Functional segregation of cortical regions underlying speech timing and articulation (original) (raw)
. Author manuscript; available in PMC: 2017 Mar 16.
SUMMARY
Spoken language is a central part of our everyday lives, but the precise roles that individual cortical regions play in the production of speech are often poorly understood. To address this issue, we focally lowered the temperature of distinct cortical regions in awake neurosurgical patients, and we relate this perturbation to changes in produced speech sequences. Using this method, we confirm that speech is highly lateralized, with the vast majority of behavioral effects seen on the left hemisphere. We then use this approach to demonstrate a clear functional dissociation between nearby cortical speech sites. Focal cooling of pars triangularis/pars opercularis (Broca’s region) and the ventral portion of the precentral gyrus (speech motor cortex) resulted in the manipulation of speech timing and articulation, respectively. Our results support a class of models that have proposed distinct processing centers underlying motor sequencing and execution for speech.
INTRODUCTION
The production of spoken language is a complex process relying upon a number of interacting brain regions (Cogan et al., 2014; Flinker et al., 2015; Guenther, 2016; Indefrey and Levelt, 2004; Price, 2010). Transcranial magnetic stimulation (Pascual-Leone et al., 1991) and focal electrical current administration (Ojemann et al., 1989; Penfield and Rasmussen, 1950) have demonstrated that two key cortical centers are necessary for speech production: the pars opercularis and pars triangularis (henceforth referred to as Broca’s region) within the left inferior frontal gyrus (IFG) and its downstream target in the precentral gyrus (speech motor cortex), but the relative contributions of these areas during speech remain elusive. While the ventral motor cortex is generally considered to control overt articulation (Bouchard et al., 2013; Guenther et al., 2006; Murphy et al., 1997; Penfield and Boldrey, 1937), a large number of functional roles have been proposed for Broca’s region (Flinker et al., 2015; Grodzinsky and Santi, 2008; Guenther, 2006; Hagoort, 2005; Hickok, 2012; Koechlin and Jubault, 2006; Musso et al., 2003; Price et al., 2011; Tettamanti and Weniger, 2006; Trupe et al., 2013), but few (if any) have been tested in a causal manner.
Here we use focal cooling of specific brain regions during the performance of vocal sequences in order to measure the impact of this selective manipulation on different characteristics of produced speech. We find that our perturbation leads to a clear double dissociation (Gough et al., 2005; Lomber et al., 2010) in which speech quality and timing are differentially modified and regionally specific. Cooling speech motor cortex leads to a striking decrease in speech quality, underscoring its role in articulation, while the same manipulation in Broca’s region leads to consistent changes in speech rate. Our findings support the idea that Broca’s region plays a key role in premotor sequencing and that specific speech-related movements are established within the primary motor cortex.
RESULTS
Cortical Cooling During Speech Production
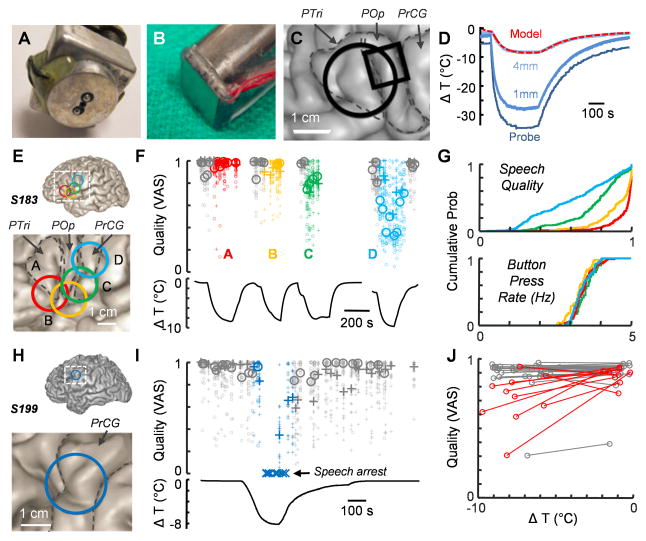
To examine the roles that individual brain regions play in speech production, we investigated the impact of a focal manipulation on the timing and articulatory quality of spoken words. Since many neural properties are highly temperature-dependent (Murphy et al., 1997; Sabatini and Regehr, 1996; Thompson et al., 1985; Volgushev et al., 2000a; Volgushev et al., 2000b), we reasoned that cortical cooling could transiently perturb circuit dynamics in the human brain, which is consistent with previous observations from simpler systems (Long and Fee, 2008; Pires and Hoy, 1992; Tang et al., 2010; Yamaguchi et al., 2008). We used two instruments to manipulate brain temperature which differed in their interface geometry (Figure 1A–C) as well the means by which cooling was achieved (see Experimental Procedures). We measured the spatiotemporal response to surface cooling (Figure 1D) (Smyth et al., 2015) in a sheep in order to estimate its effect on brain temperature at 4 mm, which roughly corresponds to the maximal depth of the gyral surface of human neocortex (Fischl and Dale, 2000).
Figure 1. Focal cooling can affect the speech quality.
(A and B) The two cooling probe types used in this study. The brain interface can be either (A) circular (2 cm diameter) or (B) square (1 cm edge).
(C) An example reconstruction from S284 in which both cooling probe types were used. The footprints of the devices are marked in black. Gyri are identified as PTri (pars triangularis), POp (pars opercularis), and PrCG (precentral gyrus).
(D) Calibration curves for the circular cooling probe with measurements taken from within the body of the device and from additional points 1 mm and 4 mm under the surface of the probe. The dashed red trace represents the modeled temperature change at a depth of 4 mm (square probe: δ = 1.5°C, λ = 2.5mm, τ = 28.3sec; circular probe: δ = 0°C, λ = 2.9mm, τ = 29.3sec, see Experimental Procedures for details).
(E) Cooling probe placements for S183.
(F) Changes in speech quality upon cooling corresponding to the four regions highlighted in (E). Pluses and circles represent the ‘counting’ and ‘days of the week’ tasks respectively. The small icons are quality scores from single listeners, and the large icons are the median values of quality scores for each vocalization across all listeners. The black curves below represent the estimated cortical temperature change.
(G) Cumulative probability histograms of individual quality scores (top) and button press rates (bottom) for each of the cooling epochs in S183 with colors corresponding to (E).
(H) Cooling probe placement for S199.
(I) Quality degradation and speech arrest following cooling the location shown in (H) with the accompanying temperature changes shown below.
(J) A population plot from 38 cooling sites in 16 subjects showing average quality and estimated temperature changes during cooling compared with control (noncooled) values. Lines colored red indicate locations in which significant quality changes were observed (p<0.001, t-test).
We next used the cooling device in patient volunteers who were undergoing awake intracranial surgery for intractable epilepsy or brain tumor resection (Table 1). In total, 22 patients enrolled in the study, and sufficient data were collected from 16 of these individuals to allow for further analysis. The cooling probe was placed at various locations within the craniotomy. We cooled 42 total areas (1–7 cooling regions per subject) while subjects produced easily generated, over-learned vocal sequences, specifically the days of the week (‘Monday’–‘Friday’) or a string of numbers (either ‘1’–‘5’ or ‘21’–‘25’). The majority of probe locations (61.9%) were aligned to language-critical sites identified with electrical stimulation-induced speech arrest (e.g. Figures 3A and S1). Cooling epochs lasted 3.7±1.6 minutes on average, and the maximum cooling level at 4 mm depth was estimated to be a decrease of 6.6±1.3°C relative to control values. As an aggregate, subjects produced a total of 783 word lists during cooling and 741 outside of cooling.
Table 1. Patient characteristics.
Demographics and experimental conditions of the 16 patients analyzed in this study. For language dominance, ψ indicates confirmation by Wada test. In other conditions, stimulation was used to suggest hemispheric dominance. Handedness was also determined in 14 patients.
| Number | Side Cooled | ID | Sex | Age (yrs) | Diagnosis | Language Dominance | Handedness | Probe |
|---|---|---|---|---|---|---|---|---|
| 1 | L | 183 | F | 39 | Epilepsy | Lψ | R | S |
| 2 | L | 187 | F | 53 | Tumor | L | R | S |
| 3 | L | 197 | F | 39 | Tumor | L | - | S |
| 4 | L | 199 | M | 71 | Tumor | L | R | S |
| 5 | R | 200 | M | 58 | Epilepsy | L | R | S |
| 6 | R | 211 | M | 58 | Epilepsy | Rψ | L | P |
| 7 | L | 234 | F | 50 | Tumor | L | R | P |
| 8 | L | 239 | F | 33 | Tumor | L | R | P |
| 9 | L | 244 | M | 63 | Tumor | L | L,R | P |
| 10 | R | 246 | M | 41 | Epilepsy | Lψ | R | P |
| 11 | R | 262 | F | 34 | Epilepsy | Lψ | L | P |
| 12 | L | 279 | F | 59 | Tumor | L | R | P,S |
| 13 | L | 284 | M | 41 | Epilepsy | Lψ | L | P,S |
| 14 | L | 299 | M | 64 | Tumor | L | R | S |
| 15 | L | 301 | M | 64 | Tumor | L | L | S |
| 16 | L | 305 | F | 56 | Tumor | L | R | S |
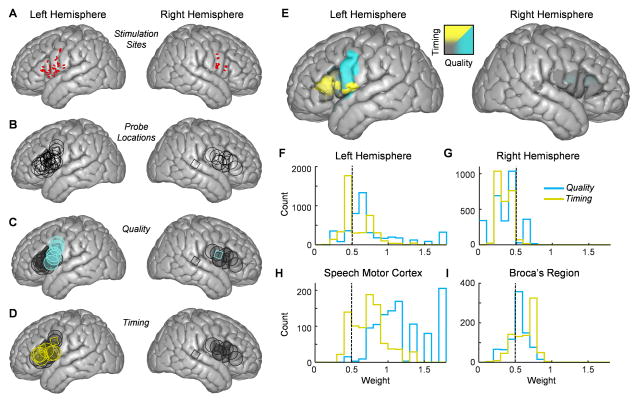
Figure 3. Functional speech maps as determined by electrical stimulation and focal cooling.
(A) Electrical stimulation mapping sites (represented by red ovals) that resulted in speech arrest for left (30 sites in 11 subjects) and right (12 sites in 4 subjects) hemispheres plotted on an ICBM template brain.
(B) Template brains on the left and right hemispheres displaying the cooling probe locations across all subjects on the left (30 sites in 12 subjects) and right (12 sites in 4 subjects) hemispheres.
(C and D) Cooling sites were designated to result in either a significant change in speech quality (C) or timing (D), indicated by blue or yellow shapes respectively. Sites that were significant for both timing and quality were designated as the category with the larger effect.
(E) A functional map showing behavioral results for both hemispheres.
(F and G) Histograms of all pixel values from speech areas on left (F) and right (G) hemispheres showing values for quality (blue) and timing (yellow).
(H and I) The distribution of timing and quality values for pixels in the speech motor cortex (H) and Broca’s region (I).
Cooling Can Alter Articulatory Quality
In some cases, cortical cooling resulted in a transient degradation of speech performance that lasted for the duration of the manipulation (Figure 1E–J, Supplemental Movies S1 and S2). To quantify the impact of cooling on speech quality for all recorded sound files, we used an online crowdsourcing approach adapted from a recently validated method (McAllister Byun et al., 2015), in which each vocalization was rated on a visual analog scale (VAS) (Munson et al., 2012) from 0 (‘Extremely degraded’) to 1 (‘Typical/Normal’). Each subject’s sound files were evaluated by 20.4 ±1.0 online participants. Ratings were found to be reliable; scores were highly correlated within crowdsourced raters (r = 0.78) and agreed with scores given by experienced listeners (r = 0.87). In one example individual (S183), a cooling device was placed at four different sites (Figure 1E), resulting in a location-specific change in the quality of speech (Figure 1F–G). When location D was cooled, the quality score covaried with cortical temperature (Figure 1F), demonstrating that cooling was capable of degrading the quality of speech in a smoothly varying manner. Importantly, the cooling protocol applied to this subject did not affect another behavior (finger tapping) that required fine motor control of different muscle groups (Figure 1G and S2). In another subject (S199), focal cooling led to a temporary speech arrest that quickly resolved once the cortical temperature returned to baseline (Figure 1H and I). In contrast to electrical stimulation (Figure 3A), such cooling-related dysfluencies were relatively rare (see Experimental Procedures for complete list), occurring in 91 out of 1567 total prompts, and cooling-related vocal errors were only consistently induced (Fisher’s exact test, p<0.01) in two other locations (S187B – some incorrect responses; S279D – list truncation). Across the population of 16 subjects (Figure 1J), cooling resulted in significant quality changes in 25.6% (10 out of 39) of all locations analyzed.
Cooling Can Affect the Timing of Speech Elements
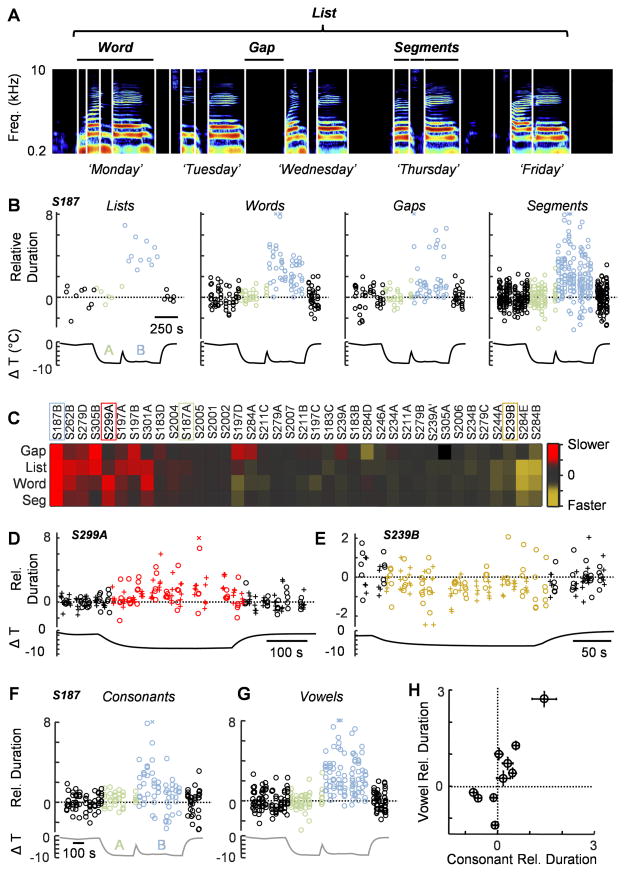
We then asked whether cooling the cortical surface would lead to changes in the timing of speech. We manually detected 29,387 reproducible spectrotemporal speech landmarks (mean and standard deviation 20.3±1.4 and 23.1±3.1 landmarks per list for ‘days of the week’ and ‘numbers’ respectively) in order to quantify the duration of different speech timescales (Figure 2A): lists, words, gaps and segments. We compared the durations of each vocalization performed in cooled and control conditions (Figure 2) by normalizing to the mean and standard deviation of controls for each vocal element (e.g., ‘T’ in ‘Tuesday’) and then pooling across all vocalizations at that timescale (e.g., all segments). We also corrected for baseline drift (Figure S3); such instability may result from changes in attention or the level of previously administered conscious sedation. Identified speech elements could either expand (Figure 2D) or compress (Figure 2E) during cooling. Typically all timescales that we measured covaried (Figure 2B and C, Supplemental Movies S3 and S4), but occasionally silent gaps would be affected differently than vocalizations (e.g. S197D). Across 16 subjects, 10 out of 39 cooling sites (25.6%) resulted in stretching of at least one timescale and 6 out of 39 (15.4%) exhibited compression, with 24 out of 39 (61.5%) resulting in no significant effects. Because selected segments were often composed of single phonemes or phonemes within an individual category, we examined whether cooling affected all of these elements equally. We identified 2,153 segments that could be collapsed into vowels (n = 978) and consonants (n = 1175) (Figure 2F–H), for 10 sites in which there was a significant change in segment duration and found that vowels showed a larger change relative to consonants (p<0.0001, Wilcoxon rank-sum test, Figure 2H).
Figure 2. Focal cooling can affect speech timing.
(A) A sonogram of the ‘days of the week’ task with a logarithmic frequency axis.
(B) Cooling can lead to a significant increase in speech across multiple timescales. For S187, cooling location B (light blue, pars opercularis and precentral gyrus) resulted in significant stretching of lists, words, gaps and segments (relative to their respective controls), while cooling location A (light green, dorsal inferior frontal gyrus) had a minimal effect. ‘X’s mark values outside the range given by the ordinate.
(C) A population plot showing the effects of cooling on the duration of gaps, lists, words, and segments for 38 sites across 15 subjects. The location IDs of the examples shown elsewhere in panels (B), (D), and (E) are demarcated with boxes. Colors indicate cooling induced changes to the mean duration of vocal elements (colorbar at right). The measurement of gaps in region A of S305 was excluded from the dataset because of unstable baseline values.
(D and E) In some cases, cooling led to either a significant increase (D, pars opercularis and precentral gyrus) or decrease (E, pars opercularis) in the duration of words.
(F and G) The timing of all consonants (F) and vowels (G) are shown for S187.
(H) The change in duration of vowels versus that of consonants for the 10 sites across 8 patients with significant changes in the timing of identified vocal segments. Error bars represent the SEM.
Assembly of Functional Speech Maps
We next investigated whether the range of behavioral effects elicited by our manipulation could be partially explained based on the location of the cooling device. One major concern in this endeavor is that the gross anatomical morphology of frontal cortical structures can vary significantly across subjects (Brett et al., 2002) and therefore a simple transfer of the center coordinates (i.e., MNI locations) of the probe positions may not accurately represent the cooled area on a standardized brain map. To address this, we normalized all cooling locations onto an ICBM (International Consortium for Brain Mapping) template brain (Mazziotta et al., 2001) using nonlinear warping to conform to the brain’s surface features (Figure S1 and S4, see Experimental Procedures), with 30 locations on the left hemisphere and 12 locations on the right hemisphere (Figure 3B). Each location could be labeled according to the primary behavioral effects elicited by cooling that site (Figure 3C–D). We then calculated the impact of our temperature manipulation on speech quality (Figure 1) and timing (Figure 2) within the canonical brain at high resolution. Each pixel (Figure S4E–F) was categorically assigned to be either ‘quality’ or ‘timing’ based on the relative values aggregated across cooling probes (Figure S5). In examining the resulting functional brain map (Figure 3E), we noticed a high degree of lateralization of both categories within Broca’s region and speech motor cortex (see Experimental Procedures for precise locations on the ICBM brain). On the right hemisphere, only 14.2% of the total area tested was shown to result in measurable changes in speech, and these locations were all categorized as alterations in speech quality (Figure 3E,G). Conversely, focal cooling administered to the left hemisphere resulted in changes in 85.0% of the total area tested (28.5% timing and 56.5% quality) (Figure 3E,F). This effect was reflected in the magnitude of quality and timing across hemispheres (p<0.0001, Wilcoxon rank-sum test). Because we interrogated the left hemisphere more completely than the right, we repeated this analysis excluding the dorsal portion of the precentral gyrus where coverage was insufficient on the right hemisphere. In this more restricted view, we continued to observe a highly significant lateralization effect (p<0.0001, Wilcoxon rank-sum test).
In addition to a lateralization of speech-related cooling effects, we also investigated the nature of changes with respect to distinct cortical regions in the left hemisphere. When we examined the surface of the left speech motor cortex, we observed a significant cooling-induced change in speech quality. Specifically, 79.3% of the sites within this region resulted in quality changes compared with 19.3% of the sites that preferentially caused changes in speech timing and 1.3% having no significant effects (Figure 3E,H). In contrast, cooling Broca’s region had the opposite impact (Figure 3E,I), with 68.6% of the gyral surface resulting in timing changes, 15.1% causing quality changes, and 16.4% failing to elicit a significant effect. These disparities could also be seen at the level of the mean timing (T) and quality (Q) values averaged across the gyral surfaces (speech motor cortex: T = 0.74±0.23, Q = 1.2±0.33; Broca’s region: T=0.62±0.15, Q=0.54±0.13) (p<0.0001, Wilcoxon rank-sum test). Additionally, in the cases where a timing effect could be seen in the speech motor cortex (Figure 3D and S5A), we noted that silent gaps were affected more strongly than words, while the reverse was true in Broca’s region (p<0.0001, one sided Wilcoxon signed rank test).
DISCUSSION
We used focal cooling to manipulate cortical dynamics, allowing us to characterize the processing underlying various stages of speech production. Although focal cooling is rarely performed in humans outside of the context of suppressing epileptic activity (Bakken et al., 2003; Brooks, 1983; Fisher, 2012; Karkar et al., 2002; Pasztor and Tomka, 1969; Smyth et al., 2015), we now demonstrate that this approach can be an effective method for localizing speech-related cortical sites. Importantly, cooling lacks many of the drawbacks of electrical stimulation mapping, such as the possibility of initiating a seizure during the procedure (Piccioni and Fanzio, 2008). Furthermore, we show that focal cooling can be used as a discovery tool to rapidly and reversibly test hypotheses concerning cortical function.
Our first finding using this method is that, while we were able to observe instances of cooling-related effects bilaterally, we found that they were primarily confined to the left hemisphere. This lateralization is consistent with clinical observations (Damasio, 1992), but opposed to recently emerging views concerning the distributed nature of speech motor control (Cogan et al., 2014; Price, 2010). In one case included in our study (S211), we were unable to see a functional effect of cooling the right pars opercularis despite the fact that the subject exhibited right language dominance. Additional data are needed to understand the anatomical organization of speech production centers in these individuals.
Within the left hemisphere, we used cooling to demonstrate a clear functional dissociation (Gelfand and Bookheimer, 2003; Gough et al., 2005) between speech motor cortex and Broca’s region. Cooling the speech motor cortex leads to changes in the quality of vocalizations. Neurons within this area display an articulator-specific topographic organization (Bouchard et al., 2013) and directly contact the motor neurons that drive speech production muscles (Simonyan, 2014). Thus, the cooling-related speech dysarticulation highlights the impact of these neuronal populations on speech kinematics. In contrast, cooling Broca’s area often led to changes in speech rate. Computational models have proposed independent signals that can control the speed of movements (Bullock and Grossberg, 1988) including speech (Guenther, 1995, 2016) and our results are consistent with the hypothesis that this computation may involve the IFG. The IFG is a heterogeneous structure (Amunts and Zilles, 2012) with a number of subregions that may carry out distinct roles. For example, studies have found differences between the dorsal IFG and other nearby regions, such as the inferior frontal sulcus (Bohland and Guenther, 2006; Myers et al., 2009) or the ventral IFG (Papoutsi et al., 2009). In future experiments, we hope to further refine our technique to perturb individual cortical subregions in order to test these observations and to unveil any additional functional organization that may exist within speech production areas.
The mechanisms by which the IFG may affect the rate of speech production are poorly understood. However, we can look for potential insight in simpler systems (Long and Fee, 2008; McKibben and Bass, 1998; Pires and Hoy, 1992; Yamaguchi et al., 2008), where cooling premotor vocal circuits has also been shown to also result to slowed vocalizations. In the songbird, a critical premotor structure forms fine-grained sequences of activity where each participating neuron is active for a single moment (approximately 10 ms) during the vocalization (Hahnloser et al., 2002). Selective cooling of this region stretches the sequence and the resultant singing behavior (Long and Fee, 2008). Because the majority of temperature-related changes reported here also involved a decrease in speech rate, we propose the intriguing possibility that at least one affected sub-part of Broca’s region is the site of sequence generation for speech production, a notion that is consistent with some previous findings (Clerget et al., 2011; Gelfand and Bookheimer, 2003; Udden and Bahlmann, 2012). A range of relevant models can be directly addressed in future experiments using high density recording techniques to measure activity at a fine spatial scale (Bouchard et al., 2013), and even at the single neuron level (Fried et al., 2014), to better understand the nature of this local processing. By adopting a sequence generation framework, we can begin to understand the mechanisms by which premotor commands are represented in Broca’s region and the processes enabling these commands to be associated with specific behavioral elements in downstream targets (Flinker et al., 2015; Lashley, 1951).
EXPERIMENTAL PROCEDURES
For details on all methods, please see Supplemental Information.
Supplementary Material
1
2
3
4
5
Acknowledgments
We thank all of the patient volunteers for their contributions to this project. Haiming Chen and Theo John Franklin Di Castri contributed research support for this project. Daniel Szeredi helped us with the software necessary to implement our crowdsourcing procedure. We acknowledge valuable discussions with David Poeppel and Daryush Mehta, and we thank Richard Tsien, David Poeppel, Kristopher Bouchard, Kristina Simonyan, Daniela Vallentin, and Georg Kosche for their comments on an earlier version of this manuscript. A talented team of neurosurgical residents at the University of Iowa Hospitals and Clinics assisted with data collection, including Adam Jackson and Daniel Hansen. This work was supported by the NIH (DC004290, DC009589, NS075044) as well as the NYSC Foundation.
Footnotes
AUTHOR CONTRIBUTIONS
M.A.L. and J.D.W.G. designed the research, and J.D.W.G., M.A.H., and M.A.L. collected data for the experiments. M.A.L. and K.A.K. wrote the paper. K.A.K. and M.A.L. were central to analyzing every aspect of the collected data, and M.A.S., R.C.C., T.M.B., N.M., H.O., M.A.H., and J.D.W.G. also contributed to this effort. Notably, T.M.B. assisted us with the crowdsourcing approach, M.A.S. and R.C.C. helped with analyzing speech timing data, and N.M. helped to design the generalized linear models used in this study. J.D.W.G., M.A.H., and H.O. assisted with neuroanatomical classifications.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amunts K, Zilles K. Architecture and organizational principles of Broca’s region. Trends Cogn Sci. 2012;16:418–426. doi: 10.1016/j.tics.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Bakken HE, Kawasaki H, Oya H, Greenlee JD, Howard MA., 3rd A device for cooling localized regions of human cerebral cortex. Technical note. Journal of neurosurgery. 2003;99:604–608. doi: 10.3171/jns.2003.99.3.0604. [DOI] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32:821–841. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- Bouchard KE, Mesgarani N, Johnson K, Chang EF. Functional organization of human sensorimotor cortex for speech articulation. Nature. 2013;495:327–332. doi: 10.1038/nature11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nat Rev Neurosci. 2002;3:243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- Brooks VB. Study of brain function by local, reversible cooling. Rev Physiol Biochem Pharmacol. 1983;95:1–109. [Google Scholar]
- Bullock D, Grossberg S. Neural dynamics of planned arm movements: emergent invariants and speed-accuracy properties during trajectory formation. Psychol Rev. 1988;95:49–90. doi: 10.1037/0033-295x.95.1.49. [DOI] [PubMed] [Google Scholar]
- Clerget E, Badets A, Duque J, Olivier E. Role of Broca’s area in motor sequence programming: a cTBS study. Neuroreport. 2011;22:965–969. doi: 10.1097/WNR.0b013e32834d87cd. [DOI] [PubMed] [Google Scholar]
- Cogan GB, Thesen T, Carlson C, Doyle W, Devinsky O, Pesaran B. Sensory-motor transformations for speech occur bilaterally. Nature. 2014;507:94–98. doi: 10.1038/nature12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. Aphasia. The New England journal of medicine. 1992;326:531–539. doi: 10.1056/NEJM199202203260806. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS. Therapeutic devices for epilepsy. Ann Neurol. 2012;71:157–168. doi: 10.1002/ana.22621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinker A, Korzeniewska A, Shestyuk AY, Franaszczuk PJ, Dronkers NF, Knight RT, Crone NE. Redefining the role of Broca’s area in speech. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:2871–2875. doi: 10.1073/pnas.1414491112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried I, Rutishauser U, Cerf M, Kreiman G. Single neuron studies of the human brain. Cambridge, MA: MIT Press; 2014. [Google Scholar]
- Gelfand JR, Bookheimer SY. Dissociating neural mechanisms of temporal sequencing and processing phonemes. Neuron. 2003;38:831–842. doi: 10.1016/s0896-6273(03)00285-x. [DOI] [PubMed] [Google Scholar]
- Gough PM, Nobre AC, Devlin JT. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J Neurosci. 2005;25:8010–8016. doi: 10.1523/JNEUROSCI.2307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzinsky Y, Santi A. The battle for Broca’s region. Trends Cogn Sci. 2008;12:474–480. doi: 10.1016/j.tics.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Guenther FH. Speech sound acquisition, coarticulation, and rate effects in a neural network model of speech production. Psychol Rev. 1995;102:594–621. doi: 10.1037/0033-295x.102.3.594. [DOI] [PubMed] [Google Scholar]
- Guenther FH. Cortical interactions underlying the production of speech sounds. Journal of communication disorders. 2006;39:350–365. doi: 10.1016/j.jcomdis.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Guenther FH. Neural control of speech. Cambridge, MA: MIT Press; 2016. [Google Scholar]
- Guenther FH, Ghosh SS, Tourville JA. Neural modeling and imaging of the cortical interactions underlying syllable production. Brain Lang. 2006;96:280–301. doi: 10.1016/j.bandl.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P. On Broca, brain, and binding: a new framework. Trends Cogn Sci. 2005;9:416–423. doi: 10.1016/j.tics.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Hahnloser RH, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature. 2002;419:65–70. doi: 10.1038/nature00974. [DOI] [PubMed] [Google Scholar]
- Hickok G. Computational neuroanatomy of speech production. Nat Rev Neurosci. 2012;13:135–145. doi: 10.1038/nrn3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Karkar KM, Garcia PA, Bateman LM, Smyth MD, Barbaro NM, Berger M. Focal cooling suppresses spontaneous epileptiform activity without changing the cortical motor threshold. Epilepsia. 2002;43:932–935. doi: 10.1046/j.1528-1157.2002.03902.x. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Jubault T. Broca’s area and the hierarchical organization of human behavior. Neuron. 2006;50:963–974. doi: 10.1016/j.neuron.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Lashley KS. The problem of serial order of behavior. In: Jeffress LA, editor. Cerebral mechanisms in behavior. New York: Wiley; 1951. pp. 112–136. [Google Scholar]
- Lomber SG, Meredith MA, Kral A. Cross-modal plasticity in specific auditory cortices underlies visual compensations in the deaf. Nat Neurosci. 2010;13:1421–1427. doi: 10.1038/nn.2653. [DOI] [PubMed] [Google Scholar]
- Long MA, Fee MS. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature. 2008;456:189–194. doi: 10.1038/nature07448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philos Trans R Soc Lond B Biol Sci. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister Byun T, Halpin PF, Szeredi D. Online crowdsourcing for efficient rating of speech: a validation study. Journal of communication disorders. 2015;53:70–83. doi: 10.1016/j.jcomdis.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKibben JR, Bass AH. Behavioral assessment of acoustic parameters relevant to signal recognition and preference in a vocal fish. J Acoust Soc Am. 1998;104:3520–3533. doi: 10.1121/1.423938. [DOI] [PubMed] [Google Scholar]
- Munson B, Johnson JM, Edwards J. The role of experience in the perception of phonetic detail in children’s speech: a comparison between speech-language pathologists and clinically untrained listeners. American journal of speech-language pathology/American Speech-Language-Hearing Association. 2012;21:124–139. doi: 10.1044/1058-0360(2011/11-0009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Corfield DR, Guz A, Fink GR, Wise RJ, Harrison J, Adams L. Cerebral areas associated with motor control of speech in humans. J Appl Physiol (1985) 1997;83:1438–1447. doi: 10.1152/jappl.1997.83.5.1438. [DOI] [PubMed] [Google Scholar]
- Musso M, Moro A, Glauche V, Rijntjes M, Reichenbach J, Buchel C, Weiller C. Broca’s area and the language instinct. Nat Neurosci. 2003;6:774–781. doi: 10.1038/nn1077. [DOI] [PubMed] [Google Scholar]
- Myers EB, Blumstein SE, Walsh E, Eliassen J. Inferior frontal regions underlie the perception of phonetic category invariance. Psychol Sci. 2009;20:895–903. doi: 10.1111/j.1467-9280.2009.02380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. Journal of neurosurgery. 1989;71:316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- Papoutsi M, de Zwart JA, Jansma JM, Pickering MJ, Bednar JA, Horwitz B. From phonemes to articulatory codes: an fMRI study of the role of Broca’s area in speech production. Cereb Cortex. 2009;19:2156–2165. doi: 10.1093/cercor/bhn239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Gates JR, Dhuna A. Induction of speech arrest and counting errors with rapid-rate transcranial magnetic stimulation. Neurology. 1991;41:697–702. doi: 10.1212/wnl.41.5.697. [DOI] [PubMed] [Google Scholar]
- Pasztor E, Tomka I. Changes of electrocorticographic activity in response to direct brain surface cooling in epileptic patients. Acta Physiol Acad Sci Hung. 1969;36:277–292. [PubMed] [Google Scholar]
- Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- Penfield W, Rasmussen T. The Cerebral Cortex of Man. New York: MacMillan; 1950. [Google Scholar]
- Piccioni F, Fanzio M. Management of anesthesia in awake craniotomy. Minerva anestesiologica. 2008;74:393–408. [PubMed] [Google Scholar]
- Pires A, Hoy RR. Temperature coupling in cricket acoustic communication. II. Localization of temperature effects on song production and recognition networks in Gryllus firmus. Journal of comparative physiology A, Sensory, neural, and behavioral physiology. 1992;171:79–92. doi: 10.1007/BF00195963. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Price CJ, Crinion JT, Macsweeney M. A Generative Model of Speech Production in Broca’s and Wernicke’s Areas. Frontiers in psychology. 2011;2:237. doi: 10.3389/fpsyg.2011.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini BL, Regehr WG. Timing of neurotransmission at fast synapses in the mammalian brain. Nature. 1996;384:170–172. doi: 10.1038/384170a0. [DOI] [PubMed] [Google Scholar]
- Simonyan K. The laryngeal motor cortex: its organization and connectivity. Curr Opin Neurobiol. 2014;28:15–21. doi: 10.1016/j.conb.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MD, Han RH, Yarbrough CK, Patterson EE, Yang XF, Miller JW, Rothman SM, D’Ambrosio R. Temperatures Achieved in Human and Canine Neocortex During Intraoperative Passive or Active Focal Cooling. Therapeutic hypothermia and temperature management. 2015;5:95–103. doi: 10.1089/ther.2014.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang LS, Goeritz ML, Caplan JS, Taylor AL, Fisek M, Marder E. Precise temperature compensation of phase in a rhythmic motor pattern. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettamanti M, Weniger D. Broca’s area: a supramodal hierarchical processor? Cortex. 2006;42:491–494. doi: 10.1016/s0010-9452(08)70384-8. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Masukawa LM, Prince DA. Temperature dependence of intrinsic membrane properties and synaptic potentials in hippocampal CA1 neurons in vitro. J Neurosci. 1985;5:817–824. doi: 10.1523/JNEUROSCI.05-03-00817.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupe LA, Varma DD, Gomez Y, Race D, Leigh R, Hillis AE, Gottesman RF. Chronic apraxia of speech and Broca’s area. Stroke. 2013;44:740–744. doi: 10.1161/STROKEAHA.112.678508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udden J, Bahlmann J. A rostro-caudal gradient of structured sequence processing in the left inferior frontal gyrus. Philos Trans R Soc Lond B Biol Sci. 2012;367:2023–2032. doi: 10.1098/rstb.2012.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgushev M, Vidyasagar TR, Chistiakova M, Eysel UT. Synaptic transmission in the neocortex during reversible cooling. Neuroscience. 2000a;98:9–22. doi: 10.1016/s0306-4522(00)00109-3. [DOI] [PubMed] [Google Scholar]
- Volgushev M, Vidyasagar TR, Chistiakova M, Yousef T, Eysel UT. Membrane properties and spike generation in rat visual cortical cells during reversible cooling. The Journal of physiology. 2000b;522(Pt 1):59–76. doi: 10.1111/j.1469-7793.2000.0059m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A, Gooler D, Herrold A, Patel S, Pong WW. Temperature-dependent regulation of vocal pattern generator. J Neurophysiol. 2008;100:3134–3143. doi: 10.1152/jn.01309.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2
3
4
5