, Bachelot T, Campone M, Dalenc F, Perez-Garcia JM, Hurvitz SA, et al. Targeting FGFR with dovitinib (TKI258): preclinical and clinical data in breast cancer. Clin Cancer Res. 2013;19:3693–702. doi: 10.1158/1078-0432.CCR-13-0190. [DOI] [PubMed] [Google Scholar]
- 13.Synapse [Internet] TCGA_Pancancer. Available from: https://www.synapse.org/Synapse:syn300013.
- 14.Broad GDAC Firehose [Internet] Available from: http://gdac.broadinstitute.org.
- 15.Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr., Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–58. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dietrich S, Glimm H, Andrulis M, von Kalle C, Ho AD, Zenz T. BRAF inhibition in refractory hairy-cell leukemia. N Engl J Med. 2012;366:2038–40. doi: 10.1056/NEJMc1202124. [DOI] [PubMed] [Google Scholar]
- 18.Banerji U, Ranson M, Schellens JHM, Esaki T, Dean E, Zivi A, et al. Results of two phase I multicenter trials of AZD5363, an inhibitor of AKT1, 2 and 3: Biomarker and early clinical evaluation in Western and Japanese patients with advanced solid tumors. Cancer Res. 2013;73:LB–66. [Google Scholar]
- 19.Hammerman PS, Sos ML, Ramos AH, Xu C, Dutt A, Zhou W, et al. Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discov. 2011;1:78–89. doi: 10.1158/2159-8274.CD-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cappuzzo F, Bemis L, Varella-Garcia M. HER2 mutation and response to trastuzumab therapy in non-small-cell lung cancer. N Engl J Med. 2006;354:2619–21. doi: 10.1056/NEJMc060020. [DOI] [PubMed] [Google Scholar]
- 21.Serra V, Vivancos A, Puente XS, Felip E, Silberschmidt D, Caratu G, et al. Clinical response to a lapatinib-based therapy for a Li-Fraumeni syndrome patient with a novel HER2V659E mutation. Cancer Discov. 2013;3:1238–44. doi: 10.1158/2159-8290.CD-13-0132. [DOI] [PubMed] [Google Scholar]
- 22.Wagle N, Grabiner BC, Van Allen EM, Hodis E, Jacobus S, Supko JG, et al. Activating mTOR mutations in a patient with an extraordinary response on a phase I trial of everolimus and pazopanib. Cancer Discov. 2014;4:546–53. doi: 10.1158/2159-8290.CD-13-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Ahmadie H, Iyer G, Hohl M, Asthana S, Inagaki A, Schultz N, et al. Synthetic Lethality in ATM-Deficient RAD50-Mutant Tumors Underlies Outlier Response to Cancer Therapy. Cancer Discov. 2014;4:1014–21. doi: 10.1158/2159-8290.CD-14-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iyer G, Hanrahan AJ, Milowsky MI, Al-Ahmadie H, Scott SN, Janakiraman M, et al. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338:221. doi: 10.1126/science.1226344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartwell LH, Szankasi P, Roberts CJ, Murray AW, Friend SH. Integrating genetic approaches into the discovery of anticancer drugs. Science. 1997;278:1064–8. doi: 10.1126/science.278.5340.1064. [DOI] [PubMed] [Google Scholar]
- 26.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, McDonald NT, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30:863–70. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doebele RC, Vaishnavi A, Capelletti M, Le AT, Kako S, Butaney M, et al. NTRK1 gene fusions as a novel oncogene target in lung cancer. J Clin Oncol. 2013;31:8023. [Google Scholar]
- 29.Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Good BM AB, McMichael JF, Su AI, Griffith O. Organizing knowledge to enable personalization of medicine in cancer. Genome Biol. 2014;15:438. doi: 10.1186/s13059-014-0438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]