Three-dimensionally preserved minute larva of a great-appendage arthropod from the early Cambrian Chengjiang biota (original) (raw)
Significance
Understanding the nature of the Cambrian radiation involves knowing not only the morphologies of adult animals but also their developmental pathways. However, fossil evidence of early larvae is rare. Here we describe a well-preserved 2-mm-long larva of the short-great-appendage arthropod Leanchoilia illecebrosa from the early Cambrian Chengjiang biota. The exceptional 3D preservation has allowed our microcomputed tomography analyses to resolve a series of rudimentary limb Anlagen in the posterior portion of the larva—an arrangement resembling that in late-stage eucrustacean metanauplii. L. illecebrosa is considered as an early representative of either chelicerates or of euarthropods as a whole. Therefore, this discovery provides fossil evidence that posthatching segment addition is a feature rooted in the ancestor of Euarthropoda.
Keywords: Cambrian radiation, 3D preservation, micro-CT, arthropod larva, Chengjiang biota
Abstract
A three-dimensionally preserved 2-mm-long larva of the arthropod Leanchoilia illecebrosa from the 520-million-year-old early Cambrian Chengjiang biota of China represents the first evidence, to our knowledge, of such an early developmental stage in a short-great-appendage (SGA) arthropod. The larva possesses a pair of three-fingered great appendages, a hypostome, and four pairs of well-developed biramous appendages. More posteriorly, a series of rudimentary limb Anlagen revealed by X-ray microcomputed tomography shows a gradient of decreasing differentiation toward the rear. This, and postembryonic segment addition at the putative growth zone, are features of late-stage metanauplii of eucrustaceans. L. illecebrosa and other SGA arthropods, however, are considered representative of early chelicerates or part of the stem lineage of all euarthropods. The larva of an early Cambrian SGA arthropod with a small number of anterior segments and their respective appendages suggests that posthatching segment addition occurred in the ancestor of Euarthropoda.
Evolutionary developmental biology (evo-devo) explains evolutionary changes in different organisms by investigating their developmental processes (1). Paleontology contributes to evo-devo by providing information that is only available in fossil organisms (2). Studies of evolutionary development in fossil arthropods, which have dominated faunas from the early Cambrian (∼520 million years ago) to the present, have focused on trilobites (3), “Orsten”-type fossil crustaceans (4–6), and Mesozoic malacostracan crustaceans (7). Due to their small size and low preservation potential, fossil evidence of the appendages of early developmental stages of arthropods are rare, and known mainly from those with the special “Orsten” type of preservation (8), i.e., with the cuticle secondarily phosphatized, from the mid-Cambrian (500–497 million years ago) (9).
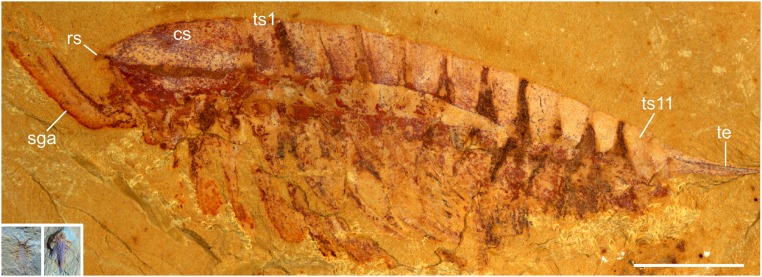
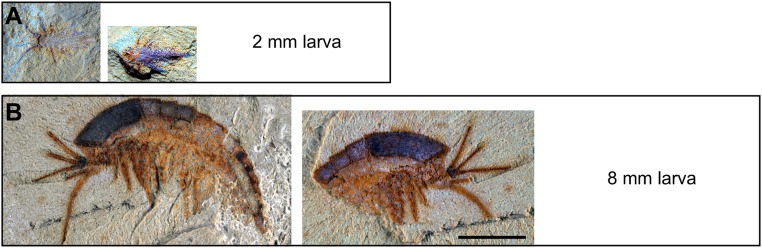
Here we describe an exceptionally preserved early developmental stage of a Cambrian arthropod from the Chengjiang biota of China. The specimen is only 2 mm long and is three-dimensionally preserved (Fig. 1, Insets). We interpret this specimen as a representative of the short-great-appendage (SGA) arthropod _Leanchoilia illecebrosa_—the most abundant SGA arthropod from this biota (10). SGA arthropods form a distinct early group characterized by prominent anteriormost appendages specialized for sensory (11) or feeding purposes (11, 12). Thus far, knowledge of L. illecebrosa is based mainly on adult specimens with a body length ranging from 20 to 46 mm (13) (Fig. 1). Specimens smaller than 20 mm are rare—only two examples, both 8 mm long, have been reported (8, 12) (Fig. S1_B_).
Fig. 1.
L. illecebrosa from the Chengjiang biota. Macrophotographs of an adult (specimen YKLP 11087) and the minute larva (Insets; specimen YKLP 11088a, b). cs, cephalic shield; rs, rostrum; sga, short great appendage; ts1 and ts11, trunk segments 1 and 11; te, telson. Insets are to the same scale as main image. (Scale bar: 5 mm.)
Fig. S1.
Two larval stages of L. illecebrosa. (A) The 2-mm-long larva described here (specimen YKLP 11088a, b). (B) An 8-mm-long larva previously reported in ref. 12 (specimen YKLP 11084a, b; reprinted with permission from ref. 12). (Scale bar: 2 mm.)
Results
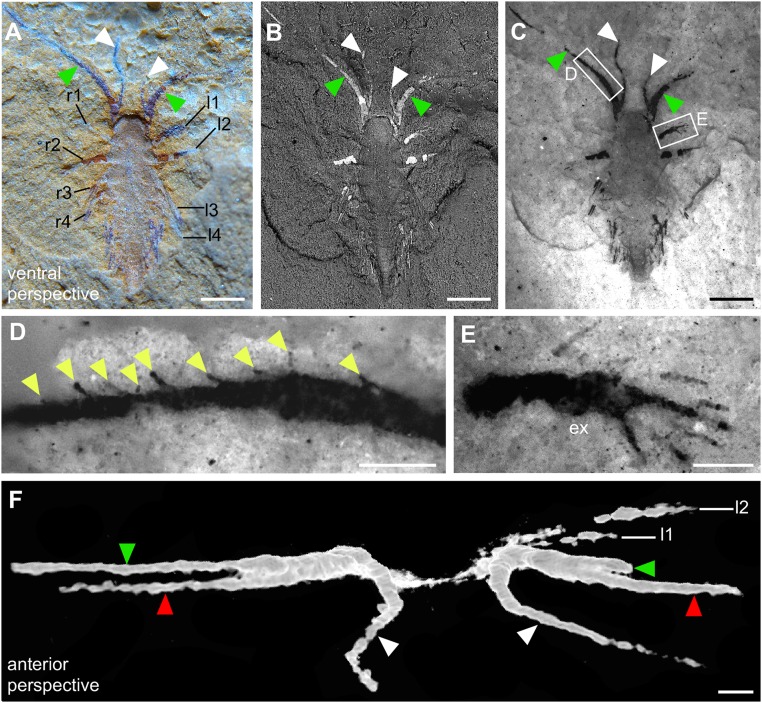
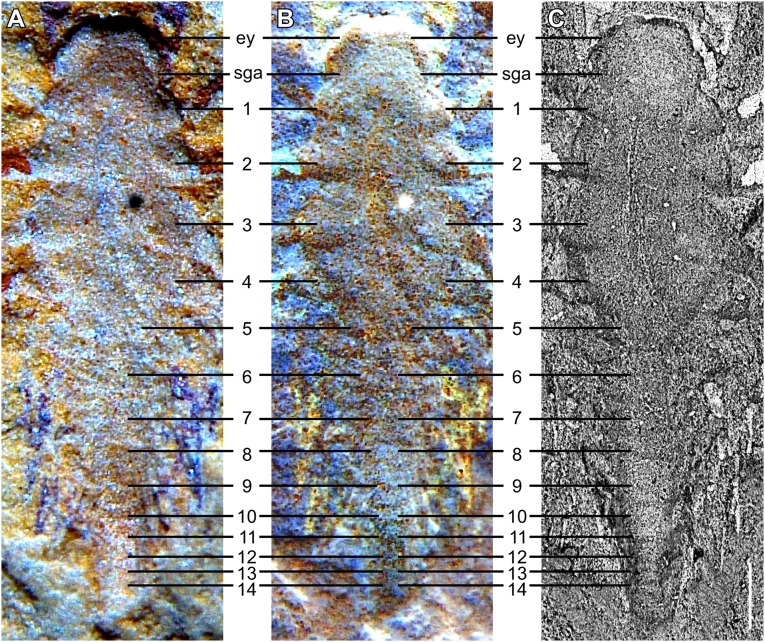
The specimen was discovered by separating two opposing slabs (YKLP 11088a, b; Fig. 1, Insets). Slab a (YKLP 11088a) exhibits the specimen from a ventral perspective, but, because the two slabs separated from each other along the dorsal side of the specimen, it shows details of the dorsal morphology (Fig. 2). The SGAs are preserved only on this slab (cf. Figs. 3 and 4), where two fingers of each SGA are exposed on the surface (Fig. 2 A_−_C). Fluorescence microscopy revealed spine-like armatures on one finger (Fig. 2 C and D) and setae along the outer edge of the exopod of the first post-SGA appendage on the left side of the animal (Fig. 2 C and E). A third finger, hidden inside the slab, was resolved with microcomputed tomography (micro-CT) (Fig. 2_F_). Body segmentation is indicated by the insertion of the appendages (Fig. 2 A and B). In addition to the segments bearing the eye and SGA, 14 post-SGA segments are identified (Fig. S2). The outline of the anterior portion of the body (from eye segment to fourth post-SGA segment) is somewhat oval, whereas the posterior portion (fifth to 14th post-SGA segment) is much narrower (Fig. S2_C_).
Fig. 2.
Minute larva of L. illecebrosa (slab a, YKLP 11088a). (A–E) Ventral perspective. (F) Anterior perspective. (A) Macrophotograph documenting two fingers of each SGA (green and white arrowheads) and four pairs of appendages (l1–l4, r1–r4) in the anterior part of the larva. (B) SEM revealing indications of body segmentation (see also Fig. S2). (C) Fluorescence microscopy showing setae or seta-like armatures in the appendages. (D) Close-up showing a row of seta-like armatures (yellow arrowheads) arising from the median margin of one of the fingers. (E) Close-up showing a paddle-like seta-bearing exopod (ex). (F) Micro-CT image revealing three fingers of each SGA (green and white arrowheads correspond to those in A_–_C, red arrowheads point to the fingers “hidden” inside the slab). l1–l4, post-SGA appendages on the left side of the animal; r1–r4, post-SGA appendages on the right side of the animal. [Scale bars: 0.5 mm (A–C); 0.1 mm (D–F).]
Fig. 3.
Minute larva of L. illecebrosa (slab b, YKLP 11088b). Dorsal perspective. (A) Macrophotograph documenting four pairs of appendages (l1–l4, r1–r4) in the oval-shaped anterior part of the larva and a much narrower posterior part ending in a dagger-like telson (te). (B) SEM additionally revealing the hypostome (hy; see Fig. 4 C and F) and the setae or seta-like armatures on the exopods (see C and D) and the telson (see F). (C) Fluorescence microscopy showing details of the setae or seta-like armatures. (D) Close-up showing setae or spines in the exopod (ex) of post-SGA appendages 3 (r3) and 4 (r4) on the right side of the larva. The basipod (bas) and endopod (en) of r4 are also shown. (E) Close-up showing setae or spines (black arrowheads) along the inner margin of the basipod of r4. (F) Close-up showing several setae along the margin of the telson. [Scale bars: 0.5 mm (A–C); 0.1 mm (D and F); 0.05 mm (E).]
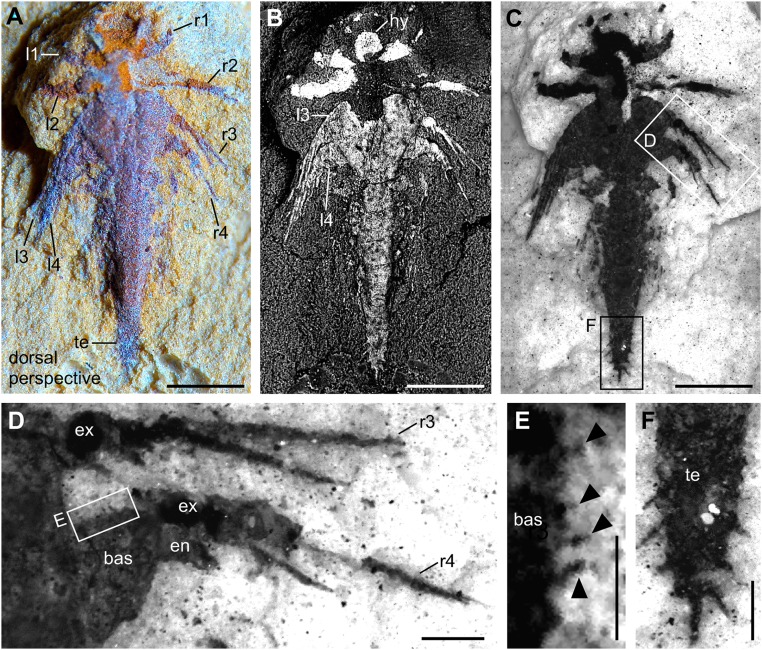
Fig. 4.
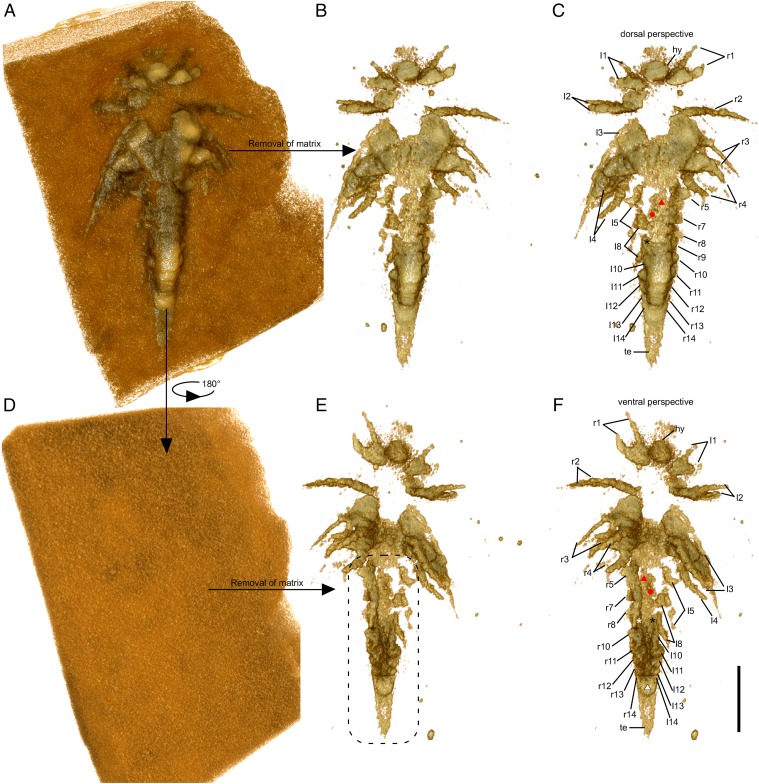
Minute larva of L. illecebrosa (slab b, YKLP 11088b). Rendering of 3D models derived from a micro-CT scan. (A–C) Dorsal perspective. (D–F) Ventral perspective. (A) Model of the larva together with the surrounding matrix. (B) Model of the larva after digitally removing the matrix. (C) Same model as in B, with interpretation. (D) Ventral side of the larva with all structures still covered by the matrix. (E) Model showing the structures on the ventral side of the larva after digital removal of the matrix. For detailed interpretation of structures in the marked region (dashed line), see Fig. S3. (F) Same model as in E, with interpretation (see also Fig. S3). Red triangle, post-SGA segment 6; red dot, post-SGA appendage 7 on the left side; white and black asterisks, post-SGA appendage 9 on the right and left side, respectively; white triangle, anal membrane. Other abbreviations are as in Figs. 1 and 2. (Scale bar: 0.5 mm.)
Fig. S2.
Body segmentation of the 2-mm-long larva of L. illecebrosa (slab a, YKLP 11088a). Ventral perspective. (A) Macrophotograph. (B) Inverted macrophotograph from A. (C) SEM image. ey, eye segment; sga, short great appendage, 1–14, post-sga segments 1–14. (Scale bar: 0.2 mm.)
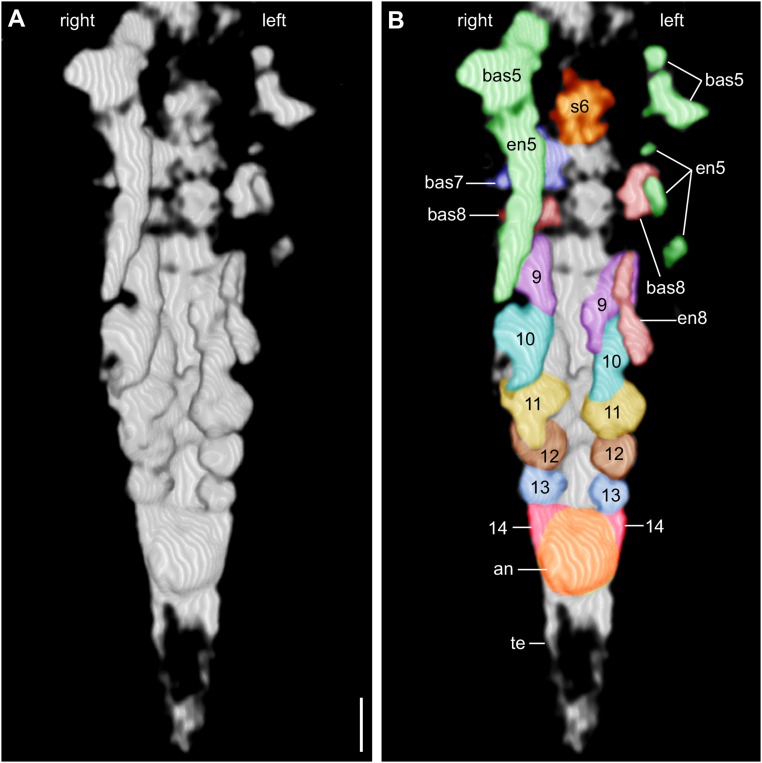
Slab b (YKLP 11088b) exhibits the animal from a dorsal perspective (Figs. 3 and 4). The anteriormost structure of the specimen shown on this slab is a somewhat rounded, 150-µm-long hypostome (Figs. 3_B_ and 4 C and F). Although the exopods of the first four pairs of biramous appendages (following the SGA) are well preserved on the surface of slabs a and b (Figs. 2_E_ and 3_D_), the endopods of these appendages are concealed within slab b and revealed by micro-CT (Fig. 4). Around seven podomeres (sensu 13), each 50–60 µm long, are evident in the endopod of post-SGA appendages 2–4 (Fig. 4 E and F). Post-SGA appendages 5 (left and right) and 8 (left) are incomplete, each exhibiting a basipod and posteriorly directed endopod consisting of several podomeres (see Fig. 4_F_ and Fig. S3). Exopods of these appendages were probably lost during fossilization, as were both branches of post-SGA appendage 6 left and right, 7 left, and 8 right (Fig. S3). All of the more posterior appendages are much less differentiated than the first eight post-SGA appendages: each of post-SGA appendages 9–13 appears as a single flap-like rudimentary Anlage, their length decreasing toward the posterior, suggesting a decreasing gradient of differentiation (Fig. S3). The rear end of the specimen is represented by a telson whose anterior part is an enlarged area (Fig. S3) that is thought to have accommodated the anus and whose posterior part is a dagger-like terminal end piece with several setae along its margin (Figs. 3 and 4). We identified a pair of flattened triangular structures (Fig. 4 E and F and Fig. S3) between the anus and appendage 13 that we interpret as rudimentary Anlagen of appendage 14.
Fig. S3.
(A) 3D model of the posterior portion of the 2-mm-long larva of L. illecebrosa rendered from micro-CT scans (slab b, YKLP 11088b). Ventral perspective. (B) Same model as in A, with interpretation. The paired appendage Anlagen 5–14 become smaller and less differentiated toward the rear. an, position of anus; bas, basipod; en, endopod; s, post-SGA segment. (Scale bar: 0.1 mm.)
Discussion
Tiny specimens such as that reported here are rare in the early Cambrian Chengjiang biota, which is dominated by flattened arthropod fossils in the size range of several centimeters (10). We interpret the specimen as a larva (sensu 12) of L. illecebrosa based on (i) the occurrence of adult specimens at the same locality (see Methods Summary); (ii) the spine-like armatures on the three-fingered SGA (Fig. 2 C and D)—a diagnostic character of the larval form of L. illecebrosa (12); and (iii) the overall morphology, including the telson with a dagger-like terminal end piece whose margin bears several setae (13) (Figs. 3 C and F and 4). However, another diagnostic feature of this species—the short, pointed rostrum that forms the front end of the head shield (10)—is absent in the larva specimen and may not have developed by this ontogenetic stage (Fig. 2 A_−_C).
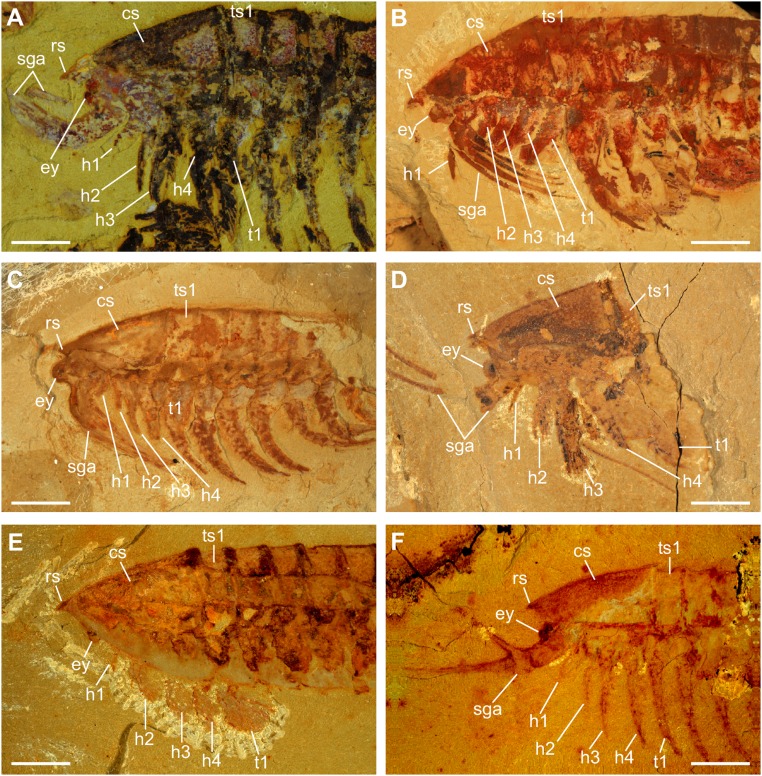
By combining the information from both slabs (see Results), we conclude that the first four pairs of biramous appendages of the larva are well developed and, most likely, fully functional. An oval space enclosed by the hypostome and the basal parts of post-SGA biramous appendages 1–4 would have played a role in feeding by gathering food items trapped by the SGAs (12) (Figs. 3_B_ and 4). The paddle-like exopods (Fig. 2_E_) were probably used for swimming, as in the adult (13). Although information about post-SGA appendages 5–8 is very limited due to the preservation of the specimen, an elongate endopod with several podomeres is evident in these appendages (Fig. S3). This suggests that post-SGA appendages 5–8 are also developed as limbs but not to the same extent as the more anterior ones (Fig. 4_F_ and Fig. S3). A significant size decrease is evident in post-SGA appendages 9–13, each of which is preserved as a small, unbranched flap (Fig. S3). Post-SGA appendage 14 is represented by just a pair of flattened triangular structures that are associated with a segment emerging from a putative growth zone located anterior to the anus. Such a strong size gradient is not observed in the appendages of the adult (10, 13) but is considered to be real, as all of the Anlagen are revealed by two or more different 3D softwares such as Drishti (Fig. 4) and Fiji (Fig. S3), and are presumably preserved in the same manner. Adults of L. illecebrosa have 11 trunk segments, each bearing a pair of biramous appendages (10, 13). Although previous interpretations identified three pairs of biramous appendages posterior to the SGA in the adult head (13), our recent investigation of new material suggests four pairs (Fig. S4). This gives a total of 15 pairs of biramous appendages in the adult, and further suggests that one additional appendage-bearing segment remained to emerge from the growth zone in the larva.
Fig. S4.
Anterior region of adult L. illecebrosa. Four pairs of biramous appendages (h1–h4) are identified in the head of various specimens. (A) YKLP 11089; (B) YKLP 11090; (C) YKLP 11091; (D) YKLP 11092; (E) YKLP 11093; (F) YKLP 11094. cs, cephalic shield; ey, eye; rs, rostrum; sga, short great appendage; t1, trunk appendage 1; ts1, trunk segment 1. (Scale bars: 3 mm.)
The developmental mode revealed by the minute larva of L. illecebrosa is similar to that in many extant eucrustaceans. Eucrustaceans (e.g., prawns, lobsters, brine shrimps, and barnacles) are so diverse morphologically that they share just a few specific and unique characters—one of which is the nauplius larva (14). Right after hatching, the nauplius (15) bears three pairs of functional appendages (14, 15). In subsequent stages, further segments and their appendages emerge from a growth zone in the posterior portion of the body (15, 16). The minute larva of L. illecebrosa resembles the late-stage metanauplius of certain eucrustaceans, especially anostracans (17), other branchiopods, and cephalocarids (14), in several aspects. First, the anterior (post-SGA segments 1–4) and posterior (post-SGA segments 5–14) portions of the body differ in outline (rounded vs. narrow and elongated; Fig. 3 and Fig. S2). This is not a result of flattening: The outline of soft-bodied fossils is not subject to distortion as they collapse during decay (18). Second, the anterior and posterior portions of the body also differ in the stage of development of the appendages (well developed and presumably fully functional vs. less developed (post-SGA appendages 5–8) and even rudimentary (post-SGA appendages 9–14); see Figs. 3 and 4 and Fig. S3.
The SGA arthropods are considered either as early representatives of chelicerates (11, 12, 19, 20) (e.g., sea spiders, horseshoe crabs, spiders, scorpions, and mites) or stem lineage representatives of all euarthropods (21) (chelicerates, myriapods, crustaceans, and insects). Therefore, this discovery represents the oldest example of well-developed appendages preserved in the larva of an early nonmandibulate arthropod—∼20 million years older than the larva of the pycnogonid Cambropycnogon klausmuelleri from the late Cambrian “Orsten” of Sweden (22). As L. illecebrosa is a euarthropod, the SGA is, most likely, homologous with the crustacean antennula and the chelicerate chelicera (21). Such forms are derived from arthropods with a larval series that involves adding body segments during development—a situation that is also suggested here for L. illecebrosa. Thus, the earliest larval stage of L. illecebrosa consists of fewer segments than the metanauplius-like example described here—reflecting the “head larva” hypothesis proposed by previous researchers (23). This hypothesis suggests that the ancestral euarthropod larva had an “antennula” plus three appendages, such as the condition in the early crustacean Henningsmoenicaris scutula (5), which became further derived to a nauplius-like larva in eucrustaceans and pycnogonids with antennula plus two appendages. An alternative, that the nauplius-like larva was ancestral in Euarthropoda, seems unlikely, as early noneucrustacean crustaceans also possessed a hatching larva with the antennula plus three pairs of appendages (5). In either case, however, the phylogenetic position of Leanchoilia (21) supports our conclusion that a larva with just a few anterior limb-bearing segments, which adds segments after hatching [anamorphosis (24)], not only occurs in eucrustaceans (8, 14) but was also present in all other major arthropod groups (25–27), i.e., in the ground pattern of Euarthropoda. Such indirect development with segment-poor larvae can be understood as the evolutionary trigger for an efficient distribution of early euarthropods.
Methods Summary
Materials.
The studied specimens (YKLP 11087, YKLP11088a, b, YKLP 11089–11094) are housed at the Yunnan Key Laboratory for Palaeobiology, Yunnan University, Kunming, China. Both specimens were collected from the Yu’anshan Member of the Chiungchussu Formation (Cambrian Series 2, Stage 3) at Mafang Village, Haikou, Kunming, Yunnan Province, China.
Imaging.
To observe as many details as possible, we used a combination of macrophotography (MP), scanning electron microscopy (SEM) and fluorescence microscopy (FM) to reveal the structures on the surface of the fossil slabs (Figs. 2 and 3). We also used X-ray micro-CT to resolve structures hidden inside the slabs (Figs. 2_F_ and 4). Digital MP images were captured with an MP-E 65-mm macro objective attached to a Canon EOS Rebel T3i digital camera. For SEM analyses, a Leo 1430 VP was used with 100-µm aperture, acceleration voltage of 30 kV, chamber pressure between 2 pa and 16 pa, and four quadrant back-scattered electron (QBSE) detector. Fluorescence photographs were generated as a composite fluorescence image with a Keyence BZ-9000 fluorescence microscope. A stack was documented for each image detail and fused to a sharp image with CombineZM/ZP; all fused images were combined to a single high-resolution image with Microsoft Image Composite Editor or the Photomerge function of Adobe Photoshop CS3. For micro-CT scanning, each slab was mounted on a holder made of tightly fitting plastic vials. A Phoenix Nanotom (GE Sensing & Inspection Technologies) cone beam CT scanner located at the Bavarian State Collection of Zoology, Munich, was used at a voltage of 140 kV and a current of 120 µA (Fig. 2_F_) and 100 kV/70 µA (Fig. 4 and Fig. S3) for 47 min each. A total of 1,440 radiographs were registered in each scan and saved as TIFF stacks for further processing with either Drishti (Figs. 2_F_ and 4) or Fiji (Fig. S3). The figures were arranged in Canvas Draw.
Acknowledgments
Y.L. thanks Prof. G. S. Boyan, Prof. G. Wörheide, and the Graduate School of Systemic Neuroscience, LMU Munich for support. We thank Prof. N. C. Hughes (University of California, Riverside) for helpful discussion. We are grateful for freely available software such as Drishti, Fiji, CombineZM, and OpenOffice. This study is funded by NSFC (U1302232 and 41372031 to X.-G.H.; 41528202 to Y.L.), and by the LMU Munich’s Institutional Strategy LMUexcellent within the framework of the German Excellence Initiative (Y.L.). J.T.H. is supported by the German Research Foundation (DFG HA 6300/3-1). C.H. is funded by the Bavarian Equal Opportunities Sponsorship of the LMU. D.E.G.B. is supported by NASA Astrobiology Institute (NNA13AA90A). M.K.H. is supported by the Studienstiftung des deutschen Volkes.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.M. is a guest editor invited by the Editorial Board.
References
- 1.Hall BK. Evo-Devo: Evolutionary developmental mechanisms. Int J Dev Biol. 2003;47(7-8):491–495. [PubMed] [Google Scholar]
- 2.Urdy S, Wilson LAB, Haug JT, Sánchez-Villagra MR. On the unique perspective of paleontology in the study of developmental evolution and biases. Biol Theory. 2013;8(3):293–311. [Google Scholar]
- 3.Hughes NC, Minelli A, Fusco G. The ontogeny of trilobite segmentation: A comparative approach. Paleobiology. 2006;32(4):602–627. [Google Scholar]
- 4.Walossek D. The Upper Cambrian Rehbachiella and the phylogeny of Branchiopoda and Crustacea. Fossils Strata. 1993;32:1–202. [Google Scholar]
- 5.Haug JT, Maas A, Waloszek D. †Henningsmoenicaris scutula, †Sandtorpia vestrogothiensis gen. et sp. nov. and heterochronic events in early crustacean evolution. Trans R Soc Edinburgh Earth Sci. 2010;100(3):311–350. [Google Scholar]
- 6.Zhang X-G, Maas A, Haug JT, Siveter DJ, Waloszek D. A eucrustacean metanauplius from the Lower Cambrian. Curr Biol. 2010;20(12):1075–1079. doi: 10.1016/j.cub.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Haug JT, Martin JW, Haug C. A 150-million-year-old crab larva and its implications for the early rise of brachyuran crabs. Nat Commun. 2015;6:6417. doi: 10.1038/ncomms7417. [DOI] [PubMed] [Google Scholar]
- 8.Waloszek D, Maas A. The evolutionary history of crustacean segmentation: A fossil-based perspective. Evol Dev. 2005;7(6):515–527. doi: 10.1111/j.1525-142X.2005.05056.x. [DOI] [PubMed] [Google Scholar]
- 9.Maas A, et al. The ‘Orsten’—More than a Cambrian Konservat-Lagerstätte yielding exceptional preservation. Palaeoworld. 2006;15(3-4):266–282. [Google Scholar]
- 10.Hou X-G, Bergström J. Arthropods of the Lower Cambrian Chengjiang fauna, southwest China. Fossils Strata. 1997;45:1–120. [Google Scholar]
- 11.Chen J-Y, Waloszek D, Maas A. A new ‘great-appendage’ arthropod from the Lower Cambrian of China and homology of chelicerate chelicerae and raptorial antero-ventral appendages. Lethaia. 2004;37(1):3–20. [Google Scholar]
- 12.Liu Y, Haug JT, Haug C, Briggs DEG, Hou X-G. A 520 million-year-old chelicerate larva. Nat Commun. 2014;5:4440. doi: 10.1038/ncomms5440. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Hou X-G, Bergström J. Chengjiang arthropod Leanchoilia illecebrosa (Hou, 1987) reconsidered. GFF. 2007;129(3):263–272. [Google Scholar]
- 14.Martin JW, Olesen J, Høeg JT. The crustacean nauplius. In: Martin JW, Olesen J, Høeg JT, editors. Atlas of Crustacean Larvae. Johns Hopkins Univ Press; Baltimore: 2014. pp. 8–16. [Google Scholar]
- 15.Haug C, Haug JT, Maas A, Waloszek D. Fossil larvae (head larvae, nauplii, and others) from the Cambrian in Orsten preservation. In: Martin JW, Olesen J, Høeg JT, editors. Atlas of Crustacean Larvae. Johns Hopkins Univ Press; Baltimore: 2014. pp. 17–26. [Google Scholar]
- 16.Dohle W, Gerberding M, Hejnol A, Scholtz G. Cell lineage, segment differentiation, and gene expression in crustaceans. In: Scholtz G, editor. Evolutionary Developmental Biology of Crustacea. A.A. Balkema; Lisse, The Netherlands: 2004. pp. 95–133. [Google Scholar]
- 17.Olesen J. Anostraca. In: Martin JW, Olesen J, Høeg JT, editors. Atlas of Crustacean Larvae. Johns Hopkins Univ Press; Baltimore: 2014. pp. 29–35. [Google Scholar]
- 18.Briggs DEG, Williams SH. The restoration of flattened fossils. Lethaia. 1981;14(2):157–164. [Google Scholar]
- 19.Tanaka G, Hou X-G, Ma X-Y, Edgecombe GD, Strausfeld NJ. Chelicerate neural ground pattern in a Cambrian great appendage arthropod. Nature. 2013;502(7471):364–367. doi: 10.1038/nature12520. [DOI] [PubMed] [Google Scholar]
- 20.Haug JT, Waloszek D, Maas A, Liu Y, Haug C. Functional morphology, ontogeny and evolution of mantis shrimp-like predators in the Cambrian. Palaeontology. 2012;55(2):369–399. [Google Scholar]
- 21.Legg DA, Sutton MD, Edgecombe GD. Arthropod fossil data increase congruence of morphological and molecular phylogenies. Nat Commun. 2013;4:2485. doi: 10.1038/ncomms3485. [DOI] [PubMed] [Google Scholar]
- 22.Waloszek D, Dunlop JA. A larval sea spider (Arthropoda: Pycnogonida) from the Upper Cambrian Orsten of Sweden, and the phylogenetic position of pycnogonids. Palaeontology. 2002;45(3):421–446. [Google Scholar]
- 23.Walossek D, Müller KJ. Cambrian ‘Orsten’-type arthropods and the phylogeny of Crustacea. In: Fortey RA, Thomas RH, editors. Arthropod Relationships. Chapman & Hall; London: 1998. pp. 139–153. [Google Scholar]
- 24.Minelli A, Fusco G. Arthropod post-embryonic development. In: Minelli A, Boxshall G, Fusco G, editors. Arthropod Biology and Evolution. Springer; Heidelberg: 2013. pp. 91–122. [Google Scholar]
- 25.Brenneis G, Arango CP, Scholtz G. Morphogenesis of Pseudopallene sp. (Pycnogonida, Callipallenidae) II: Postembryonic development. Dev Genes Evol. 2011;221(5-6):329–350. doi: 10.1007/s00427-011-0381-5. [DOI] [PubMed] [Google Scholar]
- 26.Minelli A, Sombke A. Chilopoda–Development. In: Minelli A, editor. Treatise on Zoology–Anatomy, Taxonomy, Biology–The Myriapoda I. Brill; Leiden, The Netherlands: 2011. pp. 295–308. [Google Scholar]
- 27.Tipping C. 2008. Proturans (Protura). Encyclopedia of Entomology, ed Capinera JL (Springer, New York), Vol 3, pp 3062–3064.