SPATIAL AND TEMPORAL DISTRIBUTION OF GROWTH FACTORS RECEPTORS IN THE CALLUS: IMPLICATIONS FOR IMPROVEMENT OF DISTRACTION OSTEOGENESIS (original) (raw)
. 2011 Aug;73(3-4):117–127.
ABSTRACT
Management of bone deficits by distraction osteogenesis is an appreciated but lengthy procedure. To accelerate the consolidation of newly formed distraction callus, an administration of growth factors into the distraction gap has been suggested. Changes in expression of growth factors receptors in the distracted callus during consolidation were studied in order to improve our understanding of the underlying molecular mechanisms and to provide a scientific basis for clinical application of growth factors. In a model of rat bone lengthening the expression of receptors for: vascular endothelial growth factor, transforming growth factor β1, insulin like growth factor and platelet derived growth factor were evaluated semiquantitatively with immunohistochemistry and quantitatively with real time PCR in various callus zones at zero, one and two weeks of consolidation. Overall growth factors receptors’ expression was highest at the beginning of consolidation. It was strongest in the trabecular bone and weakest in the fibrous zone. Transforming growth factor β receptor 1 was most abundant and vascular endothelial growth factor receptor 1, although scarce, showed the most consistent expression. In contrast to the osteogenic zones, the fibrous zone demonstrated a dramatic loss of the growth factors receptors over time. High growth factors receptors expression shortly after termination of the distraction may warrant the maximal callus’ response to injected growth factors. Rapid decline of growth factors receptors in the fibrous zone may imply its decreasing sensitivity to growth factors and, as a consequence, a declining osteogenic potential.
Key Words: Distraction osteogenesis, Growth factors, Flt-1, TGFβR1, IGF-1R, PDGFRα
Introduction
Management of a leg length deficit or short stature poses a major challenge. Although distraction osteogenesis is the least invasive procedure, the formation and maturation of new bone during distraction is a time consuming process. Problems related to the treatment’s duration (pin tract infection, failure of the distraction device), or to the biology itself (poor callus formation, delayed consolidation) are not uncommon.1) Therefore, it seems reasonable to seek improvements to this valuable method, thus possibly shortening its duration. Distraction osteogenesis consists of two phases – active distraction (new bone formation) and consolidation (maturation and remodeling). The optimal rate of distraction has been established both experimentally and clinically, and due to limited soft tissues regeneration must not be accelerated.2) However, it is possible to reduce time of the second phase by enhancing consolidation and improving the quality of the callus. Acceleration of consolidation has been attempted with various methods and outcomes. The newest techniques favor autogenous materials. Local injections of bone marrow, stem cells or GFs,3-6) or their combinations7) have been reported.
Autologous platelet-rich plasma abundantly provides the key stimulators of bone development, e.g., VEGF (vascular endothelial growth factor), TGFβ1 (transforming growth factor β1), IGF1 (insulin like growth factor 1) and PDGF (platelet derived growth factor), etc. In addition PRP (platelet rich plasma) is widely accessible and easily applicable to the distraction gap and has provided good outcomes in preliminary clinical trials5,6).
To most efficiently use growth factors, it is imperative to understand their cellular and molecular biology. However, bone formation is regulated not only by growth factors availability itself, but also by the functioning of their specific receptors. PDGFRα (receptor for PDGF) regulates the entire process of bone formation from hematoma briefly incurred after injury, until remodeling.8-10) It is particularly active during the inflammation and promotes bone cell proliferation. Angiogenesis is an indispensible precursor of successful osteogenesis; bone apposition depends on the adequate function of Flt-1 (receptor for VEGF).11,12) The pro-osteogenic effect assured by IGF is reduced in any case of IGF-R1 (receptor for IGF1) impairment.3,14) Osteoblast proliferation depends on TGFβR1 (receptor for TGFβ1), and all isoforms of TGFβ1 receptor are necessary for ossification, the formation of mature bone and for bone turnover.15-17)
In this study the performance of the above mentioned growth factors receptors was assessed in order to define their spatial distribution, sequence and quantity (most importantly the time of the highest receptors’ expression) during the consolidation phase of distraction osteogenesis.
We discussed alterations in growth factors receptors’ expression, especially in fibrous and bone regions of the callus, and the potential impact of these changes on bone formation and remodeling. We hypothesize that bone formation may benefit most from injected growth factors if they are applied at the times of the highest activity of their specific receptors. To the best of our knowledge, this is the first report evaluating receptors for VEGF, TGFβ1, IGF1 and PDGF in the rat model of long-bone distraction.
Materials and Methods
Model of bone distraction
The research model was designed in accordance with general guidelines for the care and use of experimental animals and was approved by the animal experiment committee of the Nagoya University Graduate School of Medicine. Thirty-two 9- week-old Sprague-Dawley rats weighing 330–380 g underwent surgery under general anesthesia. Through a lateral incision, a mini external fixator-distractor (Nagoya Screw Manufacturing Co., Ltd, Nagoya, Japan) was secured to the rat’s femoral bone using four threaded pins (Fig. 1A). The mid-third of the bone shaft was osteotomized (Fig. 1B), and the skin was closed. Full weight bearing was allowed immediately after recovering from anesthesia. Distraction was initiated after 7 days of latency at a rate of 0.375 mm twice a day, and lasted for 10 days (Fig. 1C), producing 7.5 mm of distraction callus. After termination of the distraction, callus’ consolidation was allowed for 0 (Fig. 1C), 7 and 14 (Fig. 1D) days, after which tissue samples were harvested. The animals were randomly divided into five experimental groups. In two groups (after 0 and 14 days of consolidation) distraction callus (Fig. 1E) was harvested for mRNA analyses of growth factors receptors. In the remaining three groups (after 0, 7 and 14 days of consolidation) femoral bones (Fig. 1F) were harvested for histological and immunohistochemical analysis of growth factors receptors.
Fig. 1.
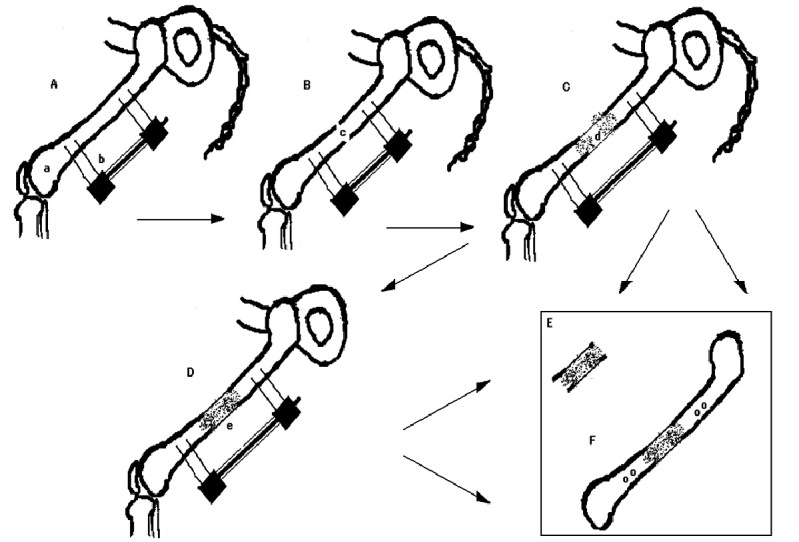
Rat model of long bone lengthening. A – insertion of an external fixator-distractor device, B – osteotomy of the femoral bone, C – distraction of femoral bone and production of the distraction callus (0 days of consolidation), D – consolidation of distraction callus (7 or 14 days), E – harvesting of distraction callus for mRNA analysis (after 0 or 14 days of consolidation), F – harvesting of whole femoral bone for immunohistochemical analysis (after 0, 7 or 14 days of consolidation); a – femoral bone, b – external fixation-distraction device, c – site of bone osteotomy, d – distracted callus, e – consolidating callus.
Histological and immunohistochemical analysis
Whole femoral bones were fixed in paraformaldehyde, embedded in paraffin, cut into 6 μm sections and mounted on slides.
Serial samples were stained with hematoxylin and eosin. The consistency of bone formation and changes in cellular characteristics during callus maturation was assessed under a light microscope with attention to four histological zones: woven and trabecular bone, fibrous tissue and chondral tissue.
For immunohistochemical analysis we used primary polyclonal anti-mouse Flt-1 (NeoMarkers, Fremont, CA, USA; catalog # RB-1527-R7) and primary rabbit polyclonal anti-human TGFβR1 (BioVision, Mountain View, CA, USA; catalog # 5636-100), IGF-1R (Spring Bioscience, Fremont, CA, USA; catalog # E12204) antibodies and PDGFRα Ab-1 (Thermo Scientific, Fremont, CA, USA; catalog # RB-1691). Bone sections were stained using the Simple Stain Rat MAX PRO Histofine (Nichirei Biosciences, Japan) and DAB (3, 3’-diaminobenzidine tetrahydrochloride) (Nichirei Biosciences, Japan). Negative controls were obtained by excluding primary antibodies from the protocols. Blinded observers evaluated immunohistochemical stains of the callus under a light microscope. Two digital microphotographs were taken of each zone: fibrous, chondral, woven and trabecular. All images were evaluated at two points (a sixteen-point study of every specimen). All relevant cells in the analyzed zones (fibroblasts, chondroblasts/chondrocytes and osteoblasts/osteocytes respectively) were counted. The stained cells were expressed as a percentage of all counted cells; a semiquantitative approach was applied. In accordance with protocols applied in previous studies,18-20) the areas were labeled by applying the following scale: – (no staining), + (≤ 25%), ++ (≤ 50%), +++ (≤ 75%), ++++ (≤ 100%) of staining. Such observations were repeated for each of the four receptors.
RNA Isolation, RT-PCR and quantitative real time analysis
For mRNA analysis each distraction callus was harvested and dry frozen. Total RNA was extracted by Trizol and purified with an RNeasy Mini Kit (QUIAGEN, Tokyo, Japan). A reverse Transcription System (Roche, New Jersey, USA) was used to obtain a total cDNA of every sample. In the Light Cycler instrument, using Light Cycler-Fast Start DNA Master SYBR Green I (Roche Molecular Biochemicals, Mannheim, Germany), one sample of total cDNA was amplified with oligonucleotide primer for GFR (designed by Nihon Gene Research Laboratories, Sendai, Japan) (Table 1). The PCR product (receptor-specific cDNA) was dyed with Loading Buffer 6x (Takara, Shiga, Japan), and underwent electrophoresis in the agarose gel to confirm the primer’s specificity by visualization of a single band. With QIAquick Spin (QUIAGEN, Tokyo, Japan), cDNA was extracted from the agarose gel. Absorption of extracted cDNA was measured with a spectrophotometer (Biophotometer Plus, Eppendorf, Germany). cDNA concentration was calculated and serial dilutions were prepared as standards. The same procedure was repeated to generate standards for other GFRs’ primers. Using the prepared standards, quantitative real-time PCR was carried out on the remaining samples in the Light Cycler instrument, with primers for GFRs and for GAPDH as a house-keeping gene. Values of Flt-1, TGFβR1, IGF-1R, and PDGFRα were calculated in respect to values for GAPDH, and further analyzed.
Table 1.
Forward (F) and reverse (R) nucleotide sequence of four growth factors receptors’ (GFRs’) primers used for quantitative real-time RT-PCR and size of product.
| GFR | Sequence (5‘->3’) | Product size |
|---|---|---|
| Flt1 F | GCCTACGTGTCCGCATTA | 114 |
| Flt1 R | GGTAGCAGGCTGGACAGTAA | |
| IGF R1 F | AGGAGGCTGAGTACCGTAAA | 144 |
| IGF R1 R | TGTCAGCTACCGTGGTGT | |
| TGFβR1 F | CCATTTGTTTGTGCACCATC | 173 |
| TGFβR1 R | ATAAGTGCAATGCAGACGAA | |
| PDGFRα F | GACAACTTGACCCTGATTGAG | 116 |
| PDGFRα R | CCGCTGTCTTCTTCCTTAG |
RESULTS
Histological examination:
A central part of the callus (fibrous zone, Fig. 2a) consisted of longitudinally aligned spindle-like fibrous cells (with regions of less organized fibroblasts). The length of the fibrous zone was decreasing with time (Fig. 3 A-C). A trabecular zone (Fig. 2b) was adjacent to the fibrous zone. The trabeculae were formed of columns of osteoblasts organized along the axis of distraction. This zone (active osteogenesis) was subject to most histological changes during consolidation. A large number of osteoblasts with big nuclei (day 0 of consolidation) was decreasing with time, yielding smaller osteocytes in a more abundant extracellular matrix (day 14 of consolidation). The most peripheral zone of the distraction callus adjacent to the osteotomy site (Fig. 2c) was occupied by disorganized woven bone (Fig. 2d) which was also invading a medullary canal in retrograde fashion. Although distraction callus forms through an intramembranous ossification, rare islands of chondrogenesis could be observed (Fig. 2e). Chondroblasts (briefly after distraction) or hypertrophied chondrocytes (later in the consolidation phase) were forming nodules within the fibrous tissue or paraperiosteally. The osteoid (similar to the woven zone) produced by periosteal reaction was surrounding the callus as a side layer (Fig. 2f).
Fig. 2.
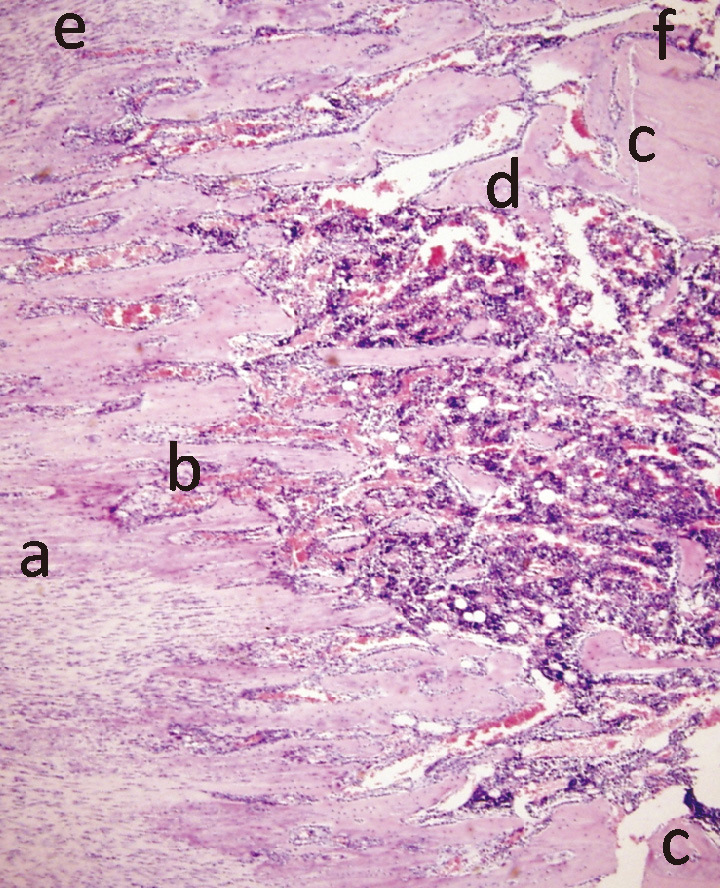
Microphotograph of a distraction callus’ regions: (a) central fibrous zone, (b) trabecular bone zone, (c) site of osteotomy with adjacent (d) woven bone zone, (e) nodules of chondral tissue, (f) zone of periosteal ossification (1:100).
Fig. 3.
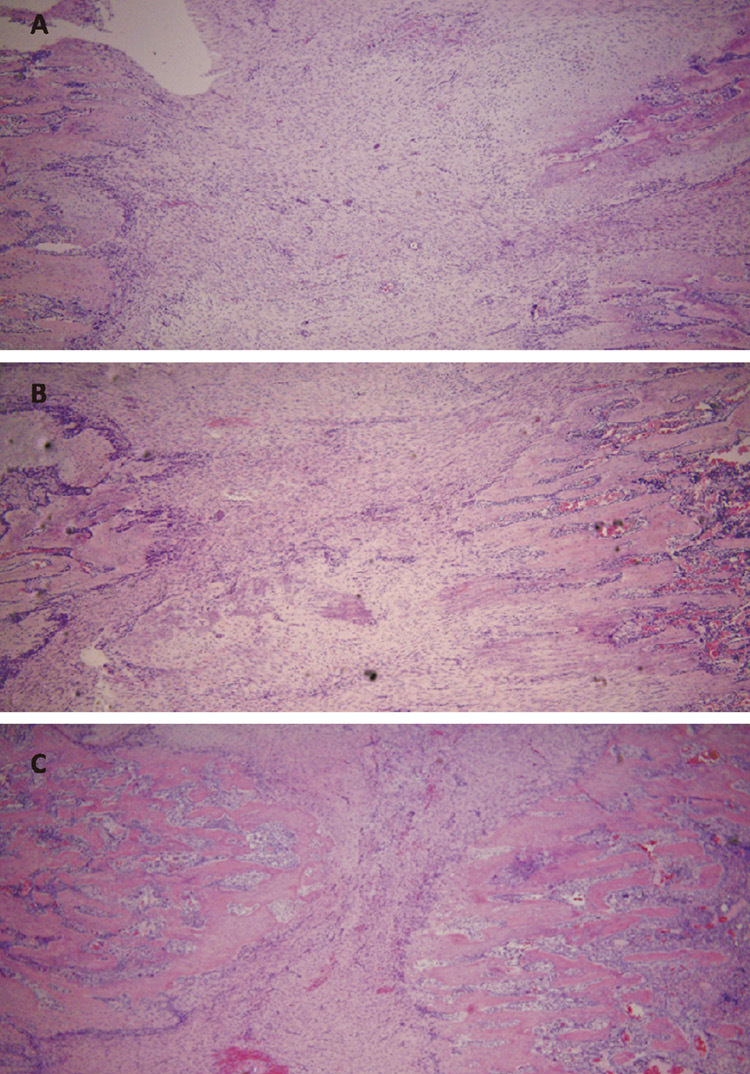
Microphotograph of the central, fibrous region of distraction callus at 0 (A), 7 (B) and 14 (C) days of consolidation. The fibrous zone decreases with time as the front of osteogenesis invades towards the center of distraction callus (1:40).
Immunohistochemical examination (Table 2):
Temporal characteristics: Expression of all receptors was most abundant at the beginning of consolidation (day 0) but showed a general decrease with time (day 7 and day 14). These changes were, however, tissue- and receptor-specific. The reduction of staining was most significant in the chondral tissue (by ++ for most receptors). In the fibrous tissue, TGFβR1 and PDGFRα had already decreased in the 7th day of consolidation. In the bone zones, the decline was in the 25% ranged and was delayed to 14 days of consolidation for most receptors.
Tissue characteristics: The average expression of all receptors was highest in the trabecular zone which was the region of most active bone formation (over 75%) and remained over 50% after 2 weeks (with exception of Flt-1). However, since the absolute number of bone cells within the trabeculae was decreasing (due to maturation from osteoblasts to osteocytes), the overall number of stained cells was reduced significantly as well. Very intensive staining was evident in the cells lining the trabeculae (up to 100%). The woven bone had a relatively strong (+++/++++) stain as well, and the periosteal ossification zone showed similar receptor characteristics (data not shown).
Table 2.
Immunohistochemical analysis of temporal changes of GFRs expression in four zones of distraction callus. An overall decrease in expression of all analyzed GFRs could be observed after 14 days of consolidation. a The virtually 100% Flt-1 staining in endothelium and trabeculae lining cells not included into average expression calculation.
| Chondral tissue | Fibrous tissue | Woven bone | Trabecular bone | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IGF-1R | TGF-βR1 | PDG-FRα | Flt-1 | IGF-1R | TGF-βR1 | PDG-FRα | Flt-1* | IGF-1R | TGF-βR1 | PDG-FRα | Flt-1 | IGF-1R | TGF-βR1 | PDG-FRα | Flt-1a | |
| 0 days | ++++ | +++ | +++ | +++ | + | +++ | +++ | + | ++++ | ++++ | ++++ | ++ | ++++ | ++++ | ++++ | +++ |
| 7 days | ++ | ++ | ++ | + | + | + | + | + | +++ | ++++ | ++++ | ++ | +++ | ++++ | ++++ | ++ |
| 14 days | + | ++ | + | + | + | + | + | + | +++ | +++ | +++ | ++ | +++ | +++ | +++ | ++ |
The least staining (++) with rapid decrease was observed in the fibrous-like tissue (Fig. 4). Interestingly, highly organized and aligned spindle-like cells had fewer detectable receptors than in the areas of less ordered fibrous tissue. Compared to Flt-1 and IGF-1R, the expression of TGFβR1 and PDGFRα in this zone was at least 50% stronger.
Fig. 4.
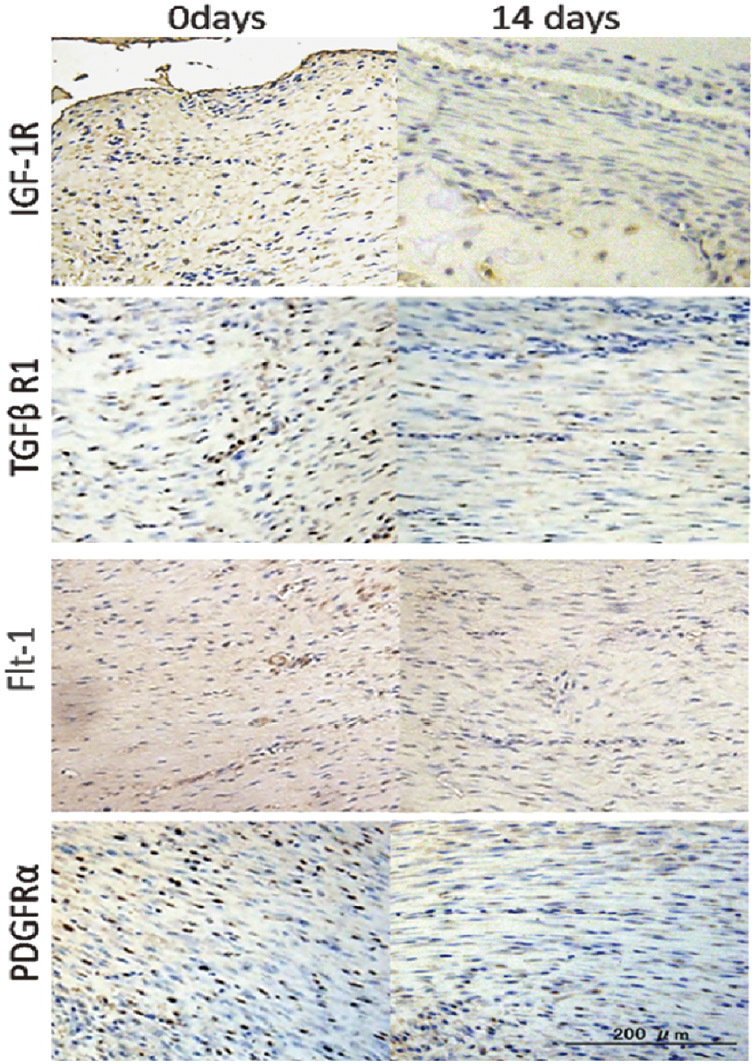
Microphotographs of immunohistological staining of IGF-1R, TGFβR1, PDGFRα and Flt-1 at 0 and 14 days of consolidation in fibrous zone. Particularly large receptor reduction was observed for TGFβR1 and PDGFRα after 14 days of consolidation.
In the immature chondroid, IGF-1R staining reached over 75%, but almost disappeared when the chondrocytes hypertrophied during consolidation. The remaining receptors also decreased in this zone, with the Flt-1 decline manifesting itself as early as already on day 7).
A number of vessels’ lumen was also highlighted immunohistochemically.
Receptor characteristics: TGFβR1 showed the most intense staining at all stages in all zones (only IGF-1R in immature chondroid exhibited stronger staining but lost its dominance after 1 week). PDGFRα represented the second most intensive staining after TGFβR1 (a weaker expression than TGFβR1 was observed only in hypertrophied chondrocytes). Both receptors decreased slowly in bony tissues but had lost their intensity after only 1 week in the fibrous zone. Except for chondral tissue, Flt-1 showed a low (+/++) but constant expression throughout consolidation. However, vessels and cells lining the surface of newly formed trabeculae were stained close to 100%, while no other receptor showed a similarly strong expression in analogous areas.
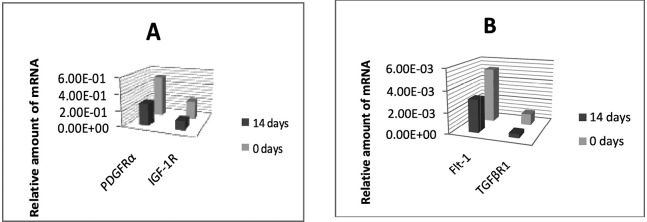
Quantitative real-time PCR (Fig. 5):
Fig. 5.
Overall decrease in average expression of mRNA for PDGFRα and IGF-1R (A) and for Flt-1 and TGFβR1 (B) from day 0 to day 14 of consolidation (by quantitative real-time RT-PCR). GAPDH was used for a control; the amount of receptors’ mRNA expression was calculated in relation to expression for GAPDH.
The overall decrease in mRNA receptors expression with time was consistent with immunohistochemical studies.
PDGFRα exhibited the strongest expression of all receptors at both 0 and 14 days of consolidation, reaching an almost two-fold reduction within those 2 weeks (5.13E-01 and 2.83E-01, respectively). IGF-1R was prevalent to Flt-1 and TGFβR1 while mRNA for Flt-1 displayed a relatively weak expression (5.20E-03 and 3.14E-03, respectively). The expression of TGFβR1 mRNA dropped from 1.06E-03 at 0 days to 3.71E-04 at 14 days.
Discussion
Growth factors are indispensible during any bone formation e.g., skeleton growth,21,22) fracture healing8) or callotasis,20,23) or development of pathologic bone.24) They control all stages of osteogenesis25) including mesenchymal cell chemotaxis,26) osteoprogenitor differentiation and proliferation,22) matrix apposition, mineralization and remodeling,23) and osteogenesis-related angiogenesis.11,27) Growth factors or growth-factors-rich PRP accelerate the consolidation of distraction gaps,4-7) bone cyst healing,28) incorporation of bone grafts29,30) and osteogenic differentiation of bone marrow.3) Higher concentrations of PRP (GFs) have even superior impacts on bone formation.31) VEGF, TGFβ, IGF and PDGF are obligatory for osteogenesis and are particularly abundant in PRP thus easily available. Therefore, receptors for these four growth factors became the scope of our investigation.
Since it was not feasible to examine receptors’ mRNA in the four callus’ zones individually, it posed a serious limitation to this study. However, consistently with the tissue-specific immunohistological examination, the mRNA analysis served to prove the overall drop in receptors’ activity. Another limitation of this study was the semiquantitative evaluation of immunohistochemical staining. The cell count was subjective, and only randomly selected sections were evaluated. Moreover, no reasonable control could be designed (neither fracture healing nor healthy bone provides a sufficient fibrous zone or zone of rapid osteogenic changes for comparison). Such a selectivity of material, subjectivity of analysis and lack of control could not provide sound basis for a statistical analysis. However, semiquantitative analysis is a recommended method for the evaluation of cellular components,18-20,32) and it allows observations of temporal changes in receptors’ profiles and permits discussion of their clinical significance. We concluded that our results might at least provide a valuable indication for an improvement of distraction osteogenesis.
The initiation and sustaining of a distraction callus formation requires the vessels’ development, in which osteogenesis follows angiogenesis. The inhibition of an Flt1 tyrosine kinase domain gene or the receptor itself reduces skeletal development.11) We have found Flt1 in all callus regions, confirming its uniform involvement. Cells lining the new trabeculae expressed an exceptionally strong VEGF dependence. Flt 1 remained mild to moderate, especially in the woven and the trabecular tissue through the experiment, what was similar to findings in an early consolidation of a distracted mandible.18)
TGFβ is stored in platelets, bone and cartilage. It guides mesenchymal cells and osteo-/chondroblasts towards the formation of mineralized, mature bone.4,16) Endochondral and intramembranous ossification involve all isoforms of TGFβ receptor,21) and only the interaction of TGFβ receptors I and II promotes bony tissue turnover.15,17) During distraction osteogenesis, a vigorous proliferous activity is dependent upon TGFβR1.33) In our experiment the woven and trabecular zones stained positively for TGFβR1 with only a slight decrease through consolidation. Such prolonged sensitivity, however, applied only to zones already differentiated towards bone. In contrast, TGFβR1 decreased early in the fibrous zone. That may imply an early loss of pro-osteogenic (consolidation) potential soon after an active distraction is terminated.
IGF is the most abundant growth factor stored in a bone matrix. Its cascade is activated in all rapidly growing tissues, it stimulates pro-osteogenic activity19) and mediates mechanical stress effects.23) It appears that the receptor itself has a greater regulatory effect than its ligand: IGF-R1 null mutation, IGF-R1 antibody,13) and age related reduction of IGF-1R14) all impair the pro-osteogenic effect. At the end of distraction we found abundant IGF-R1, especially in the osteoblasts. In distraction osteogenesis IGF-R1 is limited to bone of rich histology33) (a low proliferation rate during impaired bone formation provokes abundant IGF activity and thus receptor down-regulation). We also found a weak though persistent IGF-R1 expression in fibrous tissue. Currently there is a growing interest in IGF binding proteins, which complicates the interpretation of the ligand-receptor signaling axis.
PDGF is an ideal initiator of a wound healing. It presents in the first hours after osteotomy and remains active until the late stages of bone formation.25) During consolidation, PDGF is stored in all cells and matrix components.32) PDGFRα transduces potent mitogenic signals8) and exerts an antiapoptotic effect.10) It is present in rapidly forming heterotrophic and osteophytic bone24) and during fracture healing.26) We, for the first time, confirmed the participation of PDGFRα in distraction osteogenesis. In cell cultures, the expression of the receptor declines after cells maturation.34) Since PDGF may suppress osteogenic transformation, it is reasonable that we also found an initially very high PDGFRα participation reducing in an already formed bone (trabecular or woven). However, its rapid reduction in the fibrous zone (as seen in our study) may lead to an insufficient number of precursors ready for further osteogenic differentiation promoted by other growth factors.
Late bone formation in the distracted callus occurs through at least two mechanisms – central and peripheral. In the center, the pluripotential cells of the fibrous zone undergo osteogenic differentiation. In the periphery, the trabeculae provide an actively proliferating front of osteogenesis with pre-/ and osteoblasts invading the fibrous zone and replacing it. It seems that as soon as an active distraction is stopped, the decreased ability of the fibrous zone to respond to the pro-osteogenic factors (early decrease of receptors) may reduce central osteogenesis. During late consolidation, although receptors in the trabecular zone are preserved to some extent, growth factors seem to primarily stimulate bone maturation but no longer proliferation (a rich net of actively proliferating osteoblasts is replaced by sparsely distributed mature osteocytes). The trabecular zone loses its potential to invade the fibrous zone. Thus, both the central and peripheral consolidations decelerate. Replacement of the fibrous tissue may fail, and gap consolidation may be inefficient.
A predominantly fibrous distraction callus is encountered in various clinical situations: in congenital pseudoarthrosis, tumors, after irradiation, in osteoporotic bone, in various musculo-skeletal dysplasiae and in case of an inadequate rate of distraction. A prolonged presence of a fibrous gap is one of the most serious concerns during bone lengthening and may have dramatic consequences. Thus, particularly in a poorly forming fibrous callus, a timely administration of growth factors seems to be imperative. This study shows that the callus’ receptors are elevated shortly after the termination of distraction and may provide a good target for exogenous growth factors. Therefore administration of growth factors into the distraction gap at this point should be the most beneficial, especially for promoting an early osteoinduction within the fibrous zone. During the later consolidation it might be more difficult to enhance osteogenesis with growth factors alone, as the number of target cells (carrying the specific receptors) decreases. However the behavior of receptors after growth factor injection is unforeseeable. For example their down regulation might occur. Further studies are warranted to examine receptors’ response to exogenous growth factors. It would be essential to compare the rates of callus maturation when enhanced, with growth factors administered early or late during consolidation.
REFERENCES
- 1).Paley D: Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin Orthop Relat Res, 1990; 250: 81–104. [PubMed]
- 2).White SH, Kenwright J: The timing of distraction of an osteotomy. J Bone Joint Surg Br, 1990; 72: 356–361. [DOI] [PubMed]
- 3).van den Dolder J, Mooren R, Vloon AP, Stoelinga PJ, Jansen JA: Platelet-rich plasma: quantification of growth factor levels and the effect on growth and differentiation of rat bone marrow cells. Tissue Eng, 2006; 12: 3067–3073. [DOI] [PubMed]
- 4).Ozkan K, Eralp L, Kocaoglu M et al: The effect of transforming growth factor beta1 (TGF-beta1) on the regenerate bone in distraction osteogenesis. Growth Factors, 2007; 25: 101–107. [DOI] [PubMed]
- 5).Robiony M, Polini F, Costa F, Politi M: Osteogenesis distraction and platelet-rich plasma for bone restoration of the severely atrophic mandible: preliminary results. J Oral Maxillofac Surg, 2002; 60: 630–635. [DOI] [PubMed]
- 6).Swennen GR, Schutyser F, Mueller MC, Kramer FJ, Eulzer C, Schliephake H: Effect of platelet-rich-plasma on cranial distraction osteogenesis in sheep: preliminary clinical and radiographic results. Int J Oral Maxillofac Surg, 2005; 34: 294–304. [DOI] [PubMed]
- 7).Kitoh H, Kitakoji T, Tsuchiya H, Katoh M, Ishiguro N: Transplantation of culture expanded bone marrow cells and platelet rich plasma in distraction osteogenesis of the long bones. Bone, 2007; 40: 522–528. [DOI] [PubMed]
- 8).Tsiridis E, Upadhyay N, Giannoudis P: Molecular aspects of fracture healing: which are the important molecules? Injury, 2007; 38 Suppl 1: S11–25. [DOI] [PubMed]
- 9).Tallquist M, Kazlauskas A: PDGF signaling in cells and mice. Cytokine Growth Factor Rev, 2004; 15: 205–213. [DOI] [PubMed]
- 10).Heldin CH, Westermark B: Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev, 1999; 79: 1283–1316. [DOI] [PubMed]
- 11).Jacobsen KA, Al-Aql ZS, Wan C et al: Bone formation during distraction osteogenesis is dependent on both VEGFR1 and VEGFR2 signaling. J Bone Miner Res, 2008; 23: 596–609. [DOI] [PMC free article] [PubMed]
- 12).Otomo H, Sakai A, Uchida S et al: Flt-1 tyrosine kinase-deficient homozygous mice result in decreased trabecular bone volume with reduced osteogenic potential. Bone, 2007; 40: 1494–1501. [DOI] [PubMed]
- 13).Wang Y, Nishida S, Boudignon BM et al: IGF-I receptor is required for the anabolic actions of parathyroid hormone on bone. J Bone Miner Res, 2007; 22: 1329–1337. [DOI] [PMC free article] [PubMed]
- 14).Cao JJ, Kurimoto P, Boudignon B, Rosen C, Lima F, Halloran BP: Aging impairs IGF-I receptor activation and induces skeletal resistance to IGF-I. J Bone Miner, Res 2007; 22: 1271–1279. [DOI] [PubMed]
- 15).Steinbrech DS, Mehrara BJ, Rowe NM et al: Gene expression of TGF-beta, TGF-beta receptor, and extracellular matrix proteins during membranous bone healing in rats. Plast Reconstr Surg, 2000; 105: 2028–2038. [DOI] [PubMed]
- 16).Derynck R, Feng XH: TGF-beta receptor signaling. Biochim Biophys Acta, 1997; 1333: F105–150. [DOI] [PubMed]
- 17).Yamashita H, Ten Dijke P, Heldin CH, Miyazono K: Bone morphogenetic protein receptors. Bone, 1996; 19: 569–574. [DOI] [PubMed]
- 18).Byun JH, Park BW, Kim JR, Lee JH: Expression of vascular endothelial growth factor and its receptors after mandibular distraction osteogenesis. Int J Oral Maxillofac Surg, 2007; 36: 338–344. [DOI] [PubMed]
- 19).Haque T, Amako M, Nakada S, Lauzier D, Hamdy RC: An immunohistochemical analysis of the temporal and spatial expression of growth factors FGF 1, 2 and 18, IGF 1 and 2, and TGFbeta1 during distraction osteogenesis. Histol Histopathol, 2007; 22: 119–128. [DOI] [PubMed]
- 20).Tavakoli K, Yu Y, Shahidi S, Bonar F, Walsh WR, Poole MD: Expression of growth factors in the mandibular distraction zone: a sheep study. Br J Plast Surg, 1999; 52: 434–439. [DOI] [PubMed]
- 21).Horner A, Kemp P, Summers C et al: Expression and distribution of transforming growth factor-beta isoforms and their signaling receptors in growing human bone. Bone, 1998; 23: 95–102. [DOI] [PubMed]
- 22).Sakou T, Onishi T, Yamamoto T, Nagamine T, Sampath T, Ten Dijke P: Localization of Smads, the TGF-beta family intracellular signaling components during endochondral ossification. J Bone Miner Res, 1999; 14: 1145–1152. [DOI] [PubMed]
- 23).Weiss S, Baumgart R, Jochum M, Strasburger CJ, Bidlingmaier M: Systemic regulation of distraction osteogenesis: a cascade of biochemical factors. J Bone Miner Res, 2002; 17: 1280–1289. [DOI] [PubMed]
- 24).Horner A, Bord S, Kemp P, Grainger D, Compston JE: Distribution of platelet-derived growth factor (PDGF) A chain mRNA, protein, and PDGF-alpha receptor in rapidly forming human bone. Bone, 1996; 19: 353–362. [DOI] [PubMed]
- 25).Chaudhary LR, Hofmeister AM, Hruska KA: Differential growth factor control of bone formation through osteoprogenitor differentiation. Bone, 2004; 34: 402–411. [DOI] [PubMed]
- 26).Fujii H, Kitazawa R, Maeda S, Mizuno K, Kitazawa S: Expression of platelet-derived growth factor proteins and their receptor alpha and beta mRNAs during fracture healing in the normal mouse. Histochem Cell Biol, 1999; 112: 131–138. [DOI] [PubMed]
- 27).Weiss S, Zimmermann G, Baumgart R, Kasten P, Bidlingmaier M, Henle P: Systemic regulation of angiogenesis and matrix degradation in bone regeneration–distraction osteogenesis compared to rigid fracture healing. Bone, 2005; 37: 781–790. [DOI] [PubMed]
- 28).Cieslik-Bielecka A, Bielecki T, Gazdzik TS, Cieslik T, Szczepanski T: Improved treatment of mandibular odontogenic cysts with platelet-rich gel. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2008; 105: 423–429. [DOI] [PubMed]
- 29).Marx RE: Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg, 2004; 62: 489–496. [DOI] [PubMed]
- 30).Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR: Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 1998; 85: 638–646. [DOI] [PubMed]
- 31).Kawasumi M, Kitoh H, Siwicka KA, Ishiguro N: The effect of the platelet concentration in platelet-rich plasma gel on the regeneration of bone. J Bone Joint Surg Br, 2008; 90: 966–972. [DOI] [PubMed]
- 32).Knabe C, Nicklin S, Yu Y et al: Growth factor expression following clinical mandibular distraction osteogenesis in humans and its comparison with existing animal studies. J Craniomaxillofac Surg, 2005; 33: 361–369. [DOI] [PubMed]
- 33).Eingartner C, Coerper S, Fritz J, Gaissmaier C, Koveker G, Weise K: Growth factors in distraction osteogenesis. Immuno-histological pattern of TGF-beta1 and IGF-I in human callus induced by distraction osteogenesis. Int Orthop, 1999; 23: 253–259. [DOI] [PMC free article] [PubMed]
- 34).Yu X, Hsieh SC, Bao W, Graves DT: Temporal expression of PDGF receptors and PDGF regulatory effects on osteoblastic cells in mineralizing cultures. Am J Physiol, 1997; 272: C1709–1716. [DOI] [PubMed]