Obesogenic memory can confer long-term increases in adipose tissue but not liver inflammation and insulin resistance after weight loss (original) (raw)
Abstract
Objective
Obesity represents a major risk factor for the development of type 2 diabetes mellitus, atherosclerosis and certain cancer entities. Treatment of obesity is hindered by the long-term maintenance of initially reduced body weight, and it remains unclear whether all pathologies associated with obesity are fully reversible even upon successfully maintained weight loss.
Methods
We compared high fat diet-fed, weight reduced and lean mice in terms of body weight development, adipose tissue and liver insulin sensitivity as well as inflammatory gene expression. Moreover, we assessed similar parameters in a human cohort before and after bariatric surgery.
Results
Compared to lean animals, mice that demonstrated successful weight reduction showed increased weight gain following exposure to ad libitum control diet. However, pair-feeding weight-reduced mice with lean controls efficiently stabilized body weight, indicating that hyperphagia was the predominant cause for the observed weight regain. Additionally, whereas glucose tolerance improved rapidly after weight loss, systemic insulin resistance was retained and ameliorated only upon prolonged pair-feeding. Weight loss enhanced insulin action and resolved pro-inflammatory gene expression exclusively in the liver, whereas visceral adipose tissue displayed no significant improvement of metabolic and inflammatory parameters compared to obese mice. Similarly, bariatric surgery in humans (n = 55) resulted in massive weight reduction, improved hepatic inflammation and systemic glucose homeostasis, while adipose tissue inflammation remained unaffected and adipocyte-autonomous insulin action only exhibit minor improvements in a subgroup of patients (42%).
Conclusions
These results demonstrate that although sustained weight loss improves systemic glucose homeostasis, primarily through improved inflammation and insulin action in liver, a remarkable obesogenic memory can confer long-term increases in adipose tissue inflammation and insulin resistance in mice as well as in a significant subpopulation of obese patients.
Keywords: Obesity, Weight loss, Weight regain, Insulin resistance, Metabolic inflammation
Highlights
- •
Upon weight loss in mice liver insulin sensitivity rapidly improves. - •
Upon weight loss in mice fat retains metabolic inflammation and insulin resistance. - •
Weight gain upon successful weight reduction in mice is driven by increased food intake. - •
A proportion of human subjects undergoing bariatric surgery retain AT-inflammation.
1. Introduction
Obesity is a major health problem with increasing prevalence around the world [1], [2]. The World Health Organization estimates that each year, 2.8 million people die as a result of being overweight or obese [3]. The increased mortality can mainly be attributed to obesity-associated diseases such as type 2 diabetes mellitus (T2DM), cardiovascular disease, and some types of cancer [4]. Weight reduction represents an obesity intervention strategy, which efficiently ameliorates some associated diseases [5], [6], [7].
To successfully promote weight loss, energy intake has to fall below total energy expenditure. Increasing energy expenditure by physical exercise improves several obesity-related disorders and should therefore be an important component of any weight loss plan. However, physical exercise alone, without concomitant dietary changes, promotes only modest weight reduction [8], [9], and, importantly, only few patients successfully manage to include exercise into their daily schedule. Therefore, decreasing caloric intake remains the single most effective and cost efficient intervention strategy for obesity. Notably, for the beneficial effects, it is of little importance what exact dietary composition is used during caloric restriction, as long as weight reduction can be achieved [10], [11]. However, intensive lifestyle changes that lead to weight reduction do not necessarily reduce cardiovascular mortality in obese adults with T2DM [12]. It is therefore questionable whether weight loss is sufficient to reverse all metabolic or inflammatory changes induced by severe obesity. Moreover, although weight loss programs can result in an average body weight reduction of 10% [13], the majority of patients regain their body weight quickly [14], [15], once again placing them at high risk to develop obesity-associated diseases.
The biological mechanisms underlying this key obstacle in the treatment of obesity are poorly understood. There is evidence from human studies suggesting that weight reduction ultimately leads to a profound decrease in energy expenditure [16], [17], [18], [19]. However, whether this decrease is more than what can be explained by the changes in body composition remains controversial [20]. A disadvantage of human studies designed to assess the mechanism for weight regain is the selection bias of choosing already obese patients. Genetic, epigenetic and behavioral predispositions that led to obesity development in the first place influence weight regain and might also be responsible for some of the differences observed after weight reduction [21].
Rodent models offer the advantage of genetic similarity and the possibility to better control nutrient composition of the diet and caloric intake. However, there are only a limited number of rodent studies published so far that analyze the long-term consequences of weight loss and regain. Studies that used obesity-prone rats or embryonic glutamate injections to induce obesity fail to distinguish between the direct effects of weight reduction in obesity and changes of the animal model itself [22], [23]. Thus, the biological mechanisms behind weight regain as well as the long-term consequences of weight loss on tissue-specific insulin action and insulin secretion remain only partly understood.
In the present study, we have analyzed the effect of caloric restriction in HFD-induced obesity in mice. Our experiments revealed that weight reduction efficiently improved systemic glucose tolerance and that insulin sensitivity was improved in the liver upon weight loss but did not equally improve in adipose tissue after weight reduction. Consistently, while obesity-induced hepatic inflammation resolved upon weight loss, adipose-tissue inflammation persisted. Importantly, we also demonstrate that upon massive and sustained weight loss in humans undergoing bariatric surgery, hepatic inflammation resolves, while at least in a subgroup of obese individuals, adipose tissue inflammation persists up to 12 months of follow-up. Furthermore, adipocyte-autonomous insulin action only marginally improves despite massive improvements of systemic insulin-induced glucose disposal in these patients. These results highlight that even successful, long-term weight reduction does not consistently reverse adverse, inflammatory reactions in adipose tissue associated with obesity, thus offering a molecular basis of sustained increased cardiovascular risk despite weight reduction.
2. Methods
2.1. Animal procedures
All animal procedures and euthanasia were reviewed by the animal care committee and approved by local government authorities (Tierschutzkommission acc. §15 TSchG of the Landesamt für Natur, Umwelt und Verbraucherschutz North Rhine Westphalia) and were in accordance with NIH guidelines. Male C57BL/6 mice were acquired at 3 weeks age from Charles River Laboratory and held in a virus-free facility at 22–24 °C on a 12-h light, 12-h dark cycle. After one week of acclimatization in our animal facility, the mice were randomly assigned either a high fat diet (D12492, 60% kcal from fat, Research diets, New Brunswick, NJ, USA) or a sucrose-matched, low fat diet (D12450 J, 10% kcal from fat, Research diets, New Brunswick, NJ, USA). Body weight was measured weekly.
At 22 weeks of age, two thirds of the high fat diet-fed mice were switched to 40% caloric restriction using a caloric restriction diet (D11063001, 10% kcal of fat, Research diets, New Brunswick, NJ, USA). This diet was added to the experimental paradigm in order to ensure an equal intake of vitamins, minerals, and amino acids during the different dietary conditions, even when restricted to 60% of their prior caloric intake. For caloric restriction and pair-feeding, the rationed food was introduced once daily before the beginning of the night cycle.
2.2. Indirect calorimetry
Indirect calorimetry was performed for 120 h using an open-circuit, indirect calorimetry system including spontaneous activity by beam breaking (PhenoMaster System, TSE systems) as previously described [24].
2.3. Analysis of body composition by computer tomography
Imaging of mice was performed with a LaTheta LCT-100 (Aloka Co. LTD., Tokyo, Japan) micro computed tomography scanner. The X-ray source tube voltage was set at 50 kV with a constant 1 mA current. A holder with an inner diameter of 48 mm was used, resulting in pixel resolutions of 100 μm. Pitch was 0.5 mm and scan speed 4.5 s/slice. LaTheta software V2.10 estimates the contrast of the different tissues using differences in X-ray density. A Midazolam (5 mg/kg) and Medetomidine (0.5 mg/kg) anesthesia was used to immobilize the animals. After the procedure the anesthetics were antagonized using Flumazenil (0.5 mg/kg) and Atipamezole (2.5 mg/kg).
2.4. Glucose and insulin tolerance testing
Glucose tolerance testing (GTT) was performed in 6 h starved animals. After determination of the fasting blood glucose values, a 20% glucose solution (Glucosteril, Fresenius Kabi, Bad Homburg, Germany) was injected intraperitoneally at a dose of 10 ml/kg body weight. Blood glucose concentrations were determined at 15, 30, 60, and 120 min after injection (Bayer Contour, Bayer diabetes care, Leverkusen, Germany). For insulin tolerance testing (ITT), blood glucose values were measured in random fed mice before injecting 0.75 U/kgBW of insulin (Actrapid; Novo Nordisk A/S, Denmark), and blood glucose was measured at 15, 30 and 60 min after insulin injection.
2.5. Glucose-stimulated insulin secretion
Mice were fasted for 12 h. Blood samples were collected from mice before an i.v. injection by tail vein of 2 mg g−1 body weight of glucose (20% glucose; Glucosteril, Fresenius Kabi, Bad Homburg, Germany). Additional blood samples were collected 2, 5, 15, 30 and 60 min after the injection. Serum insulin levels were determined by enzyme-linked immunosorbent assay according to the manufacturer's instructions (mouse/rat Insulin ELISA, Crystal Chem Inc., Downers Grove, IL, USA).
2.6. Human bariatric surgery intervention study
We selected 23 Caucasian obese patients (14 women, 9 men) out of a group of 55 patients who underwent a two-step bariatric surgery strategy with gastric sleeve resection as the first step and a Roux-en-Y gastric bypass as second step 12 ± 2 months later (Table 1). At both time points, serum/plasma samples, omental and subcutaneous (sc) adipose tissue (AT) and liver biopsies were obtained. Patient selection was based on the absence (n = 23) of significant changes in the number of macrophages in omental adipose tissue ∼1 year after the first surgery. For the entire cohort, general inclusion and exclusion criteria as well as measurement of anthropometric and laboratory parameters have been reported recently [25]. Study protocols have been approved by the ethics committee of the University of Leipzig (Reg. No. 031-2006 and 017-12-23012012). All participants gave written informed consent before taking part in the study.
Table 1.
Characteristics of patients at baseline and 1 year after bariatric surgery. ATM, adipose tissue macrophages, **p < 0.01, ***p < 0.001, ****p < 0.0001 for comparisons between baseline and 1 year after surgery within the ATM changes subgroups.
| No significant reduction in omental ATM after surgery (n = 23) | Significant reduction in omental ATM after surgery (n = 32) | |||
|---|---|---|---|---|
| Baseline | 1 year after surgery | Baseline | 1 year after surgery | |
| Age (years) | 47.8 ± 8.1 | 48.8 ± 8.1 | 41.2 ± 13.9 | 42.2 ± 13.9 |
| Men/Women (n) | 9/14 | 9/14 | 8/24 | 8/24 |
| Height (m) | 172.9 ± 11.9 | 172.9 ± 11.9 | 170.3 ± 13.5 | 170.3 ± 13.5 |
| Body weight (kg) | 156.6 ± 25.0 | 105.9 ± 17.68**** | 159.4 ± 35.0 | 110.1 ± 32.32**** |
| Body mass index (kg/m2) | 52.4 ± 7.0 | 35.5 ± 5.1**** | 54.8 ± 7.6 | 37.9 ± 8.5**** |
| Fat mass (kg) | 71.4 ± 12.5 | 36.5 ± 11.1**** | 75.2 ± 10.1 | 39.4 ± 12.3**** |
| Fasting plasma glucose (mmol/l) | 7.0 ± 1.7 | 5.6 ± 1.6** | 7.4 ± 1.5 | 5.7 ± 1.8** |
| Fasting plasma insulin (pmol/l) | 215.1 ± 120 | 56.5 ± 54.3**** | 234.9 ± 158 | 69 ± 81.3**** |
| HOMA-IR | 9.7 ± 6.2 | 2.4 ± 3.4**** | 11.2 ± 7.5 | 2.5 ± 2.8**** |
| HbA1c (%) | 6.6 ± 0.9 | 5.2 ± 0.8**** | 6.8 ± 1.1 | 5.6 ± 0.8*** |
| CRP (mg/l) | 6.4 ± 3.6 | 2.3 ± 2.2**** | 6.9 ± 4.4 | 2.7 ± 2.5**** |
| Triglycerides (mmol/l) | 2.2 ± 1.1 | 1.3 ± 0.6*** | 2.3 ± 0.9 | 1.7 ± 0.5** |
2.7. Characterization of human adipose tissue and liver samples
In addition to liver biopsies, AT samples were taken from the abdominal sc and the intraabdominal omental fat depots at defined locations during surgery. Liver and AT were analyzed as a whole (immediately frozen in liquid nitrogen after explantation) for histology (only AT) and mRNA expression analyses. Additional AT samples were collected in 37 °C PBS buffer, and adipocytes were isolated by collagenase (1 mg/ml) digestion. Immediately after collagenase digestion, adipocytes were fixed with osmic acid, incubated for 48 h at 37 °C. To determine adipocyte number, 200 μl aliquots of adipocytes were fixed with osmic acid, incubated for 48 h at 37 °C and counted in a Coulter counter (Multisizer III, Beckman Coulter GmbH, Krefeld, Germany). Basal and insulin stimulated glucose uptake into isolated adipocytes was measured as previously described [26]. For histologic analyses and measurement of macrophage count, AT samples were fixed at room temperature in 4% formaldehyde and embedded in paraffin. Five-micrometer sections were mounted on glass slides, deparaffinized in xylol, and stained for CD68 using anti-CD68 monoclonal mouse antihuman antibody (Dako, Glostrup, Denmark; close PGM1 M0876, dilution 1:100). Macrophages were identified in the adipose parenchyma when cytoplasmic staining for CD68 was present along with an identifiable mononuclear nucleus and presented as the number per 100 adipocytes (percent macrophages). In AT and liver biopsies, human TNFα, IL-1β and CCL3 mRNA expression were measured by quantitative real-time RT-PCR in a fluorescent temperature cycler using TaqMan assay-on-demand kits (Applied Biosystems), and fluorescence was detected on an ABI PRISM 7000 sequence detector (Applied Biosystems, Darmstadt, Germany). TNFα, IL-1β and CCL3 mRNA expression was calculated relative to the mRNA expression of Hypoxanthine-Guanine-Phosphoribosyltransferase 1 (HPRT1).
2.8. Assessment of insulin-stimulated signaling in vivo
Mice were fasted for 6 h at the beginning of light phase. Human recombinant insulin or saline were injected intraperitoneally (2.8 iU/kgBW), and animals were sacrificed by decapitation 20 min following injections; tissues were frozen on liquid nitrogen until further processing.
2.9. Western blot analysis
Tissues were snap frozen in liquid nitrogen following dissection then thawed and homogenized in lysis buffer using a Polytron homogenizer (T10 basic ULTRA-TURRAX, IKA). Proteins were separated by SDS-PAGE and blotted onto PVDF membranes (Bio-Rad, Munich, Germany). Membranes were incubated with 1% blocking reagent (Roche, Mannheim, Germany) for 1 h, before incubating with primary antibody (1:1,000) diluted in 0.5% blocking solution overnight at 4 °C. After three washing steps with TBS-T the membranes were incubated with the respective secondary antibodies for 1 h at room temperature (peroxidase-coupled anti-rabbit A6154, Sigma–Aldrich, 1:2000, anti-mouse A4416, Sigma–Aldrich, 1:10,000). After three washing steps, the signals were visualized using Pierce ECL Western Blotting Substrate (Perbio Science, Bonn, Germany) and exposure to chemiluminescence film (Amersham, Braunschweig, Germany).
2.10. Quantitative real-time (qRT)-PCR
Brain samples were prepared using a brain slicer (Braintree Scientific, Braintree, MA, USA). All tissues were snap frozen in liquid nitrogen after dissection. RNA was isolated from tissues using RNeasy kit (Quiagen, Hilden, Deutschland) according to the manufacturer's instructions. The RNA was reverse transcribed with a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA) and amplified using TaqMan Gene Expression Master Mix with TaqMan Assay-on-demand kits (Applied Biosystems). Relative expression was determined for target mRNA (Tnf, Mm00443260_g1; Il1b, Mm01336189_m1; Il10, Mm00439616_m1; Il6, Mm00446190_m1; Ccl3, Mm00441258_m1; Pomc, Mm00435874_m1; Agrp, Mm00475829_g1). Samples were adjusted for total mRNA content by hypoxanthine-phosphoribosyltransferase (Hprt, Mm00446968_m1) mRNA quantitative PCR. Calculations were performed using a comparative method (2−ΔΔCT).
2.11. Immunohistochemistry
Immunohistochemistry of AT was performed as previously described [3], [27], [28] with an antibody directed against F4/80 (#ab6640, Abcam). The quantification of F4/80 positive crown like structures (CLS) was performed with AxioVision 4.2 (Carl Zeiss MicroImaging GmbH, Jena, Deutschland).
Insulin peroxidase staining of the pancreas was performed as described earlier [29] using an anti-insulin antibody (DAKO A0564). Analysis was performed using digital scans by Slide Scanner SCN400 (Leica, Wetzler, Germany).
2.12. Statistics
In all figures, data are presented as mean ± sem. p-values were calculated with two-tailed unpaired or paired student's t-tests or, if more than two conditions were compared, with ANOVA followed by Bonferroni post-tests. p ≤ 0.05 was considered significant.
3. Results
3.1. Establishing a murine model of weight loss and regain
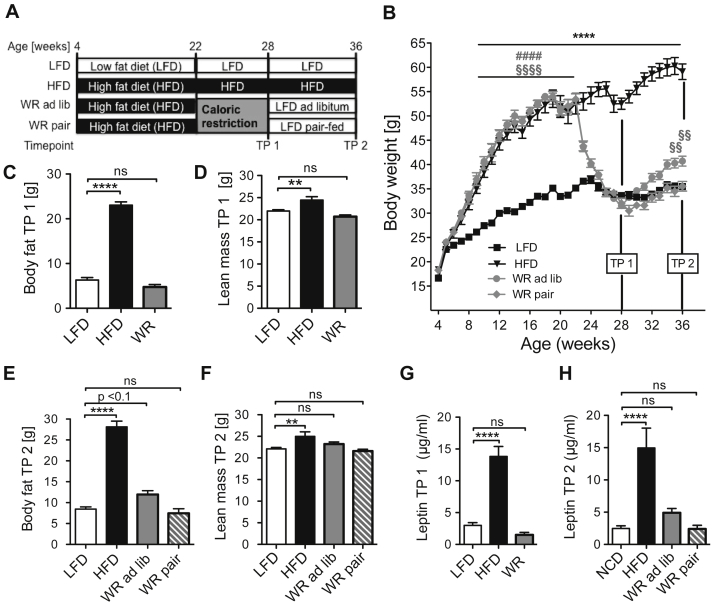
In the current study, we designed a feeding paradigm to analyze the long-term consequences of weight loss and regain, independent of genetic predisposition and individual nutritional choices. Here, wild type C57BL/6 mice were kept either on low fat diet (LFD) or high fat diet (HFD) diet. After a period of 18 weeks, by which point the HFD fed mice had developed severe obesity, a random group of obese animals was subjected to weight reduction (WR) by caloric restriction. After their body weight was reduced to the level of LFD fed controls, the WR mice were either switched to a low fat diet ad libitum (WR ad lib) or were pair-fed (WR pair) with the control group (Figure 1A). 18 weeks of HFD feeding of C57BL/6 control mice established severe obesity with more than 50% higher body weight than that of lean, normal LFD-fed control mice (Figure 1B). After 6 weeks of caloric restriction (TP 1), the WR mice reached the same body weight as the LFD-fed control mice and showed no significant differences in fat and lean mass compared to the control group (Figure 1C, D). Ad libitum exposure of these mice to LFD (WR ad lib) resulted in rapid regain of body weight and fat mass (Figure 1B, E, F). Furthermore, serum leptin concentrations, as an independent measure of adiposity, correlated well with the fat mass of the different experimental groups. While serum leptin concentrations were massively increased in HFD animals, no changes were observed at TP 1 between WR and LFD animals (Figure 1G). At TP 2, WR ad lib mice exhibited a trend towards higher serum leptin levels compared to LFD and WR pair mice (Figure 1H).
Figure 1.
Weight regain after successful weight reduction. (A) Experimental set up (B) Body weight curve (n = 16 (LFD), 7 (HFD), 10 (WR ad lib), 11 (WR pair)) (C) Lean mass after weight loss TP 1 (n = 17 (LFD), 11 (HFD), 16 (WR)) (D) Fat mass after weight loss TP 1 (n = 17 (LFD), 11 (HFD), 16 (WR)) (E) Lean after weight regain TP 2 (n = 13 (LFD), 7 (HFD), 10 (WR ad lib), 11 (WR pair)) (F) Body fat after weight regain TP 2 (n = 13 (LFD), 7 (HFD), 10 (WR ad lib), 11 (WR pair)) (G) Serum leptin levels after weight loss TP 1 (n = 8 (LFD), 7 (HFD), 8 (WR)) (H) Serum leptin levels after weight regain TP 2 (n = 13 (LFD), 7 (HFD), 10 (WR ad lib), 11 (WR pair)). §, * p < 0.05; §§, ** p < 0.01; §§§§, ****, #### p < 0.0001; (B) 2-Way-ANOVA with Bonferroni's post-test (C)–(H) 2-Way-ANOVA with Bonferroni's post-test, level of significance always compared to LFD group unless stated otherwise.
To determine whether the weight regain after weight loss can be attributed to increased food intake, the group of weight-reduced mice, which was pair-fed to the LFD-group (WR pair) was included in the experiment. In contrast to the WR ad lib group, pair-feeding did not result in an increased weight gain or alterations in lean or fat mass (Figure 1B, E, F). Collectively, these data indicate that hyperphagia might be the primary cause for weight regain after dietary body WR.
To confirm that hyperphagia was indeed the putative driver of weight regain, we directly monitored food intake in the WR ad lib mice and in animals of the LFD group. WR ad lib mice showed a daily increase in caloric intake of up to 16% compared to the LFD control group (Suppl. Figure 1a). Importantly, this increase in caloric intake was not limited to the first week of re-feeding, but persisted throughout the analyses up to 8 weeks following WR (Suppl. Figure 1a).
To identify putative molecular drivers of increased food intake following initially successful WR, we analyzed the mRNA expression of established hypothalamic regulators of food intake, namely Proopiomelanocortin (Pomc) and Agouti-related peptide (Agrp) in the different groups of animals. At the end of caloric restriction period, a higher ratio of Agrp/Pomc mRNA expression (Suppl. Figure 1b) was observed in the WR group, consistent with the perception of negative energy balance in these animals upon WR. These changes, however, did not persist beyond re-feeding, where no differences could be seen between the groups (Suppl. Figure 1c).
Despite hyperphagia being the primary cause for the observed weight regain, we sought to specifically investigate potential changes in energy expenditure under the different experimental conditions. This was of specific importance since decreases in energy expenditure are thought to underlie weight regain in humans [16], [17], [18], [19]. To this end, we performed indirect calorimetry in each group of animals. At TP 1, WR mice showed an 11% decrease of total energy expenditure during the light phase when compared to the LFD control group (Suppl. Figure 2a). However, neither WR pair nor WR ad lib mice showed any alteration in energy expenditure 8 weeks after WR at TP 2 (Suppl. Figure 2b), further confirming the pivotal role of hyperphagia in weight regain. Moreover, whereas HFD fed mice showed a strong reduction in physical activity throughout the experiment (Suppl. Figure 2c, d), after WR, WR pair and WR ad lib mice displayed a similar level of activity compared to LFD controls (Suppl. Figure 2d).
Taken together, even though energy expenditure was reduced during weight loss in mice, these changes did not persist beyond WR even in the WR pair group. Furthermore, activity was decreased in obesity but completely restored to the level of LFD-fed mice after WR.
3.2. Glucose homeostasis after weight loss
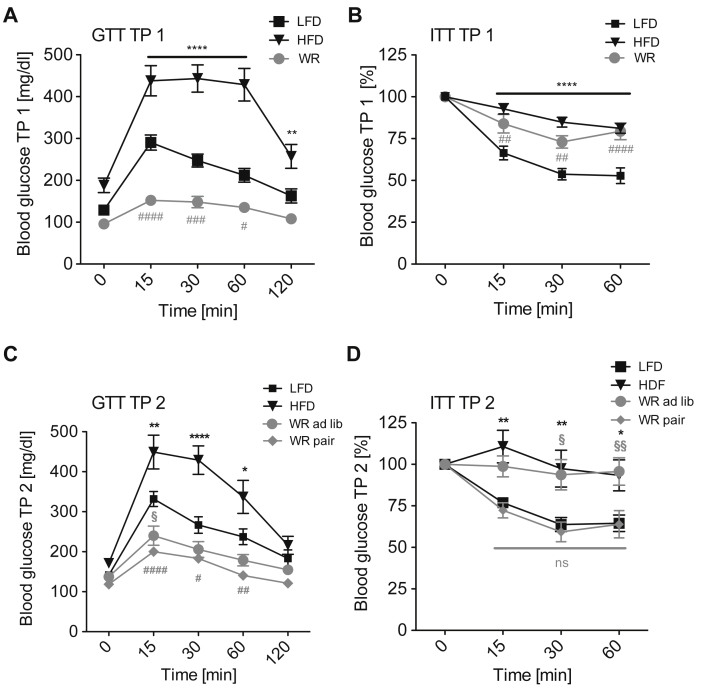
Next, we determined the effects of WR and regain on glucose homeostasis and insulin action. HFD feeding strongly impaired glucose clearance as reflected by increased blood glucose concentrations during glucose tolerance tests (GTT) (Figure 2A). WR however, potently ameliorated glucose tolerance (Figure 2A). Surprisingly, WR mice even displayed improved glucose clearance compared to the lean LFD-fed group (Figure 2A). In contrast, systemic insulin sensitivity remained decreased despite WR to an extent similar to HFD-fed, obese animals (Figure 2B).
Figure 2.
Insulin and glucose tolerance after weight reduction and regain. (A) Glucose tolerance after weight loss TP 1 (n = 16 (LFD), 10 (HFD), 15 (WR)) (B) Insulin tolerance after weight loss TP 1 (n = 15 (LFD), 14 (HFD), 15 (WR)) (C) Glucose tolerance after weight regain TP 2 (n = 12 (LFD), 8 (HFD), 10 (WR ad lib), 11 (WR pair)) (D) Insulin tolerance after weight regain TP 2 (n = 12 (LFD), 10 (HFD), 9 (WR ad lib), 11 (WR pair)). *, #, § p < 0.05; **, ##, §§ p < 0.01; ***, ###, §§§ p < 0.001; ****, #### p < 0.0001; 2-Way-ANOVA with Bonferroni's post-test, significance always compared to LFD group.
At TP 2, the improved glucose tolerance in WR mice persisted compared to the LFD controls, regardless of whether animals were WR ad lib or WR pair (Figure 2C). However, whereas insulin sensitivity of WR ad lib mice was similar to that of HFD-fed mice, insulin sensitivity was improved in WR pair mice to the level of the LFD group (Figure 2D). Thus, although glucose tolerance improves rapidly upon weight loss, the improvement of systemic insulin sensitivity requires long-term maintenance of body weight reduction.
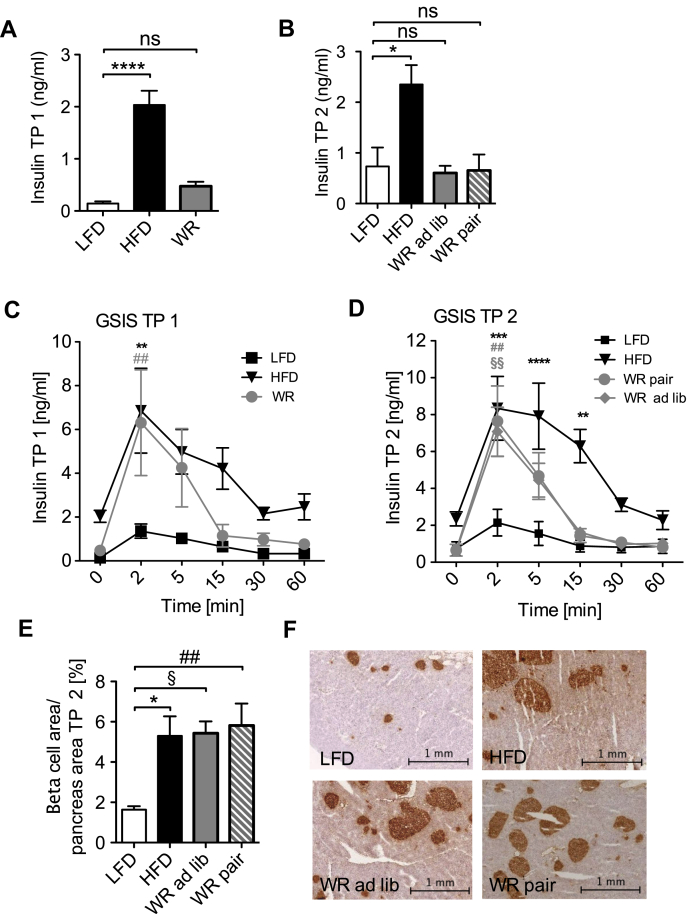
To further investigate the observed discrepancy between systemic glucose tolerance and systemic insulin sensitivity, we first determined fasted serum insulin concentrations in the different groups of mice. Whereas HFD feeding led to a strong increase of fasted serum insulin concentrations compared to LFD-fed mice, WR largely normalized serum insulin concentrations to the level of LFD mice (Figure 3A). Moreover, re-feeding did not change fasted serum insulin concentrations, either in the WR ad lib or in the WR pair group (Figure 3B).
Figure 3.
Insulin secretion and pancreatic beta-cell islets after weight loss. (A) Fasted serum insulin levels TP 1 (n = 7 (LFD), 7 (HFD), 8 (WR)) (B) Fasted serum insulin levels TP 2 (n = 5 (LFD), 7 (HFD), 6 (WR ad lib), 7 (WR pair)) (C) Glucose stimulated insulin secretion after weight loss TP 1 (n = 7 (LFD), 7 (HFD), 8 (WR)) (D) Glucose stimulated insulin secretion after weight regain TP 2 (n = 5 (LFD), 7 (HFD), 6 (WR ad lib), 7 (WR pair)) (E) Area of pancreatic beta-cells relative to total pancreatic area TP 2 (n = 5 (LFD), 4 (HFD), 5 (WR ad lib), 5 (WR pair)) (F) Insulin peroxidase immunohistochemistry of the pancreas (images are representative for each group). *, §, # p < 0.05; **, §§, ## p < 0.01; *** p < 0.001; **** p < 0.0001; (C)–(D) 2-Way-ANOVA with Bonferroni's post-test, (A), (B) & (E) 1-Way-ANOVA with Bonferroni's post-test, level of significance always compared to LFD group unless displayed otherwise.
Next, we performed an analysis of glucose-stimulated insulin secretion (GSIS) to test whether the dynamic regulation of insulin release might be altered in obesity, WR and regain. Compared to LFD-fed mice, HFD-fed mice exhibited increases both in first phase and second phase of insulin secretion during GSIS at TP 1 and TP 2, consistent with a compensatory hypersecretion of insulin in the face of massive insulin resistance (Figure 3C, D). However, compared to LFD-fed mice, WR mice also exhibited significantly elevated serum insulin levels during the first phase of GSIS, similar to what was observed in obese HFD-fed mice (Figure 3C), while the prolonged second phase of insulin secretion was comparable to that of LFD-fed mice. Importantly, these changes persisted upon re-feeding in both WR ad lib and WR pair mice (Figure 3D). In line with this, immunohistological analyses revealed increased β-cell mass in WR ad lib and WR pair mice to an extent observed in HFD-fed mice compared to LFD mice (Figure 3F).
These experiments indicate that upon weight loss and regain, pancreatic β-cell hyperplasia persists, and the resulting enhancement of insulin release likely contributes to the observed increases in glucose tolerance even when systemic insulin sensitivity is still reduced in WR animals at TP 1. In addition, at TP 2 enhanced insulin sensitivity in WR-pair mice contributes to improved glucose metabolism.
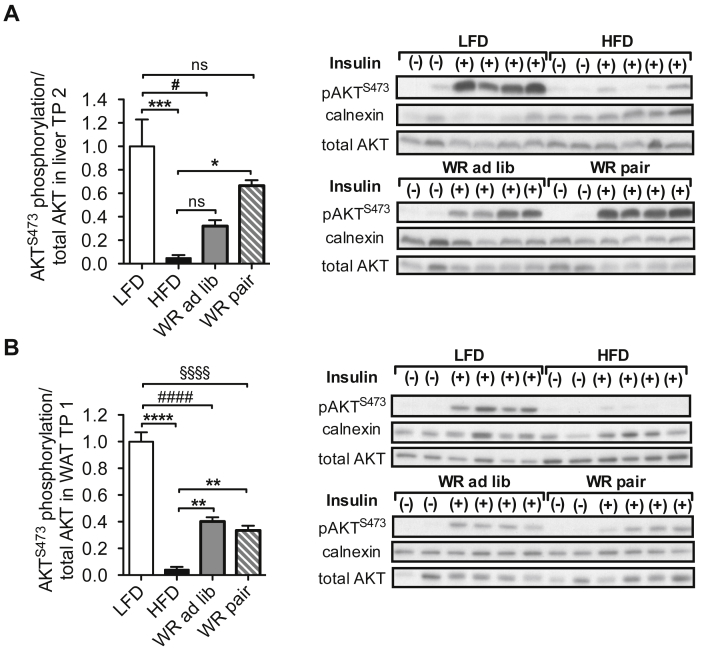
3.3. Tissue specific persistence of insulin resistance upon weight loss and regain
Next, we investigated how tissue-specific changes in insulin action may contribute to the differential manifestation of insulin resistance in WR mice, which had either retained reduced body weight (WR pair) or regained some of their body weight (WR ad lib). Therefore, we assessed activation of the insulin signaling cascade in liver and white adipose tissue (WAT) in the different groups of mice. As demonstrated previously [30], HFD feeding almost completely abolished insulin-stimulated hepatic AKT phosphorylation compared to LFD mice (Figure 4A). WR and subsequent pair-feeding largely restored insulin-stimulated AKT phosphorylation in the liver, which was no longer significantly reduced compared to LFD animals, whereas WR ad lib mice still displayed some degree of compromised hepatic insulin signaling (Figure 4A).
Figure 4.
Tissue specific persistence of insulin resistance after weight loss and regain. (A) AKT S473 phosphorylation relative to total AKT after insulin stimulation in liver tissue (n = 4 (LFD), 4 (HFD), 4 (WR ad lib), 4 (WR pair)) (B) AKT S473 phosphorylation relative to total AKT after insulin stimulation in WAT (n = 4 (LFD), 4 (HFD), 4 (WR ad lib), 4 (WR pair)). #, § p < 0.05; ** p < 0.01, *** p < 0.0001, ****, §§§§, #### p < 0.00001; 1-Way-ANOVA with Bonferroni's post-test.
In line with the observed effects of obesity on insulin signaling in liver, insulin-stimulated AKT phosphorylation was strongly reduced in WAT of obese, HFD-fed mice (Figure 4B). However, in contrast to what was observed in liver, in WAT, WR and subsequent pair-feeding only improved insulin sensitivity in WAT to a minor extent (Figure 4B). Here, WR pair mice showed similar insulin-stimulated AKT phosphorylation as WR ad lib mice (Figure 4B). Collectively, these results demonstrate that pair-feeding-mediated weight control after WR efficiently and persistently restores hepatic insulin action to the level of LFD mice. In contrast, WAT insulin sensitivity remained impaired after weight reduction even independent of weight regain.
3.4. Adipose tissue inflammation persists following weight loss
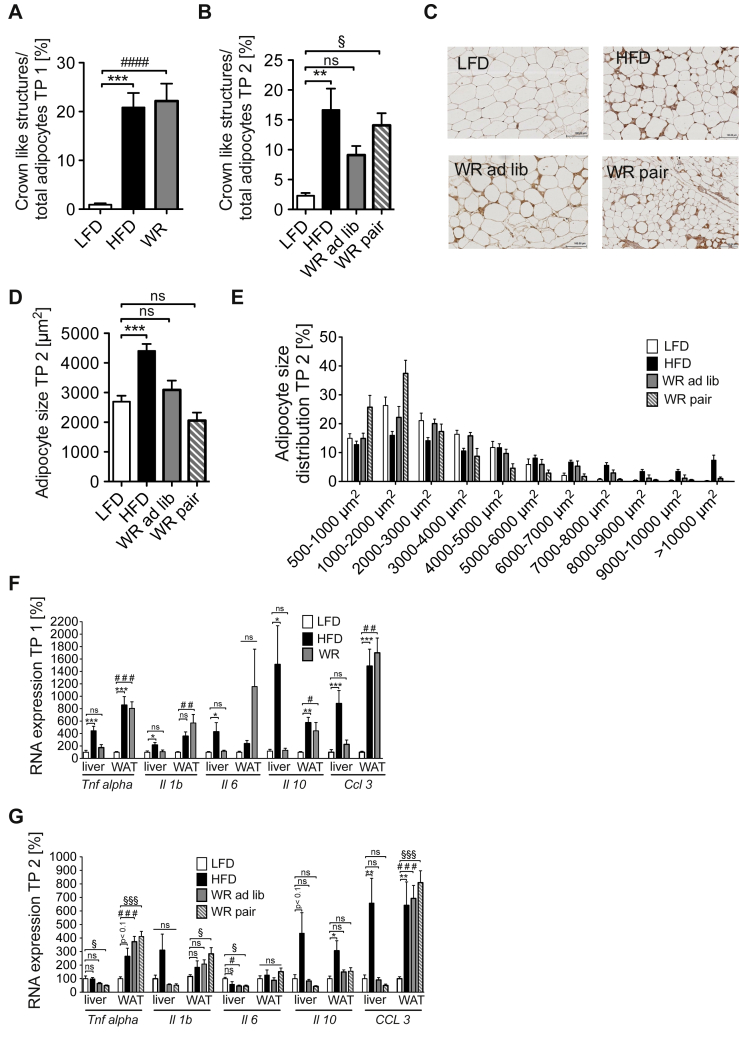
Obesity and insulin resistance are associated with the development of a chronic, low-grade inflammatory state [5], [6], [7], [31], [32], [33], [34], [35]. We first sought to investigate the microscopic changes in AT, analyzing adipocyte morphology as well as macrophage infiltration upon obesity development and subsequent weight loss. Therefore, we performed F4/80 immunohistochemistry stainings of WAT in the different groups of animals. While LFD mice showed only very few crown like structures compared to HFD animals, WR mice displayed a similar macrophage infiltration pattern as obese mice, with crown like structures being elevated 20-fold compared to the control (LFD) group (Figure 5A). Interestingly, macrophage infiltration was also elevated to the same degree in WR ad lib and WR pair mice as compared to HFD-fed obese mice even 8 weights after maintained weight loss (Figure 5B, C). At the same time, the average adipocyte size correlated well with the body weight in all groups, where HFD animals showed larger adipocytes compared to LFD animals (Figure 5C, D, E). In contrast to macrophage infiltration, however, WR normalized adipocyte size in both the WR ad lib as well as the WR pair mice (Figure 5C, D, E). These data indicate that analogous to persistent insulin resistance after prolonged weight reduction and/or regain, obesity-associated macrophage infiltration persists within AT, despite the fact that adipocyte size distribution is reduced comparable to the pre-obese state.
Figure 5.
Inflammation of adipose tissue persists after weight loss. (A) Crown like structures in white adipose tissue relative to total adipocytes after weight loss TP 1 (n = 7 (LFD), 7 (HFD), 8 (WR)) (B) Crown like structures in white adipose tissue relative to total adipocytes after weight regain TP 2 (n = 6 (LFD), 7 (HFD), 4 (WR ad lib), 7 (WR pair)) (C) Immunohistochemical staining of F4/80 positive cell in white adipose tissue after weight regain TP 2 (images representative for each group) (D) Average adipocytes size TP 2 (n = 6 (LFD), 7 (HFD), 5 (WR ad lib), 7 (WR pair)) (E) Adipocyte-size distribution TP 2 (n = 6 (LFD), 7 (HFD), 5 (WR ad lib), 7 (WR pair)) (F) Quantitative RT-PCR analysis of gene expression in WAT and liver TP 1 (n = 6–8 in each group)) (G) Quantitative RT-PCR analysis of gene expression in WAT and liver TP 2 (n = 6–8 in each group). *, #, § p < 0.05; **, ## p < 0.01; ***, ###, §§§ p < 0.001; 1-Way-ANOVA with Bonferroni's post-test.
To further investigate the persistence of low-grade inflammation of WAT after WR, we analyzed the expression of inflammatory marker genes in liver and WAT of the different dietary groups. HFD-feeding robustly induced expression of Tnf, Il1b, Il6, Il10, Ccl2 and Ccl3 in liver and WAT compared to LFD-feeding, indicating the development of obesity-associated inflammation in these tissues (Figure 5F). Interestingly, whereas WR efficiently reduced expression of the analyzed genes in liver, inflammatory gene expression was unaltered in WAT of WR mice compared to HFD-fed animals (Figure 5F).
Moreover, neither ad libitum nor pair re-feeding of WR mice led to substantial changes in hepatic and adipose tissue inflammatory gene expression at TP 2 compared to WR mice at TP 1 (Figure 5G). An exception was Il10, whose expression in WAT appeared to be sensitive to prolonged weight loss, showing a reduction in WR ad lib as well as WR pair mice (Figure 5G). These data indicate that analogous to persistent insulin resistance after prolonged weight reduction and/or regain, obesity-associated inflammatory gene expression changes were largely irreversible in WAT but not in liver.
3.5. Differential regulation of inflammation in liver and WAT upon extensive weight loss in humans
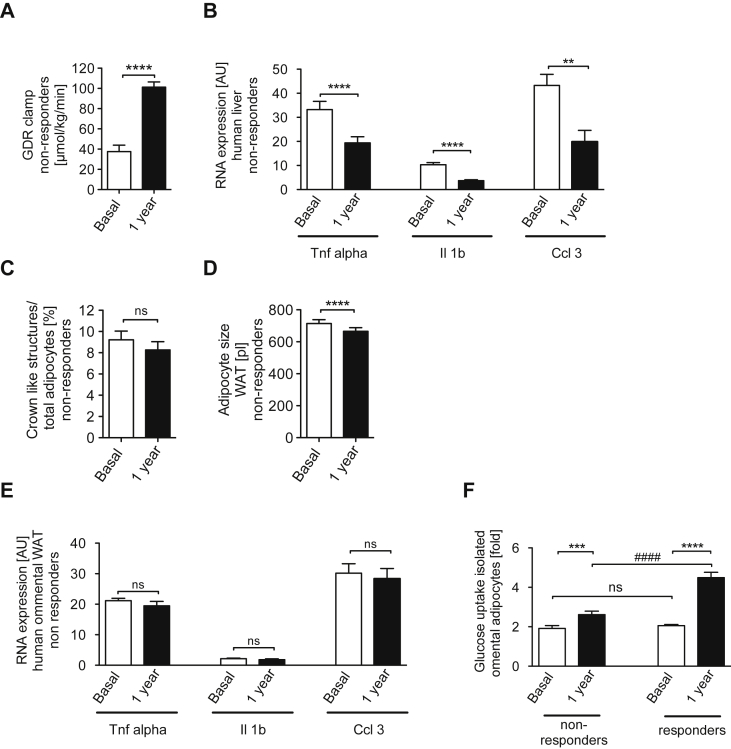
Having defined the striking difference in reversibility of obesity-induced inflammation and insulin resistance in liver and WAT upon weight loss in mice, we next aimed to investigate whether this phenomenon might also be conserved in humans. To this end, we recruited a cohort of obese human subjects, who underwent a two-step bariatric surgery. From a total of 55 individuals, we selected 23 obese patients, in which extensive weight loss did not significantly affect omental adipose tissue macrophage (ATM) counts. Importantly, biopsies were obtained both from liver and WAT before weight loss and one year after bariatric surgery. The subjects in this study had a mean baseline BMI of 53.6 ± 7.3 kg/m2 and exhibited profound insulin resistance, evidenced by a significant elevation of HOMA-IR (10.5 ± 6.8) (Table 1). Both at baseline and one year after the first step bariatric surgery, there was no significant difference in key parameters of anthropometry, glucose and lipid metabolism, insulin sensitivity or systemic inflammation between individuals with or without reduction in omental ATM counts (Table 1). In both groups, bariatric surgery successfully reduced body weight and fat mass one year after surgery (Table 1). In addition, systemic insulin sensitivity improved, as evidenced by more than doubling the systemic glucose disposal rate during euglycemic hyperinsulinemic clamps (Figure 6A) or by improved HOMA-IR (Table 1). As shown for the subgroup of individuals without significant omental ATM reduction, this improvement was accompanied by a significant reduction of hepatic _TNFα_-, _IL-1ß_- and _CCL-3_-mRNA-expression one year after the initial bariatric surgery (Figure 6B). In contrast, although adipocyte size was significantly reduced in this subgroup one year after surgery (Figure 6C), there was no significant reduction of ATM number and crown-like structures in WAT detectable after extensive weight loss (Figure 6D). Consistent with unaltered crown like structures (CLS) following weight loss, also gene expression of _TNFα_-, _IL-1ß_- and CCL-3 in WAT remained unaltered compared to the pre-weight loss analysis (Figure 6E). In the subgroup of obese individuals with significant changes in omental ATM numbers one year after bariatric surgery, we found a significant decrease in CLS of omental AT in response to weight loss (baseline: 11.3 ± 2.5% versus 1 year after surgery: 3.8 ± 3.5%, p < 0.001). Finally, the obese subgroups with or without a reduction in omental ATMs also displayed significant differences in insulin sensitivity of isolated adipocytes. Consistent with retained AT inflammation, despite massive, maintained weight loss, insulin-stimulated glucose uptake in adipocytes increased only by 37% following weight loss, compared to a 280% increase in systemic glucose disposal in the group with persistent AT inflammation (Figure 6A, F). By contrast, in the subgroup of obese patients with a significant reduction of omental CLS (n = 32), insulin-stimulated glucose uptake in adipocytes increased by ∼115% (Figure 6F). This distinction in obese individuals, who either respond with or without reduction of omental ATMs to extensive weight, suggests that in humans additional factors may contribute to the regulation of adipose tissue inflammation compared to those in mice with calorie restriction induced weight loss. However, the existence of obese individuals who do not respond to weight loss with significant reduction in visceral ATM number indicates that, similar to what we observed in a mouse model of dietary weight loss, a substantial proportion of obese human patients display a striking dichotomy between hepatic and AT inflammation and insulin sensitivity upon long term weight reduction, which will require further stratification of these patients in the future.
Figure 6.
Differential regulation of inflammation in liver and WAT upon prolonged weight loss in humans. (A) Hyperinsulinemic euglycemic clamp in non-responders before and 1 year after bariatric surgery induced weight loss (n = 11) (B) Quantitative RT-PCR analysis of gene expression in liver of non-responders before and 1 year after bariatric surgery (n = 23) (C) Adipocyte size of non-responders WAT before and 1 year after bariatric surgery (n = 23) (D)) Crown like structures in WAT of non-responders before and 1 year after bariatric surgery (n = 23) (E) Quantitative RT-PCR analysis of gene expression in WAT of non-responders before and 1 year after bariatric surgery (n = 23) (F) Insulin stimulated Glucose uptake into isolated omental adipocytes (non responders: n = 23; responders: n = 32). * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 paired student's t-test; #### p < 0.0001 unpaired student's t-test.
4. Discussion
In the current study, we introduced a feeding paradigm of high fat diet feeding and caloric restriction to assess the dynamics and mechanisms of weight regain after weight reduction in obesity. We show that weight regain after weight loss occurs independently of genetic predispositions, even if alimentation with a western high-fat diet is permanently discontinued after weight loss. In contradiction with the concept of long-term reduced thermogenesis after dieting, we found that energy expenditure is likely to have only a minor impact on weight regain, while persistent alterations in energy intake play a key role. In fact, even though a reduction of energy expenditure was present during weight reduction, this decrease was only transient. Changes of hypothalamic neuropeptide expression might be responsible for the initiation of hyperphagia, as we observed a shift towards an increased ratio of orexigenic AgRP-expression versus anorexigenic POMC-expression upon acute weight loss. However, it remains elusive which central or peripheral mechanisms are responsible for the enduring increase in food intake observed following weight loss.
In addition to assessing the principles of weight regain, our animal model offered the unique possibility to analyze the extent to which the complex metabolic and inflammatory changes seen in obesity are reversible upon weight reduction and maintenance. So far, little attention has been paid to the degree to which obese patients who have lost weight are still influenced by metabolic or inflammatory alterations developed during years of obesity. Tirosh et al. showed that an increased BMI in adolescents displays a risk for coronary heart disease independent of the BMI present before diagnosis [36]. Consistent with the notion that obesity-associated changes persist beyond weight reduction and are able to increase cardiovascular mortality, the Look Ahead Study failed to show a decrease in cardiovascular mortality after weight loss to treat obese adults with type 2 diabetes. Even though body weight, and glycated hemoglobin and waist circumference improved significantly, cardiovascular mortality remained unchanged [12].
In the present study, we were able to demonstrate that many of the complex changes in obesity are only partially reversed upon weight loss and are still present after a period of weight maintenance on a low fat diet. Thus, despite a rapid improvement in glucose tolerance after weight loss, systemic insulin resistance persists and recovers only when reduced body weight is maintained long-term. While basal fasted insulin levels are suppressed effectively, glucose stimulated insulin secretion is massively elevated, and pancreatic β-cell islets remain hyperplastic. This is consistent with the detection of robust β-cell hyperplasia in human obesity [37] and the notion of increased insulin release after weight loss in some human weight loss studies [38], [39]. However, our findings may be confounded by the fact that both during weight reduction and weight maintenance in the WR-pair animals food was provided in one meal at the beginning of light phase. It has been previously demonstrated that meal feeding affects glucose-stimulated insulin secretion [40]. Thus, the feeding pattern independent of weight loss may have affected pancreatic β-cell responses in our experiments. Finally, the results of our study are consistent with a previous report of improved glucose tolerance in fat mass matched and caloric restricted mice [41]. Nevertheless, currently employed methods for assessment of glucose metabolism following weight reduction, i.e. by glucose tolerance testing and detection of fasted glucose and insulin levels, might in fact lead clinicians to over-interpret the metabolic benefits of weight reduction.
Most strikingly, in addition to the changes seen in systemic insulin tolerance and insulin secretion, we were able to reveal complex alterations in tissue specific insulin sensitivity and inflammation after weight loss both in mice and humans. While hepatic insulin sensitivity is recovered almost completely following weight loss in the mouse model, this is paralleled by a complete resolution of hepatic pro-inflammatory gene expression both in weight-reduced mice and humans. In contrast, visceral WAT in mice and in a substantial proportion of human remains insulin resistant, even after maintenance of weight reduction. Interestingly, decreased insulin sensitivity in visceral WAT is paralleled by sustained tissue inflammation, which is observed in both mice and humans. Thus, despite the fundamentally different modalities to achieve weight loss, caloric restriction in mice and bariatric surgery in humans, each supports a consistent and unifying picture of evolutionary conserved, tissue-specific inflammatory mechanisms during weight loss. Surprisingly, few studies have addressed the long-term consequences of weight loss on inflammatory reactions in human obesity. In addition, the majority of these studies have only reported circulating concentrations of inflammatory mediators or analyzed gene expression in sc WAT. The results of these studies have been partly controversial, some showing improvements of sc WAT inflammation [42], [43], [44], [45], [46], while others failed to detect weight-loss-induced resolution of sc WAT inflammation [47]. Cancello and coworkers demonstrated that macrophage infiltration into subcutaneous AT was significantly decreased in response to weight loss, 3 months after bariatric surgery [46]. Interestingly, here we identified a subgroup of extremely obese and insulin resistant individuals (∼42%), in whom extensive weight loss and significant improvements of insulin sensitivity after bariatric surgery were not associated with significant reduction in both visceral and sc AT macrophage infiltration. Of course, we have to acknowledge that in the previous study, AT biopsies had been taken as early as 3 months after bariatric surgery, most likely during a weight reduction phase, whereas our observations are based on a follow-up at one year, presumably at a weight maintenance phase. We can, therefore, not exclude, that reduction of AT inflammation also occurred intermediately, during the early response phase to bariatric surgery in our study cohort. However, this appears unlikely in light of the results obtained in our mouse model. At least for this human subgroup the consistent, robust maintenance of inflammation in visceral WAT is consistent with maintained insulin resistance in WAT, but surprisingly not on a systemic level. Thus, future studies will have to investigate the long-term physiological consequences distinguishing both subgroups of patients and will clearly have to address the molecular basis, how immune cell infiltration in visceral WAT remains activated despite a normalization of fat cell size upon weight loss.
Footnotes
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Finucane M.M., Stevens G.A., Cowan M.J., Danaei G., Lin J.K., Paciorek C.J. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens G.A., Singh G.M., Lu Y., Danaei G., Lin J.K., Finucane M.M. National, regional, and global trends in adult overweight and obesity prevalences. Population Health Metrics. 2012;10:22. doi: 10.1186/1478-7954-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.W.H. Organisation, http://www.who.int/mediacentre/factsheets/fs311/en/index.html, 2013 (n.d.).
- 4.Flegal K.M., Graubard B.I., Williamson D.F., Gail M.H. Cause-specific excess deaths associated with underweight, overweight, and obesity. Journal of American of Medical Association. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 5.Leblanc E.S., O'Connor E., Whitlock E.P., Patnode C.D., Kapka T. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2011;155:434–447. doi: 10.7326/0003-4819-155-7-201110040-00006. [DOI] [PubMed] [Google Scholar]
- 6.Tuomilehto J., Lindström J., Eriksson J.G., Valle T.T., Hämäläinen H., Ilanne-Parikka P. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. The New England Journal of Medicine. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 7.Stevens V.J., Obarzanek E., Cook N.R., Lee I.M., Appel L.J., Smith West D. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Annals of Internal Medicine. 2001;134:1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 8.Shaw K., Gennat H., O'Rourke P., Del Mar C. Exercise for overweight or obesity. Cochrane Database of Systematic Reviews. 2006:CD003817. doi: 10.1002/14651858.CD003817.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thorogood A., Mottillo S., Shimony A., Filion K.B., Joseph L., Genest J. Isolated aerobic exercise and weight loss: a systematic review and meta-analysis of randomized controlled trials. American Journal of Medicine. 2011;124:747–755. doi: 10.1016/j.amjmed.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 10.Sacks F.M., Bray G.A., Carey V.J., Smith S.R., Ryan D.H., Anton S.D. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. The New England Journal of Medicine. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shai I., Schwarzfuchs D., Henkin Y., Shahar D.R., Witkow S., Greenberg I. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. The New England Journal of Medicine. 2008;359:229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 12.The Look AHEAD Research Group Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. The New England Journal of Medicine. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bray G.A., Bouchard C. CRC Press; 2003. Handbook of obesity. [Google Scholar]
- 14.Wing R.R., Hill J.O. Successful weight loss maintenance. Annual Review of Nutrition. 2001;21:323–341. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- 15.Wadden T.A., Stunkard A.J. Guilford Press; 2004. Handbook of obesity treatment. [Google Scholar]
- 16.Leibel R.L., Rosenbaum M., Hirsch J. Changes in energy expenditure resulting from altered body weight. The New England Journal of Medicine. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 17.Rosenbaum M., Hirsch J., Gallagher D.A., Leibel R.L. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. American Journal of Clinical Nutrition. 2008;88:906–912. doi: 10.1093/ajcn/88.4.906. [DOI] [PubMed] [Google Scholar]
- 18.Camps S.G.J.A., Verhoef S.P.M., Westerterp K.R. Weight loss, weight maintenance, and adaptive thermogenesis. American Journal of Clinical Nutrition. 2013;97:990–994. doi: 10.3945/ajcn.112.050310. [DOI] [PubMed] [Google Scholar]
- 19.Astrup A., Gøtzsche P.C., van de Werken K., Ranneries C., Toubro S., Raben A. Meta-analysis of resting metabolic rate in formerly obese subjects. American Journal of Clinical Nutrition. 1999;69:1117–1122. doi: 10.1093/ajcn/69.6.1117. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz A., Kuk J.L., Lamothe G., Doucet E. Greater than predicted decrease in resting energy expenditure and weight loss: results from a systematic review. Obesity (Silver Spring) 2012;20:2307–2310. doi: 10.1038/oby.2012.34. [DOI] [PubMed] [Google Scholar]
- 21.Ochner C.N., Barrios D.M., Lee C.D., Pi-Sunyer F.X. Biological mechanisms that promote weight regain following weight loss in obese humans. Physiology & Behavior. 2013;120:106–113. doi: 10.1016/j.physbeh.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maclean P.S. Enhanced metabolic efficiency contributes to weight regain after weight loss in obesity-prone rats. AJP: Regulatory, Integrative and Comparative Physiology. 2004;287:R1306–R1315. doi: 10.1152/ajpregu.00463.2004. [DOI] [PubMed] [Google Scholar]
- 23.Maclean P.S. Metabolic adjustments with the development, treatment, and recurrence of obesity in obesity-prone rats. AJP: Regulatory, Integrative and Comparative Physiology. 2004;287:R288–R297. doi: 10.1152/ajpregu.00010.2004. [DOI] [PubMed] [Google Scholar]
- 24.Turpin S.M., Nicholls H.T., Willmes D.M., Mourier A., Brodesser S., Wunderlich C.M. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metabolism. 2014;20:678–686. doi: 10.1016/j.cmet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Kannt A., Pfenninger A., Teichert L., Tönjes A., Dietrich A., Schön M.R. Association of nicotinamide-N-methyltransferase mRNA expression in human adipose tissue and the plasma concentration of its product, 1-methylnicotinamide, with insulin resistance. Diabetologia. 2015;58:799–808. doi: 10.1007/s00125-014-3490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kloting N., Fasshauer M., Dietrich A., Kovacs P., Schon M.R., Kern M. Insulin-sensitive obesity. AJP: Endocrinology and Metabolism. 2010;299:E506–E515. doi: 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- 27.Mauer J., Chaurasia B., Plum L., Quast T., Hampel B., Blüher M. Myeloid cell-restricted insulin receptor deficiency protects against obesity-induced inflammation and systemic insulin resistance. PLoS Genetics. 2010;6:e1000938. doi: 10.1371/journal.pgen.1000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang R., Patel D., Morris J.J., Rutschman R.L., Murray P.J. Shaping gene expression in activated and resting primary macrophages by IL-10. Journal of Immunology. 2002;169:2253–2263. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 29.Vogt M.C., Paeger L., Hess S., Steculorum S.M., Awazawa M., Hampel B. Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell. 2014;156:495–509. doi: 10.1016/j.cell.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kornfeld J.-W., Baitzel C., Könner A.C., Nicholls H.T., Vogt M.C., Herrmanns K. Obesity-induced overexpression of miR-802 impairs glucose metabolism through silencing of Hnf1b. Nature. 2013;494:111–115. doi: 10.1038/nature11793. [DOI] [PubMed] [Google Scholar]
- 31.Kahn S.E., Hull R.L., Utzschneider K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 32.Arkan M.C., Hevener A.L., Greten F.R., Maeda S., Li Z.-W., Long J.M. IKK-beta links inflammation to obesity-induced insulin resistance. Nature Medicine. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 33.Wunderlich F.T., Ströhle P., Könner A.C., Gruber S., Tovar S., Brönneke H.S. Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. Cell Metabolism. 2010;12:237–249. doi: 10.1016/j.cmet.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Xu H., Barnes G.T., Yang Q., Tan G., Yang D., Chou C.J. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. Journal of Clinical Investigation. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mauer J., Chaurasia B., Goldau J., Vogt M.C., Ruud J., Nguyen K.D. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nature Immunology. 2014;15:423–430. doi: 10.1038/ni.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tirosh A., Shai I., Afek A., Dubnov-Raz G., Ayalon N., Gordon B. Adolescent BMI trajectory and risk of diabetes versus coronary disease. The New England Journal of Medicine. 2011;364:1315–1325. doi: 10.1056/NEJMoa1006992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saisho Y., Butler A.E., Manesso E., Elashoff D., Rizza R.A., Butler P.C. β-cell mass and turnover in humans: effects of obesity and aging. Diabetes Care. 2013 Jan;36(1):111–117. doi: 10.2337/dc12-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weyer C., Hanson K., Bogardus C., Pratley R.E. Long-term changes in insulin action and insulin secretion associated with gain, loss, regain and maintenance of body weight. Diabetologia. 2000;43:36–46. doi: 10.1007/s001250050005. [DOI] [PubMed] [Google Scholar]
- 39.Holte J., Bergh T., Berne C., Wide L., Lithell H. Restored insulin sensitivity but persistently increased early insulin secretion after weight loss in obese women with polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism. 1995;80:2586–2593. doi: 10.1210/jcem.80.9.7673399. [DOI] [PubMed] [Google Scholar]
- 40.Vahl T.P., Aulinger B.A., Smith E.P., Drazen D.L., Ulrich-Lai Y., Seeley R.J. Meal feeding improves oral glucose tolerance in male rats and causes adaptations in postprandial islet hormone secretion that are independent of plasma incretins or glycemia. AJP: Endocrinology and Metabolism. 2014;307:E784–E792. doi: 10.1152/ajpendo.00339.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirchner H., Hofmann S.M., Fischer-Rosinský A., Hembree J., Abplanalp W., Ottaway N. Caloric restriction chronically impairs metabolic programming in mice. Diabetes. 2012;61:2734–2742. doi: 10.2337/db11-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montecucco F., Lenglet S., Quercioli A., Burger F., Thomas A., Lauer E. Gastric bypass in morbid obese patients is associated with reduction in adipose tissue inflammation via N-oleoylethanolamide (OEA)-mediated pathways. Thrombosis and Haemostasis. 2015;113:838–850. doi: 10.1160/TH14-06-0506. [DOI] [PubMed] [Google Scholar]
- 43.Trachta P., Dostalova I., Haluzikova D., Kasalicky M., Kavalkova P., Drapalova J. Laparoscopic sleeve gastrectomy ameliorates mRNA expression of inflammation-related genes in subcutaneous adipose tissue but not in peripheral monocytes of obese patients. Molecular and Cellular Endocrinology. 2014;383:96–102. doi: 10.1016/j.mce.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 44.Lakhdar N., Denguezli M., Zaouali M., Zbidi A., Tabka Z., Bouassida A. Diet and diet combined with chronic aerobic exercise decreases body fat mass and alters plasma and adipose tissue inflammatory markers in obese women. Inflammation. 2013;36:1239–1247. doi: 10.1007/s10753-013-9661-8. [DOI] [PubMed] [Google Scholar]
- 45.Moschen A.R., Molnar C., Geiger S., Graziadei I., Ebenbichler C.F., Weiss H. Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Gut. 2010;59:1259–1264. doi: 10.1136/gut.2010.214577. [DOI] [PubMed] [Google Scholar]
- 46.Cancello R., Henegar C., Viguerie N., Taleb S., Poitou C., Rouault C. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 47.Only C-reactive protein, but not TNF-alpha or IL6, reflects the improvement in inflammation after bariatric surgery. Obesity Surgery. 2012;22:131–139. doi: 10.1007/s11695-011-0546-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.