CGG Repeat associated non-AUG translation utilizes a cap-dependent, scanning mechanism of initiation to produce toxic proteins (original) (raw)
. Author manuscript; available in PMC: 2017 Apr 21.
SUMMARY
Repeat associated non-AUG (RAN) translation produces toxic polypeptides from nucleotide repeat expansions in the absence of an AUG start codon and contributes to neurodegenerative disorders such as ALS and Fragile X Tremor/Ataxia Syndrome (FXTAS). How RAN translation occurs is unknown. Here we define the critical sequence and initiation factors that mediate CGG repeat RAN translation in the 5′ leader of Fragile X mRNA, FMR1. Our results reveal that CGG RAN translation is 30–40% as efficient as AUG initiated translation, is m7G-cap and eIF4E-dependent, requires the eIF4A helicase, and is strongly influenced by repeat length. However, it displays a dichotomous requirement for initiation site selection between reading frames, with initiation in the +1 frame, but not the +2 frame, occurring at near-cognate start codons upstream of the repeat. These data support a model where RAN translation at CGG repeats utilizes cap-dependent ribosomal scanning, yet bypasses normal requirements for start codon selection.
Graphical abstract
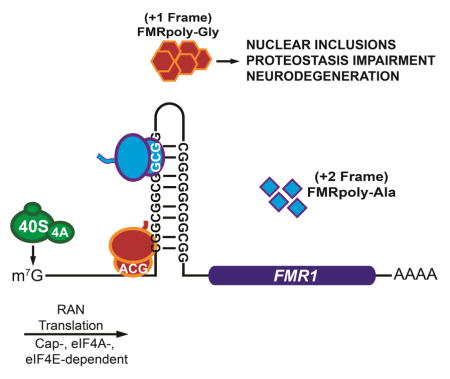
INTRODUCTION
Nucleotide repeat expansions are implicated in numerous neurodegenerative and neuromuscular diseases (Nelson et al., 2013). Repeats can elicit toxicity through a series of overlapping pathogenic cascades, including protein gain-of-function, protein loss-of-function, and RNA gain-of-function mechanisms. Recently, an unconventional form of translational initiation known as repeat associated non-AUG (RAN) translation has emerged as a contributor to repeat expansion disorder pathogenesis (Kearse and Todd, 2014; Zu et al., 2011). RAN translation produces homopolymeric or dipeptide repeat-containing proteins from nucleotide repeat expansions in the absence of an AUG start site. RAN translation occurs in multiple reading frames on the same repeat and within RNA regions typically thought of as non-coding (e.g., introns and 5′ leader sequences). Initially described in association with CAG and CUG repeats (Zu et al., 2011), RAN translation occurs in Spinocerebellar Ataxia Type 8 (SCA8), Myotonic Dystrophy (DM1), Amyotrophic Lateral Sclerosis and Frontotemporal Dementia associated with GGGGCC/CCCCGG repeats in C9orf72 (C9ALS/FTD) (Ash et al., 2013; Mori et al., 2013; Zu et al., 2013), Huntington’s Disease (HD) with CAG repeats in the Huntingtin gene (Banez-Coronel et al., 2015), and Fragile X-associated Tremor/Ataxia Syndrome (FXTAS) at CGG repeats in the Fragile X gene, FMR1 (Todd et al., 2013).
RAN translation at CGG repeats contributes to neurodegeneration in model systems. CGG repeat expansions in the 5′ leader sequence of the fragile X gene, FMR1, triggers multiple distinct diseases. The normal repeat size is approximately 30 repeats. Large expansions greater than 200 repeats result in hypermethylation and transcriptional silencing of the FMR1 locus, loss of the fragile X protein, FMRP, and development of Fragile X Syndrome (Nelson et al., 2013), the most common monogenic cause of autism and intellectual disability. In contrast, “premutation” carriers harbor between 55 and 200 repeats (Hagerman, 2013). These patients have increased FMR1 transcription and risk developing the adult onset neurodegenerative disorder Fragile X-associated Tremor/Ataxia Syndrome (FXTAS) and Fragile X-associated Premature Ovarian Insufficiency (FXPOI) (Hagerman, 2013). FXTAS symptoms are associated with development of neuronal intranuclear ubiquitinated inclusions in patient brains (Greco et al., 2006). Expanded CGG repeats elicit RAN translation in at least two reading frames, producing poly-glycine (FMRpolyG, +1 reading frame with respect to the downstream initiation site of the Fragile X protein, FMRP) and poly-alanine (FMRpolyA, +2 frame) containing proteins (Todd et al., 2013). FMRpolyG accumulates in neuronal inclusions in FXTAS patient brains and disease models (Todd et al., 2013). CGG RAN translation is required for toxicity in Drosophila and impairs protein quality control pathways (Oh et al., 2015; Todd et al., 2013), with similar findings observed in other repeat expansion disorders (Mizielinska et al., 2014; Yamakawa et al., 2015; Zhang et al., 2015).
Despite a potentially central role in multiple neurodegenerative disorders, the mechanism of RAN translation at a molecular level is unknown. Furthermore, how translation occurs in regions traditionally thought to be non-coding and in the absence of AUG start codons raises unique questions about eukaryotic translation initiation site choices and the coding potential of our genomes. Canonical eukaryotic translation typically follows a scanning model of initiation, as the 5′ m7G-cap recruits specific initiation factors and the pre-initiation complex (PIC) to the 5′ end of the mRNA. This PIC complex then scans in a 5′ to 3′ orientation in search of an AUG start codon in an appropriate sequence context (Sonenberg and Hinnebusch, 2009). The rate of initiation is strongly influenced by the flanking region around the AUG codon, commonly referred as the Kozak sequence (Kozak, 1986). Additionally, many initiation factors (eIFs) have been identified in forward genetic screens to play important roles in AUG start codon recognition and stringency (Sonenberg and Hinnebusch, 2009). Determining how these canonical translational rules intersect with RAN translational requirements could identify direct targets for future therapeutic development.
We developed a series of RAN translation-specific luciferase reporters to investigate the mechanisms mediating RAN translation at CGG repeat expansions using in vitro and cell based assays. We also assessed the efficiency of CGG RAN translation compared to canonical translation. Here we provide evidence that CGG RAN translation utilizes a cap-, eIF4E-, and eIF4A-dependent scanning mechanism to initiate at multiple near-cognate start codons just upstream of or within CGG repeats in the FMR1 mRNA, with strong influences from repeat size and sequence context. These findings suggest that RAN translation adapts canonical mechanisms for initiation at non-AUG start codons near and within CGG repeats.
RESULTS
Development of RAN translation-specific luciferase reporters
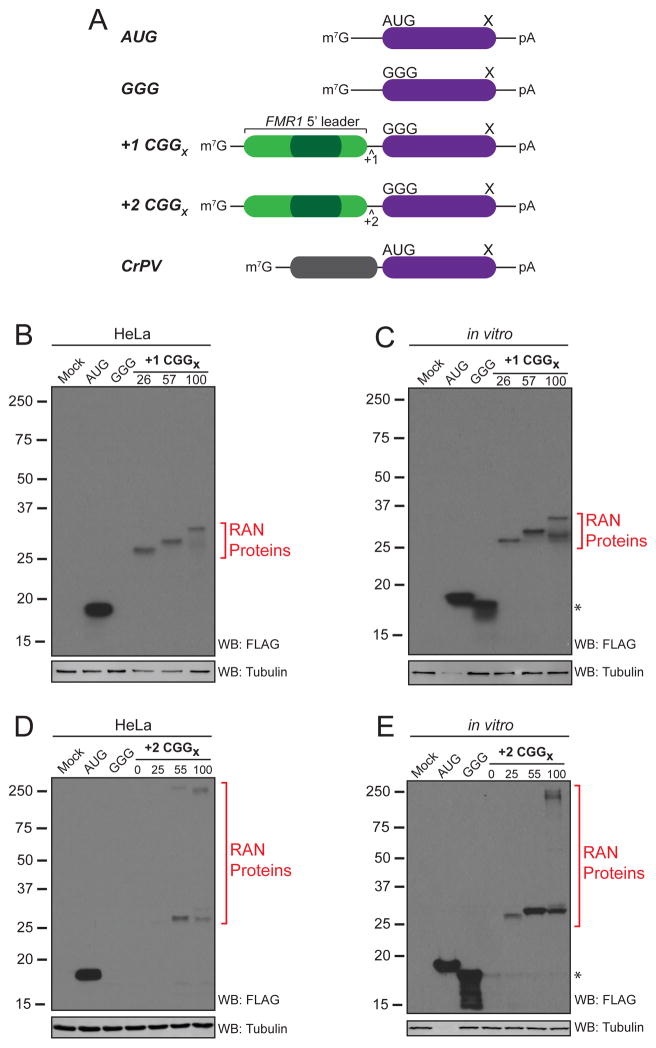
We developed a series of CGG RAN translation-specific luciferase reporters by placing the human FMR1 5′ leader sequence with 100 CGG repeats above a mutated form of nanoLuciferase (nLuc) lacking an AUG start codon (Figure 1). Unlike previous GFP reporters (Todd et al., 2013), repeats placed into the +1 (poly-Gly) or +2 (poly-Ala) reading frames only produced nLuc fusion proteins (FMRpolyG-nLuc and FMRpolyA-nLuc) in HeLa cells as well as in a mammalian in vitro translation system (Figure 1B–E). Thus, downstream quantitation of luciferase activity in assays solely reflects RAN translated nLuc fusion proteins initiated within the FMR1 5′ leader sequence.
Figure 1. A CGG repeat associated non-AUG (RAN) translation reporter.
(A) Schematic of nanoLuciferase-3xFLAG (nLuc) reporters. (B–E) Western blots of +1 CGG RAN translation reporters shows a repeat-length dependent increase in molecular weight in HeLa cells (B) and in vitro (C). A smaller molecular weight protein (*) derived from initiation within the nLuc coding sequence (Figure S1A) is detectable in vitro. +2 CGG RAN reporters produce high molecular weight proteins that run at the top of the gel, suggesting insolubility or aggregation at expanded sizes in HeLa cells (D) and in vitro (E). To avoid overexposure on Western blots, one-tenth of AUG control reporter was used to transfect HeLa cells and in vitro reactions were diluted 1:10 in sample buffer.
We observed a repeat-length dependent increase in observed molecular weight of poly-glycine proteins from the +1 CGG RAN translation-specific reporter in both translation systems (Figure 1B,C). The +2 CGG RAN translation reporter also exhibited a repeat-length dependent expression pattern by Western blot in HeLa cells and in vitro (Figure 1D,E). However, poly-alanine RAN proteins from the +2 CGG RAN translation reporter do not migrate according to their predicted molecular weight (Figure 1D,E and Figure S1B). In both HeLa cells and in vitro translation reactions, poly-alanine-nLuc fusion proteins migrate at ~25kD, with a slower migrating protein accumulating in the gel stack with expanded repeats (Figure 1D,E). This is a feature to poly-alanine containing proteins, as an identical pattern is seen with this fusion protein is generated using a canonical AUG start codon in the +2 reading frame (Figure S1B). Releasing the homopolymeric alanine polypeptide from the nLuc reporter by cleaving at an engineered protease site causes the poly-alanine peptide to adapt a more smeared electrophoretic pattern (Figure S1C), akin to what is observed in CAG RAN translation (Banez-Coronel et al., 2015; Zu et al., 2011). Neither the poly-glycine nor poly-alanine fusion to nLuc significantly altered luciferase activity (Figure S2Aa–d) and repeat length does not affect reporter mRNA or protein stability (Figure S2Ae,f).
CGG RAN translation is less efficient than canonical translation
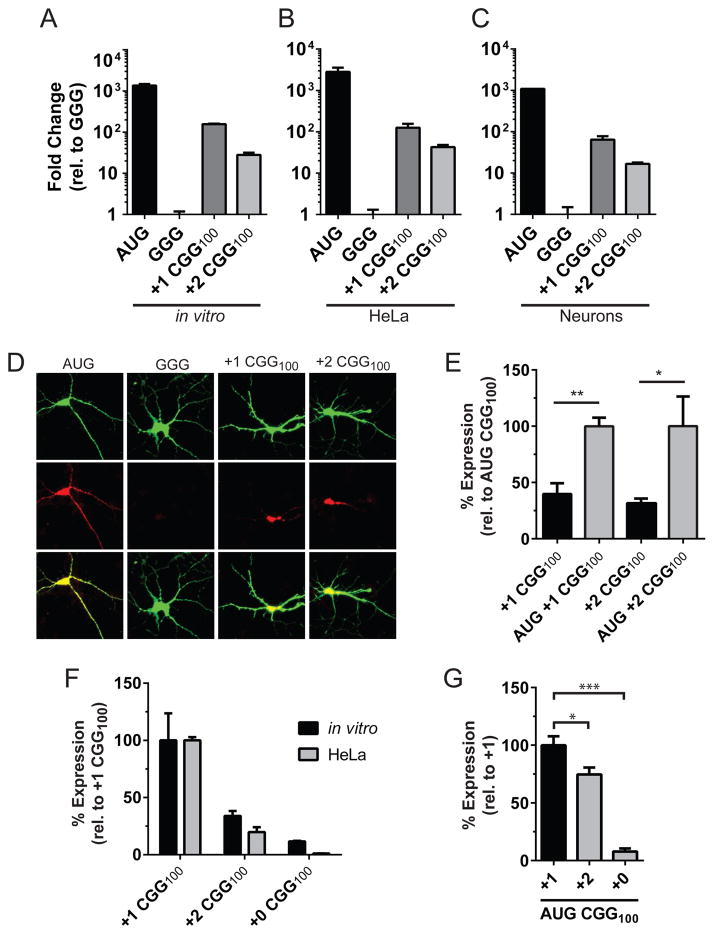
To interrogate CGG RAN translation, we first optimized in vitro translation reactions to be in the linear dynamic range of detection (Figures S2Ba,b). Luciferase activity of both CGG RAN reporters was 20–200 times higher than the control reporter, GGG-nLuc, which lacks the FMR1 5′ leader and wherein the start codon was inactivated (Figure 2A–C). mRNAs from CGG RAN reporters were translated with comparable efficiency in vitro (Figure 2A and Figure S2Bc–e) and in mRNA transfected HeLa cells (Figure 2B). To confirm the relevance of this reporter to RAN translation in neurons, we transfected plasmids expressing these vectors into rat primary cortical neurons. Luciferase activity demonstrated a clear RAN translation-specific signal in both reading frames compared to the control reporter (Figure 2C). Furthermore, immunocytochemistry shows both the +1(poly-glycine) and +2(poly-alanine) CGG RAN translation reporters were expressed and present in both the soma and dendrites (Figure 2D).
Figure 2. CCG RAN translation exhibits differential kinetics across repeat reading frames.
(A–C) Relative expression of AUG control and CGG RAN translation reporters normalized to the GGG nLuc control reporter (A) in vitro, (B) HeLa cells, and (C) in rat primary cortical neurons. (D) Representative immunostaining of control and CGG RAN translation reporters in neurons. (E) RAN translation is 30–40% as efficient in vitro as initiation from an AUG codon placed in optimal Kozak sequence context upstream of the repeat. (F) Comparison of reading frames shows +1 CGG RAN translation is more efficient in vitro and in HeLa cells. (G) Initiation at an AUG start codon placed in optimal Kozak context above CGG100 repeats translating the repeat in either the +0, +1, or +2 frame in vitro suggests differences in both initiation rate and translated repeat codon dictate overall RAN translation levels. Graphs represent mean ± S.D., n=3. *Student’s T-test, p<0.05; **Student’s T-test, p<0.01;***Student’s T-test, p<0.001.
To assess the efficiency of RAN translation at pathogenic expanded repeats compared to canonical translation initiation, we placed an AUG start codon in an optimal Kozak context above the expanded repeats. CGG RAN translation in both frames is 30–40% as efficient as canonical AUG initiation and translation through the repeat (Figure 2E). However, the two CGG RAN reading frames exhibit differences in efficiency, such that +2 CGG RAN translation is 30–50% as efficient as +1 CGG RAN translation (Figure 2F and Figure S2Bf). This differential efficiency between the +1 and +2 reading frames is largely dependent on initiation rate, as the relative translation efficiency of reporters with an AUG start codon just upstream of the repeat are only 25% different (Figure 2G). However, the translated repeat codon does have a role in dictating CGG RAN translation levels, as canonical AUG initiated translation of +0 CGG100 (CGG codon, Arg) is <10% that of a similar +1 CGG100 (GGC codon, Gly) reporter (Figure 2G).
Non-AUG initiation in the FMRP reading frame is attenuated by CGG repeats
A feature of RAN translation at CGG repeats that differs from other repeats is the apparent lack of products generated from all potential reading frames. Our initial CGG RAN translation work using GFP fusion reporters suggested that no RAN product is generated at CGG repeats in the +0 (Arginine) reading frame (Todd et al., 2013). Moreover, such a product would create an N-terminal extension on the fragile X protein, FMRP, yet no such extension has been observed in FXTAS or control patients. As with the +1 and +2 reading frames, the native (+0) reading frame lacks an AUG start codon within the FMR1 5′ leader.
Using a series of +0 reading frame nLuc reporters with different sized CGG repeats, we detected no RAN translation product at normal or expanded repeat lengths by Western analysis in HeLa cells or in vitro (Figure 2F and Figure S2Ca–c). A product could be detected if an AUG start codon (in optimal Kozak context) is inserted upstream of the repeat, but this signal was quite weak compared to AUG initiated translation in either the +1 or +2 reading frames (Figure 2G). The lack of a RAN translation product in this reading frame cannot be explained by nLuc reporter hindrance as a poly-arginine fusion does not drastically affect nLuc activity (Figure S2Ac). Intriguingly, in reporters with the FMR1 5′UTR but no CGG repeat (+0 CGG0), a product was generated in both HeLa cells and in vitro (Figure S2Ca–c). Mutational analysis of near-AUG start codons identified the site of initiation as an ACG codon upstream of the repeat site (Figure S2Cd). Luciferase activity of +0 CGGx reporters from mRNA transfected HeLa cells suggests +0 CGG reading frame translation in the FMR1 5′ leader is attenuated to the level of the negative control (GGG-nLuc) reporter at both normal (+0 CGG28) and expanded length repeats (+0 CGG100) (Figure S2Ce). This suggests that the length of the CGG repeat has a direct negative influence on +0 CGG frame translation.
CGG RAN translation requires the 5′ cap and ribosomal scanning
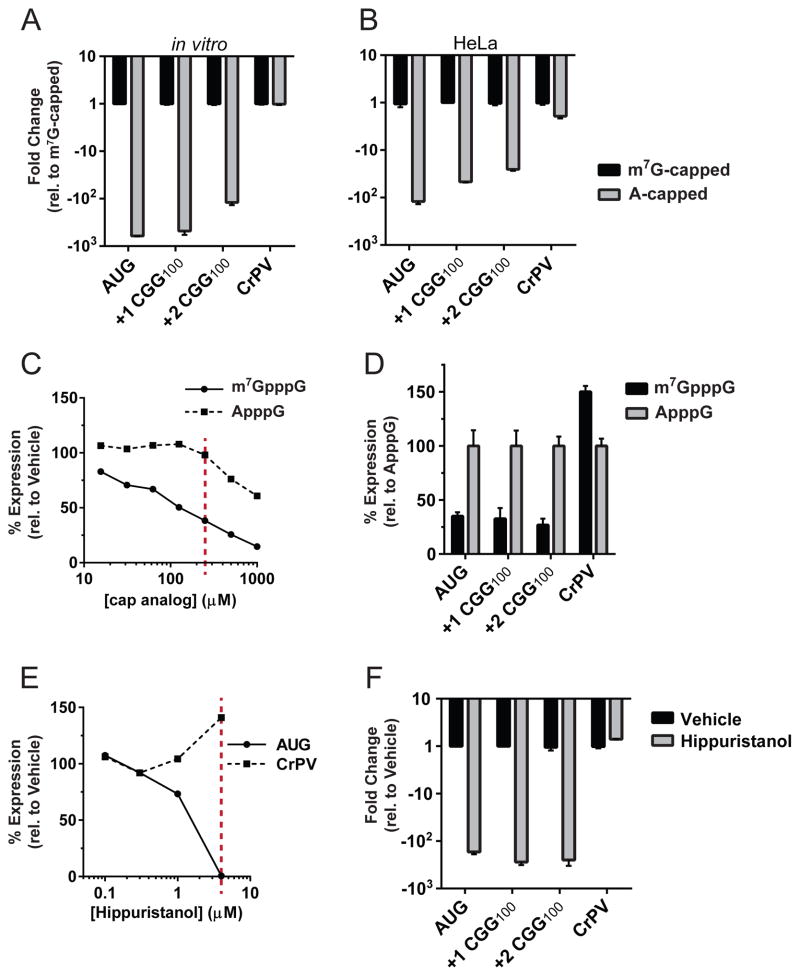
We next defined the molecular requirements for CGG RAN translation in multiple reading frames. Eukaryotic translation can be broadly classified as cap-dependent or -independent. Viruses and some stress-response mRNAs utilize internal ribosome entry sties (IRES) to bypass regulatory mechanisms at low translation states [reviewed in (Jackson et al., 2010)]. IRESs do not require the 5′ m7G-cap to recruit the translation machinery. Instead, the IRES element forms a complex secondary structure that, with only a subset or a complete absence of the canonical initiation factors (eIFs), directly recruits the 40S ribosome for initiation. FMRP translation could be mediated by an IRES in the 5′ leader, as suggested by previous work (Chiang et al., 2001). We therefore determined whether the CGG repeats allow for cap-independent translation by directly testing the necessity of the 5′ m7G-cap for RAN translation. Messenger RNAs were synthesized with a functional m7G-cap (able to bind the eIF4E cap-binding protein and elicit canonical recruitment of the translation machinery) or a non-functional A-cap (does not bind eIF4E, but protects from mRNA degradation). Control mRNAs behaved as expected: translation of the AUG reporter mRNA, which bears a short, unstructured 5′ leader sequence and a canonical AUG start codon, exhibited clear cap-dependence, whereas translation initiated by the CrPV IRES, which only requires the 40S ribosome subunit for initiation, was translated with equal efficiency regardless of the presence or absence of a m7G-cap (Figure 3A–B). Both +1 and +2 CGG RAN reporters exhibit strong cap-dependence comparable to that of the AUG control (Figure 3A–B). We next tested the necessity for the cap-binding protein eIF4E by titrating the competitive inhibitor m7G-cap analog (m7GpppG) or, as a negative control, ApppG, into the in vitro translation reaction. Expression of both CGG RAN reporters, along with the cap-dependent control, was specifically inhibited by adding the m7G-cap analog in trans (Figure 3C,D). In contrast, expression of the cap- and eIF4E-independent CrPV IRES control was increased upon addition of m7G-cap analog, likely due to a larger pool of free 40S ribosomes (Figure 3C–D and Figure S3A). Taken together, these results establish the importance of a 5′cap and eIF4E for CGG RAN translation in multiple reading frames.
Figure 3. RAN translation utilizes canonical translation machinery.
(A) Expression of the canonical translation reporter (AUG) and both CGG RAN translation reporters were reduced by 200–800 fold in vitro when A-capped compared to when m7G-capped. In contrast, expression of the cap-independent CrPV IRES reporter was similar for both A-capped and m7G-capped. (B) m7G-cap dependency in mRNA transfected HeLa cells. (C) Expression of AUG control when m7G-cap analog was titrated in vitro as a competitive inhibitor of eIF4E. ApppG cap analog was included as a negative control. (D) 250 μM m7GpppG (red line in C) reduced +1 and +2 CGG RAN translation to ~30% of control levels. (E) Dose response curve for the eIF4A-specific inhibitor hippuristanol on control reporters. (F) 4 μM hippuristanol (red line in E) inhibited CGG RAN translation. Graphs represent mean ± S.D. from 3 independent experiments. All differences are statistically significant (Student’s T-test, p<0.01 after Bonferroni correction).
The observed cap- and eIF4E-dependency indicates that a scanning mode of initiation may drive RAN translation. In Drosophila and in cell culture, repeats are only translated when placed in the 5′ leader of GFP, but not when the repeat is moved to the 3′ UTR (Todd et al., 2013). To confirm ribosome scanning is required for CGG RAN translation initiation, we examined the role of eIF4A, a helicase required for pre-initiation complex scanning during translation initiation. Addition of the eIF4A specific inhibitor, hippuristanol (Bordeleau et al., 2006) significantly reduced both canonical translation and CGG RAN translation in both the +1 and +2 reading frames, but not translation of the eIF4A-independent CrPV IRES control (Figure 3E,F and Figure S3B). This finding indicates that eIF4A plays a crucial role in RAN translation. Thus, CGG RAN translation in multiple reading frames at normal and expanded repeats utilizes a scanning mechanism requiring at least a subset of the canonical initiation factors, and is clearly separable from an IRES element-mediated initiation event.
CGG RAN translation is repeat length dependent and initiates at multiple non-AUG codons
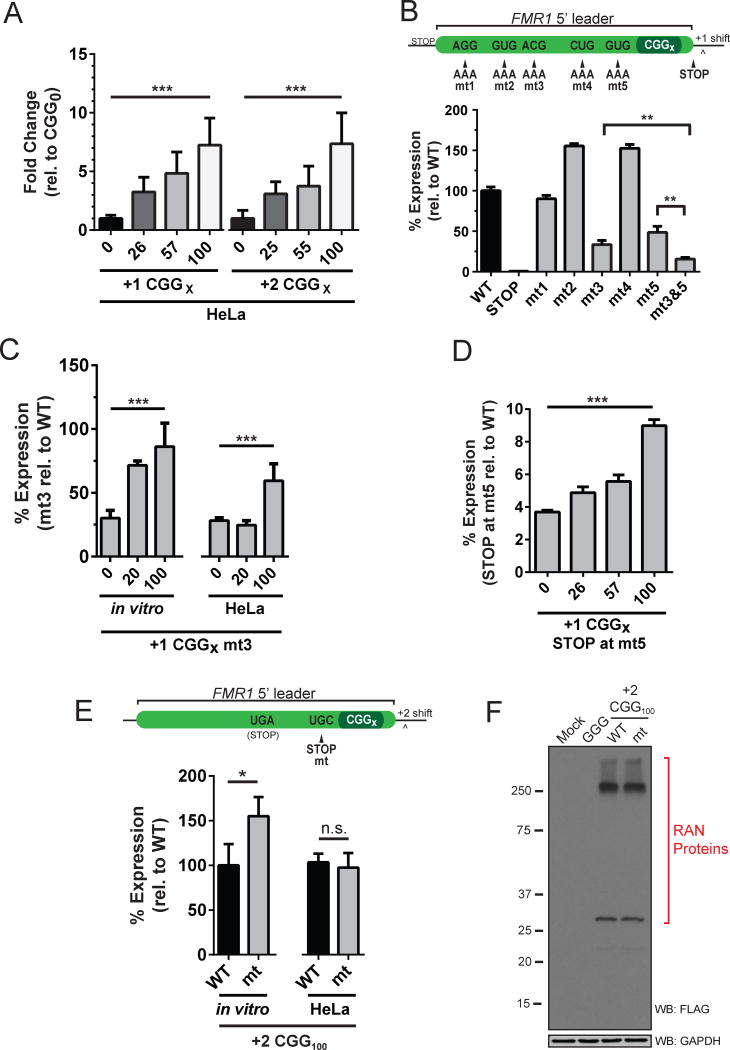
FMRpolyG inclusions were detected in frontal cortical and hippocampal neurons in FXTAS patient brain tissue, but not in age-matched controls, supporting a role in FXTAS pathology (Todd et al., 2013). To assess if FMRpolyG accumulation in FXTAS tissue could be due to increased levels of RAN translation at expanded repeats, we next measured CGG RAN translation across repeat sizes ranging from no repeats to the wild type normal range in the population (20–45) to expanded disease-associated length repeats (>55). In mRNA transfected HeLa cells, +1 and +2 CGG RAN reporter mRNAs with expanded repeats were translated at higher levels than reporters with normal length repeats (Figure 4A). Furthermore, CGG RAN translation in both reading frames was more efficient in a repeat length-dependent manner over CGG0 controls (Figure 4A). This same phenomena was even more pronounced for the +2 reading frame in vitro, as +2 CGG100 was translated ~25 times more than +2 CGG0 (Figure S4A). Unexpectedly, +1 CGG RAN translation appeared to be largely repeat-independent in vitro (Figure S4A).
Figure 4. RAN translation is repeat-dependent and initiates at separate sites in different reading frames.
(A) HeLa cells transfected with equimolar in vitro transcribed CGG RAN translation reporter mRNA harboring zero, normal, and expanded repeats. (B) Mutation analysis of near-cognate start codons in +1 CGG0 reporter identifies initiation at ACG and GUG codons upstream of the repeat. (C) Mutation of ACG to AAA (mt3) does not preclude initiation upon repeat expansion. For each repeat length, expression of reporters harboring mt3 mutations are shown as the percent signal of non-mutated (WT) reporters with the same repeat length (e.g., +1 CGG100-mt3(AAA) vs. +1 CGG100-WT(ACG)). (D) A UAG stop codon at the GUG position in mt5 eliminates 90+% of activity in vitro. This mutation is less effective at expanded CGG100 repeats. Expression of reporters harboring stop codons are shown as the percent signal of non-mutated (WT) reporters with the same number of repeats. (E) The +2 reading frame of the FMR1 5′ leader contains no near-cognate start codons between a native UAG stop codon and the CGG repeat. Insertion of a stop mutation (mt) just upstream of the CGG repeat does not impede +2 CGG RAN translation. (F) Western analysis of native and mutant +2 CGG RAN translation reporter in HeLa cells. Similar results are seen in vitro (Figure S4E). **Student’s T-test, p<0.01; ***One-way ANOVA, p<0.0001.
We next sought to identify the codon used to initiate synthesis of FMRpolyG. Mutational analysis of each near-cognate AUG codons in the +1 reading frame demonstrates that two separate non-AUG start codons upstream of the repeat, ACG and GUG, can be used for initiation (Figure 4B and Figure S4B,C). However, testing these same mutations at normal and expanded repeat lengths in vivo suggests that usage of any one specific near-cognate start codon for initiation becomes less essential in the presence of expanded repeats (Figure 4C and Figure S4D). We previously found that a UAG stop codon placed just upstream of the CGG repeat markedly reduced production of +1 CGG100 RAN products (Todd et al., 2013). To assess if a UAG stop codon mutation just upstream (i.e., 3 codons) of the repeat would terminate +1 CGG RAN translation, we tested the mutation’s effectiveness across repeat sizes. Insertion of a UAG stop codon markedly reduced expression at all repeat lengths, confirming that the majority of +1 CGG RAN translation initiates upstream of the repeat within the FMR1 leader sequence (Figure 4D). However, this stop codon is less effective at blocking RAN translation with larger repeats in vitro, suggesting that a small fraction of initiation may occur within the repeat itself at larger repeat sizes (Figure 4D).
CGG RAN translation initiation is, however, distinct between reading frames. While a stop codon substitution three codons upstream of the CGG repeat in the +1 frame strongly suppresses RAN translation in that frame (Figure 4D), an identical mutation in the +2 frame did not impede +2 CGG RAN translation in vitro or in HeLa cells (Figure 4E,F and Figure S4E). Together, our data indicate that the majority of +1 CGG RAN translation initiates upstream of the repeat at near-cognate start codons (i.e., ACG and GUG) while +2 CGG RAN translation initiates within the CGG repeat.
DISCUSSION
We developed a robust assay for studying RAN translation both in vitro and in mammalian cultured cells that is potentially applicable to multiple repeat expansion disorders. We applied this system to demonstrate specific cis and trans requirements for RAN translation at CGG repeats associated with the neurodegenerative disorder FXTAS. CGG RAN translation utilizes a cap-, eIF4E-, and eIF4A-dependent scanning mechanism of initiation across different repeat lengths and reading frames. However, important differences from both canonical translation and between the three potential CGG repeat reading frames are observed. For example, +1 CGG RAN translation initiation occurs preferentially at near-cognate start codons just 5′ to the repeat, but +2 CGG RAN translation requires no near-cognate start codon and appears to initiate predominantly or entirely within the CGG repeat itself while exhibiting a stronger dependency on repeat expansion for initiation in vitro and in vivo. In contrast, +0 CGG RAN translation of a poly-arginine product is not observed at normal or expanded repeat sizes, although a product can be generated in this reading frame from the FMR1 5′ leader sequence in the absence of any CGG repeat. These findings suggest that CGG RAN translation produces heterogeneous proteins through a dynamic process that is dependent on repeat content, repeat length, repeat reading frame, and surrounding sequence context.
CGG RAN translational initiation is strongly influenced by repeat length, consistent with work on RAN translation at other repeat expansions (Mori et al., 2013; Zu et al., 2011). A priori, this repeat length dependence could reflect secondary structures that directly recruit initiation complexes in a manner similar to IRES elements. Our results argue against CGG repeats directly recruiting initiation complexes, as +1 and +2 CGG RAN translation are 5′ cap-, eIF4E- and eIF4A-dependent. Alternatively, the secondary structure could impede preinitiation complex scanning, leading to enhanced usage of non-cognate start codons upstream or within the repeat as a mechanism for RAN translational initiation. This ability of a secondary structure to influence RAN translation would be consistent with earlier observations demonstrating that artificial stem-loops have the ability to stall scanning 43S pre-initiation complexes and favor initiation within a small window just upstream of the stem-loop at near-cognate codons (Kozak, 1990). While the exact secondary structure of CGG repeats in FMR1 is unclear (i.e., hairpin/stem-loop vs. G-quadruplex) and is likely dynamic (Napierala et al., 2005; Zumwalt et al., 2007), it clearly impairs translation at downstream AUG codons in vivo and in vitro and is predicted to form a strong impediment to scanning (Chen et al., 2003; Ludwig et al., 2011). Furthermore, the sequence upstream of the FMR1 repeat is highly GC-rich, which in itself is sufficient to slow 43S PIC scanning kinetics, thereby increasing the chance of non-AUG initiation (Kozak, 1991; van der Velden et al., 2002). Indeed, we still observe some initiation at near-cognate start codons in the FMR1 5′ leader independent of the repeat in vitro, although this effect is blunted in vivo.
Our findings in the +1 frame suggest that some aspects of CGG RAN translation are related to regulatory upstream open reading frames (uORFs) (Sachs and Geballe, 2006). We detect robust RAN translation at normal repeat sizes, both in vitro and in transfected cells in the +1 frame. This initiation appears particularly dependent on initiation at specific ACG and GUG codons just 5′ to the repeat and published work suggests that even 30 repeats influences FMRP translation kinetics (Chen et al., 2003; Ludwig et al., 2011). The observations are germane given that most uORFs are thought to act as regulatory elements which influence downstream AUG codon usage (Sachs and Geballe, 2006). While most of these characterized uORFs initiate at AUG codons, RAN translation by definition does not use an AUG. Recent ribosome profiling and mass spectroscopy studies suggest that there are potentially 1000′s of unannotated uORFs within the transcriptome, many of which utilize near-cognate codons for initiation (Calvo et al., 2009; Ingolia et al., 2011). Most of these events remain uncharacterized and are without known function. Our in-depth analysis of this single 5′ leader with a highly sensitive reporter suggests that the kinetics of translation for each transcript may be more complicated than previously thought, with initiation site choices being influenced by multiple variables and potentially occurring in multiple reading frames at different rates. Indeed, while we have delineated some of the central sequence and protein-based requirements that support translation in this 5′ leader, how the actual RAN translation start site is selected remains unclear.
An additional interesting finding arises from the lack of RAN translation in the +0 (CGG, arginine) reading frame. Surprisingly, a product can be generated in this reading frame both in vitro and in cells when the repeat is completely removed via a mechanism that appears similar to that observed for the +1 CGG reading frame. However, even normal repeat sizes markedly suppress translation of this product, and no N-terminal extensions are observed on FMRP in patients with FXTAS. We initially suspected this suppression could be due to activation of the non-stop RNA decay pathway, which detects translation of homopolymeric basic amino acids as occurs with translation of a polyA tail [reviewed by (Klauer and van Hoof, 2012). However, a mutation that enhanced translation through the repeat did not reduce RNA levels (Figure S2Cf), suggesting that alternative mechanisms may be at play here.
The finding that initiation dynamics and start site choice are significantly different in the +2 CGG reading frame is particularly interesting. Our previous data rules out a frameshift event in this reading frame (Todd et al., 2013) and placing a stop codon just upstream of the CGG repeat does not preclude +2 CGG RAN translation (Figure 4E,F and Figure S4E). This reading frame of the CGG repeat thus exhibits similar characteristics to that reported for RAN translation at other repeat expansions (Ash et al., 2013; Banez-Coronel et al., 2015; Mori et al., 2013; Zu et al., 2011; Zu et al., 2013). In this sense, it is noteworthy that the +2 CGG repeat reading remains highly dependent on cap recognition and follows a ribosome scanning model in vitro and in transfected cells. Whether these rules will similarly apply to other repeats is still unknown, but certain features of RAN translation at other repeats are at least superficially incompatible with our model (Banez-Coronel et al., 2015; Zu et al., 2011). In this larger context, our systematic comparison across reading frames suggest that RAN translation as a whole likely encompasses multiple overlapping initiation mechanisms that are influenced by variables including repeat content, reading frame and sequence context.
In summary, we provide mechanistic data across multiple models, including neurons, to support a specific mechanism for RAN translation at CGG repeats. This evidence suggests that while many aspects of canonical translational initiation are retained in CGG RAN translation, specific features are strongly dependent on the surrounding sequence, repeat length, and repeat reading frame. These features combine to loosen the requirements typically applied in AUG start codon selection and allow for non-canonical initiation. The assays utilized here should provide a robust means for studying RAN translation in other repeat-associated diseases, including CAG repeats in SCA8, DM1, HD, and GGGGCC/CCCCGG repeats in C9ORF72 ALS/FTD. By defining the mechanisms of RAN translation across different disorders, repeats and sequence contexts, we should be able to identify features that are unique to RAN translation. Such insights can then be used to develop future targets for RAN translation specific inhibition, which would have significant therapeutic potential across multiple neurodegenerative disorders.
EXPERIMENTAL PROCEDURES
Plasmid Construction
Oligonucleotides used are listed in Table S1 and cloning details are located in the supplemental experimental procedures. The nLuc coding sequence was cloned into pcDNA3.1+ from pNL1.1 for expression in both HeLa cells (CMV promoter) or in vitro (T7 promoter). The AUG of nLuc was mutated to GGG and a 3xFLAG tag was inserted by site-directed mutagenesis. The FMR1 5′ leader was inserted upstream of the GGG via BlpI/XhoI. All intervening stops were removed to make nLuc fusions. The reading frame of the repeat relative to nLuc was shifted by inserting nucleotides after the human sequence.
In vitro Translation
In vitro translation reactions (10 μL) using the Flexi Rabbit Reticulocyte Lysate System were programmed with 3 nM mRNA for 30 min at 30°C a nd then terminated by incubation on ice. Diluted reactions in Glo Lysis Buffer were then used for luciferase activity quantification using Nano-Glo. For Western blot analysis, reactions were programmed with 50 ng mRNA (saturated mRNA levels).
Reactions testing cap-, eIF4E-, and eIF4A-dependency were all performed in the presence of 21 nM competing mRNA. In testing the m7G-cap in trans, cap analogs were pre-incubated with lysate for 10 min at 30 °C before the addition of nLuc reporter mRNAs. Hippuristanol (a kind gift from Jerry Pelletier, McGill University) was added to reactions in parallel to nLuc reporter mRNAs.
HeLa Cell Culture and Transfection
For Western analysis, HeLa cells were seeded at 1.0 × 105 cells per well. At 24 hrs post seeding, cells were transfected with 500 ng plasmid per well with Viafect. 24 hrs later, cells were harvested and lysed in RIPA buffer. For luciferase assays, RNA transfections were performed with HeLa cells seeded at 1.2 × 104 cells per well. At 24 hrs post seeding, cells were transfected using Lipofectamine 2000 with 100 ng nLuc reporter mRNA and 100 ng pGL4.13 (transfection control). 24 hrs post-transfection, luciferase activity was measured using Nano-Glo and nLuc levels were normalized to FFLuc.
Supplementary Material
supplement
Highlights.
- Expanded repeats stimulate CGG Repeat Associated Non-AUG (RAN) translation
- CGG RAN translation shares initiation requirements with canonical initiation
- RAN translation displays differential start codon selection across reading frames
- Sequence context and repeat codon influence CGG RAN translation dynamics
Acknowledgments
We would like to thank Paul Hagerman (University of California – Davis) for parent FMR1 constructs, Jerry Pelletier (McGill University) for Hippuristanol, Nicolas Charlet-Burguerand and Dan Southworth for helpful discussions, Nathan Blewett and Chase Weidmann for early work, Trista Schagat for advice in reporter design, Michael Sutton and Christian Althaus for help with rat primary neuron isolation, and Hank Paulson, Sami Barmada, and Fang He for reviewing the manuscript. We apologize to colleagues whose important work we could not site secondary to manuscript size limitations. Funding for this work was provided by the NIH (K08NS069809 to P.K.T., R01NS086810 to P.K.T., and F32NS089124 to M.G.K.) and the M-Cubed Initiative (University of Michigan).
Footnotes
AUTHOR CONTRIBUTIONS
M.G.K., A.C.G., and P.K.T. designed the experiments. M.G.K. optimized and performed experiments with help from K.M.G. and A.E.L. A.K. performed the initial plasmid construction and helped with the Western analysis from HeLa cells. C.M.R. isolated, transfected, and immunostained rat primary neurons. M.G.K., A.C.G., and P.K.T wrote the manuscript with input from all authors.
DISCLAIMER
The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ash PE, Bieniek KF, Gendron TF, Caulfield T, Lin WL, Dejesus-Hernandez M, van Blitterswijk MM, Jansen-West K, Paul JW, 3rd, Rademakers R, et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77:639–646. doi: 10.1016/j.neuron.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banez-Coronel M, Ayhan F, Tarabochia AD, Zu T, Perez BA, Tusi SK, Pletnikova O, Borchelt DR, Ross CA, Margolis RL, et al. RAN Translation in Huntington Disease. Neuron. 2015;88:667–677. doi: 10.1016/j.neuron.2015.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordeleau ME, Mori A, Oberer M, Lindqvist L, Chard LS, Higa T, Belsham GJ, Wagner G, Tanaka J, Pelletier J. Functional characterization of IRESes by an inhibitor of the RNA helicase eIF4A. Nat Chem Biol. 2006;2:213–220. doi: 10.1038/nchembio776. [DOI] [PubMed] [Google Scholar]
- Calvo SE, Pagliarini DJ, Mootha VK. Upstream open reading frames cause widespread reduction of protein expression and are polymorphic among humans. Proc Natl Acad Sci U S A. 2009;106:7507–7512. doi: 10.1073/pnas.0810916106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LS, Tassone F, Sahota P, Hagerman PJ. The (CGG)n repeat element within the 5′ untranslated region of the FMR1 message provides both positive and negative cis effects on in vivo translation of a downstream reporter. Hum Mol Genet. 2003;12:3067–3074. doi: 10.1093/hmg/ddg331. [DOI] [PubMed] [Google Scholar]
- Chiang PW, Carpenter LE, Hagerman PJ. The 5′-untranslated region of the FMR1 message facilitates translation by internal ribosome entry. J Biol Chem. 2001;276:37916–37921. doi: 10.1074/jbc.M105584200. [DOI] [PubMed] [Google Scholar]
- Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, Trapp BD, Iwahashi C, Brunberg J, Grigsby J, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- Hagerman P. Fragile X-associated tremor/ataxia syndrome (FXTAS): pathology and mechanisms. Acta Neuropathol. 2013;126:1–19. doi: 10.1007/s00401-013-1138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia NT, Lareau LF, Weissman JS. Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell. 2011;147:789–802. doi: 10.1016/j.cell.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol. 2010;11:113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse MG, Todd PK. Repeat-associated non-AUG translation and its impact in neurodegenerative disease. Neurotherapeutics. 2014;11:721–731. doi: 10.1007/s13311-014-0292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauer AA, van Hoof A. Degradation of mRNAs that lack a stop codon: a decade of nonstop progress. Wiley Interdiscip Rev RNA. 2012;3:649–660. doi: 10.1002/wrna.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986;44:283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Ludwig AL, Hershey JW, Hagerman PJ. Initiation of translation of the FMR1 mRNA Occurs predominantly through 5′-end-dependent ribosomal scanning. J Mol Biol. 2011;407:21–34. doi: 10.1016/j.jmb.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizielinska S, Gronke S, Niccoli T, Ridler CE, Clayton EL, Devoy A, Moens T, Norona FE, Woollacott IO, Pietrzyk J, et al. C9orf72 repeat expansions cause neurodegeneration in Drosophila through arginine-rich proteins. Science. 2014;345:1192–1194. doi: 10.1126/science.1256800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Weng SM, Arzberger T, May S, Rentzsch K, Kremmer E, Schmid B, Kretzschmar HA, Cruts M, Van Broeckhoven C, et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science. 2013;339:1335–1338. doi: 10.1126/science.1232927. [DOI] [PubMed] [Google Scholar]
- Napierala M, Michalowski D, de Mezer M, Krzyzosiak WJ. Facile FMR1 mRNA structure regulation by interruptions in CGG repeats. Nucleic Acids Res. 2005;33:451–463. doi: 10.1093/nar/gki186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DL, Orr HT, Warren ST. The unstable repeats--three evolving faces of neurological disease. Neuron. 2013;77:825–843. doi: 10.1016/j.neuron.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SY, He F, Krans A, Frazer M, Taylor JP, Paulson HL, Todd PK. RAN translation at CGG repeats induces ubiquitin proteasome system impairment in models of fragile X-associated tremor ataxia syndrome. Hum Mol Genet. 2015;24:4317–4326. doi: 10.1093/hmg/ddv165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs MS, Geballe AP. Downstream control of upstream open reading frames. Genes Dev. 2006;20:915–921. doi: 10.1101/gad.1427006. [DOI] [PubMed] [Google Scholar]
- Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd PK, Oh SY, Krans A, He F, Sellier C, Frazer M, Renoux AJ, Chen KC, Scaglione KM, Basrur V, et al. CGG repeat-associated translation mediates neurodegeneration in fragile X tremor ataxia syndrome. Neuron. 2013;78:440–455. doi: 10.1016/j.neuron.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa M, Ito D, Honda T, Kubo K, Noda M, Nakajima K, Suzuki N. Characterization of the dipeptide repeat protein in the molecular pathogenesis of c9FTD/ALS. Hum Mol Genet. 2015;24:1630–1645. doi: 10.1093/hmg/ddu576. [DOI] [PubMed] [Google Scholar]
- Zhang K, Donnelly CJ, Haeusler AR, Grima JC, Machamer JB, Steinwald P, Daley EL, Miller SJ, Cunningham KM, Vidensky S, et al. The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature. 2015;525:56–61. doi: 10.1038/nature14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu T, Gibbens B, Doty NS, Gomes-Pereira M, Huguet A, Stone MD, Margolis J, Peterson M, Markowski TW, Ingram MA, et al. Non-ATG-initiated translation directed by microsatellite expansions. Proc Natl Acad Sci U S A. 2011;108:260–265. doi: 10.1073/pnas.1013343108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu T, Liu Y, Banez-Coronel M, Reid T, Pletnikova O, Lewis J, Miller TM, Harms MB, Falchook AE, Subramony SH, et al. RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc Natl Acad Sci U S A. 2013;110:E4968–4977. doi: 10.1073/pnas.1315438110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumwalt M, Ludwig A, Hagerman PJ, Dieckmann T. Secondary structure and dynamics of the r(CGG) repeat in the mRNA of the fragile X mental retardation 1 (FMR1) gene. RNA Biol. 2007;4:93–100. doi: 10.4161/rna.4.2.5039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplement